Nanosheet floral clusters of Fe-doped Co3O4 for high-performance supercapacitors†
Congcong
Lu
,
Yu
Yang
,
Songjun
Li
and
Maiyong
Zhu
 *
*
School of Materials Science & Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: lsjchem@ujs.edu.cn; maiyongzhu@ujs.edu.cn
First published on 1st April 2024
Abstract
Because of their superior power and energy densities, supercapacitors have garnered considerable amounts of attention in energy storage technologies. Co3O4 is considered as a regularly used electrode material for supercapacitors because of its high theoretical capacitance and outstanding electrochemical activity. However, owing to the Co3O4 electrode materials’ rapid capacity decay and low intrinsic conductivity, it is challenging to achieve high theoretical capacitance. As a result, doping Co3O4 with metal components can enhance its electrical and surface characteristics while also raising its conductivity. Through a straightforward hydrothermal reaction and subsequent thermal decomposition, a series of Fe doping into Co3O4 has been successfully realized in this paper. This generates a nanosheet floral cluster structure and exposes more active sites, and the two metal elements work in concert to improve the capacitance performance. The electrochemical performance of the Co-0.2Fe-450 electrode material appears satisfactory when the doping amount of Fe is 0.2 mmol and its thermal decomposition temperature is 450 °C. The specific capacitance at 1 A g−1 is 680 F g−1. The capacitance retention rate at 10 A g−1 current density after 5000 charge–discharge cycles is 84.67%. Notably, the assembled asymmetric supercapacitor retains 105.5% of its capacitance after 5000 cycles and has a power density of 803.13 W kg−1 and an energy density of 17.78 W h kg−1. Fe-doped Co3O4 has a strong future in energy storage, as evidenced by its exceptional electrochemical characteristics.
1. Introduction
Excessive use of traditional fossil fuels increases the cumulative emission of greenhouse gases, leading to serious disturbances in the ecological balance, and it is necessary to use new clean and renewable sources of energy and to take a green development path in order to achieve the major strategic goal of “dual carbon” for sustainable development.1–3 Research is now focused on finding appropriate energy storage devices so that power produced by sustainable energy can be easily stored and used whenever and wherever it is needed.4,5 The two most common kinds of energy storage devices are supercapacitors and batteries. Supercapacitors typically have a higher power density than batteries. They also offer the benefits of a long cycle life, excellent safety, and quick charging and discharging speeds.6–8 In particular, electrode materials are a crucial component and essential component of supercapacitors. Supercapacitor electrode materials can be broadly classified into three categories: carbon-based materials,9 conductive polymers,10 and compounds based on transition metals.11 Each category has pros and cons of its own. Therefore, the development of electrode materials with a distinctive structure and form as well as excellent performance is extremely crucial to improve the electrochemical performance of supercapacitors.12,13The most popular electrode materials for supercapacitors are transition metal oxides (TMOs),14–17 which include NiO,18 CuO,19 MnO2,20 Fe3O4,21 Co3O4,22 and others. These materials offer several benefits, including rich oxidation states, great mechanical stability, and outstanding electrochemical activity.23,24 Among them, Co3O4 is a significant metal oxide that has found widespread application as an electrode material for supercapacitors due to its high theoretical capacitance, outstanding reversible REDOX behavior, low cost, and excellent environmental friendliness.25,26 However, its actual applicability in supercapacitors is restricted due to its drawbacks, including weak conductivity, structural stacking, and poor REDOX reversibility; also, its capacitance is substantially lower than its theoretical value of 4292 F g−1.27 A variety of tactics have been used by researchers to overcome these problems. To increase the conductivity of Co3O4 electrode materials and buffer volume expansion during charge and discharge, for instance, cover carbon materials or conductive polymers. This coating method's primary drawbacks include its more complicated electrode material manufacturing, easily broken coating, and challenging large-scale production.28 Another effective strategy is to prepare composite electrode materials, which have better properties due to their synergistic effects. In an effort to enhance the electrochemical behavior of metal oxide electrode materials, researchers have recently combined metal oxides with carbon substrates. This combination increases the specific surface area and reactive site of the material.29 For example, Madhu et al.30 prepared cellular porous carbon–Co3O4 nanocomposites (PSAC/Co3O4) using seed shells as carbon sources (PSAC), which have significant specific capacitance and excellent long-term cyclic stability. Zou et al.31 fabricated a layered electrode structure (Co3O4/NGF) composed of Co3O4 and nitrogen-doped graphene foam (NGF). In electrochemical measurements, the capacitive performance of the Co3O4/NGF electrode was increased from 320 F g−1 of the original Co3O4 to 451 F g−1 with excellent rate capability. Although the composite electrode material can make up for the defects of a single material and improve the electrochemical performance, the contact resistance between the composite components may increase the total resistance of the material, and the preparation process is complex, which is not conducive to large-scale production in a short time. Doping TMOs with additional elements has shown to be a successful method for resolving these issues, as it enhances the intrinsic conductivity and electrical characteristics of TMOs while also increasing their stability.32,33 Wang et al.34 synthesized Fe-doped MnO2 nanostructures, which showed significantly improved specific capacitance, magnanimity, and cycle life compared to undoped MnO2. Co3O4 has a spinel structure, and the introduction of Fe doping can exert the synergistic effect of the two substances to modify the electronic structure of Co3O4, expose more reactive sites, and thus improve the conductivity and electrochemical performance. Wang et al.35 prepared Fe-doped Co3O4/graphene nanocomposites by a hydrothermal method. The specific capacitance reached 671 F g−1 at a current density of 5 A g−1, and the capacitance remained at 98.7% after 1000 cycles, which proved to be a significant candidate for doping.
Therefore, in this study, Fe-doped Co3O4 nanosheet floral cluster structures were prepared by a simple hydrothermal method and heat treatment reaction. Due to the doping of Fe elements and the excellent microstructure, the electrode material prepared has excellent conductivity and large specific surface area, and shows an excellent electrochemical performance in supercapacitors. The specific capacitance at 1 A g−1 in the three-electrode system can reach 680 F g−1, and after 5000 cycles of charge and discharge at 10 A g−1, the capacitance retention rate is 84.67%. To build asymmetric supercapacitors, activated carbon (AC) serves as the negative electrode and iron-doped Co3O4 as the positive electrode. Even after 5000 cycles, the capacitance retention may reach 105.5% when the power density is 803.1 W kg−1 and the energy density is 17.78 W h kg−1. The remarkable cycle stability and electrochemical performance of iron-doped Co3O4 electrode materials validate their extensive potential for use in supercapacitor energy storage devices.
2. Experimental section
2.1. Materials
Co(NO3)2·6H2O, Fe(NO3)3·9H2O, urea and ammonium fluoride are all purchased from Shanghai Aladdin Biochemical Materials Co., Ltd. All chemicals are not purified and belong to the analytical grade. Deionized water is prepared in the laboratory.2.2. Method of synthesis
The Fe-doped Co3O4 was made via a straightforward hydrothermal process. A homogeneous solution was formed by dissolving 2 mmol of Co(NO3)2·6H2O (0.582 g), 0.2 mmol of Fe(NO3)3·9H2O (0.081 g), 10 mmol of urea (0.601 g) and 6 mmol of NH4F (0.222 g) in 65 mL of deionized water and stirring for 30 minutes. The resulting solution was then transferred to a 100 mL Teflon lined reactor and heated in an oven to 120 °C for 8 h. At the end of the reaction, the solution was allowed to cool naturally to room temperature. It was then washed two to three times with ethanol and deionized water and dried under vacuum at 60 °C for 12 h. The dried powder samples were annealed under nitrogen at 450 °C for 2 h. The effects of different Fe (NO3)3·9H2O contents (0, 0.2, 0.4, and 0.6 mmol) and annealing temperatures (T = 350, 450, and 600 °C) on the electrochemical performance of supercapacitors were also investigated.2.3. Materials characterization
(1) X-ray diffraction (XRD) was performed using an XRD diffractometer (D8 ADVANCE), using JADE to characterize the crystal structure composition of the active substance. In the experimental test, the test angle is 10–80° and the scan rate is 10° min−1. (2) Using field emission scanning electron microscopy (SEM), a small quantity of the active ingredient (powder) was dissolved and ultrasonically dispersed in ethanol, bound to a conductive adhesive, sprayed with gold, and its shape was examined. (3) Transmission electron microscopy (TEM), which involves dissolving a little quantity of an active material in ethanol for ultrasonic dispersion, adding two to three drops to a copper mesh, letting it dry naturally, and using a transmission electron microscope to view the samples' internal microstructure. (4) X-ray photoelectron spectroscopy (XPS), which typically uses X-ray excitation to determine the chemical state of an active material by examining the inner or valence electrons of its atoms or molecules. (5) Specific surface area analysis and determination (BET), through the specific surface area analysis and measurement instrument to test the specific surface area and aperture distribution of active substances. (6) Fourier transform infrared absorption spectroscopy (FTIR), analytical reagent for KBr, analysis of functional group changes of active substances.2.4. Electrochemical measurements
Three electrode test: First, the active substance, acetylene black, and polyvinylidene fluoride (PVDF), were mixed uniformly in N-methylpyrrolidone (0.015, 0.003, and 0.002 g) and coated on nickel foam with a coating quantity of 3–4 mg cm−2. After pressing and drying, a working electrode was produced. An electrochemical test of the three-electrode system was performed using a 1 M KOH alkaline aqueous solution as the electrolyte, a saturated calomel electrode as the reference electrode, and a platinum foil electrode as the electrical level.Dual electrode test: commercial activated carbon (AC) is employed as the negative electrode material, and the active material working electrode that was previously created serves as the positive electrode. A series of electrochemical experiments are conducted in a 1 M KOH electrolyte, and the fabrication procedure for the negative electrode material is comparable to that for the active electrode material.
The specific capacity (C, F g−1), energy density (E, W h kg−1) and power density (P, W K g−1) are calculated as follows:36,37
 | (1) |
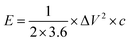 | (2) |
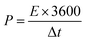 | (3) |
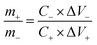 | (4) |
3. Results and discussion
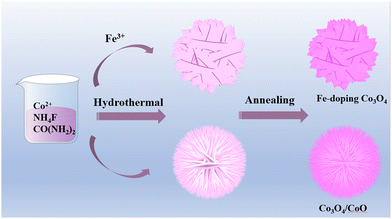
The preparation process of Fe-doped Co3O4 layered nanosheet floral cluster structure is shown in Fig. 1. First, a straightforward hydrothermal process was used to dissolve Co2+, Fe3+, urea, and ammonium fluoride in deionized water to create the CoFe precursor. Thermal reduction of Fe3+ produced the Fe-doped Co3O4 flake-like floral cluster structure after the precursor was annealed at high temperature under N2 protection. Then, without adding Fe3+, Co3O4/CoO composite sea urchin-like structures were produced. It is important that the electrochemical characteristics are greatly impacted by annealing temperature changes and the addition of various concentrations of Fe3+.The sample's crystal structure and phase composition were examined using XRD. The impact of varying doping concentrations on the crystal structure of Co3O4 at 450 °C is seen in Fig. 2a. The sample is mostly Co3O4 (PDF No. 42-1467) when the iron source is not doped, with a minor quantity of CoO (PDF No. 48-1719). The manufactured Co-0.2Fe-450 and Co-0.4Fe-450 demonstrate the presence of metal Fe in 44.8° and 44.6°. The angle moves to the left with increasing Fe doping concentration. Moreover, the Co-0.6Fe-450 sample displayed FexC at 42.5°, which can be the result of the iron and carbon atoms’ strong binding force at high temperatures, which makes it simple to create interstitial compounds. A local enlargement of the material is shown in Fig. 2b. The lattice spacing rises and the diffraction peak changes to the left as a result of the varied Fe doping replacing Co2+, indicating the successful doping of Fe elements.
The FT-IR absorption spectra of several materials following heat treatment at 450 °C are displayed in Fig. S1 (ESI†). The broad peaks centered at 3440 cm−1 in all samples represent the –OH absorbed by the water molecules in the sample, while the peaks at 1633 cm−1 represent the C–O bond's stretching vibration. Furthermore, in the Co-0Fe-450 samples, the stretching vibration absorption peaks of Co3O4 were seen at 665 cm−1 and 658 cm−1, suggesting that Co3O4 was the predominant product at this point in time.38 The Fe doped sample's characteristic peak is nearly identical to Co3O4's and does not alter Co3O4's functional group structure, suggesting that the two materials interact non-covalently and providing preliminary evidence of the material's successful production.
The chemical state and elemental composition of the samples were examined using XPS. The full XPS spectra of the Co-0Fe-450 and Co-0.2Fe-450 samples are shown in Fig. 3a. There is no signal peak of Fe in the full XPS spectrum of the Co-0Fe-450 sample, while the XPS spectrum of the Co-0.2Fe-450 sample shows the simultaneous presence of the elements Co, Fe, C, and O, which confirms that the element Fe is successfully doped into Co3O4. C1s spectra are shown in Fig. S2 (ESI†), and the peaks located at 284.8, 286.3, and 288.6 eV correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N and C–O bonds, and high-temperature thermal decomposition is the main source of the presence of C elements. The O 1s spectra of the two groups of samples are shown in Fig. 3b. The three signal peaks in the figure represent surface adsorbed water molecular bonds (H–O–H), oxygen defect bonds (M–O–H), and metal–oxygen–metal bonds (M–O–M),39 respectively. The Co 2p spectra, which have two prominent peaks with binding energies of 780.2 eV and 795.4 eV for Co 2p3/2 and Co 2p1/2, can be seen in Fig. 3c. The two main peaks can be simulated to synthesize four peaks, among which 780.1 eV and 795.2 eV correspond to Co3+, and the peaks at 781.6 eV and 796.8 eV correspond to Co2+, indicating the successful formation of Co3O4.40 Interestingly, the signal peaks of Co-0Fe-450 and Co-0.2Fe-450 samples did not shift significantly indicating that the chemical environment of the atoms inside the material had not changed. Fig. 3d is the Fe 2p spectrum, and the combined energy corresponds to two main peaks of Fe 2p3/2 and Fe 2p1/2 at 712.8 eV and 724.3 eV, where 710.7 eV and 722.0 eV correspond to Fe0, and 712.9 eV and 724.4 eV correspond to Fe2+. 715.2 eV and 727.8 eV correspond to Fe3+, and 718.2 eV and 732.7 eV are a set of satellite peaks.41 The test results of XPS further indicate that Fe is successfully doped in Co3O4.
C, C–N and C–O bonds, and high-temperature thermal decomposition is the main source of the presence of C elements. The O 1s spectra of the two groups of samples are shown in Fig. 3b. The three signal peaks in the figure represent surface adsorbed water molecular bonds (H–O–H), oxygen defect bonds (M–O–H), and metal–oxygen–metal bonds (M–O–M),39 respectively. The Co 2p spectra, which have two prominent peaks with binding energies of 780.2 eV and 795.4 eV for Co 2p3/2 and Co 2p1/2, can be seen in Fig. 3c. The two main peaks can be simulated to synthesize four peaks, among which 780.1 eV and 795.2 eV correspond to Co3+, and the peaks at 781.6 eV and 796.8 eV correspond to Co2+, indicating the successful formation of Co3O4.40 Interestingly, the signal peaks of Co-0Fe-450 and Co-0.2Fe-450 samples did not shift significantly indicating that the chemical environment of the atoms inside the material had not changed. Fig. 3d is the Fe 2p spectrum, and the combined energy corresponds to two main peaks of Fe 2p3/2 and Fe 2p1/2 at 712.8 eV and 724.3 eV, where 710.7 eV and 722.0 eV correspond to Fe0, and 712.9 eV and 724.4 eV correspond to Fe2+. 715.2 eV and 727.8 eV correspond to Fe3+, and 718.2 eV and 732.7 eV are a set of satellite peaks.41 The test results of XPS further indicate that Fe is successfully doped in Co3O4.
Using the N2 adsorption–desorption method, the specific surface area and mesoporous characteristics of several samples were examined. The outcome is displayed in Fig. 4. A type IV hysteresis loop with clear mesoporous features was seen in every sample. Co-0Fe-450, Co-0.2Fe-450, Co-0.4Fe-450, and Co-0.6Fe-450 have specific surface areas of 13.12, 43.00, 42.10, and 37.97 m2 g−1, in that order. The findings demonstrated that following Fe doping, the electrode material's specific surface area greatly increased. Dynamic ion/electron rapid transport channels are generated, and more exposed active sites are revealed. The Co-0.2Fe-450 sample has the most specific surface area of any of them. The average pore size of the four sets of samples is 13.01 nm, 9.58 nm, 8.40 nm, and 10.41 nm, respectively, as shown by the pore size distribution diagram in Fig. 4, which illustrates a mesoporous structure. The aforementioned study's findings demonstrate that high temperatures promote the formation of pore structures. Interestingly the surface area of Co-0.2Fe decreased significantly (23.87 m2 g−1) after high temperature thermal decomposition at 600 °C, which can be attributed to the fact that with the increase of the thermal decomposition temperature, the morphological structure of the Co-0.2Fe-600 samples was disrupted, shrinkage occurred and larger particles were produced (Fig. S3, ESI†). The above findings suggest that the formation of mesoporous pore structure can be induced by thermal decomposition reaction at the appropriate temperature.42,43 In addition, the nanosheet flower cluster structure formed by Fe doping can effectively increase the contact area between the electrolyte and the electrode material, which is favorable for the electrochemical performance.
 | ||
| Fig. 4 N2 adsorption desorption curves for (a) Co-0Fe-450, (b) Co-0.2Fe-450, (c) Co-0.4Fe-450, (d) Co-0.6Fe-450. (Inset: pore size distribution map). | ||
SEM was used to examine the morphology and structure of the material. As shown in Fig. 5a, in the absence of Fe doping, the Co-0Fe samples exhibit anisotropic radial nano-needle-like structures similar to those of sea urchins. With the addition of Fe, a lamellar structure was formed and the nanosheets were interspersed to form floral clusters accompanied by a small number of rod-like structures. It is noteworthy that the morphology and structure of the samples did not change significantly with the increase of iron content (Fig. 5b and Fig. S4, ESI†). As shown in Fig. S5 (ESI†), after thermal decomposition at 450 °C, the morphology of the Co-0Fe samples without Fe-doped elements changed from the original nano-needle structure to a rod-like structure, and the surface became rough. The morphology and structure of the Co-0.2Fe-450 and Co-0.4Fe-450 samples have good stability. However, the Co-0.6Fe-450 sample experienced a collapse of the structure, which may be due to the fact that the addition of more Fe elements drastically affects the morphology and structure of the product. Elemental mapping analysis of the Co-0.2Fe-450 sample (Fig. 5c) demonstrated that the Co, Fe and O elements were uniformly distributed in the Co-0.2Fe-450 sample, which further indicated that Fe was successfully doped into the product (Fig. 5d–f). In addition, we investigated the effect of different temperatures on the appearance and morphology of Co-0.2Fe samples. As can be seen from Fig. S6 (ESI†), at 350 °C, the surface of the sample became rough and a large number of particles were generated; at 600 °C, the structure of the sample collapsed, which could be attributed to the high thermal decomposition temperature, which made the structure of the sample unstable and possibly fractured. Meanwhile, due to the addition of urea and NH4F during the sample preparation, the nucleation and crystal growth processes of the samples were well regulated by the alkaline solution.
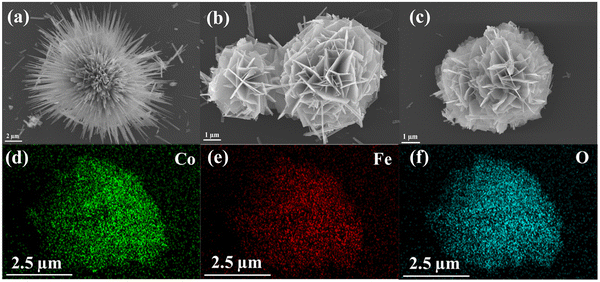 | ||
| Fig. 5 SEM images of (a) Co-0Fe, (b) Co-0.2Fe and (c) Co-0.2Fe-450. (d)–(f) Elemental mapping of Co-0.2Fe-450. | ||
Transmission electron microscopy (TEM) was used to further investigate the Co-0.2Fe-450 sample's structural properties. The floral-cluster Co-0.2Fe-450, as shown in Fig. 6a and b, was made up of ultra-thin nanosheets. The homogenous nanosheets helped to preserve the sample structure's regularity, which was in line with the SEM results. The lattice fringe of Co-0.2Fe-450 is depicted in Fig. 6c. Lattice fringes with crystal face spacing of 0.286 nm correspond to the (220) plane of Co-0.2Fe-450. The rapid Fourier transform is used to extract the selected electron diffraction pattern (SAED) in Fig. 6d from the selected region in the HRTEM. The diffraction rings in the image correspond to the (222), (440), and (422) crystal faces of Co-0.2Fe-450, respectively, which correspond to the standard XRD cards. The figure shows that polycrystal features are seen.
 | ||
| Fig. 6 (a) and (b) TEM images, (c) high-resolution TEM images, (d) selected electron diffraction patterns of Co-0.2Fe-450. | ||
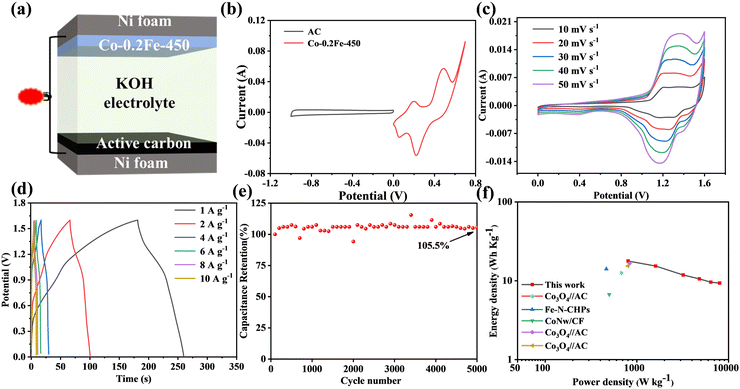
Three electrode electrochemical tests were conducted on the samples to verify that Fe doping is a successful method of improving the electrochemical characteristics of Co3O4 materials. Fig. S7 (ESI†) displays the cyclic voltammetry (CV) and constant current charge and discharge (GCD) curves for Co-0Fe, Co-0.2Fe, Co-0.4Fe, and Co-0.6Fe. The fact that each set of CV curves contains a pair of clearly visible cathode and anode peaks as well as a clearly visible charging and discharging platform in the GCD curve suggests that the materials can have some of the properties of batteries. Co-0.2Fe is selected as the precursor material due to the fact that the figure shows that it has the highest specific capacitance. The CV and GCD curves of products produced at various temperatures during the thermal decomposition process are displayed in Fig. 7a and b. It is assumed that the CV curve of Co-0.2Fe-450 has the highest specific capacitance due to its biggest closed area. The specific capacitance of Co-0.2Fe-450 is up to 680 F g−1 at 1 A g−1, as shown in the GCD curve, which is much superior to that of Co-0Fe (288 F g−1) and Co-0.2Fe-350 (590 F g−1). Co-0.2Fe (400 F g−1) and Co-0.2Fe-600 (450 F g−1) demonstrated the accuracy of the CV curve's derivation. The Nyquist diagram, which is depicted in Fig. 7c, exhibits charge transfer resistance in the high frequency zone and diffusion resistance in the low frequency region. With a steeper slope at low frequencies, the produced Co-0.2Fe-450 material displays diffusion-controlled capacitive behavior, suggesting quicker charge transfer and diffusion between the electrode material and the electrolyte. The CV curve of Co-0.2Fe-450 is shown in Fig. 7d. When the sweep speed increases from 10 mV s−1 to 50 mV s−1, the anode peak moves to high potential and the cathode peak moves to low potential, and the area of the CV curve remains basically unchanged, indicating that the Co-0.2Fe-450 electrode material has excellent magnification performance. The specific capacitance of Co-0.2Fe-450, as illustrated in Fig. 7e, is 680, 540, 424, 372, 328, and 302 F g−1, respectively, under current densities of 1, 2, 4, 6, 8, and 10 A g−1. In comparison to other comparable electrode materials indicated in Table 1, this suggests exceptional capacitive performance that is noticeably superior. As shown in Fig. S8 (ESI†), when the current density was increased from 1 A g−1 to 10 A g−1, the specific capacitance decreased from 680 F g−1 to 295.5 F g−1 with a capacitance retention of 43.5%. The Co-0.2Fe-450 sample's specific capacitance retention after 5000 cycles at a current density of 10 A g−1 is 84.67% of the starting value (Fig. 7f). Table 2 lists the stability properties of other similar materials reported in recent years, and it can be seen that Co-0.2Fe-450 has strong cycling stability. Fe doping is a simple and effective way to enhance the electrochemical performance of Co3O4 electrode materials.
| Electrode material | Electrolyte | Current density | Specific capacitance | Ref. |
|---|---|---|---|---|
| Co-0.2Fe-450 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1 A g−1 | 680 F g−1 | This work |
| Mn0.05Co2.95O4 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1 A g−1 | 80.8 F g−1 | 44 |
| Co3O4 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1 A g−1 | 523 F g−1 | 45 |
| Co3O4-T | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1 A g−1 | 216.4 F g−1 | 46 |
| Mn–Co3O4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1.5 A g−1 | 537![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F g−1 F g−1 |
47 |
| Co3O4-NLIG | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
0.5 mA cm−2 | 216.3 F g−1 | 48 |
| Co3O4/N-CP | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
— | 316.2 F g−1 | 49 |
| Fe–Co3O4 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1 A g−1 | 153 F g−1 | 50 |
| Co3O4@CSCN | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1 A g−1 | 508 F g−1 | 51 |
| Electrode material | Electrolyte | Current density | Cycle number | Capacitance retention | Ref. |
|---|---|---|---|---|---|
| Co-0.2Fe-450 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
10 A g−1 | 5000 | 84.67% | This work |
| Co3O4@N/C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
1 A g−1 | 5000 | 83% | 52 |
| N-Co3O4 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
10 A g−1 | 3000 | 80.1% | 53 |
| O67/C67/CC | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
6000 | 70% | 54 | |
| Ni–Co3O4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
5 A g−1 | 1000 | 89.3% | 54 |
| Mn–Co3O4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M KOH M KOH |
5 A g−1 | 5000 | 73.9% | 55 |
| Mn–Co3O4 | 2 M KOH | 5 A g−1 | 2000 | 71.2% | 56 |
| S-ZnCo2O4 | 2 M KOH | 5 A g−1 | 5000 | 78% | 57 |
| S-NixCo3−xO4 | 2 M KOH | 4 A g−1 | 2000 | 80.8% | 58 |
The two-electrode test is used to confirm the electrode material's practical applicability. Due to its high voltage window, commercial activated carbon (AC) is typically employed as a negative material for supercapacitors. In order to determine the mass ratio of Co-0.2Fe-450 to AC, an electrochemical test of AC was first performed in a three-electrode system. The CV curve of AC, as illustrated in Fig. S9 (ESI†), exhibits an essentially rectangular shape that is typical of carbon-based materials. There is no discernible degradation in the curve shape as sweep speed increases. The GCD curve has double electric layer characteristics and is triangular in shape. It lacks an evident charging and discharging platform. The specific capacitance under a current density of 1 A g−1 is 120 F g−1, and eqn (4) may be used to derive the mass ratio of CO-0.2Fe-450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AC = 0.18. Fig. 8a shows an asymmetric supercapacitor assembled with Co-0.2Fe-450 and AC (Co-0.2Fe-450//AC ASC). Fig. 8b shows the operating voltage window of Co-0.2Fe-450 and AC. Among them, the voltage window of Co-0.2Fe-450 electrode ranges from 0 to 0.7 V, and the potential window of AC electrode ranges from −1 to 0 V. The two have the characteristics of battery capacitor and double layer capacitor respectively when the sweep speed is 20 mV s−1, further confirming the operating voltage window of ASC, as shown in Fig. S10 (ESI†). When the voltage window increases to 1.7 V, the curve becomes more polarized, so it is determined that the ASC's operating voltage window is 0–1.6 V. CV curves of ASC at different sweep speeds are shown in Fig. 8c. With the increase of sweep speed, CV curves basically remain unchanged, which proves that ASC has excellent magnification performance. In addition, according to the shape of CV curve, it can be observed that the double layer and Faraday reaction exist simultaneously. Fig. 8d displays the ASC GCD curve at various current densities. Under current densities of 1, 2, 4, 6, 8, and 10 A g−1, the specific capacitance of ASC is 50, 44, 35, 30, 27.5, and 25 F g−1, in that order. Fig. S11 (ESI†) shows the relationship between the current density and the specific capacitance. When the current density increases from 1 A g−1 to 10 A g−1, the capacitance retention rate is 50%, which has a good magnification performance. After 5000 cycles of charging and discharging, the material activation of the ASC electrode material causes the capacitance retention rate to reach 105.5% at a current density of 10 A g−1. It has satisfactory cycle stability. Eqn (2) and (3) can be used to obtain the energy density and power density of ASC, as seen in Fig. 8f. The ASC can achieve a maximum energy density of 17.78 W h kg−1 and a power density of 803.13 W kg−1. Furthermore, the energy density can reach 3.35 W h kg−1 at a high power density of 8014.28 W kg−1. These outcomes significantly surpass those of other analogous electrode materials that have been previously documented, including Co3O4//AC,59 Fe-N-CHPs,60 Co3O4//AC,61 CoNW//CF,62 and Co3O4//AC.63The practical application of ASC is further proved.
AC = 0.18. Fig. 8a shows an asymmetric supercapacitor assembled with Co-0.2Fe-450 and AC (Co-0.2Fe-450//AC ASC). Fig. 8b shows the operating voltage window of Co-0.2Fe-450 and AC. Among them, the voltage window of Co-0.2Fe-450 electrode ranges from 0 to 0.7 V, and the potential window of AC electrode ranges from −1 to 0 V. The two have the characteristics of battery capacitor and double layer capacitor respectively when the sweep speed is 20 mV s−1, further confirming the operating voltage window of ASC, as shown in Fig. S10 (ESI†). When the voltage window increases to 1.7 V, the curve becomes more polarized, so it is determined that the ASC's operating voltage window is 0–1.6 V. CV curves of ASC at different sweep speeds are shown in Fig. 8c. With the increase of sweep speed, CV curves basically remain unchanged, which proves that ASC has excellent magnification performance. In addition, according to the shape of CV curve, it can be observed that the double layer and Faraday reaction exist simultaneously. Fig. 8d displays the ASC GCD curve at various current densities. Under current densities of 1, 2, 4, 6, 8, and 10 A g−1, the specific capacitance of ASC is 50, 44, 35, 30, 27.5, and 25 F g−1, in that order. Fig. S11 (ESI†) shows the relationship between the current density and the specific capacitance. When the current density increases from 1 A g−1 to 10 A g−1, the capacitance retention rate is 50%, which has a good magnification performance. After 5000 cycles of charging and discharging, the material activation of the ASC electrode material causes the capacitance retention rate to reach 105.5% at a current density of 10 A g−1. It has satisfactory cycle stability. Eqn (2) and (3) can be used to obtain the energy density and power density of ASC, as seen in Fig. 8f. The ASC can achieve a maximum energy density of 17.78 W h kg−1 and a power density of 803.13 W kg−1. Furthermore, the energy density can reach 3.35 W h kg−1 at a high power density of 8014.28 W kg−1. These outcomes significantly surpass those of other analogous electrode materials that have been previously documented, including Co3O4//AC,59 Fe-N-CHPs,60 Co3O4//AC,61 CoNW//CF,62 and Co3O4//AC.63The practical application of ASC is further proved.
4. Conclusions
To summarize, Fe-doped Co3O4 with a nanosheet floral cluster structure was effectively created using a straightforward hydrothermal technique and a subsequent thermal decomposition reaction. By optimizing the influence of different doped iron contents and thermal decomposition temperature on the electrochemical performance, the electrode material of the Co-0.2Fe-450 supercapacitor prepared at the best ratio and the most suitable temperature has excellent electrochemical performance. Fe doping can enhance an electrode material's electrical characteristics while exposing more reactive sites to create diffusion channels that will facilitate the movement of ions and electrons. In the three-electrode system, the specific capacitance was 680 F g−1 at a current density of 1 A g−1, and the capacitance retention rate was 84.67% after 5000 cycles. The assembled asymmetric supercapacitor (Co-0.2Fe-450//AC) has an excellent energy storage performance and cycling stability with a power density of 803.13 W kg−1 at an energy density of 17.78 W h kg−1 and a capacitance retention of 105.5% after 5000 cycles. Thus, these excellent results indicate that the synthesis strategy of Fe-doped Co3O4 can provide a feasible pathway for other doped materials.Author contributions
Design of the study and supervision of the overall project: Songjun Li, Maiyong Zhu. Performing of the experiments, collection of data, and drafting of the manuscript: Congcong Lu. Contribution to the result discussion: Congcong Lu, Yu Yang.Conflicts of interest
The authors declare no competing financial interest.References
- F. Jerez, P. B. Ramos, V. E. Córdoba, M. F. Ponce, G. G. Acosta and M. A. Bavio, Yerba mate: From waste to activated carbon for supercapacitors, J. Environ. Manage., 2023, 330, 117158 CrossRef CAS PubMed.
- W. Zhang, W. Li and S. Li, Self-template activated carbons for aqueous supercapacitors, Sustainable Mater. Technol., 2023, 36, e00582 CrossRef CAS.
- Z. Guo, X. Han, C. Zhang, S. He, K. Liu, J. Hu, W. Yang, S. Jian, S. Jiang and G. Duan, Activation of biomass-derived porous carbon for supercapacitors: A review, Chin. Chem. Lett., 2023, 109007 CrossRef.
- A. Amiri, A. Bruno and A. A. Polycarpou, Configuration-dependent stretchable all-solid-state supercapacitors and hybrid supercapacitors, Carbon Energy, 2023, 5, e320 CrossRef CAS.
- N. Choudhary, C. Li, J. Moore, N. Nagaiah, L. Zhai, Y. Jung and J. Thomas, Asymmetric supercapacitor electrodes and devices, Adv. Mater., 2017, 29, 1605336 CrossRef PubMed.
- V. Sharma, S. Biswas and A. Chandra, Need for revisiting the use of magnetic oxides as electrode materials in supercapacitors: unequivocal evidence of significant variation in specific capacitance under variable magnetic field, Adv. Energy Mater., 2018, 8, 1800573 CrossRef.
- C. Yang, S. Yun, J. Shi, M. Sun, N. Zafar, A. Arshad, Y. Zhang and L. Zhang, Tailoring the supercapacitive behaviors of Co/Zn-ZIF derived nanoporous carbon via incorporating transition metal species: a hybrid experimental-computational exploration, Chem. Eng. J., 2021, 419, 129636 CrossRef CAS.
- X. Zhao, L. Mao, Q. Cheng, J. Li, F. Liao, G. Yang, L. Xie, C. Zhao and L. Chen, Two-dimensional spinel structured Co-based materials for high performance supercapacitors: a critical review, Chem. Eng. J., 2020, 387, 124081 CrossRef CAS.
- Z. Ji, G. Tang, L. Chen, J. Zhong, Y. Chen, G. Zhu, X. Chuan, J. Zhang and X. Shen, Defect engineering of hollow porous N, S co-doped carbon spheres-derived materials for high-performance hybrid supercapacitors, Chem. Eng. J., 2024, 480, 148213 CrossRef CAS.
- T. Cheng, F. Wang, Y.-Z. Zhang, L. Li, S.-Y. Gao, X.-L. Yang, S. Wang, P.-F. Chen and W.-Y. Lai, 3D printable conductive polymer hydrogels with ultra-high conductivity and superior stretchability for free-standing elastic all-gel supercapacitors, Chem. Eng. J., 2022, 450, 138311 CrossRef CAS.
- Q. Luo, C. Lu, L. Liu and M. Zhu, A review on the synthesis of transition metal nitride nanostructures and their energy related applications, Green Energy Environ., 2023, 8, 406–437 CrossRef CAS.
- S. Zheng, X. Li, B. Yan, Q. Hu, Y. Xu, X. Xiao, H. Xue and H. Pang, Transition-metal (Fe, Co, Ni) based metal-organic frameworks for electrochemical energy storage, Adv. Energy Mater., 2017, 7, 1602733 CrossRef.
- G. A. Tafete, M. K. Abera and G. Thothadri, Review on nanocellulose-based materials for supercapacitors applications, J. Energy Storage, 2022, 48, 103938 CrossRef.
- S. A. Pawar, D. S. Patil, D. K. Nandi, M. M. Islam, T. Sakurai, S.-H. Kim and J. C. Shin, Cobalt-based metal oxide coated with ultrathin ALD-MoS2 as an electrode material for supercapacitors, Chem. Eng. J., 2022, 435, 135066 CrossRef CAS.
- G. Zhang, X. Xiao, B. Li, P. Gu, H. Xue and H. Pang, Transition metal oxides with one-dimensional/one-dimensional-analogue nanostructures for advanced supercapacitors, J. Mater. Chem. A, 2017, 5, 8155–8186 RSC.
- Y. Ma, X. Xie, W. Yang, Z. Yu, X. Sun, Y. Zhang, X. Yang, H. Kimura, C. Hou and Z. Guo, Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors, Adv. Compos. Hybrid Mater., 2021, 1–19 Search PubMed.
- R. R. Salunkhe, Y. V. Kaneti and Y. Yamauchi, Metal–organic framework-derived nanoporous metal oxides toward supercapacitor applications: progress and prospects, ACS Nano, 2017, 11, 5293–5308 CrossRef CAS PubMed.
- G. Meng, Q. Yang, X. Wu, P. Wan, Y. Li, X. Lei, X. Sun and J. Liu, Hierarchical mesoporous NiO nanoarrays with ultrahigh capacitance for aqueous hybrid supercapacitor, Nano Energy, 2016, 30, 831–839 CrossRef CAS.
- I. Hussain, S. Sahoo, T. Hussain, M. Ahmad, M. S. Javed, C. Lamiel, S. Gu, T. Kaewmaraya, M. S. Sayed and K. Zhang, Theoretical and Experimental Investigation of In Situ Grown MOF-Derived Oriented Zr-Mn-oxide and Solution-Free CuO as Hybrid Electrode for Supercapacitors, Adv. Funct. Mater., 2023, 33, 2210002 CrossRef CAS.
- L. Liu, C. Lu, Y. Ma, Y. Yang, S. Li and M. Zhu, Fe2O3@N-C@MnO2 Composite with Chinese-Chestnut Structure for High-Performance Supercapacitors, J. Electron. Mater., 2023, 52, 4988–4999 CrossRef CAS.
- C. Lu, C. Tu, Y. Yang, Y. Ma and M. Zhu, Construction of Fe3O4@Fe2P Heterostructures as Electrode Materials for Supercapacitors, Batteries, 2023, 9, 326 CrossRef CAS.
- C. Lu, L. Liu, Y. Yang, Y. Ma, Q. Luo and M. Zhu, Recent Progress in Co3O4-Based Nanomaterials for Supercapacitors, ChemNanoMat, 2023, e202200537 CrossRef CAS.
- H. Gao, S. Xin and J. B. Goodenough, The origin of superior performance of Co (OH)2 in hybrid supercapacitors, Chem, 2017, 3, 26–28 CAS.
- Q. T. Nguyen, U. T. Nakate, J. Chen, D. T. Tran and S. Park, Evolution of novel nanostructured MoCoFe-based hydroxides composites toward high-performance electrochemical applications: Overall water splitting and supercapacitor, Composites, Part B, 2023, 252, 110528 CrossRef CAS.
- W. Xu, J. Chen, M. Yu, Y. Zeng, Y. Long, X. Lu and Y. Tong, Sulphur-doped Co3O4 nanowires as an advanced negative electrode for high-energy asymmetric supercapacitors, J. Mater. Chem. A, 2016, 4, 10779–10785 RSC.
- J. Hao, S. Peng, H. Li, S. Dang, T. Qin, Y. Wen, J. Huang, F. Ma, D. Gao and F. Li, A low crystallinity oxygen-vacancy-rich Co3O4 cathode for high-performance flexible asymmetric supercapacitors, J. Mater. Chem. A, 2018, 6, 16094–16100 RSC.
- N. Tang, W. Wang, H. You, Z. Zhai, J. Hilario, L. Zeng and L. Zhang, Morphology tuning of porous CoO nanowall towards enhanced electrochemical performance as supercapacitors electrodes, Catal. Today, 2019, 330, 240–245 CrossRef CAS.
- J. Huang, J. Wei, Y. Xiao, Y. Xu, Y. Xiao, Y. Wang, L. Tan, K. Yuan and Y. Chen, When Al-doped cobalt sulfide nanosheets meet nickel nanotube arrays: a highly efficient and stable cathode for asymmetric supercapacitors, ACS Nano, 2018, 12, 3030–3041 CrossRef CAS PubMed.
- F.-N. Shi, J. Jiang, H. Xiao and X. Li, An extra-long-life supercapacitor based on NiO/C&S composite by decomposition of Ni-based coordination complex, Mater. Des., 2018, 153, 203–210 CrossRef CAS.
- R. Madhu, V. Veeramani, S.-M. Chen, A. Manikandan, A.-Y. Lo and Y.-L. Chueh, Honeycomb-like porous carbon–cobalt oxide nanocomposite for high-performance enzymeless glucose sensor and supercapacitor applications, ACS Appl. Mater. Interfaces, 2015, 7, 15812–15820 CrossRef CAS PubMed.
- Y. Zou, I. A. Kinloch and R. A. Dryfe, Mesoporous vertical Co3O4 nanosheet arrays on nitrogen-doped graphene foam with enhanced charge-storage performance, ACS Appl. Mater. Interfaces, 2015, 7, 22831–22838 CrossRef CAS PubMed.
- R. Ambare, S. Bharadwaj and B. Lokhande, Non-aqueous route spray pyrolyzed Ru: Co3O4 thin electrodes for supercapacitor application, Appl. Surf. Sci., 2015, 349, 887–896 CrossRef CAS.
- W. Liu, Z. Zhang, Y. Zhang, Y. Zheng, N. Liu, J. Su and Y. Gao, Interior and exterior decoration of transition metal oxide through Cu0/Cu+ Co-doping strategy for high-performance supercapacitor, Nano-Micro Lett., 2021, 13, 1–14 CrossRef CAS PubMed.
- Z. Wang, F. Wang, Y. Li, J. Hu, Y. Lu and M. Xu, Interlinked multiphase Fe-doped MnO2 nanostructures: A novel design for enhanced pseudocapacitive performance, Nanoscale, 2016, 8, 7309–7317 RSC.
- S. Wang, Q. Li, M. Chen, W. Pu, Y. Wu and M. Yang, Electrochemical capacitance performance of Fe-doped Co3O4/graphene nanocomposite: investigation on the effect of iron, Electrochim. Acta, 2016, 215, 473–482 CrossRef CAS.
- J. Fan, H. Zheng, A. Chen, L. Gu, X. Xie, J. Fan and Z. Ding, In-situ construction of amorphous–crystalline NiCo bimetallic oxyhydroxide for low-temperature supercapacitor device, Chem. Eng. J., 2023, 476, 146638 CrossRef CAS.
- M. Zhu, C. Lu, Y. Ma and Y. Yang, NiCo-glycolate-derived porous spherical NiCo2S4 for high-performance asymmetric supercapacitors, Appl. Organomet. Chem., 2023, 37(12), e7301 CrossRef CAS.
- A. I. Rabee, C. B. Gaid, G. A. Mekhemer and M. I. Zaki, Combined TPR, XRD, and FTIR studies on the reduction behavior of Co3O4, Mater. Chem. Phys., 2022, 289, 126367 CrossRef CAS.
- H. Sun, Y. Miao, G. Wang, X. Ren, E. Bao, X. Han, Y. Wang, X. Ma, C. Xu and H. Chen, Flower-like ZnCo2O4 microstructures with large specific surface area serve as battery-type cathode for high-performance supercapacitors, J. Energy Storage, 2023, 72, 108502 CrossRef.
- X. Huang, L. Liu, H. Gao, W. Dong, M. Yang and G. Wang, Hierarchically nanostructured MnCo2O4 as active catalysts for the synthesis of N-benzylideneaniline from benzyl alcohol and aniline, Green Chem., 2017, 19, 769–777 RSC.
- C. Jin, Z. He, Y. Zhao, Y. Pan, W. Wu, X. Wang and G. Tong, Controllable synthesis, formation mechanism, and enhanced microwave absorption of dendritic AgFe alloy/Fe3O4 nanocomposites, CrystEngComm, 2018, 20, 1997–2009 RSC.
- J. Kim, C. Young, J. Lee, Y.-U. Heo, M.-S. Park, M. S. A. Hossain, Y. Yamauchi and J. H. Kim, Nanoarchitecture of MOF-derived nanoporous functional composites for hybrid supercapacitors, J. Mater. Chem. A, 2017, 5, 15065–15072 RSC.
- S. A. Han, H. Qutaish, J. W. Lee, M. S. Park and J. H. Kim, Metal-organic framework derived porous structures towards lithium rechargeable batteries, EcoMat, 2023, 5, e12283 CrossRef CAS.
- S. Aslam, S. M. Ramay, A. Mahmood, G. M. Mustafa, S. Zawar and S. Atiq, Electrochemical performance of transition metal doped Co3O4 as electrode material for supercapacitor applications, J. Sol-Gel Sci. Technol., 2023, 105, 360–369 CrossRef CAS.
- Y. Zhao, X. Wang, H. Li, B. Qian, Y. Zhang and Y. Wu, Synthesis of Co3O4 nanospheres for enhanced photo-assisted supercapacitor, Chem. Eng. J., 2022, 431, 133981 CrossRef CAS.
- F. Wu, F. Cui, Q. Ma, J. Zhang, X. Qi and T. Cui, Facile construction of hexagonal Co3O4 as the electrode for high-performance supercapacitor, Mater. Lett., 2022, 324, 132791 CrossRef CAS.
- A. Karthikeyan and R. Mariappan, Enhancing pseudo-capacitive behavior in Mn-doped Co3O4 nanostructures for supercapacitor applications, J. Alloys Compd., 2023, 968, 172094 CrossRef CAS.
- M. Khandelwal, A. P. Nguyen, C. Van Tran and J. B. In, Simple fabrication of Co3O4 nanoparticles on N-doped laser-induced graphene for high-performance supercapacitors, RSC Adv., 2021, 11, 38547–38554 RSC.
- Z. Lin and X. Qiao, Coral-like Co3O4 decorated N-doped carbon particles as active materials for oxygen reduction reaction and supercapacitor, Sci. Rep., 2018, 8, 1802 CrossRef PubMed.
- D. Guragain, S. Karna, J. Choi, R. Bhattarai, T. P. Poudel, R. K. Gupta, X. Shen and S. R. Mishra, Electrochemical performance of iron-doped cobalt oxide hierarchical nanostructure, Processes, 2021, 9, 2176 CrossRef CAS.
- H. S. Kim, M. S. Kang and W. C. Yoo, Co3O4 Nanocrystals on Crab Shell-derived Carbon Nanofibers (Co3O4@CSCNs) for High-performance Supercapacitors, Bull. Korean Chem. Soc., 2018, 39, 327–334 CrossRef CAS.
- S. A. Al Kiey and H. N. Abdelhamid, Metal-organic frameworks (MOFs)-derived Co3O4@N-doped carbon as an electrode materials for supercapacitor, J. Energy Storage, 2022, 55, 105449 CrossRef.
- Z. Lin, X. Xie, D. Wu, X. Feng, M. Chen, X. Jia, Y. Sun, Y. Qin, Y. Qi and W. Du, N-Doped celery-based biomass carbon with tunable Co3O4 loading for enhanced-performance of solid-state supercapacitors, New J. Chem., 2022, 46, 6921–6931 RSC.
- T.-Y. Chen, T.-R. Kuo, S. Yougbaré, L.-Y. Lin and C.-Y. Xiao, Novel direct growth of ZIF-67 derived Co3O4 and N-doped carbon composites on carbon cloth as supercapacitor electrodes, J. Colloid Interface Sci., 2022, 608, 493–503 CrossRef CAS PubMed.
- H. Chen, J. Wang, F. Liao, X. Han, Y. Zhang, C. Xu and L. Gao, Uniform and porous Mn-doped Co3O4 microspheres: Solvothermal synthesis and their superior supercapacitor performances, Ceram. Int., 2019, 45, 11876–11882 CrossRef CAS.
- H. Chen, J. Wang, F. Liao, X. Han, C. Xu and Y. Zhang, Facile synthesis of porous Mn-doped Co3O4 oblique prisms as an electrode material with remarkable pseudocapacitance, Ceram. Int., 2019, 45, 8008–8016 CrossRef CAS.
- Y. Yang, C. Yang, K. Tao, Q. Ma and L. Han, Construction of S-doped ZnCo2O4 microspindles with enhanced electrochemical performance for supercapacitors, Vacuum, 2020, 181, 109740 CrossRef CAS.
- Y. Li, L. Li and F. Du, Amorphous S-doped NixCo3-xO4 for high-performance asymmetric supercapacitors, Electrochim. Acta, 2022, 434, 141326 CrossRef CAS.
- Z. Peng, W. Wang, H. Zhao, J. Qi, H. Zhang, C. Zhou, B. Yan and Z. Zhang, Size-dependent synthesis of hollow Co3O4 nanocubes derived from Co-Fe PBA as battery-type electrode materials for hybrid supercapacitors, J. Electrochem. Soc., 2021, 168, 080542 CrossRef CAS.
- K. P. Shejale and S. Y. Kim, Ultralow Fe augmented defect-prone porous carbon for highly efficient supercapacitor, Mater. Lett., 2023, 343, 134331 CrossRef CAS.
- Y. Jiang, L. Chen, H. Zhang, Q. Zhang, W. Chen, J. Zhu and D. Song, Two-dimensional Co3O4 thin sheets assembled by 3D interconnected nanoflake array framework structures with enhanced supercapacitor performance derived from coordination complexes, Chem. Eng. J., 2016, 292, 1–12 CrossRef CAS.
- P. Howli, S. Das, S. Sarkar, M. Samanta, K. Panigrahi, N. S. Das and K. K. Chattopadhyay, Co3O4 nanowires on flexible carbon fabric as a binder-free electrode for all solid-state symmetric supercapacitor, ACS Omega, 2017, 2, 4216–4226 CrossRef CAS PubMed.
- D. Guo, X. Song, F. Li, L. Tan, H. Ma, L. Zhang and Y. Zhao, Oriented synthesis of Co3O4 core-shell microspheres for high-performance asymmetric supercapacitor, Colloids Surf., A, 2018, 546, 1–8 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00170b |
| This journal is © the Partner Organisations 2024 |