 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lewis acid catalysis of phosphate-modified CaNb2O6 for xylose dehydration to furfural†
Zijian
Wang‡
ab,
Ryota
Osuga‡
 a,
Koichiro
Endo
a,
Daniele
Padovan
a,
Satoshi
Suganuma
a,
Koichiro
Endo
a,
Daniele
Padovan
a,
Satoshi
Suganuma
 a,
Atsushi
Fukuoka
a,
Atsushi
Fukuoka
 a,
Hideki
Kato
a,
Hideki
Kato
 c and
Kiyotaka
Nakajima
c and
Kiyotaka
Nakajima
 *a
*a
aInstitute for Catalysis, Hokkaido University, Kita 21 Nishi 10, Kita-ku, Sapporo, Hokkaido 001-0021, Japan. E-mail: nakajima@cat.hokudai.ac.jp
bGraduate School of Chemical Sciences and Engineering, Hokkaido University, Kita 13 Nishi 8, Kita-ku, Sapporo, Hokkaido 060-8628, Japan
cInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, 980-8577, Japan
First published on 12th March 2025
Abstract
Phosphate-modified CaNb2O6 was prepared using the amorphous metal complex method and tested as a solid catalyst for xylose dehydration to furfural. The orthorhombic CaNb2O6 consists of octahedral NbO6 and square antiprismatic CaO8, providing unique Lewis and Brønsted acid sites. These active sites exhibited a higher furfural yield compared to orthorhombic Nb2O5.
The effective use of biomass-derived sugars, such as glucose and xylose, as renewable carbon resources can contribute to achieving carbon neutrality.1 Xylose is a pentose sugar obtained via acid-catalyzed hydrolysis of xylan, the main component of hemicellulose.2 The dehydration of xylose yields furfural, which is reported as one of the top-listed biobased products by the US Department of Energy due to its high potential applications as a green solvent, fuel additive, organic fertilizer, and valuable biopolymer precursor.3 Previously, we demonstrated that the water-tolerant Lewis acid catalysis of amorphous Nb2O5 is effective for xylose dehydration, with unsaturated coordination Nb5+ centers serving as pivotal active sites.4 However, catalyst reusability remains a significant challenge.5 In catalytic sugar conversion, insoluble polymers, called humin, are usually formed via the polymerization of the substrate, intermediates, and/or products. These by-products are deposited on the surface, leading to severe deactivation. Catalyst regeneration requires calcination (>500 °C) in the presence of oxygen to remove organic deposits, which induces the crystallization of amorphous Nb2O5, thereby reducing its intrinsic activity by a decrease in the number of unsaturated Nb5+ centers, i.e., catalytic active sites.6 To overcome such thermal instability, it is essential to develop a crystalline Nb-based metal oxide catalyst that retains its activity even after the thermal post-treatment. The incorporation of additional elements into Nb2O5 to form Nb-based complex oxides changes its Lewis acidity and enhances its catalytic performance.6b,c Similarly, phosphate treatment (P-treatment) effectively tunes catalyst surface properties.4a Here, we report the catalysis of CaNb2O6, an alkaline earth metal niobate, for xylose dehydration. Orthorhombic CaNb2O6 consists of octahedral NbO6 and square antiprismatic CaO8 with edge-sharing structure, while orthorhombic Nb2O5 features edge-shared NbO6 and pentagonal bipyramidal NbO7 (Fig. 1). We hypothesized that the unstable edge-sharing structure of CaNb2O6 facilitates the formation of coordinatively unsaturated Lewis acid sites, enhancing the activity in xylose dehydration. Such a structural future is specific to CaNb2O6 compared to other alkaline earth metal niobates. The effects of phosphoric acid treatment (P-treatment) on crystalline Nb2O5 and CaNb2O6 were also examined to improve their activity.
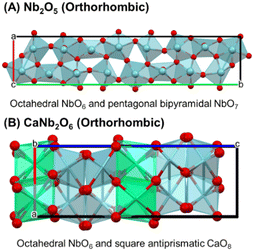 | ||
| Fig. 1 Crystalline structures of (A) Nb2O5 and (B) CaNb2O6. Red balls, blue and green sticks represent O, Nb and Ca, respectively. | ||
The amorphous metal complex (AMC) method using a water-soluble niobium peroxo complex was employed to prepare high surface area catalysts.7 The P-treatment was performed by the procedure reported in our previous papers using a 1 M H3PO4 aqueous solution.4a The detailed synthetic procedures are described in ESI.†
X-ray diffraction (XRD) measurements (Fig. S1, ESI†) revealed that the orthorhombic phases of both CaNb2O6 and Nb2O5 are retained after P-treatment. N2 adsorption–desorption measurements (Table 1) showed that CaNb2O6 has a larger Brunauer–Emmett–Teller (BET) surface area than Nb2O5, likely due to the incorporation of the light Ca element. The P contents of the catalysts were estimated using an X-ray photoelectron spectroscopy (XPS) (Fig. S2, ESI†) as 3.9 atom% for P–Nb2O5 and 2.6 atom% for P–CaNb2O6 (Table 1).
| Catalysts | S BET (m2 g−1) | Surface P contentb (atom%) | LASc (μmol g−1) | BASd (μmol g−1) |
|---|---|---|---|---|
| a Determined by N2 adsorption. b Determined by XPS. c Lewis acid site estimated by FTIR measurement of pyridine-adsorbed sample. d Brønsted acid site estimated by FTIR measurement of pyridine-adsorbed sample. | ||||
| Nb2O5 | 9 | — | 23 | n.d. |
| P–Nb2O5 | 9 | 3.9 | 14 | n.d. |
| CaNb2O6 | 23 | — | 32 | <10 |
| P–CaNb2O6 | 23 | 2.6 | 37 | 10 |
The acid properties of the catalysts were characterized by in situ infrared (IR) spectroscopy with pyridine as a basic probe molecule to quantify Brønsted acid site (BAS) and Lewis acid site (LAS).8Fig. 2(A) displays the difference IR spectra of adsorbed pyridine species on Nb2O5 and CaNb2O6 before and after P-treatment (see ESI,† Fig. S3 for O–H and C–H stretching vibrations). Both Nb2O5 and P–Nb2O5 have two specific bands at 1605 and 1444 cm−1 corresponding to typical vibrational modes of the pyridine coordinated on LAS. The P-treatment for Nb2O5 reduced LAS density (Table 1), indicating that phosphate species deactivate some LASs (Fig. 2(B)). The active LAS of amorphous Nb2O5 has been proposed as the tetrahedrally coordinated NbO4 species in our previous study,4a,b and they are fully stabilized with weakly coordinated and exchangeable H2O ligand(s). The active LAS of crystalline Nb2O5 is likely associated with the octahedrally coordinated but oxygen-defective NbO6, and a portion of these LASs lose their Lewis acidity upon the reaction with phosphoric acid. In contrast, CaNb2O6 has two apparent differences compared to Nb2O5: 1) variations in band frequency around 1600 cm−1 and 2) the presence of BAS. Two distinct bands were observed at 1608 and 1602 cm−1 for CaNb2O6 and P–CaNb2O6, which are well-known vibrational modes sensitive to metal type, coordination environment, and acid strength.8b,9 For instance, the vibrational frequency of adsorbed pyridine on octahedrally coordinated AlO6 species in γ-Al2O3 appears at a lower frequency than that of tetrahedrally coordinated AlO4 species.10 In the case of CaNb2O6, differences in the coordination environment between defects in Nb–O–Nb and in Nb–O–Ca suggest that the two observed bands can be assignable to distinct LASs. Since the vibrational band for liquid-phase pyridine is present at 1580 cm−1, the higher frequencies observed for CaNb2O6 indicate stronger interactions, i.e., stronger LASs. Consequently, CaNb2O6 has both slightly stronger and weaker LASs compared to Nb2O5. According to Pauling's principles, the edge-sharing structure is less stable than the corner-sharing structure, leading to a preferential formation of defect sites at Nb–O–Ca bonds. Another notable difference is the presence of BAS in CaNb2O6 and P–CaNb2O6. A weak band at 1542 cm−1, assignable to the vibrational mode of the pyridinium cation,8 was observed in CaNb2O6 and became pronounced in P–CaNb2O6. The numbers of BASs are summarized in Table 1. The Brønsted acidic nature of CaNb2O6 probably originate from the polarized Nb–O–Ca bond, where a proton is formed on a negatively charged oxygen atom to satisfy charge compensation. Still, the amount of BAS is negligibly small. P-treatment enhances the hydrolysis of polarized Nb–O–Ca bonds, resulting in the formation of Ca–O–PO(OH)2 species and unsaturated coordination Nb sites, as illustrated in Fig. 2(B). The process explains the increase in BASs, which can be attributed to the immobilization of phosphate species. The formation of LAS can be supported by the increased band intensity at 1602 cm−1. The unsaturated coordination Nb species formed by P-treatment are stabilized with weakly coordinated and exchangeable H2O ligand(s) and serve as LASs in acid-catalyzed reactions.
The catalytic performance of Nb2O5 and CaNb2O6 was evaluated in the dehydration of xylose (Fig. 3(A)). While CaNb2O6 showed almost the same furfural yield as Nb2O5, its furfural selectivity was low. This low furfural selectivity indicates the formation of polymerized by-product so-called humin, which is most likely catalyzed by relatively stronger LASs derived from the octahedrally coordinated Nb5+ centers in CaNb2O6. The P-treatment improved the catalytic performance of both CaNb2O6 and Nb2O5. Notably, P–CaNb2O6 exhibited higher furfural yield and selectivity compared to the parent CaNb2O6, even at almost the same levels of xylose conversion. This improvement suggests an increased rate of furfural formation accompanied by a decreased rate of humin formation. On the contrary, P–Nb2O5 showed enhanced furfural selectivity without a significant increase in furfural yield. This behavior suggests that the LAS responsible for humin formation is deactivated by the formation of phosphate moieties on the surface. The effect of P-treatment on crystalline Nb2O5 was consistent with that observed for amorphous Nb2O5, but the enhanced activity of CaNb2O6 after P-treatment cannot be explained by the deactivation of LAS that causes side reactions.
Our previous study on amorphous Nb2O5 revealed that LAS produces furfural through the stepwise dehydration of xylose, and intrinsic BAS does not participate in furfural formation (Fig. S4, ESI†).4a Assuming that crystalline Nb2O5 and CaNb2O6 follow the same reaction pathway, the high activity of P–CaNb2O6 is unlikely to result from the increased BAS after the P-treatment. Instead, the improved furfural selectivity of CaNb2O6 is primarily interpreted by the decrease of LAS that causes humin formation. In addition, the increased furfural yield is manly attributed to an increase in LAS effective for furfural formation (Table 1). We speculate the increase in both LAS and BAS to the hydrolysis of polar Ca–O–Nb bond generating phosphate-based BAS and Nb-based LAS during the P-treatment (Fig. 2B). The effect of phosphate loading on the activity of the resulting P–CaNb2O6 revealed that phosphate species immobilized via the equilibrium adsorption method are essential for enhancing the acid properties and improving the catalytic activity (Table 1 and Fig. S5, ESI†).
Control reactions were performed using reference catalysts to confirm the unique catalysis of P–CaNb2O6. A mixture of CaNb2O6 and H3PO4 improves the target product yield compared to CaNb2O6 alone. However, the product yield remained lower than that of P–CaNb2O6, which evidences that the catalysis of P–CaNb2O6 is not merely derived from the simple combination of CaNb2O6 and H3PO4. P–CaNb2O6 demonstrated a higher furfural yield than conventional homogeneous Lewis acid catalyst, Sc(OTf)3. Since Brønsted acid catalysts are known for high furfural selectivity,11 we compared the catalytic activity of typical homogeneous and heterogeneous Brønsted acid catalysts, such as H2SO4, a sulfonated polystyrene resin (Amberlyst-15) and H-beta zeolite. Although the selectivity of P–CaNb2O6 was slightly lower than that of these Brønsted acid catalysts, it still showed a higher furfural yield, highlighting its great potential as a solid acid catalyst for xylose dehydration. The time-course experiments (Fig. S6, ESI†) showed no significant difference in furfural selectivity between the two catalysts during the reaction, but the reaction rate for furfural formation with P–CaNb2O6 was larger than that with P–Nb2O5, likely due to the large amounts of LAS. Hot filtration experiments (Fig. S7, ESI†) indicate that the reaction completely stopped after the catalyst was removed, implying no obvious leaching of phosphate species during the reaction.
The reusability of heterogeneous catalysts is crucial for industrial applications and serves as a key indicator of catalyst performance. Amorphous Nb2O5 (Nb2O5-am) is a promising catalyst for xylose dehydration. To highlight the advantages of crystalline material, the reusability of Nb2O5-am and P–CaNb2O6 was evaluated. Prior to the reusability test, thermogravimetric-differential thermal analysis (TG-DTA) of fresh and spent P–CaNb2O6 was performed to determine the optimal regeneration temperature (Fig. S8, ESI†). Continuous weight loss was detected up to 500 °C in the spent catalyst, accompanied by a slight exothermic peak due to the deposited humin species, while there was almost no weight loss in the fresh catalyst. Therefore, the spent catalyst was calcined at 500 °C for 5 h in air to remove the organic deposits before reuse. Nb2O5-am decreased steeply its original activity for the first and second reuse tests (Fig. S9, ESI†), This deactivation is caused by the crystallization of amorphous Nb2O5 phase during the calcination process, because the strong acidity of Nb2O5-am is derived from its amorphous nature.6b The calcination treatment of Nb2O5-am formed orthorhombic Nb2O5 (Fig. S10, ESI†) and also decreased BET surface area from 120 to 67 m2 g−1. In contrast, P–CaNb2O6 retained its original activity even after five cycles (Fig. 3B). XRD measurements revealed no difference between fresh and spent P–CaNb2O6 (Fig. S11, ESI†), evidencing its high thermal stability towards during regeneration treatment.
Conclusions
Orthorhombic P–CaNb2O6 has proven to be an effective catalyst for the conversion of xylose to furfural. In situ IR spectroscopy using pyridine as a molecular probe revealed the presence of abundant and stronger LAS on P–CaNb2O6. Such specific surface acidity leads to its better catalytic performance. P–CaNb2O6 demonstrated excellent reusability for at least five cycles. The choice of the elements and the addition of functions through appropriate post-treatment can be an important factor in the catalytic application of crystalline metal oxides. This approach offers promising opportunities for designing efficient and durable metal oxide catalysts tailored for specific reactions.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Z. W. and K. E. carried out experiments on the synthesis and characterization of catalysts, catalytic reactions. D. P. and H. K. synthesized catalysts. Z. W. wrote the manuscript. R. O., H. K., and K. N. analysed experimental data. R. O., S. S., A. F., H. K., and K. N. revised the manuscript. K. N. supervised the project. All authors provided critical feedback and contributed to the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported financially by the following grants: JST-MIRAI Program (Grant number JPMJMl19E3), JST e-Asia Joint Research Program (JPMJSC22E3), JST-PRESTO (JPMJPR2472), JSPS Grant-in-Aid for Transformative Research Areas (A) “Hyper-Ordered Structures Science” (20H05879), JSPS Grant-in-Aid Scientific Research (B) (22H01861), JSPS Grant-in-Aid for Early-Career Scientist (24K17555), the Cooperative Research Program of Institute for Catalysis, Hokkaido University (24ES0579), and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices” (20241118).Notes and references
- K. J. Yong, T. Y. Wu, C. B. T. L. Lee, Z. J. Lee, Q. Liu, J. M. Jahim, Q. Zhou and L. Zhang, Biomass Bioenergy, 2022, 161, 106458 CrossRef CAS
.
-
(a) P. Mäki-Arvela, T. Salmi, B. Holmbom, S. Willför and D. Y. Murzin, Chem. Rev., 2011, 111, 5638 CrossRef PubMed
; (b) D. S. Naidu, S. P. Hlangothi and M. J. John, Carbohydr. Polym., 2018, 179, 28 CrossRef CAS PubMed
.
-
(a) J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC
; (b) K. Yan, G. Wu, T. Lafleur and C. Jarvis, Renewable Sustainable Energy Rev., 2014, 38, 663 CrossRef CAS
; (c) C. B. T. L. Lee and T. Y. Wu, Renewable Sustainable Energy Rev., 2021, 137, 110172 CrossRef CAS
; (d) Z. Zhang and G. W. Huber, Chem. Soc. Rev., 2018, 47, 1351 RSC
.
-
(a) N. K. Gupta, A. Fukuoka and K. Nakajima, ACS Catal., 2017, 7, 2430 CrossRef CAS
; (b) K. Nakajima, Y. Baba, R. Noma, M. Kitano, J. N. Kondo, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2011, 133, 4224 CrossRef CAS
; (c) K. Nakajima, R. Noma, M. Kitano and M. Hara, J. Mol. Catal. A: Chem., 2014, 388–389, 100 CrossRef CAS
.
-
(a) K. Nakajima, R. Noma, M. Kitano and M. Hara, J. Phys. Chem. C, 2013, 117, 16028 CrossRef CAS
; (b) R. Noma, K. Nakajima, K. Kamata, M. Kitano, S. Hayashi and M. Hara, J. Phys. Chem. C, 2015, 119, 17117 CrossRef CAS
; (c) S. M. A. H. Siddiki, Md. N. Rashed, Md. A. Ali, T. Toyao, P. Hirunsit, M. Ehara and K. Shimizu, ChemCatChem, 2019, 11, 383 CrossRef CAS
.
-
(a) M. Yang, S. Li and J. Huang, ACS Appl. Mater. Interfaces, 2021, 13, 39501 CrossRef CAS PubMed
; (b) D. Padovan, K. Endo, T. Matsumoto, T. Yokoi, A. Fukuoka, H. Kato and K. Nakajima, Small Struct., 2023, 4, 2200224 CrossRef CAS
; (c) M. Kim, S. Ronchetti, B. Onida, N. Ichikuni, A. Fukuoka, H. Kato and K. Nakajima, ChemCatChem, 2020, 12, 350 CrossRef CAS
.
-
(a) D. Dey, V. Petrykin, S. Sasaki and M. Kakihana, J. Ceram. Soc. Jpn., 2007, 115, 808 CrossRef CAS
; (b) M. Kakihana, M. Kobayashi, K. Tomita and V. Petrykin, Bull. Chem. Soc. Jpn., 2010, 83, 1285 CrossRef CAS
.
-
(a) M. Tamura, K. Shimizu and A. Satsuma, Appl. Catal., A, 2012, 433–434, 135 CrossRef CAS
; (b) M. I. Zaki, M. A. Hasan, F. A. Al-Sagheer and L. Pasupulety, Colloids Surf., A, 2001, 190, 261 CrossRef CAS
.
-
(a) G. Busca, Phys. Chem. Chem. Phys., 1999, 1, 723 RSC
; (b) A. Travert, A. Vimont, A. Sahibed-Dine, M. Daturi and J.-C. Lavalley, Appl. Catal., A, 2006, 307, 98 CrossRef CAS
.
- T. K. Phung, C. Herrera, M. Á. Larrubia, M. García-Diéguez, E. Finocchio, L. J. Alemany and G. Busca, Appl. Catal., A, 2014, 483, 41 CrossRef CAS
.
-
(a) B. M. Matsagar, M. K. Munshi, A. A. Kelkar and P. L. Dhepe, Catal. Sci. Technol., 2015, 5, 5086 RSC
; (b) B. M. Matsagar, S. A. Hossain, T. Islam, H. R. Alamri, Z. A. Alothman, Y. Yamauchi, P. L. Dhepe and K. C.-W. Wu, Sci. Rep., 2017, 7, 13508 CrossRef PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and characterization data. See DOI: https://doi.org/10.1039/d5cy00010f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |


