Recent developments in atomically precise metal nanocluster-based photocatalysts for hydrogen production
Rugma
T. P.
,
Michael N.
Pillay
 and
C. W.
Liu
and
C. W.
Liu
 *
*
Department of Chemistry, National Dong Hwa University, Hualien, 97401, Taiwan, Republic of China. E-mail: chenwei@gms.ndhu.edu.tw
First published on 7th May 2025
Abstract
Photocatalytic hydrogen production offers a sustainable approach for utilising light energy, providing a promising solution to global energy challenges. The efficiency of this process relies on developing photocatalysts with broad light responsiveness and effective charge carrier separation capabilities. Atomically precise metal nanoclusters (NCs) have emerged as a highly favourable class of materials for this role due to their unique atomic arrangements, ultrasmall size, quantum confinement effects, and plenty of surface-active sites. These exceptional properties endow NCs with semiconductor-like behaviour, allowing for the generation of electrons and holes under light excitation, thus driving the hydrogen production reaction. Moreover, their robust light-absorption properties across the UV to near-IR spectrum, coupled with tuneable optical properties controlled by their composition and structure, promise NCs as next-generation photocatalysts. This review explores recent developments in the application of NCs for photocatalytic hydrogen production, emphasising strategies to enhance charge carrier separation and transfer efficiency, as well as photostability. The discussion also highlights the challenges and future opportunities in using NCs for efficient hydrogen production.
1. Introduction
The quest for sustainable and renewable energy sources has driven significant developments in various scientific fields, including photocatalysis, a promising clean energy production avenue. In particular, photocatalytic hydrogen production, which utilises photons to generate hydrogen fuel, offers a promising solution to problems associated with the storage of hydrogen as a fuel. Creating on-demand onsite or in situ production is key to the viability of hydrogen as a fuel stock. Additionally, this process not only provides a renewable and clean alternative to fossil fuels but also has the potential to reduce greenhouse gas emissions significantly.1–3 In this context, the exploration of new materials and catalysts that can enhance the efficiency of photocatalytic hydrogen production is of paramount importance. Early research into the photocatalytic water-splitting process centered on metal oxide-based photocatalysts, which are still outstanding candidates for hydrogen generation.4 However, most metal oxides possess a relatively wide bandgap (Eg > 3.0 eV) and are only UV-light-responsive, with a large charge carrier recombination rate, hindering their practical applications. Later on, visible-light-based photocatalysts such as g-C3N4 have attracted much attention from researchers due to their effective consumption of the solar spectrum (∼43%).5 However, in their bulk form, these semiconductor catalysts also suffered from low photocatalytic activity due to several limitations: high charge carrier recombination rates, low specific surface areas and less active sites, moderate oxidation ability, and slow surface reaction kinetics. Over the years, various design strategies such as exfoliation, co-catalyst loading, band-gap engineering, morphology tuning, defect engineering, heterojunction construction, and many others have been employed to increase the photocatalytic activity of traditional semiconductor photocatalysts.6 The intense exploration for efficient photocatalysts has further led to nanoparticles (NPs), the starting point for nanocatalysis, revealing how the unique environments and quantum effects at such small scales lead to high catalytic activity.7,8 However, the heterogenous nature of NPs with no two nanoparticles having the same configuration at an atomic level, with variability in size, shape, and surface characteristics, makes it challenging to intuitively fine-tune their catalytic performance in relation to structure. Often, these discrepancies cause poor reproducibility and reduced efficiencies. Traditional semiconductor-based and nanoparticle catalysts, while widely explored, often suffer from limitations such as sluggish charge transfer, high recombination rates, and insufficient active site utilization, which hinder their overall efficiency. Moreover, the balance between stability and activity remains a persistent challenge, restricting their long-term applicability.Due to their extremely small size, numerous surface sites with unsaturated coordination, distinctive atomic arrangements, and pronounced quantum confinement effects, NCs have attracted significant interest in the field of catalysis.9–11 Their electronic distribution itself is a major property that alters their catalytic efficiency.12 In addition, the pronounced quantum confinement effect in these tiny clusters means that even a single atom's addition or removal can dramatically alter their catalytic activity. Furthermore, the precise composition, structure, and surface environment of NCs allows for in-depth exploration of catalytic mechanisms and the development of detailed structure–property relationships.13 For effective photocatalysis, an ideal catalyst must possess strong light absorption capabilities, efficiently generate photoinduced electron–hole pairs, and facilitate rapid transfer and separation of these charge carriers.14,15 The surface plasmon resonance (SPR) in conventional NPs results in distinctive optical absorption properties, usually restricted to the visible light spectrum. This limitation arises because the collective oscillation of electrons on the NP surface, characteristic of SPR, only interacts with specific wavelengths.16,17 In contrast, NCs exhibit a much broader light absorption spectrum, extending from the ultraviolet (UV) to the near-infrared (NIR) regions. This wide absorption range makes NCs particularly advantageous for applications requiring enhanced light-harvesting capabilities across a diverse range of wavelengths, including those beyond the visible spectrum.18,19
Ideally, NCs can function as single photocatalysts, capable of generating electrons and holes under light excitation due to their small-band-gap semiconductor properties. However, practical challenges, such as their ultrasmall size leading to high surface energy and the tendency for charge carrier recombination, often make it difficult to achieve efficient photocatalysis when they are used alone. As a result, NCs are more commonly employed as co-catalysts in experimental setups. In this role, they enhance the performance of semiconductor-based photocatalysts by facilitating charge separation and transfer, which improves overall photocatalytic efficiency. The utilisation of NCs in catalytic applications has seen rapid growth, with significant advancements in recent years. However, their use in photocatalysis has not yet caught up in other catalytic fields like electrocatalysis.20–23 To address the challenges posed by NCs, such as rapid charge carrier recombination and poor photostability, several synthesis methods have been explored to create NC–porous support composites, which help prevent aggregation and enhance photocatalytic performance. Recent studies have proposed coupling NCs with other materials to form heterostructures, thereby enhancing photostability and extending charge carrier lifetimes.24,25
Some recent reviews have explored the electrocatalytic and photocatalytic applications of NCs,26,27 but their role as co-catalysts in photocatalytic reactions has not been thoroughly examined. In addition, these reviews have broadly addressed the wide-ranging applications of NCs, but our focus is on photocatalytic hydrogen production from water due to its potential for simplicity and scalability. Unlike more complex systems, water-splitting using powder photocatalysts offers a facile, cost-effective approach that can be easily adapted for large-scale hydrogen production. Therefore, we believe it is essential to provide a detailed review of these studies to shed light on the recent progress, challenges, and potential of powder-based photocatalysts in hydrogen production. Additionally, the review has addressed the current challenges and future opportunities in this field. By summarising the fundamental principles of photocatalytic hydrogen production and discussing various NC-based heterostructures, this review offers a comprehensive understanding of the key role that NCs play in advancing photocatalytic hydrogen production.
2. Fundamentals of photocatalysis
2.1. Basic mechanism
Inspired by artificial photosynthesis, producing clean H2 through photocatalysis of water directly with sunlight is the most promising route for the conversion of solar energy into chemical energy.28 Water splitting consists of mainly two half-reactions: the oxidation half-reaction (OER) and the reduction half-reaction (HER). Overall water-splitting (eqn (1)) is difficult to achieve because of the positive change in the Gibbs free energy (ΔG > 0). Among both reactions, water oxidation is more challenging since it requires an extra bias (1.23 V) involving a four-electron transfer.29| 2H2O → 2H2 + O2 (E > 1.23 eV) | (1) |
The mechanism of photocatalytic water splitting for hydrogen production generally consists of three main steps: (1) light absorption by photocatalysts, which generates electron (e−)–hole (h+) pairs; (2) separation and movement of these charges to the surface of the photocatalyst; and (3) surface reactions for water reduction and oxidation (Fig. 1a).30 Semiconductor photocatalysts typically have a conduction band (CB) and a valence band (VB) separated by a band gap (Eg). When the photon energy is equal to or greater than the band gap energy, the photocatalyst absorbs light, leading to the excitation of electrons from the VB to the CB, leaving holes in the VB. The electrons in the CB drive the reduction reaction to produce hydrogen, while the holes in the VB drive the oxidation process. For the reactions to occur, the CB edge must be more negative than the H+/H2 redox potential (0 V vs. NHE), and the VB edge must be more positive than the O2/H2O redox potential (Fig. 1(b)).2 Without suitable active sites on the photocatalyst surface, electron–hole pairs can rapidly recombine within the material. Studies have shown that the recombination of photogenerated charges in the catalyst bulk happens very quickly, within picoseconds to milliseconds. Therefore, to ensure efficient charge separation and transfer, cocatalysts must quickly capture the electrons and holes and facilitate the surface reduction reactions, which are vital for successful photocatalytic water splitting.
Photocatalytic water splitting typically involves two types of systems: photochemical and photoelectrochemical.31 The most basic method is photochemical water splitting, using a semiconductor slurry. In this setup, a photocatalytic system contains an aqueous suspension of semiconductor particles exposed to light. Upon irradiation, the semiconductor absorbs photons, generating electrons in the conduction band and holes in the valence band. These charge carriers then move to the catalyst's surface, where they participate in the splitting of water molecules into hydrogen and oxygen at the active sites.32 However, one limitation of this approach is that the band gap photocatalyst must align with the water reduction and oxidation potentials, which restricts the available material choices. Additionally, comparing the hydrogen production rates of various catalysts is challenging due to the various light sources and reactor designs used in different studies. Another challenge is the simultaneous production of hydrogen and oxygen in the same reactor, which complicates the separation of the two gases. Photocatalytic water splitting can be performed using either a single catalyst or a dual-catalyst configuration, such as a tandem or Z-scheme or any of the charge transfer pathways illustrated in Fig. 2. The Z-scheme approach mimics natural photosynthesis, where the two chlorophyll centres in photosystems II and I carry out the oxidation and reduction reactions, respectively. Similarly, in a Z-scheme, water splitting occurs across two different catalysts, with each semiconductor only needing to match the potential for each half-reaction.33 This allows for a broader selection of materials compared to single-catalyst systems. To further boost photocatalytic performance, cocatalysts (such as noble metals or metal oxides) can be added to the semiconductor to serve as electron or hole sinks, facilitating water reduction and oxidation while minimising charge recombination. The water oxidation catalyst produces oxygen using the holes generated in the valence band, while the water reduction catalyst utilises the electrons for the reduction process.
 | ||
| Fig. 2 (a) One-step photoexcitation process and (b) two-step photoexcitation (Z-scheme) process for overall water-splitting. Reproduced with permission from ref. 2. Copyright 2021 Elsevier. (c) Four types of conventional semiconductor heterojunctions. | ||
2.2. Role of co-catalysts in photocatalytic hydrogen evolution
The recombination of photo-generated charge carriers, whether on the surface or within the bulk, occurs rapidly, faster than the oxidation and reduction reactions in the millisecond and microsecond range, respectively. According to research by Leytner and Hupp, about 60% of electron–hole pairs in semiconductor materials recombine within a timescale of 25 nanoseconds.34 This recombination process is a major limitation to photocatalytic efficiency. For optimal photocatalytic performance, it is crucial to effectively separate and transport the photo-generated charge carriers to the surface while minimising recombination. Enhancing the crystallinity of photocatalysts can reduce the number of defect sites, decreasing recombination rates by providing fewer sites for recombination. Additionally, using smaller crystal or particle sizes in photocatalysts can boost efficiency by shortening the distance that charge carriers must travel to reach the surface, thus reducing recombination and increasing the likelihood of charge carriers reaching reaction centres.35 To further enhance charge transfer, photocatalysts should be coupled with co-catalysts, such as noble metals or other semiconductors, which help accelerate reduction and oxidation reactions. If the energy levels of the semiconductor and co-catalyst are well aligned, these co-catalysts can significantly improve charge migration, enhancing the overall efficiency of the photocatalyst. Using cocatalysts can enhance surface reactions, lower the overpotential for hydrogen and oxygen evolution, improve charge carrier separation, and increase the stability of semiconductor materials. Cocatalysts achieve better charge separation in semiconductors by forming p–n or n–n junctions, or a metal–semiconductor junction (Schottky barrier), with the semiconductor.36–38 Based on the Femi levels and energy band positions of the loaded co-catalysts, there are mainly four types of semiconductor heterojunctions: types I, II, and III heterojunctions and the Schottky junction (Fig. 2c).39 Type II and Schottky junctions have been shown to effectively separate electrons and holes, reducing the probability of recombination. This helps to extend the lifetime of the charge carriers, leading to higher efficiency and improved performance in a variety of applications. Therefore, it is highly desirable to construct a Schottky junction and type II alignment for photocatalytic applications. The Z-scheme mechanism of charge transfer is a type II alignment process in which more energetic charge carriers are used in the reactions, and less energetic charge carriers recombine. Recently, a new mechanism called the Step-scheme (S-scheme) has been proposed. The S-scheme heterojunction system works by forming an electric field between the two semiconductors, a reduction photocatalyst (RP) and an oxidation photocatalyst (OP). The electric field induced band bending, and coulombic attraction at the interface of the OP and RP triggers the recombination of holes in the VB of the RP and electrons in the conduction band of the OP. This drives oxidation at the valence band of the OP and reduction at the conduction band of the RP, producing an efficient photocatalytic reaction.40 These junctions help separate charge carriers, allowing them to be collected or released more efficiently by the semiconductor. In general, the internal electric field created at semiconductor junctions and the Schottky barrier provides the driving force needed to separate charges, leading to more efficient hydrogen generation at the photocatalyst/cocatalyst interface. The overall performance of a photocatalytic system depends on the type and amount of cocatalyst used. Since different cocatalysts influence the reaction rate differently, selecting the appropriate cocatalyst and its optimal amount is critical to achieving maximum photocatalytic efficiency.41,422.3. Importance of metal nanoparticles as co-catalysts
The incorporation of metal nanoparticles as cocatalysts can significantly enhance the catalytic efficiency of semiconductor photocatalysts. Both noble metals, such as Pt,43 Au,44 Ag,45 and Pd,46 and abundant non-precious metals, including Cu47 and Ni,48 are among the most studied and effective cocatalysts. When metal nanoparticles are deposited on the surface of semiconductor photocatalysts (consider the traditional TiO2 photocatalyst as an example), the photogenerated electrons from the conduction band of the semiconductor can easily transfer to the metal particles due to the lower Fermi level of the metal compared to the conduction band of the photocatalyst. This creates a Schottky barrier at the interface, allowing for unidirectional electron flow from TiO2 to the cocatalyst, thereby improving the efficiency of electron utilisation and enhancing the photocatalytic activity of the semiconductor. The metal's ability to trap electrons largely depends on its work function. Among these, Pt is regarded as the most effective electron promoter due to its low Fermi level and low hydrogen evolution overpotential. As photogenerated electrons accumulate on the metal cocatalyst, the Fermi level shifts to a more negative position, favouring hydrogen production in photocatalytic water splitting. In addition to serving as cocatalysts that promote the efficient separation of photogenerated electrons, certain metals can also induce localised surface plasmon resonance (LSPR), contributing hot electrons that boost photocatalytic activity. The surface plasmon resonance effect is a physical process where the free electrons in noble metal nanoparticles oscillate in response to incident light, resulting in strong light absorption. For specific noble metals, their shape can be adjusted to achieve strong absorption in the visible spectrum and the near-infrared region. Additionally, the size of the metal nanoparticles also influences photocatalytic hydrogen evolution. Smaller particles tend to shift the Fermi level further in the negative direction, facilitating more efficient proton reduction and increasing the rate of hydrogen production.49Taking this further, NCs, due to their ultra-small size and quantum confinement effects, offer unique properties compared to traditional metal nanoparticles. Metal NCs can serve as highly active cocatalysts due to their enhanced surface area and a higher density of reactive sites, which improve the transfer and separation of charge carriers at the semiconductor interface. This, combined with the tuneable surface properties of NCs, allows for more precise control over catalytic activity. Furthermore, NCs can exhibit strong quantum size effects, where even small changes in particle size can drastically affect their electronic properties, further optimising photocatalytic efficiency. Thus, the transition from traditional metal nanoparticles to NCs could lead to breakthroughs in designing more efficient and cost-effective photocatalytic systems. The following sections provide a summary of the recent progress in utilising NCs as co-catalysts for photocatalytic hydrogen production reactions.
3. Advances in NC-based photocatalytic hydrogen production
As highlighted in the previous section, NCs exhibit distinct energy levels characterised by unique HOMO–LUMO transitions and specialised photo-absorption properties. These characteristics make them highly suitable for applications in photocatalytic hydrogen production. In recent years, significant advancements have been made in developing composite photocatalysts, mainly through integrating NCs with conventional semiconductors. This approach has opened new avenues for enhancing the efficiency of photocatalytic processes. We have listed a detailed overview of studies involving NCs photocatalysts in Table 1, including their experimental conditions, photocatalytic hydrogen production activities, and quantum yields (QYs).| Catalyst | Co-catalyst | Light source | Amount of catalyst used (g) | Sacrificial agent (vol %) | H2 activity (mmol h−1 g−1) | QY (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Pt/Pt5/CdS | Pt | 300 W Xe lamp (λ > 420 nm) | 0.005 | TEOA (20) | 13 | 25.03 (400 nm) | 50 |
| Au25(Cys)18/g-C3N4 | Au25(Cys)18 | 300 W Xe lamp (λ > 420 nm) | 0.02 | TEOA (10) | 0.32 | 0.20 (420 nm) | 52 |
| MoSe2/CdSe/Au25 (GS)18 | Au25(GS)18 | 300 W Xe lamp (λ > 420 nm) | 0.01 | Lactic acid (10) | 0.14 | 53 | |
| Cr2O3/Au25/BaLa4Ti4O15 | Au25(PET)18 | 400 W Hg lamp | 0.05 | — | ∼5 | 54 | |
| Au25/BaLa4Ti4O15 | Au25 | 400 W Hg lamp | 0.5 | — | 0.50 | 55 | |
| Au25–Cr2O3–BaLa4Ti4O15 | Au25(GS)18 | 400 W Hg lamp | 0.5 | Methanol (10) | 4.69 | 6.30 (270 nm) | 56 |
| CdS@Au/Ti3−xC2Ty | CdS@Au | 300 W Xe lamp (λ > 420 nm) | 0.1 | Lactic acid (10) | 5.37 | 16.70 (420 nm) | 57 |
| Ag44(SR)30/TiO2 | Ag44(SR)30 | 300 W Xe lamp | 0.02 | Methanol (20) | 7.40 | 59 | |
| Pt1Ag28-BTT/CoP | Pt1Ag28 | 300 W Xe lamp (λ > 420 nm) | 0.005 | TEOA (15) | 240 | 25.77 (400 nm) | 60 |
| Au12Ag32/TiO2 | Au12Ag32 | Xe lamp | 0.005 | Methanol (20) | 6.81 | 61 | |
| UiO-66-NH2-Au25(L-Cys)18 | Au25(L-Cys)18 | 300 W Xe lamp (λ > 420 nm) | 0.005 | TEOA (10) | 17.02 | 62 | |
| AuAg24@UiO-66-NH2 | AuAg24 | 300 W Xe lamp (λ > 380 nm) | 0.005 | TEA (—) | 3.60 | 63 | |
| Ni6(SR)12/TiO2 | Ni6(SR)12 | 300 W Xe lamp | 0.02 | Methanol (20) | 5.60 | 7.80 (365 nm) | 65 |
| Ni12(SR)24/g-C3N4 | Ni12(SR)24 | 300 W Xe lamp | 0.01 | TEOA (15) | 3.00 | 66 | |
| Ni6(SC2H4Ph)12/g-C3N4 | Ni6(SC2H4Ph)12 | 300 W Xe lamp (λ > 400 nm) | 0.02 | TEOA (20) | 5.87 | 8.90 (400 nm) | 67 |
| Cu20/TiO2 | Cu20 | 300 W Xe lamp | — | TEOA (—) | 13.00 | 2.32 (390 nm) | 68 |
| Cu8-MOF | — | 300 W Xe lamp (λ > 380 nm) | 0.002 | TEOA (—) | 14.10 | 69 |
Maximizing the utilisation of photo-excited electrons is crucial for achieving high-efficiency photocatalytic hydrogen production. Among the various materials explored, Pt-based compounds stand out as highly effective co-catalysts due to their exceptional capacity to facilitate the separation of photogenerated electron–hole pairs. Lu et al. made a significant contribution to this field, developing a potent Pt5/CdS photocatalyst by attaching Pt5(GS)10 (GS = glutathione) clusters onto CdS nanorods.50 This structure was analysed using X-ray absorption fine structure (XAFS) spectroscopy with synchrotron radiation, confirming the presence of monoatomic platinum distributed across the surface of the CdS nanorods to form Pt–S4 active sites (Fig. 3d). This structure promoted a marked enhancement in photocatalytic performance. When exposed to visible light (λ > 400 nm), the Pt5(GS)10 loaded CdS photocatalyst achieved a hydrogen production rate of 13 mmol h−1 g−1 as shown in Fig. 3(a), which significantly surpassed the hydrogen production activity of bare CdS nanorods. Furthermore, ultrafast transient absorption (TA) spectroscopy revealed that the atomically dispersed platinum effectively extracted photo-generated electrons from the CdS nanorods, thereby promoting efficient separation of the photo-excited charge carriers (Fig. 3(c)). This process was key to the enhanced photocatalytic activity observed in the Pt5/CdS system. In addition, DFT calculations showed that the single Pt cocatalyst in Pt5/CdS possessed an optimal ΔGH* value close to zero. Apparently, the isolated single Pt atoms present on the surface of CdS nanorods formed new active sites and improved the intrinsic activity of CdS. The photocatalytic hydrogen production activity of the composite was also investigated after calcination. Although a physical mixture of CdS NRs and Pt5(GS)10 clusters initially demonstrated a significant increase in hydrogen evolution activity, this performance was further improved with thermal treatments. The catalyst's cycling stability was notably enhanced, as shown in Fig. 3(b). The quantum efficiency was calculated to be 25.08% at 400 nm.
 | ||
| Fig. 3 (a) H2 evolution activity as a function of loading Pt5(GS)10, (b) the cycling stability of the Pt5/CdS catalysts before (red) and after photocatalytic reaction (black), (c) plausible photocatalytic mechanism of the Pt5/CdS photocatalyst, and (d) XAFS analysis (black: measured; red: fitted) of Pt5/CdS and a probable coordination configuration of Pt atoms in Pt5/CdS (inset). Reproduced with permission from ref. 50. Copyright 2022 Royal Society of Chemistry. | ||
In 2012, Shen et al.51 developed gold-modified CdS photocatalysts, revealing impressive hydrogen production. This work was the first to demonstrate the potential of gold particles for photocatalytic hydrogen production. Building on these developments, Wang et al.52 reported a heterostructure composed of Au25(L-Cys)18 NCs (L-Cys = L-cysteine) integrated with graphitic carbon nitride (g-C3N4) via a wet-impregnation method. The Au25(L-Cys)18 NCs were uniformly dispersed on the surface of g-C3N4, significantly enhancing photocatalytic hydrogen production under visible light. The highly dispersed Au25 NCs formed numerous junctions at the metal–semiconductor interface, optimising the active site distribution. This led to a remarkable increase in hydrogen production, reaching 320 μmol h−1 g−1 under visible light irradiation in an aqueous solution containing triethanolamine as a hole scavenger. The enhancement in photocatalytic performance was attributed to the effective band alignment between the metal clusters and the semiconductor, which facilitated the efficient separation of photogenerated electron–hole pairs at the interface. Moreover, the hybrid photocatalysts demonstrated excellent stability over three consecutive recycling tests. The AQY of the system, measured using monochromatic light at a wavelength of 420 ± 10 nm, was determined to be 0.2%. Recently, Yan et al.53 developed a Z-type heterojunction photocatalyst using Au25(GS)18 clusters with controlled interfacial charge. The MoSe2/CdSe/Au25 (M/C/A) catalyst was formed by modifying MoSe2 nanosheets with mercaptoacetic acid to introduce a negative charge, while CdSe quantum dots were treated with 2-aminoethanethiol to impart a positive charge. These modified components were self-assembled into a binary heterostructure, which was then combined with Au25 clusters, forming a ternary heterostructure (Fig. 4(a)). The photocatalytic hydrogen production results showed negligible activity for MoSe2 and CdSe alone and significantly improved activity for the MoSe2/CdSe (M/C) heterostructure (Fig. 4(b1–b3)). A comparison with a mechanically mixed catalyst revealed that the presence of all three components, with charge regulation, was key for maximising hydrogen production. The improved performance was attributed to MoSe2 accelerating electron transfer from CdSe and reducing electron–hole recombination. The electron transfer mechanism follows a Z-scheme: under visible light, electrons in Au25(GS)18 are excited and recombine with CdSe holes, while the remaining electrons transfer to MoSe2, enhancing the catalytic efficiency as shown in Fig. 4(c).
 | ||
| Fig. 4 (a) Synthesis route of MoSe2/CdSe/Au25, (b) hydrogen production activities of MoSe2, CdSe and Mx/C, and (c) photocatalytic mechanism over the MoSe2/CdSe/Au25 heterostructure. Reproduced with permission from ref. 53. Copyright 2023 Wiley-VCH. | ||
In NC-based heterostructures, ligands at the interface often hinder interaction between the reactants and the NCs, resulting in reduced catalytic activity. While calcination can remove some of these ligands, excessive calcination may cause NC aggregation, leading to a loss of size-dependent catalytic properties. Kawawaki et al.54 investigated the effects of temperature on ligand removal and NC size changes, using BaLa4Ti4O15-supported 2-phenylethane thiolate-protected Au25(SR)18 NCs as a model. The desorption of ligands occurs in three stages as the temperature increases: (1) dissociation of ligands from the NC surface, (2) adsorption of the resulting compounds onto the support, and (3) desorption of these compounds from the support. The phenomenon that occurs during the calcination of Au25(PET, p-MBA)18/BaLa4Ti4O15 is shown in Fig. 5(a). In this system (i.e. Au25/BaLa4Ti4O15 and Cr2O3/Au25/BaLa4Ti4O15), the Au NCs only act as the co-catalyst and not as light absorbers (Fig. 5(b)). By carefully controlling the calcination process, the authors developed a highly active water-splitting photocatalyst with good stability, as shown in Fig. 5(c). Even earlier, Negishi et al.55,56 explored the photocatalytic water-splitting capabilities of gold NCs. They deposited Au25(GS)18 onto BaLa4Ti4O15 by removing the ligand at 300 °C under vacuum conditions. The photocatalytic activity of Au25-BaLa4Ti4O15 was found to be 2.6 times higher compared to AuNP-BaLa4Ti4O15 when exposed to light.
 | ||
| Fig. 5 (a) Phenomenon that occurs during calcination of Au25(PET, p-MBA)18/BaLa4Ti4O15, (b) schematic illustration of photocatalytic water splitting using a one-step photoexcitation system where Au NCs only act as a co-catalyst, and (c) photocatalytic water-splitting activities of the composites. Reproduced with permission from ref. 54. Copyright 2021 Wiley-VCH. | ||
Alongside traditional wide-band-gap metal oxides, several new materials with photocatalytic hydrogen production activity have been recently developed. Li et al.57 used the reductive Ti vacancies in Ti3xC2Ty MXene to create a core–shell structure to form a ternary structure of CdS/Au/MXene. At the interface, they formed two Schottky barriers, facilitating charge transfer from CdS to Au NCs and from Au NCs to MXene. The optimised Au NC and MXene loadings on CdS achieved a hydrogen production rate of 5371 mmol g−1 h−1, which exceeded that of bare CdS. In addition to enhancing light absorption by serving as a photosensitiser, the NCs also acted as a small-band-gap semiconductor, enabling the formation of a heterojunction. Both Au and MXene, known for their excellent electrical conductivity, cooperatively improved the efficiency of charge separation and transfer in CdS, resulting in a greater number of electrons available for photocatalytic reactions. The apparent quantum efficiency (AQE) at 420 nm for bare CdS and the composite photocatalysts were 0.3% and 16.7%, respectively. Polymers have also proved successful in guiding the controlled assembly of NCs into specific configurations. The surface properties of these polymers often complement those of the surface ligands on NCs. Building on this, Bera et al.58 grafted gold NCs (≈2 nm) and super clusters (SCs) (≈100 nm) on two-dimensional polydopamine (PDA). AuSCs@PDA showed enhanced photostability, lower charge transfer resistance, and higher photocurrent responses than AuNCs@PDA, AuSCs, and PDA NPs, with the highest hydrogen evolution rate of 3.20 mmol g−1 h−1.
Wang et al.59 developed a type II photosystem by combining TiO2 with atomically precise Ag44(SR)30 NCs (SR = thiolate). The composite exhibited a hydrogen generation rate of 7.4 mmol h−1 g−1, which is ten times greater than that of pure TiO2 nanoparticles under the same conditions (Fig. 6(b)). In addition, the composite shows good stability, with 83% of the photocatalytic activity remaining after five cycles (Fig. 6(c)). This notable enhancement in activity is attributed to the extended photoresponse and improved charge carrier separation and transport. Ultrafast transient absorption (TA) spectroscopy revealed that Ag44 NCs function as both light absorbers and small-band-gap semiconductors under UV/vis light irradiation. Specifically, under visible light, the NCs act solely as light absorbers, while under UV/vis light, they also enhance charge separation efficiency as small-band-gap semiconductors (Fig. 6(a)). Thus, this type II photosystem enables the NCs to act as co-catalysts rather than just a photosensitiser.
 | ||
| Fig. 6 (a) Schematic energy diagram of TiO2 before (black) and after (orange) deposition of NCs, (b) hydrogen evolution activity under UV/vis light irradiation, and (c) recyclability test of the composite. Reproduced with permission from ref. 59. Copyright 2020 Wiley-VCH. | ||
Zhu et al.60 constructed a Z-scheme heterojunction based on Pt1Ag28-BTT/CoP (BTT = 1,3,5-benzenetrithiol) through the Co–S interface (Fig. 7(a)). This heterostructure shows a marked improvement in photocatalytic hydrogen production, achieving a rate of 24.89 mmol h−1 g−1 compared to bare CoP. Additionally, the stable and well-integrated interface contributes to the long-term stability of the nanocomposite, with Pt1Ag28-BTT/CoP maintaining nearly consistent catalytic activity over five recycling tests (Fig. 7(b and c)). The composite also achieved an apparent quantum yield of 25.77% at 420 nm and retained approximately 100% activity. The improved photocatalytic hydrogen production performance is attributed to the strong internal electric field and the efficient Co–S interfacial charge transfer. The inclusion of central Pt atoms in Pt1Ag28-BTT improves the band alignment with CoP, while sulfur modification on the surface creates an effective charge transport pathway, boosting photocatalytic efficiency, as shown in Fig. 7(d).
 | ||
| Fig. 7 (a) Schematic of fabrication of the Pt1Ag28-BTT/CoP heterojunction, (b) wavelength dependent hydrogen production and AQY of Pt1Ag28-BTT/CoP, (c) cycling stability of Pt1Ag28-BTT/CoP, and (d) charge transfer mechanism in Pt1Ag28-BTT/CoP. Reproduced with permission from ref. 60. Copyright 2024 Springer. | ||
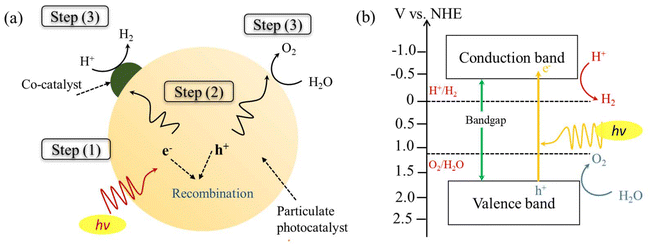
Bootharaju et al.61 synthesized Au12Ag32(SePh)30 (SePh = phenylselenolate) NCs with a unique structure comprising an Au icosahedral core and an Ag dodecahedral shell using the galvanic exchange technique. These Au12Ag32(SePh)30 NCs demonstrated superior stability and near-infrared-II photoluminescence compared to Ag44(SePh)30 NCs, their homometallic counterpart. Taking advantage of the oxygen vacancies in TiO2, the Au12Ag32 NCs were anchored onto the TiO2 surface. The well-aligned energy levels between the Au12Ag32 (SePh)30 NCs and TiO2 facilitated better separation of photogenerated charge carriers (Fig. 8(c and d)). The Au12Ag32/TiO2 composite showed a remarkable increase in photocatalytic hydrogen production, reaching 6810 μmol g−1 h−1, approximately 6.2 times higher than that of Ag44/TiO2, as displayed in Fig. 8(a). The external quantum efficiency of Au12Ag32/TiO2 was determined to be 0.89%, 0.52%, and 0.96% for photoexcitation at 365 nm, 380 nm, and 400 nm, respectively. The Au12Ag32/TiO2 catalysts maintained stability for up to four months, with only a minor decrease (approximately 6%) in photocatalytic activity (Fig. 8(b)), highlighting the importance of tailoring NCs to improve H2 production and stability.
 | ||
| Fig. 8 (a) Photocatalytic activity of the NCs and the composite photocatalysts, (b) H2 production by a fresh and aged Au12Ag32/TiO2 sample; inset bar diagram showing nearly intact photocatalytic activity of Au12Ag32/TiO2 clusters after four months of storage, and (c and d) photocatalytic H2 generation mechanism under solar irradiation. Reproduced with permission from ref. 61. Copyright 2023 Wiley-VCH. | ||
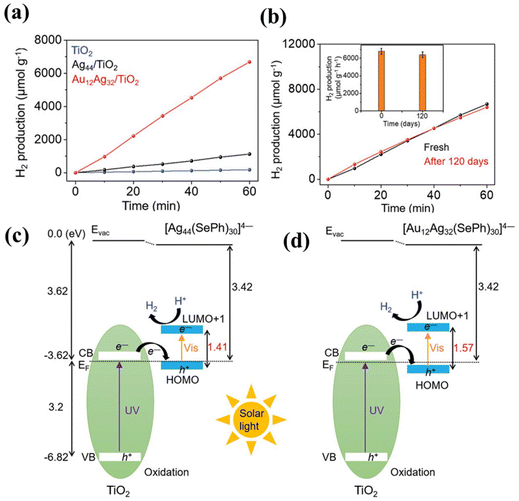
The extremely small size of NCs contributes to increased photocatalytic activity but also leads to limited stability. Some porous materials can serve as supports by accumulating these NCs on their surface, thereby reducing aggregation. Metal–organic frameworks (MOFs) and covalent organic frameworks (COFs) are examples of some widely explored porous functional supports made up of several ligands linked by covalent bonds. Their structurally adjustable nature allows for the rational design and precise control of NC deposition. Yao et al.62 developed a strategy to stabilise Au25(L-Cys)18 NCs on porous UiO-66-NH2 by creating covalent amide bonds (Fig. 9(a)). The strong metal–support interaction resulted in enhanced photocatalytic hydrogen production activity compared to the mechanically mixed UiO-66-NH2/Au25(PET)18 (PET = 2-phenylethanethiolate). As shown in Fig. 9(b), the covalently bonded UiO-66-NH2-Au25(L-Cys)18 demonstrated the best hydrogen production rate, 90 times higher than that of its bare counterpart. Moreover, the covalently bonded UiO-66-NH2-Au25(L-Cys)18 showed excellent stability (Fig. 9(c)), whereas the physically mixed UiO-66-NH2/Au25(PET)18 exhibited a significant decrease in catalytic performance during the second cycle. The improved performance is attributed to the covalent bond between UiO-66-NH2 and Au25(L-Cys)18, which strengthens the metal–support interaction, facilitating charge transfer and reducing charge carrier recombination.
 | ||
| Fig. 9 (a) Different synthesis procedures for loading Au25 NCs on UiO-66-NH2, (b) photocatalytic H2 production activities of UiO-66-NH2, UiO-66-NH2-Au25(L-Cys)18 and UiO-66-NH2/Au25(PET)18, and (c) stability runs of UiO-66-NH2-Au25(L-Cys)18 and UiO-66-NH2/Au25(PET)18. Reproduced with permission from ref. 62. Copyright 2023 Springer Nature. | ||
Recently, Wang et al.63 integrated M1Ag24(BT)x (M = Ag, Pd, Pt or Au; BT = benzenethiolate) NCs into UIO-66-NH2via electrostatic interactions (Fig. 10(a)) to evaluate the influence of the central dopant on photocatalytic performance. The authors state that the incorporation of heteroatoms into NCs could contribute to the redistribution of the surface charge and change the physicochemical properties of the NCs. MAg24 NCs are negatively charged whereas UiO-66-NH2 is positively charged, as confirmed by zeta potential analysis. Therefore, MAg24 can be electrostatically attracted onto UiO-66-NH2 to form MAg24@UiO-66-NH2. This integration led to a 5.6- and 6.4-fold increase in the photocatalytic hydrogen production rate which is far superior to that of AgNPs@UiO-66-NH2 and Ag25 supported on UiO-66-NH2 respectively, under 380 nm light in a CH3CN-H2O medium with TEOA as the sacrificial agent (Fig. 10(b)). Additionally, they demonstrate outstanding photocatalytic recyclability as shown in Fig. 10(c). X-ray photoelectron spectroscopy (XPS) under dark and illuminated conditions confirmed the existence of a Z-scheme charge transfer between M1Ag24 NCs and UiO-66-NH2 (Fig. 10(d)). The atomically precise structure allows for a detailed exploration of structure–property relationships. The AQE of AuAg24@UiO-66-NH2, measured under 380 nm light irradiation, is approximately 0.53%.
 | ||
| Fig. 10 (a) Synthesis route to fabricate MAg24@UiO-66-NH2, (b) photocatalytic hydrogen production activities of MAg24@UiO-66-NH2, (c) photocatalytic cycling performance of AuAg24@UiO-66-NH2, and (d) Z-scheme heterojunction formation at the MAg24–MOF interface. Reproduced with permission from ref. 63. Copyright 2024 Wiley-VCH. | ||
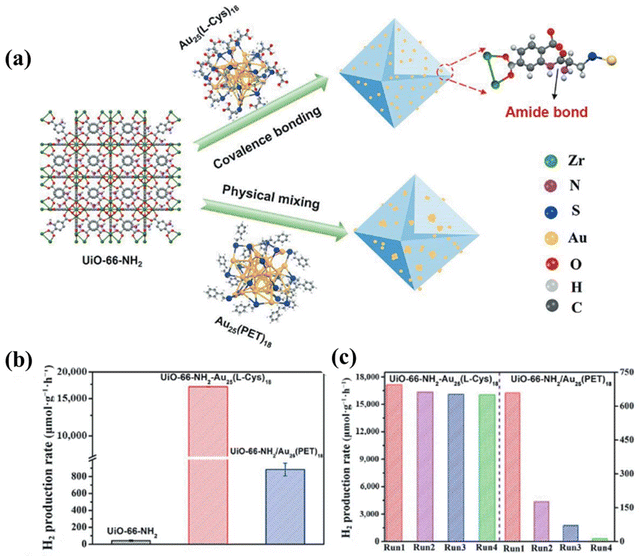
In 2013, Kagalwala et al.64 showcased the impressive photocatalytic hydrogen production activity of Ni-based clusters. They employed Ni6(SR)12 clusters as the photocatalyst, in the presence of triethylamine (TEA) scavenger and a photosensitizer. Their system achieved a turnover frequency (TOF) of 970 h−1. This study highlighted that Ni-based clusters can be good candidates for photocatalytic hydrogen production reactions. Later, Tian et al.65 incorporated Ni6(BT)12 clusters onto TiO2, creating a composite catalyst that markedly improved photocatalytic hydrogen production under simulated sunlight in a water–methanol medium. The Ni6/TiO2 photocatalyst retained its structure after the photocatalytic process as confirmed by the diffusion reflectance spectroscopy (DRS), X-ray photoelectron spectroscopy (XPS) and powder X-ray diffraction (PXRD) studies that were carried out before and after the photocatalytic reactions. Ni6(SR)12 exhibited distinct absorption peaks in the UV-visible spectrum (Fig. 11(a)). Fig. 11(c) illustrates the photocatalytic hydrogen production efficiency of catalysts with varying Ni6 loadings, with greater cluster content leading to improved hydrogen production activity. It was observed that visible light significantly improves the hydrogen evolution efficiency of the Ni6/TiO2 composite. However, visible light alone does not activate the photocatalytic activity of the Ni6/TiO2 composite, as the amount of hydrogen produced under these conditions remains negligible even after 8 hours of light exposure (Fig. 11(b)). A dynamic electron transfer process from TiO2 to Ni6(SCH2Ph)12 NCs was proposed, where the photogenerated electrons of TiO2 transfer to the near HOMO occupied orbitals of Ni6(SCH2Ph)12 (Fig. 11(d)). Such a charge transfer process is similar to the Z-scheme mechanism in heterojunction photocatalysis. The AQE for hydrogen evolution by the Ni6/TiO2 photocatalyst is 7.8% at 365 nm, although this high performance is dependent on the presence of a sacrificial reagent, with no significant hydrogen evolution observed without it.
 | ||
| Fig. 11 (a) UV/vis absorption spectrum of Ni6(SR)12, (b) H2 activity over Ni6/TiO2 under different light irradiation, (c) hydrogen evolution activity with varying cluster loadings under a solar simulator, (d) charge transfer mechanism in Ni6/TiO2, (e) Ni–S toroidal geometry, and (f) the full molecular structure of Ni12(SR)24 (atom notations: Ni, blue; yellow, S; gray, C; and white, H). Reproduced with permission from ref. 65 and 66. Copyright 2021 Elsevier and American Chemical Society. | ||
Later, they examined the influence of the passivation layer on photocatalytic performance, using g-C3N4, which has delocalized π bonds, incorporating Ni12(SR)24 as the cocatalyst. The Ni12 cluster consisted of a ring structure, protected by methylbenzene thiolate ligands (Fig. 11e and f). The cluster's absorption in the UV-visible range primarily resulted from electronic transition within the Ni–S ring structure and from the cluster core to the benzene ring's π-bonds in the ligands. Upon exposure to UV light, electrons migrated from the core of the ring structure to the benzene ring present in the ligand. Neither Ni12 nor g-C3N4 exhibited photocatalytic activity in their bare form, but Ni12-modified g-C3N4 demonstrated a high hydrogen evolution rate, which increased with higher cluster loading.66 In comparison, Wei et al.67 utilized a smaller hexanuclear Ni cluster of the type [Ni6(SC2H4Ph)12] (Ni6) as a cocatalyst supported on g-C3N4 nanosheets. The electrostatic interaction between the Ni6 clusters and g-C3N4 nanosheets led to the formation of a stable Ni6/g-C3N4 hybrid photocatalyst. Under visible light irradiation, in the presence of the triethanolamine scavenger, the photocatalytic hydrogen evolution activity of Ni6/g-C3N4 significantly increased, reaching 5.87 mmol h−1 g−1. Upon illumination, photogenerated electrons were transferred from Ni6 to the surface of g-C3N4, resulting in a substantial improvement over bare g-C3N4. The AQY of the Ni6/g-C3N4 catalyst was determined to be 8.9% at 400 nm.
Cu-based clusters have also demonstrated remarkable catalytic properties for photochemical H2 production. Cao et al.68 introduced [Cu20O1(C20H24O2)12(CH3COO)6] (UJN-Cu20), stabilized by ethinylestradiol ligands and acetate anions (Fig. 12(a)). They combined UJN-Cu20 with TiO2 nanosheets to create a highly efficient composite for hydrogen evolution photocatalysis. When tested with varying loadings of UJN-Cu20, and under a 300 W xenon lamp simulating sunlight, the UJN-Cu20@TiO2 composite significantly outperformed the bare materials, which produced only trace amounts of hydrogen. The optimal H2 production rate of 13 mmol g−1 h−1 was achieved with 2% UJN-Cu20 loading (Fig. 12(b)). The photocurrent response curve as shown in Fig. 12(d) revealed that while both TiO2 and UJN-Cu20 showed weak individual responses, the composite exhibited a substantial increase in photoelectric response. This indicated that the combination of UJN-Cu20 clusters and TiO2 greatly enhanced the separation of photogenerated electrons and holes. A Z-type photocatalytic system was constructed, significantly promoting this separation process, as shown in the electron transfer mechanism diagram (Fig. 12(c)).
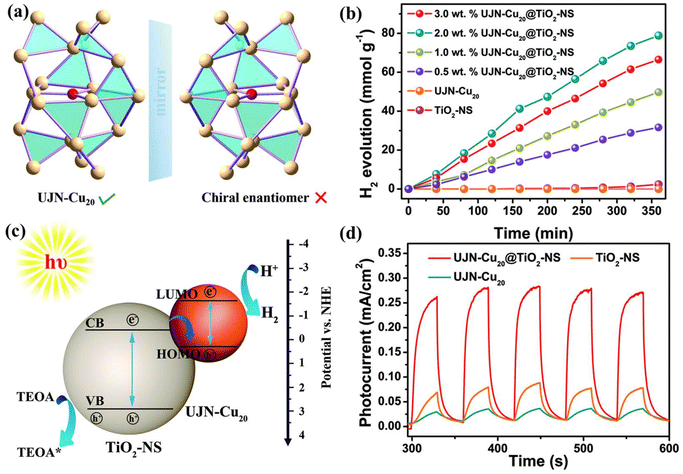 | ||
| Fig. 12 (a) The intrinsically chiral structure of O@Cu20 in UJN-Cu20, (b) photocatalytic activities of UJN-Cu20@TiO2 containing different wt% UJN-Cu20, (c) photocatalytic H2 production mechanism of UJN-Cu20@TiO2, and (d) photocurrent measurements of TiO2, UJN-Cu20 and UJN-Cu20@TiO2. Reproduced with permission from ref. 68. Copyright 2021 Royal Society of chemistry. | ||
Xu et al.69 fabricated Cu8(SN)4(tBuS)4 (SN = 4-(4-pyridinyl) thiazole-2-thiol) with a well-defined structure. They then utilized this cluster as a metal node to construct a copper-based MOF. In this setup, SN acted as an organic linker/Janus type ligand with dual coordination sites, with the thiolate and pyridyl directing the self-assembly of the solid-state framework (Fig. 13(a)). The MOF, when tested with fluorescein and triethylamine as photosensitizers and sacrificial agents, exhibited good photocatalytic hydrogen production performance (Fig. 13b). Furthermore, the Cu-MOF demonstrated relatively good stability, with performance maintained over three cycles (Fig. 13c). The addition of fluorescein significantly improved the photocurrent response of the Cu-MOF, suggesting effective electron transfer from the photosensitizer to the catalyst and efficient separation and movement of photogenerated electrons. Compared to Cu8(SN)4(tBuS)4 units, the ordered porous framework of the Cu-MOF facilitated more efficient charge transfer, contributing to its superior photocatalytic activity.
 | ||
| Fig. 13 (a) Synthesis procedure of copper cluster-based MOFs via secondary assembly of copper cluster units, (b) photocatalytic H2 evolution performance of CCMOF and the Cu8SN4 cluster, and (c) cycling stability of CCMOF. Reproduced with permission from ref. 69. Copyright 2023 Royal Society of chemistry. | ||
Cao et al.70 developed a series of anion templated clusters by introducing [MoOS3]2− units into the CuI clusters to form a series of atomically precise MoVI–CuI bimetallic clusters of [Cu6(MoOS3)2(C6H5(CH2)S)2(P(C6H4-R)3)4]·xCH3CN ((C6H5(CH2)S = benzyl mercaptan; P(C6H4-R)3 = triphenylphosphine; R = H, CH3, or F)). This modification not only enhances the structural stability of the Cu clusters but also introduces additional active sites. When these clusters were combined with Fe3O4, the resulting composite catalyst showed remarkable catalytic performance and stability in photocatalytic hydrogen production. The improved performance is due to the electronic effects of the ligands, which fine-tune the HOMO–LUMO levels of the clusters. This innovative copper cluster design provides a significant boost to photocatalytic hydrogen production. Moreover, three similar Cu–Mo heterometal clusters, incorporating [MoOS3]2− units and Cu(I) thiolate clusters with various substituents (CH3, H, or F), were engineered to adjust the electron effects of the stabilizing ligands. The photocatalytic hydrogen evolution activity of these clusters is closely related to their LUMO and HOMO energy levels, which control the efficiency of photo-generated charge carrier transfer in the photocatalytic process.
Zhang et al.71 incorporated thiomolybdate [Mo2S12]2− NCs onto TiO2via a simple impregnation method. The composite photocatalyst showed an improved hydrogen evolution rate when exposed to light. The optimized composite achieves a hydrogen evolution rate of 213.1 μmol h−1 g−1, approximately 51 times greater than that of pure TiO2. The authors attributed the enhanced photocatalytic activity to the close contact formed between [Mo2S12]2− and TiO2 which enhances the separation of electron–hole pairs thus extending the charge carrier lifetime. Additionally, the abundant bridging sulfur atoms in [Mo2S12]2− serve as active sites for hydrogen evolution, further enhancing the hydrogen production rate. Wang et al.72 developed supertetrahedral T4 NCs of the type [Cd3In17Se31]5, which functioned as both visible light absorber and co-catalysts to aid in charge separation. When combined with TiO2, the [Cd3In17Se31]5/TiO2 composite demonstrated a significant enhancement in photocatalytic hydrogen production, reaching 328.2 μmol g−1 h−1 in a reaction solution containing triethanolamine as the sacrificial agent. The [Cd3In17Se31]5/TiO2 composite showed good stability, maintaining its performance with minimal change after 50 hours of photocatalytic hydrogen production testing. Wu et al.73 successfully incorporated a series of ultra-small Cd4−xZnxIn16S35 (x: 0–4) metal chalcogenide supertetrahedral NCs onto g-C3N4 to create a 0D/2D heterostructure for photocatalytic hydrogen production. The MCSNs, with their adjustable Cd ratios, allowed fine-tuning of the energy band structure, leading to improved electron–hole pair separation and reduced charge transfer resistance. The optimized Cd1Zn3/g-C3N4 heterostructure achieved a hydrogen production rate of 288 μmol g−1 h−1, approximately seven times higher than that of pure g-C3N4.
4. Challenges and future directions
Given their extraordinary ability to respond to a wide range of light wavelengths and their discrete, molecular-like energy levels, NCs have emerged as highly promising candidates for photocatalytic hydrogen production. Their unique optical and electronic properties offer great advantages for enhancing photocatalytic activity. Despite the substantial progress made in the research on NCs for photocatalytic hydrogen production, a comprehensive understanding of the precise scaling relationships remains a challenge. This gap in expertise limits our ability to fully exploit the potential of these materials. Furthermore, NC-based catalysts face several other practical challenges that impede their widespread application. One major issue is the difficulty in manipulating NCs at the atomic scale. Achieving precise control over their size and structure is crucial for optimizing their photocatalytic performance, but current techniques are often inadequate or impractical for such fine-tuning. Another critical concern is the stability of NCs under photocatalytic experimental conditions. A significant number of NCs are vulnerable to degradation upon exposure to light. For NCs to be effectively utilized in photocatalytic processes, they must maintain their reliability and performance over time, particularly under the harsh conditions often associated with photocatalytic reactions. To address these challenges, it is essential to explore and develop advanced strategies for designing efficient NC-based photocatalysts for hydrogen production. Future research should focus on improving the atomic-level control of NCs, enhancing their stability, and expanding our understanding of their fundamental properties. By resolving these issues, we can unlock the full potential of NCs and advance their practical applications in photocatalytic hydrogen production. In this section, we aim to outline future directions for overcoming the challenges in designing efficient NC based photocatalysts.4.1. Improving light absorption
Light absorption is an important aspect of photocatalysis, which mainly depends on photons to start chemical reactions. Many studies have relied on photosensitizers to enhance the light absorption capabilities of NCs in photocatalytic experiments. While this approach has been effective, it introduces additional complexity and potential issues with stability. Instead, researchers should focus on improving the intrinsic light absorption properties of NCs themselves. Recent progress in research has made NCs absorb visible light, but if we consider the real-world scenario, a major part of sunlight falls into the infrared spectrum, which remains underutilized. Therefore, more improvements will likely focus on expanding the light absorption range of NCs to include infrared light. This could involve controlling the size and composition of NCs or modifying their surface to better capture and use more of the photon energy. Such progress could make photocatalysts much more efficient. In addition, all the NC-based photocatalysts discussed in Table 1 have been evaluated under Xe lamps, in some cases with a cut-off filter. However, for practical applications, it is necessary to shift toward sunlight as the light source. There are an increasing number of reports now focused on sunlight-driven reactions to better mirror real-world conditions.744.2. NC-composite design strategies
Integrating NCs with semiconductors to form heterostructures is a promising strategy for improving both charge carrier separation and NC stability. Recent studies have focused on traditional photocatalysts, such as metal oxides, metal sulfides, and carbon nitrides. Additionally, porous materials like MOFs and COFs have been explored as supports for anchoring NCs. There are possibilities to explore more advanced materials such as metal carbides, perovskites, layered materials, metal oxynitrides, etc. to further enhance the photocatalytic performance and stability of NCs. Wet impregnation, electrostatic attraction and mechanical mixing are the commonly used methods for depositing NCs onto the supports. Wet impregnation often involves immersing a support material in a metal precursor solution, followed by drying and thermal treatment to facilitate composite formation.75 However, this method often results in poor dispersion and aggregation due to uncontrolled nucleation. Additionally, weak metal–support interactions can lead to NC leaching, reducing long-term stability. On the other hand, electrostatic attraction relies on surface charge differences between NCs and the support material, allowing for deposition via electrostatic interactions. Yan et al.53 constructed a ternary MoSe2/CdSe/Au25 photocatalyst via electrostatic interactions and compared the hydrogen production activity with a mechanically mixed catalyst of the three phases. The heterostructure formed by the electrostatic attraction route produced more hydrogen compared to the mechanically mixed catalyst. Similarly, Yao et al.62 reported that the covalently bonded UiO-66-NH2-Au25(L-Cys)18 showed excellent stability compared to a physically mixed UiO-66-NH2/Au25(PET)18 which showed a decreased catalytic performance during the second cycle of the photocatalytic test. These studies show that it is necessary to explore new strategies for integrating NCs on to support materials. Alternative strategies such as in situ synthesis76 and photodeposition77 methods can be explored which are widely studied for traditional photocatalyst-based heterostructures. These alternative approaches may provide improved control over NC dispersion, charge carrier dynamics, and catalytic stability.4.3. Exploring the role of ligands
Identifying the actual catalytic sites on the NCs and selecting ligands that enhance photoinduced charge transfer are crucial. For example, in the case of the hybrid of Au25(GS)18 with g-C3N4, band gap narrowing was observed, showing a moderate tuning of the band structure of the composite. The authors attributed this to the interaction between glutathiolate ligands and the surface of g-C3N4 with the support of XPS analysis.52 Similarly, improved hydrogen production activity of Ni6(SCH2Ph)12@TiO2 was ascribed to the efficient electron transfer from TiO2 to the NC, assisted by the benzyl group of the thiolate ligands.65 Furthermore, in Ni12(SPhCH3)24 modified g-C3N4, photogenerated electrons on the phenyl groups of the ligands promote the electron injection from the NC to the conduction band of g-C3N4, resulting in efficient charge carrier separation.66 These studies indicate vast potential to further explore the role of ligands in improving the photogenerated charge carrier efficiency that ultimately can lead to improved hydrogen production activity.In some cases, the stability of NCs when exposed to light remains a major challenge. Key issues include the detachment of ligands, the nature of any detached fragments, and how these changes affect the catalytic performance. One approach is the development of advanced surface ligands that can protect NCs from photodegradation without compromising their catalytic or optical properties. Ligands play a crucial role in both the morphology and surface interactions. One primary function of the ligand is to provide solubility and improved dispersion in composites or the catalytic system. This could be a key area for atomically precise NCs, providing extremely uniform catalysts or composites. In addition, water-soluble NCs could be a beneficial aspect to explore both from a performance-based standpoint and in alignment with the sustainable energy goals. These water-soluble NCs can improve stability and dispersion in aqueous systems, which are ideal for photocatalytic water splitting. Research into the design of NCs with enhanced thermal and chemical stability will be essential for pushing the boundaries of their performance in real-world applications, where long-term stability is critical.
4.4. Structure–activity relationships
The relationship between structure and properties in NC-based photocatalysis is crucial for optimizing performance. The atomic precision of NCs allows for fine-tuning of active sites, which directly influences light absorption and charge transfer efficiency. Size-dependent quantum effects in NCs alter electronic properties like bandgap energy, enabling better control over photocatalytic activity. Additionally, surface ligands and core–shell structures affect the electronic environment and interaction with reactants, while structural features like exposed facets enhance charge separation and prevent recombination. Focusing on such structure–property correlation will lead to significant leaps in future research.4.5. Achieving overall water-splitting
NCs have shown promising advancement in photocatalytic hydrogen production reactions. Nevertheless, in most of the studies reported, hole scavengers are used to eliminate the oxidizing holes created in the valence band, which helps reduce electron–hole recombination and makes better use of photogenerated electrons for hydrogen generation. While this method boosts hydrogen production, it only addresses half of the water-splitting reaction. It also comes with higher costs and potential environmental concerns. The goal is to split water into hydrogen and oxygen (overall water-splitting) without using scavengers so that photogenerated charge carriers are used efficiently to produce hydrogen and oxygen in the right balance. This is the direction that future photocatalytic research should be aiming for.4.6. Non-noble metal NCs
A significant drawback of current NC-based photocatalysis is the heavy reliance on noble metals such as Au, Pt, and Ag. These noble metals are not only costly but also have limited availability, which constrains the practical application of photocatalytic technologies. Therefore, it is important to create metal-based NCs that are cost-effective, abundant, and demonstrate comparable or superior photocatalytic activity. Future research should explore different materials such as transition metals and alloys, and optimize their electronic and structural properties to improve the light absorption properties and photocatalytic activity while also developing scalable synthesis routes to reduce costs and optimize production. Nanocluster-based photocatalysis could attain long-term viability and wider applicability by redirecting attention towards these more sustainable approaches.4.7. A standardized measure of efficiency
In the process of photocatalytic hydrogen production, the choice of a standard measurement technique, or set of conditions is vital. Currently a large variation in the conditions used to record photocatalytic performance has created a challenge for a thorough comparative analysis. Some researchers report process efficiencies in terms of product yields per unit mass of photocatalyst per hour (mmol h−1 g−1) while others rely on quantum efficiencies or TOFs; rarely are all parameters reported. Although, theoretically, each individual parameter is suitable for evaluating the efficiency of the photocatalytic hydrogen production process, the inconsistency in experimental apparatus and conditions can hinder direct comparison. This issue underlines a broader challenge within the field of photocatalysis, which is the lack of a standardized testing protocol.5. Conclusion
We have summarised the recent developments in NCs for photocatalytic hydrogen production. The well-defined structure of NCs provides a significant advantage to explore the relationship between structure and photocatalytic activity. Researchers have concentrated on key aspects of catalyst design, including the light absorption capabilities, proper energy level alignment to enhance charge separation and transfer, and modifying the surface-active sites of NCs. Notable achievements have been made in the application of NCs for photocatalytic H2 production. However, a comprehensive understanding of the precise scaling relationships remains incomplete. NC-based catalysts continue to face several challenges in practical applications. One major difficulty is the precise control of NCs at the atomic level. Additionally, ensuring the stability of NCs remains a critical issue for their effective use in photocatalysis. Despite ongoing difficulties and challenges, extensive research efforts have been dedicated to addressing these issues. It is anticipated that significant advancements in NCs photocatalysis will be realized in the coming years.Author contributions
All authors contributed equally to the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this perspective review article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science and Technology Council of Taiwan (113-2123-M-259-001).References
- S. N. Reddy, S. Nanda, D.-V. N. Vo, T. D. Nguyen, V.-H. Nguyen, B. Abdullah and P. Nguyen-Tri, New Dimensions in Production and Utilization of Hydrogen, Elsevier, 2020, pp. 1–20 Search PubMed.
- S. K. Lakhera, A. Rajan, T. P. Rugma and B. Neppolian, Renewable Sustainable Energy Rev., 2021, 152, 111694 CrossRef CAS.
- T. P. Rugma, A. P. Varghese, K. P. Kangeyan, G. A. Shiny and S. K. Lakhera, in Green Hydrogen Economy for Environmental Sustainability. Volume 1: Fundamentals and Feedstocks, 2024, pp. 49–81 Search PubMed.
- M. Z. Rahman, F. Raziq, H. Zhang and J. Gascon, Angew. Chem., Int. Ed., 2023, 62, e202305385 CrossRef CAS PubMed.
- W.-H. Chen, J. E. Lee, S.-H. Jang, S.-S. Lam, G. H. Rhee, K.-J. Jeon, M. Hussain and Y.-K. Park, Int. J. Energy Res., 2022, 46, 5467–5477 CrossRef CAS.
- M. Sohail, S. Rauf, M. Irfan, A. Hayat, M. M. Alghamdi, A. A. El-Zahhar, D. Ghernaout, Y. Al-Hadeethi and W. Lv, Nanoscale Adv., 2024, 6, 1286–1330 RSC.
- T. S. Rodrigues, A. G. M. da Silva and P. H. C. Camargo, J. Mater. Chem. A, 2019, 7, 5857–5874 RSC.
- S. B. Somwanshi, S. B. Somvanshi and P. B. Kharat, J. Phys.:Conf. Ser., 2020, 1644, 012046 CrossRef.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- X. Kang, Y. Li, M. Zhu and R. Jin, Chem. Soc. Rev., 2020, 49, 6443–6514 RSC.
- R. Jin, G. Li, S. Sharma, Y. Li and X. Du, Chem. Rev., 2021, 121, 567–648 CrossRef CAS PubMed.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- L. Liu and A. Corma, Chem. Rev., 2023, 123, 4855–4933 CrossRef CAS PubMed.
- C. Gao, J. Low, R. Long, T. Kong, J. Zhu and Y. Xiong, Chem. Rev., 2020, 120, 12175–12216 CrossRef CAS PubMed.
- J. Low, C. Zhang, J. Ma, D. Y. Murzin and Y. Xiong, Trends Chem., 2023, 5, 121–132 CrossRef CAS.
- M. Sayed, J. Yu, G. Liu and M. Jaroniec, Chem. Rev., 2022, 122, 10484–10537 CrossRef CAS PubMed.
- M. Herran, A. Sousa-Castillo, C. Fan, S. Lee, W. Xie, M. Doblinger, B. Auguie and E. Cortes, Adv. Funct. Mater., 2022, 32, 2203418 CrossRef CAS.
- R. P. B. Silalahi, T.-H. Chiu, J.-H. Kao, C.-Y. Wu, C.-W. Yin, Y.-C. Liu, Y. J. Chen, J.-Y. Saillard, M.-H. Chiang and C. W. Liu, Inorg. Chem., 2021, 60, 10799–10807 CrossRef PubMed.
- A. V. Artem'ev and C. W. Liu, Chem. Commun., 2023, 59, 7182–7195 RSC.
- X. Cai, G. Li, W. Hu and Y. Zhu, ACS Catal., 2022, 12, 10638–10653 CrossRef CAS.
- D. Yang, J. Wang, Q. Wang, Z. Yuan, Y. Dai, C. Zhou, X. Wan, Q. Zhang and Y. Yang, ACS Nano, 2022, 16, 15681–15704 CrossRef CAS PubMed.
- X. Yuan and M. Zhu, Inorg. Chem. Front., 2023, 10, 3995–4007 RSC.
- T.-H. Chiu, J.-H. Liao, R. P. B. Silalahi, M. N. Pillay and C. W. Liu, Nanoscale Horiz., 2024, 9, 675–692 RSC.
- H. Wang, X. Liu, W. Yang, G. Mao, Z. Meng, Z. Wu and H.-L. Jiang, J. Am. Chem. Soc., 2022, 144, 22008–22017 CrossRef CAS PubMed.
- Q. You, H. Wang, Y. Zhao, W. Fan, W. Gu, H.-L. Jiang and Z. Wu, J. Am. Chem. Soc., 2024, 146, 9026–9035 CrossRef CAS PubMed.
- Y. Du, C. Li, Y. Dai, H. Yin and M. Zhu, Nanoscale Horiz., 2024, 9, 1262–1278 RSC.
- T. Song, X. Cai and Y. Zhu, Nanoscale, 2024, 16, 13834–13846 RSC.
- J. Qi, W. Zhang and R. Cao, Adv. Energy Mater., 2018, 8, 1701620 CrossRef.
- K. Maeda and K. Domen, J. Phys. Chem. C, 2007, 111, 7851–7861 CrossRef CAS.
- N. Xiao, S. Li, X. Li, L. Ge, Y. Gao and N. Li, Chin. J. Catal., 2020, 41, 642–671 CrossRef CAS.
- R. Abe, J. Photochem. Photobiol., C, 2010, 11, 179–209 CrossRef CAS.
- T. Grewe, M. Meggouh and H. Tüysüz, Chem. – Asian J., 2016, 11, 22–42 CrossRef CAS PubMed.
- E. S. Andreiadis, M. Chavarot-Kerlidou, M. Fontecave and V. Artero, Photochem. Photobiol., 2011, 87, 946–964 CrossRef CAS PubMed.
- S. Leytner and J. T. Hupp, Chem. Phys. Lett., 2000, 330, 231–236 CrossRef CAS.
- Y. Goto, T. Hisatomi, Q. Wang, T. Higashi, K. Ishikiriyama, T. Maeda, Y. Sakata, S. Okunaka, H. Tokudome, M. Katayama, S. Akiyama, H. Nishiyama, Y. Inoue, T. Takewaki, T. Setoyama, T. Minegishi, T. Takata, T. Yamada and K. Domen, Joule, 2018, 2, 509–520 CrossRef CAS.
- T. P. Rugma, S. Kumar Lakhera, T. Sahoo and N. Bernaurdshaw, FlatChem, 2022, 31, 100330 CrossRef.
- T. P. Rugma, B. S. Krishna, K. P. Kangeyan, N. Bernaurdshaw, A. S. AlArifi and S. K. Lakhera, Sustainable Energy Fuels, 2024, 8, 4461–4471 RSC.
- R. T P., B. Neppolian and S. K. Lakhera, Mater. Today Sustain., 2023, 24, 100513 Search PubMed.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef PubMed.
- A. Shabbir, S. Sardar and A. Mumtaz, J. Alloys Compd., 2024, 1003, 175683 CrossRef CAS.
- B. Bakbolat, C. Daulbayev, F. Sultanov, R. Beissenov, A. Umirzakov, A. Mereke, A. Bekbaev and I. Chuprakov, J. Nanomater., 2020, 10, 1790 CrossRef CAS PubMed.
- H. H. Do, D. L. T. Nguyen, X. C. Nguyen, T.-H. Le, T. P. Nguyen, Q. T. Trinh, S. H. Ahn, D.-V. N. Vo, S. Y. Kim and Q. V. Le, Arabian J. Chem., 2020, 13, 3653–3671 CrossRef CAS.
- M. V. Dozzi, G. L. Chiarello, M. Pedroni, S. Livraghi, E. Giamello and E. Selli, Appl. Catal., B, 2017, 209, 417–428 CrossRef CAS.
- P. Wang, Y. Cao, S. Xu and H. Yu, Appl. Surf. Sci., 2020, 532, 147420 CrossRef CAS.
- N. L. Reddy, S. Kumar, V. Krishnan, M. Sathish and M. V. Shankar, J. Catal., 2017, 350, 226–239 CrossRef CAS.
- F. Platero, A. López-Martín, A. Caballero, T. C. Rojas, M. Nolan and G. Colón, ACS Appl. Nano Mater., 2021, 4, 3204–3219 CrossRef CAS.
- J. Zhu, G. Cheng, J. Xiong, W. Li and S. Dou, Sol. RRL, 2019, 3, 1900256 CrossRef.
- S. K. Lakhera, T. P. Rugma, H. Y. Hafeez and B. Neppolian, ChemSusChem, 2019, 12, 4293–4303 CrossRef CAS PubMed.
- X. Jiang and M. Fuji, J. Jpn. Soc. Powder Powder Metall., 2023, 70, 203–212 CrossRef CAS.
- X. Lu, A. Tong, D. Luo, F. Jiang, J. Wei, Y. Huang, Z. Jiang, Z. Lu and Y. Ni, J. Mater. Chem. A, 2022, 10, 4594–4600 RSC.
- P. Shen, S. Zhao, D. Su, Y. Li and A. Orlov, Appl. Catal., B, 2012, 126, 153–160 CrossRef CAS.
- C. Wang, P. Lv, D. Xue, Y. Cai, X. Yan, L. Xu, J. Fang and Y. Yang, ACS Sustainable Chem. Eng., 2018, 6, 8447–8457 CrossRef CAS.
- X. Yan, X.-Y. Fu and F.-X. Xiao, Adv. Funct. Mater., 2023, 33, 2303737 CrossRef CAS.
- T. Kawawaki, Y. Kataoka, M. Hirata, Y. Akinaga, R. Takahata, K. Wakamatsu, Y. Fujiki, M. Kataoka, S. Kikkawa, A. S. Alotabi, S. Hossain, D. J. Osborn, T. Teranishi, G. G. Andersson, G. F. Metha, S. Yamazoe and Y. Negishi, Angew. Chem., Int. Ed., 2021, 60, 21340–21350 CrossRef CAS PubMed.
- Y. Negishi, M. Mizuno, M. Hirayama, M. Omatoi, T. Takayama, A. Iwase and A. Kudo, Nanoscale, 2013, 5, 7188–7192 RSC.
- W. Kurashige, R. Kumazawa, D. Ishii, R. Hayashi, Y. Niihori, S. Hossain, L. V. Nair, T. Takayama, A. Iwase, S. Yamazoe, T. Tsukuda, A. Kudo and Y. Negishi, J. Phys. Chem. C, 2018, 122, 13669–13681 CrossRef CAS.
- Z. Li, W. Huang, J. Liu, K. Lv and Q. Li, ACS Catal., 2021, 11, 8510–8520 CrossRef CAS.
- D. Bera, S. Mahata, M. Biswas, K. Kumari, S. Rakshit, Nonappa, S. Ghosh and N. Goswami, Small, 2025, 21, 2406551 CrossRef CAS PubMed.
- Y. Wang, X.-H. Liu, Q. Wang, M. Quick, S. A. Kovalenko, Q.-Y. Chen, N. Koch and N. Pinna, Angew. Chem., Int. Ed., 2020, 59, 7748–7754 CrossRef CAS PubMed.
- Q. Zhu, H. Shen, C. Han, L. Huang, Y. Zhou, Y. Du, X. Kang and M. Zhu, Nano Res., 2024, 17, 5002–5010 CrossRef CAS.
- M. S. Bootharaju, C. W. Lee, G. Deng, H. Kim, K. Lee, S. Lee, H. Chang, S. Lee, Y.-E. Sung, J. S. Yoo, N. Zheng and T. Hyeon, Adv. Mater., 2023, 35, 2207765 CrossRef CAS PubMed.
- A. Yao, Y. Du, M. Han, Y. Wang, J. Hu, Q. Zhu, H. Sheng and M. Zhu, Nano Res., 2023, 16, 1527–1532 CrossRef CAS.
- H. Wang, X. Zhang, W. Zhang, M. Zhou and H.-L. Jiang, Angew. Chem., Int. Ed., 2024, 63, e202401443 CrossRef CAS PubMed.
- H. N. Kagalwala, E. Gottlieb, G. Li, T. Li, R. Jin and S. Bernhard, Inorg. Chem., 2013, 52, 9094–9101 CrossRef CAS PubMed.
- F. Tian, J. Chen, F. Chen, Y. Liu, Y. Xu and R. Chen, Appl. Catal., B, 2021, 292, 120158 CrossRef CAS.
- F. Tian, X. Huang, W. Li, Y. An, G. Li and R. Chen, ACS Catal., 2023, 13, 12186–12196 CrossRef CAS.
- J. Wei, R. Zhao, D. Luo, X. Lu, W. Dong, Y. Huang, X. Cheng and Y. Ni, J. Colloid Interface Sci., 2023, 631, 212–221 CrossRef CAS PubMed.
- Y.-D. Cao, H.-P. Hao, H.-S. Liu, D. Yin, M.-L. Wang, G.-G. Gao, L.-L. Fan and H. Liu, Nanoscale, 2021, 13, 16182–16188 RSC.
- Y. Xu, Q.-G. Dong, J.-P. Dong, H. Zhang, B. Li, R. Wang and S.-Q. Zang, Chem. Commun., 2023, 59, 3067–3070 RSC.
- Y.-D. Cao, D. Yin, S. Li, X.-Y. Dong, Y. Feng, H. Liu, L.-L. Fan, G.-G. Gao and S.-Q. Zang, Angew. Chem., Int. Ed., 2023, 62, e202307678 CrossRef CAS PubMed.
- R. Zhang, K. Gong, F. Du and S. Cao, Int. J. Hydrogen Energy, 2022, 47, 19570–19579 CrossRef CAS.
- Y. Wang, J. Li, Q. Hu, M. Hao, Y. Liu, L. Gong, R. Li and X. Huang, ACS Appl. Mater. Interfaces, 2021, 13, 40562–40570 CrossRef CAS PubMed.
- Z. Wu, X.-L. Wang, X. Wang, X. Xu, D.-S. Li and T. Wu, Chem. Eng. J., 2021, 426, 131216 CrossRef CAS.
- T. Hisatomi and K. Domen, Nat. Catal., 2019, 2, 387–399 CrossRef CAS.
- B. A. T. Mehrabadi, S. Eskandari, U. Khan, R. D. White and J. R. Regalbuto, in Adv. Catal, ed. C. Song, Academic Press, 2017, vol. 61, pp. 1–35 Search PubMed.
- J. Wu, J. Du, X. He and X. Guo, ChemCatChem, 2024, 16, e202301234 CrossRef CAS.
- K. Wenderich and G. Mul, Chem. Rev., 2016, 116, 14587–14619 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |