Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of culture vessel materials on biomanufacturing of dendritic cell-based immunotherapies in closed systems
Jiyu Jessica Tian a,
Hamid Ebrahimi Orimi
a,
Hamid Ebrahimi Orimi a,
Natalie Fekete
a,
Natalie Fekete b,
Nicolas Droletc,
Katie Campbell
b,
Nicolas Droletc,
Katie Campbell c,
Michel L. Tremblay
c,
Michel L. Tremblay d,
Linda Peltier
d,
Linda Peltier e,
Pierre Laneuville
e,
Pierre Laneuville e,
Pierre-Luc Girard-Lauriault
e,
Pierre-Luc Girard-Lauriault a and
Corinne A. Hoesli
a and
Corinne A. Hoesli *af
*af
aDepartment of Chemical Engineering, McGill University, Montreal, Canada. E-mail: corinne.hoesli@mcgill.ca; Tel: +5143984275
bAlliance for Regenerative Medicine, Washington D.C., USA
cSaint-Gobain Ceramics & Plastics, Inc., Northborough Research and Development Center, Northborough, Massachusetts, USA
dRosalind and Morris Goodman Cancer Institute, McGill University, Montreal, Canada
eThe Research Institute of McGill University Health Center, Montreal, Canada
fDepartment of Biological and Biomedical Engineering, McGill University, Montreal, Canada
First published on 1st July 2025
Abstract
Dendritic cell-based immunotherapy is a promising strategy to treat malignant diseases. In vitro manufacturing of dendritic cells conventionally relies on culturing primary monocytes in dishes or vessels made from tissue culture treated polystyrene. For clinical applications, the implementation of closed culture vessels such as cell culture bags is highly desirable to minimize the risks of contamination and allow automated fluid handling. However, this transition typically represents a significant change in substrate surface properties which can impact cell–surface interactions. This review provides an overview of closed culture systems for dendritic cell therapy product biomanufacturing and describes how material selection can impact cell–surface interactions and thereby the resulting cell fate decisions. Gaining a fundamental understanding of cell culture vessel material surface properties, how proteins adsorb to these materials, and how monocyte-derived dendritic cells may adhere or interact with these surfaces can help guide closed cell culture vessel selection.
Dendritic cells (DCs) play a crucial role in mediating communication and regulation between the innate and adaptive immune systems, which makes them a promising vehicle for various applications, including cancer treatment. DC-based cancer immunotherapies harness the antigen-presenting capacity of DCs to reengage the immune system's anti-tumor responses after being administered to the patient. DC-based immunotherapies can be manufactured through in vitro differentiation and maturation from their progenitors, most commonly peripheral blood primary monocytes. Tissue culture polystyrene (TCPS) has been the most commonly used material for disposable labware for cell culture applications since the 1960s.1 Traditional polystyrene culture systems such as T-flasks and multi-well plates have an open configuration, which requires the vessels to be opened and closed during manual manipulations, introducing chances for contamination. In the context of the production of clinical cellular therapies in accordance with current good manufacturing practice (cGMP) regulations,2,3 closed culture systems such as single-use culture bags are preferred due to the greatly minimized risks of contamination in closed manufacturing systems.
The transition towards bag cell culture vessels entails significant changes in the polymers used and their surface properties. Upon contact with the culture vessel, proteins present in the medium rapidly adsorb onto the surface and form a protein adlayer covering the base substrate. Therefore, it is widely thought that cells interact with surface-adsorbed proteins rather than directly with the surface itself.4 Substrate surface properties determine how and which proteins found in cell culture media adsorb to surfaces,5 consequently controlling the behavior of various cell types when they come into contact with the surface.6–9 The plastics most commonly used to form cell culture bags are flexible and gas-permeable polymers such as fluoropolymers and polyolefins.5 Our earlier review10 has extensively documented comparative studies between monocyte-derived dendritic cells (Mo-DCs) cultured in suspension bags versus conventional polystyrene vessels, reviewing key quality attributes including maturation phenotype, cell yield, viability, and functional parameters (e.g. IL-12 secretion profile, phagocytic capacity, and migratory potential). However, reported outcomes varied among different research groups.10 Many authors reported no significant differences between Mo-DCs generated in bags versus flasks,11–14 whereas some studies demonstrated a diminished yield and functional abilities of bag-generated Mo-DCs cultured in suspension.15,16 These varying observations could be attributed to proprietary surface treatments used by different vendors and lot-to-lot variations in vessel manufacturing, factors that are often overlooked. Furthermore, despite the intrinsic hydrophobicity and bio-inertness of fluoropolymers, we and others observed monocyte and Mo-DC adhesion onto fluoropolymer surfaces.13,15 This observation could reconcile apparently divergent findings on Mo-DC bag culture outcomes, since it is often unclear whether both the adherent and the non-adherent cell fraction were harvested prior to analyses. Moreover, slight changes in material properties may change the proportion of adherent Mo-DCs given their facultative adherent nature.
To inform the engineering of culture substrate materials, it is critical to identify the underlying mechanisms governing monocyte and Mo-DC interactions with synthetic polymeric surfaces, which will enable researchers to tailor the fractions between adherent and non-adherent Mo-DC populations. This review discusses the ongoing efforts from both the immunological and material engineering perspectives to optimize the performance of Mo-DC immunotherapies. This review also highlights the effects of surface properties on cell–surface interaction mechanisms, which play an integral role in cell mechanotransduction and affect the final cell fate decisions.17 Other critical materials considerations such as mass transfer and extractables/leachables profiles are also important factors in vessel material selection, which have been comprehensively covered in our earlier review and will not be the focus of this current work.10 While several emerging biomaterial platforms such as hydrogels, 3D scaffolds, and nanostructured surfaces are being explored for their potential to enhance DC culture and function, their application remains largely limited to research settings. Polyethylene glycol-based hydrogels can support the differentiation and maturation of the human leukemia monocytic cell line THP-1 into DCs, with tunable matrix stiffness influencing DC phenotype and enabling efficient recovery of viable cells.18 Similarly, collagen-based 3D scaffolds can promote differentiation of bone marrow cells into regulatory DC subsets.19 Titanium surfaces with defined nano- and micro-topographies can modulate DC adhesion and activation.20 Despite their promise, these materials are not yet widely adopted in cGMP-compliant manufacturing or in the design of closed culture systems. This review hence focuses on polymeric materials such as fluoropolymers and polyolefins used to fabricate closed culture systems, which currently represent the most practical and scalable options for Mo-DC biomanufacturing. Biomaterials that remain in research settings are beyond the scope of this review.
Introduction to Mo-DC cancer immunotherapy
Since their discovery by Cohn and Steinman in 1973,21 DCs have been widely recognized as the most potent professional antigen-presenting cells (APCs). While other APC populations such as macrophages also possess the ability to uptake and process antigens, DCs are the only cell type in circulation capable of migrating to the lymph nodes to activate naïve T cells, thus serving as the bridge between innate and adaptive immunity.22,23 The pivotal role of DCs in triggering antigen-specific adaptive immunity places them at the core of immune system regulation. This makes DCs a powerful vaccination platform for treating diseases that are resistant to conventional therapies.Since the first documented attempt to use DCs as therapeutic vehicles in 1995, over 445 active or completed clinical trials utilizing DCs as a therapeutic drug to treat cancer have been registered worldwide on ClinicalTrials.gov as of August 2024 (search criteria include: conditions/disease: cancer; other terms: dendritic cells; study status: active not recruiting or completed). The first US Food and Drug Administration (FDA) approved cellular therapy, sipuleucel-T, is a personalized, autologous DC vaccine for the treatment of advanced-stage prostate cancer.24 The goal of DC-based cancer immunotherapy is to exploit the antigen presentation capacity of DCs to reengage the tumor-specific immune responses mediated by cytotoxic T lymphocytes. In addition, DC-based vaccines have a favorable safety profile due to their low immune-related toxicity (e.g. risk of inducing autoimmunity or cytokine storm) compared with other immunotherapeutic strategies such as cytokine or antibody therapies.22,25 In addition to oncology, Mo-DC immunotherapies are also being studied towards the treatment of autoimmune disease and chronic viral infections such as HIV.26
Basics of DC immunobiology
Normally, immature DCs are developed in the bone marrow, then released into the bloodstream to patrol peripheral tissues. Upon encountering exogenous pathogens (recognized by pattern recognition receptors such as toll-like receptors (TLRs)), immature DCs phagocytose the pathogens and promptly activate the maturation process. Mature DCs downregulate phagocytosis, increase secretion of pro-inflammatory cytokines (e.g. interleukin-12, IL-12), and upregulate expression of major histocompatibility complex (MHC) molecules (e.g. human leukocyte antigen HLA-DR, an MHC class II molecule) and co-stimulatory molecules (e.g. CD80, CD86 and CD40). Maturation is also associated with the upregulated expression of C–C chemokine receptor 7, which drives the homing of mature DCs into a nearby secondary lymphoid organ such as the draining lymph nodes.23,27,28Once in the draining lymph nodes, DCs engage the T-cell receptors (TCRs) and present the processed antigens to naïve T cells in complex with MHC molecules. DCs are known for cross-presenting exogenous antigens to engage the CD8+ T cell immunity. DCs present antigens on MHC class I molecules to naïve or memory CD8+ T cells, while on MHC class II molecules to CD4+ T cells.29,30 The interaction between antigen-MHC complex and CD3-TCR complex provides the antigen-specific “signal 1” needed for T cell activation. “Signal 2” involves cross-talk between pairs of counter co-stimulatory molecules present on the surface of DCs and T cells, more specifically, between CD80–CD28, CD86–CD28 and CD40–CD40L.30 This co-stimulatory signal is antigen-nonspecific but indispensable for the full activation and survival of T cells.30 In addition, DCs secrete a variety of cytokines contributing to the “signal 3” that instructs the polarization of activated T cells.31,32 The particular cytokines produced by DCs depend on the specific DC subset and the nature of the pathogen that DCs encounter. For instance, IL-12 released by DCs (often in synergy with IL-18) polarizes CD4+ T cells towards T helper 1 (Th1) response.31,33 In contrast, production of IL-4 by T cells upon interaction with DCs promotes Th2 polarization.31 TGF-β secreted by DCs in combination with IL-6 is known to induce Th17 development from naïve T cells.31 DC secretion of IL-23 also sustains and expands Th17 responses after initial differentiation. Conversely, in the absence of IL-6, DC-derived TGF-β supports regulatory T cell (Treg) development which can be reinforced by IL-10 secreted by tolerogenic DCs.31 Therefore, DC-derived polarizing cytokines provide the crucial third signal that directs T cell fate. Upon receiving all three signals, namely antigen presentation, co-stimulation, and polarizing cytokine cues from DCs, naïve T cells are activated, leading to proliferation and differentiation into antigen-specific memory and effector T cells. CD4+ T cells give rise to Th cells, while CD8+ T cells differentiate into cytotoxic T lymphocytes (CTLs).27 In malignancies, anti-tumor immunity is primarily mediated by CD8+ CTLs, with CD4+ Th cells providing vital support to the proper functioning of CD8+ CTLs. Effector CD4+ and CD8+ T cells localize to the tumor sites, where they eliminate tumor cells through cytotoxic activities and production of effector cytokines.30
Human blood DCs consist of heterogenous subsets that are typically classified by ontogeny into conventional DC type 1 (cDC1), conventional DC type 2 (cDC2), plasmacytoid DCs (pDCs), Mo-DC and DC3.34,35 DC precursors arise from the bone marrow. CD34+ hematopoietic stem cells give rise to granulocyte–monocyte–dendritic-cell progenitors (GMDPs), from which an IRF8hi branch becomes the common DC progenitors (CDPs) that yield pre-cDCs and pDCs.34,36 Notably, studies in mice have shown that pDC precursors can originate not only from myeloid CDPs but also from lymphoid progenitors. Whether this dual origin holds true for human pDCs remains to be determined.34 Separately, IRF8low GMDPs give rise to monocytes, which differentiate into Mo-DCs in inflamed tissues or in response to cytokines such as GM-CSF. These cells closely resemble cDCs morphologically and share many surface markers and genes associated with classical DC identity, including the transcription factor IRF4.34,35 In recent years, single cell analyses have identified a CD14+ inflammatory-type DC, often termed DC3, which arises along the monocyte-related pathway from IRF8low GMDPs.34 cDCs, in particular cDC1s, are central coordinators to induce CTL-mediated anti-tumor immunity. cDC1s possess superior ability to capture and cross-present exogenous antigens on MHC class I, thereby efficiently priming CD8+ T cells, whereas cDC2s are more proficient at presenting antigens via MHC class II to activate CD4+ T cells.37 In fact, cDC1s are identified as the most potent APC subset for inducing robust activation of CD8+ T cells ex vivo. However, they are not unique in this capacity, as inflammatory Mo-DCs and cDC2s have also been shown to cross-present antigens to prime CD8+ T cells under appropriate conditions.34,38 Moreover, cDC1s are potent inducers of Th1 response, while cDC2s are specialized in Th2 and Th17 skewing.39 On the other hand, pDCs mainly participate in the immune responses to viral infections primarily by secreting type I interferons.34,39 Their role in anti-tumor immunity is less characterised than the cDCs.
Ex vivo generation of clinical-grade DCs for cancer immunotherapy
DCs constitute approximately 0.16–0.68% of naturally circulating leukocytes in human peripheral blood.40 Due to their low occurrence frequency, DCs used to manufacture cancer immunotherapies are commonly differentiated ex vivo from a progenitor cell source, normally CD34+ hematopoietic stem cells or CD14+ peripheral blood monocytes either from autologous (from the patients themselves) or allogeneic (from a donor) sources.27Differentiating autologous monocytes in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF) remains the most commonly used preparation method to generate DCs in clinical settings.26,41 Fig. 1 illustrates the Mo-DC differentiation protocol currently adopted by our research group. The differentiation phase usually lasts 5 to 7 days to generate immature Mo-DCs, followed by stimulation with a maturation cocktail for 48 hours to produce mature Mo-DCs.22 The composition of the maturation cocktail is not fully standardized and varies among research groups. In early-generation trials, the gold standard typically included TNF-α, IL-1β, and IL-6, combined with prostaglandin E2 (PGE2).25,41 Despite the effect of PGE2 in enhancing DC migration, it also limits IL-12 secretion by DCs, being a key in inducing Th1 immune responses.41 Significant work has been devoted to developing alternative maturation cocktails such as IFN-γ with CD40L (to trigger the CD40-CD40L co-stimulatory pathway)25 or activating the TLRs using agonists such as poly[I:C] (against TLR3), resiquimod (against TLR7/8) and 3-O-deacylated monophosphoryl lipid A (MPLA, against TLR4).41
 | ||
| Fig. 1 Mo-DC differentiation and maturation protocol adopted by our research group. Created with BioRender. | ||
Antigen loading represents the last but critical step in obtaining a functional Mo-DC-based cancer vaccine, as it specifies the target for the CTL-mediated anti-tumor immunity. This step can be done during or after the maturation phase by pulsing the Mo-DCs with tumor-associated antigen (TAA) peptides or electroporation of the antigen mRNA.41,42 Shared or defined TAAs were typically used in earlier trials to generate broadly applicable cancer vaccines, where the efficacy is typically limited due to the loss of epitope expression caused by the high mutational rate of tumor cells.27,41 Recent advances in bioinformatics tools and RNA sequencing have made it possible to identify patient-specific neoantigens, which can be used as targets to manufacture personalized cancer vaccines that might exhibit improved clinical benefits.28,41 The antigen-loaded Mo-DCs are then administered to patients to reengage the anti-tumor immunity mediated by CD8+ CTLs.
Recent advances in DC cancer immunotherapy and future directions
The immunogenicity of Mo-DC-based cancer vaccines has been proven in clinical studies. The outcomes of a meta-analysis showed that following infusion of a DC-based vaccine, 77% of patients with prostate cancer and 61% of patients with renal cell carcinoma elicited immune responses.25,43 In addition, patients receiving DC immunotherapy treatment had at least a 20% increase in median overall survival.25 As an example, in the phase III IMPACT study, patients infused with the landmark sipuleucel-T showed a 4-month overall survival benefit versus patients treated with a placebo.44Despite the proven immunogenicity and survival benefit of Mo-DC cancer immunotherapies, the overall clinical objective response rate (a direct measurement of drug anti-tumor activity) remains poor.25,41 Less than 5% of patients achieved an objective response in the IMPACT study of sipuleucel-T.25,44 The clinical benefit is limited by the immunosuppressive microenvironment that Mo-DCs experience after infusion. Immunosuppressive factors secreted by tumor cells such as IL-10, transforming growth factor-β and arginase I could impair antigen processing by Mo-DCs, and/or lead to the development of tolerogenic DCs stimulating proliferation of regulatory T cells over conventional T cells, thereby blunting the anti-tumor immunity.41
Borges et al. have summarized the latest advances in preclinical and clinical DC antitumor vaccine development, highlighting current research efforts to overcome this challenge.45 One approach is to optimize DC differentiation and maturation protocols to generate Mo-DCs with enhanced immunostimulatory capacities through enhanced IL-12 secretion which has been recognized as a crucial determinant of clinical anti-tumor activity.25,28 Refined maturation cocktails that incorporate potent adjuvants (e.g. TLR agonists or damage-associated molecular patterns) have been used to further activate DCs and improve antigen cross-presentation.45 Researchers have also experimented with genetic engineering of DCs, for example, programming them to express TAAs or secrete IL-12 constitutively to bolster vaccine potency.45–47 Another approach utilizes Langerhans cell-type DCs derived from hematopoietic stem cells or monocytes which are highly efficient in stimulating CTLs.25,48 Beyond traditional ex vivo vaccines, novel delivery strategies are emerging. In situ vaccination techniques aim to recruit and load DCs directly within the patient, for instance by using oncolytic viruses or injectable biomaterial scaffolds at the tumor site that provide both tumor antigens and danger signals. This approach could help circumvent the immunosuppressive microenvironment and simplify vaccine logistics.45 Another trend in optimizing clinical performance is through combination therapy. The synergistic interactions between DC vaccination and other cancer treatments (such as immune checkpoint blockers, adoptive T cell transfer and certain chemotherapies) could be leveraged to unleash the potential of DC-based immunotherapies.25,41,45
Moving towards cGMP-compliant immunotherapy manufacturing
Clinical success of Mo-DCs depends not only on the cytokines, peptides and other signaling factors added to cultures, but also on the feasibility to create adequate microenvironments in cGMP-compliant biomanufacturing processes. The basal medium and vessel type applied in the transition from research to clinic can impact Mo-DC yields, quality and process reproducibility.Considerations of the culture medium: serum-free, chemically defined formulations
The standard medium formulation used in traditional Mo-DC culture is the basal medium Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS). FBS is the liquid fraction of clotted blood from bovine fetus after the removal of cells and clotting factors. FBS contains an abundance of components such as proteins and growth factors that are conducive to cell growth,49 although its ill-defined composition signifies a high batch-to-batch variability and inferior experimental reproducibility. Including FBS in the medium formulation also makes the culture more prone to contamination and transmission of harmful zoonotic pathogens such as prions, viruses and mycoplasma.50,51 Accordingly, manufacturers have developed medium formulations devoid of animal-derived serum which are preferred in clinical applications, and required in many countries.52Serum-free medium can be further classified into the following subgroups depending on the components present in the medium: xeno-free (does not contain non-human animal components but might contain human-sourced crude protein fractions such as human serum albumin), animal component-free (does not contain any animal-sourced components including from humans), protein-free (entirely devoid of proteins or polypeptides) and chemically defined (does not contain any chemically undefined components such as crude protein fractions but could contain highly purified components such as recombinant proteins).50 GMP-grade media provide traceability and validation of all components in the recipe. Table 1 presents several serum-free Mo-DC medium formulations that are commercially available. While the exact composition of serum-free media remains proprietary, most of these media contain basal components (glucose, amino acids, lipids, vitamins, buffers, inorganic salts), a fraction of human plasma, as well as recombinant proteins tailored to Mo-DC culture.
| Company | Product name | Classification |
|---|---|---|
| STEMCELL Technologies | ImmunoCult™-ACF dendritic cell medium | Serum-free, animal component-free |
| CellGenix | CellGenix® GMP dendritic cell medium | Serum-free, GMP-grade |
| PromoCell | Dendritic cell generation medium XF | Serum-free, xeno-free |
| R&D systems | StemXVivo serum-free dendritic cell base media | Serum-free |
| ThermoFisher | CTS AIM V medium | Serum-free, fully defined |
| Lonza | TheraPEAK™ X-VIVO™ 15 | Serum-free, GMP-grade |
Considerations of the culture system: functionally closed culture bags
Closed culture systems such as single-use cell culture bags offer significant advantages in clinical applications by minimizing contamination risk, facilitating scalability, ensuring regulatory compliance, and reducing the need for manual handling. Fig. 2 provides an overview of the available closed culture platforms for each step in the manufacturing process of Mo-DC immunotherapies. | ||
| Fig. 2 Overview of the Mo-DC manufacturing process and the corresponding closed culture systems. Created with BioRender. | ||
Among monocyte enrichment systems,26 only two closed systems are currently available in the market. The Elutra (Terumo BCT) enables monocyte enrichment from leukapheresis product based on counterflow centrifugal elutriation. The instrument applies a step-wisely increasing flow over cell layers in the opposite direction of the centrifugal force, enabling separation of cells based on cell size and density. The Elutra makes use of disposable tubing sets and sealed elutriation chambers, allowing fast selection of monocytes with high recovery rate.26,53,54 The CliniMACS (Miltenyi Biotec) is another closed-system platform that enriches monocytes by conducting a positive immunomagnetic selection with anti-CD14 antibodies labelled with magnetic particles. This method can be costly due to the use of antibodies but it is GMP-compliant and can yield high purifies of 99 ± 2%.26,55,56 Miltenyi Biotec also offers a clinical-scale cell processing solution that has integrated monocyte enrichment, culture, and antigen loading by electroporation in an automated platform; the CliniMACS Prodigy®. This device incorporates a culture chamber with temperature and CO2 control (named the CentriCult), which enables the differentiation of monocytes following their isolation with the CliniMACS technology in a functionally closed system.42,57 The size of the CentriCult chamber can limit the Prodigy® throughput and scalability for certain applications.
Cell culture bags are the most commonly used vessel for culture, differentiation and antigen loading of Mo-DCs. Commercially available cell culture bags are primarily made from polyolefins or fluoropolymers. These polymers emerged as excellent substrates for vessel design because of their high-performance properties, such as mechanical flexibility, gas permeability, chemical resistance, low leachable profile, and the ability to be melt-extruded or thermoformed.58,59 Fluorinated ethylene propylene (FEP) is one of the most commonly used fluoropolymers to manufacture cell culture bags; examples include the VueLife® C and AC series (Saint-Gobain), and PermaLife™ (OriGen Biomedical). Polyolefins, particularly polyethylene and polypropylene, are also widely used, with examples including the MACS GMP Cell Expansion Bag (Miltenyi Biotec) and Corning™ Cell Expansion Bags. These culture bags are mechanically flexible, which can be easily scaled and docked to other cell processing units through integrated tubing, facilitating cellular therapy production in a functionally closed bioreactor system.10 Several manufacturers such as Saint-Gobain and Meissner offer customization options for bag shape, size and port configurations to meet specific processing requirements.
Recent advances in bioreactor systems have further enhanced the capabilities of cell therapy manufacturing.60 Aside from the Prodigy®, Cocoon™ platform (Lonza) is another emerging all-in-one device, which uses a single-use, transportable cassette to carry out cell transfection/transduction and expansion in a closed, automated fashion. While Cocoon does not in its current design perform initial cell separation or final product formulation like the Prodigy, Cocoon units contain adaptable internal fluidics and unit operations. Cocoon systems can also readily be scaled out and networked in parallel under unified electronic control for concurrent large-scale production.60,61 Innovative bioreactors with engineered culture substrates have also been developed to support high-density cell expansion in closed environments. An example is the Quantum® Cell Expansion System (Terumo), a hollow-fiber perfusion bioreactor that is functionally closed and automated.60,62 The Quantum®'s cartridge contains thousands of semi-permeable capillaries that provide a high surface-area, 3D scaffold for cell growth while perfusing nutrients and oxygen. This design enables very high cell densities and has proven effective for immune cell cultivation. The emergence of advanced bioreactor systems highlights the critical role of implementing functionally closed systems, which are essential for translating DC-based and other cell therapies into scalable, GMP-compliant, and clinically viable treatments.
Effect of culture surface on cell–surface interaction and cell fate decision
Impact of surface properties on cell behavior at the substrate interface
Substrate physiochemical properties such as surface charge, chemical makeup and hydrophilicity largely determine protein adsorption phenomena. The type, amount and conformation of proteins constituting the protein adlayer in turn control the behavior of cells when they come into contact with the surface. In general, protein adsorption onto a solid surface is governed by the interfacial free energy between the surface and the liquid atop.6 Some authors described a competition between adhesion-inhibiting globular proteins (e.g. albumin) and large pro-adhesion proteins (e.g. fibronectin) for adsorption onto solid surfaces.6,7 Hydrophilic substrates with high surface free energy (lower interfacial free energy) were observed to favor the adsorption of pro-adhesion proteins, whilst hydrophobic surfaces were found to have a higher amount of surface-adsorbed albumin.6,7 Hydrophilic substrates with a higher amount of surface-adsorbed fibronectin significantly enhanced adhesion and proliferation of fibroblasts as compared to the hydrophobic controls.7Monocyte adhesion mechanisms: lessons learned from studies on polystyrene
We and others observed that a fraction of monocytes adhered to FEP surface despite its intrinsic hydrophobicity.13,15 The initial adhesion phase, which represents the first stage in in vitro cell culture, is crucial for cell mechanotransduction and significantly affects cell behavior, function and final fate decisions.17 The mechanisms by which monocytes adhere to hydrophobic surfaces including fluoropolymers still remain to be elucidated. Previous studies on monocyte adhesion mechanisms, summarized in Table 2, have mostly been conducted on polystyrene-based surfaces. Cellular attachment to the culture substrate is proposed to proceed through the binding of cell adhesion molecules, particularly integrin receptors, to adhesive protein ligands that are adsorbed on the substrate surface4,63,64 (Fig. 3). Integrins along with selectins, cadherins and members of the immunoglobulin superfamily make up the four major groups of cell adhesion molecules. Selectins, cadherins and immunoglobulin superfamily members typically act mainly as cell–cell adhesion mediators, while integrins are identified as the major receptors for extracellular matrix proteins, which facilitate cell–surface interactions.63,65 Integrins are heterodimeric adhesion receptors that are formed through the non-covalent association of two type I transmembrane glycoproteins, termed the α- and the β-subunit.66,67 Human peripheral blood monocytes have been characterized for the expression of nine integrin heterodimers: α1β1, α3β1, α4β1, α5β1, αLβ2, αMβ2, αXβ2, αVβ3,68 and αDβ2.69 Table 3 outlines their respective ligands and reported expression levels. | ||
| Fig. 3 Mechanisms of cell–protein–surface interactions in monocyte initial adhesion and differentiation into Mo-DCs. | ||
| Author | Cell type | Surface | Observations |
|---|---|---|---|
Babaei et al., 2018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 83 |
U937, NB4, monocytes | N- and O-rich PPCs | • The O- and N-rich organic coatings promoted cell adhesion, but only if the coatings contain a minimum functional group content. |
| • The number of adherent monocytes was proportional to functional group composition (COOH, NH2) in the coatings. | |||
| • Podosomes were observed on nearly all surfaces that promoted cell adhesion. | |||
| • Presence of albumin on the PPC-coated surfaces above the defined critical concentration might indicate the adhesion of monocytes to the PPCs. | |||
| • Main conclusion: modifying the nitrogen content in the PPCs could turn a surface from adhesion-deterrent to adhesion-promoting for specific cell types, a phenomenon that could be exploited to select certain cell types from a mixture by exposing the mixture to a PPC containing a particular nitrogen content. | |||
Sándor et al., 2016![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
Monocytes, Mo-DCs | TCPS coated with fibrinogen | • Blocking αX resulted in a significant (∼25%) reduction in monocyte adhesion. |
| • Blocking αM had a slight but not significant reduction in adhesion. However, blocking αM significantly decreased monocyte adhesion strength. | |||
| • Main conclusion: αXβ2 dominates adhesion of monocytes to fibrinogen over αMβ2. | |||
McNally and Anderson, 2002![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71 |
Monocytes | ProNectin F-coated TCPS | • EDTA and EGTA at 10 mM either completely or nearly completely inhibited initial monocyte adhesion. |
| • Addition of RGD peptides in the media had no impact on initial monocyte adhesion. | |||
| • Anti-β1 antibodies (clone JB1a & 6S6) and anti-β3 antibody (clone B3A) did not impact initial monocyte adhesion, whereas anti-β2 antibodies (clone YFC118.3 & MHM23) either partially or completely inhibited monocyte adhesion. | |||
| • Main conclusion: initial monocyte adhesion is likely mediated by β2 integrins, and independent of pathways binding to RGD-containing proteins. | |||
Shen et al., 2001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
Monocytes | 4 polystyrene-based surfaces (PS, TCPS, Primaria, ULA), pre-incubated with 1% plasma | • Monocyte adhesion linearly and positively correlated with the amount of fibrinogen adsorbed on each tested surface. |
| • Addition of EDTA before seeding the cells reduced monocyte adhesion in a dose-dependent manner. Monocyte adhesion was completely inhibited at 2 mM EDTA. | |||
| • Addition of EDTA after the cells started to adhere only partially reduced adhesion and cannot completely inhibit adhesion even at high EDTA concentrations. | |||
| • Main conclusion: initial monocyte adhesion to polystyrene-based surfaces is mediated by fibrinogen-binding integrins, possibly αMβ2; initial adhesion has an important effect on long-term adhesion. | |||
Garnotel et al., 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 72 72 |
Monocytes | TCPS coated with acid-soluble or pepsin-digested collagen I | • Adhesion was reduced by 85% with 5 mM EDTA. |
| • Antibodies against αX (clone FK-24 and 5-HCl-3) and β2 (clone MEM-48 and MHM-23) significantly reduced monocyte adhesion. | |||
| • Slight but significant adhesion reduction was observed by blocking β1 (with clone P4C10). | |||
| • No significant inhibition was observed by blocking αL and αM. | |||
| • Main conclusion: monocytes interact with type I collagen through αXβ2. | |||
Patarroyo et al., 1988![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
Monocytes | TCPS | • Blocking of the β2 subunit alone with mAbs 60.3 showed an inhibitory effect. |
| • Blocking of the α subunits, αX (with anti-Leu-M5), αM (with 60.1), and αL (anti-LFA-1) individually showed either limited or minimal inhibitory effects, but combination of the three showed inhibitory effect that was comparable to blocking β2 with 60.3. | |||
| • Main conclusion: β2 integrin, either alone or associate with αX, αM, or αL mediates monocyte adhesion. However, blocking of β2 did not exhibit full inhibition of monocyte adhesion to plastics, indicating the existence of β2-independent adhesion mechanisms. | |||
McNally and Anderson (1994)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74 |
Monocytes | Modified polystyrene-based surfaces (fluorinated, siliconized, nitrogenated, oxygenated) | • Blocking of β2 integrins (60.3, MHM23) inhibited initial monocyte adhesion to all four tested surfaces. Blocking of αM (60.1) also exhibited a partial inhibitory effect. |
| • Adhesion to surfaces reduced by 50–100% when complement component C3-depleted serum was used, but restored when C3 was replenished, indicating interactions between C3 and αM/β2 promote monocyte adhesion. | |||
| • Adsorbed fibrinogen reduced effectiveness of mAbs tested. | |||
| • Main conclusion: alternate adhesion pathways may be adopted depending on the propensities of adhesion-mediating components present on different surfaces. |
| β subunit | α subunit | Common ligands | Reported expression level |
|---|---|---|---|
| β1 (CD29) | α1 (CD49a) | • Laminin, collagen | • Low expression: MFI ratio to isotype control (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)84 8)84 |
| • Recognize GFOGER sequence in collagen | |||
| α3 (CD49c) | • Fibronectin, laminin, collagen, epiligrin etc. | • Low expression: MFI ratio to isotype control (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)84 8)84 |
|
| • Recognize RGD sequence | |||
| α4 (CD49d) | • Fibronectin by recognizing domains in CS-1, CS-5 regions | • Low expression: MFI ratio to isotype control (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)84 8)84 |
|
| • VCAM-1 | |||
| α5 (CD49e) | • Fibronectin & fibrinogen | • Mid-low expression: MFI ratio to isotype control (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)84 8)84 |
|
| • Recognize RGD sequence | |||
| β2 (CD18) | αL (CD11a) | • ICAM 1-4 | • High expression: MFI ratio to isotype control (180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)84 8)84 |
• High expression: MFI ratio to isotype control (230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50)69 50)69 |
|||
| • High expression85 | |||
| αM (CD11b) | • More than 40 dissimilar ligands | • High expression, MFI ratio to isotype control (155![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)84 8)84 |
|
| • Complements iC3b, C4b | • Mid-high expression, MFI ratio to isotype control (120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), similar to CD14 50), similar to CD14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
||
| • Fibrinogen | • High expression85 | ||
| • ICAMs | • Ratio of CD11b/CD11c: 7.1; CD11b& CD11c competes for binding to fibrinogen75 | ||
| αX (CD11c) | • Similar binding specificity as CD11b due to sequence similarity (e.g. iC3b, ICAM-1) | • Mid-low expression: MFI ratio to isotype control (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8)84 8)84 |
|
| • Fibrinogen by binding G-P-R | • Mid-high expression: MFI ratio to isotype control (120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), similar to CD14 50), similar to CD14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
||
| • High expression but relatively lower than CD11a & CD11b85 | |||
| αD (CD11d) | • ICAM-3, VCAM-1 | • Mid-high expression: MFI ratio to isotype control (150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), similar to CD14 50), similar to CD14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 69 69 |
|
| • Newly discovered, proposed to bind to fibronectin, fibrinogen, vitronectin like CD11b. | • High expression but relatively lower than CD11a & CD11b85 | ||
| β3 (CD61) | αV (CD51) | • Vitronectin, fibronectin, laminin, collagen. | • High expression of β3 subunit; both β3 and αV upregulated upon adhesion to type I collagen & fibronectin68 |
| • Recognize R-G-D sequence |
Ethylenediaminetetraacetic acid (EDTA) is a chelator well-known for inhibiting integrin-mediated adhesion by binding to divalent cations including Ca2+, Mg2+ and Mn2+ in such a way that these cations are prevented from coordinating integrin ligation. Monocyte adhesion to surfaces can be completely or nearly fully inhibited at EDTA concentrations varying from 2 to 10 mM.70–72 Anti-integrin monoclonal antibodies were frequently utilized to identify specific integrins involved in monocyte adhesion to polystyrene-based surfaces. Numerous studies point to the β2 integrin subfamily as the major monocyte-surface adhesion mediator.70–75 Blocking individual α subunits (αX, αM, and αL) associated with the β2 subunit resulted in limited inhibition of monocyte adhesion, but simultaneous blocking of all three α subunits produced an inhibitory effect comparable to blocking the β2 subunit, suggesting the involvement of all three heterodimers in adhesion.73 The contribution of each heterodimer varies depending on the availability of adhesion-mediating proteins in the media that are adsorbed on the surfaces. For instance, monocytes interact with type I collagen exclusively through αXβ2,72 while αMβ2 is reported to mediate adhesion via adsorbed C3 fragments (C3bi) and serum components such as fibrinogen and factor X.71,74 Studies show that blocking αM or αX reduces monocyte adhesion on fibrinogen-coated surfaces, but a more pronounced effect was observed for αX.75 RGD-binding integrins (α4β1, α5β1, and αVβ3) are expressed by human monocytes, suggesting a role in monocyte adhesion.68,76 Monocyte adhesion to synthetic RGD-containing copolymers (which happens exclusively through RGD-binding mechanisms) was less effective than adhesion to fibrinogen-coated surfaces, indicating that the context in which RGD is presented, for example, the presence of other cell receptor binding sites, play a decisive role in cell attachment to fibronectin.77 RGD-containing fibronectin fragments cannot disrupt already established monocyte adhesion.71 Hence, different integrin pathways are likely adopted depending on the specific composition of protein adlayer on the surface.
Integrin-independent adhesion mechanisms such as electrostatic and hydrophobic interactions should also be considered.78–80 Positively charged surfaces can facilitate the adhesion of various cell types,78,79,81,82 generally believed to be due to the electrostatic attraction between the positively charged substrates and the typically negatively charged cell membranes.78,79 For instance, the typically non-adherent human monocyte cell line U937 has been observed to adhere to cationic polymer-coated surfaces when these surfaces exceed a certain threshold of surface charge and primary amine group concentration.79,83 However, surface charge density alone cannot fully predict electrostatic interactions because proteins adsorb to surfaces on time scales that are often much shorter than cell settling and adhesion.4,5 The screening effect of electrolytes in the culture media also reduces the net attractive force.78,79 These phenomena complicate cell–surface electrostatic interactions, often involving more than simple attraction between opposite charges. For instance, in serum-containing media, positively charged substrates may also enhance adhesion by adsorbing proteins in favorable conformations that increase binding accessibility by integrins80 rather than through simple attractive forces. Studies have shown similar adhesion levels for HT-1080 and HeLa cells on cationic, anionic, and nonionic substrates in serum-free media, suggesting that electrostatic interactions are not solely dependent on surface charge.78
Phenotype and function of bag- vs. flask-generated Mo-DCs
The impact of culture vessel materials on ex vivo-derived Mo-DCs has been reviewed in our earlier publication10 and presented in Table 4. Most authors reported no significant differences between Mo-DCs generated in FEP bags versus polystyrene flasks.11–13 In our hands, Mo-DCs cultured in FEP bags were observed to have comparable viability, phenotype, cytokine secretion profile and CD8+ T cell stimulatory capacity as those cultured in TCPS flasks.13 Some authors, on the other hand, reported a diminished secretion of IL-12 by FEP bag-cultured Mo-DCs.15,86 Some authors also studied the performance of hydrophobic bags composed of polyolefins. Mo-DCs generated in the MACS GMP bags of the CliniMACS Prodigy® system were reported to be phenotypically and functionally equivalent to those generated by standard flask culture.42 Tan et al. reported a considerably lower DC yield and post-cryopreservation viability in polyolefin bags as compared to polystyrene flasks,16 whereas Guyre et al. suggested that bag-generated Mo-DCs were superior in yield, viability and functions.87 Variations in Mo-DC culture media, vessels and handling could account for the discrepancies observed between groups.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10)
10)
| Culture method | DC viability (%) | Maturation markers (CD80, CD83, CD86, CCR7) | Cytokine secretion | Functionality |
|---|---|---|---|---|
| Fluoropolymer bag vs. polystyrene flask (review of multiple studies by Fekete et al., 2018)10 | Comparable viability in bag and flask | Similar expression of DC maturation markers in both systems; only minor phenotype differences noted (e.g., slight variation in CD1a) | Mixed findings across studies – some report significantly lower IL-12 production in bag cultures, while others find no difference | Mo-DCs generated in closed bags are largely phenotypically and functionally similar to those from flasks. Bags can lead to reduced IL-12 (and IL-10) production in some cases, but bags offer advantages in a cGMP context. |
| Hydrophobic gas-permeable bag vs. plastic flask (Guyre et al., 2002)87 | Higher viability observed in bag-cultured DCs: >90% in bag vs. lower in flask | Typical DC markers (CD80, CD83, CD86) were expressed in both; bag-cultured DCs showed a mature phenotype with only slight differences, and MHC I/II remained high in both | IL-12 increased during maturation in both bag and flask; no significant difference after normalization | DCs produced in bags were at least as potent (if not more) in antigen presentation and T cell stimulation as flask-derived DCs. |
| FEP Teflon bag vs. polystyrene flask (Kurlander et al., 2006)15 | Comparable viability in both conditions | CD83 and CCR7 maturation marker levels were similar in bag- and flask-derived DCs; overall phenotype indistinguishable | Significantly lower IL-12 (p70) secretion in bag culture during DC maturation; IL-10 was likewise reduced in bag (quantitative reduction noted, exact pg mL−1 not given) | Bag-derived DCs showed no loss in migratory response or ability to expand antigen-specific CD8+ T cells. |
| Clinical-grade bag vs. plate culture (Rouas et al., 2010)86 | Not reported (both methods yielded viable mature DCs; no noted viability issues) | Mature DC phenotype achieved in both: high expression of CD80, CD83, CD86 | Bag-DCs secreted negligible IL-12; in contrast, plate-generated DCs produced robust IL-12 | Bag-DCs failed to initiate effective Th1 responses likely due to an altered activation profile (NF-κB and β-catenin preactivation, down-regulation of IL-12/costimulatory genes). |
| Adherent plastic dish vs. non-adherent dish (Sauter et al., 2019)98 | No significant change between adherent vs. low-attach culture | CD80, CD86, and CCR7 expression were significantly decreased in suspension (bag-like) culture compared to standard adherent culture | >10 fold lower IL-12 (p40 subunit) secretion from DCs on non-adherent surface (LPS-matured) vs. adherent surface. IL-10 and TNF-α were similarly >10-fold lower with non-adherence | DCs matured on non-adherent dishes induced antigen-specific T cells similarly to adherent-cultured DCs. |
| FEP bag vs. TCPS multi-well plate (Bastien et al., 2020)13 | No significant difference in final DC viability (bag: 75 ± 11% vs. plate: 62 ± 3%) | Comparable maturation marker profile – upregulation of CD80 (∼40% of viable cells), CD83 (∼30%), CD86 (∼80%) was equivalent in bag- and flask-grown DCs by day 9 | No significant difference in IL-12 secretion was observed upon maturation on day 9 between DCs cultured in FEP (median: 5000 pg mL−1) vs. TCPS (median: 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1); IL-10 was similarly in the same order of magnitude 000 pg mL−1); IL-10 was similarly in the same order of magnitude |
Bag vs. plate cultured Mo-DCs expanded CMV-specific CD8+ T cells with similar fold expansion and CD25 expression on both day 7 and day 14. Both culture methods yielded T cells with upregulated CD107a and granzyme B expression. |
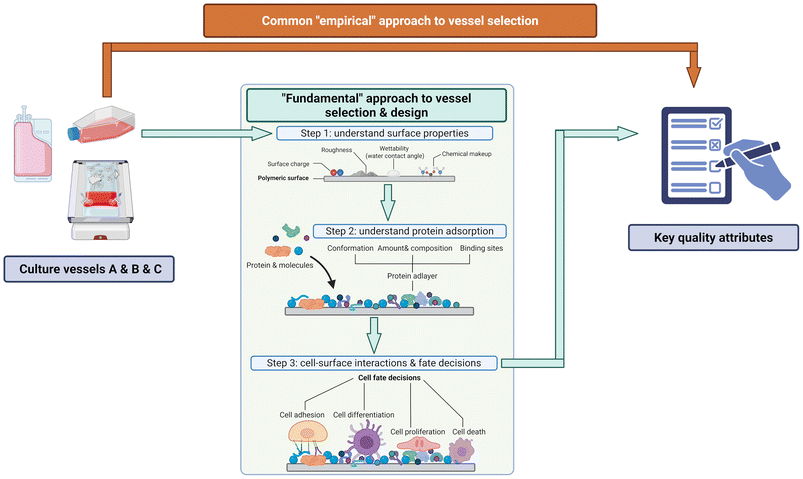
Current comparative studies of Mo-DCs cultured in flasks versus bags often lack comprehensive characterization of the culture materials used. Many studies utilize commercially available culture vessels without conducting surface characterization assays, overlooking potential lot-to-lot variations or differences between vendors (e.g., TCPS from Corning versus Falcon may undergo different surface treatments). To enable more systematic and truly comparative studies, a fundamental, stepwise approach is necessary for culture vessel selection (Fig. 4): 1. characterize surface properties such as surface charge, wettability, roughness, and surface chemistry using surface characterization techniques (e.g., scanning electron microscopy, X-ray photoelectron spectroscopy). 2. Analyze protein adsorption phenomena and characterize the resulting protein adlayer properties. 3. Study cell-surface interactions and subsequent cellular behaviors. This fundamental approach would enable more comprehensive and reproducible comparative studies by addressing important intermediate steps that are often neglected in current research, and provide informative vessel design and selection guidelines.
Current gap in knowledge on the effect of cell adhesion on the differentiation and maturation of Mo-DCs
Another source of variability in reported Mo-DC yield and quality is whether the product being characterized includes only the adherent fraction, only the suspension fraction, or both. The fraction of adherent cells and their characteristics differs from one culture material to another (e.g. between untreated polystyrene, TCPS, olefins, FEP, etc.). Some groups harvest only the suspension cell fraction as qualified Mo-DCs and regarded the adherent fraction as macrophage-like cells.12,88,89 Conversely, other groups documented that adherent Mo-DCs were functionally equivalent or even superior to the non-adherent cells.90,91 Traditional protocols rely on the adherence of monocytes to TCPS,92,93 whereas more recent research efforts have demonstrated that the generation of mature Mo-DCs could completely be carried out in suspension cultures.10,13,94 The MicroDEN® (Corning Life Sciences), a closed and automated perfusion system, was successfully employed to generate immature Mo-DCs, showing that Mo-DC differentiation could also be performed under perfusion.95Certain studies have demonstrated the critical roles played by integrin binding during cell adhesion in deciding the cell fate of monocytes and monocyte-derived cell types.68,76,96,97 Gonzalez et al. observed that binding of CD61/CD51 and CD29/CD49e to plasma proteins drove the Mo-DC differentiation in extracorporeal photochemotherapy.76 Rezzonico et al. reported that ligation of CD11b or CD11c rapidly stimulated high production of IL-1β.96 However, to date, it is still unclear whether cell adhesion is beneficial for the differentiation and maturation of a facultative adherent cell type such as Mo-DCs.10 The mechanotransduction and downstream signaling pathways that are triggered during the adhesion process remain to be systematically uncovered, which will be essential to understand the biological machinery underlying how cell–surface interactions would impact the final attributes of Mo-DC products. Although the benefits of adhesion are not yet fully understood, culture materials can be engineered through surface modifications to either promote or inhibit cell adhesion, enabling the generation of homogeneous populations and facilitating more controlled comparative studies.
Engineering tailored Mo-DC culture vessels using surface treatments
Synthetic polymers such as polystyrene and fluoropolymers have been broadly used in biological applications including in vitro cell culture owing to their excellent bulk properties such as mechanical strength, chemical resistance and biocompatibility.99,100 A surface treatment allows tuning of the surface properties without affecting the favorable bulk properties.Chemical surface treatment
Chemical surface modification techniques are the traditional methods for surface treatment due to their minimal need for specialized equipment. These techniques typically involve treating polymeric materials with gaseous or, more commonly, liquid reagents to introduce reactive functional groups on the surface through reactions such as hydrolysis, aminolysis, fluorination, oxidation, or reduction.101,102 Numerous studies have documented the chemical treatment of various polymeric materials towards cell culture applications.101–103Kou et al. found that polymethacrylate polymers with higher surface carbon content correlated with enhanced DC maturation, whereas rich surface oxygen preserved a less inflammatory, immature DC state.104 Self-assembled monolayers presenting polar terminal groups (–OH, –COOH, –NH2) cause modest DC maturation. In contrast, Mo-DCs on CH3-terminated monolayers unexpectedly released high levels of TNF-α and IL-6 despite being the least mature, likely due to increased apoptosis and altered integrin signaling on the non-polar surface.105 DCs cultured on clinical titanium substrate that were sandblasted and acid etched expressed higher levels of maturation markers and pro-inflammatory cytokines compared to those cultured on smooth tissue culture plastic controls.106 A principal component analysis demonstrated that non-stimulating surface property and the resulting immature DC phenotype are associated with high surface hydrophilicity and surface oxygen content.106 These studies indicate that surface modifications can tune the balance of stimulatory vs. tolerogenic signals received by DCs during culture. By choosing “stimulatory” surface treatments, researchers can enhance the immunogenicity of Mo-DCs prepared for therapy.
Plasma surface treatment
Plasma surface treatment is considered a prominent alternative to wet chemistry for cell culture vessel treatment due to its high reproducibility, versatility, minimal waste production and solvent-free nature.99,100 Plasma is often referred to as the fourth state of matter.100,107 When gas molecules are provided with sufficient energy, some or all molecules will have gained enough energy to ionize, resulting in a mixture of ions, free electrons, radicals, photons, and neutral species that is so-called a plasma. Plasma is generally classified into two main categories: thermal and non-thermal plasmas.100 For the surface treatment of polymeric substrates, non-thermal low-temperature plasma is mostly used as it does not impose thermal damage to the polymers. There exist different interaction modes between a plasma and a surface which gives rise to various plasma modification techniques including plasma polymerization, plasma-induced grafting (of chemical functionalities), plasma activation, sputtering and etching, and plasma syn-irradiation. The underlying principles and reaction mechanisms of these different techniques have been extensively reviewed elsewhere.99,100Applying an electrical potential remains the most commonly used strategy to generate non-thermal gaseous plasma, which is generally referred to as electrical discharge plasma.108,109 Corona discharge is perhaps the best example of electrical discharge plasma that is widely used in the treatment of TCPS culture ware by several manufacturers, including Nunclon Delta® (Thermo Scientific) and CellBIND® (Corning). Electrical discharge processes come in two basic types: low-pressure (or vacuum) and atmospheric discharge.108 Low-pressure plasma systems employ a vacuum chamber, whereby ambient gas in the chamber is pumped out and then filled with the desired process gas at a pre-set and controlled pressure. Atmospheric plasma, with the example of corona discharge, is generated by applying a high voltage to an electrode in the form of a wire or a sharp tip.107,110 As air or surrounding gas passes through the electrode, a fraction of the gas molecules is ionized, leading to the formation of a corona discharge that bombards the treated surface to introduce oxidation products.101 Corona discharge is widely used in the manufacturing of culture vessels due to its simplicity, lower operational costs, and lack of need for specialized equipment like vacuum chambers. However, it offers limited control over the physicochemical characteristics of the treated surfaces and is reported to have a short stability period on polyolefins.101,111
Plasma polymerization employs a polymerizable monomer in either gas or liquid state at the plasma discharge, which gets converted into reactive fragments that form a highly cross-linked, thin plasma polymer coating (PPC) upon reacting with a surface.100 Hydrocarbons are frequently used as the monomer of choice. Considering the important roles of oxygen- and nitrogen-containing functional groups in the context of biological applications, one or more heteroatom gas sources rich in oxygen or nitrogen elements (e.g. carbon dioxide and ammonia) are often mixed with a polymerizable hydrocarbon gas (e.g. ethylene and butadiene) to form the precursor gas mixture.112 The resulting active species condense onto the substrate, forming an organic thin-film coating with oxygen and/or nitrogen-containing functionalities implanted in the cross-linked hydrocarbon backbone. The concentration of implanted functional groups can be controlled by adjusting the deposition process parameters such as the hydrocarbon-to-heteroatom gas ratio.112
A set of oxygen-rich and nitrogen-rich PPCs have been developed and characterized to study their potential in biological and biomedical applications.83,108,112,113 It has been reported extensively that the introduction of biologically relevant functionalities such as primary amines (–NH2), hydroxyl (–OH), and carboxyl (–COOH) groups led to enhanced adhesion of various cell types.7,8,114 Past studies by our team revealed that the number of adherent monocytes correlated to the concentration of functional groups present on the surface.83 On the other hand, a low-adhesion coating such as a PPC carrying methyl (–CH3) can be applied if minimizing cell adhesion is favored.8 These studies reveal that material surface properties play decisive roles in controlling cell adhesion at the substrate interface, presenting a prospect to potentially modulate cell–surface interactions by tuning the substrate surface chemistry with surface modification techniques.
Peptide or protein surface modification
The surface modifications described above influence which proteins adsorb and how, which in turn can affect the interactions between Mo-DCs and surfaces. However, another approach is to attach bioactive compounds, such as cell adhesion and receptor-interacting molecules to the surfaces. This bioconjugation typically requires functional group precursors to immobilize biomolecules through covalent grafting (for example, via prior plasma treatment).101 Our group has successfully employed this method to capture and expand endothelial progenitor cells on peptide-functionalized polystyrene surfaces.115,116 Studies have also demonstrated the use of peptide grafting to modulate immune cell interactions and responses.117,118 For example, RGD-grafted surfaces have been shown to enhance the adhesion and activation (elevated expression of CD86, MHC-II and intracellular IL-10) of dendritic cells.119 Additionally, this strategy could potentially be used to create a completely non-adhesive Mo-DC substrate by grafting proteins or polymers that block adhesion. Similarly, polymers such as poly(N-isopropylacrylamide) (PNIPAM), which undergo temperature-responsive phase transitions, have been used to enable conditional cell detachment or adherence based on environmental stimuli.120Surface topography and mechanical stimuli
Researchers are also increasingly exploring biomechanical engineering to direct cellular responses. Mennens et al. observed that immature DCs cultured on polyacrylamide substrates of varying stiffness exhibited differential C-type lectin expression and antigen internalization.121 Quartey et al.'s recent review highlights how DCs sense nanoscale features of their microenvironment through integrin-mediated mechanotransduction and adapt their antigen uptake, migration, and maturation to mechanical stimuli introduced by modifying the surfaces with 3D hydrogels or ECM-mimetic substrates.122 As an example, DCs cultured in 3D hydrogels with aligned collagen fibers exhibited a reprogrammed metabolic profile and greater maturation compared to those in non-aligned matrices.122 We recommend that readers refer to Quartey et al. for an extensive review of emerging biomaterial platforms such as hydrogels, 3D scaffolds, and nanostructured surfaces incorporating mechanotransduction and matrix biology, all of which offer promise in designing DC culture vessels.In addition to static surface modifications, fluid shear stress (FSS) has emerged as an orthogonal mechanical cue to activate Mo-DCs in ex vivo culture. Dombroski et al. used a cone-and-plate device to expose immature DCs to circulatory-level FSS and observed increased secretion of pro-inflammatory cytokines, upregulation of MHC I/II and costimulatory markers, as well as activation of NF-κB and cFos phosphorylation relative to static controls.123 These findings indicate that optimized shear forces can amplify immunogenicity without substantially compromising cell health.123 Hence, dynamic substrates that apply cyclic strain or introduce fluid flow, such as perfusion bioreactors, rocking platforms or in-line pressure and shear sensors could potentially be incorporated into the manufacturing processes.
Translating engineered surfaces into clinical culture vessels
Surface engineering offers a powerful strategy to optimize DC vaccines by modulating the culture environment at the cell–material interface. By engineering surface chemistry, topography, and stiffness, researchers have been able to modulate DC maturation markers, cytokine profiles, and immunogenic potential in ways directly relevant to vaccine efficacy.124In cGMP-compliant manufacturing, any techniques employed to modulate surface properties must minimize leachable and extractable compounds and be compatible with rigorous cleaning, sterilization, and lot-to-lot reproducibility. At the same time, tailored culture vessels with specialized coatings or proprietary materials may enhance Mo-DC potency but can also increase production costs and limit accessibility. Understanding how vessel selection and surface treatment influence critical quality attributes while balancing cGMP requirements and cost will be essential for transitioning DC vaccines into clinical trials and ensuring broad, sustainable access to these therapies.
Conclusion
With the rapid expansion of the global cell therapy market, the impact of in vitro cell culture on the final therapeutic efficacy has gained much traction and the selection of appropriate culture vessels has become an important consideration in the product development strategy. Despite their appealing prospects, Mo-DC cancer immunotherapies have only shown limited benefits in clinical trials. Strategically designed culture vessels together with ongoing advances in culture preparation methods represent auspicious means that may overcome the challenge presented by the limited clinical performance. In fact, Mo-DC generation is associated with high batch-to-batch variability, which is another challenge currently faced in the field. This is, in part, a result of the poor control over cell–surface interactions during cell preparation. The cell–surface interaction mechanisms presented in this review constitute the first step in the cascades to fully elucidate the impact of in vitro cell culture on Mo-DC cell fate, and will inform the design and development of next-generation biomaterials. Novel surface modification techniques can be employed to fine tune vessel surface properties, either enhancing or inhibiting cell adhesion mechanisms to better control cell behavior at the substrate interface. By optimizing these interactions, it is possible to improve both in vitro manufacturing efficiency and the in vivo therapeutic efficacy, and ultimately leading to more consistent and scalable production methods for clinical applications.Author contributions
Jiyu Jessica Tian: conceptualization, writing – original draft, writing – review and editing. Hamid Ebrahimi Orimi: writing – review and editing. Natalie Fekete: writing – review and editing. Nicolas Drolet: writing – review and editing. Katie Campbell: writing – review and editing. Michel L. Tremblay: writing – review and editing. Linda Peltier: writing – review and editing. Pierre Laneuville: writing – review and editing. Pierre-Luc Girard-Lauriault: conceptualization, writing – review and editing, supervision. Corinne A. Hoesli: conceptualization, writing – review and editing, supervision, project administration, funding acquisition.Conflicts of interest
J. Tian was employed by STEMCELL Technologies while the review was revised. N. Fekete was employed by Saint-Gobain at the time the review was initially written. K. Campbell and N. Drolet were employed by Saint-Gobain at the time the review was written or reviewed. M. L. Temblay is co-founder and chief scientific officer of Kanyr Pharma. C. H. is co-founder and shareholder of Capcyte Biotherapeutics and CellTerix Biomedical.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
J. Tian was funded by the Fonds de recherche du Quebec – Nature et technologies Master's scholarship, program: Bourses de maîtrise en recherche. Adhésion des monocytes aux plastiques de culture cellulaire pendant la culture, dans le cadre de la production d'immunothérapie cellulaire contre le cancer; fund number: 298275, available from: https://doi.org/10.69777/298275. This work was funded through Collaborative Research and Development (CRD) grant between Saint-Gobain Ceramics & Plastics, Inc., Canada's National Sciences and Engineering Research Council Collaborative Research and Development Grants (NSERC), MEDTEQ – a Quebec Consortium for Industrial Research and Innovation in Medical Technology and MITACS. We thank Balaji Ramachandran, Praveen Kumar Raju and Lisa Danielczak for their insightful discussions and comments. We express our gratitude for the following networks for activities in support of this project: Quebec Cell, Tissue and Gene Therapy Network – ThéCell (a thematic network supported by the Fonds de recherche du Québec – Santé), Réseaux thématiques de recherche, 2024-2028, DOI: https://doi.org/10.69777/337725.; PROTEO (The Quebec Network for Research on Protein Function), Regroupements stratégiques / à maturation, 2024-2030, DOI: https://doi.org/10.69777/341121; CQMF/QCAM (Quebec Centre for Advanced Materials) Regroupements stratégiques / à maturation, 2024-2030, DOI: https://doi.org/10.69777/341666; Canada's SCN (Stem Cell Network).References
- M. J. Lerman, J. Lembong, S. Muramoto, G. Gillen and J. P. Fisher, The Evolution of Polystyrene as a Cell Culture Material, Tissue Eng., Part B, 2018, 24(5), 359–372 CrossRef CAS PubMed.
- J. Chisholm, C. Ruff and S. Viswanathan, Current state of Health Canada regulation for cellular and gene therapy products: potential cures on the horizon, Cytotherapy, 2019, 21(7), 686–698 CrossRef PubMed.
- P. Bedford, J. Jy, L. Collins and S. Keizer, Considering Cell Therapy Product “Good Manufacturing Practice” Status, Front. Med., 2018, 5, 118 CrossRef.
- C. J. Wilson, R. E. Clegg, D. I. Leavesley and M. J. Pearcy, Mediation of biomaterial-cell interactions by adsorbed proteins: a review, Tissue Eng., 2005, 11(1–2), 1–18 CrossRef CAS PubMed.
- A. Wargenau, N. Fekete, A. V. Beland, G. Sabbatier, O. M. Bowden and M. D. Boulanger, et al., Protein film formation on cell culture surfaces investigated by quartz crystal microbalance with dissipation monitoring and atomic force microscopy, Colloids Surf., B, 2019, 183, 110447 Search PubMed.
- A. Carré and V. Lacarrière, How Substrate Properties Control Cell Adhesion. A Physical–Chemical Approach, J. Adhes. Sci. Technol., 2010, 24(5), 815–830 CrossRef.
- M. Zelzer, D. Albutt, M. R. Alexander and N. A. Russell, The Role of Albumin and Fibronectin in the Adhesion of Fibroblasts to Plasma Polymer Surfaces, Plasma Processes Polym., 2012, 9(2), 149–156 CrossRef CAS.
- Y. Arima and H. Iwata, Effects of surface functional groups on protein adsorption and subsequent cell adhesion using self-assembled monolayers, J. Mater. Chem., 2007, 17(38), 4079–4087 Search PubMed.
- H. M. Rostam, S. Singh, F. Salazar, P. Magennis, A. Hook and T. Singh, et al., The impact of surface chemistry modification on macrophage polarisation, Immunobiology, 2016, 221(11), 1237–1246 CrossRef CAS.
- N. Fekete, A. V. Béland, K. Campbell, S. L. Clark and C. A. Hoesli, Bags versus flasks: a comparison of cell culture systems for the production of dendritic cell-based immunotherapies, Transfusion, 2018, 58(7), 1800–1813 CrossRef CAS.
- E. C. C. Wong, S. M. Lee, K. Hines, J. Lee, C. S. Carter and W. Kopp, et al., Development of a closed-system process for clinical-scale generation of DCs: evaluation of two monocyte-enrichment methods and two culture containers, Cytotherapy, 2002, 4(1), 65–76 CrossRef CAS.
- Y. Suen, S. M. Lee, F. Aono, S. Hou, M. Loudovaris and G. Ofstein, et al., Comparison of monocyte enrichment by immuno-magnetic depletion or adherence for the clinical-scale generation of DC, Cytotherapy, 2001, 3(5), 365–375 CrossRef CAS.
- J. P. Bastien, N. Fekete, A. V. Beland, M. P. Lachambre, V. Laforte and D. Juncker, et al., Closing the system: production of viral antigen-presenting dendritic cells eliciting specific CD8+ T cell activation in fluorinated ethylene propylene cell culture bags, J. Transl. Med., 2020, 18(1), 383 CrossRef CAS PubMed.
- N. Fekete, G. Sabbatier, A. Wargenau, A. Béland, S. Xu and N. Tufenkji, et al., Effect of fluoropolymer-based culture vessel surface on monocyte differentiation, Cytotherapy, 2018, 20(5), S109 CrossRef.
- R. J. Kurlander, A. Tawab, Y. Fan, C. S. Carter and E. J. Read, A functional comparison of mature human dendritic cells prepared in fluorinated ethylene-propylene bags or polystyrene flasks, Transfusion, 2006, 46(9), 1494–1504 CrossRef CAS PubMed.
- Y. F. Tan, G. C. Sim, A. Habsah, C. F. Leong and S. K. Cheong, Experimental production of clinical-grade dendritic cell vaccine for acute myeloid leukemia, Malays. J. Pathol., 2008, 30(2), 73–79 Search PubMed.
- A. Ahmad Khalili and M. R. Ahmad, A Review of Cell Adhesion Studies for Biomedical and Biological Applications, Int. J. Mol. Sci., 2015, 16(8), 18149–18184 CrossRef.
- J. Choi and C. S. Ki, Differentiation, maturation, and collection of THP-1-derived dendritic cells based on a PEG hydrogel culture platform, Biotechnol. Lett., 2024, 46(2), 235–247 CrossRef CAS PubMed.
- Y. Fang, B. Wang, Y. Zhao, Z. Xiao, J. Li and Y. Cui, et al., Collagen scaffold microenvironments modulate cell lineage commitment for differentiation of bone marrow cells into regulatory dendritic cells, Sci. Rep., 2017, 7, 42049 CrossRef CAS.
- Y. Yang, Y. Lin, R. Xu, Z. Zhang, W. Zeng and Q. Xu, et al., Micro/Nanostructured Topography on Titanium Orchestrates Dendritic Cell Adhesion and Activation via β2 Integrin-FAK Signals, Int. J. Nanomed., 2022, 17, 5117–5136 Search PubMed.
- R. M. Steinman and Z. A. Cohn, IDENTIFICATION OF A NOVEL CELL TYPE IN PERIPHERAL LYMPHOID ORGANS OF MICE, J. Exp. Med., 1973, 137(5), 1142–1162 CrossRef CAS.
- I. Y. Filin, K. V. Kitaeva, C. S. Rutland, A. A. Rizvanov and V. V. Solovyeva, Recent Advances in Experimental Dendritic Cell Vaccines for Cancer, Front. Oncol., 2021, 11, 730824 CrossRef CAS PubMed.
- A. Gardner, Á. de Mingo Pulido and B. Ruffell, Dendritic Cells and Their Role in Immunotherapy, Front. Immunol., 2020, 11, 924 CrossRef CAS PubMed.
- Sipuleucel-T: APC 8015, APC-8015, prostate cancer vaccine–Dendreon, Drugs R&D, 2006, 7(3), 197–201, DOI:10.2165/00126839-200607030-00006.
- S. Anguille, E. L. Smits, E. Lion, V. F. van Tendeloo and Z. N. Berneman, Clinical use of dendritic cells for cancer therapy, Lancet Oncol., 2014, 15(7), e257–e267 CrossRef CAS PubMed.
- E. L. Hopewell and C. Cox, Manufacturing Dendritic Cells for Immunotherapy: Monocyte Enrichment, Mol. Ther. – Methods Clin. Dev., 2020, 16, 155–160 CrossRef CAS.
- K. Palucka and J. Banchereau, Cancer immunotherapy via dendritic cells, Nat. Rev. Cancer, 2012, 12(4), 265–277 CrossRef CAS PubMed.
- J. A. Cintolo, J. Datta, S. J. Mathew and B. J. Czerniecki, Dendritic cell-based vaccines: barriers and opportunities, Future Oncol., 2012, 8(10), 1273–1299 CrossRef CAS PubMed.
- C. Fu and A. Jiang, Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment, Front. Immunol., 2018, 9, 3059 CrossRef CAS.
- Z. Hu, P. A. Ott and C. J. Wu, Towards personalized, tumour-specific, therapeutic vaccines for cancer, Nat. Rev. Immunol., 2018, 18(3), 168–182 CrossRef CAS.
- I. Gutcher and B. Becher, APC-derived cytokines and T cell polarization in autoimmune inflammation, J. Clin. Invest., 2007, 117(5), 1119–1127 CrossRef CAS.
- B. León, A model of Th2 differentiation based on polarizing cytokine repression, Trends Immunol., 2023, 44(6), 399–407 CrossRef PubMed.
- N. G. Jacobson, S. J. Szabo, R. M. Weber-Nordt, Z. Zhong, R. D. Schreiber and J. E. Darnell Jr, et al., Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4, J. Exp. Med., 1995, 181(5), 1755–1762 CrossRef CAS PubMed.
- E. Segura, Human dendritic cell subsets: An updated view of their ontogeny and functional specialization, Eur. J. Immunol., 2022, 52(11), 1759–1767 CrossRef CAS PubMed.
- C. R. Perez and M. De Palma, Engineering dendritic cell vaccines to improve cancer immunotherapy, Nat. Commun., 2019, 10, 5408 CrossRef.
- M. Guilliams, F. Ginhoux, C. Jakubzick, S. H. Naik, N. Onai and B. U. Schraml, et al., Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny, Nat. Rev. Immunol., 2014, 14(8), 571–578 CrossRef CAS PubMed.
- F. M. Cruz, J. D. Colbert, E. Merino, B. A. Kriegsman and K. L. Rock, The biology and underlying mechanisms of cross-presentation of exogenous antigens on MHC I molecules, Annu. Rev. Immunol., 2017, 35, 149–176 CrossRef CAS PubMed.
- S. Kuhn, J. Yang and F. Ronchese, Monocyte-Derived Dendritic Cells Are Essential for CD8+ T Cell Activation and Antitumor Responses After Local Immunotherapy, Front. Immunol., 2015, 6, 584 Search PubMed.
- J. Liu, X. Zhang, Y. Cheng and X. Cao, Dendritic cell migration in inflammation and immunity, Cell. Mol. Immunol., 2021, 18(11), 2461–2471 CrossRef CAS PubMed.
- J. Haller Hasskamp, J. L. Zapas and E. G. Elias, Dendritic cell counts in the peripheral blood of healthy adults, Am. J. Hematol., 2005, 78(4), 314–315 CrossRef.
- B. Mastelic-Gavillet, K. Balint, C. Boudousquie, P. O. Gannon and L. E. Kandalaft, Personalized Dendritic Cell Vaccines—Recent Breakthroughs and Encouraging Clinical Results, Front. Immunol., 2019, 10, 766 CrossRef CAS PubMed.
- M. Erdmann, U. Uslu, M. Wiesinger, M. Brüning, T. Altmann and E. Strasser, et al., Automated closed-system manufacturing of human monocyte-derived dendritic cells for cancer immunotherapy, J. Immunol. Methods, 2018, 463, 89–96 CrossRef CAS.
- A. Draube, N. Klein-González, S. Mattheus, C. Brillant, M. Hellmich and A. Engert, et al., Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis, PLoS One, 2011, 6(4), e18801 CrossRef CAS.
- P. W. Kantoff, C. S. Higano, N. D. Shore, E. R. Berger, E. J. Small and D. F. Penson, et al., Sipuleucel-T immunotherapy for castration-resistant prostate cancer, N. Engl. J. Med., 2010, 363(5), 411–422 Search PubMed.
- F. Borges, R. S. Laureano, I. Vanmeerbeek, J. Sprooten, O. Demeulenaere and J. Govaerts, et al., Trial watch: anticancer vaccination with dendritic cells, OncoImmunology, 2024, 13(1), 2412876 CrossRef.
- J. Han and H. Wang, Cytokine-overexpressing dendritic cells for cancer immunotherapy, Exp. Mol. Med., 2024, 56(12), 2559–2568 CrossRef CAS.
- B. De Keersmaecker, S. Claerhout, J. Carrasco, I. Bar, J. Corthals and S. Wilgenhof, et al., TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: link between T-cell activation and clinical responses in advanced melanoma, J. Immunother. Cancer, 2020, 8(1), e000329 CrossRef PubMed.
- D. J. Chung, S. Sharma, M. Rangesa, S. DeWolf, Y. Elhanati and K. Perica, et al., Langerhans dendritic cell vaccine bearing mRNA-encoded tumor antigens induces antimyeloma immunity after autotransplant, Blood Adv., 2022, 6(5), 1547–1558 CrossRef CAS PubMed.
- D. Y. Lee, S. Y. Lee, S. H. Yun, J. W. Jeong, J. H. Kim and H. W. Kim, et al., Review of the Current Research on Fetal Bovine Serum and the Development of Cultured Meat, Food Sci. Anim. Resour., 2022, 42(5), 775–799 Search PubMed.
- T. Yao and Y. Asayama, Animal-cell culture media: History, characteristics, and current issues, Reprod. Med. Biol., 2017, 16(2), 99–117 CrossRef PubMed.
- J. Keenan, D. Pearson and M. Clynes, The role of recombinant proteins in the developmentof serum-free media, Cytotechnology, 2006, 50(1), 49 CrossRef CAS PubMed.
- G. M. Minonzio and E. Linetsky, The Use of Fetal Bovine Serum in Cellular Products For Clinical Applications: Commentary, CellR4, 2014, 2(6), e1307 Search PubMed.
- S. Kim, H. O. Kim, E. J. Baek, Y. Choi, H. S. Kim and M. G. Lee, Monocyte enrichment from leukapheresis products by using the Elutra cell separator, Transfusion, 2007, 47(12), 2290–2296 CrossRef PubMed.
- D. F. Stroncek, V. Fellowes, C. Pham, H. Khuu, D. H. Fowler and L. V. Wood, et al., Counter-flow elutriation of clinical peripheral blood mononuclear cell concentrates for the production of dendritic and T cell therapies, J. Transl. Med., 2014, 12(1), 241 CrossRef PubMed.
- J. D. M. Campbell, C. Piechaczek, G. Winkels, E. Schwamborn, D. Micheli and S. Hennemann, et al., Isolation and Generation of Clinical-Grade Dendritic Cells Using the CliniMACS System. in Adoptive Immunotherapy: Methods and Protocols, ed. B. Ludewig and M. W. Hoffmann, Humana Press, Totowa, NJ, 2005, pp. 55–70, [cited 2023 Jan 27]. (Methods in Molecular MedicineTM). Available from: DOI:10.1385/1-59259-862-5:055.
- S. Nava, M. Dossena, S. Pogliani, S. Pellegatta, C. Antozzi and F. Baggi, et al., An Optimized Method for Manufacturing a Clinical Scale Dendritic Cell-Based Vaccine for the Treatment of Glioblastoma, PLoS One, 2012, 7(12), e52301 CrossRef CAS.
- M. Apel, M. Brüning, M. Granzin, M. Essl, J. Stuth and J. Blaschke, et al., Integrated Clinical Scale Manufacturing System for Cellular Products Derived by Magnetic Cell Separation, Centrifugation and Cell Culture, Chem. Ing. Tech., 2013, 85(1–2), 103–110 CrossRef CAS.
- H. Teng, Overview of the Development of the Fluoropolymer Industry, Appl. Sci., 2012, 2(2), 496–512 CrossRef.
- S. Ebnesajjad, 5 - Introduction to Thermoplastic Fluoropolymers, in Introduction to Fluoropolymers, ed. S. Ebnesajjad, William Andrew Publishing, Oxford, 2nd edn, 2021, pp. 43–61. (Plastics Design Library) Search PubMed.
- I. Ganeeva, E. Zmievskaya, A. Valiullina, A. Kudriaeva, R. Miftakhova and A. Rybalov, et al., Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies, Bioengineering, 2022, 9(12), 808 CrossRef CAS PubMed.
- A. Melocchi, B. Schmittlein, S. Sadhu, S. Nayak, A. Lares and M. Uboldi, et al., Automated manufacturing of cell therapies, J. Controlled Release, 2025, 381, 113561 Search PubMed.
- C. H. Hulme, C. Mennan, H. S. McCarthy, R. Davies, T. Lan and L. Rix, et al., A comprehensive review of quantum bioreactor cell manufacture: Research and clinical applications, Cytotherapy, 2023, 25(10), 1017–1026 Search PubMed.
- H. Harjunpää, M. Llort Asens, C. Guenther and S. C. Fagerholm, Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment, Front. Immunol., 2019, 10, 1078 CrossRef PubMed.
- A. J. García, Get a grip: integrins in cell-biomaterial interactions, Biomaterials, 2005, 26(36), 7525–7529 CrossRef PubMed.
- C. Wu, Focal adhesion: a focal point in current cell biology and molecular medicine, Cell Adhes. Migr., 2007, 1(1), 13–18 Search PubMed.
- E. H. J. Danen, Integrins: An Overview of Structural and Functional Aspects, in Madame Curie Bioscience Database, Landes Bioscience, Austin (TX), 2000–2013 Search PubMed.
- I. D. Campbell and M. J. Humphries, Integrin structure, activation, and interactions, Cold Spring Harbor Perspect. Biol., 2011, 3(3), a004994 Search PubMed.
- S. Brilha, R. Wysoczanski, A. M. Whittington, J. S. Friedland and J. C. Porter, Monocyte Adhesion, Migration, and Extracellular Matrix Breakdown Are Regulated by Integrin αVβ3 in Mycobacterium tuberculosis Infection, J. Immunol., 2017, 199(3), 982–991 CrossRef CAS.
- Y. Miyazaki, A. Vieira-de-Abreu, E. S. Harris, A. M. Shah, A. S. Weyrich and H. C. Castro-Faria-Neto, et al., Integrin αDβ2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling, PLoS One, 2014, 9(11), e112770 CrossRef PubMed.
- M. Shen and T. A. Horbett, The effects of surface chemistry and adsorbed proteins on monocyte/macrophage adhesion to chemically modified polystyrene surfaces, J. Biomed. Mater. Res., 2001, 57(3), 336–345 CrossRef CAS.
- A. K. McNally and J. M. Anderson, Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation, Am. J. Pathol., 2002, 160(2), 621–630 Search PubMed.
- R. Garnotel, L. Rittié, S. Poitevin, J. C. Monboisse, P. Nguyen and G. Potron, et al., Human Blood Monocytes Interact with Type I Collagen Through αxβ2 Integrin (CD11c-CD18, gp150-95), J. Immunol., 2000, 164(11), 5928–5934 CrossRef CAS PubMed.
- M. Patarroyo, J. Prieto, P. G. Beatty, E. A. Clark and C. G. Gahmberg, Adhesion-mediating molecules of human monocytes, Cell. Immunol., 1988, 113(2), 278–289 CrossRef CAS PubMed.
- A. K. McNally and J. M. Anderson, Complement C3 participation in monocyte adhesion to different surfaces, Proc. Natl. Acad. Sci. U. S. A., 1994, 91(21), 10119–10123 CrossRef CAS PubMed.
- N. Sándor, S. Lukácsi, R. Ungai-Salánki, N. Orgován, B. Szabó and R. Horváth, et al., CD11c/CD18 Dominates Adhesion of Human Monocytes, Macrophages and Dendritic Cells over CD11b/CD18, PLoS One, 2016, 11(9), e0163120 CrossRef PubMed.
- A. L. Gonzalez, C. L. Berger, J. Remington, M. Girardi, R. E. Tigelaar and R. L. Edelson, Integrin-driven monocyte to dendritic cell conversion in modified extracorporeal photochemotherapy, Clin. Exp. Immunol., 2014, 175(3), 449–457 CrossRef CAS PubMed.
- N. Orgovan, R. Ungai-Salánki, S. Lukácsi, N. Sándor, Z. Bajtay and A. Erdei, et al., Adhesion kinetics of human primary monocytes, dendritic cells, and macrophages: Dynamic cell adhesion measurements with a label-free optical biosensor and their comparison with end-point assays, Biointerphases, 2016, 11(3), 031001 CrossRef PubMed.
- T. Hoshiba, C. Yoshikawa and K. Sakakibara, Characterization of Initial Cell Adhesion on Charged Polymer Substrates in Serum-Containing and Serum-Free Media, Langmuir, 2018, 34(13), 4043–4051 Search PubMed.
- M. R. Wertheimer, A. St-Georges-Robillard, S. Lerouge, F. Mwale, B. Elkin and C. Oehr, et al., Amine-Rich Organic Thin Films for Cell Culture: Possible Electrostatic Effects in Cell–Surface Interactions, Jpn. J. Appl. Phys., 2012, 51(11S), 11PJ04 Search PubMed.
- L. Bacakova, E. Filova, M. Parizek, T. Ruml and V. Svorcik, Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants, Biotechnol. Adv., 2011, 29(6), 739–767 CrossRef CAS PubMed.
- H. Y. Chang, W. L. Kao, Y. W. You, Y. H. Chu, K. J. Chu and P. J. Chen, et al., Effect of surface potential on epithelial cell adhesion, proliferation and morphology, Colloids Surf., B, 2016, 141, 179–186 CrossRef CAS PubMed.
- A. Rosenhahn, J. A. Finlay, M. E. Pettit, A. Ward, W. Wirges and R. Gerhard, et al., Zeta potential of motile spores of the green alga Ulva linza and the influence of electrostatic interactions on spore settlement and adhesion strength, Biointerphases, 2009, 4(1), 7–11 CrossRef PubMed.
- S. Babaei, N. Fekete, C. A. Hoesli and P. L. Girard-Lauriault, Adhesion of human monocytes to oxygen- and nitrogen- containing plasma polymers: Effect of surface chemistry and protein adsorption, Colloids Surf., B, 2018, 162, 362–369 CrossRef CAS PubMed.
- C. Ammon, S. P. Meyer, L. Schwarzfischer, S. W. Krause, R. Andreesen and M. Kreutz, Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells, Immunology, 2000, 100(3), 364–369 Search PubMed.
- L. Schittenhelm, C. M. Hilkens and V. L. Morrison, β2 Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function, Front. Immunol., 2017, 8, 1866 CrossRef PubMed.
- R. Rouas, H. Akl, H. Fayyad-Kazan, N. El Zein, B. Badran and B. Nowak, et al., Dendritic Cells Generated in Clinical Grade Bags Strongly Differ in Immune Functionality When Compared With Classical DCs Generated in Plates, J. Immunother., 2010, 33(4), 352 CrossRef CAS PubMed.
- C. A. Guyre, J. L. Fisher, M. G. Waugh, P. K. Wallace, C. G. Tretter and M. S. Ernstoff, et al., Advantages of hydrophobic culture bags over flasks for the generation of monocyte-derived dendritic cells for clinical applications, J. Immunol. Methods, 2002, 262(1–2), 85–94 CrossRef CAS PubMed.
- J. Wang, X. Dai, C. Hsu, C. Ming, Y. He and J. Zhang, et al., Discrimination of the heterogeneity of bone marrow-derived dendritic cells, Mol. Med. Rep., 2017, 16(5), 6787–6793 CrossRef CAS PubMed.
- V. Pullarkat, R. Lau, S. M. Lee, J. G. Bender and J. S. Weber, Large-scale monocyte enrichment coupled with a closed culture system for the generation of human dendritic cells, J. Immunol. Methods, 2002, 267(2), 173–183 CrossRef CAS PubMed.
- H. J. Yi and G. X. Lu, Adherent and non-adherent dendritic cells are equivalently qualified in GM-CSF, IL-4 and TNF-α culture system, Cell. Immunol., 2012, 277(1–2), 44–48 Search PubMed.
- G. B. Li and G. X. Lu, Adherent cells in granulocyte-macrophage colony-stimulating factor-induced bone marrow-derived dendritic cell culture system are qualified dendritic cells, Cell. Immunol., 2010, 264(1), 4–6 CrossRef CAS PubMed.
- N. Romani, D. Reider, M. Heuer, S. Ebner, E. Kämpgen and B. Eibl, et al., Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability, J. Immunol. Methods, 1996, 196(2), 137–151 CrossRef CAS PubMed.
- W. F. Pickl, O. Majdic, P. Kohl, J. Stöckl, E. Riedl and C. Scheinecker, et al., Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes, J. Immunol., 1996, 157(9), 3850–3859 CrossRef CAS.
- R. V. Sorg, Z. Ozcan, T. Brefort, J. Fischer, R. Ackermann and M. Müller, et al., Clinical-scale generation of dendritic cells in a closed system, J. Immunother., 2003, 26(4), 374–383 CrossRef PubMed.
- A. Kozbial, L. Bhandary, B. B. Collier, C. S. Eickhoff, D. Hoft and S. K. Murthy, Automated generation of immature dendritic cells in a single-use system, J. Immunol. Methods, 2018, 457, 53–65 CrossRef CAS PubMed.
- R. Rezzonico, R. Chicheportiche, V. Imbert and J. M. Dayer, Engagement of CD11b and CD11c β2 integrin by antibodies or soluble CD23 induces IL-1β production on primary human monocytes through mitogen-activated protein kinase–dependent pathways, Blood, 2000, 95(12), 3868–3877 Search PubMed.
- M. Reyes-Reyes, N. Mora, G. Gonzalez and C. Rosales, beta1 and beta2 integrins activate different signalling pathways in monocytes, Biochem. J., 2002, 363(Pt 2), 273–280 CrossRef CAS PubMed.
- A. Sauter, D. H. Yi, Y. Li, S. Roersma and S. Appel, The Culture Dish Surface Influences the Phenotype and Cytokine Production of Human Monocyte-Derived Dendritic Cells, Front. Immunol., 2019, 10, 2352 CrossRef CAS PubMed.
- A. Solouk, B. G. Cousins, H. Mirzadeh and A. M. Seifalian, Application of plasma surface modification techniques to improve hemocompatibility of vascular grafts: A review, Biotechnol. Appl. Biochem., 2011, 58(5), 311–327 CrossRef CAS PubMed.
- T. Jacobs, R. Morent, N. De Geyter, P. Dubruel and C. Leys, Plasma Surface Modification of Biomedical Polymers: Influence on Cell-Material Interaction, Plasma Chem. Plasma Process., 2012, 32(5), 1039–1073 CrossRef CAS.
- J. M. Goddard and J. H. Hotchkiss, Polymer surface modification for the attachment of bioactive compounds, Prog. Polym. Sci., 2007, 32(7), 698–725 CrossRef CAS.
- D. Hetemi and J. Pinson, in Surface Modification of Polymers, ed. J. Pinson, and D. Thiry, John Wiley & Sons, Ltd, 1st edn, 2019, pp. 211–240, Available from: https://onlinelibrary.wiley.com/doi/10.1002/9783527819249 Search PubMed.
- A. P. Kharitonov and L. N. Kharitonova, Surface modification of polymers by direct fluorination: A convenient approach to improve commercial properties of polymeric articles, Pure Appl. Chem., 2009, 81(3), 451–471 Search PubMed.
- P. M. Kou, N. Pallassana, R. Bowden, B. Cunningham, A. Joy and J. Kohn, et al., Predicting biomaterial property-dendritic cell phenotype relationships from the multivariate analysis of responses to polymethacrylates, Biomaterials, 2012, 33(6), 1699–1713 Search PubMed.
- S. P. Shankar, T. A. Petrie, A. J. García and J. E. Babensee, DENDRITIC CELL RESPONSES TO SELF-ASSEMBLED MONOLAYERS OF DEFINED CHEMISTRIES, J. Biomed. Mater. Res., Part A, 2010, 92(4), 1487–1499 CrossRef PubMed.
- P. M. Kou, Z. Schwartz, B. D. Boyan and J. E. Babensee, Dendritic cell responses to surface properties of clinical titanium surfaces, Acta Biomater., 2011, 7(3), 1354–1363 CrossRef CAS PubMed.
- A. Bogaerts, E. Neyts, R. Gijbels and J. van der Mullen, Gas discharge plasmas and their applications, Spectrochim. Acta, Part B, 2002, 57(4), 609–658 CrossRef.
- P. L. Girard-Lauriault, P. Desjardins, W. E. S. Unger, A. Lippitz and M. R. Wertheimer, Chemical Characterisation of Nitrogen-Rich Plasma-Polymer Films Deposited in Dielectric Barrier Discharges at Atmospheric Pressure, Plasma Processes Polym., 2008, 5(7), 631–644 Search PubMed.
- H. Conrads and M. Schmidt, Plasma generation and plasma sources - IOPscience, Plasma Sources Sci. Technol., 2000, 9(4), 441 CrossRef CAS.
- Y. Vadikkeettil, Y. Subramaniam, R. Murugan, P. V. Ananthapadmanabhan, J. Mostaghimi and L. Pershin, et al., Plasma assisted decomposition and reforming of greenhouse gases: A review of current status and emerging trends, Renewable Sustainable Energy Rev., 2022, 161, 112343 Search PubMed.
- M. Ozdemir, C. U. Yurteri and H. Sadikoglu, Physical polymer surface modification methods and applications in food packaging polymers, Crit. Rev. Food Sci. Nutr., 1999, 39(5), 457–477 CrossRef CAS PubMed.
- S. Babaei and P. L. Girard-Lauriault, Tuning the Surface Properties of Oxygen-Rich and Nitrogen-Rich Plasma Polymers: Functional Groups and Surface Charge, Plasma Chem. Plasma Process., 2016, 36(2), 651–666 CrossRef CAS.
- S. Ghafouri, S. Abdijahed, S. Farivar, S. I. Hosseini, F. Rezaei and A. Ardeshirylajimi, et al., Study on Physio-chemical Properties of plasma polymerization in C2H2/N2 plasma and Their Impact on COL X, Sci. Rep., 2017, 7(1), 9149 CrossRef PubMed.
- Y. Wang, Y. Ji, Y. Zhao, Y. Kong, M. Gao and Q. Feng, et al., Effects of surface functional groups on proliferation and biofunction of Schwann cells, J. Biomater. Appl., 2016, 30(10), 1494–1504 CrossRef CAS PubMed.
- M. A. Elkhodiry, M. D. Boulanger, O. Bashth, J. F. Tanguay, G. Laroche and C. A. Hoesli, Isolating and expanding endothelial progenitor cells from peripheral blood on peptide-functionalized polystyrene surfaces, Biotechnol. Bioeng., 2019, 116(10), 2598–2609 Search PubMed.
- O. S. Bashth, M. A. Elkhodiry, G. Laroche and C. A. Hoesli, Surface grafting of Fc-binding peptides as a simple platform to immobilize and identify antibodies that selectively capture circulating endothelial progenitor cells, Biomater. Sci., 2020, 8(19), 5465–5475 RSC.
- A. S. Chung, H. Waldeck, D. R. Schmidt and W. J. Kao, Monocyte inflammatory and matrix remodeling response modulated by grafted ECM-derived ligand concentration, J. Biomed. Mater. Res., Part A, 2009, 91(3), 742–752 CrossRef PubMed.
- W. J. Kao, Evaluation of protein-modulated macrophage behavior on biomaterials: designing biomimetic materials for cellular engineering, Biomaterials, 1999, 20(23), 2213–2221 CrossRef CAS PubMed.
- A. P. Acharya, N. V. Dolgova, N. M. Moore, C. Q. Xia, M. J. Clare-Salzler and M. L. Becker, et al., The modulation of dendritic cell integrin binding and activation by RGD-peptide density gradient substrates, Biomaterials, 2010, 31(29), 7444–7454 CrossRef CAS PubMed.
- A. Guerron, H. T. Phan, C. Peñaloza-Arias, D. Brambilla, V. G. Roullin and S. Giasson, Selectively triggered cell detachment from poly(N-isopropylacrylamide) microgel functionalized substrates, Colloids Surf., B, 2022, 217, 112699 CrossRef CAS.
- S. F. B. Mennens, M. Bolomini-Vittori, J. Weiden, B. Joosten, A. Cambi and K. van den Dries, Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells, Sci. Rep., 2017, 7(1), 17511 CrossRef PubMed.
- B. C. Quartey, G. Torres, M. ElGindi, A. Alatoom, J. Sapudom and J. C. Teo, Tug of war: Understanding the dynamic interplay of tumor biomechanical environment on dendritic cell function, Mechanobiol. Med., 2024, 2(3), 100068 CrossRef PubMed.
- J. A. Dombroski, S. J. Rowland, A. R. Fabiano, S. V. Knoblauch, J. M. Hope and M. R. King, Fluid shear stress enhances dendritic cell activation, Immunobiology, 2023, 228(6), 152744 CrossRef CAS PubMed.
- S. Wang, Y. Chen, Z. Ling, J. Li, J. Hu and F. He, et al., The role of dendritic cells in the immunomodulation to implanted biomaterials, Int. J. Oral Sci., 2022, 14(1), 1–15 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |