Open Access Article
Open Access ArticleA swift and efficient approach to boron-functionalized scaffolds: borylation of alkenes and alkynes using a carbon nanotube–copper ferrite catalyst†
Mateus P.
Nunes
 ab,
Luana A.
Machado
ab,
Luana A.
Machado
 a,
Hállen D. R.
Calado
a,
Hállen D. R.
Calado
 a,
Guilherme A. M.
Jardim
a,
Guilherme A. M.
Jardim
 a,
Joel A.
Tchuiteng Kouatchou
a,
Joel A.
Tchuiteng Kouatchou
 b,
Valérie
Geertsen
b,
Valérie
Geertsen
 c,
Youzhu
Yuan
c,
Youzhu
Yuan
 d,
Eric
Doris
d,
Eric
Doris
 *b,
Eufrânio N.
da Silva Júnior
*b,
Eufrânio N.
da Silva Júnior
 *a and
Edmond
Gravel
*a and
Edmond
Gravel
 *b
*b
aInstitute of Exact Sciences, Department of Chemistry, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais 31270-901, Brazil. E-mail: eufranio@ufmg.br
bUniversité Paris-Saclay, CEA, INRAE, Département Médicaments et Technologies pour la Santé (DMTS), SCBM, 91191 Gif-sur-Yvette, France. E-mail: eric.doris@cea.fr; edmond.gravel@cea.fr
cUniversité Paris-Saclay, CEA, CNRS, NIMBE, 91191 Gif-sur-Yvette, France
dState Key Laboratory of Physical Chemistry of Solid Surfaces, National Engineering Laboratory for Green Chemical Productions of Alcohols-Ethers-Esters, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China
First published on 28th April 2025
Abstract
We introduce herein a carbon nanotube–CuFe2O4-catalyzed borylation reaction of alkenes and alkynes as a practical and sustainable approach. Our system operates with low catalytic loading (0.05 mol%), under air, and the heterogeneous catalyst can be recycled via simple centrifugation. The methodology is of broad scope with a variety of substituted alkenes and alkynes.
Boron-based molecules are ubiquitous in chemistry, where they are often used as synthetic intermediates and reagents, but also in biomedical applications.1 Herbert C. Brown's research on boranes and borohydrides in the 1940s and 1950s2 has sparked renewed interest in organoboron compounds, which boomed with the discovery of the Suzuki–Miyaura coupling reaction in 1979.3 The latter transformation prompted research groups not only to further develop palladium-based coupling chemistry,4 but also to explore better ways of accessing boronic acids and boronic esters.5 On the other hand, compounds containing a C(sp2)–B bond are important motifs in medicinal chemistry, as they are versatile building blocks in drug discovery.6 Accordingly, the pharmaceutical industry is actively searching for robust methodologies applied to the synthesis of C(sp2)–B substrates, prioritizing economic viability and sustainability.7
Over the last decade, some of us have been working on the design of carbon nanotube (CNT)-based nanohybrid catalysts to activate chemical processes in a robust and sustainable way.8 CNTs were decorated with various transition metal nanoparticles, such as ruthenium,9 gold,10 platinum,11 or rhodium.12 More recently, we showed that CNTs bearing bimetallic rhodium/ruthenium nanoparticles have unique characteristics in promoting reactions such as hydrophosphinylations,13 hydrothiolations,14 and also hydroborations15 in excellent yields, with low catalyst loading (Scheme 1a). However, the above hydroboration reaction involved air- and moisture-sensitive pinacolborane, which necessitated an experimental set-up under inert atmosphere. Similarly, the use of noble metals (Ru and Rh) as catalytic materials was not fully satisfactory. With these features in mind, we hypothesized that the use cheaper and more accessible metals (e.g. copper)16 on CNTs, combined with bench-stable boron sources (e.g. bis(pinacolato)diboron – B2pin2), could provide a more sustainable route to borylated molecules (Scheme 1b).
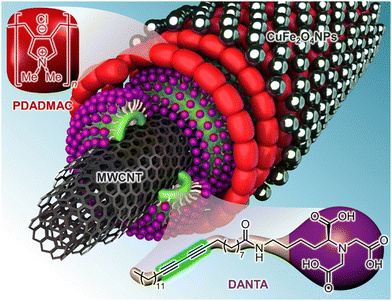
The copper-based catalyst was assembled using our previously reported procedure.17 Briefly, the grafting of copper ferrite (CuFe2O4) nanoparticles at the surface of carbon nanotubes involved a layer-by-layer approach in four steps: i) anionic DANTA amphiphiles were first adsorbed onto multiwalled carbon nanotubes (MWCNTs), forming a primary layer; ii) the diacetylene motifs within the amphiphile's lipophilic chains were photopolymerized to reinforce the supramolecular architecture; iii) a secondary cationic polymer (PDADMAC) layer was deposited; and finally iv) pre-synthesized CuFe2O4 nanoparticles were added to provide access to the CuFe2O4CNT nanohybrid (Fig. 1). The polyammonium network in this structure created a 3D framework that secured and stabilized the nanoparticles. The heterogeneous CuFe2O4CNT catalyst was recovered as an aqueous suspension, and copper concentration was quantified using inductively coupled plasma mass spectrometry (ICP-MS, [Cu] = 1.2 mM).
With the nanotube-based catalyst in hands, we set a model reaction involving phenylacetylene (1a) as the alkyne partner, B2pin2 as the boron source, CuFe2O4CNT (0.05 mol% loading) as the catalyst, Cs2CO3 as the base, and MeOH as the solvent (see ESI† for the solvent exchange procedure). Satisfactorily, after 20 h at 50 °C, the target compound 2a was obtained in 61% yield (Table 1, entry 1). Yet, better yields could be obtained by changing the base for a stronger one such as NaOMe (72%, entry 2), KOtBu (72%, entry 3), or NaOtBu (80%, entry 4) that provided the best results. Working at room temperature with NaOtBu led to a decrease in the recovered yield of 2a (36%, entry 5), and increasing temperature to 70 °C (entry 6) did not improve the yield significantly (81%). Changing the solvent for EtOH (entry 7, traces) or THF (entry 8, 20% yield) had a detrimental effect on the overall conversion. The 0.05 mol% loading represents a good compromise between sustainability and efficacy. We also tested lower loadings and found that similar conversions could be obtained with values as low as 0.01 mol%, but with a much longer reaction time (72 h vs. 24 h).
| Entry | Solvent | Base (mol%) | T (°C) | Yielda% |
|---|---|---|---|---|
| General reaction conditions: solvent (1 mL); 1a (0.5 mmol); B2pin2 (1.1 equiv.).a Isolated yields; r.t. = room temperature. | ||||
| 1 | MeOH | Cs2CI3 (10) | 50 | 61 |
| 2 | MeOH | NaOMe (10) | 50 | 72 |
| 3 | MeOH | KOtBu (10) | 50 | 72 |
| 4 | MeOH | NaO t Bu (10) | 50 | 80 |
| 5 | MeOH | NaOtBu (10) | r.t. | 36 |
| 6 | MeOH | NaOtBu (10) | 70 | 81 |
| 7 | EtOH | NaOtBu (10) | 50 | Trace |
| 8 | THF | NaOtBu (10) | 50 | 20 |
The optimized reaction conditions were then applied to different alkene and alkyne substrates to investigate the scope of the transformation (Scheme 2). Variations in the aromatic ring of alkyne 1a by insertion of electron-donating or withdrawing groups resulted in variable reaction yields. A methoxy group in para position of the aromatic ring led to a small decrease in yield (2b, 74% yield). The same comment applies to methoxy in the ortho position (2c, 50% yield). Replacing the para methoxy with less donating methyl group resulted in a slight decrease in yield (66% for 2d). A more substantial drop was observed with bromine at the para position (33% yield for 2e) and fluorine at the para (2f, 56% yield) or meta position (2g, 61% yield). While the presence of other para electron-withdrawing groups like –CF3 and phenyl only slightly lowered the yield (67% yield for 2h and 66% yield for 2i), the reaction was severely hampered by the two –CF3 groups of 1j, yielding only trace amounts of 2j.
 | ||
| Scheme 2 Scope of the CuFe2O4CNT-catalyzed borylation reaction of alkenes and alkynes. NR: no reaction. | ||
The reactivity of alkyl-substituted alkynes was next studied. We observed that the CuFe2O4CNT borylation reaction was compatible with a wide variety of functional groups borne by the alkyl chain, including phenyl (2k, 76% yield), halogens such as bromine (2l, 50% yield) or chlorine 2m (55% yield), primary hydroxyl group (2n, 68% yield) and tertiary OH (2o, 88% yield). Interestingly, in the latter case the reaction was not affected by the bulkiness of the nearby tertiary alcohol. The same comment applies to cyclic alcohol 2p (77% yield). The reaction also proceeded smoothly with cyclopropyl alkynes (2q, 72% yield) and unsubstituted aliphatic alkynes (2r, 78% yield). In all the above examples, the borylation reaction is taking place in an anti-Markovnikov fashion, leading exclusively to the C-terminal-borylated product and with trans-configuration. Although active on terminal alkynes, our system failed to promote the borylation of internal alkynes (e.g. diphenylacetylene).
As the borylation reaction was effective on alkynes, we next attempted performing the same transformation on alkenes. Borylations proceeded in average to good yields for styrene and norbornene, with derivatives 2s and 2t obtained in 56% and 77% yield, respectively. However, for linear 1-decene 1u, only traces of the product 2u were detected, and the reaction with cyclohexene 1v did not proceed at all. It appears that only activated alkenes, i.e. those with aromatic conjugation or internal strain, are suitable for the CuFe2O4CNT-catalyzed borylation.
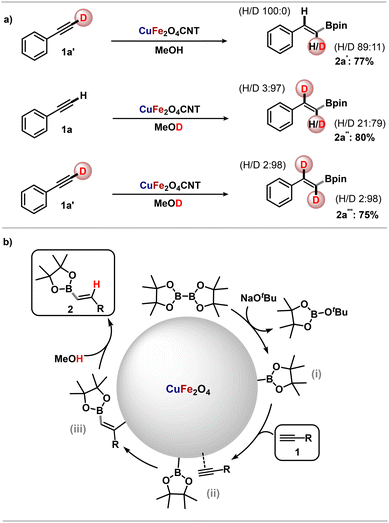
To better apprehend the reaction mechanism, experiments involving isotopic labelling with deuterium were carried out (Scheme 3a). The use of deuterated phenylacetylene 1a′ resulted in the formation of the deuterated product 2a′ in 77% yield, with however some loss of deuterium at the α position to boron. In fact, while the starting phenylacetylene 1a′ was fully deuterated (ca. 96%), isotopic enrichment of 2a′ was only 11%. This suggests some rapid H–D exchange between deuterated phenylacetylene and the methanolic solvent, under our reaction conditions. This was confirmed by conducting the reaction with authentic phenylacetylene 1a but in deuterated methanol. In this case we detected the incorporation of deuterium not only at the expected benzylic position (ca. 97% isotopic enrichment), but also α to boron (ca. 79% isotopic enrichment), suggesting H–D exchange on phenylacetylene and leading to compound 2a′′ in 80% yield. Incorporation of deuterium at the benzylic position suggests the occurrence of a vinyl-metal intermediate that is protonated by the solvent in the last step. Finally, the reaction of deuterated phenylacetylene 1a′ as the alkyne source in MeOD led to the formation of doubly labelled 2a′′′ in 75% yield and with high isotopic enrichment (ca. 98%) at the two positions. Of note, in the above transformations we did not observe any kinetic isotope effect, suggesting the rate-determining step does not involve the dissociation of either X–H(D) bond.
Based on previous works18 and our deuterium labelling experiments, a possible reaction mechanism is proposed in Scheme 3b. The initial reaction between B2pin2 and sodium tert-butoxide generates BpinOtBu and intermediate (i) by addition of the other Bpin to the catalyst. This step is followed by coordination of the alkyne at the surface of the catalyst, where interactions with Bpin takes place (ii). Migratory insertion of the alkyne into the catalyst-Bpin bond generates intermediate (iii) whose protonation by methanol releases the desired borylated product. The catalytically active species in this CuFe2O4CNT-mediated transformation is likely copper, with the contribution of iron acting as a mild coordinating unit that activates the alkyne (or the alkene) in a synergistic fashion.19
The use of carbon nanotubes as platforms for the anchoring of metal nanoparticles permitted heterogenization of copper-ferrite and its recycling. To demonstrate recyclability of CuFe2O4CNT, the reaction with 1a was repeated 6 times using a simple recycling protocol consisting in a mild centrifugation. The solvent phase containing the product was collected and the catalyst could be reused by adding fresh solvent and reagents. Of note, the Cu content of the solvent phase was measured by ICP-MS and was found to be below quantification thresholds, confirming that no significant leaching occurred during the catalytic cycle. In each successive reaction, borylated compound 2a was obtained with nearly the same yield and within the same reaction time (Scheme 4a). Synthetic applicability of vinyl boronate ester 2a was illustrated by performing a Suzuki reaction20 leading to compound 3a in 65% yield, and conversion into vinyl-ether 3b in 45% yield in the presence of copper acetate in ethanol21 (Scheme 4b).
Conclusions
We report a heterogeneous carbon nanotube–copper ferrite-catalyzed borylation of alkynes and alkenes, achieving good yields and excellent recyclability, as the heterogeneous catalyst maintains its efficiency even after six cycles. This approach offers a practical, economical and sustainable method for synthesizing boron-based building blocks. The system presents significant advantages over existing techniques (see Table S1†), including low catalytic loadings, use of cheap base metals that are acting in a synergistic fashion to activate the alkyne partner (Fe) and promote the coupling reaction (Cu), use of an air- and moisture-tolerant boron source, and recovery/reuse of the CuFe2O4CNT-catalyst.Data availability
The authors confirm that the data supporting the findings of this study are available within the manuscript and its ESI.†Author contributions
Investigation, methodology and validation: M. P. N., L. A. M., J. A. T. K., V. G. and Y. Y.; writing – original draft: G. A. M. J. and E. N. S. J.; data curation, visualization, conceptualization, funding acquisition, project administration, writing – reviewing and editing: H. D. R. C., G. A. M. J., E. D., E. N. S. J. and E. G.; all authors revised and agreed with the present form of the paper.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is partly supported by a public grant overseen by the French National Research Agency (ANR) as part of the “Investissements d'Avenir” program, through the “ADI 2020′′ project funded by the IDEX Paris-Saclay, ANR-11-IDEX-0003-02 (PhD grant to M. P. N.). The Paris-Saclay University is acknowledged for the award of the “Jean d'Alembert” fellowship to E. N. S. J. (France 2030 program ANR-11-IDEX-0003). We also thank CNPq, CAPES, and FAPEMIG for support. E. N. S. J. and G. A. M. J. thanks CNPq (PQ 309774/2020-9, 405052/2021-9, 151294/2022-4, 200115/2022-7, 441404/2023-5, 421655/2023-2, 421655-2023-2), CAPES Finance Code 001, FAPEMIG (APQ-00724-23, APQ-01538-24, APQ-04401-23 and TEC-RED-00081-23). L. A. M. thanks CNPq (402980/2024-7, 151734/2024-0 and FAPEMIG (APQ-03607-17). Special thanks to CAPES-COFECUB project No. 88881.878986/2023-01.References
- (a) H. C. Brown and B. Singaram, Acc. Chem. Res., 1988, 21, 287–293 CrossRef CAS; (b) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS; (c) D. G. Hall, Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2005, ISBN: 978-3-527-30618-8 CrossRef; (d) B. C. Das, P. Thapa, R. Karki, C. Schinke, S. Das, S. Kambhampati, S. K. Banerjee, P. V. Veldhuizen, A. Verma, L. M. Weiss and T. Evans, Future Med. Chem., 2013, 5, 653–676 CrossRef CAS PubMed.
- (a) H. C. Brown and B. C. Subba Rao, J. Am. Chem. Soc., 1956, 78, 2582–2588 CrossRef CAS; (b) H. C. Brown and B. C. Subba Rao, J. Org. Chem., 1957, 22, 1136–1137 CrossRef CAS; (c) H. C. Brown, Science, 1980, 210, 485–492 CrossRef CAS PubMed.
- N. Miyaura and A. Suzuki, J. Chem. Soc., Chem. Commun., 1979, 866–867 RSC.
- (a) J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508–524 CrossRef; (b) R. F. Heck, J. Am. Chem. Soc., 1968, 90, 5518–5526 CrossRef CAS; (c) Y. Nakao, A. K. Sahoo, H. Imanaka, A. Yada and T. Hiyama, Pure Appl. Chem., 2006, 78, 435–440 CrossRef CAS.
- (a) S. Darses and J.-P. Genêt, Chem. Rev., 2008, 108, 288–325 CrossRef CAS PubMed; (b) I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 110, 890–931 CrossRef CAS PubMed.
- (a) S. J. Baker, C. Z. Ding, T. Akama, Y.-K. Zhang, V. Hernandez and Y. Xia, Future Med. Chem., 2009, 1, 1275–1288 CrossRef CAS PubMed; (b) H. S. Ban and H. Nakamura, Chem. Rec., 2015, 15, 616–635 CrossRef CAS PubMed; (c) G. F. S. Fernandes, W. A. Denny and J. L. Dos Santos, Eur. J. Med. Chem., 2019, 179, 791–804 CrossRef CAS PubMed.
- (a) P. K. Verma, M. L. Shegavi, S. K. Bose and K. Geetharani, Org. Biomol. Chem., 2018, 16, 857–873 RSC; (b) S. G. Koenig, D. K. Leahy and A. S. Wells, Org. Process Res. Dev., 2018, 22, 1344–1359 CrossRef CAS; (c) J. D. Hayler, D. K. Leahy and E. M. Simmons, Organometallics, 2019, 38, 36–46 CrossRef CAS; (d) S. Guria, M. Md M. Hassana and B. Chattopadhyay, Org. Chem. Front., 2024, 11, 929–953 RSC; (e) N. Compagno, N. Lucchetti, A. Palmisano, R. Profeta and A. Scarso, J. Org. Chem., 2024, 89, 12452–12461 CrossRef CAS PubMed.
- J. John, E. Gravel, A. Hagège, H. Li, T. Gacoin and E. Doris, Angew. Chem., Int. Ed., 2011, 50, 7533–7536 CrossRef CAS PubMed.
- (a) D. V. Jawale, E. Gravel, C. Boudet, N. Shah, V. Geertsen, H. Li, I. N. N. Namboothiri and E. Doris, Chem. Commun., 2015, 51, 1739–1742 RSC; (b) P. Basu, P. Prakash, E. Gravel, N. Shah, K. Bera, E. Doris and I. N. N. Namboothiri, ChemCatChem, 2016, 8, 1298–1302 CrossRef CAS; (c) R. G. Almeida, R. L. de Carvalho, M. P. Nunes, R. S. Gomes, L. F. Pedrosa, C. A. de Simone, E. Gopi, V. Geertsen, E. Gravel, E. Doris and E. N. da Silva Júnior, Catal. Sci. Technol., 2019, 9, 2742–2748 RSC.
- (a) R. Kumar, E. Gravel, A. Hagège, H. Li, D. V. Jawale, D. Verma, I. N. N. Namboothiri and E. Doris, Nanoscale, 2013, 5, 6491–6497 RSC; (b) R. Kumar, E. Gravel, A. Hagège, H. Li, D. Verma, I. N. N. Namboothiri and E. Doris, ChemCatChem, 2013, 5, 3571–3575 CrossRef CAS; (c) D. V. Jawale, E. Gravel, V. Geertsen, H. Li, N. Shah, I. N. N. Namboothiri and E. Doris, ChemCatChem, 2014, 6, 719–723 CrossRef CAS; (d) N. Shah, E. Gravel, D. V. Jawale, E. Doris and I. N. N. Namboothiri, ChemCatChem, 2014, 6, 2201–2205 CrossRef CAS; (e) D. V. Jawale, E. Gravel, E. Villemin, N. Shah, V. Geertsen, I. N. N. Namboothiri and Eric Doris, Chem. Commun., 2014, 50, 15251–15254 RSC; (f) S. Donck, E. Gravel, N. Shah, D. V. Jawale, E. Doris and I. N. N. Namboothiri, RSC Adv., 2015, 5, 50865–50868 RSC; (g) E. Villemin, E. Gravel, D. V. Jawale, P. Prakash, I. N. N. Namboothiri and E. Doris, Macromol. Chem. Phys., 2015, 216, 2398–2403 CrossRef CAS; (h) N. Shah, P. Basu, P. Prakash, S. Donck, E. Gravel, I. N. N. Namboothiri and E. Doris, Nanomaterials, 2016, 6, 37–44 CrossRef PubMed; (i) P. Prakash, E. Gravel, H. Li, F. Miserque, A. Habert, M. den Hertog, W. L. Ling, I. N. N. Namboothiri and E. Doris, Catal. Sci. Technol., 2016, 6, 6476–6479 RSC; (j) E. Gopi, E. Gravel and E. Doris, Nanoscale Adv., 2019, 1, 1181–1185 RSC; (k) E. Gopi, V. Geertsen, E. Gravel and E. Doris, ChemCatChem, 2019, 11, 5758–5761 CrossRef CAS.
- D. V. Jawale, V. Geertsen, F. Miserque, P. Berthault, E. Gravel and E. Doris, Green Chem., 2021, 23, 815–820 RSC.
- (a) D. V. Jawale, E. Gravel, N. Shah, V. Dauvois, H. Li, I. N. N. Namboothiri and E. Doris, Chem. – Eur. J., 2015, 21, 7039–7042 CrossRef CAS PubMed; (b) S. Donck, E. Gravel, A. Li, P. Prakash, N. Shah, J. Leroy, H. Li, I. N. N. Namboothiri and E. Doris, Catal. Sci. Technol., 2015, 5, 4542–4546 RSC; (c) P. Prakash, E. Gravel, D.-V. Nguyen, I. N. N. Namboothiri and E. Doris, ChemCatChem, 2017, 9, 2091–2094 CrossRef CAS; (d) R. L. de Carvalho, G. A. M. Jardim, A. C. C. Santos, M. H. Araujo, W. X. C. Oliveira, A. C. S. Bombaça, R. F. S. Menna-Barreto, E. Gopi, E. Gravel, E. Doris and E. N. da Silva Júnior, Chem. – Eur. J., 2018, 24, 15227–15235 CrossRef CAS PubMed.
- D. V. Jawale, F. Fossard, F. Miserque, V. Geertsen, E. Doris and E. Gravel, Catal. Sci. Technol., 2022, 12, 4983–4987 RSC.
- D. V. Jawale, J. A. Tchuiteng Kouatchou, F. Fossard, F. Miserque, V. Geertsen, E. Gravel and E. Doris, Green Chem., 2022, 24, 1231–1237 RSC.
- M. P. Nunes, D. V. Jawale, F. G. Delolo, M. H. Araujo, E. Gravel, E. Doris and E. N. da Silva Júnior, Chem. Commun., 2023, 59, 2763–2766 RSC.
- (a) B. Mohan and K. H. Park, Appl. Catal., A, 2016, 519, 78–84 CrossRef CAS; (b) X. Zeng, C. Gong, H. Guo, H. Xu, J. Zhang and J. Xie, New J. Chem., 2018, 42, 17346–17350 RSC; (c) M. L. Shegavi, S. Saini, R. Bhawar, M. D. Vishwantha and S. K. Bose, Adv. Synth. Catal., 2021, 363, 2408–2416 CrossRef CAS.
- P. Prakash, R. Kumar, F. Miserque, V. Geertsen, E. Gravel and E. Doris, Chem. Commun., 2018, 54, 3644–3647 RSC.
- (a) B. Wang, L. Gao, H. Yang and G. Zheng, ACS Appl. Mater. Interfaces, 2021, 13, 47530–47540 CrossRef CAS PubMed; (b) B. Mohan and K. H. Park, Appl. Catal., A, 2016, 519, 78–84 CrossRef CAS; (c) S. Tao, Y. Wang, Q. Pan, J. Zhao, Q. Bu, F. Chen, J. Liu, B. Dai, D. Wei and N. Liu, Green Chem., 2023, 25, 6704–6716 RSC; (d) C. Gunanathan, M. Hölscher, F. Pan and W. Leitner, J. Am. Chem. Soc., 2012, 134, 14349–14352 CrossRef CAS PubMed; (e) X. Zeng, C. Gong, H. Guo, H. Xu, J. Zhanga and J. Xie, New J. Chem., 2018, 42, 17346 RSC.
- High oxidation state iron species have been shown to act as Lewis acids, coordinating to alkenes/alkynes: (a) M. D. Greenhalgh, A. S. Jones and S. P. Thomas, ChemCatChem, 2015, 7, 190–222 CrossRef CAS; (b) E. Vrancken and J. M. Campagne, Organic Transformations Promoted by Lewis Acid Iron Catalysts, in PATAI'S Chemistry of Functional Groups, ed. Z. Rappoport, 2013 Search PubMed.
- H. H. Rau and N. S. Werner, Bioorg. Med. Chem. Lett., 2018, 28, 2693–2696 CrossRef CAS PubMed.
- X. Shi, S. Li and L. Wu, Angew. Chem., Int. Ed., 2019, 58, 16167–16171 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cy00335k |
| This journal is © The Royal Society of Chemistry 2025 |