Achieving balanced UV SHG responses, optical band gaps and birefringence in rare earth compounds Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Er, Ho, Dy, Eu)†
Shihua
Ma
a,
Lei
Geng
 *a,
Baozhu
Zhu
a and
Changyu
Meng
*b
*a,
Baozhu
Zhu
a and
Changyu
Meng
*b
aSchool of Physics and Physical Engineering, Qufu Normal University, Qufu 273165, China. E-mail: lgeng@qfnu.edu.cn
bGuangxi Key Laboratory of Agricultural Resources Chemistry and Biotechnology, Department of Chemistry and Food Science, Yulin Normal University, Yulin 537000, China. E-mail: mengchyu_2007@163.com
First published on 15th April 2025
Abstract
A series of rare earth iodate sulfate UV compounds, Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Er, Ho, Dy, Eu), have been successfully synthesized by the hydrothermal method at 200 °C. These isostructural compounds all crystallize in the noncentrosymmetric space group P212121 (no. 19) and feature a neutral three-dimensional Ln(IO3)(SO4) framework which is composed of 2D cationic Ln[SO4]+ layers bridged by anionic [IO3]− trigonal pyramids through sharing corner oxygen atoms. Under 1064 nm laser irradiation, Y(IO3)(SO4)·3H2O exhibits a second-harmonic generation (SHG) with an efficiency of 0.7 × KDP@1064 nm. Furthermore, Y(IO3)(SO4)·3H2O has a moderate birefringence (0.118@532 nm) and a large band gap (4.60 eV) and may be a potential UV nonlinear optical material. For Eu(IO3)(SO4)·3H2O, it emits intense photoluminescence peaks at 594 nm and 617 nm when excited under 393 nm near-ultraviolet light, showing promising applications as red phosphors of white-LEDs. The current study elucidates that the incorporation of highly anisotropic lone-paired (IO3)− units into highly isotropic (SO4)2− sulfate groups can achieve balanced SHG responses, optical band gaps and birefringence, facilitating the development of novel iodate sulfate crystals for UV nonlinear optical applications.
Introduction
Rare earth compounds have multifunctional applications in luminescence, ceramic materials, catalysts, nonlinear laser crystals, etc.1–5 From an atomic viewpoint, rare earth elements have fully occupied outermost and second outermost layers filled with electrons, and empty or singly occupied 5d orbitals, and the internal 4f orbitals have an increasing number of electrons as the atomic number increases. Additionally, rare earth elements exhibit flexible coordination capabilities, enabling them to combine with oxygen atoms to form various polyhedral LnOx compounds (x = 6–10). These structures often exhibit distortions or noncentrosymmetric arrangements, which are highly advantageous for the formation of nonlinear optical crystals.6–10 The recently synthesized noncentrosymmetric rare earth compounds, such as REI5O14 (RE = Y and Gd) (14 × and 15 × KDP), Cs2YB3O6F2 (5.6 × KDP), and Y3F(SeO3)4 (5.5 × KDP), exhibit strong second-harmonic generation (SHG) efficiency, making them promising candidates for nonlinear optical materials.11–13 Several strategies have been employed to synthesize compounds with noncentrosymmetric structures. These include the introduction of second-order Jahn–Teller (SOJT) distortion d0 transition metal (TM) cations (Mo6+, V5+, Nb5+, Ti4+, etc.), as seen in K3Nb3Ge2O13 and Li2TiTeO6;14,15 the use of stereochemically active lone-pair (SCALP) cations (Bi3+, Pb2+, etc.), as in BiO(IO3) and Cs2Bi2O(Ge2O7);16,17 the incorporation of strongly electronegative F− anions, as in CaCe(IO3)3(IO3F)F and [GaF(H2O)][IO3F];18,19 and the mixing of different types of anionic groups, as in Cd2(IO3)(PO4) and AgBi(SO4)(IO3)2.20,21I5+ ions may exhibit significant optical anisotropy due to the presence of lone-pair electrons, which strongly influence the SHG and birefringence properties of crystals. Sulfates and phosphates typically exhibit shorter UV absorption edges, which are significantly favorable for achieving large band gaps in compounds.22–24 In rare earth iodate sulfates, due to the rich coordination environment of rare earth elements, many structures have been found such as Ln(IO3)(SO4) (Ln = La–Gd, except Pm), Ln2(IO3)3(SO4)OH·3H2O (Ln = Sm, Eu, Dy), and Ce(IO3)2(SO4).25–27 In several previous studies, heterovalent substitution has been recognized as an efficient way to synthesize non-centrosymmetric structures, for instance, from Pb2TiOF(SeO3)2Cl to Pb2GaF2(SeO3)2Cl,28,29 from CeF2(SO4)·H2O to Ce(IO3)2(SO4),27 and from α- and β-Ba2[VO2F2(IO3)2](IO3) to α- and β-Ba2[GaF4(IO3)2](IO3).30,31 Building on the known crystal structures based on the above strategy, we experimentally introduced lone-paired [IO3−] groups into rare earth sulfates via the hydrothermal method, and successfully synthesized a new series of rare earth iodate sulfate compounds Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Er, Ho, Dy, Eu) with noncentrosymmetric structures and large UV absorption edges. Among these structures, Y(IO3)(SO4)·3H2O exhibits a wide band gap of 4.6 eV, a large SHG efficiency of 0.7 × KDP@1064 nm, and type-I phase matching, fully achieving balanced UV SHG efficiency and birefringence. In this study, the synthesis, crystal structures, infrared and UV–vis–NIR spectra, and fluorescence spectra of Ln(IO3)(SO4)·3H2O will be reported and discussed. This study demonstrates that combining large anisotropic [IO3−] groups with isotropic sulfates is an effective strategy for achieving a balance between UV SHG efficiency and birefringence properties.
Results and discussion
Crystal structure
Crystallographic data and refinement results are summarized in Table S1,† while atomic coordinates and bond valence sums (BVS) are provided in Table S2.† Selected bond lengths and bond angles are listed in Table S3.† The phase purity of the synthesized materials Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Er, Ho, Dy, Eu) was confirmed by XRD data comparison (Fig. S1†). EDS results show that the crystals contain elements and proportions consistent with the chemical formula (Fig. S2†). The crystal structures of the six crystals are isostructural, exemplified here by Y(IO3)(SO4)·3H2O, which crystallizes in the noncentrosymmetric space group P212121 (19). The asymmetric unit of Y(IO3)(SO4)·3H2O consists of one Y atom, one I atom, one S atom, ten oxygen atoms, and six hydrogen atoms. The I5+ cation is connected to three oxygens atoms, forming a distorted trigonal pyramid with bond lengths from 1.787(4) to 1.804(4) Å (Fig. 1a). The S6+ cation is connected to four oxygen atoms to form a [SO4] tetrahedron with bond lengths from 1.449(4) to 1.485(4) Å. The Y3+ cation is coordinated by eight oxygen atoms to form the [YO8] polyhedron with Y–O bond lengths from 2.262(4) to 2.450(4) Å. The overall structure is composed of three structural units, i.e., [IO3], [SO4], and [YO8], with each [YO8] polyhedron linked to three [IO3] trigonal pyramids, three [SO4] tetrahedra, and two H2O through sharing corner oxygen atoms with the monodentate method (Fig. 1b). It is observed along the a-axis that free water molecules are trapped in the cavity structure formed by four [YO8], two [IO3], and two [SO4] units. The calculated BVS of Y, I, and S in Y(IO3)(SO4)·3H2O are +3.337, +5.268, and +6.095, respectively, closely matching their formal values and confirming the structural rationality of Y(IO3)(SO4)·3H2O. The local dipole moments were calculated to analyze the geometric distortion of [IO3], [SO4], and [LnO8] units for compounds Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Eu) (Table S4†). For Y(IO3)(SO4)·3H2O, the calculated dipole moments of [IO3], [SO4], and [YO8] units are 27.8823 D, 1.1702 D, and 4.3600 D, respectively. For Gd(IO3)(SO4)·3H2O, the dipole moments of [IO3], [SO4], and [GdO8] units are 27.8973 D, 1.1840 D, and 3.6735 D, respectively. For Eu(IO3)(SO4)·3H2O, the dipole moments of the [IO3], [SO4] and [EuO8] units are 27.7063 D, 1.2442 D, and 4.7849 D, respectively. Clearly, the distortions of the trigonal pyramidal [IO3] and tetrahedral [SO4] units are similar in these compounds, while the distortions of the [LnO8] units follow the trend [EuO8] > [YO8] > [LnO8], consistent with the SHG efficiency measurements.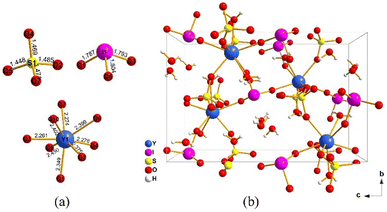 | ||
| Fig. 1 The coordination environment of [SO4], [IO3], and [YO8] units (a) and the crystal structure of Y(IO3)(SO4)·3H2O viewed down the a-axis (b). | ||
From a structural evolution perspective, rare earth sulfates Y(IO3)(SO4)·3H2O can be considered derivatives formed by introducing an [IO3] group into the parent structure of Y2(SO4)3·8H2O.32 As is evident from Fig. 2, Y2(SO4)3·8H2O has a two-dimensional neutral layer structure consisting of [YO8] and [IO3] functional units as well as crystal water. There are two types of macrocyclic components in the two-dimensional layer of Y2(SO4)3·8H2O including a larger eight-membered ring composed of four [SO4] tetrahedra and four [YO8] polyhedra and a smaller four-membered ring composed of two [SO4] tetrahedra and two [YO8] polyhedral alternatively arranged along the a-axis direction. However, for the title compound Y(IO3)(SO4)·3H2O, with the participation of [IO3], the two kinds of macrocyclic Y–S–O rings converge into only one kind of six-membered ring, consisting of three [SO4] tetrahedra and three [YO8] polyhedra. The [IO3] trigonal pyramids further bridge the adjacent two-dimensional Y(SO4) layers into a three-dimensional framework of Y(IO3)(SO4)·3H2O. It is noteworthy that the introduction of [IO3] into Y2(SO4)3·8H2O not only increases the dimensionality of the crystal structure but also facilitates the structural conversion from centrosymmetric to noncentrosymmetric space groups.
Infrared and UV–vis–NIR spectra
The infrared spectra of Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Er, Ho, Dy, Eu) are presented in Fig. S3.† All compounds have six characteristic absorption bands observed around 3300–3600, 1620–1650, 1100–900, 870–770, 660–500, and 500–400 cm−1, showing a variety of symmetric and antisymmetric vibrations of O–H, S–O, and I–O bonds. Two absorption bands in the ranges of 3300–3600 and 1620–1650 cm−1 are attributed to the O–H bonds from hydroxyl groups, indicating the presence of crystal water molecules in the crystals. The absorption band at 1100–900 cm−1 is assigned to the symmetric stretching ν1 modes of [SO4]. The absorption spectrum in the range of 870–770 cm−1 can be attributed to the antisymmetric stretching ν3 mode of [IO3]. The range of 660–500 cm−1 is attributed to the antisymmetric bending ν4 mode of [SO4], and the absorption spectra in the range of 500–400 cm−1 can be attributed to the combined action of [IO3−] and [SO4]. These assignments are consistent with previous literature.33The UV–vis–NIR diffuse reflectance spectra of Ln(IO3)(SO4)·3H2O (Ln = Er, Ho, Dy, Eu) display characteristic electronic excitation, which is assigned and labeled in their respective spectra (Fig. S4†).34–36 The band gaps of Ln(IO3)(SO4)·3H2O (Ln = Er, Ho, Dy, Eu) were determined to be 4.51 eV (Dy), 4.51 eV (Er), 4.52 eV (Ho), and 4.42 eV (Eu) by making tangent lines at the absorption edges (Fig. S5†). As shown in Fig. 3, the band gaps of Y(IO3)(SO4)·3H2O and Gd(IO3)(SO4)·3H2O are 4.6 eV and 4.55 eV, respectively. These values are higher than those of most iodate-based materials, such as Bi2O(SO4)(IO3)2 (2.40 eV),37 KBi(IO3)3(OH) (3.5 eV),38 KBi(SeO4)(IO3)Cl (3.9 eV),39 and Bi(IO3)(SO4) (3.91 eV).33 This demonstrates that Y(IO3)(SO4)·3H2O and Gd(IO3)(SO4)·3H2O are promising candidates for UV optical materials.
Fluorescence
Due to the imperative necessity of f-block cations in the field of photoluminescence, we investigated the excitation and emission spectra of the compounds Ln(IO3)(SO4)·3H2O (Ln = Eu, Dy, Ho, Er) (Fig. 4). Under 393 nm near-ultraviolet excitation, Eu(IO3)(SO4)·3H2O exhibits two emission peaks: the strongest at 594 nm and the next strongest at 617 nm, corresponding to the 5D0 → 7F1 and 5D0 → 7F2 transitions of Eu3+ ions, respectively (Fig. 4a).40 The excitation spectrum of Dy(IO3)(SO4)·3H2O displays multiple absorption peaks at 324 nm (6P3/2), 350 nm (6P7/2), 365 nm (6P7/2), 386 nm (4F7/2 + 4I13/2), and 449 nm (4I15/2) when the emission is monitored at 578 nm. However, under 350 nm excitation, only two main emission bands are observed: the strongest emission peak at 578 nm (4F7/2 → 6H13/2) and a secondary emission peak at 490 nm (4F9/2 → 6H15/2) (Fig. 4b). In the case of Ho3+ ions in Ho(IO3)(SO4)·3H2O, a strong and narrow emission peak at 396 nm is observed when monitored at 439 nm emission wavelength (Fig. 4c).41 Similarly, under 470 nm near-ultraviolet excitation, the Er3+ ions in Er(IO3)(SO4)·3H2O exhibit a strong and narrow emission peak at 517 nm (Fig. 4d).42,43SHG properties
The SHG responses of the six crystals were measured under 1064 nm radiation using the conventional Kurtz–Perry method. As shown in Fig. 5 and Fig. S6,† although all six crystals crystallize in noncentrosymmetric space groups, the SHG effect for Er(IO3)(SO4)·3H2O, Dy(IO3)(SO4)·3H2O, and Ho(IO3)(SO4)·3H2O is too weak to be measured. In contrast, Y(IO3)(SO4)·3H2O and Gd(IO3)(SO4)·3H2O exhibit their largest SHG effect at particle sizes of 100–150 μm, with SHG intensities of 0.7 × KDP and 0.9 × KDP, respectively. Meanwhile, Eu(IO3)(SO4)·3H2O reaches its maximum SHG intensity of about 1.1 × KDP at particle sizes of 180–250 μm. However, neither Gd(IO3)(SO4)·3H2O nor Eu(IO3)(SO4)·3H2O can achieve phase matching, as indicated by the trend of SHG intensities versus particle sizes. The SHG intensities of Y(IO3)(SO4)·3H2O increase with the increase of particle sizes and substantially remain unchanged at larger size ranges, suggesting the phase-matching ability of the crystal. Calculations of dipole moments reveal that the [IO3] group makes a prominent contribution to the SHG response of the crystals. The calculated dipole moment of the [EuO8] polyhedron is 4.7849 D, largest among the three crystals (Ln = Y, Gd, Eu) and the experimentally determined SHG intensity of Eu(IO3)(SO4)·3H2O is also the highest. These results highlight the significant contribution of the [LnO8] polyhedra to the SHG responses in the Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Eu) systems, which should not be overlooked. Given that the SHG effect and band gap are two critical optical parameters for evaluating nonlinear optical materials, we compared the SHG responses and band gaps of Y(IO3)(SO4)·3H2O and Gd(IO3)(SO4)·3H2O with some selected iodate sulfate crystals (Table 1). | ||
| Fig. 5 SHG intensity for samples Ln(IO3)(SO4)·3H2O (Ln = Y, Gd and Eu) and standard KDP with different particle sizes. | ||
| Crystal | Space group | SHG response (×KDP) | Band gaps (eV) |
|---|---|---|---|
Bi2O(SO4)(IO3)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
P21/n | 0 | 2.40 |
| Ba2Ce(IO3)8(H2O)44 | Pna21 | 0.2 | 2.44 |
BaZr(IO3)6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
0 | 3.1 |
| KBi(IO3)3(OH)38 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
0 | 3.5 |
| Bi(IO3)(SO4)33 | P21/c | 0 | 3.91 |
CdBi(IO3)(SO4)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
P21/c | 0 | 4.03 |
Rb2SO4·SbF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
P212121 | 0.3 | 4.15 |
| NaGaI3O9F47 | P21/c | 0 | 4.27 |
K2SO4·SbF3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
P212121 | 0.1 | 4.44 |
Na7(IO3)(SO4)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
P212121 | 0.5 | 4.83 |
| Gd(IO 3 )(SO 4 )·3H 2 O | P212121 | 0.8 | 4.55 |
| Y(IO 3 )(SO 4 )·3H 2 O | P212121 | 0.7 | 4.6 |
Theoretical analysis
To understand the microscopic mechanisms underlying the optical properties of Y(IO3)(SO4)·3H2O, theoretical calculations have been carried out using the first principles method within the framework of plane-wave-based pseudopotential density-functional theory (DFT). The calculated band structure of compound Y(IO3)(SO4)·3H2O in Fig. 6a reveals an indirect band gap of 4.034 eV, which is slightly lower than the experimental value of 4.60 eV obtained from UV–vis–NIR diffuse reflectance spectra. This discrepancy arises from the shortcoming of the conventional DFT-GGA-PBE functional, which tends to lower the conduction band energy levels, leading to an underestimation of the calculated band gap.49–52 From the total density of states (TDOS) and partial density of states (PDOS) of Y(IO3)(SO4)·3H2O, it is evident that the top of the valence band (VB) is primarily contributed by the I-5s5p and O-2p orbitals. In contrast, the bottom of the conduction band (CB) is mainly contributed by the I-5p, O-2p, and Y-4d orbitals (Fig. 6b). Since the optical properties depend closely on the electron transitions from the VB to the CB, the results of theoretical analysis suggest that the synergistic interaction between the [YO8] polyhedra and [IO3] trigonal pyramids is the primary origin of the optical absorption in Y(IO3)(SO4)·3H2O.The birefringence of Y(IO3)(SO4)·3H2O is significantly enhanced by the introduction of highly anisotropic [IO3] units. For comparison, the calculated birefringence of Y2(SO4)3·8H2O is only 0.004 @ 532 nm, whereas the refractive index of Y(IO3)(SO4)·3H2O is calculated to be 0.118 @ 532 nm (Fig. 7). Furthermore, the birefringence of Y(IO3)(SO4)·3H2O exceeds those of most metal sulfate materials, such as RbY(SO4)2·4H2O (0.045 @ 546 nm),53 Hg3O2SO4 (0.100 @ 546 nm),54 and RbSbSO4Cl2 (0.110 @ 1064 nm).55 The sufficiently large birefringence of 0.118 at 532 nm for Y(IO3)(SO4)·3H2O explains its phase-matchable SHG responses.
Conclusions
In summary, we successfully synthesized a series of rare earth iodate sulfates Ln(IO3)(SO4)·3H2O (Ln = Y, Gd, Er, Ho, Dy, Eu) with noncentrosymmetric structures. Among these, Eu(IO3)(SO4)·3H2O exhibits a large SHG response of 1.1 × KDP at particle sizes of 180–250 μm, while Gd(IO3)(SO4)·3H2O and Y(IO3)(SO4)·3H2O show SHG responses of 0.9 × and 0.7 × KDP at particle sizes of 100–150 μm, respectively. The three-in-one combination of rare earth, iodate, and sulfate functional units results in a comprehensive enhancement of both the SHG effect and optical band gap. The findings of this work provide a feasible design strategy for exploring new nonlinear optical materials that simultaneously exhibit large SHG responses and wide band gaps. Although the other three compounds, Ln(IO3)(SO4)·3H2O (Ln = Er, Ho, Dy), do not display SHG responses, they demonstrate interesting fluorescence properties and can serve as host crystals of rare earth dopants, making them promising candidates for next-generation lighting materials.Data availability
Crystallographic data have been deposited at the CCDC with deposition numbers 2431211–2431216.† The data supporting this article have been included as part of the ESI,† including synthesis and property characterization; details of crystallographic refinement; atomic coordinates and BVS; bond lengths and angles; dipole moments; PXRD patterns; EDS; infrared spectroscopy; UV–vis–NIR diffuse reflectance; and SHG intensity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the Natural Science Foundation of Shandong Province (ZR2020ME021), the Program of High-level Talents Research Funding of Yulin Normal University (G2020ZK20 and G2020ZK21), and the Project of the Cultivation of Middle-aged and Young Backbone Teachers in Guangxi Colleges and Universities (1000 Plan).References
- E. E. Oyeka, M. J. Winiarski, H. Swiatek, W. Balliew, C. D. McMillen, M. L. Liang, M. I. Sorolla and T. T. Tran, Ln2(SeO3)2(SO4)(H2O)2 (Ln = Sm, Dy, Yb): A Mixed-Ligand Pathway to New Lanthanide(III) Multifunctional Materials Featuring Nonlinear Optical and Magnetic Anisotropy Properties, Angew. Chem., Int. Ed., 2022, 61, e202213499 CrossRef CAS.
- C. K. Aslani, V. V. Klepov, M. A. A. Aslani and H. C. Z. Loye, Hydrothermal Synthesis of New Iodates Ln2(IO3)3(IO4) (Ln = La, Nd, Pr) Containing the Tetraoxoiodate(V) Anion: Creation of Luminescence Properties by Doping with Eu, Dy, and Tb, Cryst. Growth Des., 2021, 21, 4707–4712 CrossRef CAS.
- N. Haldar, T. Mondal, T. Das, D. Sarkar, M. Pal, A. H. Seikh and C. K. Ghosh, A truncated octahedron NaCe(MoO4)2 nanostructure: a potential material for blue emission and acetone sensing, Mater. Adv., 2024, 5, 4480–4490 RSC.
- Y. Suffren, O. Leynaud, P. Plaindoux, A. Brenier and I. Gautier-Luneau, Differences and Similarities between Lanthanum and Rare-Earth Iodate Anhydrous Polymorphs: Structures, Thermal Behaviors, and Luminescent Properties, Inorg. Chem., 2016, 55, 11264–11272 CrossRef CAS.
- L. Geng, C. Y. Meng and Q. Yan, Polar lanthanide copper iodates LnCu(IO3)5 (Ln = La, Ce, Pr, and Nd): Synthesis, crystal structure and characterization, J. Solid State Chem., 2022, 308, 122934 CrossRef CAS.
- J. Song, C. G. Li, J. M. Jiao, Y. H. She, W. L. Zhao, F. Liang, N. Ye, Z. G. Hu and Y. C. Wu, KNa2La2(BO3)3: a shortite-type lanthanide borate exhibiting strong nonlinear optical activity induced by isolated [BO3] triangles and distorted [LaO9] polyhedra, Inorg. Chem. Front., 2023, 10, 5488–5495 RSC.
- P. F. Li, C. L. Hu, B. X. Li, F. Kong and J. G. Mao, Y(HSeO3)(SeO3)(H2O)·(H2O) and Y2(SeO3)2(SeO4)(H2O)2·(H2O)0.75: Two yttrium selenites with a short UV cut-off edge explored from pure selenite compounds, J. Alloys Compd., 2023, 959, 170570 CrossRef CAS.
- A. M. Li, D. K. Xu, H. Lin, S. H. Yang, Y. Z. Shao and Y. L. Zhang, NaGd(MoO4)2 nanocrystals with diverse morphologies: controlled synthesis, growth mechanism, photoluminescence and thermometric properties, Sci. Rep., 2016, 6, 31366 CrossRef CAS PubMed.
- Q. Wu, X. Liu, S. R. Xu, H. B. Pi and Y. J. Li, Five New Silver Rare–Earth Iodates, AgLn(IO3)4 (Ln = Pr, Sm, Dy, Er and Tm): Synthesis, Structures and Properties, ChemistrySelect, 2019, 4, 8709–8712 CrossRef CAS.
- P. F. Li, C. L. Hu, J. G. Mao and F. Kong, A UV non-hydrogen pure selenite nonlinear optical material for achieving balanced properties through framework-optimized structural transformation, Mater. Horiz., 2024, 11, 1704–1709 RSC.
- J. Chen, C. L. Hu, F. F. Mao, B. P. Yang, X. H. Zhang and J. G. Mao, REI5O14 (RE = Y and Gd): Promising SHG Materials Featuring the Semicircle-Shaped I5O143− Polyiodate Anion, Angew. Chem., Int. Ed., 2019, 58, 11666–11669 CrossRef CAS PubMed.
- H. P. Wu, Z. J. Wei, Z. G. Hu, J. Y. Wang, Y. C. Wu and H. W. Yu, Assembly of π-Conjugated [B3O6] Units by Mer-Isomer [YO3F3] Octahedra to Design a UV Nonlinear Optical Material, Cs2YB3O6F2, Angew. Chem., Int. Ed., 2024, 63, e202406318 CrossRef CAS PubMed.
- P. F. Li, C. L. Hu, F. Kong and J. G. Mao, The First UV Nonlinear Optical Selenite Material: Fluorination Control in CaYF(SeO3)2 and Y3F(SeO3)4, Angew. Chem., Int. Ed., 2023, 62, e202301420 CrossRef CAS PubMed.
- C. Chen, J. Chen, G. Yang, H. Tian, M. Luo, T. Yan, Z. Hu, J. Wang, Y. Wu, N. Ye and G. Peng, Intense d-p Hybridization in Nb3O15 Tripolymer Induced the Largest Second Harmonic Generation Response and Birefringence in Germanates, Angew. Chem., Int. Ed., 2023, 62, e202217039 CrossRef PubMed.
- X. L. Du, X. J. Guo, Z. L. Gao, F. A. Liu, F. F. Guo, S. Y. Wang, H. Y. Wang, Y. X. Sun and X. T. Tao, Li2MTeO6(M = Ti, Sn): Mid-Infrared Nonlinear Optical Crystal with Strong Second Harmonic Generation Response and Wide Transparency Range, Angew. Chem., Int. Ed., 2021, 60, 23320–23326 CrossRef CAS PubMed.
- S. D. Nguyen, J. Yeon, S. H. Kim and P. S. Halasyamani, BiO(IO3): A New Polar iodate that Exhibits an Aurivillius-Type (Bi2O2)2+ Layer and a Large SHG Response, J. Am. Chem. Soc., 2011, 133, 12422–12425 CrossRef CAS PubMed.
- R. L. Tang, C. L. Hu, B. L. Wu, Z. Fang, Y. Chen and J. G. Mao, Cs2Bi2O(Ge2O7) (CBGO): A Larger SHG Effect Induced by Synergistic Polarizations of BiO5 Polyhedra and GeO4 Tetrahedra, Angew. Chem., Int. Ed., 2019, 58, 15358–15361 CrossRef CAS PubMed.
- N. Ma, C. L. Hu, J. Chen, Z. Fang, Y. Huang, B. X. Li and J. G. Mao, CaCe(IO3)3(IO3F)F: a promising nonlinear optical material containing both IO3− and IO3F2− anions, Inorg. Chem. Front., 2022, 9, 5478–5485 RSC.
- Q. M. Huang, C. L. Hu, B. P. Yang, Z. Fang, Y. Lin, J. Chen, B. X. Li and J. G. Mao, [GaF(H2O)][IO3F]: a promising NLO material obtained by anisotropic polycation substitution, Chem. Sci., 2021, 12, 9333–9338 RSC.
- Q. Q. Chen, C. L. Hu, L. J. Yao, J. Chen, M. Y. Cao, B. X. Li and J. G. Mao, Cd2(IO3)(PO4) and Cd1.62Mg0.38(IO3)(PO4): metal iodate-phosphates with large SHG responses and wide band gaps, Chem. Commun., 2022, 58, 7694–7697 RSC.
- H. M. Liu, Q. C. Wu, L. L. Liu, Z. S. Lin, P. S. Halasyamani, X. G. Chen and J. G. Qin, AgBi(SO4)(IO3)2: aliovalent substitution induces structure dimensional upgrade and second harmonic generation enhancement, Chem. Commun., 2021, 57, 3712–3715 RSC.
- Y. Q. Li, F. Liang, S. G. Zhao, L. N. Li, Z. Y. Wu, Q. R. Ding, S. Liu, Z. S. Lin, M. C. Hong and J. H. Luo, Two Non-π-Conjugated Deep-UV Nonlinear Optical Sulfates, J. Am. Chem. Soc., 2019, 141, 3833–3837 CrossRef CAS PubMed.
- Y. Q. Li, S. G. Zhao, P. Shan, X. F. Li, Q. R. Ding, S. Liu, Z. Y. Wu, S. S. Wang, L. N. Li and J. H. Luo, Li8NaRb3(SO4)6·2H2O as a new sulfate deep-ultraviolet nonlinear optical material, J. Mater. Chem. C, 2018, 6, 12240–12244 RSC.
- X. H. Dong, Y. Long, X. Y. Zhao, L. Huang, H. M. Zeng, Z. E. Lin, X. Wang and G. H. Zou, A6Sb4F12(SO4)3 (A = Rb, Cs): Two Novel Antimony Fluoride Sulfates with Unique Crown-like Clusters, Inorg. Chem., 2020, 59, 8345–8352 CrossRef CAS PubMed.
- H. J. Lu, X. J. Guo, Y. X. Wang, K. Diefenbach, L. H. Chen, J. Q. Wang, J. Lin and S. A. Wang, Size-dependent selective crystallization using an inorganic mixed-oxoanion system for lanthanide separation, Dalton Trans., 2019, 48, 12808–12811 RSC.
- C. K. Aslani, V. V. Klepov and H. C. zur Loye, Hydrothermal synthesis of new mixed-oxoanion materials: Rare earth iodate sulfates Sm(IO3)(SO4) and Ln2(IO3)3(SO4)OH·3H2O (Ln = Sm, Eu, Dy), Solid State Sci., 2022, 129, 106918 CrossRef CAS.
- T. H. Wu, X. X. Jiang, Y. R. Zhang, Z. J. Wang, H. Y. Sha, C. Wu, Z. S. Lin, Z. P. Huang, X. F. Long, M. G. Humphrey and C. Zhang, From CeF2(SO4)·H2O to Ce(IO3)2(SO4): Defluorinated Homovalent Substitution for Strong Second-Harmonic-Generation Effect and Sufficient Birefringence, Chem. Mater., 2021, 33, 9317–9325 CrossRef CAS.
- X. L. Cao, C. L. Hu, X. Xu, F. Kong and J. G. Mao, Pb2TiOF(SeO3)2Cl and Pb2NbO2(SeO3)2Cl: small changes in structure induced a very large SHG enhancement, Chem. Commun., 2013, 49, 9965–9967 RSC.
- F. G. You, F. Liang, Q. Huang, Z. G. Hu, Y. C. Wu and Z. S. Lin, Pb2GaF2(SeO3)2Cl: Band Engineering Strategy by Aliovalent Substitution for Enlarging Bandgap while Keeping Strong Second Harmonic Generation Response, J. Am. Chem. Soc., 2019, 141, 748–752 CrossRef CAS PubMed.
- H. W. Yu, M. L. Nisbet and K. R. Poeppelmeier, Assisting the Effective Design of Polar Iodates with Early Transition-Metal Oxide Fluoride Anions, J. Am. Chem. Soc., 2018, 140, 8868–8876 CrossRef CAS PubMed.
- J. Chen, C. L. Hu, F. F. Mao, J. H. Feng and J. G. Mao, A Facile Route to Nonlinear Optical Materials: Three-Site Aliovalent Substitution Involving One Cation and Two Anions, Angew. Chem., Int. Ed., 2019, 58, 2098–2102 CrossRef CAS PubMed.
- P. Held and M. Wickleder, Yttrium(III) sulfate octahydrate, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2003, 59, i98–i100 CrossRef CAS.
- Y. L. Li, C. L. Hu, J. Chen and J. G. Mao, Two bismuth iodate sulfates with enhanced optical anisotropy, Dalton Trans., 2021, 50, 16139–16146 RSC.
- W. T. Carnall, P. R. Fields and K. Rajnak, Spectral Intensities of the Trivalent Lanthanides and Actinides in Solution. II. Pm3+, Sm3+, Eu3+, Gd3+, Tb3+, Dy3+, and Ho3+, J. Chem. Phys., 1968, 49, 4412–4423 CrossRef CAS.
- W. T. Carnall, P. R. Fields and K. Rajnak, Electronic Energy Levels of the Trivalent Lanthanide Aquo Ions. IV. Eu3+, J. Chem. Phys., 1968, 49, 4450–4455 CrossRef CAS.
- M. Y. Qie, J. Lin, F. Kong, M. A. Silver, Z. H. Yue, X. M. Wang, L. J. Zhang, H. L. Bao, T. E. Albrecht-Schmitt and J. Q. Wang, A Large Family of Centrosymmetric and Chiral f-Element-Bearing Iodate Selenates Exhibiting Coordination Number and Dimensional Reductions, Inorg. Chem., 2018, 57, 1676–1683 CrossRef CAS PubMed.
- L. Shi, D. J. Mei, J. L. Xu and Y. D. Wu, Bi2O(XO4)(IO3)2 (X = S, Se, Cr): Three-dimensional frameworks containing [Bi4O2]8+ clusters, Solid State Sci., 2017, 63, 54–61 CrossRef CAS.
- Y. J. Jia, Y. G. Chen, T. Wang, Y. Guo, X. F. Guan and X. M. Zhang, KBi(IO3)3(OH) and NaBi(IO3)4: from the centrosymmetric chain to a noncentrosymmetric double layer, Dalton Trans., 2019, 48, 10320–10326 RSC.
- L. Geng, S. H. Ma, B. Z. Zhu, W. F. Li, K. Y. Jiang, H. Y. Lu, Q. Yan and C. Y. Meng, KBi(SeO4)(IO3)Cl: A mixed-anion oxychloride with three types of building units in one structure, J. Solid State Chem., 2024, 329, 124385 CrossRef CAS.
- A. S. Souza, Y. A. R. Oliveira and M. A. Couto dos Santos, Enhanced approach to the Eu3+ ion 5D0→7F0 transition intensity, Opt. Mater., 2013, 35, 1633–1635 CrossRef CAS.
- Z. G. Feng, H. P. Xia, C. Wang, Z. X. Zhang, D. S. Jiang, J. Zhang, S. N. He, Q. Y. Tang, Q. G. Sheng, X. M. Gu, Y. P. Zhang, B. J. Chen and H. C. Jiang, Fluorescence spectra of Na5Lu9F32 single crystals co-doped with Ho3+/Tm3+ grown by Bridgman method, Chem. Phys. Lett., 2016, 652, 68–72 CrossRef CAS.
- J. A. Sanz-García, G. Lifante-Pedrola, J. E. M. Santiuste and E. Cantelar, Dual luminescent nano-thermometry based on the selective excitation of optical centers in CaF2:Er3+ nanoparticles, J. Alloys Compd., 2025, 1010, 177529 CrossRef.
- C. Tian, J. Ruan, X. J. Zhao, J. J. Han and C. Liu, Structure, spectroscopic properties and optical temperature-sensing behavior of glass-ceramics containing polymorphic CaTa2O6:Er3+/Yb3+ nanocrystals, J. Mater. Chem. C, 2024, 12, 16594–16607 RSC.
- X. Y. Zhang, X. H. Zhang, B. P. Yang and J. G. Mao, A new polar alkaline earth-rare earth iodate: Ba2Ce(IO3)8(H2O), Dalton Trans., 2023, 52, 4423–4428 RSC.
- H. S. Ahn, D. W. Lee and K. M. Ok, Dimensionality variations in new zirconium iodates: hydrothermal syntheses, structural determination, and characterization of BaZr(IO3)6 and K2Zr(IO3)6, Dalton Trans., 2014, 43, 10456–10461 RSC.
- F. F. He, L. Wang, C. F. Hu, J. Zhou, Q. Li, L. Huang, D. J. Gao, J. Bi, X. Wang and G. H. Zou, Cation-tuned synthesis of the A2SO4.SbF3 (A = Na+, NH4+, K+, Rb+) family with nonlinear optical properties, Dalton Trans., 2018, 47, 17486–17492 RSC.
- D. D. Wang, P. F. Gong, X. Y. Zhang, Z. S. Lin, Z. G. Hu and Y. C. Wu, NaGaI3O9F: a new alkali metal gallium iodate combined with IO3− and IO3F2− units, Dalton Trans., 2021, 50, 11562–11567 RSC.
- M. M. Ding, H. W. Yu, Z. G. Hu, J. Y. Wang and Y. C. Wu, Na7(IO3)(SO4)3: the first noncentrosymmetric alkaline-metal iodate-sulfate with isolated [IO3] and [SO4] units, Chem. Commun., 2021, 57, 9598–9601 RSC.
- Q. Q. Chen, C. L. Hu, B. X. Li and J. G. Mao, [M(OH)2]3(IO3)(SeO4)·H2O (M = Ga and In): metal iodate–selenate nonlinear optical materials with a hexagonal tungsten oxide-type topology, Inorg. Chem. Front., 2023, 10, 3121–3130 RSC.
- L. Geng, C. Y. Meng, H. Y. Lu, Z. Z. Luo, C. S. Lin and W. D. Cheng, Bi2Te(IO3)O5Cl: a novel polar iodate oxychloride exhibiting a second-order nonlinear optical response, Dalton Trans., 2015, 44, 2469–2475 RSC.
- Q. Li, H. N. Liu, H. W. Yu, Z. G. Hu, J. Y. Wang, Y. C. Wu and H. P. Wu, Alignment of Λ-Shaped Basic Building Units to Construct One New KMoO3(IO3) Polar Polymorph, Inorg. Chem., 2023, 62, 3896–3903 CrossRef CAS PubMed.
- C. Y. Meng, L. Geng, W. T. Chen, M. F. Wei, K. Dai, H. Y. Lu and W. D. Cheng, Syntheses, structures, and characterizations of a new second-order nonlinear optical material: Pb2(SeO3)(NO3)2, J. Alloys Compd., 2015, 640, 39–44 CrossRef CAS.
- W. Lu, Y. Shen and Q. Wu, RbY(SO4)2·4H2O: A new ultraviolet birefringent crystal, J. Solid State Chem., 2024, 333, 124546 CrossRef CAS.
- X. Dong, L. Huang, H. Zeng, Z. Lin, K. M. Ok and G. Zou, High-Performance Sulfate Optical Materials Exhibiting Giant Second Harmonic Generation and Large Birefringence, Angew. Chem., Int. Ed., 2022, 61, e202116790 CrossRef CAS PubMed.
- F. He, Y. Deng, X. Zhao, L. Huang, D. Gao, J. Bi, X. Wang and G. Zou, RbSbSO4Cl2: an excellent sulfate nonlinear optical material generated due to the synergistic effect of three asymmetric chromophores, J. Mater. Chem. C, 2019, 7, 5748–5754 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, property characterization and crystallographic data. CCDC 2431211-2431216. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00613a |
| This journal is © The Royal Society of Chemistry 2025 |





