DOI:
10.1039/D4DT02957G
(Perspective)
Dalton Trans., 2025,
54, 9803-9834
Zeolitic imidazolate framework-67-derived chalcogenides as electrode materials for supercapacitors†
Received
23rd October 2024
, Accepted 22nd April 2025
First published on 12th May 2025
Abstract
With the rapid development of new energy technologies, hybrid supercapacitors have received widespread attention owing to their advantages of high power density, fast charging/discharging rate and long cycle life. In this case, the selection and design of electrode materials are the key to improving the energy storage performance of supercapacitors. Herein, zeolitic imidazolate framework-67 (ZIF-67) is presented as a good candidate material for the fabrication of supercapacitor electrodes because of its controllable pore size, constant cavity size and large specific area. Moreover, pristine ZIF-67 and ZIF-67-derived porous carbon have shown exemplary performances in supercapacitors. However, they belong to the class of electric double layer capacitor materials and have a lower magnitude of energy storage compared with pseudocapacitor materials. Therefore, to improve the energy density of hybrid supercapacitors, other ZIF-67 derivatives need to be explored, especially chalcogenides. This review mainly reports the application of ZIF-67-derived transition metal chalcogenides (TMCs, C including Oxide, Sulfide, Selenide, Telluride) in supercapacitors. Moreover, the strategies for the preparation of ZIF-67-derived TMCs and their electrochemical performance in supercapacitors are further discussed. Finally, the remaining challenges and future perspectives are highlighted.
1. Introduction
With the continuous development of society and the economy, energy storage has become a key element in achieving energy sustainability goals, leading to energy and cost savings.1–3 Thus, supercapacitors have attracted widespread attention from scientific researchers owing to their advantages of high power density, fast charging/discharging rate and long cycle life.4–9 However, their relatively low energy density limits their further practical application.10–12 Therefore, increasing research has been conducted on hybrid supercapacitors because of their charge-storage mechanisms, including both electric double layer capacitance and pseudocapacitance, resulting in better cycling stability than pseudocapacitors and higher energy density than electric double layer capacitors (EDLCs).13–18 Moreover, there has been growing demand for both high energy and high power densities in the same material.19–23
As a new class of porous crystal materials, metal organic frameworks (MOFs), which are composed of organic ligands and metal cations linked via coordination bonds, have been widely studied in the field of nanotechnology and energy storage because of their inherent advantages such as abundant porosity, high specific surface area and controllable topology.24–26 Moreover, with the development of various synthetic strategies, many new MOFs with a specific topology or architecture have been manufactured and applied as supercapacitor electrode materials.27,28 In recent years, an increasing number of MOFs have been used as templates or precursors to generate MOF derivatives, such as porous carbon,29 metal-doped carbon, hydroxides, phosphides30 and chalcogenides (O,31 S,32 Se,33 and Te34). Furthermore, it has been proven that the size, shape and composition of MOF derivatives can be effectively controlled on the basis of their precursors.35,36 Moreover, various conductive matrices (conductive polymers,37 carbon-based materials38,39 and MXene materials40,41) have been combined with pristine MOFs or MOF derivatives to improve their conductivity and structural stability during long-term cycling, resulting in promising cycling stability, better rate performance and high specific capacitance/capacity.34 However, some of these MOFs are unstable in water and alkaline solutions, which limit their use in supercapacitors.42
As a member of metal–organic frameworks, zeolitic imidazolate framework-67 (ZIF-67) is a good candidate material for the fabrication of supercapacitors (as shown in Fig. S1†) because of its controllable pore size, high chemical stability and thermal stability.43,44 The ZIF-67 material (as shown in Fig. 1) is composed of imidazole and Co2+, which can provide a large specific area and abundant oxidation active sites as an electrode material precursor, together with a constant cavity and thermal stability.45 Thus, ZIF-67 has been considered as a promising electrode material for supercapacitors. For example, Xie et al.46 successfully prepared one-dimensional ZIF-67, which exhibited a much higher charge storage capacity, rate capability and cycling stability than two-dimensional and three-dimensional ZIF-67 because of its abundant linker-missing defects and favorable exposure of cobalt ions as redox active sites. Moreover, due to the drawback of low conductivity of pristine ZIF-67, ZIF-67-derived porous carbon materials are also considered good EDLC materials for supercapacitors. Torad et al.47 synthesized nanoporous carbon via the one-step direct carbonization of ZIF-67, which showed a good electrochemical performance of 238 F g−1 at 20 mV s−1 in 0.5 M H2SO4 electrolyte. However, both pristine ZIF-67 (Table S1†) and ZIF-67-derived porous carbon (Table S2†) have a low energy density because they are EDLC materials. Therefore, it is necessary to develop new ZIF-67-derived pseudocapacitors materials to improve the energy density of hybrid supercapacitors.
 |
| | Fig. 1 Schematic of the structure of ZIF-67, showing the connection between its various atoms. | |
As typical pseudocapacitor materials, transition metal chalcogenides (TMCs, C including O, S, Se, and Te) have been widely researched and applied in hybrid supercapacitors, exhibiting both high energy density and power density.34 Thus, in this review, we focus on ZIF-67-derived TMCs as electrode materials for application in supercapacitors. With the development of single-metal compounds to multi-metal compounds and composite materials, their preparation technology and electrochemical performances in supercapacitors are discussed to highlight the relevant challenges that need to be resolved in the future, enabling the research and development of high-performance materials. Furthermore, significantly, this review can guide the direction of future work based on the comparison of the electrochemical performance of various derivatives in supercapacitors.
2. ZIF-67-derived transition metal oxides
Metal oxide materials derived from ZIF-67 possess a high reversible capacity and superior rate and cycle performance, making them excellent electrode materials25 (as shown in Table 1), which can be easily obtained by thermal treatment, while their charge storage mechanisms obey pseudocapacitance behavior.48 Moreover, in the future, the class of transition metal oxides will play a crucial role in the development of cost-effective, high-powered, and environment-friendly energy storage.2
Table 1 Summary of the electrochemical characteristics of ZIF-67-derived oxide materials determined using three-electrode measurements
| Electrode materials |
Specific capacity |
Electrolyte |
Potential window (CV) |
Capacity retention |
Cyclic stability |
Ref. |
| Co3O4 |
504 F g−1 at 5 mV s−1 |
6 M KOH |
−0.1–0.4 V (Ag/AgCl) |
52% (5 to 200 mV s−1) |
|
49
|
| Co3O4 |
1110 F g−1 at 1.25 A g−1 |
3 M KOH |
0–0.5 V (Hg/HgO) |
39.4% (1.25 to 12.5 A g−1) |
95.1% (6000) |
50
|
| Co3O4–NTA |
2.26 C cm−2 at 2 mA cm−2 |
2 M KOH |
0–0.6 V |
46.9% (2 to 40 mA cm−2) |
96.9% (5000) |
51
|
| Co3O4/3DGN/NF |
321 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
76.6% (1 to 20 A g−1) |
88% (2000) |
52
|
| Co3O4/C@MoS2 |
1076 F g−1 at 1 A g−1 |
2 M KOH |
0–0.7 V (Ag/AgCl) |
76.9% (1 to 10 A g−1) |
64.5% (5000) |
53
|
| Co3O4–CeO2 |
1288.3 F g−1 at 2.5 A g−1 |
3 M KOH |
0–0.55 V (Hg/HgO) |
53.3% (2.5 to 12.5 A g−1) |
>96.7% (6000) |
54
|
| M–Co3O4 |
1216.4 F g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (Hg/HgO) |
76.1% (1 to 20 A g−1) |
|
55
|
| NixCo3−xO4/CNTs |
668 F g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (Ag/AgCl) |
91.6% (1 to 10 A g−1) |
91.2% (2000) |
56
|
| MnCo2O4@Co3O4 |
1440 C cm−2 at 1 mA cm−2 |
2 M KOH |
0–0.6 V (SCE) |
36% (1 to 10 mA cm−2) |
82.76% (8000) |
57
|
| Co3O4/C |
875.6 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Ag/AgCl) |
|
87.8% (1000) |
58
|
| Co3O4@N-pC |
422.8 F g−1 at 1 A g−1 |
1 M KOH |
−0.2–0.8 V (SCE) |
60.5% (1 to 5 A g−1) |
87.9% (5000) |
59
|
| rGO/Co3O4 |
688 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (SCE) |
64.3% (1 to 10 A g−1) |
79.7% (1000) |
60
|
| Co3O4/Co(OH)2 |
184.9 mA h g−1 at 1 A g−1 |
1 M KOH |
−0.1–0.4 V (Ag/AgCl) |
76% (1 to 16 A g−1) |
|
61
|
| Co3O4/MCS |
1409.5 F g−1 at 0.5 A g−1 |
1 M KOH |
0–0.65 V (SCE) |
93.5% (0.5 to 1 A g−1) |
93.2% (1000) |
62
|
| Au@Co3O4 |
763 F g−1 at 1 A g−1 |
2 M KOH |
0.2–0.7 V |
76% (1 to 10 A g−1) |
83.7% (5000) |
63
|
| ZCCO |
701 C g−1 at 2 A g−1 |
3 M KOH |
−0.1–0.55 V (Hg/HgO) |
61% (2 to 60 A g−1) |
93.6% (6000) |
64
|
| NiCo LDH/Co3O4 |
1393.9 F g−1 at 1 A g−1 |
3 M KOH |
0–0.5 V (Ag/AgCl) |
71.3% (1 to 15 A g−1) |
88.4% (5000) |
65
|
| NS–Co3O4@PC |
940 F g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
42.5% (1 to 20 A g−1) |
|
66
|
| Co3O4@MnO2 |
413 F g−1 at 0.5 A g−1 |
1 M LiOH |
0–0.6 V (Ag/AgCl) |
41% (0.5 to 10 A g−1) |
110% (2000) |
67
|
| NiMoO4@Co3O4/CA |
436.9 C g−1 at 0.5 A g−1 |
2 M KOH |
0–0.6 V (SCE) |
70.7% (0.5 to 5 A g−1) |
82.7% (5000) |
68
|
| 3D-H CoWO4/NF |
1395 F g−1 at 0.5 A g−1 |
6 M KOH |
0–0.6 V (SCE) |
32.3% (0.5 to 40 A g−1) |
89% (3000) |
69
|
| Co2V2O7/G |
276.5 C g−1 at 1 A g−1 |
1 M KOH |
0–0.6 V (Ag/AgCl) |
62.5% (1 to 20 A g−1) |
93% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
70
|
| Z-CoO/RGO |
275 F g−1 at 1 A g−1 |
6 M KOH |
−1–0 V (Ag/AgCl) |
53.% (1 to 10 A g−1) |
|
71
|
| Fe-doped Co3O4 |
1535.8 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
|
88% (8000) |
72
|
| Co3O4/NiCo2O4 |
770 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Ag/AgCl) |
84% (1 to 20 A g−1) |
70% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
73
|
| Co3O4/NiNH |
583 C g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
82% (1 to 20 A g−1) |
|
74
|
| Co3O4/N-rGA |
1485 F g−1 at 1 A g−1 |
6 M KOH |
0–0.4 V (SCE) |
61% (1 to 20 A g−1) |
93.4% (5000) |
75
|
| NPC-60 |
594.8 mF cm−2 at 5 mV s−1 |
6 M KOH |
0–0.45 V (SCE) |
|
64% (2000) |
76
|
| Co3O4@C |
703.3 F g−1 at 1 A g−1 |
1 M KOH |
0–0.8 V (SCE) |
82.7% (1 to 10 A g−1) |
100% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
77
|
| ZnO/Co3O4/NiO |
1119.11 C g−1 at 1 A g−1 |
2 M KOH |
−0.1–0.7 V (SCE) |
72.92% (1 to 10 A g−1) |
93.75% (5000) |
78
|
| Co3O4@Co/NC-HN |
273.9 mA h g−1 at 1 A g−1 |
3 M KOH |
0–0.75 V (Hg/HgO) |
47.6% (1 to 10 A g−1) |
86.3% (3000) |
79
|
| Co3O4@NPC/Ni |
525 F g−1 at 1.5 A g−1 |
1 M KOH |
0–0.6 V (Ag/AgCl) |
42.9% (1.5 to 5 A g−1) |
81% (8000) |
80
|
| Porous Co3O4–CNTs |
875 F g−1 at 5 mV s−1 |
6 M KOH |
0–0.5 V (SCE) |
|
98% (1000) |
38
|
| CoOx/carbon |
1660.4 F g−1 at 1 A g−1 |
1 M KOH |
−0.1–0.4 V (Ag/AgCl) |
22% (1 to 20 A g−1) |
95.9% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
81
|
| Co3O4@NiO |
1017 F g−1 at 1 A g−1 |
|
0–0.6 V (SCE) |
55.9% (1 to 25 A g−1) |
90.1% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
82
|
| Co3O4 HPCs |
140 F g−1 at 1 A g−1 |
|
0–0.5 V (Hg/HgO) |
49.5% (1 to 15 A g−1) |
|
83
|
| Co3O4@N-doped C |
709 F g−1 at 1 A g−1 |
1 M KOH |
0–0.4 V (Ag/AgCl) |
43% (1 to 10 A g−1) |
83% (5000) |
84
|
| α-Ni(Co)S@NiCoO2 |
502.4 mA h g−1 at 1 A g−1 |
2 M KOH |
−0.2–0.7 V (Ag/AgCl) |
45.7% (1 to 20 A g−1) |
|
85
|
| Co–O–NSs@CFC |
842 F g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Ag/AgCl) |
40% (1 to 20 A g−1) |
96.4% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
86
|
| CoVO-HNC |
427.64 F g−1 at 1 A g−1 |
3 M KOH |
0–0.6 V (Hg/HgO) |
88% (1 to 10 A g−1) |
89.38% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
87
|
| Co3O4@CoNi2S4-20/CC |
1798 F g−1 at 1 A g−1 |
1 M KOH |
0–0.6 V (Ag/AgCl) |
77.9% (1 to 10 A g−1) |
|
88
|
| Ni–Co LDH@Co3O4 Nc |
1866 F g−1 at 2 A g−1 |
1 M KOH |
0–0.6 V (Ag/AgCl) |
80% (2 to 40 A g−1) |
|
89
|
| Co3O4@C |
251 F g−1 at 1 A g−1 |
1 M KOH |
0–0.6 V (Hg/HgO) |
84% (1 to 5 A g−1) |
90% (5000) |
90
|
| NiCo2O4/graphene |
1365 F g−1 at 1 A g−1 |
1 M KOH |
0–0.65 V (Ag/AgCl) |
51.5% (1 to 10 A g−1) |
89.11% (2000) |
91
|
| Ni/Co-CN |
1701 F g−1 at 1 A g−1 |
3 M KOH |
0–0.5 V (Ag/AgCl) |
61.9% (1 to 10 A g−1) |
|
92
|
| Co3O4@MnO2 |
768 C g−1 at 1 A g−1 |
1 M KOH |
0–0.6 V (Hg/HgO) |
|
86% (5000) |
93
|
| Co(OH)F@Co3O4/NF |
543 F g−1 at 1 A g−1 |
3 M KOH |
0–0.7 V |
57.8% (1 to 10 A g−1) |
88.34% (5000) |
94
|
| Co3O4@NC |
1144 F g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
45.4% (1 to 20 A g−1) |
91.6% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
95
|
| CoMoO4@Co3O4 |
2003 F g−1 at 1 A g−1 |
3 M KOH |
−0.1–0.6 V (Ag/AgCl) |
|
68% (5000) |
96
|
| Co3O4@SiO2/PPy |
107.7 F g−1 at 0.6 A g−1 |
1 M KOH |
−0.55–0.45 V (Ag/AgCl) |
|
|
97
|
| MWCNTx@Co3O4 |
206.89 F g−1 at 1 A g−1 |
3 M KOH |
0–0.6 V (Ag/AgCl) |
92.6% (1 to 5 A g−1) |
78.9% (1000) |
98
|
| HCT-2@Co3O4@SnS2 |
439 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
72.1% (1 to 20 A g−1) |
63.5% (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
99
|
| Co3O4–VG–CC |
1360 F g−1 at 2.5 A g−1 |
2 M KOH |
0–0.8 V (Hg/HgO) |
90% (2.5 to 50 A g−1) |
|
100
|
| Co3O4/NiCo2O4 DSNCs |
236.18 C g−1 at 1 mA cm−2 |
2 M KOH |
0–0.6 V (SCE) |
63.1% (1 to 20 mA cm−2) |
103.43% (5000) |
101
|
| Carbon/TiO2/Co3O4 |
481.7 F g−1 at 1 A g−1 |
3 M KOH |
−0.05–0.55 V (Hg/HgO) |
78.9% (1 to 10 A g−1) |
91.6% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
102
|
| Co3O4–NiCo2O4@CF |
166.25 F g−1 at 1 A g−1 |
1 M KOH |
−0.5–0.5 V (Ag/AgCl) |
91% (1 to 2.5 A g−1) |
|
103
|
| CoOx/CNF |
750 mF cm−2 at 10 mV s−1 |
6 M KOH |
0–1 V |
81% (10 to 100 mV s−1) |
91% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
104
|
| NM-CH/ZnO@Co3O4 CNCs |
1179 F g−1 at 0.5 A g−1 |
1 M KOH |
0–0.7 V (Ag/AgCl) |
72.5% (0.5 to 20 A g−1) |
98% (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
105
|
| CoFe2O4–Fe@NC-700 |
3960.9 F g−1 at 1 A g−1 |
6 M KOH |
−0.2–0.7 V (SCE) |
|
|
106
|
| Co3O4@N-pc/PEDOT |
305.3 F g−1 at 0.6 A g−1 |
1 M KOH |
0–0.45 V (Ag/AgCl) |
|
84% (3000) |
107
|
| CuCo2O4@40-rGO |
762 F g−1 at 0.5 A g−1 |
6 M KOH |
0–0.6 V (Ag/AgCl) |
71.6% (0.5 to 20 A g−1) |
108% (5000) |
108
|
| Pt–NiCo2O4/C |
1665.3 F g−1 at 1 A g−1 |
6 M KOH |
0–0.4 V (SCE) |
|
94.49% (3500) |
109
|
FeNiCo ZIF-67 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
532.7 F g−1 at 1 A g−1 |
3 M KOH |
−0.3–0.25 V (Ag/AgCl) |
41.4% (1 to 10 A g−1) |
91% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
110
|
| Co3O4–carbon |
574.44 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Ag/AgCl) |
52.6% (1 to 20 A g−1) |
94.7% (1000) |
111
|
2.1 Single metal oxides
Salunkhe et al.49 prepared both nanoporous carbon and nanoporous cobalt oxide materials from a single ZIF-67 precursor by optimizing the annealing conditions, as shown in Fig. 2a. The resulting ZIF-derived carbon possessed highly graphitic walls and a high specific surface area of 350 m2 g−1, while the resulting ZIF-derived nanoporous Co3O4 possessed a high specific area of 148 m2 g−1. Also, when both materials were tested in a three-electrode system, they showed high capacitance values (272 and 504 F g−1, respectively, at a scan rate of 5 mV s−1). Also, Zhang et al.50 successfully constructed a supercapacitor by adopting porous hollow Co3O4 derived from the ZIF-67 rhombic dodecahedral structure as the electrode material, which showed a large specific capacitance of 1100 F g−1 and retained more than 95.1% of its specific capacitance after 6000 continuous charge–discharge cycles. Meanwhile, Guo et al.58 reported an effective approach to synthesize cohesive porous Co3O4/C materials using a ZIF-67 single-source precursor via two-step calcination. As a supercapacitor electrode material, the Co3O4/C composite exhibited a higher specific capacitance (875.6 F g−1 at 1 A g−1) and better cycling stability (capacitance retention of 87.8% after 1000 cycles at 6 A g−1) compared to pure Co3O4 (245.3 F g−1 and 78.4% under the same conditions). In addition, it showed good cycling stability with a capacitance retention of 82% after 1000 cycles at 6 A g−1 in a symmetric supercapacitor device. The better electrochemical performance of the composite can be attributed to the improvement in conductivity, larger surface area for more active sites, and the ameliorative volume expansion of Co3O4 by the stable structure of Co3O4 nanoparticles well embedded in the partially graphitized carbon matrix during the long cycling process. To compare the influence of the order of the two-step calcination in the oxide/carbon composite materials, Chen et al.112 synthesized six active materials including carbonized ZIF67 (C67), oxidized C67 (C67/O), oxidized ZIF67-covered C67 (C67/O67), oxidized ZIF67 (O67), carbonized O67 (O67/C) and carbonized ZIF67-covered O67 (O67/C67) (shown in Fig. 2b). Among them, the C67/O electrode showed the highest capacitance value of 332.3 mF cm−2 at 20 mV s−1 due to the suitable N-doped graphite and Co3O4 composition as well as the porous particle-assembled polyhedron structure. Similarly, as shown in Fig. 2c, Shu et al.77 grew flake-like ZIF-67 on carbon cloth, and subsequently synthesized Co3O4@C by adopting a similar two-step thermal conversion. As a result, this strategy effectively prevents structural collapse and defect formation, which are usually caused by the direct oxidation of ZIF-67 nanosheets. In supercapacitor tests, Co3O4@C-500 showed an appreciable specific capacitance (703.3 F g−1 at 1 A g−1) and excellent cycling stability with the capacitance retention of 100% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1. Moreover, the Co3O4@C-500//reduced graphene oxide (RGO) asymmetric supercapacitor (ASC) exhibited an energy density of 43.99 W h kg−1 at a power density of 824.8 W kg−1, and excellent cycling performance with a capacity retention of 88% after 10
000 cycles at 10 A g−1. Moreover, the Co3O4@C-500//reduced graphene oxide (RGO) asymmetric supercapacitor (ASC) exhibited an energy density of 43.99 W h kg−1 at a power density of 824.8 W kg−1, and excellent cycling performance with a capacity retention of 88% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This excellent performance was attributed to the rational composition control and unique structure of Co3O4 nanoparticles embedded in the carbon skeleton, which resulted in short electrolyte diffusion channels as well as electron transport distance and effectively reduced the negative effect of volume change in the electrode materials during the charge/discharge process. Alternatively, Li et al.81 prepared tree-like CoOx/carbon on Au surface via a chemical vapor deposition method and two-step treatment, as shown in Fig. 2d. The as-prepared CoOx/carbon composite arrays provided a less tortuous pathway for ion diffusion, high pseudocapacitance from the transition metal oxide, and good electrical conductivity from carbon. Moreover, the absence of adhesives in array electrodes is beneficial for the promotion of the electrochemical performance. Subsequently, Wang et al.95 changed the order of the two-step thermal treatment method to obtain Co3O4/NC. Co3O4/NC exhibited a specific capacitance of 1144 F g−1 at a current density of 1 A g−1 (much higher than the specific capacitance of pure Co3O4), and its retention rate was 45.4% at a current density of 20 A g−1. In addition, the asymmetric supercapacitor (ASC) device consisting of Co3O4/NC composite electrodes and activated carbon produced the highest energy density of 40 W h kg−1 at a power density of 1037 W kg−1. At a current density of 5 A g−1, the capacitor retained 87.6% of its initial capacitance after 10
000 cycles. This excellent performance was attributed to the rational composition control and unique structure of Co3O4 nanoparticles embedded in the carbon skeleton, which resulted in short electrolyte diffusion channels as well as electron transport distance and effectively reduced the negative effect of volume change in the electrode materials during the charge/discharge process. Alternatively, Li et al.81 prepared tree-like CoOx/carbon on Au surface via a chemical vapor deposition method and two-step treatment, as shown in Fig. 2d. The as-prepared CoOx/carbon composite arrays provided a less tortuous pathway for ion diffusion, high pseudocapacitance from the transition metal oxide, and good electrical conductivity from carbon. Moreover, the absence of adhesives in array electrodes is beneficial for the promotion of the electrochemical performance. Subsequently, Wang et al.95 changed the order of the two-step thermal treatment method to obtain Co3O4/NC. Co3O4/NC exhibited a specific capacitance of 1144 F g−1 at a current density of 1 A g−1 (much higher than the specific capacitance of pure Co3O4), and its retention rate was 45.4% at a current density of 20 A g−1. In addition, the asymmetric supercapacitor (ASC) device consisting of Co3O4/NC composite electrodes and activated carbon produced the highest energy density of 40 W h kg−1 at a power density of 1037 W kg−1. At a current density of 5 A g−1, the capacitor retained 87.6% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles.
000 charge/discharge cycles.
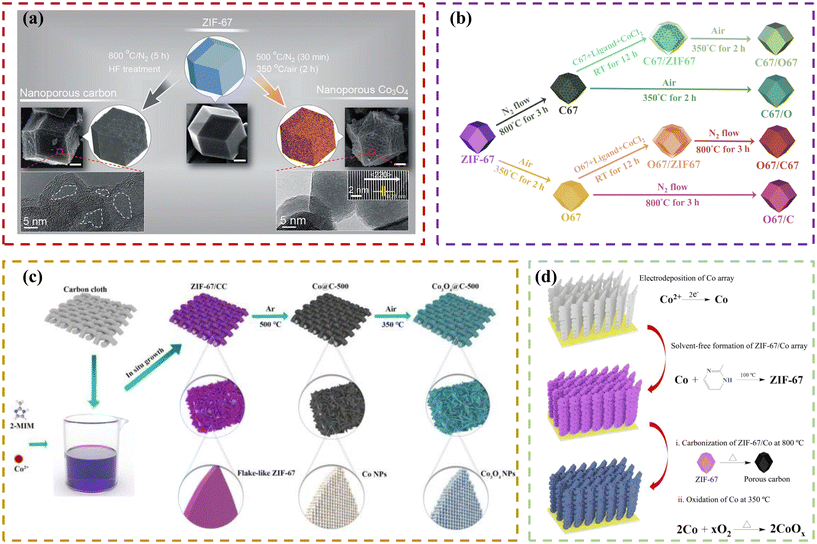 |
| | Fig. 2 (a) Schematic of the process for the preparation of nanoporous carbon and nanoporous Co3O4 from a ZIF-67 polyhedron as the single precursor by optimized thermal treatment. The actual SEM images and high-resolution TEM images of the respective materials are shown below their illustrations. Reprinted with permission from ref. 49, Copyright 2015, the American Chemical Society. (b) Illustration of the process for the synthesis of C67, C67/O67, C67/O, O67, O67/C67 and O67/C. Reprinted with permission from ref. 112, Copyright 2021, Elsevier Ltd. (c) Schematic of the formation of Co3O4@C nanosheet array. Reprinted with permission from ref. 77, Copyright 2021, Elsevier B.V. (d) Schematic of the process for the preparation of CoOx/carbon arrays. Reprinted with permission from ref. 81, Copyright 2021, the American Chemical Society. | |
2.2 Multi-metal oxides
The doping of other metal ions in the precursor ZIF-67 can be performed to derive more transition metal oxides. Also, multi-metal oxides can provide a higher theoretical capacitance because of the greater number of changes in their valence state. Chu et al.69 designed a three-dimensional hollow CoWO4 composite grown on Ni-foam (3D-H-CoWO4/NF) based on a flower-like ZIF-67 utilizing a facile dipping and hydrothermal approach. 3D-H-CoWO4/NF not only possessed a large specific area and rich active sites, but also accommodated the volume expansion/contraction during the charge/discharge processes. Additionally, its unique structure facilitated fast electron/ion transport, resulting in a high specific capacitance of 1395 F g−1 and an excellent cycle stability with 89% retention after 3000 cycles and superior rate property. Furthermore, 3D-H CoWO4/NF could be used as a cathode to assemble an asymmetric supercapacitor (ASC), and 3D-H CoWO4/NF//AC showed a good energy density (29.0 W h kg−1). Similarly, Le et al.70 designed Co2V2O7/graphene composites through an in situ ion exchange reaction, as shown in Fig. 3g. The morphological and compositional characterization confirmed that ZIF-67 was successfully transformed into the nanoparticle-assembled hollow Co2V2O7 nanocages, which were densely distributed on graphene (shown in Fig. 3a–f). Benefiting from the well-designed structure and compositions, the electrochemical tests indicated that the as-prepared Co2V2O7/graphene electrode exhibited a high specific capacity of 276.5 C g−1 at 1 A g−1, good rate capability (Fig. 3h), and remarkably long cycling stability (93% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). The asymmetric supercapacitor devices assembled with Co2V2O7/graphene and reduced graphene oxides delivered a high energy density of 25.7 W h kg−1 at a power density of 663.5 W kg−1 and excellent long cycling stability.
000 cycles). The asymmetric supercapacitor devices assembled with Co2V2O7/graphene and reduced graphene oxides delivered a high energy density of 25.7 W h kg−1 at a power density of 663.5 W kg−1 and excellent long cycling stability.
 |
| | Fig. 3 (a and b) SEM images of ZIF-67/G; (c) SEM image, (d and e) TEM images, and (f) HR-TEM image of Co2V2O7/G. (g) Schematic of the fabrication process for Co2V2O7/G. (h) Specific capacitance of Co2V2O7, Co2V2O7/G and Co3O4/G electrodes at different current densities. Reprinted with permission from ref. 70, Copyright 2020, The Royal Society of Chemistry. | |
Moreover, the doping of metal ions in the process of forming the precursor ZIF-67 or in situ ion exchange reaction of the precursor ZIF-67 is also an important approach to obtain multi-metal oxides. Cheng et al.72 synthesized three-dimensional porous iron-doped Co3O4 using a single bimetallic metal–organic framework, FeCo-ZIF-67 (Fig. 4a). The optimal 3D porous Fe-doped Co3O4 possessed a high capacitance of 767.9 C g−1 at 1 A g−1. Also, the Fe-doped Co3O4//N-doped carbon HSC device achieved a desirable specific energy (37 W h kg−1) and power (750 W kg−1), and satisfactory cycling stability (90% retention after 4000 cycles). Similarly, Venkatesh et al.91 reported the synthesis of NiCo2O4 nanosheets based on NiCo-MOFs and combined them with graphene oxide due to its outstanding mechanical support and electronic conductivity. The NiCo2O4 nanosheets/graphene nanosheets achieved a high specific capacitance of 1365 F g−1 at 1 A g−1 and excellent capacity retention of 89.11% over 2000 cycles at 10 A g−1. As shown in Fig. 4b, Zheng et al.106 used pure ZIF-67 as the precursor to fabricate CoFe2O4–Fe@NC particles with a high surface area, which helped to accelerate ion and charge transfer. The specific capacitance of the CoFe2O4–Fe@NC composite carbonized at 700 °C reached 3960.9 F g−1 at 1 A g−1. When this composite was combined with activated carbon (AC) to construct an asymmetric supercapacitor (ASC), a density of energy of up to 84.9 W h kg−1 was attained at a power capacity of 291.6 W kg−1. Moreover, this composite maintained a capacitance retention of up to 94.9% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. At the same time, Semerci et al.108 prepared CuCo2O4@rGO nanomaterials using a step-wise method, which involved the annealing of the CoCu-LDH@rGO precursor derived from ZIF-67@rGO. At a graphene concentration of 4.1%, the composite named CuCo2O4@40-rGO exhibited the best performance among the CuCo2O4@rGO composites (Fig. 4c–f). A specific capacity of 762 F g−1 (381 C g−1) was recorded at a current density of 0.5 A g−1 and an excellent rate performance of 71.6%, revealing an excellent capacitive performance and reversibility. Moreover, the electrochemical performance of CuCo2O4@40-rGO was evaluated as a cathodic electrode and biomass-derived activated carbon (AC) as the negative electrode. The hybrid supercapacitor device showed an energy density of 20.7 W h kg−1 at a power density of 0.43 kW kg−1 with a working potential window of 0–1.7 V, as well as 98.8% capacity retention over 10
000 cycles. At the same time, Semerci et al.108 prepared CuCo2O4@rGO nanomaterials using a step-wise method, which involved the annealing of the CoCu-LDH@rGO precursor derived from ZIF-67@rGO. At a graphene concentration of 4.1%, the composite named CuCo2O4@40-rGO exhibited the best performance among the CuCo2O4@rGO composites (Fig. 4c–f). A specific capacity of 762 F g−1 (381 C g−1) was recorded at a current density of 0.5 A g−1 and an excellent rate performance of 71.6%, revealing an excellent capacitive performance and reversibility. Moreover, the electrochemical performance of CuCo2O4@40-rGO was evaluated as a cathodic electrode and biomass-derived activated carbon (AC) as the negative electrode. The hybrid supercapacitor device showed an energy density of 20.7 W h kg−1 at a power density of 0.43 kW kg−1 with a working potential window of 0–1.7 V, as well as 98.8% capacity retention over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Also, Wei et al.54 reported the synthesis of hybrid porous Co3O4–CeO2 hollow polyhedrons via a simple cation-exchange route, followed by heat treatment. When utilized as the electrode material for supercapacitors, the hybrid porous Co3O4–CeO2 hollow polyhedrons delivered a large specific capacitance of 1288.3 F g−1 at 2.5 A g−1 and a remarkably long cycling stability (<3.3% loss after 6000 cycles). Furthermore, an asymmetric supercapacitor (ASC) device based on the hybrid porous Co3O4–CeO2 hollow polyhedrons was assembled. The ASC device possessed an energy density of 54.9 W h kg−1, which retained 44.2 W h kg−1 even at a power density of 5100 W kg−1, indicating its promising application in electrochemical energy storage.
000 cycles. Also, Wei et al.54 reported the synthesis of hybrid porous Co3O4–CeO2 hollow polyhedrons via a simple cation-exchange route, followed by heat treatment. When utilized as the electrode material for supercapacitors, the hybrid porous Co3O4–CeO2 hollow polyhedrons delivered a large specific capacitance of 1288.3 F g−1 at 2.5 A g−1 and a remarkably long cycling stability (<3.3% loss after 6000 cycles). Furthermore, an asymmetric supercapacitor (ASC) device based on the hybrid porous Co3O4–CeO2 hollow polyhedrons was assembled. The ASC device possessed an energy density of 54.9 W h kg−1, which retained 44.2 W h kg−1 even at a power density of 5100 W kg−1, indicating its promising application in electrochemical energy storage.
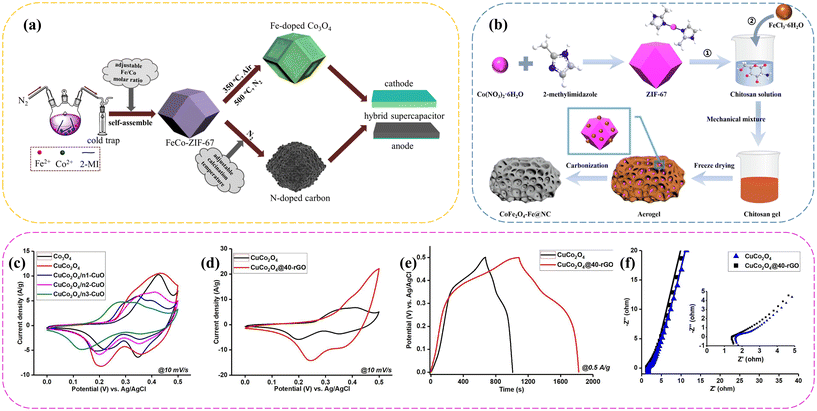 |
| | Fig. 4 (a) Schematic of the design of Fe-doped Co3O4 and N-doped carbon. Reprinted with permission from ref. 72, Copyright 2020, Elsevier Inc. (b) Procedure for the synthesis of the CoFe2O4–Fe@NC composite material. Reprinted with permission from ref. 106, Copyright 2024 Elsevier Inc. (c) CV curves of the metal oxide electrodes at a scan rate of 10 mV s−1; (d) CV curves of CuCo2O4 and CuCo2O4@40-rGO at 10 mV s−1; (e) GCD curves of CuCo2O4 and CuCo2O4@40-rGO at a current density of 0.5 A g−1; and (f) Nyquist plots of CuCo2O4 and CuCo2O4@40-rGO. Reprinted with permission from ref. 108, Copyright 2024, Elsevier B.V. | |
2.3 Composite materials
It is estimated that particle-shaped metal oxides usually experience high internal resistance, and consequently reduced charge transfer due to the agglomeration of the particles. In this regard, ZIF-67-derived oxides are usually composited with other materials to improve their electrochemical properties, such as carbon materials and conductive polymers to improve their conductivity or other transition metal compounds to achieve a higher specific capacitance.
Deng et al.52 successfully prepared honeycomb-like porous Co3O4 grown on three-dimensional graphene network/nickel foam (Co3O4/3DGN/NF) via a facile solution growth process with subsequent annealing treatment, as shown in Fig. 5a. Fig. 5b shows Co3O4/3DGN grown on Ni foam, which possessed a large specific surface area, as shown in Fig. 5c. Also, the Co3O4/3DGN/NF electrode delivered a high specific capacitance (321 F g−1 at 1 A g−1) and excellent long-cycling stability (88% of its maximum capacitance after 2000 charge–discharge cycles). Furthermore, the Co3O4/3DGN/NF electrode exhibited the maximum energy density of 7.5 W h kg−1 with the power density of 794 W kg−1 and retained 4.1 W h kg−1 at the power density of 15 kW kg−1 in a two-electrode system. Its enhanced electrochemical properties can be attributed to the unique nanostructure of Co3O4 with admirable pseudocapacitance performance and the intimate integration of graphene with Co3O4 and the Ni foam matrix, which not only enhanced the electron conductivity for fast electron and ion transport but also provided a high specific surface area and excellent structural stability. Similarly, Lin et al.60 obtained rGO/Co3O4via the in situ growth of ZIF-67 polyhedra in the presence of graphene oxide, followed by thermal annealing (Fig. 5d). The resultant rGO/Co3O4 composites consisted of a continuously-conductive double-network constructed from graphene sheets and derived N-doped carbons from ZIF-67, showing a large specific surface area of 523 m2 g−1. The as-fabricated symmetrical supercapacitor based on rGO/Co3O4 exhibited a high specific capacitance of 277.5 F g−1 at 25 A g−1 (as compared in Fig. 5e–g) and an energy density of 24.7 W h kg−1 at a power density of up to 40 kW kg−1. The supercapacitor also retained 87.5% of its initial capacitance over 5000 cycles at 5 A g−1. The large capacitance, high energy density, and excellent cycling stability for rGO/Co3O4 can be attributed to the 3D double conductive network from the 2D graphene sheets and porous channels of the pseudo-capacitive Co3O4 polyhedra. Moreover, Zha et al.71 initially synthesized ZIF-67-derived CoO, and then formed a composite with reduced graphene oxide through the hydrothermal method. As shown in Fig. 5h–j, Z-CoO/RGO exhibited an outstanding mass specific capacitance (275 F g−1 at 1 A g−1) and excellent resistance characteristic due to the synergistic effect between ZIF-67 and RGO. Besides graphene, Yang et al.98 reported the preparation of ZIF-67-derived cobalt oxide/carbon nanotube (MWCNTx@Co3O4) composites by calcining the MWCNTx@ZIF-67 precursor in one step. Benefiting from the homogeneous conductive carbon nanotubes, the synthesized MWCNTx@Co3O4 electrode displayed a maximum specific capacitance of 206.89 F g−1 at 1 A g−1.
 |
| | Fig. 5 (a) Schematic of the fabrication process for Co3O4/3DGN/NF; (b) TEM image of Co3O4/3DGN/NF; and (c) pore size distribution of Co3O4/3DGN/NF. Reprinted with permission from ref. 52, Copyright 2016, Elsevier B.V. (d) Schematic of the synthesis route for rGO/Co3O4 composite; (e) CV curves of rGO, Co3O4, and rGO/Co3O4 at 20 mV s−1; (f) CV curves of rGO/Co3O4 at different scan rates; and (g) GCD curves of rGO/Co3O4 at different current densities. Reprinted with permission from ref. 60, Copyright 2019, The Royal Society of Chemistry. (h) Nyquist impedance plots, (i) CV curves and (j) GCD curves of ZIF-67, Z-CoO, ZIF-67/RGO and Z-CoO/RGO. Reprinted with permission from ref. 71, Copyright 2020, Elsevier Ltd. | |
Also, Chen's group97 reported the synthesis and characterization of nanocomposites via a two-step method using polypyrrole (PPy) and Co3O4@SiO2 in the presence of an oxidizing agent. As shown in Fig. 6a, Co3O4@SiO2/PPy was obtained by coating PPy on Co3O4@SiO2, which was prepared by annealing SiO2-coated ZIF-67 at high temperature. Comparing Fig. 6b with Fig. 6c and d, it can be seen that PPy was well combined with Co3O4@SiO2. Also, the results showed that the specific capacitance of Co3O4@SiO2 and Co3O4@SiO2/PPy (2 mL) was 33.36 F g−1 and 107.7 F g−1 at 0.6 A g−1, indicating that the addition of PPy greatly improved the electrochemical performance of Co3O4@SiO2 (as shown in Fig. 6e and f). This may be due to synergy between Co3O4@SiO2 and the PPy polymer, where the addition of PPy may help construct more ion channels in Co3O4@SiO2 to enhance the ion transport. Subsequently, the next year, Chen's group107 again reported the preparation of the Co3O4@N-pc/PEDOT composite as an electrode material for supercapacitors. Employing ZIF-67@ZIF-8 as the precursor, the obtained Co3O4@N-pc/PEDOT composite exhibited the highest specific capacitance of 305.3 F g−1 at 0.6 A g−1, which is significantly superior to that of PEDOT (63.6 F g−1), ZIF-67@ZIF-8-400 (91.4 F g−1), ZIF-67 (43.8 F g−1) and Co3O4@N-pc-400 (154.8 F g−1). Further, Co3O4@N-pc/PEDOT (4 mL) was used to construct a symmetric supercapacitor, which delivered outstanding values of energy (20.03 W h kg−1) and power density (415.17 W kg−1).
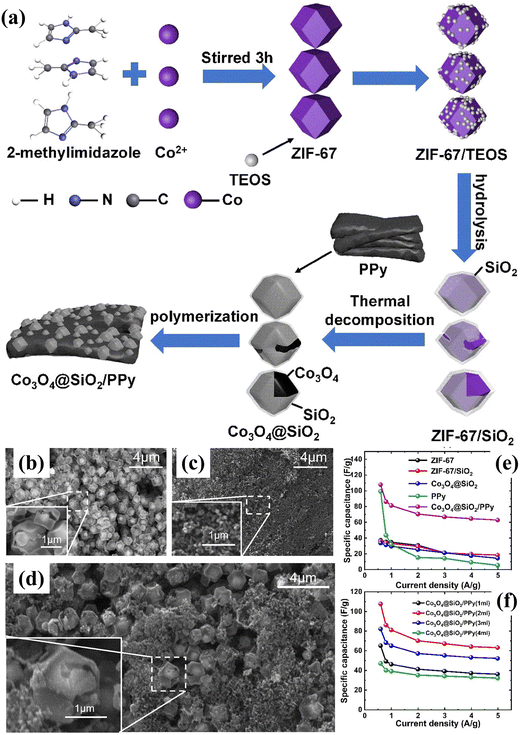 |
| | Fig. 6 (a) Schematic illustration of synthetic procedure for ZIF-67, ZIF-67/SiO2, Co3O4@SiO2 and Co3O4@SiO2/PPy; SEM images of (b) Co3O4@SiO2, (c) PPy and (d) Co3O4@SiO2/PPy (2 mL); (e) specific capacitance as a function of current density for ZIF-67, ZIF-67/SiO2, Co3O4@SiO2, PPy and Co3O4@SiO2/PPy; and (f) specific capacitance as a function of the current density for Co3O4@SiO2 and PPy in different proportions. Reprinted with permission from ref. 97, Copyright 2023, Springer Nature. | |
Wang et al.53 used ZIF-67 and (NH4)2MoS4 as the precursors of Co3O4/C and MoS2, respectively, to obtain hierarchical mesoporous Co3O4/C@MoS2 core–shell structure materials via calcination and solvothermal methods. Co3O4/C@MoS2 exhibited a high specific capacitance (1076 F g−1 at 1 A g−1), rate capability (76.9% capacitance retention at 10 A g−1) and cyclic stability (64.5% capacitance retention after 5000 cycles at 10 A g−1) due to the presence of MoS2, greatly influencing the electrochemical performances of the obtained core–shell materials, which were better than that of Co3O4/C. Xu et al.67 reported the synthesis of a hollow Co3O4@MnO2 cubic nanomaterial derived from the ZIF-67@Mn-ZIF sacrificial precursor. It demonstrated high performances, such as specific capacitance of 413 F g−1 at the current density of 0.5 A g−1, and when the current density increased from 0.5 to 10 A g−1 (20 times increase), it still exhibited ∼41% retention of its initial capacitance. Similarly, Babu et al.93 also synthesized a type of Co3O4@MnO2 nanosheets using ZIF-67 and provided an effective pathway for rapidly transporting electrons and ions, as shown in Fig. 7a. As a result, the ZIF-67-derived Co3O4@MnO2-3 electrode material showed a high specific capacitance of 768 C g−1 at 1 A g−1 with outstanding cycling stability (86% retention after 5000 cycles) and the porous structure of the material had a good BET surface area of 160.8 m2 g−1 according to Fig. 7b and c. As a hybrid supercapacitor, Co3O4@MnO2-3//activated carbon exhibited a high specific capacitance (82.9 C g−1) and long cycle life (85.5% retention after 5000 cycles). Moreover, a high energy density of 60.17 W h kg−1 and power density of 2674.37 W kg−1 were achieved. Additionally, as shown in Fig. 7d, Dai et al.65 modified ZIF-67-derived Co3O4 with NiCo-layered double hydroxide nanosheets to obtain an outstanding electrode composite material. The specific capacitance of NiCo LDH/50 mg Co3O4 was 1393.9 F g−1 at 1 A g−1 with outstanding cycle stability (88.4% up to 5000 cycles) compared with other electrodes, as shown in Fig. 7e. An aqueous asymmetric supercapacitor (ASC) was assembled by employing NiCo LDH/50 mg Co3O4 as the positive electrode and activated carbon (AC) as the negative electrode, which delivered a voltage window of 1.5 V and a high energy density of 46.4 W h kg−1 at a power density of 750.4 W kg−1.
 |
| | Fig. 7 (a) Schematic diagram of the synthesis of Co3O4@MnO2 core–shell structure; (b) BET analysis and (c) BJH pore size distribution curves of ZIF-67-derived Co3O4, Co3O4@MnO2-1, Co3O4@MnO2-2 and Co3O4@MnO2-3. Reprinted with permission from ref. 93, Copyright 2022, The Royal Society of Chemistry. (d) Schematic of the processes for the preparation of NiCo LDH/Co3O4; and (e) comparison of the specific capacitance of Co3O4, NiCo LDH, NiCo LDH/Co3O4 Mix, NiCo LDH/20 mg Co3O4, NiCo LDH/50 mg Co3O4, NiCo LDH/80 mg Co3O4 and NiCo LDH/110 mg Co3O4 at different current densities. Reprinted with permission from ref. 65, Copyright 2019, Springer Nature. | |
Considering two-electrode systems, El-Deen's group89 further applied Ni–Co LDH@Co3O4 nanocubes (prepared as shown in Fig. 8a) in an asymmetric solid-state supercapacitor device (ASC) and constructed the device based on a full cell with the Ni–Co LDH@Co3O4 nanocubes and three-dimensional spongy graphene. When tested in a two-electrode system, as shown in Fig. 8b–e, the ASC delivered the highest energy density of 66.7 W h kg−1 with a power density of 800 W kg−1 and superior recyclability over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cycles with a holding capacitance retention ratio of 90.5% and coulombic efficiency of 99.8%. Recently, Wang et al.99 reported the preparation of hollow carbon tube@Co3O4@SnS2 composites by growing ZIF-67 and SnS2 nanosheets on a PMMA/PAN/Zn2+ electrospun film as the precursors (Fig. 8f). The unique inner core–shell and the outer-shell SnS2 contributed to the excellent characteristics, including abundant pores and channels for rapid ion transport and storage, high specific surface area, improved electrical conductivity, and additional electroactive sites for the faradaic reaction. Owing to the synergy between the unique 1D porous hollow structure and the different components, the as-fabricated HCT-2@Co3O4@SnS2 electrode exhibited a high specific capacitance of 439 F g−1 at 1 A g−1. Moreover, as shown in Fig. 8g, the assembled flexible supercapacitor also demonstrated a remarkable energy density of 40.22 W h kg−1, the corresponding power density of 750.22 W kg−1, and long cycle life (Fig. 8j). In addition, no structural deformation and capacitance loss were observed in the bent devices, as shown in Fig. 8h and i.
500 cycles with a holding capacitance retention ratio of 90.5% and coulombic efficiency of 99.8%. Recently, Wang et al.99 reported the preparation of hollow carbon tube@Co3O4@SnS2 composites by growing ZIF-67 and SnS2 nanosheets on a PMMA/PAN/Zn2+ electrospun film as the precursors (Fig. 8f). The unique inner core–shell and the outer-shell SnS2 contributed to the excellent characteristics, including abundant pores and channels for rapid ion transport and storage, high specific surface area, improved electrical conductivity, and additional electroactive sites for the faradaic reaction. Owing to the synergy between the unique 1D porous hollow structure and the different components, the as-fabricated HCT-2@Co3O4@SnS2 electrode exhibited a high specific capacitance of 439 F g−1 at 1 A g−1. Moreover, as shown in Fig. 8g, the assembled flexible supercapacitor also demonstrated a remarkable energy density of 40.22 W h kg−1, the corresponding power density of 750.22 W kg−1, and long cycle life (Fig. 8j). In addition, no structural deformation and capacitance loss were observed in the bent devices, as shown in Fig. 8h and i.
 |
| | Fig. 8 (a) Schematic process for the fabrication of Ni–Co LDH@Co3O4 nanocube; (b) CV profiles of 3DSGr and Ni–Co LDH@Co3O4 nanocube electrodes at a scan rate of 10 mV s−1; (c) CV plots of the ASC at various scan rates; (d) GCD curves of Ni–Co LDH@Co3O4 nanocube//3DSGr device at various current densities; and (e) capacitance measured for the prepared ASC device. Reprinted with permission from ref. 89, Copyright 2022, Elsevier B.V. (f) Schematic illustration of the preparation of HCT-2@Co3O4@SnS2; (g) schematic illustration of HCT-2@Co3O4@SnS2//AC device; (h) flexible device bending at various angles; (i) GCD plots of the ASC device at various bending conditions; and (j) cycling stability of the device at 10 A g−1. Reprinted with permission from ref. 99, Copyright 2023, Elsevier B.V. | |
3. ZIF-67-derived transition metal sulfides
As a type of pseudocapacitor material, sulfides possess larger specific capacitance values, narrower band gaps, lower melting points, higher conductivity and richer redox sites compared to metal oxides. However, they still has some challenges, which limit their further research such as side reactions during the electrochemical measurement process, susceptibility to oxidization and structural destruction.34 Thus, to solve the above-mentioned problems, the design of the material structure needs to be studied, while ZIF-67 is regarded as a good precursor to obtain transition metal sulfides with unique structures113 (Table 2).
Table 2 Summary of the electrochemical characteristics of ZIF-67-derived sulfide materials in three-electrode measurements
| Electrode materials |
Specific capacity |
Electrolyte |
Potential window (CV) |
Capacity retention |
Cyclic stability |
Ref. |
| CoS-NP/CoS-NS |
980 F g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (SCE) |
60% (1–20 A g−1) |
89% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
114
|
| CoNi2S4 |
1890 F g−1 at 4 A g−1 |
2 M KOH |
0–0.5 V (Ag/AgCl) |
81.2% (4–20 A g−1) |
71.6% (5000) |
32
|
| Co9S8@N–C@MoS2 |
410 F g−1 at 10 A g−1 |
3 M KOH |
0–0.6 V (Hg/HgO) |
79.8% (0.5–20 A g−1) |
101.7% (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
115
|
| NiCo2S4 |
939 C g−1 at 1 A g−1 |
6 M KOH |
−0.1–0.6 V (SCE) |
75.8% (1–10 A g−1) |
|
116
|
| CNT/CoS |
2173.1 F g−1 at 5 A g−1 |
6 M KOH |
−0.07–0.63 (Hg/HgO) |
65% (5–20 A g−1) |
|
117
|
| GH@NC@Co9S8 |
842.4 F cm−2 at 1 A g−1 |
6 M KOH |
0–0.5 V (Ag/AgCl) |
65.5% (1–10 A g−1) |
|
118
|
| MnO2@NiCo-LDH/CoS2 |
1547 F g−1 at 1 A g−1 |
2 M KOH |
0–0.55 V (SCE) |
76.9% (1–10 A g−1) |
82.3% (2000) |
119
|
| NiCo2S4 |
1232 F g−1 at 2.1 A g−1 |
3 M KOH |
0–0.65 V (Hg/HgO) |
55% (2.1–41.5 A g−1) |
80% (8000) |
120
|
| CoS2 |
375.2 C g−1 at 1 A g−1 |
2 M KOH |
−0.2–0.6 V (Hg/HgO) |
70% (1–20 A g−1) |
92.1% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
121
|
| Mo-doped CoS HNC |
781 F g−1 at 0.5 A g−1 |
2 M KOH |
0–0.6 V (SCE) |
52% (0.5–10 A g−1) |
46.8% (5000) |
122
|
| CoSx/Ni–Co LDH |
1562 F g−1 at 1 A g−1 |
2 M KOH |
−01–0.55 V (SCE) |
65.4% (1–20 A g−1) |
76.64% (5000) |
123
|
| CoSx@NiCo-LDH |
680.8 C g−1 at 1 A g−1 |
3 M KOH |
0–0.6 V (Ag/AgCl) |
76% (1–10 A g−1) |
80% (3000) |
124
|
| NiCo2S4 |
1382 F g−1 at 1 A g−1 |
2 M KOH |
0–0.5 V (Ag/AgCl) |
83.6% (1–10 A g−1) |
70% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
125
|
| CuCo2S4 |
1344 F g−1 at 1 A g−1 |
3 M KOH |
0–0.6 V (Hg/HgO) |
71.3% (1–30 A g−1) |
93.1% (5000) |
126
|
| Co3S4-HNCs@PPy |
1706 F g−1 at 1 A g−1 |
2 M KOH |
−0.1–0.8 V (SCE) |
73.2% (1–10 A g−1) |
68.1% (5000) |
37
|
| Co3S4@NiO |
187.93 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (SCE) |
74.1% (1–20 A g−1) |
92.6% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
127
|
| MnCo2S4/Co9S8 |
1058 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
47.6% (1–10 A g−1) |
|
128
|
| Co3S4/WS2 |
412.7 F g−1 at 1 A g−1 |
1 M H2SO4 |
0–1 V (Ag/AgCl) |
|
94.3% (2000) |
129
|
| NiCo2S4 |
1350 F g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (Hg/HgO) |
70% (1–20 A g−1) |
|
130
|
| Co–W–S@N, S-PC |
1001 F g−1 at 2 A g−1 |
2 M KOH |
0–0.5 V (SCE) |
45% (2–20 A g−1) |
|
131
|
| Co3S4/PANI |
1106 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (SCE) |
66.55% (1–10 A g−1) |
86% (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
132
|
| rGO/CoSx–rGO/rGO |
460 F g−1 at 1 A g−1 |
1 M KOH |
0–0.5 V (SCE) |
30% (1–16 A g−1) |
85% (5000) |
133
|
| NiCoS@PPy |
2316.6 F g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (SCE) |
60.8% (1–10 A g−1) |
|
134
|
| α-MnS@Co3S4 |
283.3 mA h g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Ag/AgCl) |
81.5% (1–25 A g−1) |
92.7% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
135
|
| Co9S8/NF |
369.1 mA h g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
75.5% (1–20 A g−1) |
75% (5000) |
136
|
| CoNi2S4/MXene/NF |
933 C g−1 at 1 A g−1 |
3 M KOH |
0–0.7 V (Hg/HgO) |
70.2% (1–10 A g−1) |
|
137
|
| NiCo-LDH/Co9S8 |
1654 F g−1 at 2 A g−1 |
3 M KOH |
0–0.5 V (SCE) |
83.3% (2–20 A g−1) |
82.5% (5000) |
138
|
| CoNi2S4 |
2448 F g−1 at 1 A g−1 |
3 M KOH |
0–0.43 C |
63.3% (1–10 A g−1) |
|
139
|
| hollow tube@sheets NiCo2S4 |
3227.94 F g−1 at 2 A g−1 |
2 M KOH |
0–0.7 V (SCE) |
59% (2–10 A g−1) |
83.92% (5000) |
140
|
| Al-doped Co9S8@NG |
736 C g−1 at 1 A g−1 |
2 M KOH |
−0.2–0.6 V (Ag/AgCl) |
71% (1–40 A g−1) |
92% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
141
|
| MSZ@NF |
4840 mF cm−2 at 1 mA cm−2 |
2 M KOH |
0–0.6 V |
|
81.3% (5000) |
142
|
| Co1−xS/HNPSCS |
1058.9 C g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (SCE) |
74.3% (1–20 A g−1) |
|
143
|
| Co3S4/Ti3C2Tx |
602 F g−1 at 1 A g−1 |
3 M KOH |
0–0.6 V (Hg/HgO) |
81.6% (1–10 A g−1) |
|
144
|
| CC/Co9S8/PPy |
429 F g−1 at 1 mA cm−2 |
2 M KOH |
−0.2–0.6 V (SCE) |
66.6% (1–16 mA cm−2) |
83.5% (2500) |
145
|
| rGO@CuCo2S4@CoS2/NF |
2447.1 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
55.2% (1–10 A g−1) |
79.2% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
146
|
| CoS/NC |
789 F g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
80.2% (1–20 A g−1) |
92.8% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
147
|
| MoS2@CoS2 |
950 F g−1 at 1 A g−1 |
2 M KOH |
−0.2–0.8 V (SCE) |
75.6% (1–10 A g−1) |
94.6% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
148
|
| N-Co3S4–GN/CNT |
1158 F g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
86% (1–10 A g−1) |
97.2% (4000) |
149
|
| Co-NTC@Co3S4 |
3117.3 F g−1 at 1 A g−1 |
1 M KOH |
0–0.45 V (SCE) |
80% (1–10 A g−1) |
87% (1000) |
150
|
| NiS2/CoS2@C |
1373 C g−1 at 1 A g−1 |
6 M KOH |
0–0.7 V (SCE) |
68.6% (1–20 A g−1) |
100.2% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
151
|
| V2O5@Co3S4 |
1493.6 F g−1 at 1 A g−1 |
2 M KOH |
0–0.5 V (SCE) |
57.9% (1–20 A g−1) |
75.1% (7000) |
152
|
| CoNi2S4/MXene |
751 C g−1 at 1 A g−1 |
3 M KOH |
0–0.7 V |
73.2% (1–10 A g−1) |
72.1% (5000) |
153
|
| Co3S4@CoCH/NF |
2697.1 F g−1 at 2 A g−1 |
3 M KOH |
−0.05–0.55 V (SCE) |
84.4% (2–20 A g−1) |
79.8% (8000) |
154
|
| NiCo2S4/Co3S4 |
8.43 F cm−2 at 2 mA cm−2 |
3 M KOH |
0–0.7 V (SCE) |
24.9% (2–50 mA cm−2) |
60% (5000) |
155
|
| Co4S3/Ni3S2@MoS2 |
1238 F g−1 at 1 A g−1 |
1 M KOH |
−0.1–0.8 V (Ag/AgCl) |
56.9% (1–8 A g−1) |
85% (2000) |
156
|
| NF/FeNi2S4@Co9S8–rGO |
1308 C g−1 at 1 A g−1 |
6 M KOH |
0–0.7 V (Ag/AgCl) |
83.6% (1–36 A g−1) |
93.75 (8500) |
157
|
| CoS2@C |
1151 F g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (SCE) |
73.4% (1–10 A g−1) |
85.58% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
158
|
| Ni(OH)2/CoS/CC |
561.6 mA h g−1 at 1 A g−1 |
2 M KOH |
0–0.8 V (Hg/HgO) |
70.8% (1–32 A g−1) |
|
159
|
| NiCo2S4 |
408 mA h g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
51.1% (1–20 A g−1) |
84.3% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
160
|
| rGO/NiCo2S4 |
171 mA h g−1 at 1 A g−1 |
2 M KOH |
0–0.7 V (Hg/HgO) |
71.3% (1–10 A g−1) |
85% (2000) |
161
|
| CoS2@gC/rGO |
1188 F g−1 at 1 A g−1 |
1 M KOH |
0–0.4 V (Ag/AgCl) |
57% (1–20 A g−1) |
76% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
162
|
| CoSx@CoNi2S4/CC |
1970 F g−1 at 1 A g−1 |
1 M KOH |
0–0.6 V (Hg/HgO) |
92% (1–10 A g−1) |
|
163
|
| Co9S8@ZnGa2S4 |
1018 C g−1 at 5 mA cm−2 |
3 M KOH |
−0.05–0.55 V (Hg/HgO) |
65% (5–187.5 mA cm−2) |
93.5% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
164
|
| Ni–Co–Mo–S/CNT |
1728.6 F g−1 at 1 A g−1 |
2 M KOH |
−0.1–0.8 V (Hg/HgO) |
55.8% (1–10 A g−1) |
|
165
|
| HN2CMS |
928.1 C g−1 at 1 A g−1 |
3 M KOH |
0–0.7 V (Hg/HgO) |
52.3% (1–10 A g−1) |
85% (5000) |
166
|
| HCCoS@CNT |
1250 F g−1 at 0.5 A g−1 |
3 M KOH |
0–0.5 V (SCE) |
69.6% (1–10 A g−1) |
90.4% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
167
|
| Co3S4/NiS2/Cu2S |
464.16 C g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Ag/AgCl) |
|
|
168
|
| CoNi2S4/carbon/MXene |
1221.6 F g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
79.1% (1–20 A g−1) |
63.96% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
40
|
| Ni3S4@Co3S4 |
747.3 C g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
77% (1–10 A g−1) |
|
169
|
| NiCo2S4/MXene/NC |
1786 F g−1 at 1 A g−1 |
3 M KOH |
−0.2–0.7 V (Hg/HgO) |
52% (1–40 A g−1) |
110.5% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
170
|
| MoS2/Co3S4/NPC |
612 F g−1 at 1 mV s−1 |
1 M KOH |
0–0.45 V (Ag/AgCl) |
|
88.59% (9000) |
171
|
| Co3S4 |
668 F g−1 at 1 A g−1 |
1 M KOH |
0–0.7 V (SCE) |
52.8% (1–10 A g−1) |
86.4% (5000) |
172
|
| CoNiS |
598.8 C g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
59.9% (1–30 A g−1) |
70.1% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
173
|
| Co3S4–Mo15S19 |
3283 F g−1 at 1 A g− |
2 M KOH |
0–0.5 V (Ag/AgCl) |
77.7% (1–10 A g−1) |
77% (2500) |
174
|
| Au/ZnS@Co3S4@Ni3S2 |
302.6 mA h g−1 at 1 A g−1 |
3 M KOH |
0–0.6 V (SCE) |
70.3% (1–10 A g−1) |
|
175
|
| ZnCo2S4@ZIF-67 |
447.25 F g−1 at 1 A g−1 |
1 M KOH |
−0.1–0.6 V (Hg/HgO) |
40.3% (1–7 A g−1) |
|
176
|
| CoNi2S4/C-CNTs |
1314.6 C g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (Hg/HgO) |
72.1% (1–20 A g−1) |
78% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
177
|
| P–Co3S4 HNCs |
499.1 C g−1 at 1 A g−1 |
6 M KOH |
0–0.55 V (Hg/HgO) |
|
88.6% (5000) |
178
|
| MoS2/CoMoS4/C |
426.5 F g−1 at 1 A g−1 |
2 M KOH |
0–0.6 V (Hg/HgO) |
73.3% (1–20 A g−1) |
93% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
179
|
| MoNiCo–S |
3125.1 F g−1 at 1 A g−1 |
2 M KOH |
0–0.7 V (Hg/HgO) |
47.6% (1–30 A g−1) |
72.9% (5000) |
180
|
| S-hollow ZIF-67 and NiCo-LDH |
3744.4 F g−1 at 1 A g−1 |
6 M KOH |
−0.2–0.6 V (Hg/HgO) |
68.6% (1–10 A g−1) |
82% (3000) |
181
|
| Mo–CoS2 |
1382.6 F g−1 at 0.5 A g−1 |
6 M KOH |
0–0.7 V (Hg/HgO) |
49.0% (0.5–10 A g−1) |
75.75% (1000) |
182
|
3.1 Single metal sulfides
Hu et al.114 demonstrated the template-induced formation of double-shelled hollow structures with CoS-nanoparticle-assembled nanoboxes surrounded by outer CoS-nanosheet-constructed shells (CoS-NP/CoS-NS DSNBs). Delicate manipulation of the template-induced reaction between ZIF-67 and water led to the formation of ZIF-67/Co(OH)2-nanosheet yolk–shelled structures, which were then transformed into CoS-NP/CoS-NS DSNBs through the reaction with Na2S, as shown in Fig. 9a. As a result of the unique assembly of subunits with different dimensionalities, the CoS-NP/CoS-NS DSNBs possessed a high specific surface area, suitable mesopores, and good structural robustness (Fig. 9b and c), which contributed to their exceptional performance as a battery-type electrode (a specific capacitance of 980 F g−1 at 1 A g−1 and 89% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in Fig. 9d). Additionally, as shown in Fig. 9e, Jia et al.121 reported the designed synthesis of porous quadruple-shelled CoS2 hollow dodecahedrons with concave surfaces through a template-induced formation process. As the result of the combined advantages of the materials and structures (Fig. 9f), when evaluating as electrodes for supercapacitors, the porous quadruple-shelled CoS2 showed a high specific capacity of 375.2 C g−1 with a good rate performance and excellent stability with 92.1% retention after 10
000 cycles in Fig. 9d). Additionally, as shown in Fig. 9e, Jia et al.121 reported the designed synthesis of porous quadruple-shelled CoS2 hollow dodecahedrons with concave surfaces through a template-induced formation process. As the result of the combined advantages of the materials and structures (Fig. 9f), when evaluating as electrodes for supercapacitors, the porous quadruple-shelled CoS2 showed a high specific capacity of 375.2 C g−1 with a good rate performance and excellent stability with 92.1% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Moreover, the assembled quadruple-shelled CoS2//active carbon asymmetric supercapacitor achieved a high energy density of 52.1 W h kg−1, excellent specific capacitance (146 F g−1 at 0.5 A g−1), and electrochemical cycling stability (89% retention after 5000 cycles). Moreover, Peng et al.136 successfully synthesized hierarchical porous Co9S8 nanowire arrays grown on Ni foam via a multi-step method for use in supercapacitors (Fig. 9g). Introducing ZIF-67 is favorable for the formation of hierarchical pores and small active powder. Also, it is beneficial to provide a large surface area and short diffusion channels, as shown in Fig. 9h, which can reduce the volume expansion and ensure the stability of the architecture. ZIF-derived Co9S8 displayed a large specific capacitance because of its abundant redox sites and high electrical conductivity. The Co9S8/NF electrode possessed outstanding electrochemical properties with the specific capacitance of 369.1 mA h g−1 at 1 A g−1 and 75.5% retention at 20 A g−1. After 5000 cycles, its showed 75% capacitance retention at 20 A g−1. Furthermore, a button-type asymmetric supercapacitor (ASC) was assembled with Co9S8/NF as the positive electrode, which displayed the maximum specific energy of 48.2 W h kg−1 at the specific power of 799.8 W kg−1 and excellent cycling life with 77% retention of its initial capacitance after 5000 cycles. Wang et al.147 applied chemical vapor deposition (CVD) treatment to obtain a CoS/nitrogen-doped carbon (CoS/NC) composite with a hollow cage-like polyhedron structure formed by the combination of CoS nanosheets and nitrogen-doped carbon skeleton. It exhibited a specific capacitance of 789 F g−1 at a current density of 1 A g−1 and a rate capacity of 80.2% at a current density of 20 A g−1. The CoS/NC-700//AC capacitor showed the highest energy density of 32.8 W h kg−1 when the power density was 620.0 W kg−1 and it retained 89.2% of its initial capacitance after 10
000 cycles. Moreover, the assembled quadruple-shelled CoS2//active carbon asymmetric supercapacitor achieved a high energy density of 52.1 W h kg−1, excellent specific capacitance (146 F g−1 at 0.5 A g−1), and electrochemical cycling stability (89% retention after 5000 cycles). Moreover, Peng et al.136 successfully synthesized hierarchical porous Co9S8 nanowire arrays grown on Ni foam via a multi-step method for use in supercapacitors (Fig. 9g). Introducing ZIF-67 is favorable for the formation of hierarchical pores and small active powder. Also, it is beneficial to provide a large surface area and short diffusion channels, as shown in Fig. 9h, which can reduce the volume expansion and ensure the stability of the architecture. ZIF-derived Co9S8 displayed a large specific capacitance because of its abundant redox sites and high electrical conductivity. The Co9S8/NF electrode possessed outstanding electrochemical properties with the specific capacitance of 369.1 mA h g−1 at 1 A g−1 and 75.5% retention at 20 A g−1. After 5000 cycles, its showed 75% capacitance retention at 20 A g−1. Furthermore, a button-type asymmetric supercapacitor (ASC) was assembled with Co9S8/NF as the positive electrode, which displayed the maximum specific energy of 48.2 W h kg−1 at the specific power of 799.8 W kg−1 and excellent cycling life with 77% retention of its initial capacitance after 5000 cycles. Wang et al.147 applied chemical vapor deposition (CVD) treatment to obtain a CoS/nitrogen-doped carbon (CoS/NC) composite with a hollow cage-like polyhedron structure formed by the combination of CoS nanosheets and nitrogen-doped carbon skeleton. It exhibited a specific capacitance of 789 F g−1 at a current density of 1 A g−1 and a rate capacity of 80.2% at a current density of 20 A g−1. The CoS/NC-700//AC capacitor showed the highest energy density of 32.8 W h kg−1 when the power density was 620.0 W kg−1 and it retained 89.2% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles at a current density of 5 A g−1. Meanwhile, Wei et al.158 constructed CoS2 and Co3S4 nanoparticles anchored on a carbon skeleton (CoS2@C and Co3S4@C) depending on if an oxidation process occurred, respectively (Fig. 9i). The reactions are represented by the following equations:
000 charge–discharge cycles at a current density of 5 A g−1. Meanwhile, Wei et al.158 constructed CoS2 and Co3S4 nanoparticles anchored on a carbon skeleton (CoS2@C and Co3S4@C) depending on if an oxidation process occurred, respectively (Fig. 9i). The reactions are represented by the following equations:| | | CoS2 + OH− ↔ CoS2OH + H2O + e− | (1) |
| | | CoS2OH + OH− ↔ CoS2O + H2O + e− | (2) |
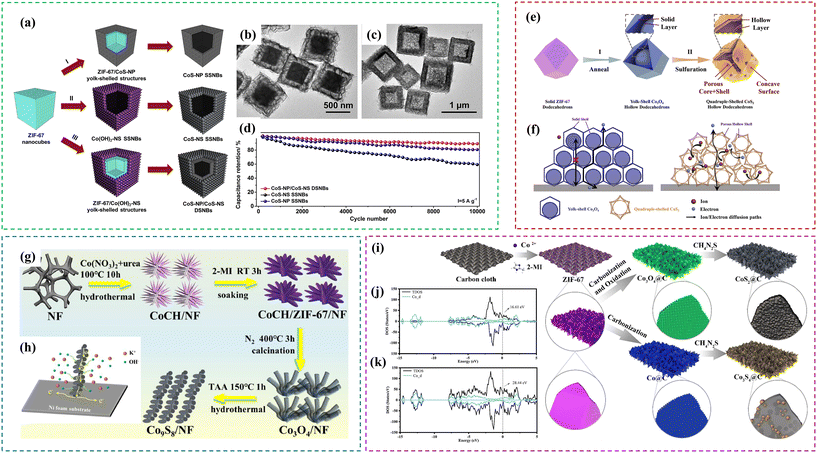 |
| | Fig. 9 (a) Procedure for the synthesis of CoS-NP SSNBs, CoS-NS SSNBs and CoS-NP/CoS-NS DSNBs; (b) TEM image of ZIF-67/Co(OH)2-NS yolk–shelled structures; (c) TEM image of CoS-NP/CoS-NS DSNBs; and (d) comparison of the cycling performance of the three CoS hollow nanostructures at 5 A g−1. Reprinted with permission from ref. 114, Copyright 2016, Elsevier Inc. (e) Schematic of the fabrication of quadruple-shelled CoS2 hollow dodecahedron electrode; (f) schematic of the reasons for the enhanced performance of quadruple-shelled CoS2 hollow dodecahedrons compared with yolk–shell Co3O4 hollow dodecahedrons. Reprinted with permission from ref. 121, Copyright 2019, Elsevier Ltd. (g) Schematic of the synthetic process of Co9S8/NF and (h) schematic structure and mechanism of Co9S8/NF. Reprinted with permission from ref. 136, Copyright 2021, Elsevier Ltd. (i) Process for the construction of CoS2 and Co3S4 and total density of states (TDOS) of (j) Co3S4 and (k) CoS2. Reprinted with permission from ref. 158, Copyright 2022, Elsevier Ltd. | |
Compared with the inhomogeneous distribution of Co3S4@C nanoparticles, a larger specific surface area is provided by numerous CoS2@C nanoparticles embedded in the 2D carbon skeleton and connected into sheets through effectively morphological construction, which provides more redox channels and active sites for electrochemical reactions. Thus, the CoS2@C electrode presented a superior electrochemical performance with a high specific capacitance of 1151 F g−1 at 1 A g−1, which is 1.69-times larger than that of Co3S4@C. Comparing Fig. 9j and k, density functional theory (DFT) calculations illustrated that CoS2 can transfer much more charge and has higher electronic activity in the process of OH− adsorption compared with Co3S4, proving that CoS2 has better conductivity. Moreover, an asymmetric supercapacitor was constructed using CoS2@C//reduced graphene oxide (RGO), which displayed a high energy density of 46.52 W h kg−1 at a power density of 800 W kg−1.
3.2 Multi-metal sulfides
Alternatively, multi-metal sulfides have richer redox-active sites, higher electronic conductivity and synergistic effects between different metal ions. Therefore, numerous studies have been devoted to designing multi-metal sulfides using ZIF-67 as a template. For instance, the Ni ion, similar to the Co ion, is usually used as a dopant ion to obtain multi-metal chalcogenides derived from ZIF-67. Guo et al.116 reported the growth of well-aligned NiCo2S4 (shown in Fig. 10a–c) and CoS2 nanoarrays with a hollow/porous configuration on a conductive matrix, which significantly increase the number of electroactive sites, shortened the charge/ion diffusion length, and enhanced the mass/electron transfer. Consequently, the obtained NiCo2S4 possessed an excellent specific capacitance of 939 C g−1, a fast charge/discharge rate, and a favorable life span. Similarly, Liu et al.120 synthesized NiCo2S4 hollow nanocages assembled by ultrathin nanosheets through a facile solid-state chemical sulfuration method, as shown in Fig. 10d. Also, as shown in Fig. 10e and f, the obtained hollow NiCo2S4 possessed inner cavities and large ultrathin nanosheets for both short ion diffusion distance and rich interfacial active sites, which can dynamically speed up faradaic reactions. A specific capacitance of 1232 F g−1 was obtained at a current density of 2 A g−1 for the NiCo2S4 electrode. Notably, compared with Co–Ni LDH, as shown in Fig. 10g and h, the NiCo2S4 electrode yielded an impressive rate capability and excellent cycling stability (80% capacitance retention after 8000 charge–discharge cycles), mainly due to its robust porous architecture and good electronic conductivity. The aqueous asymmetric supercapacitor assembled with NiCo2S4 as the positive electrode also delivered a high energy density of 41.4 W h kg−1 at a power density of 689.5 W kg−1. Moreover, Cai et al.125 changed the order of the sulfidation and cation exchange to obtain CoSx hollow cages first, and subsequently obtained NiCo2S4 by adding Ni2+, as shown in Fig. 10i and j. Compared with CoSx, as shown in Fig. 10k and l, the NiCo2S4 hollow cages exhibited an appealing capacitance of 1382 F g−1 at 1 A g−1. Furthermore, asymmetric supercapacitors (ASCs) were fabricated with the synthesized NiCo2S4 and active carbon (AC) as the electrodes. The ASCs showed an energy density of 35.3 W h kg−1 at a power density of 750 W kg−1 and outstanding cycling stability of 79% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
 |
| | Fig. 10 (a) Schematic of the process for the synthesis of the composite and fabrication of the asymmetric supercapacitor and SEM images of (b) NiCo-LDHs and (c) NiCo2S4. Reprinted with permission from ref. 116, Copyright 2018, the American Chemical Society. (d) Schematic of the fabrication of NiCo2S4; TEM images of (e) Co–Ni LDH and (f) NiCo2S4; (g) specific capacitance versus current density and (h) Nyquist plots of Co–Ni LDH and NiCo2S4. Reprinted with permission from ref. 120, Copyright 2019, Springer Nature. (i) Schematic showing the fabrication of NiCo2S4 hollow cages; (j) EDS mapping of NiCo2S4; (k) specific capacitance at different current densities and (l) Nyquist plots of NiCo2S4 and CoSx. Reprinted with permission from ref. 125, Copyright 2019, Elsevier B.V. | |
In the case of other metal ions, Yang et al.122 doped Mo in ZIF-67 in the presence of Na2MoO4, and subsequently obtained an Mo-doped CoS hollow nanocage structure (Mo-doped CoS HNC) via a sulfurization process. As shown in Fig. 11a–d, the obtained Mo-doped CoS HNC exhibited an enhanced specific capacitance (781.0 F g−1 at 0.5 A g−1) compared with the control CoS HNC (387.1 F g−1) and CoMoO4–Co(OH)2 HNC (285.1 F g−1). Furthermore, it also showed a superior rate capacity of 52% at a 20-fold increase in current density (10 A g−1). The asymmetric supercapacitor (ASC) device assembled using the Mo-doped CoS HNC as the positive electrode and activated carbon (AC) as the negative electrode displayed a high energy density of 27.7 W h kg−1 at a power density of 799.9 W kg−1 with excellent cycling stability, maintaining 88% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The excellent electrochemical performance is attributed to the unique ternary metal sulfide hollow nanocage structure having a large surface area, facile diffusion of ions, good conductivity, and rich redox reactions as well as the synergistic effect between Mo and Co ions. Also, Zhao et al.126 presented the synthesis and characterization of ZIF-67-derived mesoporous copper cobalt sulfide (CuCo2S4) prepared via simple reflux and solvothermal reactions. The obtained CuCo2S4 electrode showed a high specific capacity of up to 672 C g−1 (1344 F g−1) at 1 A g−1. Additionally, Goda et al.141 reported Al doping to improve the conductivity of cobalt sulfide. A novel type of core/sell architecture was successfully designed from aluminum-doped cobalt sulfide encapsulated by nitrogen-doped graphene (Al-doped Co9S8@NG) through the solvothermal/sulfuration of the ZIF-67 structure, subsequent wrapping with a PPy layer and calcination in argon gas at various temperatures. Interestingly, integrating the morphology and composition merits endowed the Al-doped Co9S8@NG600 electrode with highly enhanced supercapacitive features, as shown in Fig. 11e and f. The electrode attained a superior specific capacity of about 736 C g−1 at an applied current density of 1 A g−1, ultra-long cycle stability of 92% after 10
000 cycles. The excellent electrochemical performance is attributed to the unique ternary metal sulfide hollow nanocage structure having a large surface area, facile diffusion of ions, good conductivity, and rich redox reactions as well as the synergistic effect between Mo and Co ions. Also, Zhao et al.126 presented the synthesis and characterization of ZIF-67-derived mesoporous copper cobalt sulfide (CuCo2S4) prepared via simple reflux and solvothermal reactions. The obtained CuCo2S4 electrode showed a high specific capacity of up to 672 C g−1 (1344 F g−1) at 1 A g−1. Additionally, Goda et al.141 reported Al doping to improve the conductivity of cobalt sulfide. A novel type of core/sell architecture was successfully designed from aluminum-doped cobalt sulfide encapsulated by nitrogen-doped graphene (Al-doped Co9S8@NG) through the solvothermal/sulfuration of the ZIF-67 structure, subsequent wrapping with a PPy layer and calcination in argon gas at various temperatures. Interestingly, integrating the morphology and composition merits endowed the Al-doped Co9S8@NG600 electrode with highly enhanced supercapacitive features, as shown in Fig. 11e and f. The electrode attained a superior specific capacity of about 736 C g−1 at an applied current density of 1 A g−1, ultra-long cycle stability of 92% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and a remarkable retention rate (71%). Subsequently, a solid-state AsSC device with Al-doped Co9S8@NG core/shell as the positive electrode and activated PANI-derived carbon nanorods (ACNRs) as the negative electrode was assembled. The device exhibited outstanding specific capacitance, energy density, and power density values of 134 F g−1, 53.3 W h kg−1, and 0.954 kW kg−1, respectively, with a considerable cycle life stability of 93% after consuming 10
000 cycles, and a remarkable retention rate (71%). Subsequently, a solid-state AsSC device with Al-doped Co9S8@NG core/shell as the positive electrode and activated PANI-derived carbon nanorods (ACNRs) as the negative electrode was assembled. The device exhibited outstanding specific capacitance, energy density, and power density values of 134 F g−1, 53.3 W h kg−1, and 0.954 kW kg−1, respectively, with a considerable cycle life stability of 93% after consuming 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Further, when two AsSC devices were linked in series, a multicolor LED could be lit for 25 s, demonstrating their availability for making modern portable electronics, as shown in Fig. 11g–i.
000 cycles. Further, when two AsSC devices were linked in series, a multicolor LED could be lit for 25 s, demonstrating their availability for making modern portable electronics, as shown in Fig. 11g–i.
 |
| | Fig. 11 (a) CV curves, (b) GCD curves, (c) specific capacitance and (d) Nyquist plots of CoS HNC, CoMoO4–Co(OH)2 HNC and Mo-doped CoS HNC. Reprinted with permission from ref. 122, Copyright 2019, The Royal Society of Chemistry. (e) CV curves and of NG600, Al–Co9S8, Al–Co9S8@NG600 and Al–Co9S8@NG900; (f) specific capacitance of Al–Co9S8, Al–Co9S8@NG600 and Al–Co9S8@NG900; (j–i) photographs of AsSC devices linked in series to light a red LED. Reprinted with permission from ref. 141, Copyright 2021, Elsevier B.V. | |
3.3 Composite materials
As mentioned, ZIF-67-derived sulfides usually exhibit the drawbacks of limited rate capability and poor cyclic stability. Accordingly, composite materials are being developed as promising electrode materials because rationally designed structures can improve the cycling stability and rate capability and facilitate electronic transport in electrodes.42 Jian et al.117 designed a hybrid structure of a cobalt sulfide nanocage derived from ZIF-67 and interconnected by carbon nanotubes (CNT/CoS). The carbon nanotube/ZIF-67 (CNT/ZIF-67) nanocomposites with controlled ZIF-67 particle sizes were systematically studied by varying the mass ratio of CNTs to ZIF-67 during crystallization, followed by subsequent sulfurization with thioacetamide (Fig. 12a). Benefiting from the porous nanocage architecture and conductive CNTs, the optimized CNT/CoS nanocage exhibited an excellent electrochemical performance with an outstanding specific capacitance (2173.1 F g−1 at 5 A g−1) and high rate capability (65% retention at 20 A g−1), as shown in Fig. 12b and c. More importantly, the fabricated symmetric supercapacitor exhibited an energy density of 23.3 W h kg−1 at a power density of 3382.2 W kg−1 and impressive long-term stability (96.6% retention after 5000 cycles). Additionally, Zhou et al.140 constructed hollow tube@sheet NiCo2S4 core–shell nanoarrays by in situ growing ZIF-67 on Co-precursor nanorod arrays and sequentially performing anion-exchange (S2−) and cation-exchange (Ni2+) (Fig. 12d). The well-defined nanostructures could shorten the ion transport path in the charging–discharging process and increase the specific surface area and electrochemical active sites, improving the electrochemical performance. Therefore, the unique tube@sheet NiCo2S4 core–shell nanoarrays exhibited an intriguing electrochemical performance and showed an excellent areal capacitance of 11.3 F cm−2 (3227.94 F g−1) at a current density of 2 mA cm−2 (2 A g−1) (Fig. 12e and f). The assembled asymmetric supercapacitor device delivered a high energy density of 0.42 mW h cm−2 at a power density of 2.1 mW cm−2 and displayed outstanding cyclic stability (90.2% retention after 5000 cycles). Similarly, Zhang et al.149 proposed a ZIF-67 derived, nitrogen-doped, graphene-coated and carbon nanotube-interlinked 3D Co3S4/C conductive network (N–Co3S4-GN/CNT), as shown in Fig. 12g, for high-performance supercapacitors. By controlling the mass ratio of ZIF-67, melamine and g-C3N4, various microstructures with determined electrochemical performances could be achieved. The nanocomposites synthesized with the component mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NCSC-211) were proven to have the most excellent comprehensive electrochemical properties, as shown in Fig. 12h. In the NCSC-211 nanostructure, multi-layer graphene functions as conductive shells for improving the cycling performance by alleviating the pulverization caused by volume change, the thick CNTs act as conductive bridges and agglomeration spacers, which increase the electron conductivity and provide more active sites for redox reaction, and doped-nitrogen offers enhanced electrolyte wettability and faster electron transfer. Consequently, the NCSC-211 electrode displayed a specific capacitance of 1158 F g−1 at 1 A g−1, rate capability of 86% at 10 A g−1 and extraordinary cycling stability with 97.2% capacitive retention after 4000 cycles. Moreover, Li et al.165 demonstrated the synthesis of Mo-ZIF-67/CNT composites through mechanical stirring and co-precipitation. After that, Ni, Co and Mo trimetallic sulfide/CNT composites (Ni–Co–Mo–S/CNT) were produced through further ion exchange/etching and vulcanization processes. The material had multiple valence states of three different metal ions, providing a large number of oxidation–reduction sites. The doping of CNTs endowed it with a unique coral-like structure, which helped to form its huge specific surface area and large mesopores. The electrode exhibited an impressive performance with a specific capacitance of 1728.6 F g−1 at a current density of 1 A g−1. It also had a good rate capability, maintaining 55.8% of its specific capacitance when the current density increased tenfold. Moreover, Yan et al.167 developed an in situ self-assembly strategy to prepare hollow composites using ZIF-67 and carbon nanotubes, and subsequently converted ZIF-67 to cobalt sulfide via a sulfurization strategy (Fig. 12i). The unique hollow composites with different dimensions, as shown in Fig. 12j and k, exhibited a synergistic effect between subunits, resulting in excellent electrochemical properties. The impact of particle cavity channel size on electrochemical properties was evaluated systematically. As a result, the obtained CoS@CNT with hollow cattail-shape structures (HCCoS@CNT) exhibited a high specific capacitance (1250 F g−1) and cyclic stability (90.4% for over 10
1 (NCSC-211) were proven to have the most excellent comprehensive electrochemical properties, as shown in Fig. 12h. In the NCSC-211 nanostructure, multi-layer graphene functions as conductive shells for improving the cycling performance by alleviating the pulverization caused by volume change, the thick CNTs act as conductive bridges and agglomeration spacers, which increase the electron conductivity and provide more active sites for redox reaction, and doped-nitrogen offers enhanced electrolyte wettability and faster electron transfer. Consequently, the NCSC-211 electrode displayed a specific capacitance of 1158 F g−1 at 1 A g−1, rate capability of 86% at 10 A g−1 and extraordinary cycling stability with 97.2% capacitive retention after 4000 cycles. Moreover, Li et al.165 demonstrated the synthesis of Mo-ZIF-67/CNT composites through mechanical stirring and co-precipitation. After that, Ni, Co and Mo trimetallic sulfide/CNT composites (Ni–Co–Mo–S/CNT) were produced through further ion exchange/etching and vulcanization processes. The material had multiple valence states of three different metal ions, providing a large number of oxidation–reduction sites. The doping of CNTs endowed it with a unique coral-like structure, which helped to form its huge specific surface area and large mesopores. The electrode exhibited an impressive performance with a specific capacitance of 1728.6 F g−1 at a current density of 1 A g−1. It also had a good rate capability, maintaining 55.8% of its specific capacitance when the current density increased tenfold. Moreover, Yan et al.167 developed an in situ self-assembly strategy to prepare hollow composites using ZIF-67 and carbon nanotubes, and subsequently converted ZIF-67 to cobalt sulfide via a sulfurization strategy (Fig. 12i). The unique hollow composites with different dimensions, as shown in Fig. 12j and k, exhibited a synergistic effect between subunits, resulting in excellent electrochemical properties. The impact of particle cavity channel size on electrochemical properties was evaluated systematically. As a result, the obtained CoS@CNT with hollow cattail-shape structures (HCCoS@CNT) exhibited a high specific capacitance (1250 F g−1) and cyclic stability (90.4% for over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1) (as shown in Fig. 12l–o).
000 cycles at 10 A g−1) (as shown in Fig. 12l–o).
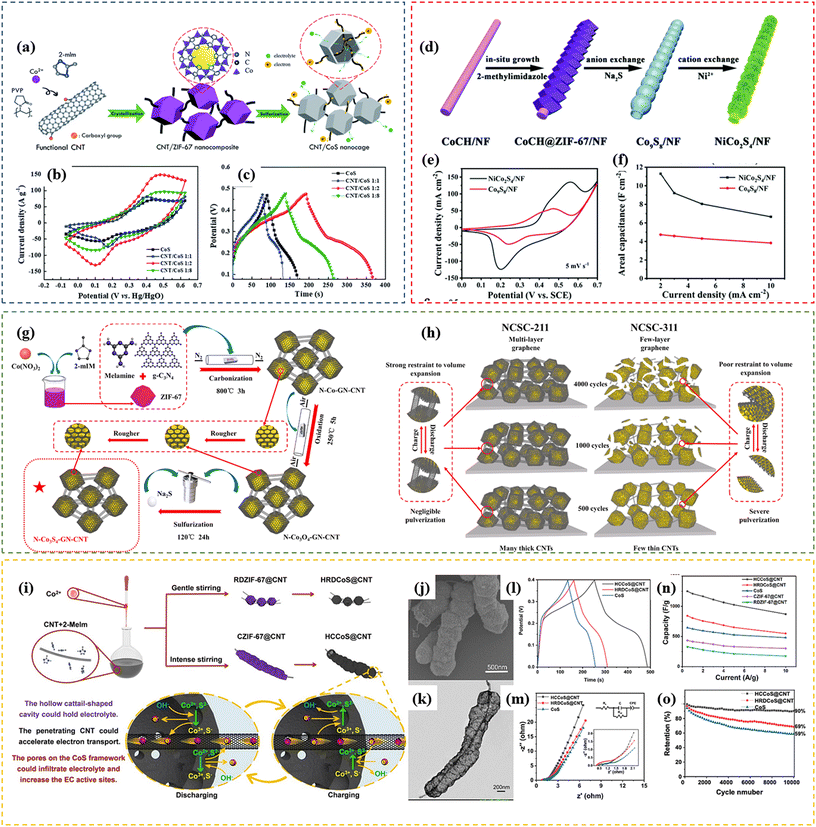 |
| | Fig. 12 (a) Schematic of the synthesis of hybrid CNT/CoS nanocages; (b) CV curves at 100 mV s−1 and (c) GCD curves at 5 A g−1 for CoS, CNT/CoS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CNT/CoS (1 1), CNT/CoS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CNT/CoS (1 2) and CNT/CoS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Reprinted with permission from ref. 117, Copyright 2019, The Royal Society of Chemistry. (d) Synthetic procedure for NiCo2S4/NF; (e) CV curves at 5 mV s−1 and (f) specific capacitance of Co9S8/NF and NiCo2S4/NF. Reprinted with permission from ref. 140, Copyright 2021, The Royal Society of Chemistry. (g) Schematic diagram of the construction of N–Co3S4-GN/CNT nanostructure and (h) schematic of the nanostructure changes in NCSC-211 and NCSC-311 after 500, 1000 and 4000 cycles. Reprinted with permission from ref. 149, Copyright 2022, Elsevier Ltd. (i) Schematic representation showing the various synthesis procedures for generating CoS@CNT structures and the electrochemical process occurring on hierarchical pore-structured HCCoS@CNT. Gentle stirring resulted in the formation of HRDCoS@CNT with a rhombic dodecahedron-shaped morphology by controlling the stirring speed to 200 rpm, while intense stirring resulted in the fabrication of HCCoS@CNT with a cattail-shaped morphology by controlling the stirring speed at 1000 rpm. (j) SEM image and (k) TEM image of HCCoS@CNT and (l) GCD curves at 2 A g−1, (m) Nyquist plots, (n) specific capacitance and (o) cycling stability at 10 A g−1 of CoS, HCCoS@CNT and HRDCoS@CNT. Reprinted with permission from ref. 167, Copyright 2023, Elsevier B.V. 8). Reprinted with permission from ref. 117, Copyright 2019, The Royal Society of Chemistry. (d) Synthetic procedure for NiCo2S4/NF; (e) CV curves at 5 mV s−1 and (f) specific capacitance of Co9S8/NF and NiCo2S4/NF. Reprinted with permission from ref. 140, Copyright 2021, The Royal Society of Chemistry. (g) Schematic diagram of the construction of N–Co3S4-GN/CNT nanostructure and (h) schematic of the nanostructure changes in NCSC-211 and NCSC-311 after 500, 1000 and 4000 cycles. Reprinted with permission from ref. 149, Copyright 2022, Elsevier Ltd. (i) Schematic representation showing the various synthesis procedures for generating CoS@CNT structures and the electrochemical process occurring on hierarchical pore-structured HCCoS@CNT. Gentle stirring resulted in the formation of HRDCoS@CNT with a rhombic dodecahedron-shaped morphology by controlling the stirring speed to 200 rpm, while intense stirring resulted in the fabrication of HCCoS@CNT with a cattail-shaped morphology by controlling the stirring speed at 1000 rpm. (j) SEM image and (k) TEM image of HCCoS@CNT and (l) GCD curves at 2 A g−1, (m) Nyquist plots, (n) specific capacitance and (o) cycling stability at 10 A g−1 of CoS, HCCoS@CNT and HRDCoS@CNT. Reprinted with permission from ref. 167, Copyright 2023, Elsevier B.V. | |
Besides carbon nanotubes, graphene is also a good conductive carbon material for improving the electrochemical properties of ZIF-67-derived transition metal sulfides. Wu et al.133 reported the preparation of a sandwich-like GO/CoSx–rGO/GO hybrid film via a three-step process and the resulting rGO/CoSx–rGO/rGO hybrid film was shown to have a good electrochemical performance because it combined the good pseudocapacitor property of cobalt sulfide and good conductivity and electric double layer capacitor property of rGO. Similarly, Reddy et al.162 added a calcination process and obtained CoS2@gC/rGO by CVD treatment, as shown in Fig. 13a. The CoS2@gC/rGO composite exhibited a specific capacitance of 1188 F g−1, cyclic stability of 76% and 99% coulombic efficiency (Fig. 13b–d). An all-solid-state asymmetric supercapacitor device was fabricated with CoS2@gC/rGO and hydrothermally reduced graphene oxide (hrGO) as the positive and negative electrodes, respectively. The device exhibited a high specific capacitance of 233 F g−1 at a current density of 1.5 A g−1 and delivered a high energy density of 82.88 W h kg−1 at a power density of 1199.56 W kg−1. Particularly, the device conserved an energy density of 42.44 W h kg−1 even at a high-power density of 7999.9 W kg−1. Additionally, it showed good cyclic stability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of repeated charging/discharging, suggesting its potential for practical supercapacitor applications. Moreover, Wu et al.161 designed the rGO/NiCo2S4 material by using GO and ZIF-67 as the template and precursor, which increased the specific surface area and provided active sites for redox reactions and shortened ion transport pathways. The synthesized rGO-10/NiCo2S4 exhibited excellent electrochemical properties as an electrode material, which had a high specific capacity of 171 mA h g−1 and 121.9 mA h g−1 (1 A g−1 and 10 A g−1). The as-obtained rGO-10/NiCo2S4//AC asymmetric supercapacitor (ASC) delivered a power density of 870 W kg−1 at the specific energy of 41 W h kg−1, with 88.2% capacitance retention after 5000 cycles at 5 A g−1.
000 cycles of repeated charging/discharging, suggesting its potential for practical supercapacitor applications. Moreover, Wu et al.161 designed the rGO/NiCo2S4 material by using GO and ZIF-67 as the template and precursor, which increased the specific surface area and provided active sites for redox reactions and shortened ion transport pathways. The synthesized rGO-10/NiCo2S4 exhibited excellent electrochemical properties as an electrode material, which had a high specific capacity of 171 mA h g−1 and 121.9 mA h g−1 (1 A g−1 and 10 A g−1). The as-obtained rGO-10/NiCo2S4//AC asymmetric supercapacitor (ASC) delivered a power density of 870 W kg−1 at the specific energy of 41 W h kg−1, with 88.2% capacitance retention after 5000 cycles at 5 A g−1.
 |
| | Fig. 13 (a) Schematic of the synthesis of CoS2@gC/rGO, the insert is optimized configuration of CoS2(200) and total density of states (TDOS) with Co 3d contributions of CoS2 (blue and yellow colors represent Co and S atoms, respectively); and (b) CV curves at 25 mV s−1, (c) GCD curves at 1 A g−1 and (d) specific capacitance of Co@gC@rGO, CoSx@gC@rGO and CoS2@gC@rGO. Reprinted with permission from ref. 162, Copyright 2023 Elsevier B.V. | |
As a representative member of the emerging 2D MXenes, Ti3C2Tx-based supercapacitor materials have been comprehensively reviewed and demonstrated to be beneficial for the conductivity and the structural stability of ZIF-67-derived transition metal sulfides.183 For example, Ruan et al.137 synthesized Ti3C2 MXene-wrapped CoNi2S4 nanosheets grown on nickel foam, as shown in Fig. 14a and b, which delivered a very high specific capacity of 933 C g−1 at 1 A g−1 and good rate performance (Fig. 14c) due to the fact that the unique dendrite morphology can provide many exposed electro-active sites and the wrapped conductive MXene can facilitate charge transfer. An asymmetric supercapacitor was assembled using CoNi2S4/MXene/NF and RGO as positive and negative electrodes, respectively, which delivered a high energy density of 30.5 W h kg−1 without deteriorating its high power density (1587 W kg−1). Also, Luo et al.144 obtained Co3S4/Ti3C2Tx powder by hydrothermal reaction and the electrode containing Co3S4/Ti3C2Tx as the active material exhibited a very high specific capacitance of 602 F g−1 at 1 A g−1, which was 2.6-times higher than of the ZIF-67/Ti3C2Tx-containing electrode. The supercapacitor based on Co3S4/Ti3C2Tx maintained 81.6% of its original capacitance even at a current density of 10 A g−1. Thus, the introduction of Ti3C2Tx in ZIF-67 not only improved the electrical conductivity of the hybrid material but also its structural stability, which acted as a support. The asymmetric Co3S4/Ti3C2Tx//AC supercapacitor (ASC) containing activated carbon (AC) as the anode showed a high energy density of 44.9 W h kg−1 at a power density of 800.3 W kg−1. This ASC also demonstrated a high rate performance (79.2%) and excellent cycling stability (with 88.3% capacitance retention and 99.23% coulombic efficiency after 5000 cycles). Similarly, Qi et al.153 reported that CoNi2S4/MXene derived from ZIF-67/MXene and NiCo-LDH/MXene exhibited an outperforming specific capacitance (751 C g−1 at 1 A g−1), which was much higher than that of pure CoNi2S4 (600 C g−1 at 1 A g−1). An asymmetric supercapacitor (CoNi2S4/MXene//reduced graphene oxide (RGO)) was assembled, which delivered a high energy density of 33.8 W h kg−1 and excellent cycling performance. As shown in Fig. 14d, Jiao et al.40 added a carbonization process to improve the conductivity and structural stability of the obtained CoNi2S4/carbon/MXene (Fig. 14e). CoNi2S4/carbon/MXene exhibited the specific capacitance of 1221.6 F g−1 (169.7 mA h g−1) at 1 A g−1 and still maintained a high capacitance of 966.4 F g−1 (134.2 mA h g−1) at 20 A g−1 (79.1% of the former), with a better cycling stability compared with other electrodes, as shown in Fig. 14f. After assembling it with activated carbon into an asymmetric supercapacitor, the device delivered a high energy density of 19.82 W h kg−1 at a power density of 469.31 W kg−1 and maintained about 71.17% of its initial capacitance after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 20 A g−1, representing excellent cycle stability and rate capability. Moreover, Li et al.170 fabricated hierarchical hollow core–shell microspheres of NiCo2S4/MXene/N-doped carbon (NiCo2S4/MXene/NC) using dual templates of polyethylene microspheres and ZIF-67 (Fig. 14g). The integration of MXene and N-doped carbon significantly enhanced the electronic conductivity of the electrode, while the construction of hollow spherical structures effectively alleviated the instability of NiCo2S4/NC during the charge–discharge cycles, leading to an excellent charge storage performance with a high specific capacitance (1786 F g−1 at 1 A g−1) and impressive cycling stability (over 100% capacitance retention after 10
000 cycles at a current density of 20 A g−1, representing excellent cycle stability and rate capability. Moreover, Li et al.170 fabricated hierarchical hollow core–shell microspheres of NiCo2S4/MXene/N-doped carbon (NiCo2S4/MXene/NC) using dual templates of polyethylene microspheres and ZIF-67 (Fig. 14g). The integration of MXene and N-doped carbon significantly enhanced the electronic conductivity of the electrode, while the construction of hollow spherical structures effectively alleviated the instability of NiCo2S4/NC during the charge–discharge cycles, leading to an excellent charge storage performance with a high specific capacitance (1786 F g−1 at 1 A g−1) and impressive cycling stability (over 100% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). The corresponding hybrid supercapacitor displayed a high specific capacitance (190 F g−1 at 1 A g−1) with a maximum energy density of 67 W h kg−1 at 796 W kg−1 and great cycling stability (over 80% capacitance retention after 10
000 cycles). The corresponding hybrid supercapacitor displayed a high specific capacitance (190 F g−1 at 1 A g−1) with a maximum energy density of 67 W h kg−1 at 796 W kg−1 and great cycling stability (over 80% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). As shown in Fig. 14h–k, density functional theory (DFT) calculations revealed that addition of MXene enhanced the conductivity of NiCo2S4.
000 cycles). As shown in Fig. 14h–k, density functional theory (DFT) calculations revealed that addition of MXene enhanced the conductivity of NiCo2S4.
 |
| | Fig. 14 (a) Schematic of synthetic process for dendrite CoNi2S4/MXene/NF; (b) SEM image of CoNi2S4/MXene/NF; and (c) specific capacitance of ZIF-67/NF, NiCo-LDH/NF, Ni–Co–S/NF and CoNi2S4/MXene/NF. Reprinted with permission from ref. 137, Copyright 2021, Elsevier B.V. (d) Schematic description of the formation of the CNS/C/MXene composites; (e) TEM images of CNS/C/MXene; and (f) cycling stability of CNS, CNS/C, CNS/MXene and CNS/C/MXene. Reprinted with permission from ref. 40, Copyright 2024, Elsevier Ltd. (g) Schematic diagram of the preparation of NiCo2S4/MXene/C; (h) constructed models of NiCo2S4 and NiCo2S4/MXene for calculation; (i) TDOS of NiCo2S4 and NiCo2S4/MXene; and PDOS of (j) NiCo2S4 and (k) NiCo2S4/MXene. Reprinted with permission from ref. 170, Copyright 2024, Elsevier B.V. | |
Cheng et al.37 inlaid Co3S4 hollow nanocages (HNCs) derived from ZIF-67 on polypyrrole (PPy) tubes, forming a Co3S4-HNCs@PPy hybrid with intertwined “Co3S4-to-PPy-to-Co3S4” conductive networks via a facile solution method. The as-synthesized Co3S4-HNCs@PPy showed outstanding electrochemical activity (1706 F g−1 at 1 A g−1) together with a high rate capability (73.2% retention at 10 A g−1), which was significantly superior to individual Co3S4-HNCs, PPy or a physical mixture of Co3S4 and PPy. Remarkably, the asymmetric supercapacitor based on Co3S4-HNCs@PPy delivered a high energy density of 50.5 W h kg−1 (at 849.1 W kg−1) with high durability (82.8% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). The outstanding supercapacitor property can be attributed to the synergistic advantages of the intertwined “Co3S4-to-PPy-to-Co3S4” networks including rich reactive sites, shortened charge diffusion pathway and enhanced charge transfer and mechanical stability. Similarly, Zhao et al.134 grew the ZIF-67-derived porous CoNi2S4 on intercrosslinked PPy tubes to obtain the NiCoS@PPy hybrid via a solution sulfidation (as shown in Fig. 15a). The NiCoS@PPy electrode achieved an ultrahigh specific capacitance of 2316.6 and 1409.5 F g−1 at 1 and 10 A g−1, largely outperforming the control NiCoS or NiCo-LDH@PPy (Fig. 15b–d). Furthermore, the fabricated ASC utilizing NiCoS@PPy as the cathode displayed an outstanding energy storage capability (34.4 W h kg−1 at 799 W kg−1) and splendid cyclic life (retaining 84% initial capacitance after 8500 cycles). Additionally, Wu et al.145 initially assembled ZIF-67 on carbon cloth, which was further hydrothermally converted to Co9S8, and subsequently PPy was coated on the surface of CC/Co9S8via an electrodeposition process. The obtained CC/Co9S8/PPy electrode showed 83.5% capacity retention after 2500 charge–discharge cycles due to the buffered volume change of Co9S8 during the redox process by the introduction of the PPy shell. Moreover, Xu et al.132 used polyaniline (PANI) as a growth substrate for ZIF-67 as a transfer scaffold of electrons and worked as a wire to connect nanoparticles, improving the conductivity of ZIF-67. The sulfides (Co3S4/PANI) obtained by sulfurization exhibited a high specific capacitance of up to 11 times that of ZIF-67 at 1 A g−1. Also, the assembled asymmetric supercapacitors (ASC) device exhibited a high specific energy of 40.75 W h kg−1 at a specific power of 800 W kg−1 and displayed super cycling stability. After 20
000 cycles). The outstanding supercapacitor property can be attributed to the synergistic advantages of the intertwined “Co3S4-to-PPy-to-Co3S4” networks including rich reactive sites, shortened charge diffusion pathway and enhanced charge transfer and mechanical stability. Similarly, Zhao et al.134 grew the ZIF-67-derived porous CoNi2S4 on intercrosslinked PPy tubes to obtain the NiCoS@PPy hybrid via a solution sulfidation (as shown in Fig. 15a). The NiCoS@PPy electrode achieved an ultrahigh specific capacitance of 2316.6 and 1409.5 F g−1 at 1 and 10 A g−1, largely outperforming the control NiCoS or NiCo-LDH@PPy (Fig. 15b–d). Furthermore, the fabricated ASC utilizing NiCoS@PPy as the cathode displayed an outstanding energy storage capability (34.4 W h kg−1 at 799 W kg−1) and splendid cyclic life (retaining 84% initial capacitance after 8500 cycles). Additionally, Wu et al.145 initially assembled ZIF-67 on carbon cloth, which was further hydrothermally converted to Co9S8, and subsequently PPy was coated on the surface of CC/Co9S8via an electrodeposition process. The obtained CC/Co9S8/PPy electrode showed 83.5% capacity retention after 2500 charge–discharge cycles due to the buffered volume change of Co9S8 during the redox process by the introduction of the PPy shell. Moreover, Xu et al.132 used polyaniline (PANI) as a growth substrate for ZIF-67 as a transfer scaffold of electrons and worked as a wire to connect nanoparticles, improving the conductivity of ZIF-67. The sulfides (Co3S4/PANI) obtained by sulfurization exhibited a high specific capacitance of up to 11 times that of ZIF-67 at 1 A g−1. Also, the assembled asymmetric supercapacitors (ASC) device exhibited a high specific energy of 40.75 W h kg−1 at a specific power of 800 W kg−1 and displayed super cycling stability. After 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of charge and discharge tests, it still maintained 88% of its initial capacitance at a higher current density of 5 A g−1.
000 cycles of charge and discharge tests, it still maintained 88% of its initial capacitance at a higher current density of 5 A g−1.
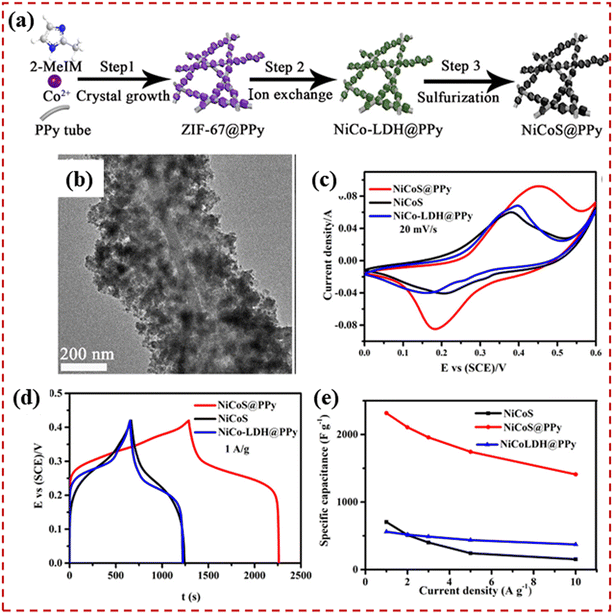 |
| | Fig. 15 (a) Procedure for the preparation of porous NiCoS@PPy; (b) TEM image of NiCoS@PPy; (c) CV curves at 20 mV s−1, (d) GCD curves at 1 A g−1 and (e) specific capacitance of NiCoS, NiCo-LDH@PPy and NiCoS@PPy. Reprinted with permission from ref. 134, Copyright 2021, the American Chemical Society. | |
Combining ZIF-67-derived transition metal sulfides with other compounds is an effective strategy to improve their electrochemical performance by taking advantage of their synergistic effect. Hou et al.115 successfully fabricated a core–shell-structured Co9S8@N–C@MoS2 nanocube through a sulfuration process based on ZIF. Due to their improved electrical conductivity and large surface area, the Co9S8@N–C@MoS2 nanocubes with a core–shell heterostructure exhibited a better electrochemical performance in supercapacitors compared with Co9S8 and delivered a high specific capacitance of 410 F g−1 at the current density of 10 A g−1 after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with excellent cycling stability (101.7% of the initial value). Similarly, Saha et al.174 used ZIF-67 and Mo-precursor to obtain Co3S4–Mo15S19 (CMS/NF) via one-pot hydrothermal sulfurization (Fig. 16a). The unique stellate-shaped architecture of the CMS/NF microflowers enhanced the exposure of the redox active sites, improving the charge storage and exhibiting superior performance. On account of the synergy of the metal ions and stellate-shaped hierarchical architecture, the CMS/NF hybrid sulfide showed an excellent specific capacitance value of 3283 F g−1 at a current density of 1 A g−1 with a capacitance retention of 77.7% at 10 A g−1. The asymmetric supercapacitor device based on CMS/NF hybrid sulfide with aqueous KOH electrolyte exhibited a high specific energy of 40.8 W h kg−1 at 400 W kg−1 with excellent cycling life of 81% after 5000 cycles with ∼100% coulombic efficiency. Also, according to the total density of states, as shown in Fig. 16b and c, it was concluded that compared to the pristine structures, the hybrid system had enhanced states close to the Fermi level, confirming the improvement in conductivity.
000 cycles with excellent cycling stability (101.7% of the initial value). Similarly, Saha et al.174 used ZIF-67 and Mo-precursor to obtain Co3S4–Mo15S19 (CMS/NF) via one-pot hydrothermal sulfurization (Fig. 16a). The unique stellate-shaped architecture of the CMS/NF microflowers enhanced the exposure of the redox active sites, improving the charge storage and exhibiting superior performance. On account of the synergy of the metal ions and stellate-shaped hierarchical architecture, the CMS/NF hybrid sulfide showed an excellent specific capacitance value of 3283 F g−1 at a current density of 1 A g−1 with a capacitance retention of 77.7% at 10 A g−1. The asymmetric supercapacitor device based on CMS/NF hybrid sulfide with aqueous KOH electrolyte exhibited a high specific energy of 40.8 W h kg−1 at 400 W kg−1 with excellent cycling life of 81% after 5000 cycles with ∼100% coulombic efficiency. Also, according to the total density of states, as shown in Fig. 16b and c, it was concluded that compared to the pristine structures, the hybrid system had enhanced states close to the Fermi level, confirming the improvement in conductivity.
 |
| | Fig. 16 (a) Schematic of the synthesis process for CMS/NF; (b) DFT optimized structure of Co3S4–Mo15S19, where red, violet, and yellow colour represent Mo, Co, and S atoms, respectively; and (c) total density of states for (i) pristine Mo15S19, (ii) pristine Co3S4, and (iii) Co3S4–Mo15S19 hybrid structure. Reprinted with permission from ref. 174, Copyright 2024, Elsevier Ltd. (d) Schematic diagram of the synthesis of MnO2@NiCo-LDH/CoS2; (e) TEM image of MnO2@NiCo-LDH/CoS2; (f) CV curves at 1 mV s−1, (g) GCD curves at 1 A g−1, (h) specific capacitance and (i) Nyquist plots of MnO2, MnO2@NiCo-LDH and MnO2@NiCo-LDH/CoS2. Reprinted with permission from ref. 119, Copyright 2019, The Royal Society of Chemistry. (j) Schematic illustration of the fabrication process of CoSx/Ni–Co LDH nanocages. Reprinted with permission from ref. 123, Copyright 2019 Elsevier B.V.; (k) schematic diagram showing the construction of V2O5@Co3S4; (l and m) TEM images of V2O5@Co3S4; and (n) specific capacitance of the V2O5, Co3S4-5 h, V2O5@Co3S4-3 h, V2O5@Co3S4-4 h, V2O5@Co3S4-5 h and V2O5@Co3S4-7 h at 1–20 A g−1. Reprinted with permission from ref. 152, Copyright 2022, Elsevier B.V. | |
Wang et al.119 designed new one-dimensional hierarchical hollow nickel–cobalt layered double hydroxide nanocages assembled on MnO2 nanotubes with uniformly dispersed CoS2 nanoparticles, MnO2@NiCo-LDH/CoS2, using ZIF-67 as the template via multiple hydrothermal and sulfuration process, as shown in Fig. 16d. Fig. 16e shows the TEM image of MnO2@NiCo-LDH/CoS2. Compared with other electrode in Fig. 16f–i, the MnO2@NiCo-LDH/CoS2 electrode materials have a high specific capacitance of 1547 F g−1 at a current density of 1 A g−1 and 1189 F g−1 at 10 A g−1, exhibiting high rate performance (76.9%) and high stability (82.3%). Also, Zardkhoshoui et al.135 synthesized nanosheet-assembled hollow α-MnS@Co3S4 spheres (NSH-MCS) with special morphology using ZIF-67 grown on manganese–glycerate (Mn–G) solid spheres as the precursor. The special structure can provide rich mass/electron transfer channels, and meanwhile prevent the accumulation of nanosheets. Taking advantage of these great merits, the NSH-MCS-based electrode exhibited appealing electrochemical features including an impressive capacity value of 283.3 mA h g−1 (1019.9 C g−1) at 1 A g−1 with the desirable rate performance of 81.5% at 25 A g−1 and significant longevity of 92.7% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 15 A g−1. Also, the hybrid supercapacitor assembled with NSH-MCS as the positive electrode and AC (activated carbon) as the negative electrode exhibited a desirable performance, such as a good energy density (54.9 W h kg−1 at 753 W kg−1) and excellent longevity of 90.5% after 10
000 cycles at 15 A g−1. Also, the hybrid supercapacitor assembled with NSH-MCS as the positive electrode and AC (activated carbon) as the negative electrode exhibited a desirable performance, such as a good energy density (54.9 W h kg−1 at 753 W kg−1) and excellent longevity of 90.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 15 A g−1. Additionally, as shown in Fig. 16j, Guan et al.123 prepared CoSx/Ni–Co LDH via the partial sulfuration of ZIF-67 and etching and precipitation of the added nickel ions. The prepared CoSx/Ni–Co LDH nanocages consisted of a hollow rhombic dodecahedral morphology with many nanosheet arrays on their shell. When used as the electrode material for electrochemical capacitors, the CoSx/Ni–Co LDH nanocages delivered a specific capacitance of 1562 F g−1 at a current density of 1 A g−1. In addition, the asymmetric supercapacitor assembled with CoSx/Ni–Co LDH as the cathode and activated carbon (AC) as the anode showed a high energy density of 35.8 W h kg−1 at a power density of 800 W kg−1 and an excellent cycling performance with the retention rate of 94.56% after 10
000 cycles at 15 A g−1. Additionally, as shown in Fig. 16j, Guan et al.123 prepared CoSx/Ni–Co LDH via the partial sulfuration of ZIF-67 and etching and precipitation of the added nickel ions. The prepared CoSx/Ni–Co LDH nanocages consisted of a hollow rhombic dodecahedral morphology with many nanosheet arrays on their shell. When used as the electrode material for electrochemical capacitors, the CoSx/Ni–Co LDH nanocages delivered a specific capacitance of 1562 F g−1 at a current density of 1 A g−1. In addition, the asymmetric supercapacitor assembled with CoSx/Ni–Co LDH as the cathode and activated carbon (AC) as the anode showed a high energy density of 35.8 W h kg−1 at a power density of 800 W kg−1 and an excellent cycling performance with the retention rate of 94.56% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, suggesting its potential application in high-performance electrochemical capacitors. These exceptional electrochemical properties can be attributed to the unique structure and synergistic effects between the metal sulfide and the bimetallic hydroxide. Similarly, Wang et al.159 directly grew ZIF-67-derived CoS with Ni(OH)2 nanosheets on carbon cloth (Ni(OH)2/CoS/CC) and the composites possessed a superior performance (561.6 mA h g−1 at 1 A g−1) to the Ni(OH)2/CC electrode (199.4 mA h g−1). The hybrid supercapacitor (HSC) with Ni(OH)2/CoS/CC as the cathode and activated carbon (AC) as the anode (Ni(OH)2/CoS/CC//AC) provided a remarkable energy density of 90.8 W h kg−1 at 800 W kg−1 and maintained 59.7 W h kg−1 even at 25
000 cycles, suggesting its potential application in high-performance electrochemical capacitors. These exceptional electrochemical properties can be attributed to the unique structure and synergistic effects between the metal sulfide and the bimetallic hydroxide. Similarly, Wang et al.159 directly grew ZIF-67-derived CoS with Ni(OH)2 nanosheets on carbon cloth (Ni(OH)2/CoS/CC) and the composites possessed a superior performance (561.6 mA h g−1 at 1 A g−1) to the Ni(OH)2/CC electrode (199.4 mA h g−1). The hybrid supercapacitor (HSC) with Ni(OH)2/CoS/CC as the cathode and activated carbon (AC) as the anode (Ni(OH)2/CoS/CC//AC) provided a remarkable energy density of 90.8 W h kg−1 at 800 W kg−1 and maintained 59.7 W h kg−1 even at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 W kg−1, which is higher than that of most of the reported Ni(OH)2-related devices, and possessed a marvelous capacity retention of 92.2% over 10
600 W kg−1, which is higher than that of most of the reported Ni(OH)2-related devices, and possessed a marvelous capacity retention of 92.2% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles. The outstanding electrochemical performance of Ni(OH)2/CoS/CC was chiefly due to the mediation of the Co2+/Co3+ redox cycle for the rapid conversion of Ni2+ into Ni3+, which greatly boosted the charge-transfer efficiency in the supercapacitor and methanol electro-oxidation. Moreover, Xue et al.152 used one-dimensional (1D) V2O5 nanowires as a flexible backbone to string ZIF-67-derived hollow Co3S4 three-dimensional (3D) nanopholyhedra to build robust core–shell 1D@3D V2O5@Co3S4 nanocomposites (Fig. 16k). As shown in Fig. 16l and m, the 1D V2O5 nanowires facilitated efficient electron transportation between the Co3S4 nanopolyhedra. The hollow/porous structure of Co3S4 not only afforded substantial electroactive sites, but also shortened the charge transport pathway. With these advantages, the optimal V2O5@Co3S4-5 h displayed enhanced electrochemical properties compared with the control V2O5 or Co3S4-5 h in a three-electrode system, as shown in Fig. 16n. Furthermore, the asymmetric supercapacitor made from V2O5@Co3S4-5 h exhibited a high energy density (40.7 W h kg−1 at 800 W kg−1) with outstanding cycle durability, maintaining 85.9% of its initial capacitance after 10
000 charge–discharge cycles. The outstanding electrochemical performance of Ni(OH)2/CoS/CC was chiefly due to the mediation of the Co2+/Co3+ redox cycle for the rapid conversion of Ni2+ into Ni3+, which greatly boosted the charge-transfer efficiency in the supercapacitor and methanol electro-oxidation. Moreover, Xue et al.152 used one-dimensional (1D) V2O5 nanowires as a flexible backbone to string ZIF-67-derived hollow Co3S4 three-dimensional (3D) nanopholyhedra to build robust core–shell 1D@3D V2O5@Co3S4 nanocomposites (Fig. 16k). As shown in Fig. 16l and m, the 1D V2O5 nanowires facilitated efficient electron transportation between the Co3S4 nanopolyhedra. The hollow/porous structure of Co3S4 not only afforded substantial electroactive sites, but also shortened the charge transport pathway. With these advantages, the optimal V2O5@Co3S4-5 h displayed enhanced electrochemical properties compared with the control V2O5 or Co3S4-5 h in a three-electrode system, as shown in Fig. 16n. Furthermore, the asymmetric supercapacitor made from V2O5@Co3S4-5 h exhibited a high energy density (40.7 W h kg−1 at 800 W kg−1) with outstanding cycle durability, maintaining 85.9% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
4. ZIF-67-derived transition metal selenides
Similar to sulfides, ZIF-67-derived transition metal selenides also have the advantages of improved redox-active sites, high specific capacity, better electronic conductivity and thermal and mechanical stability (as shown in Table 3). Additionally, as a type of nucleophile, Se not only has a faster reaction speed with active oxygen but also is easier to be restored than the S–O bond due to the existence of a π bond in the Se–O bond, which effectively avoids the occurrence of permanent oxidation and improves the cycling stability of selenides.34 Therefore, ZIF-67-derived transition metal selenides have attracted increasing attention in supercapacitor application.
Table 3 Summary of the electrochemical characteristics of ZIF-67-derived selenide materials in three-electrode measurements
| Electrode materials |
Specific capacity |
Electrolyte |
Potential window (CV) |
Capacity retention |
Cyclic stability |
Ref. |
| Co–Zn–Se@CNTs–CNFs |
1891 F g−1 at 1 A g−1 |
6 M KOH |
0–0.7 V (Hg/HgO) |
52.4% (1 to 30 A g−1) |
97.2% (5000) |
184
|
| H–Ni–Co–Se |
1175 F g−1 at 1 A g−1 |
6 M KOH |
−0.2–0.6 V (SCE) |
72.8% (1 to 10 A g−1) |
89.3% (2000) |
185
|
| NiSe2/CoSe2 |
1668 F g−1 at 1 A g−1 |
3 M KOH |
0–0.7 V (Hg/HgO) |
82.8% (1 to 20 A g−1) |
87.2% (5000) |
186
|
| P–(Ni, Co)Se2 |
755 C g−1 at 2 mA cm−2 |
3 M KOH |
0–0.6 V (Hg/HgO) |
86.4% (2 to 30 mA cm−2) |
80.1% (3000) |
187
|
| NixCo1−xSe2/CNFs/CoO@CC |
207.8 mA h g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
|
104.1% (5000) |
188
|
| CoSe2/GO |
108.31 mA h g−1 at 1 A g−1 |
2 M KOH |
−0.2–0.6 V (SCE) |
61.6% (1 to 10 A g−1) |
91.2% (5000) |
189
|
| CoSe2/C |
462 F g−1 at 5 A g−1 |
2 M KOH |
0–0.8 V (SCE) |
63.3% (1 to 10 A g−1) |
100% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
190
|
| NiCoSe2/C |
232.6 mA h g−1 at 1 A g−1 |
2 M KOH |
0–0.5 V (SCE) |
72.6% (1 to 10 A g−1) |
88.3% (5000) |
191
|
| CMS–DSHNCs |
1029.8 C g−1 at 2 A g−1 |
3 M KOH |
−0.3–0.7 V (Ag/AgCl) |
76.14% (2 to 50 A g−1) |
95.2% (8000) |
192
|
| CoNiSe2/Fe–CoNiSe2 |
1091.2 C g−1 at 1 A g−1 |
3 M KOH |
0–0.5 V (Ag/AgCl) |
55% (1 to 20 A g−1) |
85% (5000) |
193
|
| MXene@CoSe2/Ni3Se4 |
283 mA h g−1 at 1 A g−1 |
3 M KOH |
0–0.5 V (Hg/HgO) |
62% (1 to 10 A g−1) |
80% (5000) |
194
|
| H–NiCoSe2/NC |
1131 C g−1 at 1 A g−1 |
3 M KOH |
−0.1–0.8 V (Ag/AgCl) |
59.2% (1 to 10 A g−1) |
90.2% (6000) |
195
|
| CoSe2 |
269.4 mA h g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
83.7% (1 to 20 A g−1) |
83.4% (5000) |
196
|
| CoSe2/NC |
554.4 F g−1 at 1 A g−1 |
2 M KOH |
0–0.8 V (Hg/HgO) |
78% (1 to 10 A g−1) |
92% (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
197
|
| CoSe/NC |
746 F g−1 at 2 mV s−1 |
3 M KOH |
0–0.5 V (Ag/AgCl) |
9.5% (2 to 50 mV s−1) |
82.3% (4000) |
198
|
| M–(Ni, Co)–Se@CNFs/CC |
378.9 mA h g−1 at 1 A g−1 |
6 M KOH |
0–0.5 V (Hg/HgO) |
72.8% (1 to 10 A g−1) |
|
199
|
| CoSe2@NiMn-LDH@Cu1.8Se |
7064 mF cm−2 at 2 mA cm−2 |
6 M KOH |
0–0.5 V (Hg/HgO) |
|
80.11% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
200
|
| CFP/PNCNF(Se10)/PEDOT@300 |
378.1 F g−1 at 1 A g−1 |
1 M H2SO4 |
0–0.7 V (Ag/AgCl) |
19% (1 to 32 A g−1) |
|
33
|
| 60-MoSe2/(Ni,Co)Se2 |
359.9 mA h g−1 at 1 A g−1 |
6 M KOH |
0–0.6 V (Hg/HgO) |
82.3% (1 to 10 A g−1) |
83.7% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
201
|
| NiSe/Ni3Se2/CoSe/MoSe2 |
15.49 F cm−2 at 5 mA cm−2 |
6 M KOH |
−0.2–0.8 V |
55.1% (5 to 50 mA cm−2) |
96.5% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
202
|
4.1 Single metal selenides
Shaukat et al.198 synthesized CoSe/NC based on ZIF-67 as the precursor and varying amounts of selenium powder by high-temperature treatment. Compared with the other electrodes with different amounts of Se, CoSe-1 (ZIF-67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se 1
Se 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed an excellent electrochemical performance with the highest specific capacitance (746 F g−1 at 2 mV s−1), showing a cyclic capability of 82.3% after 4000 cycles because of the optimized ratio of cobalt and selenium in it. At the same time, Wang et al.197 obtained CoSe2/NC via a similar strategy at various temperatures. In a three-electrode system with 2 M KOH as the electrolyte, CoSe2/NC-1 h possessed a high capacity of 554.4 F g−1 at 1 A g−1 and excellent cycling stability (92% capacity retention after 21
1) showed an excellent electrochemical performance with the highest specific capacitance (746 F g−1 at 2 mV s−1), showing a cyclic capability of 82.3% after 4000 cycles because of the optimized ratio of cobalt and selenium in it. At the same time, Wang et al.197 obtained CoSe2/NC via a similar strategy at various temperatures. In a three-electrode system with 2 M KOH as the electrolyte, CoSe2/NC-1 h possessed a high capacity of 554.4 F g−1 at 1 A g−1 and excellent cycling stability (92% capacity retention after 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). In addition, a flexible solid-state supercapacitor was assembled with CoSe2/NC-1 h as the positive electrode and AC as the negative electrode. The power density was 800 W kg−1 at 1 A g−1, and the cycling stability was tested at 91.53% after 6000 cycles at 2 A g−1. Additionally, Wei's group190 grew CoSe2 on carbon cloth using leaf-like ZIF-67 as the precursor by a two-step calcination method (Fig. 17a–c). The novel CoSe2/carbon (CoSe2/C) electrode showed an excellent electrochemical performance (Fig. 17d and e), with a specific capacitance of 462 F g−1 at a current density of 5 A g−1, and 100% capacitance retention at 10 A g−1 after 10
000 cycles). In addition, a flexible solid-state supercapacitor was assembled with CoSe2/NC-1 h as the positive electrode and AC as the negative electrode. The power density was 800 W kg−1 at 1 A g−1, and the cycling stability was tested at 91.53% after 6000 cycles at 2 A g−1. Additionally, Wei's group190 grew CoSe2 on carbon cloth using leaf-like ZIF-67 as the precursor by a two-step calcination method (Fig. 17a–c). The novel CoSe2/carbon (CoSe2/C) electrode showed an excellent electrochemical performance (Fig. 17d and e), with a specific capacitance of 462 F g−1 at a current density of 5 A g−1, and 100% capacitance retention at 10 A g−1 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Meanwhile, an asymmetric supercapacitor was assembled, which exhibited an energy density of 20.6 W h kg−1 with a power density of 698.8 W kg−1 and outstanding cycling stability. Moreover, Fan et al.196 prepared porous CoSe2 nanosheets on nickel foam via the hydrothermal method (Fig. 17f and g). The unique nanosheet array structure could provide a highly active surface, large superficial area and fast ion transport channels. This was mainly attributed to the fact that the reaction at different hydrothermal temperatures can provide different nanosheet structures. In addition, the incorporated ZIF-67 backbone provided a pathway for rapid electron transfer and accommodated the volume expansion of the selenide during charge–discharge processes. As shown in Fig. 17h–k, due to its distinct porous structure, the CoSe2-180 electrode showed a high specific capacity of 269.4 mA h g−1 at 1 A g−1 and a distinguished retention rate of 83.7% at 20 A g−1. After 5000 cycles, the specific capacity was maintained at 83.4% of the initial value. Moreover, the asymmetric supercapacitor (ASC) device was assembled with CoSe2-180 as the positive electrode. It displayed a favorable electrochemical performance with the maximum specific energy of 45.6 W h kg−1 at a specific power of 800.8 W kg−1 and original capacitance retention rate of 81.5% after 5000 cycles.
000 cycles. Meanwhile, an asymmetric supercapacitor was assembled, which exhibited an energy density of 20.6 W h kg−1 with a power density of 698.8 W kg−1 and outstanding cycling stability. Moreover, Fan et al.196 prepared porous CoSe2 nanosheets on nickel foam via the hydrothermal method (Fig. 17f and g). The unique nanosheet array structure could provide a highly active surface, large superficial area and fast ion transport channels. This was mainly attributed to the fact that the reaction at different hydrothermal temperatures can provide different nanosheet structures. In addition, the incorporated ZIF-67 backbone provided a pathway for rapid electron transfer and accommodated the volume expansion of the selenide during charge–discharge processes. As shown in Fig. 17h–k, due to its distinct porous structure, the CoSe2-180 electrode showed a high specific capacity of 269.4 mA h g−1 at 1 A g−1 and a distinguished retention rate of 83.7% at 20 A g−1. After 5000 cycles, the specific capacity was maintained at 83.4% of the initial value. Moreover, the asymmetric supercapacitor (ASC) device was assembled with CoSe2-180 as the positive electrode. It displayed a favorable electrochemical performance with the maximum specific energy of 45.6 W h kg−1 at a specific power of 800.8 W kg−1 and original capacitance retention rate of 81.5% after 5000 cycles.
 |
| | Fig. 17 (a) Schematic of the CoSe2/C lamellar array; (b and c) SEM images of CoSe2/C; (d) CV curves at 50 mV s−1 and (e) GCD curves at 5 A g−1 of CoSe2 and CoSe2/C. Reprinted with permission from ref. 190, Copyright 2021, Elsevier B.V. (f) Schematic of the CoSe2 electrode preparation process; (g) high-resolution XPS patterns of Se 3d; (h) CV curves at 10 mV s−1, (i) specific capacitance, (j) Nyquist plots and (k) cycling performance of CoSe2-T electrode. Reprinted with permission from ref. 196, Copyright 2023, The Royal Society of Chemistry. | |
4.2 Multi-metal selenides
Also, Tan et al.185 successfully assembled nickel cobalt selenide (H–Ni–Co–Se) nanoarrays with a hollow structure from pentagon-like Ni/Co-ZIF on Ni foam by employing a sequential chemical etching and selenylation strategy. The hollow configuration grown on Ni-foam could offer a rich electroactive region, shorten the charge/ion diffusion length and enhance the mass/electrons transfer. Consequently, the as-prepared H–Ni–Co–Se delivered a favorable specific capacitance of 1175 F g−1 at 1 A g−1, fast charge/discharge rate and excellent long-term stability. Similarly, Wang et al.191 reported the preparation of a hollow NiCoSe2/C through a step-by-step derivatization method as a high-performance supercapacitor electrode. Hollow nickel–cobalt bimetallic layered hydroxide (NiCo-LDH) derived from ZIF-67 was selenized to NiCoSe2via in situ selenylation, which was then coated with a layer of carbon via the hydrothermal method using glucose as the carbon source. The electrochemical performances of the as-synthesized NiCoSe2/C were investigated, and the results indicated that the hollow NiCoSe2/C exhibited a considerable specific capacity of 232.6 mA h g−1 at 1 A g−1, good rate capability of 72.6% and high capacity retention of 88.3% after 5000 cycles. Compared with ZIF-67, NiCo-LDH and NiCoSe2, the greatly enhanced electrochemical performances of NiCoSe2/C can be attributed to the hollow nanostructure of NiCoSe2, enhanced electrical conductivity by the introduction of Se and C, and inhibited volume changes during the redox cycling from the protection of the carbon layer. Salehan et al.195 also synthesized a cobalt–nickel selenide nitrogen-doped carbon (H-CoNiSe2/NC) hollow polyhedral composite structure and the presence of the NC structure in the proposed composite could simultaneously lead to improved conductivity and reduce the volume effect created during the cycling procedure. The H–CoNiSe2/NC electrode provided a high specific capacity (1131 C g−1 at 1.0 A g−1) and outstanding cyclic stability (90.2% retention after 6000 cycles). In addition, the H–CoNiSe2/NC//AC hybrid supercapacitor delivered an ultrahigh energy density and power density (81.9 W h kg−1 at 900 W kg−1, respectively) and excellent cyclic stability (92.1% of the initial capacitance after 6000 cycles). Additionally, Zong et al.187 successfully designed and fabricated P–(Ni, Co)Se2 nanoarrays on activated carbon cloth with PBA nanocubes anchored on nanoflakes–nanowires via a facile hydrothermal method, followed precipitate reaction and phosphorization/selenylation treatment (Fig. 18a). The continuous hollow PBA nanocubes and appropriate incorporation of P in (Ni, Co)Se2 could greatly increase the active surface area and enhance the ion diffusion and charge transfer. The reaction is shown as the following equations:| | | (Ni, Co)Se2 + 2OH− ↔ NiSeOH + CoSeOH + e− | (3) |
| | | NiSeOH + OH− ↔ NiSeO + H2O + e− | (4) |
| | | CoSeOH + OH− ↔ CoSeO + H2O + e− | (5) |
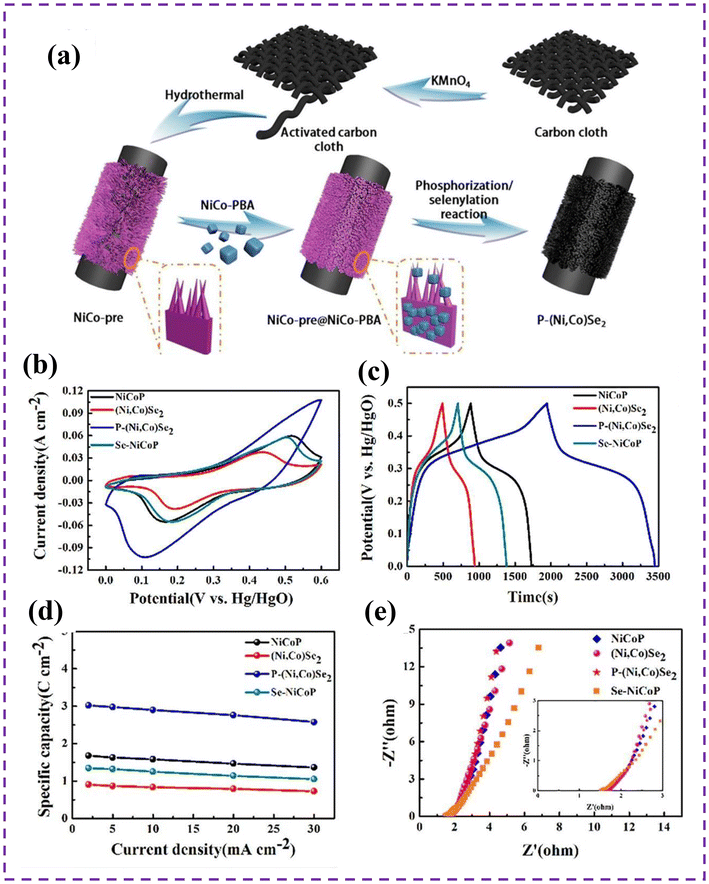 |
| | Fig. 18 (a) Schematic of the procedure for the fabrication of P–(Ni, Co)Se2 NAs on activated carbon cloth; (b) CV curves at 10 mV s−1, (c) GCD curves at 2 mA cm−2, (d) specific capacitance and (e) Nyquist plots of NiCoP, (Ni, Co)Se2, P–(Ni, Co)Se2 and Se–NiCoP. Reprinted with permission from ref. 187, Copyright 2019, Elsevier B.V. | |
The resultant P–(Ni, Co)Se2 NA electrode delivered a high areal capacity of 3.02 C cm−2 (a specific capacity of 755 C g−1) at 2 mA cm−2 and a good rate capability (2.61 C cm−2 at 30 mA cm−2), as shown in Fig. 18b–e. Furthermore, an all-solid-state hybrid supercapacitor device was also constructed using P–(Ni, Co)Se2 NAs and ZIF-8-derived carbon as the positive electrode and negative electrode, respectively, which showed a high energy density of 45.0 W h kg−1 at the power density of 446.3 W kg−1. Moreover, Andikaey et al.193 developed trimetallic CoNiSe2/Fe–CoNiSe2 yolk–shell nanoboxes (YSBs) using ZIF-67/NiCoFe Prussian blue analogue (PBA) precursors. Structural engineering of the PBA framework with Co–Fe oxide increased the topological complexity of the nanostructure. The energy storage activity was effectively improved because of the open structure and larger surface area. The presence of trimetallic selenides in CoNiSe2/Fe–CoNiSe2 YSBs improved the electronic structures, and thus the electrochemical performances, and enhanced conductivity of the nanostructure compared with its oxide counterparts. As a result, it exhibited an attractive electrochemical performance when used as an electrode for battery-type supercapacitors. CoNiSe2/Fe–CoNiSe2 yolk–shell nanoboxes showed a high specific capacity of 1091.2 C g−1 at 1 A g−1, good acceleration performance (55% at 20 A g−1), and excellent cycle stability (85% recovery after 5000 charge–discharge cycles). Furthermore, the CoNiSe2/Fe–CoNiSe2 YSB//AC hybrid SC demonstrated an energy density above 76.5 W h kg−1 at a power density of 2378.7 W kg−1.
4.3 Composite materials
Furthermore, Lv et al.184 reported the preparation of hierarchical “tube-on-fiber” nanostructures composed of carbon nanotubes (CNTs) on carbon nanofibers (CNFs) and impregnated with mixed-metal selenide nanoparticles (Co–Zn–Se@CNTs–CNFs) as high-performance supercapacitors. Co–Zn hybrid zeolitic imidazolate framework-67 (Co–Zn ZIF-67) was electrospun with polyacrylonitrile (PAN) to form nanofibers, which were sequentially thermally treated and subjected to selenylation. The “tube-on-fiber” structure was designed to confine the Co–Zn mixed-metal selenide nanoparticles and prevented their agglomeration. Extruded CNTs rooted in carbon nanofibers further improved the electronic conductivity. The mixed-metal selenide allowed more accommodation space and faradaic reactions compared to the single metal selenide. Based on these merits, the hierarchical Co–Zn–Se@CNTs–CNFs exhibited a high specific capacity of 1040.1 C g−1 (1891 F g−1) at 1 A g−1 with an impressive rate performance in supercapacitors. Furthermore, a hybrid supercapacitor was fabricated with Co–Zn–Se@CNTs–CNFs as the cathode and porous carbon nanofibers as the anode (denoted as Co–Zn–Se@CNTs–CNFs//PCNFs). It delivered a superior energy and power density of 61.4 W h kg−1 and 754.4 W kg−1, respectively, and meanwhile retained an energy density of 31.7 W h kg−1 with the working power of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421.6 W kg−1. In addition, the assembled supercapacitor device displayed an excellent capacity retention of 88.6% after 8000 cycles at 5 A g−1. Also, Wang et al.189 reported the facile synthesis of graphene oxide (GO)-supported CoSe2 (CoSe2/GO) derived from ZIF-67 via the hydrothermal method. CoSe2/GO exhibited a considerable specific capacity (108.31 mA h g−1 at 1 A g−1) and high cyclic stability (capacity retention of 91.2% after 5000 cycles). Compared with GO and CoSe2 alone, the greatly enhanced electrochemical performances of CoSe2/GO can be attributed to the synergistic effect of GO and CoSe2. Additionally, Yang et al.194 synthesized ZIF-67-derived CoSe2/Ni3Se4 nanosheets anchored vertically on an MXene substrate, which enhanced the overall conductivity. Furthermore, the close combination between the CoSe2/Ni3Se4 nanosheets and MXene nanosheets promoted a faster charge transfer rate and improved the durability of the structure (Fig. 19a). Consequently, as an electrode material for supercapacitors, the honeycomb-like MXene@CoSe2/Ni3Se4 achieved a high specific capacitance (283 mA h g−1 at 1 A g−1, as shown in Fig. 19b–e) and an outstanding capacitance retention rate with 80% after 5000 cycles. Moreover, Shi's group reported the synthesis of M–(Ni, Co)–Se@CNFs/CC199 and NixCo1−xSe2/CNFs/CoO@CC188 derived from ZIF-L for hybrid supercapacitors, respectively. M–(Ni, Co)–Se@CNFs/CC exhibited a specific capacitance of 378.9 mA h g−1 at 1 A g−1, while that of NixCo1−xSe2/CNFs/CoO@CC was 207.8 mA h g−1, and both displayed a superior performance and stability.
421.6 W kg−1. In addition, the assembled supercapacitor device displayed an excellent capacity retention of 88.6% after 8000 cycles at 5 A g−1. Also, Wang et al.189 reported the facile synthesis of graphene oxide (GO)-supported CoSe2 (CoSe2/GO) derived from ZIF-67 via the hydrothermal method. CoSe2/GO exhibited a considerable specific capacity (108.31 mA h g−1 at 1 A g−1) and high cyclic stability (capacity retention of 91.2% after 5000 cycles). Compared with GO and CoSe2 alone, the greatly enhanced electrochemical performances of CoSe2/GO can be attributed to the synergistic effect of GO and CoSe2. Additionally, Yang et al.194 synthesized ZIF-67-derived CoSe2/Ni3Se4 nanosheets anchored vertically on an MXene substrate, which enhanced the overall conductivity. Furthermore, the close combination between the CoSe2/Ni3Se4 nanosheets and MXene nanosheets promoted a faster charge transfer rate and improved the durability of the structure (Fig. 19a). Consequently, as an electrode material for supercapacitors, the honeycomb-like MXene@CoSe2/Ni3Se4 achieved a high specific capacitance (283 mA h g−1 at 1 A g−1, as shown in Fig. 19b–e) and an outstanding capacitance retention rate with 80% after 5000 cycles. Moreover, Shi's group reported the synthesis of M–(Ni, Co)–Se@CNFs/CC199 and NixCo1−xSe2/CNFs/CoO@CC188 derived from ZIF-L for hybrid supercapacitors, respectively. M–(Ni, Co)–Se@CNFs/CC exhibited a specific capacitance of 378.9 mA h g−1 at 1 A g−1, while that of NixCo1−xSe2/CNFs/CoO@CC was 207.8 mA h g−1, and both displayed a superior performance and stability.
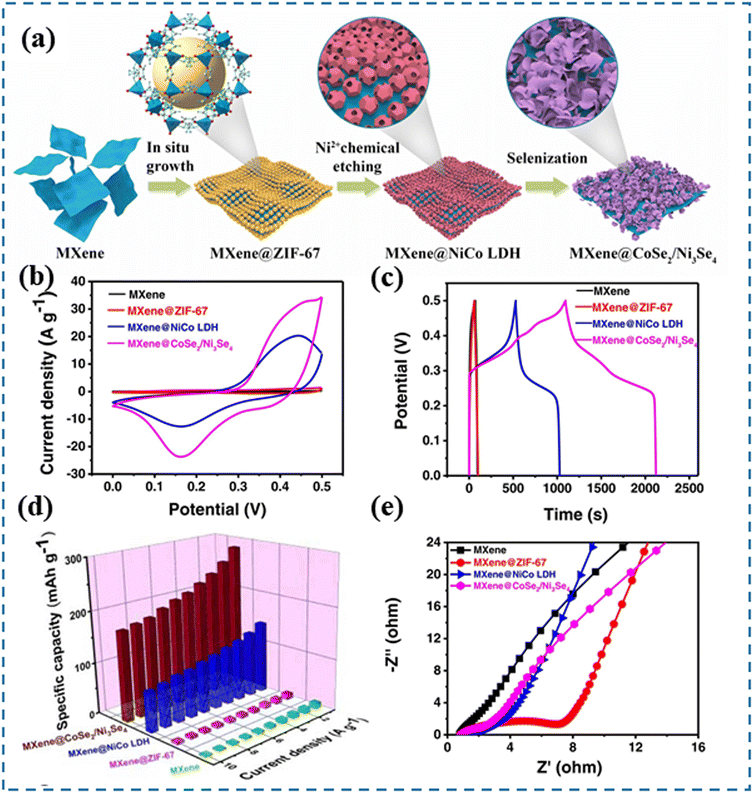 |
| | Fig. 19 (a) Schematic of the synthesis of the hierarchical 2D MXene@CoSe2/Ni3Se4 nanosheets and (b) CV curves at 5 mV s−1, (c) GCD curves at 1 A g−1, (d) specific capacitance and (e) Nyquist plots of MXene, MXene@ZIF-67, MXene@NiCo-LDH and MXene@CoSe2/Ni3Se4. Reprinted with permission from ref. 194, Copyright 2022, Elsevier B.V. | |
Meanwhile, Zhang et al.200 synthesized a wire-sheet-particle hierarchical hetero-structured CoSe2@NiMn-layered double hydroxide (NiMn-LDH)@Cu1.8Se/Copper foam (CF) electrode via a phase pseudomorphic transformation process achieved by the selective selenization of Cu and Co elements (Fig. 20a). Benefiting from the stable support structure of CuBr2, the large specific surface area of NiMn-LDH, and the excellent conductivity of CoSe2, the prepared binder-free electrode showed excellent electrochemical properties. The CoSe2@NiMn-LDH@Cu1.8Se hybrid electrode exhibited a superior specific areal capacitance of 7064 mF cm−2 at 2 mA cm−2 and a stable cyclic performance with 80.11% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 20b–e). Furthermore, the assembled CoSe2@NiMn-LDH@Cu1.8Se/CF//AC (activated carbon) asymmetric supercapacitor (ASC) achieved an energy density of 36.6 W h kg−1 at the power density of 760.6 W kg−1 and retained 87.35% of its initial capacitance after 5000 cycles. Also, Guo et al.201 synthesized MoSe2/(Ni, Co)Se2 hollow structures derived from PMo12@ZIF-67 precursor by an Ni2+ etching and selenization process (Fig. 20f). In the three-electrode test, as shown in Fig. 20g–j, the developed 60-MoSe2/(Ni, Co)Se2 electrode material exhibited an excellent specific capacity of 359.9 mA h g−1 at a current density of 1 A g−1, and a capacitance retention rate of 83.7% after 10
000 cycles (Fig. 20b–e). Furthermore, the assembled CoSe2@NiMn-LDH@Cu1.8Se/CF//AC (activated carbon) asymmetric supercapacitor (ASC) achieved an energy density of 36.6 W h kg−1 at the power density of 760.6 W kg−1 and retained 87.35% of its initial capacitance after 5000 cycles. Also, Guo et al.201 synthesized MoSe2/(Ni, Co)Se2 hollow structures derived from PMo12@ZIF-67 precursor by an Ni2+ etching and selenization process (Fig. 20f). In the three-electrode test, as shown in Fig. 20g–j, the developed 60-MoSe2/(Ni, Co)Se2 electrode material exhibited an excellent specific capacity of 359.9 mA h g−1 at a current density of 1 A g−1, and a capacitance retention rate of 83.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In addition, the asymmetric supercapacitor (ASC) assembled with 60-MoSe2/(Ni, Co)Se2 as the positive electrode and activated carbon as the negative electrode exhibited an excellent energy density of 40.3 W h kg−1 at a power density of 800.9 W kg−1.
000 cycles. In addition, the asymmetric supercapacitor (ASC) assembled with 60-MoSe2/(Ni, Co)Se2 as the positive electrode and activated carbon as the negative electrode exhibited an excellent energy density of 40.3 W h kg−1 at a power density of 800.9 W kg−1.
 |
| | Fig. 20 (a) Schematic depiction of the stepwise formation process of CoSe2@NiMn-LDH@Cu1.8Se/CF nanosheet arrays derived from ZIF-67-covered CuBr2 nanorod arrays via selective phase pseudomorphic transformation; (b) schematic of capacitance process of CoSe2@NiMn-LDH@Cu1.8Se/CF; and (c) CV curves, (d) GCD curves and (e) specific capacitance of CoSe2@NiMn-LDH@Cu1.8Se/CF, ZIF-67@NiMn-LDH@CuBr2/CF, NiMn-LDH@CuBr2/CF, CuBr2/CF, Cu(OH)2/CF and CuO/CF. Reprinted with permission from ref. 200, Copyright 2023, Elsevier Inc. (f) Schematic illustration of procedure for the preparation of MoSe2/(Ni, Co)Se2 hollow nanomaterials; and (g) CV curves at 15 mV s−1, (h) GCD curves at 1 A g−1, (i) specific capacitance and (j) Nyquist plots of (Ni, Co)Se2, 25-MoSe2/(Ni, Co)Se2, 60-MoSe2/(Ni, Co)Se2 and 100-MoSe2/(Ni, Co)Se2. Reprinted with permission from ref. 201, Copyright 2024, Elsevier B.V. | |
5. ZIF-67-derived transition metal tellurides
Similar to sulfides and selenides, tellurides are a newly explored class of chalcogenides in supercapacitors, which exhibit improved metallic properties, larger lattice parameters and higher degree of covalency in anion–metal bonding than sulfides and selenides.203 Therefore, ZIF-67-derived transition metal tellurides are also new materials worth exploring.
Kshetri et al.204 reported the fabrication of a ZIF-67-derived CoTe–carbon porous-structured composite on nickel foam (CoTe@C–NiF) by high-temperature treatment (Fig. 21a). As shown in Fig. 21b–e, in the negative potential regions, the CoTe@C–NiF hybrid electrode exhibited a maximum areal capacitance of 307.5 mF cm−2 at a current density of 1 mA cm−2 and retained 162.0 mF cm−2 at a high current density of 20 mA cm−2, resulting in a rate capability of 52.03%. However, in the positive potential region, the same electrode showed areal capacitances of 1038 mF cm−2 and 920 mF cm−2, respectively, at the same current densities, resulting in a rate capability of 88.63%. In addition, CoTe@C–NiF showed long-term electrochemical stability during the charge–discharge process in both the negative and positive potential window. In the negative potential window, it showed 86.78% and 98.57% retention of areal capacitance and coulombic efficiency, respectively. Similarly, in the positive window, 87.50% and 98.97% retention of areal capacitance and coulombic efficiency was observed after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles, respectively.
000 charge–discharge cycles, respectively.
 |
| | Fig. 21 (a) Schematic for the synthesis of CoTe@C–NiF hybrid electrode; and (b and c) specific capacitance and (d and e) cycling stability of CoTe@C–NiF in the negative and positive potential window, respectively. Reprinted with permission from ref. 204, Copyright 2021, Elsevier Ltd. | |
6. Summary and perspectives
6.1 Summary
Supercapacitors (SCs) are generally regarded as promising electrochemical devices in the field of energy storage due to their advantages of fast charge–discharge rate and long cyclic term. However, the low energy density of SCs limits their further application. In this case, electrode materials, as one of the components of SCs, play an important role in the electrochemical performance of energy storage devices. Thus, to improve the energy density of SC devices, it is essential to look for or synthesize new electrode materials. Recently, ZIF-67 has attracted considerable attention as an electrode material for SC application because of its controllable pore rate, constant cavity and large specific area. Also, pristine ZIF-67 and ZIF-67 derivatives such as porous carbon, metal-doped carbon, hydroxides (Table S3†), phosphides (Table S4†) and chalcogenides (including oxides, sulfides, selenides and tellurides) have shown exemplary performances in supercapacitors but still have some challenges that need to be overcome. Therefore, in this review, we discussed ZIF-67-derived chalcogenides as electrode materials for SCs including their preparation strategies, micro structure and electrochemical properties. Generally, as a typical pseudocapacitor materials, ZIF-67-derived chalcogenides have a larger specific capacitance and higher energy density than pristine ZIF-67 and ZIF-67-derived carbon materials. However, that still have some disadvantages such as the slightly lower capacity for oxides with high structural stability and volume expansion and structural destruction during long-term cycling for sulfides, selenides and tellurides. Therefore, researchers have developed many strategies to solve these questions. For instance, from single metal compounds to multi-metal compounds, the specific capacitance of chalcogenides can be improved greatly due to the changes in their chemical properties. Also, doping metal ions can provide more active sites for electrochemical reactions. Additionally, combining chalcogenides with other materials such as carbon materials (carbon nanotubes and graphene), conductive polymers (PPy, PANI, and PEDOT), 2D MXenes and other transition metal compounds is also an effective way to improve their conductivity and structural stability. Many of the studies mentioned herein have proven the feasibility of these methods.
6.2 Perspectives
ZIF-67 is desirable for a wide range of applications owing to its tailorable surface area, porosity, precise pore size and composition control as key considerations. However, the weak inherent conductivity and structural instability of pristine ZIF-67 restricts its usage in electrochemical applications. ZIF-67-derived chalcogenides have emerged as viable materials for supercapacitor applications due to the strong control of their morphology, functionalization, doping of heteroatoms, enhanced surface area and structural modification. However, there is still many aspects of ZIF-67-derived chalcogenides for SC application worth researching.
• ZIF-67-derived tellurides exhibit excellent electrochemical properties because of their improved metallic properties, large lattice parameters and high degree of covalency in anion–metal bonding. However, there are still few studies about ZIF-67-derived transition metal tellurides. Therefore, more work on tellurides needs further research in the future.
• There are still certain shortcomings in the current common preparation methods, as follows: (1) the coprecipitation method is the simultaneous precipitation of soluble and micro-components from the same solution by adsorption, encapsulation or mechanical trapping to form mixed crystals. However, ZIF-67 prepared by this method has poor uniformity unless premium solvents and surfactant are used. (2) The hydrothermal/solvothermal method is a synthesis process in which chemical regents and solvents are heated inside a high-pressure steel vessel, but has lower yields. (3) Annealing/carbonization is a high-temperature procedure that requires a strict atmosphere and sometimes destroys the structure of ZIF-67. Therefore, it is imperative to improve existing methods and adopt new methods. Surfactants are essential for the coprecipitation method; optimization of the parameters for the hydrothermal/solvothermal and annealing/carbonization methods, such as time, temperature and atmosphere, should be evaluated; and microwave/ultrasonic wave-assisted techniques have become increasingly popular because they cause atoms and molecules to oscillate, effectively initiating chemical processes. Moreover, the electrochemical method is also a good synthesis process due to its quicker synthesis conditions and greater control. Hence, there is a need to evaluate the synthesis of ZIF-67 and its derivatives.
• ZIF-67-derived chalcogenides have a complex composition, including local crystal/amorphous inorganic compounds and organic frameworks/carbon, which increases the difficulty of characterizing the microstructure of these materials. Thus, to further analyze the evolution of substances and structures in material synthesis and electrochemical reactions, organic/inorganic characterization methods should be combined for comprehensive analysis.
• Similarly, due to the complex composition of ZIF-67-derived chalcogenides, they are different from pure chalcogenides in the mechanism of energy storage/conversion systems. Therefore, in situ measurements, such as Raman spectroscopy, X-ray diffraction, X-ray absorption spectroscopy, ambient-pressure XPS and electrochemical mass spectrometry, may be useful. Meanwhile, in the process of theoretical calculation, compared to pure chalcogenides, the construction of the model must also consider the existence of frameworks, which may increase the complexity of the work.
• Flexible and wearable micro-supercapacitors and micro-batteries based on ZIF-67-derived chalcogenides with controlled properties together with safe, reliable and intelligent electronic properties also need to be further investigated.
Overall, we believe that the further design and synthesis of ZIF-67-derived chalcogenides need to be studied for meeting the requirements of supercapacitor application.
Author contributions
Lidong Jiao: investigation, formal analysis, writing-original draft, and visualization. Mingshu Zhao: conceptualization, methodology, supervision, funding acquisition, and writing-review & editing. Qingyang Zheng: investigation. Qingyi Ren: investigation and validation. Zhou Su: validation. Min Li: investigation. Feng Li: formal analysis.
Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors acknowledge Hunan Province Natural Science Fund-Regional Joint Fund (2024JJ7029) and Horizontal Project of Chinese Academy of Science Institute of Metals-Xi'an Jiaotong University (20241068). The authors also thank Mr Jun Li at Changde Collaborative Innovation Research Institute Co., Ltd for the collaborative support.
References
- T. M. I. Mahlia, T. J. Saktisandan, A. Jannifar, M. H. Hasan and H. S. C. Matseelar, Renewable Sustainable Energy Rev., 2014, 33, 532–545 CrossRef.
- Lichchhavi, A. Kanwade and P. M. Shirage, J. Energy Storage, 2022, 55, 105692 CrossRef.
- S. Y. Liu, J. Yang, P. Chen, M. Wang, S. J. He, L. Wang and J. S. Qiu, Electrochem. Energy Rev., 2024, 7, 25 CrossRef CAS.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 RSC.
- N. Choudhary, C. Li, J. Moore, N. Nagaiah, L. Zhai, Y. Jung and J. Thomas, Adv. Mater., 2017, 29, 1605336 CrossRef PubMed.
- Z. Wu, L. Li, J.-M. Yan and X.-B. Zhang, Adv. Sci., 2017, 4, 1600382 CrossRef PubMed.
- Poonam, K. Sharma, A. Arora and S. K. Tripathi, J. Energy Storage, 2019, 21, 801–825 CrossRef.
- P. Forouzandeh, V. Kumaravel and S. C. Pillai, Catalysts, 2020, 10, 969 CrossRef CAS.
- Reenu, Sonia, L. Phor, A. Kumar and S. Chahal, J. Energy Storage, 2024, 84, 110698 CrossRef.
- A. Afir, S. M. H. Rahman, A. T. Azad, J. Zaini, M. A. Islan and A. K. Azad, J. Energy Storage, 2019, 25, 100852 CrossRef.
- P. Lamba, P. Singh, P. Singh, P. Singh, Bharti, A. Kumar, M. Gupta and Y. Kumar, J. Energy Storage, 2022, 48, 103871 CrossRef.
- W. J. Fan, F. X. Wang, X. S. Xiong, B. Y. Song, T. Wang, X. B. Cheng, Z. Zhu, J. R. He, Y. K. Liu and Y. P. Wu, NPG Asia Mater., 2024, 16, 18 CrossRef CAS.
- K. Chhetri, A. Adhikari, J. Kunwar, D. Acharya, R. M. Bhattarai, Y. S. Mok, A. Adhikari, A. P. Yadav and H. Y. Kim, Int. J. Energy Res., 2023, 2023, 8885207 CrossRef.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Adv. Sci., 2017, 4, 1600539 CrossRef PubMed.
- Y. Jiang and J. Liu, Energy Environ. Mater., 2019, 2, 30–37 CrossRef.
- A. Muzaffar, M. B. Ahamed, K. Deshmukh and J. Thirumalai, Renewable Sustainable Energy Rev., 2019, 101, 123–145 CrossRef CAS.
- Q. Wu, T. He, Y. Zhang, J. Zhang, Z. Wang, Y. Liu, L. Zhao, Y. Wu and F. Ran, J. Mater. Chem. A, 2021, 9, 24094–24147 RSC.
- M. Z. Iqbal and U. Aziz, J. Energy Storage, 2022, 46, 103823 CrossRef.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- J. Yan, Q. Wang, T. Wei and Z. Fan, Adv. Energy Mater., 2014, 4, 1300816 CrossRef.
- Y. Zeng, M. Yu, Y. Meng, P. Fang, X. Lu and Y. Tong, Adv. Energy Mater., 2016, 6, 1601053 CrossRef.
- W. Raza, F. Ali, N. Raza, Y. Luo, K.-H. Kim, J. Yang, S. Kumar, A. Mehmood and E. E. Kwon, Nano Energy, 2018, 52, 441–473 CrossRef CAS.
- D. P. Chatterjee and A. K. Nandi, J. Mater. Chem. A, 2021, 9, 15880–15918 RSC.
- S. Zheng, X. Li, B. Yan, Q. Hu, Y. Xu, X. Xiao, H. Xue and H. Pang, Adv. Energy Mater., 2017, 7, 1602733 CrossRef.
- Y. Li, Y. Xu, W. Yang, W. Shen, H. Xue and H. Pang, Small, 2018, 14, 1704435 CrossRef PubMed.
- B. Xu, H. Zhang, H. Mei and D. Sun, Coord. Chem. Rev., 2020, 420, 213438 CrossRef CAS.
- K.-B. Wang, Q. Xun and Q. Zhang, EnergyChem, 2020, 2, 100025 CrossRef.
- L. Xu, Y. K. Xi, W. B. Li, Z. L. Hua, J. H. Peng, J. H. Hu, J. J. Zhou, P. L. Zhang, J. J. Wang, W. W. Wang, H. L. Ding, W. Q. Wang, W. X. Ji, Y. Yang, X. C. Xu, L. Y. Chen and X. F. Li, Nano Energy, 2022, 91, 106630 CrossRef CAS.
- R. R. Salunkhe, Y. V. Kaneti, J. Kim, J. H. Kim and Y. Yamauchi, Acc. Chem. Res., 2016, 49, 2796–2806 CrossRef CAS PubMed.
- J. A. Feng, J. Y. Li, H. W. Zhang, W. D. Liu, Z. H. Lin, T. Y. Wang, B. Sun, X. X. Zhao, F. Y. Wang and J. J. Song, Energy Mater., 2023, 3, 300001 CrossRef CAS.
- R. R. Salunkhe, Y. V. Kaneti and Y. Yamauchi, ACS Nano, 2017, 11, 5293–5308 CrossRef CAS PubMed.
- Q. Wang, F. Gao, B. Xu, F. Cai, F. Zhan, F. Gao and Q. Wang, Chem. Eng. J., 2017, 327, 387–396 CrossRef CAS.
- S. Karingula, S. Kummari, Y. G. Kotagiri, T. Bhookya and K. V. Gobi, Small, 2024, 20, 2400812 CrossRef CAS PubMed.
- C. Lamiel, I. Hussain, H. Rabiee, O. R. Ogunsakin and K. Zhang, Coord. Chem. Rev., 2023, 480, 215030 CrossRef CAS.
- Y. Peng, J. Xu, J. Xu, J. Ma, Y. Bai, S. Cao, S. Zhang and H. Pang, Adv. Colloid Interface Sci., 2022, 307, 102732 CrossRef CAS PubMed.
- A. Zhang, Q. Zhang, H. Fu, H. Zong and H. Guo, Small, 2023, 19, 2303911 CrossRef CAS PubMed.
- Q. Cheng, C. Yang, K. Tao and L. Han, Electrochim. Acta, 2020, 341, 136042 CrossRef CAS.
- K. Qu, Z. Sun, C. Shi, W. Wang, L. Xiao, J. Tian, Z. Huang and Z. Guo, Adv. Compos. Hybrid Mater., 2021, 4, 670–683 CrossRef CAS.
- J. X. Zhao, Y. Zhang, H. Y. Lu, Y. F. Wang, X. D. Liu, H. M. K. Sari, J. H. Peng, S. F. Chen, X. F. Li, Y. J. Zhang, X. L. Sun and B. G. Xu, Nano Lett., 2022, 22, 1198–1206 CrossRef CAS PubMed.
- L. Jiao, M. Zhao, Z. Su, M. Shi, M. Li and F. Li, Electrochim. Acta, 2024, 479, 143888 CrossRef CAS.
- J. Qin, Z. Yang, F. Xing, L. Zhang, H. Zhang and Z. Wu, Electrochem. Energy Rev., 2023, 6, 9 CrossRef CAS.
- A. M. Mohamed, M. Ramadan and N. K. Allam, J. Energy Storage, 2021, 34, 102195 CrossRef.
- D. G. Wang, Z. B. Liang, S. Gao, C. Qu and R. G. Zou, Coord. Chem. Rev., 2020, 404, 213093 CrossRef CAS.
- K. B. Wang, Q. Xun and Q. C. Zhang, EnergyChem, 2020, 2, 100025 CrossRef.
- A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed.
- Y. Xie, Z. Yang, M. Cao, Y. Feng and J. Yao, Electrochim. Acta, 2024, 488, 144222 CrossRef CAS.
- N. L. Torad, R. R. Salunkhe, Y. Li, H. Hamoudi, M. Imura, Y. Sakka, C.-C. Hu and Y. Yamauchi, Chem. – Eur. J., 2014, 20, 7895–7900 CrossRef CAS PubMed.
- S. A. Delbari, L. S. Ghadimi, R. Hadi, S. Farhoudian, M. Nedaei, A. Babapoor, A. S. Namini, L. Quyet Van, M. Shokouhimehr, M. S. Asl and M. Mohammadi, J. Alloys Compd., 2021, 857, 158281 CrossRef CAS.
- R. R. Salunkhe, J. Tang, Y. Kamachi, T. Nakato, J. H. Kim and Y. Yamauchi, ACS Nano, 2015, 9, 6288–6296 CrossRef CAS PubMed.
- Y.-Z. Zhang, Y. Wang, Y.-L. Xie, T. Cheng, W.-Y. Lai, H. Pang and W. Huang, Nanoscale, 2014, 6, 14354–14359 RSC.
- D. Yu, L. Ge, B. Wu, L. Wu, H. Wang and T. Xu, J. Mater. Chem. A, 2015, 3, 16688–16694 RSC.
- X. Deng, J. Li, S. Zhu, F. He, C. He, E. Liu, C. Shi, Q. Li and N. Zhao, J. Alloys Compd., 2017, 693, 16–24 CrossRef CAS.
- B. Wang, W. Tan, R. Fu, H. Mao, Y. Kong, Y. Qin and Y. Tao, Synth. Met., 2017, 233, 101–110 CrossRef CAS.
- C. Wei, K. Liu, J. Tao, X. Kang, H. Hou, C. Cheng and D. Zhang, Chem. – Asian J., 2018, 13, 111–117 CrossRef CAS PubMed.
- G. Wei, Z. Zhou, X. Zhao, W. Zhang and C. An, ACS Appl. Mater. Interfaces, 2018, 10, 23721–23730 CrossRef CAS PubMed.
- B. Xue, K. Li, S. Gu and J. Lu, J. Colloid Interface Sci., 2018, 530, 233–242 CrossRef CAS PubMed.
- J.-J. Zhou, X. Han, K. Tao, Q. Li, Y.-L. Li, C. Chen and L. Han, Chem. Eng. J., 2018, 354, 875–884 CrossRef CAS.
- S. Guo, X. Xu, J. Liu, Q. Zhang and H. Wang, J. Electrochem. Soc., 2019, 166, A960–A967 CrossRef CAS.
- M. Zhu, Q. Chen, J. Kan, J. Tang, W. Wei, J. Lin and S. Li, Energy Technol., 2019, 7, 1800963 CrossRef.
- G. Lin, Y. Jiang, C. He, Z. Huang, X. Zhang and Y. Yang, Dalton Trans., 2019, 48, 5773–5778 RSC.
- G. Lee and J. Jang, J. Power Sources, 2019, 423, 115–124 CrossRef CAS.
- X. Han, S. Liu, L. Shi, S. Li, Y. Li, Y. Wang, W. Chen, X. Zhao and Y. Zhao, Ceram. Int., 2019, 45, 14634–14641 CrossRef CAS.
- F. Wei, W. Liu, X. Zhang, X. Liu, Y. Sui and J. Qi, Mater. Lett., 2019, 255, 126534 CrossRef CAS.
- F. Saleki, A. Mohammadi, S. E. Moosavifard, A. Hafizi and M. R. Rahimpour, J. Colloid Interface Sci., 2019, 556, 83–91 CrossRef CAS PubMed.
- X. Dai, Y. Dai, J. Lu, L. Pu, W. Wang, J. Jin, F. Ma and N. Tie, Ionics, 2019, 26, 2501–2511 CrossRef.
- Y. Ding, Y. Peng, S. Chen, Z. Li, X. Zhang, P. Falaras and L. Hu, Appl. Surf. Sci., 2019, 495, 163502 CrossRef.
- J. Xu, C. Xu, Y. Zhao, J. Wu and J. Hu, Front. Chem., 2019, 7, 831 CrossRef CAS PubMed.
- M. Wang, J. Zhang, X. Yi, B. Liu, X. Zhao and X. Liu, Beilstein J. Nanotechnol., 2020, 11, 240–251 CrossRef CAS PubMed.
- D. Chu, D. Guo, B. Xiao, L. Tan, H. Ma, H. Pang, X. Wang and Y. Jiang, Chem. – Asian J., 2020, 15, 1750–1755 CrossRef CAS PubMed.
- K. Le, M. Gao, D. Xu, Z. Wang, G. Wang, G. Lu, W. Liu, F. Wang and J. Liu, Inorg. Chem. Front., 2020, 7, 3646–3656 RSC.
- X. Zha, Z. Wu, Z. Cheng, W. Yang, J. Li, Y. Chen, L. He, E. Zhou and Y. Yang, Energy, 2021, 220, 119696 CrossRef CAS.
- L. Cheng, Q. Zhang, M. Xu, Q. Zhai and C. Zhang, J. Colloid Interface Sci., 2021, 583, 299–309 CrossRef CAS PubMed.
- A. Rashti, X. Lu, A. Dobson, E. Hassani, F. Feyzbar-Khalkhali-Nejad, K. He and T.-S. Oh, ACS Appl. Energy Mater., 2021, 4, 1537–1547 CrossRef CAS.
- W. Hong, Y. Li, Y. Wu, G. Li and L. Jia, Mater. Chem. Front., 2021, 5, 1438–1447 RSC.
- J. Mao, M. Ge, I. W. P. Chen, Y. H. Ng, T. Zhu, H. Liu, J. Huang, W. Cai and Y. Lai, Mater. Chem. Front., 2021, 5, 2742–2748 RSC.
- L. Xiao, H. Qi, K. Qu, C. Shi, Y. Cheng, Z. Sun, B. Yuan, Z. Huang, D. Pan and Z. Guo, Adv. Compos. Hybrid Mater., 2021, 4, 306–316 CrossRef CAS.
- T. Shu, H. Wang, Q. Li, Z. Feng, F. Wei, K. X. Yao, Z. Sun, J. Qi and Y. Sui, Chem. Eng. J., 2021, 419, 129631 CrossRef CAS.
- L. Zhu, C. Hao, S. Zhou, X. Wang, T. Zhou and Y. Guo, J. Materiomics, 2021, 7, 708–720 CrossRef.
- L. Wang, X. Li, S. Xiong, H. Lin, Y. Xu, Y. Jiao and J. Chen, J. Colloid Interface Sci., 2021, 600, 58–71 CrossRef CAS PubMed.
- R. K. Devi, G. Muthusankar, S.-M. Chen and G. Gopalakrishnan, Microchim. Acta, 2021, 188, 196 CrossRef CAS PubMed.
- Y. Li, H. Xie, J. Li, Y. Yamauchi and J. Henzie, ACS Appl. Mater. Interfaces, 2021, 13, 41649–41656 CrossRef CAS PubMed.
- Y.-F. Ren, Z.-L. He, H.-Z. Zhao and T. Zhu, Rare Met., 2022, 41, 830–835 CrossRef CAS.
- H. Zhang, B. Yan, C. Zhou, J. Wang, H. Duan, D. Zhang and H. Zhao, Energy Fuels, 2021, 35, 16925–16932 CrossRef CAS.
- S. A. Al kiey and H. N. Abdelhamid, J. Energy Storage, 2022, 55, 105449 CrossRef.
- L. Xie, M. Li, P. Zhu, X. Xiao, Z. Jia, Z. Wei and J. Jiang, J. Alloys Compd., 2022, 898, 162861 CrossRef CAS.
- A. Mateen, M. S. Javed, S. Khan, A. Saleem, M. K. Majeed, A. J. Khan, M. F. Tahir, M. A. Ahmad, M. A. Assiri and K.-Q. Peng, J. Energy Storage, 2022, 49, 104150 CrossRef.
- C. Li, D. Ma and Q. Zhu, Nanomaterials, 2022, 12, 848 CrossRef CAS PubMed.
- Z. Duan, X.-R. Shi, C. Sun, W. Lin, S. Huang, X. Zhang, M. Huang, Z. Yang and S. Xu, Electrochim. Acta, 2022, 412, 140139 CrossRef CAS.
- A. G. El-Deen, M. K. Abdel-Sattar and N. K. Allam, Appl. Surf. Sci., 2022, 587, 152548 CrossRef CAS.
- H. Gong, S. Bie, J. Zhang, X. Ke, X. Wang, J. Liang, N. Wu, Q. Zhang, C. Luo and Y. Jia, Nanomaterials, 2022, 12, 1571 CrossRef CAS PubMed.
- K. Venkatesh, C. Karuppiah, R. Palani, G. Periyasamy, S. K. Ramaraj and C.-C. Yang, Mater. Lett., 2022, 323, 132609 CrossRef CAS.
- L. Chen, J. Wan, X. Feng, H. Shi and P. Liu, J. Alloys Compd., 2022, 918, 165769 CrossRef CAS.
- S. K. Babu, B. Gunasekaran, M. Sridharan and T. Vijayakumar, RSC Adv., 2022, 12, 28818–28830 RSC.
- Y. He, F. Hu, D. Liu, X. He, Q. Li, Y. Sui, J. Qi and Y. Wang, J. Energy Storage, 2023, 58, 106377 CrossRef.
- H. Wang, W. Li, D. Liu, G. Liu, X. An, J. Liu, C. Zhou, H. Zhang and G. Wang, J. Electroanal. Chem., 2023, 930, 117152 CrossRef CAS.
- H. Su, Q. Wang, J. Dai and W. Li, J. Mater. Sci.: Mater. Electron., 2023, 34, 403 CrossRef CAS.
- L. Xiao, J. Zhou, Z. Yu, H. Dong and Y. Chen, J. Mater. Sci.: Mater. Electron., 2023, 34, 474 CrossRef CAS.
- C. Yang, W. Li, X. Liu, X. Song, H. Li and L. Tan, Molecules, 2023, 28, 3177 CrossRef CAS PubMed.
- J. Wang, Y. Huang, X. Du, S. Zhang and M. Zong, Chem. Eng. J., 2023, 464, 142741 CrossRef CAS.
- M. He, G. J. H. Melvin, M. Wang, W. Fan, J. Lin, X. Chen, K. Xu, C. Yuan, Y. Zhang, F. Zhang and Z. Wang, J. Energy Storage, 2023, 70, 108055 CrossRef.
- J. Liu, Y. N. Meng, D. Yu, C. Guo, L. Liu and X. Liu, ChemistrySelect, 2023, 8, e202301597 CrossRef CAS.
- H. Xu, Q. Hu, T. Zhao, J. Zhu, Z. Lian and X. Jin, Carbohydr. Polym., 2024, 326, 121641 CrossRef CAS PubMed.
- D. Liu, J. Wang, M. Wang, R. Sultana, L. Cui, X. Zhang and Y. Han, J. Electroanal. Chem., 2024, 961, 118236 CrossRef CAS.
- B. Joshi, S. Kim, E. Samuel, J. Huh, M. S. Almoiqli, K. N. Alharbi and S. S. Yoon, J. Mater. Sci. Technol., 2024, 200, 83–92 CrossRef CAS.
- S. Prabu, M. Vinu, K.-Y. Chiang and M. R. Pallavolu, J. Colloid Interface Sci., 2024, 669, 624–636 CrossRef CAS PubMed.
- L. Zheng, S. Gao, S. Yao, Y. Huang, S. Zhai, J. Hao, X. Fu, Q. An and Z. Xiao, J. Colloid Interface Sci., 2024, 674, 735–744 CrossRef CAS PubMed.
- Z. Li, J. Zhou, L. Xiao, Y. Wang, S. Zhou and Y. Chen, J. Alloys Compd., 2024, 1003, 175553 CrossRef CAS.
- F. Semerci and H. Esgin, J. Electroanal. Chem., 2024, 970, 118550 CrossRef CAS.
- L. Zhu, H.-Y. Xia, C.-Y. Zhang, Y. Geng, Z. Li, L. Shu, J.-R. Bai and Y.-P. Wen, Int. J. Electrochem. Sci., 2024, 19, 100752 CrossRef CAS.
- S. Rao, G. Ali, S. Khalid, R. Naz, F. J. Iftikhar and S. Bashir, Inorg. Chem. Commun., 2024, 170, 113211 CrossRef CAS.
- S. Y. Cheng, T. H. Wang, Z. Zhang, L. Li, Y. Qing and Y. Q. Wu, Chem. Eng.
Sci., 2025, 306, 121234 CrossRef CAS.
- T.-Y. Chen, L.-Y. Lin, D.-S. Geng and P.-Y. Lee, Electrochim. Acta, 2021, 376, 137986 CrossRef CAS.
- X.-Y. Yu, L. Yu and X. W. Lou, Adv. Energy Mater., 2016, 6, 1501333 CrossRef.
- H. Hu, B. Y. Guan and X. W. Lou, Chem, 2016, 1, 102–113 CAS.
- X. Hou, Y. Zhang, Q. Dong, Y. Hong, Y. Liu, W. Wang, J. Shao, W. Si and X. Dong, ACS Appl. Energy Mater., 2018, 1, 3513–3520 CrossRef CAS.
- D. Guo, X. Song, L. Tan, H. Ma, H. Pang, X. Wang and L. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 42621–42629 CrossRef CAS PubMed.
- S.-L. Jian, L.-Y. Hsiao, M.-H. Yeh and K.-C. Ho, J. Mater. Chem. A, 2019, 7, 1479–1490 RSC.
- H. Niu, Y. Zhang, Y. Liu, B. Luo, N. Xin and W. Shi, J. Mater. Chem. A, 2019, 7, 8503–8509 RSC.
- X. Wang, F. Huang, F. Rong, P. He, R. Que and S. P. Jiang, J. Mater. Chem. A, 2019, 7, 12018–12028 RSC.
- G. Liu, H. Zhang, J. Li, Y. Liu and M. Wang, J. Mater. Sci., 2019, 54, 9666–9678 CrossRef CAS.
- H. Jia, Z. Wang, X. Zheng, Y. Cai, J. Lin, H. Liang, J. Qi, J. Cao, J. Feng and W. Fei, Electrochim. Acta, 2019, 312, 54–61 CrossRef CAS.
- Z. Yang, Q. Ma, L. Han and K. Tao, Inorg. Chem. Front., 2019, 6, 2178–2184 RSC.
- X. Guan, M. Huang, L. Yang, G. Wang and X. Guan, Chem. Eng. J., 2019, 372, 151–162 CrossRef CAS.
- X. Liu, L. Ye, Y. Du and L. Zhao, Mater. Lett., 2020, 258, 126812 CrossRef CAS.
- P. Cai, T. Liu, L. Zhang, B. Cheng and J. Yu, Appl. Surf. Sci., 2020, 504, 144501 CrossRef CAS.
- Y. Zhao, C. Huang, Y. He, X. Wu, R. Ge, X. Zu, S. Li and L. Qiao, J. Power Sources, 2020, 456, 228023 CrossRef CAS.
- S. Hou, Y. Lian, Y. Bai, Q. Zhou, C. Ban, Z. Wang, J. Zhao and H. Zhang, Electrochim. Acta, 2020, 341, 136053 CrossRef CAS.
- X. Wei, H. Wu and L. Li, J. Taiwan Inst. Chem. Eng., 2020, 111, 198–204 CrossRef CAS.
- V. Shrivastav, S. Sundriyal, P. Goel, V. Shrivastav, U. K. Tiwari and A. Deep, Electrochim. Acta, 2020, 345, 136194 CrossRef CAS.
- X. Li, Y. Fu, H. Ma, X. Liu, L. Li, J. Ma, C. Liang, M. Jin, Y. Hua and C. Wang, J. Chem. Sci., 2020, 132, 101 CrossRef CAS.
- A. M. Mohamed, A. O. A. El Naga, T. Zaki, H. B. Hassan and N. K. Allam, ACS Appl. Energy Mater., 2020, 3, 8064–8074 CrossRef CAS.
- M. Xu, H. Guo, T. Zhang, J. Zhang, X. Wang and W. Yang, J. Energy Storage, 2021, 35, 102303 CrossRef.
- C.-L. Wu and D.-H. Chen, J. Alloys Compd., 2021, 872, 159702 CrossRef CAS.
- X. Zhao, Q. Ma, K. Tao and L. Han, ACS Appl. Energy Mater., 2021, 4, 4199–4207 CrossRef CAS.
- A. M. Zardkhoshoui, B. Ameri and S. S. H. Davarani, Chem. Eng. J., 2021, 422, 129953 CrossRef.
- Z. Peng, H. Zou, W. Yang, Z. Feng and S. Chen, J. Energy Storage, 2021, 40, 102697 CrossRef.
- C. Ruan, D. Zhu, J. Qi, Q. Meng, F. Wei, Y. Ren, Y. Sui and H. Zhang, Surf. Interfaces, 2021, 25, 101274 CrossRef CAS.
- Z. Li, Y. Huang, Z. Zhang, J. Wang, X. Han, G. Zhang and Y. Li, J. Colloid Interface Sci., 2021, 604, 340–349 CrossRef CAS PubMed.
- L. Chen, J. Wan, L. Fan, Y. Wei and J. Zou, Appl. Surf. Sci., 2021, 570, 151174 CrossRef CAS.
- J. Zhou, Y. Wang, J. Zhou, K. Chen and L. Han, Dalton Trans., 2021, 50, 15129–15139 RSC.
- E. S. Goda, A. U. Rehman, B. Pandit, A. A.-S. Eissa, S. E. Hong and K. R. Yoon, Chem. Eng. J., 2022, 428, 132470 CrossRef CAS.
- L. Ma, Y. Miao, M. Zhang, Y. Sui, J. Qi, F. Wei, Q. Meng, L. Pang, Y. Ren, B. Xiao, X. Xue and Z. Sun, J. Mater. Sci.: Mater. Electron., 2022, 33, 1930–1941 CrossRef CAS.
- S. Yang, Z. Hao, S. Zhang, Y. Gao, X. Li, J. Peng, L. Li and X. Li, ACS Appl. Energy Mater., 2022, 5, 1436–1446 CrossRef CAS.
- L. Luo, Y. Zhou, W. Yan, G. Du, M. Fan and W. Zhao, J. Colloid Interface Sci., 2022, 615, 282–292 CrossRef CAS PubMed.
- X. Wu, C. Wang, Z. Wang, Y. Qin and Y. Kong, Synth. Met., 2022, 286, 117034 CrossRef CAS.
- J. Pan, S. Li, L. Zhang, T. Yu, F. Li, W. Zhang, J. Wang, D. Zhang, Y. Yu and X. Li, J. Energy Storage, 2022, 47, 103550 CrossRef.
- X. Wang, W. Li, Y. Xue, J. Liu, Y. Yue, C. Zhou, K. Zhu, D. Cao, Y. Chen and G. Wang, J. Energy Storage, 2022, 50, 104220 CrossRef.
- Y. Li, H. Wang, T. Shu, J. Yuan, G. Lu, B. Lin, Z. Gao, F. Wei, C. Ma, J. Qi and Y. Sui, J. Energy Storage, 2022, 51, 104299 CrossRef.
- S. Zhang, K. Wang, H. Chen, H. Liu, L. Yang, Y. Chen and H. Li, Carbon, 2022, 194, 10–22 CrossRef CAS.
- X. Liu, X. Zhang, Q. Yin, R. Liu, N. Liu, J. Hu, F. Shen and X. Zhu, CrystEngComm, 2022, 24, 3621–3629 RSC.
- S. Li, Y. Yang, Z. Hu, S. Li, F. Ding, X. Xiao, P. Si and J. Ulstrup, Electrochim. Acta, 2022, 424, 140604 CrossRef CAS.
- Z. Xue, K. Tao and L. Han, Appl. Surf. Sci., 2022, 600, 154076 CrossRef CAS.
- J.-Q. Qi, M.-Y. Huang, C.-Y. Ruan, D.-D. Zhu, L. Zhu, F.-X. Wei, Y.-W. Sui and Q.-K. Meng, Rare Met., 2022, 41, 4116–4126 CrossRef CAS.
- W. Zhang, Z. Shi, H. Wang, S. Sun and S. Yao, J. Alloys Compd., 2022, 919, 165828 CrossRef CAS.
- Q. Chen, Z. Huang, W. Zhao, K. Tao, G. Li and L. Han, J. Alloys Compd., 2023, 937, 168279 CrossRef CAS.
- C. Qu, J. Cao, Y. Chen, M. Wei, H. Fan, X. Liu, X. Li, Q. Wu, B. Feng and L. Yang, Int. J. Hydrogen Energy, 2023, 48, 648–661 CrossRef CAS.
- A. Mohammadi Zardkhoshoui, B. Ameri and S. Saeed Hosseiny Davarani, Chem. Eng. J., 2023, 470, 144132 CrossRef CAS.
- F. Wei, Y. Li, H. Wang, T. Shu, J. Yuan, G. Lu, B. Lin, Z. Gao, Q. Wang, J. Qi and Y. Sui, J. Energy Storage, 2023, 60, 106551 CrossRef.
- G. Wang, Z. Xu, Z. Li, Y. Ding, R. Ge, M. Xiang, G. Wang and Z. Yan, Electrochim. Acta, 2023, 443, 141980 CrossRef CAS.
- X. Wang, Y. Xu, X. An, E. Xu, J. Liu, C. Zhou, W. Li, Y. Chen and G. Wang, Int. J. Hydrogen Energy, 2023, 48, 9945–9956 CrossRef CAS.
- S. Wu, X. Xu, X. Sun, S. Zhou, C. Liu, P. Zhang, S. Fu, H. Zhang, S. Pang, X. Wang and Q. Yang, Diamond Relat. Mater., 2023, 136, 109946 CrossRef CAS.
- P. A. K. Reddy, H. Han, K. C. Kim and S. Bae, Chem. Eng. J., 2023, 471, 144608 CrossRef CAS.
- F. Cao, X.-R. Shi, P. Wang, W. Zhao, M. Huang, J. Hu, S. Xu and G. Zhao, Vacuum, 2023, 216, 112461 CrossRef CAS.
- S. K. Kaverlavani, S. E. Moosavifard, Y. K. Mishra, F. Gity, M. Sadeghipari, S. Y. Hosseini, R. A. Pour and M. Hajmirzaheydarali, J. Energy Storage, 2023, 72, 108793 CrossRef.
- Y. Li, Z. Qiu, M. Qu, Y. Wang, H. Wang and J. Jiang, J. Energy Storage, 2023, 73, 108997 CrossRef.
- J. Chen, X. Sun, W. Kong, Q. Yu, Y. Long, T. Zhou, C. Hu, Y. Dai, J. Gong, L. Pu, H. Zhang and W. Wang, J. Energy Storage, 2023, 73, 109164 CrossRef.
- M. Yan, W. Yao, R. K. Kankala, M. Xiao, J. Ye, Y. Huang, Y. Yang and X. Zhang, Appl. Surf. Sci., 2024, 645, 158757 CrossRef CAS.
- S. Mohapatra, H. T. Das, B. Chandra Tripathy and N. Das, ACS Appl. Nano Mater., 2024, 7, 3249–3259 CrossRef CAS.
- J. Xu, H. Guo, M. Wang, Y. Hao, J. Tian, H. Ren, Y. Liu, B. Ren and W. Yang, Dalton Trans., 2024, 53, 4479–4491 RSC.
- B. Li, L. Zhang, Z. Zhao, Y. Zou, B. Chen, X. Fu, F. Wang, S. Long, W. Guo, J. Liang and M. Ye, Chem. Eng. J., 2024, 487, 150730 CrossRef CAS.
- M. Raza, N. Iqbal, T. Noor, I. Shaukat, R. Ahmad, J. Gao and Z. A. Ghazi, Mater. Res. Bull., 2024, 176, 112830 CrossRef CAS.
- M. Zhu, Y. Yang, X. Zhang, Y. Liang and J. Tang, Appl. Organomet. Chem., 2024, 38, e7533 CrossRef CAS.
- J. Qi, H. Duan, Z. Peng, J. Wang, B. Ma, Z. Yuan and H. Zhang, Energy Fuels, 2024, 38, 13379–13389 CrossRef CAS.
- S. Saha, M. Kandasamy, D. Potphode, C. S. Sharma, B. Chakraborty, N. Hameed and N. Salim, J. Energy Storage, 2024, 99, 113294 CrossRef.
- S. Yang, M. Xiao, M. Xiao, W. Zhang, X. Niu, X. Du, X. Hui, Z. Song and X. Gao, J. Power Sources, 2024, 623, 235424 CrossRef CAS.
- S. Ramesh, I. Rabani, K. Senthilkumar, Y. Haldorai, M. Selvaraj, Y. S. Seo, J. H. Kim and H. S. Kim, J. Electroanal. Chem., 2024, 974, 118749 CrossRef.
- X. B. Mao, H. T. Guan, J. F. Zhou, W. W. Zhou, X. B. Shi, X. Xu and Y. F. Gao, J. Energy Storage, 2025, 105, 114751 CrossRef.
- S. Liu, X. J. Liu, K. Chen and C. G. Xue, Mater. Lett., 2025, 382, 137930 CrossRef CAS.
- Y. F. Mo, T. Feng, C. H. Zhang, Z. S. Zhang, P. Zhao, Z. H. Liu and G. Liu, J. Energy Storage, 2025, 108, 115136 CrossRef.
- C. Hao, J. Z. Tan, Z. J. Lv, M. J. Jiang, C. H. Ni, Y. R. Shen and X. H. Wang, J. Colloid Interface Sci., 2025, 684, 262–276 CrossRef CAS PubMed.
- B. Li, J. Li, P. T. Li, N. Zhang, H. S. Shang and B. Zhang, J. Alloys Compd., 2025, 1017, 179128 CrossRef CAS.
- Y.-X. Lai, R.-Y. Li and C. Young, ChemPhysChem, 2025, e202400910, DOI:10.1002/cphc.202400910.
- R. Ma, Z. Chen, D. Zhao, X. Zhang, J. Zhuo, Y. Yin, X. Wang, G. Yang and F. Yi, J. Mater. Chem. A, 2021, 9, 11501–11529 RSC.
- L.-P. Lv, C. Zhi, Y. Gao, X. Yin, Y. Hu, D. Crespy and Y. Wang, Nanoscale, 2019, 11, 13996–14009 RSC.
- L. C. Tan, D. X. Guo, D. W. Chu, J. Yu, L. L. Zhang, J. Yu and J. Wang, J. Electroanal. Chem., 2019, 851, 6 CrossRef.
- G. Qu, X. Zhang, G. Xiang, Y. Wei, J. Yin, Z. Wang, X. Zhang and X. Xu, Chin. Chem. Lett., 2020, 31, 2007–2012 CrossRef CAS.
- Q. Zong, Y. L. Zhu, Q. Q. Wang, H. Yang, Q. L. Zhang, J. H. Zhan and W. Du, Chem. Eng. J., 2020, 392, 123664 CrossRef CAS.
- L. Sun, Y. Liu, M. Yan, Q. J. Yang, X. Y. Liu and W. D. Shi, Chem. Eng. J., 2022, 431, 133472 CrossRef CAS.
- C. Wang, W. Zheng, Z. Wang, Z.-Z. Yin, Y. Qin and Y. Kong, J. Electroanal. Chem., 2021, 901, 115759 CrossRef CAS.
- H. Wang, T. Shu, J. Yuan, Y. Li, B. Lin, F. Wei, J. Qi and Y. Sui, Colloids Surf., A, 2022, 633, 127789 CrossRef CAS.
- C. Wang, Z. Wang, D. Wu, W. Cai, Y. Qin and Y. Kong, J. Electroanal. Chem., 2022, 905, 115976 CrossRef CAS.
- M. Amiri, S. S. H. Davarani, S. E. Moosavifard and Y.-Q. Fu, J. Colloid Interface Sci., 2022, 616, 141–151 CrossRef CAS PubMed.
- Z. Andikaey, A. A. Ensafi, B. Rezaei and J.-S. Hu, Electrochim. Acta, 2022, 417, 140388 CrossRef.
- Y. Yang, X. Huang, C. Sheng, Y. Pan, Y. Huang and X. Wang, J. Alloys Compd., 2022, 920, 165908 CrossRef CAS.
- P. Salehan, A. A. Ensafi, Z. Andikaey and B. Rezaei, Sci. Rep., 2023, 13, 2070 CrossRef CAS PubMed.
- J. Fan, Z. Peng, M. Chen, W. Yang, H. Zou and S. Chen, Dalton Trans., 2023, 52, 6782–6790 RSC.
- X. Wang, C. Qian, T. Zou, H. Ding, F. Jiang, H. Li, H. Cao, Z. Fang, Y. Xu, J. Liu and Y. Zhu, J. Electroanal. Chem., 2023, 943, 117612 CrossRef CAS.
- I. Shaukat, N. Iqbal, T. Noor, M. Raza and R. Ahmad, Energy Fuels, 2023, 37, 16150–16159 CrossRef CAS.
- L. Sun, Y. Liu, B. F. Luo, F. R. Yan, X. Y. Liu, F. F. Zhu and W. D. Shi, Chem. Eng. J., 2023, 454, 140088 CrossRef CAS.
- Q. Zhang, S. Liu, J. Huang, H. Fu, Q. Fan, H. Zong, H. Guo and A. Zhang, J. Colloid Interface Sci., 2024, 655, 273–285 CrossRef CAS PubMed.
- H. Guo, H. Ren, J. Tian, J. Xu, Y. Hao, L. Peng, Y. Liu and W. Yang, J. Alloys Compd., 2024, 1000, 175107 CrossRef CAS.
- W. Dong, X. Y. Li, E. R. Ye, X. C. Xu, X. Zhang, F. Yang, D. Shen, X. D. Hong and S. B. Yang, J. Energy Storage, 2025, 114, 115870 CrossRef.
- L. Hu, L. Li, Y. Y. Zhang, X. H. Tan, H. Yang, X. M. Lin and Y. X. Tong, J. Mater. Sci. Technol., 2022, 127, 124–132 CrossRef CAS.
- T. Kshetri, T. I. Singh, Y. S. Lee, D. D. Khumujam, N. H. Kim and J. H. Lee, Composites, Part B, 2021, 211, 108624 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *a,
Qingyang
Zheng
b,
Qingyi
Ren
a,
Zhou
Su
a,
Min
Li
a and
Feng
Li
a
*a,
Qingyang
Zheng
b,
Qingyi
Ren
a,
Zhou
Su
a,
Min
Li
a and
Feng
Li
a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1. Moreover, the Co3O4@C-500//reduced graphene oxide (RGO) asymmetric supercapacitor (ASC) exhibited an energy density of 43.99 W h kg−1 at a power density of 824.8 W kg−1, and excellent cycling performance with a capacity retention of 88% after 10
000 cycles at 10 A g−1. Moreover, the Co3O4@C-500//reduced graphene oxide (RGO) asymmetric supercapacitor (ASC) exhibited an energy density of 43.99 W h kg−1 at a power density of 824.8 W kg−1, and excellent cycling performance with a capacity retention of 88% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This excellent performance was attributed to the rational composition control and unique structure of Co3O4 nanoparticles embedded in the carbon skeleton, which resulted in short electrolyte diffusion channels as well as electron transport distance and effectively reduced the negative effect of volume change in the electrode materials during the charge/discharge process. Alternatively, Li et al.81 prepared tree-like CoOx/carbon on Au surface via a chemical vapor deposition method and two-step treatment, as shown in Fig. 2d. The as-prepared CoOx/carbon composite arrays provided a less tortuous pathway for ion diffusion, high pseudocapacitance from the transition metal oxide, and good electrical conductivity from carbon. Moreover, the absence of adhesives in array electrodes is beneficial for the promotion of the electrochemical performance. Subsequently, Wang et al.95 changed the order of the two-step thermal treatment method to obtain Co3O4/NC. Co3O4/NC exhibited a specific capacitance of 1144 F g−1 at a current density of 1 A g−1 (much higher than the specific capacitance of pure Co3O4), and its retention rate was 45.4% at a current density of 20 A g−1. In addition, the asymmetric supercapacitor (ASC) device consisting of Co3O4/NC composite electrodes and activated carbon produced the highest energy density of 40 W h kg−1 at a power density of 1037 W kg−1. At a current density of 5 A g−1, the capacitor retained 87.6% of its initial capacitance after 10
000 cycles. This excellent performance was attributed to the rational composition control and unique structure of Co3O4 nanoparticles embedded in the carbon skeleton, which resulted in short electrolyte diffusion channels as well as electron transport distance and effectively reduced the negative effect of volume change in the electrode materials during the charge/discharge process. Alternatively, Li et al.81 prepared tree-like CoOx/carbon on Au surface via a chemical vapor deposition method and two-step treatment, as shown in Fig. 2d. The as-prepared CoOx/carbon composite arrays provided a less tortuous pathway for ion diffusion, high pseudocapacitance from the transition metal oxide, and good electrical conductivity from carbon. Moreover, the absence of adhesives in array electrodes is beneficial for the promotion of the electrochemical performance. Subsequently, Wang et al.95 changed the order of the two-step thermal treatment method to obtain Co3O4/NC. Co3O4/NC exhibited a specific capacitance of 1144 F g−1 at a current density of 1 A g−1 (much higher than the specific capacitance of pure Co3O4), and its retention rate was 45.4% at a current density of 20 A g−1. In addition, the asymmetric supercapacitor (ASC) device consisting of Co3O4/NC composite electrodes and activated carbon produced the highest energy density of 40 W h kg−1 at a power density of 1037 W kg−1. At a current density of 5 A g−1, the capacitor retained 87.6% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles.
000 charge/discharge cycles.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). The asymmetric supercapacitor devices assembled with Co2V2O7/graphene and reduced graphene oxides delivered a high energy density of 25.7 W h kg−1 at a power density of 663.5 W kg−1 and excellent long cycling stability.
000 cycles). The asymmetric supercapacitor devices assembled with Co2V2O7/graphene and reduced graphene oxides delivered a high energy density of 25.7 W h kg−1 at a power density of 663.5 W kg−1 and excellent long cycling stability.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. At the same time, Semerci et al.108 prepared CuCo2O4@rGO nanomaterials using a step-wise method, which involved the annealing of the CoCu-LDH@rGO precursor derived from ZIF-67@rGO. At a graphene concentration of 4.1%, the composite named CuCo2O4@40-rGO exhibited the best performance among the CuCo2O4@rGO composites (Fig. 4c–f). A specific capacity of 762 F g−1 (381 C g−1) was recorded at a current density of 0.5 A g−1 and an excellent rate performance of 71.6%, revealing an excellent capacitive performance and reversibility. Moreover, the electrochemical performance of CuCo2O4@40-rGO was evaluated as a cathodic electrode and biomass-derived activated carbon (AC) as the negative electrode. The hybrid supercapacitor device showed an energy density of 20.7 W h kg−1 at a power density of 0.43 kW kg−1 with a working potential window of 0–1.7 V, as well as 98.8% capacity retention over 10
000 cycles. At the same time, Semerci et al.108 prepared CuCo2O4@rGO nanomaterials using a step-wise method, which involved the annealing of the CoCu-LDH@rGO precursor derived from ZIF-67@rGO. At a graphene concentration of 4.1%, the composite named CuCo2O4@40-rGO exhibited the best performance among the CuCo2O4@rGO composites (Fig. 4c–f). A specific capacity of 762 F g−1 (381 C g−1) was recorded at a current density of 0.5 A g−1 and an excellent rate performance of 71.6%, revealing an excellent capacitive performance and reversibility. Moreover, the electrochemical performance of CuCo2O4@40-rGO was evaluated as a cathodic electrode and biomass-derived activated carbon (AC) as the negative electrode. The hybrid supercapacitor device showed an energy density of 20.7 W h kg−1 at a power density of 0.43 kW kg−1 with a working potential window of 0–1.7 V, as well as 98.8% capacity retention over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Also, Wei et al.54 reported the synthesis of hybrid porous Co3O4–CeO2 hollow polyhedrons via a simple cation-exchange route, followed by heat treatment. When utilized as the electrode material for supercapacitors, the hybrid porous Co3O4–CeO2 hollow polyhedrons delivered a large specific capacitance of 1288.3 F g−1 at 2.5 A g−1 and a remarkably long cycling stability (<3.3% loss after 6000 cycles). Furthermore, an asymmetric supercapacitor (ASC) device based on the hybrid porous Co3O4–CeO2 hollow polyhedrons was assembled. The ASC device possessed an energy density of 54.9 W h kg−1, which retained 44.2 W h kg−1 even at a power density of 5100 W kg−1, indicating its promising application in electrochemical energy storage.
000 cycles. Also, Wei et al.54 reported the synthesis of hybrid porous Co3O4–CeO2 hollow polyhedrons via a simple cation-exchange route, followed by heat treatment. When utilized as the electrode material for supercapacitors, the hybrid porous Co3O4–CeO2 hollow polyhedrons delivered a large specific capacitance of 1288.3 F g−1 at 2.5 A g−1 and a remarkably long cycling stability (<3.3% loss after 6000 cycles). Furthermore, an asymmetric supercapacitor (ASC) device based on the hybrid porous Co3O4–CeO2 hollow polyhedrons was assembled. The ASC device possessed an energy density of 54.9 W h kg−1, which retained 44.2 W h kg−1 even at a power density of 5100 W kg−1, indicating its promising application in electrochemical energy storage.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cycles with a holding capacitance retention ratio of 90.5% and coulombic efficiency of 99.8%. Recently, Wang et al.99 reported the preparation of hollow carbon tube@Co3O4@SnS2 composites by growing ZIF-67 and SnS2 nanosheets on a PMMA/PAN/Zn2+ electrospun film as the precursors (Fig. 8f). The unique inner core–shell and the outer-shell SnS2 contributed to the excellent characteristics, including abundant pores and channels for rapid ion transport and storage, high specific surface area, improved electrical conductivity, and additional electroactive sites for the faradaic reaction. Owing to the synergy between the unique 1D porous hollow structure and the different components, the as-fabricated HCT-2@Co3O4@SnS2 electrode exhibited a high specific capacitance of 439 F g−1 at 1 A g−1. Moreover, as shown in Fig. 8g, the assembled flexible supercapacitor also demonstrated a remarkable energy density of 40.22 W h kg−1, the corresponding power density of 750.22 W kg−1, and long cycle life (Fig. 8j). In addition, no structural deformation and capacitance loss were observed in the bent devices, as shown in Fig. 8h and i.
500 cycles with a holding capacitance retention ratio of 90.5% and coulombic efficiency of 99.8%. Recently, Wang et al.99 reported the preparation of hollow carbon tube@Co3O4@SnS2 composites by growing ZIF-67 and SnS2 nanosheets on a PMMA/PAN/Zn2+ electrospun film as the precursors (Fig. 8f). The unique inner core–shell and the outer-shell SnS2 contributed to the excellent characteristics, including abundant pores and channels for rapid ion transport and storage, high specific surface area, improved electrical conductivity, and additional electroactive sites for the faradaic reaction. Owing to the synergy between the unique 1D porous hollow structure and the different components, the as-fabricated HCT-2@Co3O4@SnS2 electrode exhibited a high specific capacitance of 439 F g−1 at 1 A g−1. Moreover, as shown in Fig. 8g, the assembled flexible supercapacitor also demonstrated a remarkable energy density of 40.22 W h kg−1, the corresponding power density of 750.22 W kg−1, and long cycle life (Fig. 8j). In addition, no structural deformation and capacitance loss were observed in the bent devices, as shown in Fig. 8h and i.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles in Fig. 9d). Additionally, as shown in Fig. 9e, Jia et al.121 reported the designed synthesis of porous quadruple-shelled CoS2 hollow dodecahedrons with concave surfaces through a template-induced formation process. As the result of the combined advantages of the materials and structures (Fig. 9f), when evaluating as electrodes for supercapacitors, the porous quadruple-shelled CoS2 showed a high specific capacity of 375.2 C g−1 with a good rate performance and excellent stability with 92.1% retention after 10
000 cycles in Fig. 9d). Additionally, as shown in Fig. 9e, Jia et al.121 reported the designed synthesis of porous quadruple-shelled CoS2 hollow dodecahedrons with concave surfaces through a template-induced formation process. As the result of the combined advantages of the materials and structures (Fig. 9f), when evaluating as electrodes for supercapacitors, the porous quadruple-shelled CoS2 showed a high specific capacity of 375.2 C g−1 with a good rate performance and excellent stability with 92.1% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Moreover, the assembled quadruple-shelled CoS2//active carbon asymmetric supercapacitor achieved a high energy density of 52.1 W h kg−1, excellent specific capacitance (146 F g−1 at 0.5 A g−1), and electrochemical cycling stability (89% retention after 5000 cycles). Moreover, Peng et al.136 successfully synthesized hierarchical porous Co9S8 nanowire arrays grown on Ni foam via a multi-step method for use in supercapacitors (Fig. 9g). Introducing ZIF-67 is favorable for the formation of hierarchical pores and small active powder. Also, it is beneficial to provide a large surface area and short diffusion channels, as shown in Fig. 9h, which can reduce the volume expansion and ensure the stability of the architecture. ZIF-derived Co9S8 displayed a large specific capacitance because of its abundant redox sites and high electrical conductivity. The Co9S8/NF electrode possessed outstanding electrochemical properties with the specific capacitance of 369.1 mA h g−1 at 1 A g−1 and 75.5% retention at 20 A g−1. After 5000 cycles, its showed 75% capacitance retention at 20 A g−1. Furthermore, a button-type asymmetric supercapacitor (ASC) was assembled with Co9S8/NF as the positive electrode, which displayed the maximum specific energy of 48.2 W h kg−1 at the specific power of 799.8 W kg−1 and excellent cycling life with 77% retention of its initial capacitance after 5000 cycles. Wang et al.147 applied chemical vapor deposition (CVD) treatment to obtain a CoS/nitrogen-doped carbon (CoS/NC) composite with a hollow cage-like polyhedron structure formed by the combination of CoS nanosheets and nitrogen-doped carbon skeleton. It exhibited a specific capacitance of 789 F g−1 at a current density of 1 A g−1 and a rate capacity of 80.2% at a current density of 20 A g−1. The CoS/NC-700//AC capacitor showed the highest energy density of 32.8 W h kg−1 when the power density was 620.0 W kg−1 and it retained 89.2% of its initial capacitance after 10
000 cycles. Moreover, the assembled quadruple-shelled CoS2//active carbon asymmetric supercapacitor achieved a high energy density of 52.1 W h kg−1, excellent specific capacitance (146 F g−1 at 0.5 A g−1), and electrochemical cycling stability (89% retention after 5000 cycles). Moreover, Peng et al.136 successfully synthesized hierarchical porous Co9S8 nanowire arrays grown on Ni foam via a multi-step method for use in supercapacitors (Fig. 9g). Introducing ZIF-67 is favorable for the formation of hierarchical pores and small active powder. Also, it is beneficial to provide a large surface area and short diffusion channels, as shown in Fig. 9h, which can reduce the volume expansion and ensure the stability of the architecture. ZIF-derived Co9S8 displayed a large specific capacitance because of its abundant redox sites and high electrical conductivity. The Co9S8/NF electrode possessed outstanding electrochemical properties with the specific capacitance of 369.1 mA h g−1 at 1 A g−1 and 75.5% retention at 20 A g−1. After 5000 cycles, its showed 75% capacitance retention at 20 A g−1. Furthermore, a button-type asymmetric supercapacitor (ASC) was assembled with Co9S8/NF as the positive electrode, which displayed the maximum specific energy of 48.2 W h kg−1 at the specific power of 799.8 W kg−1 and excellent cycling life with 77% retention of its initial capacitance after 5000 cycles. Wang et al.147 applied chemical vapor deposition (CVD) treatment to obtain a CoS/nitrogen-doped carbon (CoS/NC) composite with a hollow cage-like polyhedron structure formed by the combination of CoS nanosheets and nitrogen-doped carbon skeleton. It exhibited a specific capacitance of 789 F g−1 at a current density of 1 A g−1 and a rate capacity of 80.2% at a current density of 20 A g−1. The CoS/NC-700//AC capacitor showed the highest energy density of 32.8 W h kg−1 when the power density was 620.0 W kg−1 and it retained 89.2% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles at a current density of 5 A g−1. Meanwhile, Wei et al.158 constructed CoS2 and Co3S4 nanoparticles anchored on a carbon skeleton (CoS2@C and Co3S4@C) depending on if an oxidation process occurred, respectively (Fig. 9i). The reactions are represented by the following equations:
000 charge–discharge cycles at a current density of 5 A g−1. Meanwhile, Wei et al.158 constructed CoS2 and Co3S4 nanoparticles anchored on a carbon skeleton (CoS2@C and Co3S4@C) depending on if an oxidation process occurred, respectively (Fig. 9i). The reactions are represented by the following equations:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The excellent electrochemical performance is attributed to the unique ternary metal sulfide hollow nanocage structure having a large surface area, facile diffusion of ions, good conductivity, and rich redox reactions as well as the synergistic effect between Mo and Co ions. Also, Zhao et al.126 presented the synthesis and characterization of ZIF-67-derived mesoporous copper cobalt sulfide (CuCo2S4) prepared via simple reflux and solvothermal reactions. The obtained CuCo2S4 electrode showed a high specific capacity of up to 672 C g−1 (1344 F g−1) at 1 A g−1. Additionally, Goda et al.141 reported Al doping to improve the conductivity of cobalt sulfide. A novel type of core/sell architecture was successfully designed from aluminum-doped cobalt sulfide encapsulated by nitrogen-doped graphene (Al-doped Co9S8@NG) through the solvothermal/sulfuration of the ZIF-67 structure, subsequent wrapping with a PPy layer and calcination in argon gas at various temperatures. Interestingly, integrating the morphology and composition merits endowed the Al-doped Co9S8@NG600 electrode with highly enhanced supercapacitive features, as shown in Fig. 11e and f. The electrode attained a superior specific capacity of about 736 C g−1 at an applied current density of 1 A g−1, ultra-long cycle stability of 92% after 10
000 cycles. The excellent electrochemical performance is attributed to the unique ternary metal sulfide hollow nanocage structure having a large surface area, facile diffusion of ions, good conductivity, and rich redox reactions as well as the synergistic effect between Mo and Co ions. Also, Zhao et al.126 presented the synthesis and characterization of ZIF-67-derived mesoporous copper cobalt sulfide (CuCo2S4) prepared via simple reflux and solvothermal reactions. The obtained CuCo2S4 electrode showed a high specific capacity of up to 672 C g−1 (1344 F g−1) at 1 A g−1. Additionally, Goda et al.141 reported Al doping to improve the conductivity of cobalt sulfide. A novel type of core/sell architecture was successfully designed from aluminum-doped cobalt sulfide encapsulated by nitrogen-doped graphene (Al-doped Co9S8@NG) through the solvothermal/sulfuration of the ZIF-67 structure, subsequent wrapping with a PPy layer and calcination in argon gas at various temperatures. Interestingly, integrating the morphology and composition merits endowed the Al-doped Co9S8@NG600 electrode with highly enhanced supercapacitive features, as shown in Fig. 11e and f. The electrode attained a superior specific capacity of about 736 C g−1 at an applied current density of 1 A g−1, ultra-long cycle stability of 92% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and a remarkable retention rate (71%). Subsequently, a solid-state AsSC device with Al-doped Co9S8@NG core/shell as the positive electrode and activated PANI-derived carbon nanorods (ACNRs) as the negative electrode was assembled. The device exhibited outstanding specific capacitance, energy density, and power density values of 134 F g−1, 53.3 W h kg−1, and 0.954 kW kg−1, respectively, with a considerable cycle life stability of 93% after consuming 10
000 cycles, and a remarkable retention rate (71%). Subsequently, a solid-state AsSC device with Al-doped Co9S8@NG core/shell as the positive electrode and activated PANI-derived carbon nanorods (ACNRs) as the negative electrode was assembled. The device exhibited outstanding specific capacitance, energy density, and power density values of 134 F g−1, 53.3 W h kg−1, and 0.954 kW kg−1, respectively, with a considerable cycle life stability of 93% after consuming 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Further, when two AsSC devices were linked in series, a multicolor LED could be lit for 25 s, demonstrating their availability for making modern portable electronics, as shown in Fig. 11g–i.
000 cycles. Further, when two AsSC devices were linked in series, a multicolor LED could be lit for 25 s, demonstrating their availability for making modern portable electronics, as shown in Fig. 11g–i.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (NCSC-211) were proven to have the most excellent comprehensive electrochemical properties, as shown in Fig. 12h. In the NCSC-211 nanostructure, multi-layer graphene functions as conductive shells for improving the cycling performance by alleviating the pulverization caused by volume change, the thick CNTs act as conductive bridges and agglomeration spacers, which increase the electron conductivity and provide more active sites for redox reaction, and doped-nitrogen offers enhanced electrolyte wettability and faster electron transfer. Consequently, the NCSC-211 electrode displayed a specific capacitance of 1158 F g−1 at 1 A g−1, rate capability of 86% at 10 A g−1 and extraordinary cycling stability with 97.2% capacitive retention after 4000 cycles. Moreover, Li et al.165 demonstrated the synthesis of Mo-ZIF-67/CNT composites through mechanical stirring and co-precipitation. After that, Ni, Co and Mo trimetallic sulfide/CNT composites (Ni–Co–Mo–S/CNT) were produced through further ion exchange/etching and vulcanization processes. The material had multiple valence states of three different metal ions, providing a large number of oxidation–reduction sites. The doping of CNTs endowed it with a unique coral-like structure, which helped to form its huge specific surface area and large mesopores. The electrode exhibited an impressive performance with a specific capacitance of 1728.6 F g−1 at a current density of 1 A g−1. It also had a good rate capability, maintaining 55.8% of its specific capacitance when the current density increased tenfold. Moreover, Yan et al.167 developed an in situ self-assembly strategy to prepare hollow composites using ZIF-67 and carbon nanotubes, and subsequently converted ZIF-67 to cobalt sulfide via a sulfurization strategy (Fig. 12i). The unique hollow composites with different dimensions, as shown in Fig. 12j and k, exhibited a synergistic effect between subunits, resulting in excellent electrochemical properties. The impact of particle cavity channel size on electrochemical properties was evaluated systematically. As a result, the obtained CoS@CNT with hollow cattail-shape structures (HCCoS@CNT) exhibited a high specific capacitance (1250 F g−1) and cyclic stability (90.4% for over 10
1 (NCSC-211) were proven to have the most excellent comprehensive electrochemical properties, as shown in Fig. 12h. In the NCSC-211 nanostructure, multi-layer graphene functions as conductive shells for improving the cycling performance by alleviating the pulverization caused by volume change, the thick CNTs act as conductive bridges and agglomeration spacers, which increase the electron conductivity and provide more active sites for redox reaction, and doped-nitrogen offers enhanced electrolyte wettability and faster electron transfer. Consequently, the NCSC-211 electrode displayed a specific capacitance of 1158 F g−1 at 1 A g−1, rate capability of 86% at 10 A g−1 and extraordinary cycling stability with 97.2% capacitive retention after 4000 cycles. Moreover, Li et al.165 demonstrated the synthesis of Mo-ZIF-67/CNT composites through mechanical stirring and co-precipitation. After that, Ni, Co and Mo trimetallic sulfide/CNT composites (Ni–Co–Mo–S/CNT) were produced through further ion exchange/etching and vulcanization processes. The material had multiple valence states of three different metal ions, providing a large number of oxidation–reduction sites. The doping of CNTs endowed it with a unique coral-like structure, which helped to form its huge specific surface area and large mesopores. The electrode exhibited an impressive performance with a specific capacitance of 1728.6 F g−1 at a current density of 1 A g−1. It also had a good rate capability, maintaining 55.8% of its specific capacitance when the current density increased tenfold. Moreover, Yan et al.167 developed an in situ self-assembly strategy to prepare hollow composites using ZIF-67 and carbon nanotubes, and subsequently converted ZIF-67 to cobalt sulfide via a sulfurization strategy (Fig. 12i). The unique hollow composites with different dimensions, as shown in Fig. 12j and k, exhibited a synergistic effect between subunits, resulting in excellent electrochemical properties. The impact of particle cavity channel size on electrochemical properties was evaluated systematically. As a result, the obtained CoS@CNT with hollow cattail-shape structures (HCCoS@CNT) exhibited a high specific capacitance (1250 F g−1) and cyclic stability (90.4% for over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1) (as shown in Fig. 12l–o).
000 cycles at 10 A g−1) (as shown in Fig. 12l–o).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CNT/CoS (1
1), CNT/CoS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CNT/CoS (1
2) and CNT/CoS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8). Reprinted with permission from ref. 117, Copyright 2019, The Royal Society of Chemistry. (d) Synthetic procedure for NiCo2S4/NF; (e) CV curves at 5 mV s−1 and (f) specific capacitance of Co9S8/NF and NiCo2S4/NF. Reprinted with permission from ref. 140, Copyright 2021, The Royal Society of Chemistry. (g) Schematic diagram of the construction of N–Co3S4-GN/CNT nanostructure and (h) schematic of the nanostructure changes in NCSC-211 and NCSC-311 after 500, 1000 and 4000 cycles. Reprinted with permission from ref. 149, Copyright 2022, Elsevier Ltd. (i) Schematic representation showing the various synthesis procedures for generating CoS@CNT structures and the electrochemical process occurring on hierarchical pore-structured HCCoS@CNT. Gentle stirring resulted in the formation of HRDCoS@CNT with a rhombic dodecahedron-shaped morphology by controlling the stirring speed to 200 rpm, while intense stirring resulted in the fabrication of HCCoS@CNT with a cattail-shaped morphology by controlling the stirring speed at 1000 rpm. (j) SEM image and (k) TEM image of HCCoS@CNT and (l) GCD curves at 2 A g−1, (m) Nyquist plots, (n) specific capacitance and (o) cycling stability at 10 A g−1 of CoS, HCCoS@CNT and HRDCoS@CNT. Reprinted with permission from ref. 167, Copyright 2023, Elsevier B.V.
8). Reprinted with permission from ref. 117, Copyright 2019, The Royal Society of Chemistry. (d) Synthetic procedure for NiCo2S4/NF; (e) CV curves at 5 mV s−1 and (f) specific capacitance of Co9S8/NF and NiCo2S4/NF. Reprinted with permission from ref. 140, Copyright 2021, The Royal Society of Chemistry. (g) Schematic diagram of the construction of N–Co3S4-GN/CNT nanostructure and (h) schematic of the nanostructure changes in NCSC-211 and NCSC-311 after 500, 1000 and 4000 cycles. Reprinted with permission from ref. 149, Copyright 2022, Elsevier Ltd. (i) Schematic representation showing the various synthesis procedures for generating CoS@CNT structures and the electrochemical process occurring on hierarchical pore-structured HCCoS@CNT. Gentle stirring resulted in the formation of HRDCoS@CNT with a rhombic dodecahedron-shaped morphology by controlling the stirring speed to 200 rpm, while intense stirring resulted in the fabrication of HCCoS@CNT with a cattail-shaped morphology by controlling the stirring speed at 1000 rpm. (j) SEM image and (k) TEM image of HCCoS@CNT and (l) GCD curves at 2 A g−1, (m) Nyquist plots, (n) specific capacitance and (o) cycling stability at 10 A g−1 of CoS, HCCoS@CNT and HRDCoS@CNT. Reprinted with permission from ref. 167, Copyright 2023, Elsevier B.V.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of repeated charging/discharging, suggesting its potential for practical supercapacitor applications. Moreover, Wu et al.161 designed the rGO/NiCo2S4 material by using GO and ZIF-67 as the template and precursor, which increased the specific surface area and provided active sites for redox reactions and shortened ion transport pathways. The synthesized rGO-10/NiCo2S4 exhibited excellent electrochemical properties as an electrode material, which had a high specific capacity of 171 mA h g−1 and 121.9 mA h g−1 (1 A g−1 and 10 A g−1). The as-obtained rGO-10/NiCo2S4//AC asymmetric supercapacitor (ASC) delivered a power density of 870 W kg−1 at the specific energy of 41 W h kg−1, with 88.2% capacitance retention after 5000 cycles at 5 A g−1.
000 cycles of repeated charging/discharging, suggesting its potential for practical supercapacitor applications. Moreover, Wu et al.161 designed the rGO/NiCo2S4 material by using GO and ZIF-67 as the template and precursor, which increased the specific surface area and provided active sites for redox reactions and shortened ion transport pathways. The synthesized rGO-10/NiCo2S4 exhibited excellent electrochemical properties as an electrode material, which had a high specific capacity of 171 mA h g−1 and 121.9 mA h g−1 (1 A g−1 and 10 A g−1). The as-obtained rGO-10/NiCo2S4//AC asymmetric supercapacitor (ASC) delivered a power density of 870 W kg−1 at the specific energy of 41 W h kg−1, with 88.2% capacitance retention after 5000 cycles at 5 A g−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a current density of 20 A g−1, representing excellent cycle stability and rate capability. Moreover, Li et al.170 fabricated hierarchical hollow core–shell microspheres of NiCo2S4/MXene/N-doped carbon (NiCo2S4/MXene/NC) using dual templates of polyethylene microspheres and ZIF-67 (Fig. 14g). The integration of MXene and N-doped carbon significantly enhanced the electronic conductivity of the electrode, while the construction of hollow spherical structures effectively alleviated the instability of NiCo2S4/NC during the charge–discharge cycles, leading to an excellent charge storage performance with a high specific capacitance (1786 F g−1 at 1 A g−1) and impressive cycling stability (over 100% capacitance retention after 10
000 cycles at a current density of 20 A g−1, representing excellent cycle stability and rate capability. Moreover, Li et al.170 fabricated hierarchical hollow core–shell microspheres of NiCo2S4/MXene/N-doped carbon (NiCo2S4/MXene/NC) using dual templates of polyethylene microspheres and ZIF-67 (Fig. 14g). The integration of MXene and N-doped carbon significantly enhanced the electronic conductivity of the electrode, while the construction of hollow spherical structures effectively alleviated the instability of NiCo2S4/NC during the charge–discharge cycles, leading to an excellent charge storage performance with a high specific capacitance (1786 F g−1 at 1 A g−1) and impressive cycling stability (over 100% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). The corresponding hybrid supercapacitor displayed a high specific capacitance (190 F g−1 at 1 A g−1) with a maximum energy density of 67 W h kg−1 at 796 W kg−1 and great cycling stability (over 80% capacitance retention after 10
000 cycles). The corresponding hybrid supercapacitor displayed a high specific capacitance (190 F g−1 at 1 A g−1) with a maximum energy density of 67 W h kg−1 at 796 W kg−1 and great cycling stability (over 80% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). As shown in Fig. 14h–k, density functional theory (DFT) calculations revealed that addition of MXene enhanced the conductivity of NiCo2S4.
000 cycles). As shown in Fig. 14h–k, density functional theory (DFT) calculations revealed that addition of MXene enhanced the conductivity of NiCo2S4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). The outstanding supercapacitor property can be attributed to the synergistic advantages of the intertwined “Co3S4-to-PPy-to-Co3S4” networks including rich reactive sites, shortened charge diffusion pathway and enhanced charge transfer and mechanical stability. Similarly, Zhao et al.134 grew the ZIF-67-derived porous CoNi2S4 on intercrosslinked PPy tubes to obtain the NiCoS@PPy hybrid via a solution sulfidation (as shown in Fig. 15a). The NiCoS@PPy electrode achieved an ultrahigh specific capacitance of 2316.6 and 1409.5 F g−1 at 1 and 10 A g−1, largely outperforming the control NiCoS or NiCo-LDH@PPy (Fig. 15b–d). Furthermore, the fabricated ASC utilizing NiCoS@PPy as the cathode displayed an outstanding energy storage capability (34.4 W h kg−1 at 799 W kg−1) and splendid cyclic life (retaining 84% initial capacitance after 8500 cycles). Additionally, Wu et al.145 initially assembled ZIF-67 on carbon cloth, which was further hydrothermally converted to Co9S8, and subsequently PPy was coated on the surface of CC/Co9S8via an electrodeposition process. The obtained CC/Co9S8/PPy electrode showed 83.5% capacity retention after 2500 charge–discharge cycles due to the buffered volume change of Co9S8 during the redox process by the introduction of the PPy shell. Moreover, Xu et al.132 used polyaniline (PANI) as a growth substrate for ZIF-67 as a transfer scaffold of electrons and worked as a wire to connect nanoparticles, improving the conductivity of ZIF-67. The sulfides (Co3S4/PANI) obtained by sulfurization exhibited a high specific capacitance of up to 11 times that of ZIF-67 at 1 A g−1. Also, the assembled asymmetric supercapacitors (ASC) device exhibited a high specific energy of 40.75 W h kg−1 at a specific power of 800 W kg−1 and displayed super cycling stability. After 20
000 cycles). The outstanding supercapacitor property can be attributed to the synergistic advantages of the intertwined “Co3S4-to-PPy-to-Co3S4” networks including rich reactive sites, shortened charge diffusion pathway and enhanced charge transfer and mechanical stability. Similarly, Zhao et al.134 grew the ZIF-67-derived porous CoNi2S4 on intercrosslinked PPy tubes to obtain the NiCoS@PPy hybrid via a solution sulfidation (as shown in Fig. 15a). The NiCoS@PPy electrode achieved an ultrahigh specific capacitance of 2316.6 and 1409.5 F g−1 at 1 and 10 A g−1, largely outperforming the control NiCoS or NiCo-LDH@PPy (Fig. 15b–d). Furthermore, the fabricated ASC utilizing NiCoS@PPy as the cathode displayed an outstanding energy storage capability (34.4 W h kg−1 at 799 W kg−1) and splendid cyclic life (retaining 84% initial capacitance after 8500 cycles). Additionally, Wu et al.145 initially assembled ZIF-67 on carbon cloth, which was further hydrothermally converted to Co9S8, and subsequently PPy was coated on the surface of CC/Co9S8via an electrodeposition process. The obtained CC/Co9S8/PPy electrode showed 83.5% capacity retention after 2500 charge–discharge cycles due to the buffered volume change of Co9S8 during the redox process by the introduction of the PPy shell. Moreover, Xu et al.132 used polyaniline (PANI) as a growth substrate for ZIF-67 as a transfer scaffold of electrons and worked as a wire to connect nanoparticles, improving the conductivity of ZIF-67. The sulfides (Co3S4/PANI) obtained by sulfurization exhibited a high specific capacitance of up to 11 times that of ZIF-67 at 1 A g−1. Also, the assembled asymmetric supercapacitors (ASC) device exhibited a high specific energy of 40.75 W h kg−1 at a specific power of 800 W kg−1 and displayed super cycling stability. After 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of charge and discharge tests, it still maintained 88% of its initial capacitance at a higher current density of 5 A g−1.
000 cycles of charge and discharge tests, it still maintained 88% of its initial capacitance at a higher current density of 5 A g−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with excellent cycling stability (101.7% of the initial value). Similarly, Saha et al.174 used ZIF-67 and Mo-precursor to obtain Co3S4–Mo15S19 (CMS/NF) via one-pot hydrothermal sulfurization (Fig. 16a). The unique stellate-shaped architecture of the CMS/NF microflowers enhanced the exposure of the redox active sites, improving the charge storage and exhibiting superior performance. On account of the synergy of the metal ions and stellate-shaped hierarchical architecture, the CMS/NF hybrid sulfide showed an excellent specific capacitance value of 3283 F g−1 at a current density of 1 A g−1 with a capacitance retention of 77.7% at 10 A g−1. The asymmetric supercapacitor device based on CMS/NF hybrid sulfide with aqueous KOH electrolyte exhibited a high specific energy of 40.8 W h kg−1 at 400 W kg−1 with excellent cycling life of 81% after 5000 cycles with ∼100% coulombic efficiency. Also, according to the total density of states, as shown in Fig. 16b and c, it was concluded that compared to the pristine structures, the hybrid system had enhanced states close to the Fermi level, confirming the improvement in conductivity.
000 cycles with excellent cycling stability (101.7% of the initial value). Similarly, Saha et al.174 used ZIF-67 and Mo-precursor to obtain Co3S4–Mo15S19 (CMS/NF) via one-pot hydrothermal sulfurization (Fig. 16a). The unique stellate-shaped architecture of the CMS/NF microflowers enhanced the exposure of the redox active sites, improving the charge storage and exhibiting superior performance. On account of the synergy of the metal ions and stellate-shaped hierarchical architecture, the CMS/NF hybrid sulfide showed an excellent specific capacitance value of 3283 F g−1 at a current density of 1 A g−1 with a capacitance retention of 77.7% at 10 A g−1. The asymmetric supercapacitor device based on CMS/NF hybrid sulfide with aqueous KOH electrolyte exhibited a high specific energy of 40.8 W h kg−1 at 400 W kg−1 with excellent cycling life of 81% after 5000 cycles with ∼100% coulombic efficiency. Also, according to the total density of states, as shown in Fig. 16b and c, it was concluded that compared to the pristine structures, the hybrid system had enhanced states close to the Fermi level, confirming the improvement in conductivity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 15 A g−1. Also, the hybrid supercapacitor assembled with NSH-MCS as the positive electrode and AC (activated carbon) as the negative electrode exhibited a desirable performance, such as a good energy density (54.9 W h kg−1 at 753 W kg−1) and excellent longevity of 90.5% after 10
000 cycles at 15 A g−1. Also, the hybrid supercapacitor assembled with NSH-MCS as the positive electrode and AC (activated carbon) as the negative electrode exhibited a desirable performance, such as a good energy density (54.9 W h kg−1 at 753 W kg−1) and excellent longevity of 90.5% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 15 A g−1. Additionally, as shown in Fig. 16j, Guan et al.123 prepared CoSx/Ni–Co LDH via the partial sulfuration of ZIF-67 and etching and precipitation of the added nickel ions. The prepared CoSx/Ni–Co LDH nanocages consisted of a hollow rhombic dodecahedral morphology with many nanosheet arrays on their shell. When used as the electrode material for electrochemical capacitors, the CoSx/Ni–Co LDH nanocages delivered a specific capacitance of 1562 F g−1 at a current density of 1 A g−1. In addition, the asymmetric supercapacitor assembled with CoSx/Ni–Co LDH as the cathode and activated carbon (AC) as the anode showed a high energy density of 35.8 W h kg−1 at a power density of 800 W kg−1 and an excellent cycling performance with the retention rate of 94.56% after 10
000 cycles at 15 A g−1. Additionally, as shown in Fig. 16j, Guan et al.123 prepared CoSx/Ni–Co LDH via the partial sulfuration of ZIF-67 and etching and precipitation of the added nickel ions. The prepared CoSx/Ni–Co LDH nanocages consisted of a hollow rhombic dodecahedral morphology with many nanosheet arrays on their shell. When used as the electrode material for electrochemical capacitors, the CoSx/Ni–Co LDH nanocages delivered a specific capacitance of 1562 F g−1 at a current density of 1 A g−1. In addition, the asymmetric supercapacitor assembled with CoSx/Ni–Co LDH as the cathode and activated carbon (AC) as the anode showed a high energy density of 35.8 W h kg−1 at a power density of 800 W kg−1 and an excellent cycling performance with the retention rate of 94.56% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, suggesting its potential application in high-performance electrochemical capacitors. These exceptional electrochemical properties can be attributed to the unique structure and synergistic effects between the metal sulfide and the bimetallic hydroxide. Similarly, Wang et al.159 directly grew ZIF-67-derived CoS with Ni(OH)2 nanosheets on carbon cloth (Ni(OH)2/CoS/CC) and the composites possessed a superior performance (561.6 mA h g−1 at 1 A g−1) to the Ni(OH)2/CC electrode (199.4 mA h g−1). The hybrid supercapacitor (HSC) with Ni(OH)2/CoS/CC as the cathode and activated carbon (AC) as the anode (Ni(OH)2/CoS/CC//AC) provided a remarkable energy density of 90.8 W h kg−1 at 800 W kg−1 and maintained 59.7 W h kg−1 even at 25
000 cycles, suggesting its potential application in high-performance electrochemical capacitors. These exceptional electrochemical properties can be attributed to the unique structure and synergistic effects between the metal sulfide and the bimetallic hydroxide. Similarly, Wang et al.159 directly grew ZIF-67-derived CoS with Ni(OH)2 nanosheets on carbon cloth (Ni(OH)2/CoS/CC) and the composites possessed a superior performance (561.6 mA h g−1 at 1 A g−1) to the Ni(OH)2/CC electrode (199.4 mA h g−1). The hybrid supercapacitor (HSC) with Ni(OH)2/CoS/CC as the cathode and activated carbon (AC) as the anode (Ni(OH)2/CoS/CC//AC) provided a remarkable energy density of 90.8 W h kg−1 at 800 W kg−1 and maintained 59.7 W h kg−1 even at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 W kg−1, which is higher than that of most of the reported Ni(OH)2-related devices, and possessed a marvelous capacity retention of 92.2% over 10
600 W kg−1, which is higher than that of most of the reported Ni(OH)2-related devices, and possessed a marvelous capacity retention of 92.2% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles. The outstanding electrochemical performance of Ni(OH)2/CoS/CC was chiefly due to the mediation of the Co2+/Co3+ redox cycle for the rapid conversion of Ni2+ into Ni3+, which greatly boosted the charge-transfer efficiency in the supercapacitor and methanol electro-oxidation. Moreover, Xue et al.152 used one-dimensional (1D) V2O5 nanowires as a flexible backbone to string ZIF-67-derived hollow Co3S4 three-dimensional (3D) nanopholyhedra to build robust core–shell 1D@3D V2O5@Co3S4 nanocomposites (Fig. 16k). As shown in Fig. 16l and m, the 1D V2O5 nanowires facilitated efficient electron transportation between the Co3S4 nanopolyhedra. The hollow/porous structure of Co3S4 not only afforded substantial electroactive sites, but also shortened the charge transport pathway. With these advantages, the optimal V2O5@Co3S4-5 h displayed enhanced electrochemical properties compared with the control V2O5 or Co3S4-5 h in a three-electrode system, as shown in Fig. 16n. Furthermore, the asymmetric supercapacitor made from V2O5@Co3S4-5 h exhibited a high energy density (40.7 W h kg−1 at 800 W kg−1) with outstanding cycle durability, maintaining 85.9% of its initial capacitance after 10
000 charge–discharge cycles. The outstanding electrochemical performance of Ni(OH)2/CoS/CC was chiefly due to the mediation of the Co2+/Co3+ redox cycle for the rapid conversion of Ni2+ into Ni3+, which greatly boosted the charge-transfer efficiency in the supercapacitor and methanol electro-oxidation. Moreover, Xue et al.152 used one-dimensional (1D) V2O5 nanowires as a flexible backbone to string ZIF-67-derived hollow Co3S4 three-dimensional (3D) nanopholyhedra to build robust core–shell 1D@3D V2O5@Co3S4 nanocomposites (Fig. 16k). As shown in Fig. 16l and m, the 1D V2O5 nanowires facilitated efficient electron transportation between the Co3S4 nanopolyhedra. The hollow/porous structure of Co3S4 not only afforded substantial electroactive sites, but also shortened the charge transport pathway. With these advantages, the optimal V2O5@Co3S4-5 h displayed enhanced electrochemical properties compared with the control V2O5 or Co3S4-5 h in a three-electrode system, as shown in Fig. 16n. Furthermore, the asymmetric supercapacitor made from V2O5@Co3S4-5 h exhibited a high energy density (40.7 W h kg−1 at 800 W kg−1) with outstanding cycle durability, maintaining 85.9% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)
000)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se 1
Se 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed an excellent electrochemical performance with the highest specific capacitance (746 F g−1 at 2 mV s−1), showing a cyclic capability of 82.3% after 4000 cycles because of the optimized ratio of cobalt and selenium in it. At the same time, Wang et al.197 obtained CoSe2/NC via a similar strategy at various temperatures. In a three-electrode system with 2 M KOH as the electrolyte, CoSe2/NC-1 h possessed a high capacity of 554.4 F g−1 at 1 A g−1 and excellent cycling stability (92% capacity retention after 21
1) showed an excellent electrochemical performance with the highest specific capacitance (746 F g−1 at 2 mV s−1), showing a cyclic capability of 82.3% after 4000 cycles because of the optimized ratio of cobalt and selenium in it. At the same time, Wang et al.197 obtained CoSe2/NC via a similar strategy at various temperatures. In a three-electrode system with 2 M KOH as the electrolyte, CoSe2/NC-1 h possessed a high capacity of 554.4 F g−1 at 1 A g−1 and excellent cycling stability (92% capacity retention after 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles). In addition, a flexible solid-state supercapacitor was assembled with CoSe2/NC-1 h as the positive electrode and AC as the negative electrode. The power density was 800 W kg−1 at 1 A g−1, and the cycling stability was tested at 91.53% after 6000 cycles at 2 A g−1. Additionally, Wei's group190 grew CoSe2 on carbon cloth using leaf-like ZIF-67 as the precursor by a two-step calcination method (Fig. 17a–c). The novel CoSe2/carbon (CoSe2/C) electrode showed an excellent electrochemical performance (Fig. 17d and e), with a specific capacitance of 462 F g−1 at a current density of 5 A g−1, and 100% capacitance retention at 10 A g−1 after 10
000 cycles). In addition, a flexible solid-state supercapacitor was assembled with CoSe2/NC-1 h as the positive electrode and AC as the negative electrode. The power density was 800 W kg−1 at 1 A g−1, and the cycling stability was tested at 91.53% after 6000 cycles at 2 A g−1. Additionally, Wei's group190 grew CoSe2 on carbon cloth using leaf-like ZIF-67 as the precursor by a two-step calcination method (Fig. 17a–c). The novel CoSe2/carbon (CoSe2/C) electrode showed an excellent electrochemical performance (Fig. 17d and e), with a specific capacitance of 462 F g−1 at a current density of 5 A g−1, and 100% capacitance retention at 10 A g−1 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Meanwhile, an asymmetric supercapacitor was assembled, which exhibited an energy density of 20.6 W h kg−1 with a power density of 698.8 W kg−1 and outstanding cycling stability. Moreover, Fan et al.196 prepared porous CoSe2 nanosheets on nickel foam via the hydrothermal method (Fig. 17f and g). The unique nanosheet array structure could provide a highly active surface, large superficial area and fast ion transport channels. This was mainly attributed to the fact that the reaction at different hydrothermal temperatures can provide different nanosheet structures. In addition, the incorporated ZIF-67 backbone provided a pathway for rapid electron transfer and accommodated the volume expansion of the selenide during charge–discharge processes. As shown in Fig. 17h–k, due to its distinct porous structure, the CoSe2-180 electrode showed a high specific capacity of 269.4 mA h g−1 at 1 A g−1 and a distinguished retention rate of 83.7% at 20 A g−1. After 5000 cycles, the specific capacity was maintained at 83.4% of the initial value. Moreover, the asymmetric supercapacitor (ASC) device was assembled with CoSe2-180 as the positive electrode. It displayed a favorable electrochemical performance with the maximum specific energy of 45.6 W h kg−1 at a specific power of 800.8 W kg−1 and original capacitance retention rate of 81.5% after 5000 cycles.
000 cycles. Meanwhile, an asymmetric supercapacitor was assembled, which exhibited an energy density of 20.6 W h kg−1 with a power density of 698.8 W kg−1 and outstanding cycling stability. Moreover, Fan et al.196 prepared porous CoSe2 nanosheets on nickel foam via the hydrothermal method (Fig. 17f and g). The unique nanosheet array structure could provide a highly active surface, large superficial area and fast ion transport channels. This was mainly attributed to the fact that the reaction at different hydrothermal temperatures can provide different nanosheet structures. In addition, the incorporated ZIF-67 backbone provided a pathway for rapid electron transfer and accommodated the volume expansion of the selenide during charge–discharge processes. As shown in Fig. 17h–k, due to its distinct porous structure, the CoSe2-180 electrode showed a high specific capacity of 269.4 mA h g−1 at 1 A g−1 and a distinguished retention rate of 83.7% at 20 A g−1. After 5000 cycles, the specific capacity was maintained at 83.4% of the initial value. Moreover, the asymmetric supercapacitor (ASC) device was assembled with CoSe2-180 as the positive electrode. It displayed a favorable electrochemical performance with the maximum specific energy of 45.6 W h kg−1 at a specific power of 800.8 W kg−1 and original capacitance retention rate of 81.5% after 5000 cycles.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421.6 W kg−1. In addition, the assembled supercapacitor device displayed an excellent capacity retention of 88.6% after 8000 cycles at 5 A g−1. Also, Wang et al.189 reported the facile synthesis of graphene oxide (GO)-supported CoSe2 (CoSe2/GO) derived from ZIF-67 via the hydrothermal method. CoSe2/GO exhibited a considerable specific capacity (108.31 mA h g−1 at 1 A g−1) and high cyclic stability (capacity retention of 91.2% after 5000 cycles). Compared with GO and CoSe2 alone, the greatly enhanced electrochemical performances of CoSe2/GO can be attributed to the synergistic effect of GO and CoSe2. Additionally, Yang et al.194 synthesized ZIF-67-derived CoSe2/Ni3Se4 nanosheets anchored vertically on an MXene substrate, which enhanced the overall conductivity. Furthermore, the close combination between the CoSe2/Ni3Se4 nanosheets and MXene nanosheets promoted a faster charge transfer rate and improved the durability of the structure (Fig. 19a). Consequently, as an electrode material for supercapacitors, the honeycomb-like MXene@CoSe2/Ni3Se4 achieved a high specific capacitance (283 mA h g−1 at 1 A g−1, as shown in Fig. 19b–e) and an outstanding capacitance retention rate with 80% after 5000 cycles. Moreover, Shi's group reported the synthesis of M–(Ni, Co)–Se@CNFs/CC199 and NixCo1−xSe2/CNFs/CoO@CC188 derived from ZIF-L for hybrid supercapacitors, respectively. M–(Ni, Co)–Se@CNFs/CC exhibited a specific capacitance of 378.9 mA h g−1 at 1 A g−1, while that of NixCo1−xSe2/CNFs/CoO@CC was 207.8 mA h g−1, and both displayed a superior performance and stability.
421.6 W kg−1. In addition, the assembled supercapacitor device displayed an excellent capacity retention of 88.6% after 8000 cycles at 5 A g−1. Also, Wang et al.189 reported the facile synthesis of graphene oxide (GO)-supported CoSe2 (CoSe2/GO) derived from ZIF-67 via the hydrothermal method. CoSe2/GO exhibited a considerable specific capacity (108.31 mA h g−1 at 1 A g−1) and high cyclic stability (capacity retention of 91.2% after 5000 cycles). Compared with GO and CoSe2 alone, the greatly enhanced electrochemical performances of CoSe2/GO can be attributed to the synergistic effect of GO and CoSe2. Additionally, Yang et al.194 synthesized ZIF-67-derived CoSe2/Ni3Se4 nanosheets anchored vertically on an MXene substrate, which enhanced the overall conductivity. Furthermore, the close combination between the CoSe2/Ni3Se4 nanosheets and MXene nanosheets promoted a faster charge transfer rate and improved the durability of the structure (Fig. 19a). Consequently, as an electrode material for supercapacitors, the honeycomb-like MXene@CoSe2/Ni3Se4 achieved a high specific capacitance (283 mA h g−1 at 1 A g−1, as shown in Fig. 19b–e) and an outstanding capacitance retention rate with 80% after 5000 cycles. Moreover, Shi's group reported the synthesis of M–(Ni, Co)–Se@CNFs/CC199 and NixCo1−xSe2/CNFs/CoO@CC188 derived from ZIF-L for hybrid supercapacitors, respectively. M–(Ni, Co)–Se@CNFs/CC exhibited a specific capacitance of 378.9 mA h g−1 at 1 A g−1, while that of NixCo1−xSe2/CNFs/CoO@CC was 207.8 mA h g−1, and both displayed a superior performance and stability.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 20b–e). Furthermore, the assembled CoSe2@NiMn-LDH@Cu1.8Se/CF//AC (activated carbon) asymmetric supercapacitor (ASC) achieved an energy density of 36.6 W h kg−1 at the power density of 760.6 W kg−1 and retained 87.35% of its initial capacitance after 5000 cycles. Also, Guo et al.201 synthesized MoSe2/(Ni, Co)Se2 hollow structures derived from PMo12@ZIF-67 precursor by an Ni2+ etching and selenization process (Fig. 20f). In the three-electrode test, as shown in Fig. 20g–j, the developed 60-MoSe2/(Ni, Co)Se2 electrode material exhibited an excellent specific capacity of 359.9 mA h g−1 at a current density of 1 A g−1, and a capacitance retention rate of 83.7% after 10
000 cycles (Fig. 20b–e). Furthermore, the assembled CoSe2@NiMn-LDH@Cu1.8Se/CF//AC (activated carbon) asymmetric supercapacitor (ASC) achieved an energy density of 36.6 W h kg−1 at the power density of 760.6 W kg−1 and retained 87.35% of its initial capacitance after 5000 cycles. Also, Guo et al.201 synthesized MoSe2/(Ni, Co)Se2 hollow structures derived from PMo12@ZIF-67 precursor by an Ni2+ etching and selenization process (Fig. 20f). In the three-electrode test, as shown in Fig. 20g–j, the developed 60-MoSe2/(Ni, Co)Se2 electrode material exhibited an excellent specific capacity of 359.9 mA h g−1 at a current density of 1 A g−1, and a capacitance retention rate of 83.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In addition, the asymmetric supercapacitor (ASC) assembled with 60-MoSe2/(Ni, Co)Se2 as the positive electrode and activated carbon as the negative electrode exhibited an excellent energy density of 40.3 W h kg−1 at a power density of 800.9 W kg−1.
000 cycles. In addition, the asymmetric supercapacitor (ASC) assembled with 60-MoSe2/(Ni, Co)Se2 as the positive electrode and activated carbon as the negative electrode exhibited an excellent energy density of 40.3 W h kg−1 at a power density of 800.9 W kg−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles, respectively.
000 charge–discharge cycles, respectively.

