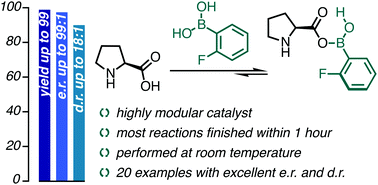
In this work, we exploit our strategy of in situ secondary-sphere modification of organocatalysts to improve the reactivity and selectivity of amino catalysts. Herein, the carboxylic acid moiety of proline was targeted as a site for modification under catalytic reaction conditions with boronic acids. Intermolecular aldol reactions between aromatic aldehydes and cyclopentanone were selected as a proof-of-concept because cyclopentanone as an aldol donor was often associated with decreased selectivity compared to its 6-membered ring analog, hexanone. Our secondary-sphere modification strategy, using naturally occurring L-proline amino acid, enabled reactions at room temperature with high levels of diastereo- and enantio-selectivity and short reaction times. NMR and HR-MS studies shed light on the nature of the catalyst structure and on the role of water in our reactions.