DOI:
10.1039/D5DT00157A
(Paper)
Dalton Trans., 2025,
54, 9494-9502
The impact of second coordination sphere functional group extension on product selectivity for manganese bipyridyl CO2 reduction electrocatalysts†
Received
20th January 2025
, Accepted 20th May 2025
First published on 27th May 2025
Introduction
The [Ni–Fe]CODH enzyme has inspired many studies of molecular transition metal catalyst designs for electrocatalytic CO2 reduction,1–3 in particular toward the role of secondary and outer coordination sphere effects on enhancing catalytic performance.4–7 By extending the second coordination sphere functionality one can potentially mimic the qualities of enzymatic co-factors and allosteric effects found in nature by including extended hydrogen bonding (H-bonding) networks, and through-space electrostatic effects. This strategy has seen successful application in electrocatalytic proton,8–10 CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11–24 and CO25 reduction as well as CO2 hydrogenation26–32 catalysis. One of the first studies highlighting the role of the outer coordination sphere was for the purpose of electrocatalytic H2 oxidation where [NiII(PCy2NGly2)2]2+ catalysts (where P2N2 = 1,5-diaza-3,7-diphosphacyclooctane) were functionalized with glycine. This was found to facilitate proton transfer from the outer carboxylate groups to the pendant amino groups in the second coordination sphere, enabling electrocatalytic H2 oxidation over a wide range of pH values (0.1–9.0).33 The outer coordination sphere of a homogenous transition metal complex is understandably less well defined than the inner and second coordination spheres. An outer coordination sphere may take the form of bulk solvent and electrolyte and any co-factors dissolved therein, each of which can potentially participate with the inner and second coordination sphere of a transition metal complex along its reaction coordinate.19,22,34,35 For example, the 1-ethyl-3-methylimidazolium tetracyanoborate, [EMIm][TCB], ionic liquid electrolyte was found to increase the rate of CO2 to CO reduction for the [fac-ReCl(2,2′-bipyridine)(CO)3] pre-catalyst at a reduced overpotential through an outer sphere [EMIm]+|bpy˙− π-stacking interaction.12 Gotico et al. later demonstrated a similar rate enhancement upon covalently incorporating a urea second coordination sphere at an Fe(III)Cl meso-tetraphenylporphyrin pre-catalyst.17 This was later attributed to multipoint hydrogen-bond templating of the bicarbonate anion in the second coordination sphere at the rate-determining C–OH bond-cleavage transition state.36 Also noteworthy is how Liu and McCrory encapsulated the cobalt phthalocyanine (CoPc) complex within a hydrophobic poly-4-vinylpyridine (P4VP) membrane thereby increasing CO product selectivity through optimized proton delivery in the outer coordination sphere.18,37 Williams et al. similarly enhanced CO2 reduction rates by introducing triazolium proton relays at the periphery of a related zinc porphyrin catalyst.20 It should never be overlooked, however, that second and outer coordination sphere effects at any catalytically active transition metal site are primarily dependent upon the core catalyst itself, i.e. the active metal site and its inner coordination sphere; and in the case of proton-coupled electron transfer (PCET) based catalysts, the proton donor activity and its transition state participation play another key role. In particular, group VII Mn(I) and Re(I) polypyridyl tricarbonyl catalysts have played a pivotal part in the study of second coordination sphere effects for CO2 reduction.38–59 The four Mn(I) bipyridyl tricarbonyl complexes presented in this study each contain an ortho-arylester second coordination sphere functional group at the 6,6′-positions of a [fac-MnI(R2-bpy)(CO)3(CH3CN)]+ pre-catalyst. This is inspired by our prior work that established the capability of an ortho-arylether second coordination sphere at promoting the low overpotential protonation-first pathway for electrocatalytic CO2 to CO conversion by this highly popular class of homogenous molecular catalyst.43 Switching to the o-arylester second coordination sphere maintains this low overpotential catalyst activation, whilst also allowing for facile extension beyond the second coordination sphere functionality. In reference to the o-phenylacetate substituted bpy ligand of [1-CH3CN]+, the tert-butyloxycarbonyl protected alanine (N-boc-ala) and o-methoxybenzoate groups of pre-catalysts [2-CH3CN]+ and [3-CH3CN]+, were here chosen to investigate how extension of the second coordination sphere H-bonding environment impacts the thermodynamic and kinetic performance of the catalyst as well as its product selectivity (Chart 1). The 2,6-dimethylphenylester group was also studied in [4-CH3CN]+ to probe any steric influence extending from the o-arylester second coordination sphere in this series of catalysts. It is important to note that, due to functionalization of the 6,6′-phenyl rings of the bpy ligands at just a single ortho-position, upon coordination at Mn a mixture of syn,syn, syn,anti and anti,anti atropisomers are possible with respect to the stereochemistry of the o-phenyl substituents and the Mn–CH3CN site where CO2 and H+ binding, and catalytic activity is anticipated to occur.
11–24 and CO25 reduction as well as CO2 hydrogenation26–32 catalysis. One of the first studies highlighting the role of the outer coordination sphere was for the purpose of electrocatalytic H2 oxidation where [NiII(PCy2NGly2)2]2+ catalysts (where P2N2 = 1,5-diaza-3,7-diphosphacyclooctane) were functionalized with glycine. This was found to facilitate proton transfer from the outer carboxylate groups to the pendant amino groups in the second coordination sphere, enabling electrocatalytic H2 oxidation over a wide range of pH values (0.1–9.0).33 The outer coordination sphere of a homogenous transition metal complex is understandably less well defined than the inner and second coordination spheres. An outer coordination sphere may take the form of bulk solvent and electrolyte and any co-factors dissolved therein, each of which can potentially participate with the inner and second coordination sphere of a transition metal complex along its reaction coordinate.19,22,34,35 For example, the 1-ethyl-3-methylimidazolium tetracyanoborate, [EMIm][TCB], ionic liquid electrolyte was found to increase the rate of CO2 to CO reduction for the [fac-ReCl(2,2′-bipyridine)(CO)3] pre-catalyst at a reduced overpotential through an outer sphere [EMIm]+|bpy˙− π-stacking interaction.12 Gotico et al. later demonstrated a similar rate enhancement upon covalently incorporating a urea second coordination sphere at an Fe(III)Cl meso-tetraphenylporphyrin pre-catalyst.17 This was later attributed to multipoint hydrogen-bond templating of the bicarbonate anion in the second coordination sphere at the rate-determining C–OH bond-cleavage transition state.36 Also noteworthy is how Liu and McCrory encapsulated the cobalt phthalocyanine (CoPc) complex within a hydrophobic poly-4-vinylpyridine (P4VP) membrane thereby increasing CO product selectivity through optimized proton delivery in the outer coordination sphere.18,37 Williams et al. similarly enhanced CO2 reduction rates by introducing triazolium proton relays at the periphery of a related zinc porphyrin catalyst.20 It should never be overlooked, however, that second and outer coordination sphere effects at any catalytically active transition metal site are primarily dependent upon the core catalyst itself, i.e. the active metal site and its inner coordination sphere; and in the case of proton-coupled electron transfer (PCET) based catalysts, the proton donor activity and its transition state participation play another key role. In particular, group VII Mn(I) and Re(I) polypyridyl tricarbonyl catalysts have played a pivotal part in the study of second coordination sphere effects for CO2 reduction.38–59 The four Mn(I) bipyridyl tricarbonyl complexes presented in this study each contain an ortho-arylester second coordination sphere functional group at the 6,6′-positions of a [fac-MnI(R2-bpy)(CO)3(CH3CN)]+ pre-catalyst. This is inspired by our prior work that established the capability of an ortho-arylether second coordination sphere at promoting the low overpotential protonation-first pathway for electrocatalytic CO2 to CO conversion by this highly popular class of homogenous molecular catalyst.43 Switching to the o-arylester second coordination sphere maintains this low overpotential catalyst activation, whilst also allowing for facile extension beyond the second coordination sphere functionality. In reference to the o-phenylacetate substituted bpy ligand of [1-CH3CN]+, the tert-butyloxycarbonyl protected alanine (N-boc-ala) and o-methoxybenzoate groups of pre-catalysts [2-CH3CN]+ and [3-CH3CN]+, were here chosen to investigate how extension of the second coordination sphere H-bonding environment impacts the thermodynamic and kinetic performance of the catalyst as well as its product selectivity (Chart 1). The 2,6-dimethylphenylester group was also studied in [4-CH3CN]+ to probe any steric influence extending from the o-arylester second coordination sphere in this series of catalysts. It is important to note that, due to functionalization of the 6,6′-phenyl rings of the bpy ligands at just a single ortho-position, upon coordination at Mn a mixture of syn,syn, syn,anti and anti,anti atropisomers are possible with respect to the stereochemistry of the o-phenyl substituents and the Mn–CH3CN site where CO2 and H+ binding, and catalytic activity is anticipated to occur.
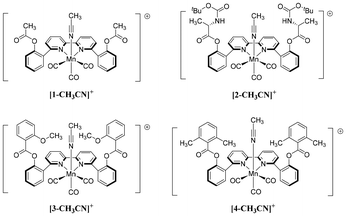 |
| | Chart 1 Manganese(I) facial tricarbonyl pre-catalyst complexes with 6,6′-substituted 2,2′-bipyridine ligands used in this study. For brevity, only the syn,syn-atropisomers are drawn. | |
Results
Synthesis
All 6,6′-disubstituted bipyridine ligands are previously unreported and prepared by conducting Steglich esterification on the 6,6′-bis(2-hydroxyphenyl)-2,2′-bipyridine intermediate using dicyclohexyl carbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) with either acetic acid, N-boc-alanine, o-methoxybenzoic acid or 2,6-dimethylbenzoic acid in dichloromethane solvent at room temperature (Scheme 1).
 |
| | Scheme 1 Summary of the reaction sequence employed for ligand synthesis. | |
Synthesis of the [fac-MnI(OTf)(N^N)(CO)3] complexes was conducted following a previously published procedure where freshly prepared [MnI(OTf)(CO)5] and the respective ligand were heated in weakly coordinating THF to give the target compounds in good yields.43 A full description of all synthetic procedures is provided in the ESI.† Once dissolved in acetonitrile, the weakly coordinating triflate anion is displaced by a solvent molecule, thus all complexes are forthwith referred to as [1-CH3CN]+, [2-CH3CN]+, [3-CH3CN]+ and [4-CH3CN]+. As discussed above, a mixture of atropisomers are likely with respect to the stereochemical location of the o-phenyl substituents and the Mn–CH3CN solvation site where CO2 binding at catalytic activity is anticipated to occur. This is evident in the 1H NMR spectra of all Mn complexes. For example, the terminal methyl moieties of the acetate groups in [1-CH3CN]+ appears as two closely spaced singlets (ESI Fig. 7†), the alanine –NH and –CH protons in [2-CH3CN]+ appear as multiplets (ESI Fig. 10†), while the spectra for the bulkier [3-CH3CN]+ and [4-CH3CN]+ appear broad an unresolved (ESI Figs. 13 and 16†).
FTIR spectroscopy
Each of the [fac-Mn(N^N)(CO)3(CH3CN)]+ solvated complexes exhibit almost identical v(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) IR absorption bands, characteristic of the fac-tricarbonyl manganese core, with a symmetric stretch at 2044 cm−1 and a broad asymmetric v(C
O) IR absorption bands, characteristic of the fac-tricarbonyl manganese core, with a symmetric stretch at 2044 cm−1 and a broad asymmetric v(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) stretch at lower wavenumber 1957–1958 cm−1. [1-CH3CN]+ appears to impart a slight descent in symmetry from a C3 to Cs point group as its low wavenumber v(C
O) stretch at lower wavenumber 1957–1958 cm−1. [1-CH3CN]+ appears to impart a slight descent in symmetry from a C3 to Cs point group as its low wavenumber v(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) band is almost resolved into two individual peaks evident at 1958 and 1949(sh) cm−1. Characteristic v(C
O) band is almost resolved into two individual peaks evident at 1958 and 1949(sh) cm−1. Characteristic v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretches of each o-arylester group at the bpy ligands are also observed for all four complexes. [1-CH3CN]+ exhibits a v(C
O) stretches of each o-arylester group at the bpy ligands are also observed for all four complexes. [1-CH3CN]+ exhibits a v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretch at 1768 cm−1, with a shoulder at 1752 cm−1. A similar absorption profile is exhibited by [3-CH3CN]+ at 1747 and 1729(sh) cm−1, and [4-CH3CN]+ at 1769 and 1755(sh) cm−1. The shoulder observed for each of these v(C
O) stretch at 1768 cm−1, with a shoulder at 1752 cm−1. A similar absorption profile is exhibited by [3-CH3CN]+ at 1747 and 1729(sh) cm−1, and [4-CH3CN]+ at 1769 and 1755(sh) cm−1. The shoulder observed for each of these v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretches is tentatively attributed to the presence of atropisomers as suggested also by their 1H NMR spectra. [2-CH3CN]+ is unique in that it exhibits two independent stretches at 1772 and 1713 cm−1 attributed to the o-arylester and N-boc ester groups, respectively.60 FTIR spectra are illustrated in Fig. 1 and tabulated below alongside infrared spectroelectrochemical (IRSEC) data (Table 2).
O) stretches is tentatively attributed to the presence of atropisomers as suggested also by their 1H NMR spectra. [2-CH3CN]+ is unique in that it exhibits two independent stretches at 1772 and 1713 cm−1 attributed to the o-arylester and N-boc ester groups, respectively.60 FTIR spectra are illustrated in Fig. 1 and tabulated below alongside infrared spectroelectrochemical (IRSEC) data (Table 2).
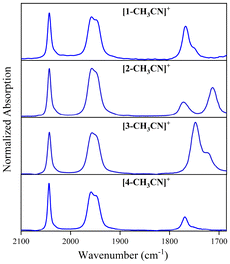 |
| | Fig. 1 FTIR absorption spectra of all four recorded in acetonitrile. | |
Voltammetry under 1 atm Ar
We have previously established that the benchmark [MnI(bpy)(CO)3(CH3CN)]+ pre-catalyst, and its 6,6′-subsituted derivatives, exhibit a two-electron activation at the first reduction wave (eqn (1)–(3)).56 For brevity the core five-coordinate [fac-Mn(R2bpy)(CO)3] structure is abbreviated here as [Mn] with [Mn–X] indicating the variable sixth coordination site in eqn (1)–(5). The two-electron reduced [MnI(bpy)(CO)3]− product, lacking any steric bulk, undergoes a subsequent bimolecular parent-child comproportionation chemical reaction to form the [Mn–Mn]0 dimer (eqn (4)).53,61 A second reduction wave occurs at −1.83 V for [MnI(bpy)(CO)3(CH3CN)]+ attributed to reduction of this dimer for quantitative formation of the two-electron reduced five-coordinate 18-valence electron active catalyst [Mn]− (eqn (5)).| | | [Mn–CH3CN]+ + e− ⇌ [Mn–CH3CN]0 | (1) |
| | | [Mn–CH3CN]0 ⇌ [Mn]0 + CH3CN | (2) |
| | | [Mn–CH3CN]+ + [Mn]− ⇌ [Mn–Mn]0 + CH3CN | (4) |
| | | [Mn–Mn]0 + 2e− ⇌ 2[Mn]− | (5) |
Consistent with prior literature, the presence of steric bulk in complexes [1-CH3CN]+–[4-CH3CN]+ from their 6,6′-bpy aryl substituents prevents the comproportionation reaction (eqn (4)) mitigating any [Mn–Mn]0 dimer formation.43,48,56–59,62 The assignment of a two-electron reduction to the first reduction wave of all four complexes was also confirmed by IRSEC studies presented below. Scan rate dependent voltammetry of this reduction wave confirms its reversibility for [1-CH3CN]+ and quasi-reversibility for the remaining complexes (ESI Fig. 17–24†). The slight negative shifts relative to the [MnI(bpy)(CO)3(CH3CN)]+ pre-catalyst is consistent with an inductive donating influence of the 6,6′-bisaryl substituents raising their bpy(π*) LUMO levels by 0.07–0.08 eV. A third reduction is observed at more negative potentials. This is likely irrelevant for electrocatalysis investigations presented below, at least for complexes [1-CH3CN]+ and [2-CH3CN]+. However, the positive shift of this third reduction for [3-CH3CN]+ and the presence of three additional reduction peaks for [4-CH3CN]+ is perhaps related to reduction of their more conjugated ester groups. This appears to have a strong impact on catalysis investigations (vide infra). A summary of all electrochemical data is provided in Table 1, with cyclic voltammograms recorded under non-catalytic conditions (0.1 M [Bu4N][PF6] acetonitrile supporting electrolyte under 1 atm of argon), presented in Fig. 2.
 |
| | Fig. 2 Cyclic voltammograms of 1 mM [1-CH3CN]+, [2-CH3CN]+, [3-CH3CN]+ and [4-CH3CN]+ recorded in argon-saturated 0.1 M [Bu4N][PF6] acetonitrile supporting electrolyte at a glassy carbon electrode with a scan rate of 0.1 V s−1. | |
Table 1 Electrochemical data derived from cyclic voltammetry of 1 mM catalysts solutions in 0.1 M [Bu4N][PF6] acetonitrile electrolyte under 1 atm of argon at a glassy carbon disc working electrode. All potentials were recorded at υ = 0.1 V s−1 and are reported versus the ferricenium/ferrocene (Fc+/0) redox couple
| |
E
pa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
E
pa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
E
pc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
E
pc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
|
Irreversible Mn(I/II).
Oxidation of the two-electron reduced [Mn]− species.
Two-electron reduction.
Irreversible.
|
|
[1-CH3CN]+
|
+1.00 |
−1.50 |
−1.55 |
−2.78 |
|
[2-CH3CN]+
|
+1.04 |
−1.52 |
−1.55 |
−2.85 |
|
[3-CH3CN]+
|
+0.73 |
−1.51 |
−1.56 |
−2.48 |
|
[4-CH3CN]+
|
+0.82 |
−1.50 |
−1.54 |
−1.90, −2.28, −2.80 |
Infrared spectroelectrochemistry
IRSEC experiments were conducted to confirm the mechanism by which catalyst activation is occurring. Evidence of the six-coordinate, one-electron reduced neutral species [1-CH3CN]0 and [2-CH3CN]0 was observed en route to quantitative formation of their two-electron reduced active catalyst derivatives confirming an ECE activation mechanism (eqn (1)–(3)). Unfortunately, the quality of IRSEC spectra was poor due to reduced solubility of all reduction products for each pre-catalyst. For this reason, identification of [3-CH3CN]0 and [4-CH3CN]0 was not feasible. Exemplar IRSEC spectra are provided in Fig. 3 for the N-boc-ala functionalized pre-catalyst [2-CH3CN]+.
 |
| | Fig. 3 IRSEC results upon controlled potential electrolysis of [2-CH3CN]+ recorded in argon-saturated 0.1 M [Bu4N][PF6] acetonitrile supporting electrolyte. | |
Initial electrolysis of [2-CH3CN]+ at −1.53 V exhibits depletion of the parent complex at 2043 and 1957 cm−1 with concurrent grow-in of the one-electron reduced six-coordinate species [2-CH3CN]0 at 2023, 1931 and 1910 cm−1 (eqn (1)). Weak evidence of the two-electron reduced species [2]− appeared to grow-in over an extended electrolysis time at 1807 cm−1. Quantitative formation of [2]− was observed at 1895 and 1807 cm−1 by applying a more negative potential of −2.00 V. A weak depletion of the parent N-boc v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretches was also observed upon formation of [2]−, however this region of the spectrum was mostly obscured by strong electrolyte absorption. Comparable IRSEC results were recorded for all four complexes (Table 2) and is provided in the ESI (Fig. 25–27†).
O) stretches was also observed upon formation of [2]−, however this region of the spectrum was mostly obscured by strong electrolyte absorption. Comparable IRSEC results were recorded for all four complexes (Table 2) and is provided in the ESI (Fig. 25–27†).
Table 2 IRSEC-derived FTIR absorption data recorded in 0.1 M [Bu4N][PF6] acetonitrile electrolyte for all complexes, summarizing v(CO) stretching frequencies
| |
v(CO) cm−1 |
Low energy ligand v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) absorption bands are obscured by strong electrolyte absorption for one- and two-electron reduced species in IRSEC experiments. O) absorption bands are obscured by strong electrolyte absorption for one- and two-electron reduced species in IRSEC experiments.
|
|
[1-CH3CN]+
|
2043, 1958, 1949(sh), 1768a, 1752(sh)a |
|
[1-CH3CN]0
|
2023, 1925 |
|
[1]−
|
1911, 1812 |
|
[2-CH3CN]+
|
2043, 1957, 1772a, 1713a |
|
[2-CH3CN]0
|
2023, 1931, 1910 |
|
[2]−
|
1895, 1807 |
|
[3-CH3CN]+
|
2043, 1957, 1747a, 1729(sh)a |
|
[3]−
|
1912, 1808 |
|
[4-CH3CN]+
|
2044, 1958, 1948(sh), 1769a, 1755(sh)a |
|
[4]−
|
1913, 1813 |
Voltammetry under 1 atm CO2 with Brønsted acid
Each catalyst was studied under 1 atm CO2 in the presence of optimum H2O and PhOH. Brønsted acid titration experiments were performed to determine the concentration of each acid at which proton dependence would not be rate-limiting. Furthermore, once optimum Brønsted acid concentrations were reached, scan rate dependence experiments were performed to reach steady-state conditions at which CO2 consumption is also not rate-limiting to allow determination of the maximum turnover frequencies (TOFmax). The turnover frequency was determined using the ratio of catalytic current (icat) and non-catalytic faradaic current (ip) according to eqn (6)| |  | (6) |
where F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 s A mol−1), υ is the scan rate (V s−1), R is the universal gas constant (8.3145 V A s K−1 mol−1), T is the temperature (298 K).63 The first reduction wave was used as a reference point for both ip and np (i.e. np = 2 for a two electron reduction). The number of electrons required for a single catalytic cycle is equivalent for each of the CO, HCO2− and H2 producing catalytic cycles (i.e. ncat = 2). The maximum turnover frequency (TOFmax) was estimated by plotting TOF vs. scan rate.
485 s A mol−1), υ is the scan rate (V s−1), R is the universal gas constant (8.3145 V A s K−1 mol−1), T is the temperature (298 K).63 The first reduction wave was used as a reference point for both ip and np (i.e. np = 2 for a two electron reduction). The number of electrons required for a single catalytic cycle is equivalent for each of the CO, HCO2− and H2 producing catalytic cycles (i.e. ncat = 2). The maximum turnover frequency (TOFmax) was estimated by plotting TOF vs. scan rate.
As discussed above, all four catalysts are activated by a two-electron reduction to the five coordinate 18-electron [Mn]− anions, primed for CO2 and/or proton binding to form the key [Mn–CO2H] and [Mn–H] intermediates. Optimum voltammetry at υ = 0.1 V s−1 for all four catalysts comparing both H2O and PhOH proton sources is summarized in Table 4 below and illustrated in Fig. 4. Two catalytic waves grow-in in the presence of optimum PhOH (1.5 M) as the proton source. Consistent with our prior studies using a bulky proton donor, growth of the low-overpotential protonation-first pathway appears to be most prominent for [1-CH3CN]+ and [2-CH3CN]+, which arguably possess lesser steric bulk.
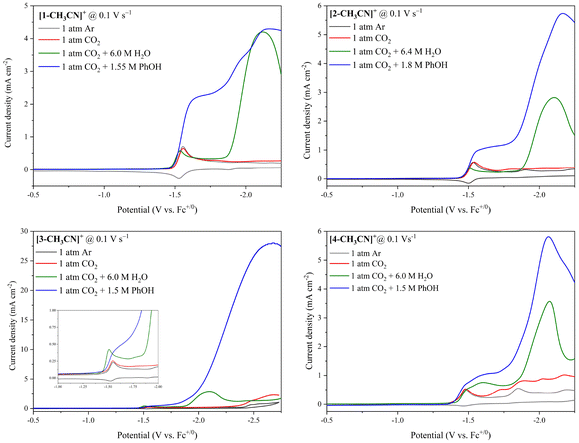 |
| | Fig. 4 Linear sweep voltammetry of all four complexes recorded at 1 mM concentration in 0.1 M [Bu4N][PF6] acetonitrile electrolyte. Each plot includes cyclic voltammetry under 1 atm Ar, overlaid with linear sweep voltammetry in CO2 saturated electrolyte with the addition of optimum concentrations of H2O and PhOH. | |
Controlled potential electrolysis
To probe the product selectivity of each catalyst, controlled potential electrolysis (CPE) experiments were conducted at Ecat/2 of each catalytic wave under 1 atm CO2 in the presence of an optimum concentration of H2O or PhOH Brønsted acid proton sources. A summary of CPE parameters and faradaic yields is presented below in Table 3.
Table 3 Summary of controlled potential electrolysis data. Experimental conditions: 5 mL of 1 mM catalyst in 0.1 M [Bu4N][PF6] acetonitrile supporting electrolyte with optimum H2O and PhOH concentrations under 1 atm CO2
| |
HAa (M) |
E
applied (V vs. Fc+/0) |
Faradaic yield CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCO2− HCO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 (%) H2 (%) |
|
Refers to the concentration of Brønsted acid [HA] not be confused with [H+].
E
applied was taken from the peak maximum due to the weak catalytic current.
|
|
[1-CH3CN]+
|
H2O (6.0 M) |
−1.96 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| PhOH (1.5 M) |
−1.55 |
97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| PhOH (1.5 M) |
−1.93 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
|
[2-CH3CN]+
|
H2O (6.4 M) |
−1.96 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| PhOH (1.8 M) |
−1.51 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| PhOH (1.8 M) |
−1.94 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
[3-CH3CN]+
|
H2O (6.0 M) |
−1.60b |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| H2O (6.0 M) |
−1.96 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 58 |
| PhOH (1.5 M) |
−1.50 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| PhOH (1.5 M) |
−2.25 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
|
[4-CH3CN]+
|
H2O (6.0 M) |
−1.92 |
63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
| PhOH (1.5 M) |
−1.60b |
66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| PhOH (1.5 M) |
−1.93 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
Table 4 Summary of electrocatalysis data derived from linear sweep voltammogram experiments for both low and high overpotential pathways
| |
|
[1-CH3CN]+
|
[2-CH3CN]+
|
[3-CH3CN]+
|
[4-CH3CN]+
|
| H2O |
PhOH |
H2O |
PhOH |
H2O |
PhOH |
H2O |
PhOH |
| Low η |
E
cat/2 (V) |
— |
−1.55 |
— |
−1.51 |
−1.47 |
−1.50 |
— |
−1.45 |
| [HA] (M) |
— |
1.5 |
— |
1.8 |
6.0 |
1.5 |
— |
1.5 |
| TOFmax (s−1) |
— |
58 |
— |
35 |
6 |
5 |
— |
82 |
| High η |
E
cat/2 (V) |
−1.96 |
−1.93 |
−1.96 |
−1.94 |
−1.96 |
−2.25 |
−1.92 |
−1.93 |
| [HA] (M) |
6.0 |
1.5 |
6.4 |
1.8 |
6.0 |
1.5 |
6.0 |
1.5 |
| TOFmax (s−1) |
167 |
235 |
127 |
598 |
78 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 451 451 |
586 |
1846 |
Of the four catalysts investigated only the simple methylester second coordination sphere functional group in [1-CH3CN]+ maintains a high selectivity for CO production across all CPE experimental conditions. Specifically, [1-CH3CN]+ exhibits FECO = 99% at high-overpotential (Ecat/2 = −1.96 V) in the presence of 6.0 M H2O. In the presence of just 1.5 M PhOH, [1-CH3CN]+ exhibits FECO = 97% and 100% at its low overpotential (Ecat/2 = −1.55 V) and high overpotential (Ecat/2 = −1.93 V) catalytic waves, respectively. With such high selectivity for CO production the low and high overpotential catalytic waves of [1-CH3CN]+ can be definitively attributed to the PT-ET (aka protonation-first) and ET-PT (aka reduction-first) pathways of the CO2-to-CO conversion catalytic cycle (re. Scheme 2 below). This observation further emphasizes the unique capacity of an o-aryl aprotic O-atom Brønsted base at promoting the highly desired low-overpotential PT-ET pathway for CO2-to-CO conversion by Mn polypyridyl electrocatalysts.58
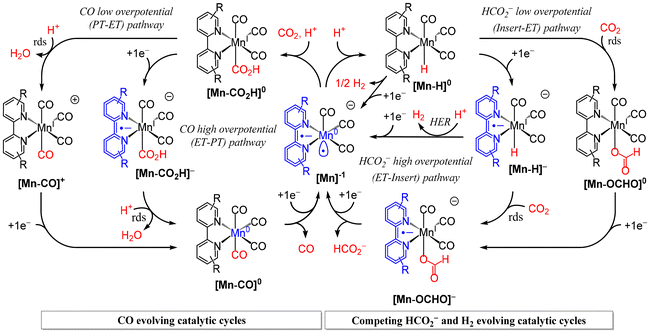 |
| | Scheme 2 A summary of competing catalytic pathways exhibited by the Mn polypyridyl class of CO2 reduction electrocatalysts for CO, HCO2− and H2 production. Left side: Low-overpotential (PT-ET) and high-overpotential (ET-PT) CO evolving pathways. Right side: Low-overpotential (insert-ET) and high-overpotential (ET-insert) pathways for HCO2− production, and competing hydrogen evolution reaction (HER) pathways. | |
Discussion
To correctly discern the data here presented the relevant competing catalytic pathways exhibited by the Mn polypyridyl class of CO2 reduction electrocatalysts must first be fully appreciated. Presented below are the established low- and high-overpotential catalytic pathways for each of the CO, HCO2− and H2 products generated from the two-electron activated catalyst [Mn]−; for brevity the core five-coordinate [fac-Mn(R2bpy)(CO)3] structure is abbreviated as [Mn] with [Mn–X] indicating the variable sixth coordination site throughout the catalytic cycle (Scheme 2). Formal Mn and R2bpy oxidation states throughout the catalytic cycle are also illustrated in Scheme 2. The CO producing pathways originate from CO2 binding to the active catalyst [Mn]−, in the presence of a proton source, to generate the metallocarboxylic acid intermediate [Mn–CO2H]0. This intermediate may directly undergo rate-determining proton induced C–OH bond cleavage to evolve H2O alongside the [MnI–CO]+ cation. Reduction of this cation to [MnI–CO]0 requires a potential less negative than initial catalyst activation, hence the onset of catalytic current for this low-overpotential PT-ET (aka protonation-first) pathway for CO evolution being coincident with the concerted two-electron catalyst activation reduction wave. The corresponding high-overpotential ET-PT (aka reduction-first) CO producing pathway exhibits an onset of catalytic current at higher overpotential concomitant with one-electron reduction of the metallocarboxylic acid intermediate to generate the [Mn–CO2H]− anion. Rate-determining proton induced C–OH bond cleavage at the [Mn–CO2H]− anion is more facile hence the consistently higher catalytic rates for this ET-PT pathway relative to is low-overpotential PT-ET pathway. Should protonation of the [Mn]− active catalyst be favoured over CO2 binding, [Mn–H]0 is formed initiating entry to the competing HCO2− and H2 evolving pathways. CO2 insertion can occur, prior to reduction, along the low-overpotential (insert-ET) pathway, with an overpotential dictated by the [Mn–CO2H]0/− reduction potential. Alternatively, the high-overpotential HCO2− producing pathway has an overpotential dictated by the [Mn–H]0/− reduction, followed by CO2 insertion (ET-insert). The low-overpotential insert-ET formate pathway is kinetically inferior but has the advantage of eliminating competitive H2 production relative to protonation of the anionic [Mn–H]− intermediate along the ET-insert pathway. It should also be noted that bimolecular H2 production is known to occur from two equivalents of the neutral [Mn–H]0 intermediate, at least with the non-sterically hindered bpy ligand.12
The first take away from this study is how the simplest o-arylester second coordination sphere functionality in pre-catalyst [1-CH3CN]+ exhibits a rare example of the highly sought after low overpotential PT-ET catalytic pathway for selective CO production. However, despite the presence of an optimized PhOH concentration (1.5 M), the TOFmax values for both its low overpotential PT-ET (TOFmax = 58 s−1, Ecat/2 = −1.55 V) and high overpotential ET-PT (TOFmax = 235 s−1, Ecat/2 = −1.93 V) CO producing pathways are relatively low in comparison to our previously reported o-arylether analogues. That being said, the goal of this study was primarily to probe the impact of extending this second coordination sphere functionality on catalyst performance from the perspective of thermodynamics, kinetics and selectivity.
Second coordination sphere extension with the N-boc arylester system in pre-catalyst [2-CH3CN]+ also exhibits highly selective CO production at low overpotential consistent with the PT-ET pathway in the presence of 1.8 M PhOH (TOFmax = 35 s−1, Ecat/2 = −1.51 V). The slight reduction in catalytic rate relative to [1-CH3CN]+ is likely due to steric bulk and crowding of the catalyst active site. Intriguingly, however, at higher overpotential (Ecat/2 = −1.94 V) under otherwise identical conditions this same catalyst exhibits an almost equal faradaic yield for HCO2− production with negligible evidence for H2 evolution. This suggests an almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split with respect to the selectivity of the activated catalyst [2]− at binding CO2 or H+ to form the critical metallocarboxylic acid [2-CO2H] or metal-hydride [2-H] intermediates along the CO or HCO2−vs. H2 catalytic pathways (Scheme 2). [2-CH3CN]+ exhibits a very similar product distribution of
1 split with respect to the selectivity of the activated catalyst [2]− at binding CO2 or H+ to form the critical metallocarboxylic acid [2-CO2H] or metal-hydride [2-H] intermediates along the CO or HCO2−vs. H2 catalytic pathways (Scheme 2). [2-CH3CN]+ exhibits a very similar product distribution of 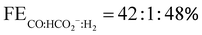 in the presence of optimum H2O (6.4 M) at high overpotential (Ecat/2 = −1.96 V). Of great significant for each of these high overpotential observations of equitable CO vs. HCO2− formation is the negligible production of H2. As the high-overpotentials required are consistent with HCO2− production along the ET-insert pathway, via the one-electron reduced manganese hydride intermediate [2-H]−, this suggests that the N-boc terminated o-arylester second coordination sphere of [2-CH3CN]+ favors CO2 insertion over protonation of [2-H]− to maintain a high selectivity for HCO2− production. This observation is indeed consistent with related complexes which also contain pendant amino functionalities in their SCS.19,22,48 Estimated hydricities of the neutral [Mn–H] catalyst, using Kubiak's linear correlation method with two-electron reduction potentials, of all four complexes are in the range of
in the presence of optimum H2O (6.4 M) at high overpotential (Ecat/2 = −1.96 V). Of great significant for each of these high overpotential observations of equitable CO vs. HCO2− formation is the negligible production of H2. As the high-overpotentials required are consistent with HCO2− production along the ET-insert pathway, via the one-electron reduced manganese hydride intermediate [2-H]−, this suggests that the N-boc terminated o-arylester second coordination sphere of [2-CH3CN]+ favors CO2 insertion over protonation of [2-H]− to maintain a high selectivity for HCO2− production. This observation is indeed consistent with related complexes which also contain pendant amino functionalities in their SCS.19,22,48 Estimated hydricities of the neutral [Mn–H] catalyst, using Kubiak's linear correlation method with two-electron reduction potentials, of all four complexes are in the range of  .64 This is slightly endergonic of the 44 kcal mol−1 assumed necessary for HCO2− formation and may also explain the low yields of H2 at low overpotential. Hydricity of the reduced [Mn–H]− intermediate has been estimated at ∼14 kcal mol−1 for a catalyst that exhibited 71% HCO2− selectivity and which bears pendant amine second coordination sphere, comparable in many respects to [2-CH3CN]+.48
.64 This is slightly endergonic of the 44 kcal mol−1 assumed necessary for HCO2− formation and may also explain the low yields of H2 at low overpotential. Hydricity of the reduced [Mn–H]− intermediate has been estimated at ∼14 kcal mol−1 for a catalyst that exhibited 71% HCO2− selectivity and which bears pendant amine second coordination sphere, comparable in many respects to [2-CH3CN]+.48
In contrast, the decreased selectivity imparted by the o-methoxybenzoate second coordination sphere in [3-CH3CN]+ relative to the N-boc arylester in [2-CH3CN]+, in the presence of either PhOH or H2O, is striking. It is also worth noting the more negative Ecat/2 of the large high-overpotential catalytic wave of [3-CH3CN]+ at −2.25 V in the presence of PhOH. Considering how the three-electron reduced derivative may be accessed at Epc = −2.48 V (Table 1) this contrasting reactivity could possibly be associated with a three-electron activated catalyst thereby mitigating any desired control of product selectivity. However, an alternative explanation may be that the product selectivity of [3-CH3CN]+ is simply dictated by the steric bulk of the extended second coordination sphere structure with limited influence of the second coordination sphere.57 Controlled potential electrolysis studies of the bulky [4-CH3CN]+ catalyst do indicate that CO2 binding is favoured but significant H2 evolution is still observed in this case. Although computed transition state geometries suggest the Mn active site is accessible in the syn,anti atropisomers of [3-CH3CN]+ and [4-CH3CN]+ (ESI Fig. 42–47†), not knowing the ratio of atropisomers in solution prevents any real conclusions to be made at this time with respect to the balance of steric vs. second coordination sphere effects on their product distribution.
To further investigate the influence of extended functionality at the second-coordination sphere, we performed density functional theory (DFT) calculations at the MN15 level of theory in conjunction with the SMD continuum solvation model for acetonitrile. For computational efficiency only the syn,anti atropisomers were calculated, and the terminal tert-butyl substituents of the N-boc protecting groups were modelled as methyl groups (see Computational methods in the ESI† for details). Using theory to predict product selectivities at high overpotential is extremely challenging due to the competition between several pathways leading to CO, HCO2− and H2 production. This is further compounded by the unknown ratio of atropisomers in each CPE experiment which likely exhibit varying product distributions. Thus, our primary aim in utilizing theoretical calculations in this work focussed on a qualitative assessment for the disparate product selectivities of just [2-CH3CN]+ and [3-CH3CN]+. The energetics of their transition state structures for [Mn–CO2]− and [Mn–H] formation, and Insert-ET versus ET-insert pathways for HCO2− and H2 production, were analyzed. The free energy of activation (ΔG‡) values, assuming fully separated reactants in solution, are illustrated in Fig. 5 for [2-CH3CN]+, (for tabulated data vs.[3-CH3CN]+ see ESI Table 1†).
 |
| | Fig. 5 A comparison of relative free energies of activation (ΔG‡) values in kcal mol−1 for selected kinetic steps in Scheme 2 for [2-CH3CN]+ with PhOH as the Brønsted acid source. | |
The computed ΔG‡s indicate that CO2 binding to doubly reduced [2]− is significantly favored over protonation by PhOH to generate [2-H], however, the activation enthalpies (ESI Table 1,† ΔH‡) are nearly isothermic which is more consistent with the almost equal product distribution of CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCO2− in controlled potential electrolysis. Once the [2-H]− intermediate is formed, HCO2− formation by electrophilic attack of CO2 is favored over H2 formation in line with our experimental observations (Fig. 5c). Theoretical data for [3-CH3CN]+ was inconsistent with our CPE experiments, however, as pointed out earlier, this may likely be due to three-electron activation of pre-catalyst [3-CH3CN]+ leading to excessive H2 production, or alternatively steric crowding of the active site hindering CO2 binding at [3]− or insertion at [3-H]− thereby enhancing the H2 yield.
HCO2− in controlled potential electrolysis. Once the [2-H]− intermediate is formed, HCO2− formation by electrophilic attack of CO2 is favored over H2 formation in line with our experimental observations (Fig. 5c). Theoretical data for [3-CH3CN]+ was inconsistent with our CPE experiments, however, as pointed out earlier, this may likely be due to three-electron activation of pre-catalyst [3-CH3CN]+ leading to excessive H2 production, or alternatively steric crowding of the active site hindering CO2 binding at [3]− or insertion at [3-H]− thereby enhancing the H2 yield.
Conclusions
This study demonstrates that the introduction of distal, outer coordination sphere, H-bonding functionality at the periphery of a homogeneous transition metal complex active site, can have a strong influence on product selectivity for proton-coupled electrocatalytic CO2 reduction. Although the bulky o-methoxybenzoate group diminishes the product selectivity for CO2 reduction, extension of the CO selective acetate SCS with the tertiary amine N-Boc-ala group shifts the reaction pathway toward a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the [Mn–CO2H] and [Mn–H] intermediates with a surprising selectivity for CO2 insertion at [Mn–H] along the competing HCO2−vs. H2 pathways as corroborated by computational studies.
1 ratio of the [Mn–CO2H] and [Mn–H] intermediates with a surprising selectivity for CO2 insertion at [Mn–H] along the competing HCO2−vs. H2 pathways as corroborated by computational studies.
Author contributions
The manuscript was drafted through the contributions of all authors: L. S., V. B. and R. S. experimental studies and data analysis; M. Z. E. computational analysis; J. R. data analysis, L. S., V. B. and R. S. supervision and project management.
Data availability
The data supporting this article have been included as part of the ESI.†
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
JR thanks the National Science Foundation for support under Grant No. CHE-1800062. The work at Brookhaven National Laboratory (M. Z. E.) was carried out under contract DE-SC0012704 with the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, and utilized computational resources at the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, and the Scientific Data and Computing Center, a component of the Computational Science Initiative, at Brookhaven National Laboratory under Contract No. DE-SC0012704.
References
- H. Takeda, C. Cometto, O. Ishitani and M. Robert, ACS Catal., 2017, 7, 70–88 CrossRef CAS.
- A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. DuBois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer and G. L. Waldrop, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- F. Franco, C. Rettenmaier, H. S. Jeon and B. Roldan Cuenya, Chem. Soc. Rev., 2020, 49, 6884–6946 RSC.
- A. W. Nichols and C. W. Machan, Front. Chem., 2019, 7, 397 CrossRef CAS PubMed.
- S. Amanullah, P. Saha, A. Nayek, M. E. Ahmed and A. Dey, Chem. Soc. Rev., 2021, 50, 3755–3823 RSC.
- S. Sinha, C. K. Williams and J. Jiang, iScience, 2022, 25, 103628 CrossRef CAS PubMed.
- S. T. Stripp, B. R. Duffus, V. Fourmond, C. Léger, S. Leimküshler, S. Hirota, Y. L. Hu, A. Jasniewski, H. Ogata and M. W. Ribbe, Chem. Rev., 2022, 122, 11900–11973 CrossRef CAS PubMed.
- M. Rakowski DuBois and D. L. DuBois, Chem. Soc. Rev., 2009, 38, 62–72 RSC.
- S. K. Mandal, S. Ray and J. Choudhury, ChemCatChem, 2024, 16, e202401149 CrossRef.
- S. Diyali, N. Diyali and B. Biswas, Coord. Chem. Rev., 2024, 500, 215496 CrossRef CAS.
- J. P. Collin, A. Jouaiti and J. P. Sauvage, Inorg. Chem., 1988, 27, 1986–1990 CrossRef CAS.
- D. C. Grills, Y. Matsubara, Y. Kuwahara, S. R. Golisz, D. A. Kurtz and B. A. Mello, J. Phys. Chem. Lett., 2014, 5, 2033–2038 CrossRef CAS PubMed.
- W. W. Kramer and C. C. L. McCrory, Chem. Sci., 2016, 7, 2506–2515 RSC.
- J. Honores, D. Quezada, M. García, K. Calfumán, J. P. Muena, M. J. Aguirre, M. C. Arévalo and M. Isaacs, Green Chem., 2017, 19, 1155–1162 RSC.
- E. M. Nichols, J. S. Derrick, S. K. Nistanaki, P. T. Smith and C. J. Chang, Chem. Sci., 2018, 9, 2952–2960 RSC.
- E. M. Nichols and C. J. Chang, Organometallics, 2019, 38, 1213–1218 CrossRef CAS.
- P. Gotico, B. Boitrel, R. Guillot, M. Sircoglou, A. Quaranta, Z. Halime, W. Leibl and A. Aukauloo, Angew. Chem., Int. Ed., 2019, 58, 4504–4509 CrossRef CAS PubMed.
- Y. Liu and C. C. L. McCrory, Nat. Commun., 2019, 10, 1683 CrossRef PubMed.
- M. Bhattacharya, S. Sebghati, R. T. VanderLinden and C. T. Saouma, J. Am. Chem. Soc., 2020, 142, 17589–17597 CrossRef CAS PubMed.
- C. K. Williams, A. Lashgari, J. Chai and J. J. Jiang, ChemSusChem, 2020, 13, 3412–3417 CrossRef CAS PubMed.
- Y. S. Liu, A. Deb, K.
Y. Leung, W. X. Nie, W. S. Dean, J. E. Penner-Hahn and C. C. L. McCrory, Dalton Trans., 2020, 49, 16329–16339 RSC.
- M. R. Madsen, M. H. Rønne, M. Heuschen, D. Golo, M. S. G. Ahlquist, T. Skrydstrup, S. U. Pedersen and K. Daasbjerg, J. Am. Chem. Soc., 2021, 143, 20491–20500 CrossRef CAS PubMed.
- H. J. Dai, R. Cui, C. S. Chen, J. T. Song, J. H. Li, L. Z. Dong, C. Y. Yu, W. F. Jiang and Y. F. Zhou, Chem. – Eur. J., 2023, 29, e202300879 CrossRef CAS PubMed.
- K. Teindl, B. O. Patrick and E. M. Nichols, J. Am. Chem. Soc., 2023, 145, 17176–17186 CrossRef CAS PubMed.
- S. Patra, S. Bhunia, S. Ghosh and A. Dey, ACS Catal., 2024, 14, 7299–7307 CrossRef CAS.
- J. T. Bays, N. Priyadarshani, M. S. Jeletic, E. B. Hulley, D. L. Miller, J. C. Linehan and W. J. Shaw, ACS Catal., 2014, 4, 3663–3670 CrossRef CAS.
- A. P. Walsh, J. A. Laureanti, S. Katipamula, G. M. Chambers, N. Priyadarshani, S. Lense, J. T. Bays, J. C. Linehan and W. J. Shaw, Faraday Discuss., 2019, 215, 123–140 RSC.
- A. Dubey, L. Nencini, R. R. Fayzullin, C. Nervi and J. R. Khusnutdinova, ACS Catal., 2017, 7, 3864–3868 CrossRef CAS.
- D. Wei, R. Sang, P. Sponholz, H. Junge and M. Beller, Nat. Energy, 2022, 7, 438–447 CrossRef CAS.
- L. K. Oliemuller, C. E. Moore and C. M. Thomas, Inorg. Chem., 2023, 62, 13997–14009 CrossRef CAS PubMed.
- S. Kostera, G. Manca and L. Gonsalvi, Chem. – Eur. J., 2023, 29, e202302642 CrossRef CAS PubMed.
- S. Kostera and L. Gonsalvi, ChemCatChem, 2024, 16, e202301391 CrossRef CAS.
- A. Dutta, S. Lense, J. Hou, M. H. Engelhard, J. A. S. Roberts and W. J. Shaw, J. Am. Chem. Soc., 2013, 135, 18490–18496 CrossRef CAS PubMed.
- G. Marcandalli, M. C. O. Monteiro, A. Goyal and M. T. M. Koper, Acc. Chem. Res., 2022, 55, 1900–1911 CrossRef CAS PubMed.
- X. Li and J. J. Warren, Dalton Trans., 2025, 54, 2086–2092 RSC.
- J. S. Derrick, M. Loipersberger, S. K. Nistanaki, A. V. Rothweiler, M. Head-Gordon, E. M. Nichols and C. J. Chang, J. Am. Chem. Soc., 2022, 144, 11656–11663 CrossRef CAS PubMed.
- W. W. Kramer and C. C. L. McCrory, Chem. Sci., 2016, 7, 2506–2515 RSC.
- F. Franco, C. Cometto, F. F. Vallana, F. Sordello, E. Priola, C. Minero, C. Nervi and R. Gobetto, Chem. Commun., 2014, 50, 14670–14673 RSC.
- C. W. Machan, S. A. Chabolla, J. Yin, M. K. Gilson, F. A. Tezcan and C. P. Kubiak, J. Am. Chem. Soc., 2014, 136, 14598–14607 CrossRef CAS PubMed.
- J. Agarwal, T. W. Shaw, H. F. Schaefer III and A. B. Bocarsly, Inorg. Chem., 2015, 54, 5285–5294 CrossRef CAS PubMed.
- C. W. Machan and C. P. Kubiak, Dalton Trans., 2016, 45, 15942–15950 RSC.
- F. Franco, C. Cometto, L. Nencini, C. Barolo, F. Sordello, C. Minero, J. Fiedler, M. Robert, R. Gobetto and C. Nervi, Chem. – Eur. J., 2017, 23, 4782–4793 CrossRef CAS PubMed.
- K. T. Ngo, M. McKinnon, B. Mahanti, R. Narayanan, D. C. Grills, M. Z. Ertem and J. Rochford, J. Am. Chem. Soc., 2017, 139, 2604–2618 CrossRef CAS PubMed.
- E. Haviv, D. Azaiza-Dabbah, R. Carmieli, L. Avram, J. M. L. Martin and R. Neumann, J. Am. Chem. Soc., 2018, 140, 12451–12456 CrossRef CAS PubMed.
- S. Sung, X. H. Li, L. M. Wolf, J. R. Meeder, N. S. Bhuvanesh, K. A. Grice, J. A. Panetier and M. Nippe, J. Am. Chem. Soc., 2019, 141, 6569–6582 CrossRef CAS PubMed.
- L. Rotundo, D. E. Polyansky, R. Gobetto, D. C. Grills, E. Fujita, C. Nervi and G. F. Manbeck, Inorg. Chem., 2020, 59, 12187–12199 CrossRef CAS PubMed.
- K. Talukdar, S. S. Roy, E. Amatya, E. A. Sleeper, P. Le Magueres and J. W. Jurss, Inorg. Chem., 2020, 59, 6087–6099 CrossRef CAS PubMed.
- M. H. Rønne, D. Cho, M. R. Madsen, J. B. Jakobsen, S. Eom, É. Escoudé, H. C. D. Hammershøj, D. U. Nielsen, S. U. Pedersen, M.-H. Baik, T. Skrydstrup and K. Daasbjerg, J. Am. Chem. Soc., 2020, 142, 4265–4275 CrossRef PubMed.
- M. R. Madsen, J. B. Jakobsen, M. H. Ronne, H. Liang, H. C. D. Hammershoj, P. Norby, S. U. Pedersen, T. Skrydstrup and K. Daasbjerg, Organometallics, 2020, 39, 1480–1490 CrossRef CAS.
- S. S. Roy, K. Talukdar and J. W. Jurss, ChemSusChem, 2021, 14, 662–670 CrossRef CAS PubMed.
- X. H. Li and J. A. Panetier, Phys. Chem. Chem. Phys., 2021, 23, 14940–14951 RSC.
- Y. Yang, M. Z. Ertem and L. L. Duan, Chem. Sci., 2021, 12, 4779–4788 RSC.
- M. H. Ronne, M. R. Madsen, T. Skrydstrup, S. U. Pedersen and K. Daasbjerg, ChemElectroChem, 2021, 8, 2108–2114 CrossRef.
- L. Rotundo, D. C. Grills, R. Gobetto, E. Priola, C. Nervi, D. E. Polyansky and E. Fujita, ChemPhotoChem, 2021, 5, 526–537 CrossRef CAS.
- S. S. Roy, K. Talukdar, S. T. Sahil and J. W. Jurss, Polyhedron, 2022, 224, 115976 CrossRef.
- V. Blasczak, M. McKinnon, L. Suntrup, N. A. Aminudin, B. Reed, S. Groysman, M. Z. Ertem, D. C. Grills and J. Rochford, Inorg. Chem., 2022, 61, 15784–15800 CrossRef CAS PubMed.
- W. W. Hong, M. Luthra, J. B. Jakobsen, M. R. Madsen, A. C. Castro, H. C. D. Hammershoj, S. U. Pedersen, D. Balcells, T. Skrydstrup, K. Daasbjerg and A. Nova, ACS Catal., 2023, 13, 3109–3119 CrossRef CAS PubMed.
- V. Blasczak, A. Murphy, L. Suntrup, K. T. Ngo, B. Reed, S. Groysman, D. C. Grills and J. Rochford, ChemCatChem, 2024, 16, e202301388 CrossRef CAS.
- W. Hong, J. B. Jakobsen, D. Golo, M. R. Madsen, M. S. G. Ahlquist, T. Skrydstrup, S. U. Pedersen and K. Daasbjerg, ChemElectroChem, 2024, 11, e202300553 CrossRef CAS.
- Y. Hiraga, S. Chaki, Y. Uyama, R. Hoshide, T. Karaki, D. Nagata, K. Yoshimoto and S. Niwayama, Organics, 2022, 3, 38–58 CrossRef CAS.
- J. O. Taylor, Y. Wang and F. Hartl, ChemCatChem, 2020, 12, 386–393 CrossRef CAS.
- M. D. Sampson, A. D. Nguyen, K. A. Grice, C. E. Moore, A. L. Rheingold and C. P. Kubiak, J. Am. Chem. Soc., 2014, 136, 5460–5471 CrossRef CAS PubMed.
- D. C. Grills, M. Z. Ertem, M. McKinnon, K. T. Ngo and J. Rochford, Coord. Chem. Rev., 2018, 374, 173–217 CrossRef CAS.
- K. M. Waldie, A. L. Ostericher, M. H. Reineke, A. F. Sasayama and C. P. Kubiak, ACS Catal., 2018, 8, 1313–1324 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, HRMS and NMR data, additional voltammetry and IRSEC data, computed geometries. See DOI: https://doi.org/10.1039/d5dt00157a |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCO2− product selectivity of ∼1
HCO2− product selectivity of ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is observed at the high overpotential catalytic wave (for both H2O and PhOH acids). Computed enthalpy and free energy of activation parameters suggest that selective CO2 insertion at the manganese hydride transition state is favored, over protonation, consistent with negligible hydrogen production during controlled potential electrolysis studies.
1 is observed at the high overpotential catalytic wave (for both H2O and PhOH acids). Computed enthalpy and free energy of activation parameters suggest that selective CO2 insertion at the manganese hydride transition state is favored, over protonation, consistent with negligible hydrogen production during controlled potential electrolysis studies.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11–24 and CO25 reduction as well as CO2 hydrogenation26–32 catalysis. One of the first studies highlighting the role of the outer coordination sphere was for the purpose of electrocatalytic H2 oxidation where [NiII(PCy2NGly2)2]2+ catalysts (where P2N2 = 1,5-diaza-3,7-diphosphacyclooctane) were functionalized with glycine. This was found to facilitate proton transfer from the outer carboxylate groups to the pendant amino groups in the second coordination sphere, enabling electrocatalytic H2 oxidation over a wide range of pH values (0.1–9.0).33 The outer coordination sphere of a homogenous transition metal complex is understandably less well defined than the inner and second coordination spheres. An outer coordination sphere may take the form of bulk solvent and electrolyte and any co-factors dissolved therein, each of which can potentially participate with the inner and second coordination sphere of a transition metal complex along its reaction coordinate.19,22,34,35 For example, the 1-ethyl-3-methylimidazolium tetracyanoborate, [EMIm][TCB], ionic liquid electrolyte was found to increase the rate of CO2 to CO reduction for the [fac-ReCl(2,2′-bipyridine)(CO)3] pre-catalyst at a reduced overpotential through an outer sphere [EMIm]+|bpy˙− π-stacking interaction.12 Gotico et al. later demonstrated a similar rate enhancement upon covalently incorporating a urea second coordination sphere at an Fe(III)Cl meso-tetraphenylporphyrin pre-catalyst.17 This was later attributed to multipoint hydrogen-bond templating of the bicarbonate anion in the second coordination sphere at the rate-determining C–OH bond-cleavage transition state.36 Also noteworthy is how Liu and McCrory encapsulated the cobalt phthalocyanine (CoPc) complex within a hydrophobic poly-4-vinylpyridine (P4VP) membrane thereby increasing CO product selectivity through optimized proton delivery in the outer coordination sphere.18,37 Williams et al. similarly enhanced CO2 reduction rates by introducing triazolium proton relays at the periphery of a related zinc porphyrin catalyst.20 It should never be overlooked, however, that second and outer coordination sphere effects at any catalytically active transition metal site are primarily dependent upon the core catalyst itself, i.e. the active metal site and its inner coordination sphere; and in the case of proton-coupled electron transfer (PCET) based catalysts, the proton donor activity and its transition state participation play another key role. In particular, group VII Mn(I) and Re(I) polypyridyl tricarbonyl catalysts have played a pivotal part in the study of second coordination sphere effects for CO2 reduction.38–59 The four Mn(I) bipyridyl tricarbonyl complexes presented in this study each contain an ortho-arylester second coordination sphere functional group at the 6,6′-positions of a [fac-MnI(R2-bpy)(CO)3(CH3CN)]+ pre-catalyst. This is inspired by our prior work that established the capability of an ortho-arylether second coordination sphere at promoting the low overpotential protonation-first pathway for electrocatalytic CO2 to CO conversion by this highly popular class of homogenous molecular catalyst.43 Switching to the o-arylester second coordination sphere maintains this low overpotential catalyst activation, whilst also allowing for facile extension beyond the second coordination sphere functionality. In reference to the o-phenylacetate substituted bpy ligand of [1-CH3CN]+, the tert-butyloxycarbonyl protected alanine (N-boc-ala) and o-methoxybenzoate groups of pre-catalysts [2-CH3CN]+ and [3-CH3CN]+, were here chosen to investigate how extension of the second coordination sphere H-bonding environment impacts the thermodynamic and kinetic performance of the catalyst as well as its product selectivity (Chart 1). The 2,6-dimethylphenylester group was also studied in [4-CH3CN]+ to probe any steric influence extending from the o-arylester second coordination sphere in this series of catalysts. It is important to note that, due to functionalization of the 6,6′-phenyl rings of the bpy ligands at just a single ortho-position, upon coordination at Mn a mixture of syn,syn, syn,anti and anti,anti atropisomers are possible with respect to the stereochemistry of the o-phenyl substituents and the Mn–CH3CN site where CO2 and H+ binding, and catalytic activity is anticipated to occur.
11–24 and CO25 reduction as well as CO2 hydrogenation26–32 catalysis. One of the first studies highlighting the role of the outer coordination sphere was for the purpose of electrocatalytic H2 oxidation where [NiII(PCy2NGly2)2]2+ catalysts (where P2N2 = 1,5-diaza-3,7-diphosphacyclooctane) were functionalized with glycine. This was found to facilitate proton transfer from the outer carboxylate groups to the pendant amino groups in the second coordination sphere, enabling electrocatalytic H2 oxidation over a wide range of pH values (0.1–9.0).33 The outer coordination sphere of a homogenous transition metal complex is understandably less well defined than the inner and second coordination spheres. An outer coordination sphere may take the form of bulk solvent and electrolyte and any co-factors dissolved therein, each of which can potentially participate with the inner and second coordination sphere of a transition metal complex along its reaction coordinate.19,22,34,35 For example, the 1-ethyl-3-methylimidazolium tetracyanoborate, [EMIm][TCB], ionic liquid electrolyte was found to increase the rate of CO2 to CO reduction for the [fac-ReCl(2,2′-bipyridine)(CO)3] pre-catalyst at a reduced overpotential through an outer sphere [EMIm]+|bpy˙− π-stacking interaction.12 Gotico et al. later demonstrated a similar rate enhancement upon covalently incorporating a urea second coordination sphere at an Fe(III)Cl meso-tetraphenylporphyrin pre-catalyst.17 This was later attributed to multipoint hydrogen-bond templating of the bicarbonate anion in the second coordination sphere at the rate-determining C–OH bond-cleavage transition state.36 Also noteworthy is how Liu and McCrory encapsulated the cobalt phthalocyanine (CoPc) complex within a hydrophobic poly-4-vinylpyridine (P4VP) membrane thereby increasing CO product selectivity through optimized proton delivery in the outer coordination sphere.18,37 Williams et al. similarly enhanced CO2 reduction rates by introducing triazolium proton relays at the periphery of a related zinc porphyrin catalyst.20 It should never be overlooked, however, that second and outer coordination sphere effects at any catalytically active transition metal site are primarily dependent upon the core catalyst itself, i.e. the active metal site and its inner coordination sphere; and in the case of proton-coupled electron transfer (PCET) based catalysts, the proton donor activity and its transition state participation play another key role. In particular, group VII Mn(I) and Re(I) polypyridyl tricarbonyl catalysts have played a pivotal part in the study of second coordination sphere effects for CO2 reduction.38–59 The four Mn(I) bipyridyl tricarbonyl complexes presented in this study each contain an ortho-arylester second coordination sphere functional group at the 6,6′-positions of a [fac-MnI(R2-bpy)(CO)3(CH3CN)]+ pre-catalyst. This is inspired by our prior work that established the capability of an ortho-arylether second coordination sphere at promoting the low overpotential protonation-first pathway for electrocatalytic CO2 to CO conversion by this highly popular class of homogenous molecular catalyst.43 Switching to the o-arylester second coordination sphere maintains this low overpotential catalyst activation, whilst also allowing for facile extension beyond the second coordination sphere functionality. In reference to the o-phenylacetate substituted bpy ligand of [1-CH3CN]+, the tert-butyloxycarbonyl protected alanine (N-boc-ala) and o-methoxybenzoate groups of pre-catalysts [2-CH3CN]+ and [3-CH3CN]+, were here chosen to investigate how extension of the second coordination sphere H-bonding environment impacts the thermodynamic and kinetic performance of the catalyst as well as its product selectivity (Chart 1). The 2,6-dimethylphenylester group was also studied in [4-CH3CN]+ to probe any steric influence extending from the o-arylester second coordination sphere in this series of catalysts. It is important to note that, due to functionalization of the 6,6′-phenyl rings of the bpy ligands at just a single ortho-position, upon coordination at Mn a mixture of syn,syn, syn,anti and anti,anti atropisomers are possible with respect to the stereochemistry of the o-phenyl substituents and the Mn–CH3CN site where CO2 and H+ binding, and catalytic activity is anticipated to occur.

![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) IR absorption bands, characteristic of the fac-tricarbonyl manganese core, with a symmetric stretch at 2044 cm−1 and a broad asymmetric v(C
O) IR absorption bands, characteristic of the fac-tricarbonyl manganese core, with a symmetric stretch at 2044 cm−1 and a broad asymmetric v(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) stretch at lower wavenumber 1957–1958 cm−1. [1-CH3CN]+ appears to impart a slight descent in symmetry from a C3 to Cs point group as its low wavenumber v(C
O) stretch at lower wavenumber 1957–1958 cm−1. [1-CH3CN]+ appears to impart a slight descent in symmetry from a C3 to Cs point group as its low wavenumber v(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O) band is almost resolved into two individual peaks evident at 1958 and 1949(sh) cm−1. Characteristic v(C
O) band is almost resolved into two individual peaks evident at 1958 and 1949(sh) cm−1. Characteristic v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretches of each o-arylester group at the bpy ligands are also observed for all four complexes. [1-CH3CN]+ exhibits a v(C
O) stretches of each o-arylester group at the bpy ligands are also observed for all four complexes. [1-CH3CN]+ exhibits a v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretch at 1768 cm−1, with a shoulder at 1752 cm−1. A similar absorption profile is exhibited by [3-CH3CN]+ at 1747 and 1729(sh) cm−1, and [4-CH3CN]+ at 1769 and 1755(sh) cm−1. The shoulder observed for each of these v(C
O) stretch at 1768 cm−1, with a shoulder at 1752 cm−1. A similar absorption profile is exhibited by [3-CH3CN]+ at 1747 and 1729(sh) cm−1, and [4-CH3CN]+ at 1769 and 1755(sh) cm−1. The shoulder observed for each of these v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretches is tentatively attributed to the presence of atropisomers as suggested also by their 1H NMR spectra. [2-CH3CN]+ is unique in that it exhibits two independent stretches at 1772 and 1713 cm−1 attributed to the o-arylester and N-boc ester groups, respectively.60 FTIR spectra are illustrated in Fig. 1 and tabulated below alongside infrared spectroelectrochemical (IRSEC) data (Table 2).
O) stretches is tentatively attributed to the presence of atropisomers as suggested also by their 1H NMR spectra. [2-CH3CN]+ is unique in that it exhibits two independent stretches at 1772 and 1713 cm−1 attributed to the o-arylester and N-boc ester groups, respectively.60 FTIR spectra are illustrated in Fig. 1 and tabulated below alongside infrared spectroelectrochemical (IRSEC) data (Table 2).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretches was also observed upon formation of [2]−, however this region of the spectrum was mostly obscured by strong electrolyte absorption. Comparable IRSEC results were recorded for all four complexes (Table 2) and is provided in the ESI (Fig. 25–27†).
O) stretches was also observed upon formation of [2]−, however this region of the spectrum was mostly obscured by strong electrolyte absorption. Comparable IRSEC results were recorded for all four complexes (Table 2) and is provided in the ESI (Fig. 25–27†).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) absorption bands are obscured by strong electrolyte absorption for one- and two-electron reduced species in IRSEC experiments.
O) absorption bands are obscured by strong electrolyte absorption for one- and two-electron reduced species in IRSEC experiments.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 s A mol−1), υ is the scan rate (V s−1), R is the universal gas constant (8.3145 V A s K−1 mol−1), T is the temperature (298 K).63 The first reduction wave was used as a reference point for both ip and np (i.e. np = 2 for a two electron reduction). The number of electrons required for a single catalytic cycle is equivalent for each of the CO, HCO2− and H2 producing catalytic cycles (i.e. ncat = 2). The maximum turnover frequency (TOFmax) was estimated by plotting TOF vs. scan rate.
485 s A mol−1), υ is the scan rate (V s−1), R is the universal gas constant (8.3145 V A s K−1 mol−1), T is the temperature (298 K).63 The first reduction wave was used as a reference point for both ip and np (i.e. np = 2 for a two electron reduction). The number of electrons required for a single catalytic cycle is equivalent for each of the CO, HCO2− and H2 producing catalytic cycles (i.e. ncat = 2). The maximum turnover frequency (TOFmax) was estimated by plotting TOF vs. scan rate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCO2−
HCO2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 (%)
H2 (%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 451
451![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split with respect to the selectivity of the activated catalyst [2]− at binding CO2 or H+ to form the critical metallocarboxylic acid [2-CO2H] or metal-hydride [2-H] intermediates along the CO or HCO2−vs. H2 catalytic pathways (Scheme 2). [2-CH3CN]+ exhibits a very similar product distribution of
1 split with respect to the selectivity of the activated catalyst [2]− at binding CO2 or H+ to form the critical metallocarboxylic acid [2-CO2H] or metal-hydride [2-H] intermediates along the CO or HCO2−vs. H2 catalytic pathways (Scheme 2). [2-CH3CN]+ exhibits a very similar product distribution of 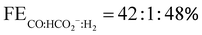 in the presence of optimum H2O (6.4 M) at high overpotential (Ecat/2 = −1.96 V). Of great significant for each of these high overpotential observations of equitable CO vs. HCO2− formation is the negligible production of H2. As the high-overpotentials required are consistent with HCO2− production along the ET-insert pathway, via the one-electron reduced manganese hydride intermediate [2-H]−, this suggests that the N-boc terminated o-arylester second coordination sphere of [2-CH3CN]+ favors CO2 insertion over protonation of [2-H]− to maintain a high selectivity for HCO2− production. This observation is indeed consistent with related complexes which also contain pendant amino functionalities in their SCS.19,22,48 Estimated hydricities of the neutral [Mn–H] catalyst, using Kubiak's linear correlation method with two-electron reduction potentials, of all four complexes are in the range of
in the presence of optimum H2O (6.4 M) at high overpotential (Ecat/2 = −1.96 V). Of great significant for each of these high overpotential observations of equitable CO vs. HCO2− formation is the negligible production of H2. As the high-overpotentials required are consistent with HCO2− production along the ET-insert pathway, via the one-electron reduced manganese hydride intermediate [2-H]−, this suggests that the N-boc terminated o-arylester second coordination sphere of [2-CH3CN]+ favors CO2 insertion over protonation of [2-H]− to maintain a high selectivity for HCO2− production. This observation is indeed consistent with related complexes which also contain pendant amino functionalities in their SCS.19,22,48 Estimated hydricities of the neutral [Mn–H] catalyst, using Kubiak's linear correlation method with two-electron reduction potentials, of all four complexes are in the range of  .64 This is slightly endergonic of the 44 kcal mol−1 assumed necessary for HCO2− formation and may also explain the low yields of H2 at low overpotential. Hydricity of the reduced [Mn–H]− intermediate has been estimated at ∼14 kcal mol−1 for a catalyst that exhibited 71% HCO2− selectivity and which bears pendant amine second coordination sphere, comparable in many respects to [2-CH3CN]+.48
.64 This is slightly endergonic of the 44 kcal mol−1 assumed necessary for HCO2− formation and may also explain the low yields of H2 at low overpotential. Hydricity of the reduced [Mn–H]− intermediate has been estimated at ∼14 kcal mol−1 for a catalyst that exhibited 71% HCO2− selectivity and which bears pendant amine second coordination sphere, comparable in many respects to [2-CH3CN]+.48
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCO2− in controlled potential electrolysis. Once the [2-H]− intermediate is formed, HCO2− formation by electrophilic attack of CO2 is favored over H2 formation in line with our experimental observations (Fig. 5c). Theoretical data for [3-CH3CN]+ was inconsistent with our CPE experiments, however, as pointed out earlier, this may likely be due to three-electron activation of pre-catalyst [3-CH3CN]+ leading to excessive H2 production, or alternatively steric crowding of the active site hindering CO2 binding at [3]− or insertion at [3-H]− thereby enhancing the H2 yield.
HCO2− in controlled potential electrolysis. Once the [2-H]− intermediate is formed, HCO2− formation by electrophilic attack of CO2 is favored over H2 formation in line with our experimental observations (Fig. 5c). Theoretical data for [3-CH3CN]+ was inconsistent with our CPE experiments, however, as pointed out earlier, this may likely be due to three-electron activation of pre-catalyst [3-CH3CN]+ leading to excessive H2 production, or alternatively steric crowding of the active site hindering CO2 binding at [3]− or insertion at [3-H]− thereby enhancing the H2 yield.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the [Mn–CO2H] and [Mn–H] intermediates with a surprising selectivity for CO2 insertion at [Mn–H] along the competing HCO2−vs. H2 pathways as corroborated by computational studies.
1 ratio of the [Mn–CO2H] and [Mn–H] intermediates with a surprising selectivity for CO2 insertion at [Mn–H] along the competing HCO2−vs. H2 pathways as corroborated by computational studies.





