Open Access Article
Open Access ArticleC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) S bond activation by an annulated 1,4,2-diazaborole†
S bond activation by an annulated 1,4,2-diazaborole†
Vignesh
Pattathil
 and
Conor
Pranckevicius
and
Conor
Pranckevicius
 *
*
Department of Chemistry, Charles E. Fipke Centre for Innovative Research, University of British Columbia, Okanagan Campus, 3247 University Way, Kelowna, BC, Canada. E-mail: conor.pranckevicius@ubc.ca
First published on 17th April 2025
Abstract
The reaction of an ambiphilic 1,4,2-diazaborole with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds results in formal (3 + 2) cycloaddition and has allowed the synthesis of a family of 1,3,2-oxazaborole and 1,3,2-thiazaborole derivatives. Computational calculations have indicated a dipolar mechanism where the π bond is concertedly activated via the Lewis acidic boron centre and the nucleophilic C5 position of the 1,4,2-diazaborole. In the case of methylisothiocyanate, preference for C
S bonds results in formal (3 + 2) cycloaddition and has allowed the synthesis of a family of 1,3,2-oxazaborole and 1,3,2-thiazaborole derivatives. Computational calculations have indicated a dipolar mechanism where the π bond is concertedly activated via the Lewis acidic boron centre and the nucleophilic C5 position of the 1,4,2-diazaborole. In the case of methylisothiocyanate, preference for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S over C
S over C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N addition is observed, and has been rationalized according to mechanistic calculations. A spirocyclic bis(1,3,2-thiazaborole) has been observed from the double activation of CS2.
N addition is observed, and has been rationalized according to mechanistic calculations. A spirocyclic bis(1,3,2-thiazaborole) has been observed from the double activation of CS2.
Introduction
The activation of π-bonds by ambiphilic metal centres according to the Dewar-Chatt-Duncanson model1–3 is a cornerstone of transition metal catalysis, facilitating the hydroelementation,4–11 metathesis,12–19 and catenation20–26 of unsaturated bonds. This interaction involves the donation of electrons from a π bond of an unsaturated substrate to a vacant metal orbital and simultaneous backdonation from the metal to a vacant substrate π* orbital. This has the net effect of lengthening and weakening the bond and lowering its thermodynamic barrier to reactivity. An essential component of this activation is the ambiphilicity of the metal centre, serving as both a Lewis acid and base in this interaction.This concept was extended to bimolecular main group systems through the advent of frustrated Lewis pairs,27–30 where non-interacting Lewis acid/base pairs have been shown to activate challenging substrates such as hydrogen,31–34 C–H bonds,35–38 as well as a wealth of π-bonded species in the absence of transition metals.39–44 More recently, ambiphilic boron heterocycles have been shown to activate a large variety of unsaturated species such as alkenes, alkynes, carbonyls, and arenes45–52 and their applications in catalytic CO2 hydroboration and N-formylation have recently been reported.53
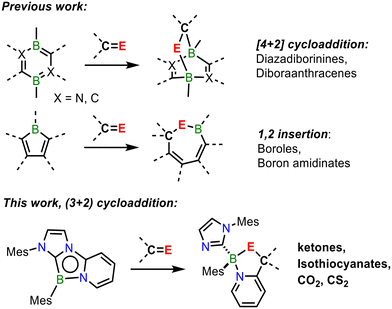
Despite the multitude of reports involving diboron systems, aromatic B1-heterocycles have received considerably less attention as platforms for bond activation.54–57 Aromatic 1,4,2-diazaboroles were first prepared by Kinjo and co-workers,58 and were shown to be boron nucleophiles, participating in a B-centred electrophilic fluorination reaction with Selectfluor. Our research group recently reported a novel tricyclic 1,4,2-diazaborole featuring a dearomatized annulated pyridyl group.59 This species displays markedly increased reactivity with respect to monocyclic analogues, and its reaction with various borane Lewis acids resulted in the formation of ring-expanded 1,4,2,5-diazadiborinine derivatives via a 1,3-dipolar addition. These observations made us question whether this reactivity could be extended to heterocycle formation via the activation of polar unsaturated bonds. Herein, we report the activation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds by an annulated 1,4,2-diazaborole to form 1,3,2-oxazaborole and 1,3,2-thiazaborole derivatives. Notably, while other borole derivatives have been previously observed to react with C
S bonds by an annulated 1,4,2-diazaborole to form 1,3,2-oxazaborole and 1,3,2-thiazaborole derivatives. Notably, while other borole derivatives have been previously observed to react with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/S bonds via 1,2-insertion to form ring-expanded products,51,60,61 we here report the first examples of (3 + 2) cycloaddition reactions with these unsaturated species (Scheme 1).
O/S bonds via 1,2-insertion to form ring-expanded products,51,60,61 we here report the first examples of (3 + 2) cycloaddition reactions with these unsaturated species (Scheme 1).
Results and discussion
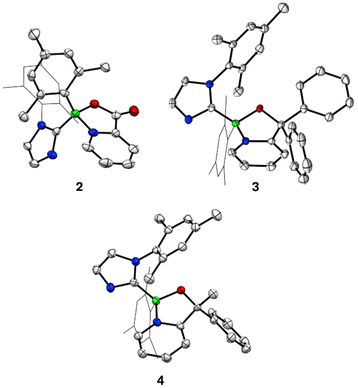
In an initial experiment, a solution of the annulated 1,4,2-diazaborole 1 in benzene was placed under an atmosphere of CO2 and was heated at 80 °C overnight, resulting in a colour change from deep red to pale brown. Removal of the solvent, followed by washing of the residue with n-hexane yielded a colourless solid. An 11B NMR spectrum of this material revealed a single resonance at 7.0 ppm. 1H NMR indicated a C1 symmetric molecule and the presence of 2-pyridyl and mesitylimidazol-2-yl moieties. The appearance of a new peak at 163.4 ppm in the 13C{1H} NMR suggested the incorporation of the CO2 moiety via esterification. A single crystal X-ray diffraction study revealed the product 2 as a 1,3,2-oxazaborole derivative (Scheme 2, Fig. 1).To shed light on the mechanism of this transformation, the reaction of 1 with CO2 was investigated using DFT methods (M062X-D3/Def2SVP). The first transition state is a rate-limiting (3 + 2) cycloaddition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond across the B2–C5 positions of the 1,4,2-diazaborole 1. This transition state is asynchronous yet concerted, where the C–C bond is formed slightly before the B–O bond (Fig. S26 in the ESI†). This intermediate subsequently undergoes loss of the imidazole moiety via C–N bond cleavage, concomitant with re-aromatization of pyridine ring to form the 1,3,2-oxazaborole derivative 2.
O bond across the B2–C5 positions of the 1,4,2-diazaborole 1. This transition state is asynchronous yet concerted, where the C–C bond is formed slightly before the B–O bond (Fig. S26 in the ESI†). This intermediate subsequently undergoes loss of the imidazole moiety via C–N bond cleavage, concomitant with re-aromatization of pyridine ring to form the 1,3,2-oxazaborole derivative 2.
To explore the scope of this novel bond activation, the reaction of 1 with ketones was next attempted. Treatment of a benzene solution of 1 with benzophenone at 75 °C cleanly resulted in the formation of a new product after 24 h. Similar to 2, 1H NMR indicated a C1 symmetric product bearing both mesitylimidazol-2-yl and 2-pyridyl moieties. 11B NMR revealed a single resonance at 9.2 ppm. A single crystal X-ray diffraction study indicated the product 3 is similarly derived from cycloaddition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond across the C–N–B linkage of 1 (Scheme 2, Fig. 1). A reaction with acetophenone (60 °C for 24 h) also afforded the analogous compound 4, and its structure was confirmed crystallographically (Scheme 2, Fig. 1). Notably, only one diastereomer of compound 4 was observed. Somewhat surprisingly, the reaction of 1 with these bulky ketones were observably faster than with CO2, suggesting that these latter reactions are facilitated by the relative electrophilicity of the ketone carbonyl centres. Accordingly, no reactivity was observed of 1 with esters, amides, isocyanates, or carbodiimides under more forcing conditions. This observation is also in line with the calculated reaction barriers for these transformations (21.6 kcal mol−1 for 2, 17.4 kcal mol−1 for 4) (M062X-D3/Def2SVP). In all cases, these compounds are generated as racemic mixtures.
O bond across the C–N–B linkage of 1 (Scheme 2, Fig. 1). A reaction with acetophenone (60 °C for 24 h) also afforded the analogous compound 4, and its structure was confirmed crystallographically (Scheme 2, Fig. 1). Notably, only one diastereomer of compound 4 was observed. Somewhat surprisingly, the reaction of 1 with these bulky ketones were observably faster than with CO2, suggesting that these latter reactions are facilitated by the relative electrophilicity of the ketone carbonyl centres. Accordingly, no reactivity was observed of 1 with esters, amides, isocyanates, or carbodiimides under more forcing conditions. This observation is also in line with the calculated reaction barriers for these transformations (21.6 kcal mol−1 for 2, 17.4 kcal mol−1 for 4) (M062X-D3/Def2SVP). In all cases, these compounds are generated as racemic mixtures.
This reactivity was next extended to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonded species in the form of isothiocyanates. The presence of both C
S bonded species in the form of isothiocyanates. The presence of both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
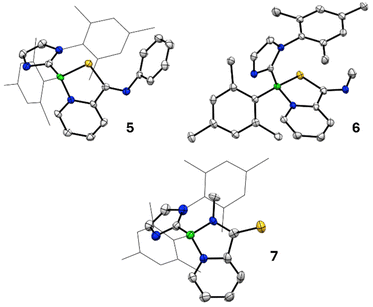
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds in the same molecule was particularly intriguing due to the possibility of regioisomerism in the reaction products. An equimolar reaction of 1 and phenylisothiocyanate at room temperature over the course of 1 hour cleanly afforded a new product 5 with an 11B NMR resonance at 3.7 ppm. A single crystal X-ray diffraction study revealed 5 to be the 1,3,2-thiazaborole derivative (Scheme 3, Fig. 2) derived from selective (3 + 2) cycloaddition of the C
S bonds in the same molecule was particularly intriguing due to the possibility of regioisomerism in the reaction products. An equimolar reaction of 1 and phenylisothiocyanate at room temperature over the course of 1 hour cleanly afforded a new product 5 with an 11B NMR resonance at 3.7 ppm. A single crystal X-ray diffraction study revealed 5 to be the 1,3,2-thiazaborole derivative (Scheme 3, Fig. 2) derived from selective (3 + 2) cycloaddition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond. Interestingly, preferential C
S bond. Interestingly, preferential C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond activation of isothiocyanates had been previously observed with boroles by Martin and co-workers.61 In these systems, it is proposed that adduct formation between the Lewis acidic B centre and the nucleophilic N atom precedes 1,2 insertion, resulting in the selective C
N bond activation of isothiocyanates had been previously observed with boroles by Martin and co-workers.61 In these systems, it is proposed that adduct formation between the Lewis acidic B centre and the nucleophilic N atom precedes 1,2 insertion, resulting in the selective C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N incorporation in the ring expanded products. Conversely, it has been calculated in the formation of 5 that C–C bond formation occurs first via nucleophilic attack of the C5 position of 1 on the isothiocyanate carbon (Fig. S26†). The bulkier N–Ph moiety likely results in steric preference for subsequent B–S bond formation and the exclusive formation of a 1,3,2-thiazaborole in this case.
N incorporation in the ring expanded products. Conversely, it has been calculated in the formation of 5 that C–C bond formation occurs first via nucleophilic attack of the C5 position of 1 on the isothiocyanate carbon (Fig. S26†). The bulkier N–Ph moiety likely results in steric preference for subsequent B–S bond formation and the exclusive formation of a 1,3,2-thiazaborole in this case.
We next examined the reactivity of a sterically unencumbered isothiocyanate. A reaction of 1 with MeNCS under analogous conditions produced a mixture of two products clearly identifiable by 1H NMR in an approximate ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An 11B NMR spectrum revealed a peak at 3.1 ppm corresponding to the major product and an additional minor resonance at 1.4 ppm. When single crystals were grown directly from the reaction mixture, a three-component co-crystal was obtained. The first component was free MeNCS; the second component was the C
1. An 11B NMR spectrum revealed a peak at 3.1 ppm corresponding to the major product and an additional minor resonance at 1.4 ppm. When single crystals were grown directly from the reaction mixture, a three-component co-crystal was obtained. The first component was free MeNCS; the second component was the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S activated 1,3,2-thiazaborole isomer 6 (Scheme 3, Fig. 2). The final component was substitutionally disordered, the minor contributor of which (39%) is also compound 6. The major contributor (61%) of this component is the C
S activated 1,3,2-thiazaborole isomer 6 (Scheme 3, Fig. 2). The final component was substitutionally disordered, the minor contributor of which (39%) is also compound 6. The major contributor (61%) of this component is the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N activated 1,3,2-diazaborole isomer 7 (Scheme 3, Fig. 2), indicating that C
N activated 1,3,2-diazaborole isomer 7 (Scheme 3, Fig. 2), indicating that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N activation is competitive with C
N activation is competitive with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S activation in this case. As these compounds co-crystallize, attempts to purify 7 from the reaction mixture were unfortunately unsuccessful. However, compound 6 could be isolated through repeated recrystallization and was fully characterized.
S activation in this case. As these compounds co-crystallize, attempts to purify 7 from the reaction mixture were unfortunately unsuccessful. However, compound 6 could be isolated through repeated recrystallization and was fully characterized.
The formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N versus C
N versus C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S activated products in the reaction of 1 with MeNCS was evaluated computationally (Fig. 3). The formation of compound 6 follows a similar mechanistic pathway as 2, 4, and 5 involving a concerted (3 + 2) cycloaddition of the C
S activated products in the reaction of 1 with MeNCS was evaluated computationally (Fig. 3). The formation of compound 6 follows a similar mechanistic pathway as 2, 4, and 5 involving a concerted (3 + 2) cycloaddition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond with an energy barrier of 20.6 kcal mol−1 (Fig. 3). However, the formation of 7 was determined to follow a stepwise mechanism involving distinct C–C (TS 1) and C–N (TS 1′) bond formation steps. Despite the greater thermodynamic stability of 7, the barrier to formation of 6 (20.6 kcal mol−1) is lower than that of 7 (23.9 kcal mol−1), which is in good agreement with the observed reaction selectivity.
S bond with an energy barrier of 20.6 kcal mol−1 (Fig. 3). However, the formation of 7 was determined to follow a stepwise mechanism involving distinct C–C (TS 1) and C–N (TS 1′) bond formation steps. Despite the greater thermodynamic stability of 7, the barrier to formation of 6 (20.6 kcal mol−1) is lower than that of 7 (23.9 kcal mol−1), which is in good agreement with the observed reaction selectivity.
 | ||
| Fig. 3 Computed reaction pathways for the formation of 6 and 7 from 1 and MeNCS (M062X-D3/Def2SVP). Values are given in kcal/mol. | ||
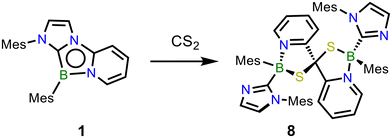
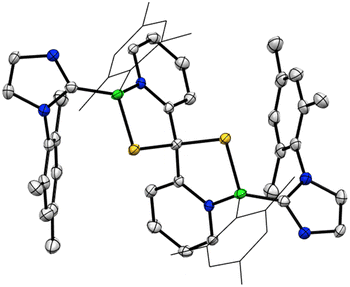
Given the propensity of 1 to activate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds, its reactivity with CS2 was next evaluated. This more strongly electrophilic compound resulted in an instantaneous reaction at ambient temperature to form a new product 8, which crystallized directly from the reaction mixture (Scheme 4). An 11B NMR spectrum revealed a new resonance at 5.7 ppm. A single crystal X-ray diffraction study revealed 8 to be a spiro compound bearing two fused 1,3,2-thiazaborole rings that share a common carbon centre (Fig. 4). This reaction is highly selective toward the double addition product 8 – a deficiency of CS2 in this reaction results in the formation of a mixture of 8 and unreacted 1. Mechanistically, it was found the formation of 8 also proceeds similarly via a double (3 + 2) cycloaddition of both C
S bonds, its reactivity with CS2 was next evaluated. This more strongly electrophilic compound resulted in an instantaneous reaction at ambient temperature to form a new product 8, which crystallized directly from the reaction mixture (Scheme 4). An 11B NMR spectrum revealed a new resonance at 5.7 ppm. A single crystal X-ray diffraction study revealed 8 to be a spiro compound bearing two fused 1,3,2-thiazaborole rings that share a common carbon centre (Fig. 4). This reaction is highly selective toward the double addition product 8 – a deficiency of CS2 in this reaction results in the formation of a mixture of 8 and unreacted 1. Mechanistically, it was found the formation of 8 also proceeds similarly via a double (3 + 2) cycloaddition of both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S moieties across two equivalents of 1 (Fig. S26†).
S moieties across two equivalents of 1 (Fig. S26†).
Conclusion
In summary, we have determined the reactivity of an annulated 1,4,2-diazaborole 1 with various C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonded species to form rare examples of 1,3,2-oxazaboroles and 1,3,2-thiazaboroles via (3 + 2) cycloaddition. These transformations constitute a unique reaction pathway in the activation of π bonds by boron heterocycles, and display unusual regioselectivity in the preferential activation of C
S bonded species to form rare examples of 1,3,2-oxazaboroles and 1,3,2-thiazaboroles via (3 + 2) cycloaddition. These transformations constitute a unique reaction pathway in the activation of π bonds by boron heterocycles, and display unusual regioselectivity in the preferential activation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds of isothiocyanates.
S bonds of isothiocyanates.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 2, 3, 4, 5, 6, [6·(6/7)], and 8 has been deposited at the CCDC under accession numbers 2431184, 2431182, 2431179, 2431181, 2431190, 2431186, and 2431178 and can be obtained from https://www.ccdc.cam.ac.uk/Conflicts of interest
There are no conflicts to declare.Acknowledgements
C.P. thanks The University of British Columbia's Advanced Research Computing for computing facilities. C.P acknowledges NSERC Discovery Grant RGPIN-2021-03056 and CFI JELF/BCKDF 41570 for funding.References
- J. Chatt and L. A. Duncanson, J. Chem. Soc., 1953, 2939–2947 RSC.
- J. Chatt, L. A. Duncanson and L. M. Venanzi, J. Chem. Soc., 1955, 4456–4460 RSC.
- D. M. P. Mingos, J. Organomet. Chem., 2001, 635, 1–8 CrossRef CAS.
- J. R. Hummel, J. A. Boerth and J. A. Ellman, Chem. Rev., 2017, 117, 9163–9227 CrossRef CAS PubMed.
- C.-H. Jun, Chem. Soc. Rev., 2004, 33, 610–618 RSC.
- R. K. Dhungana, S. Kc, P. Basnet and R. Giri, TCR, 2018, 18, 1314–1340 CAS.
- J. S. Yadav, A. Antony, T. S. Rao and B. V. Subba Reddy, J. Organomet. Chem., 2011, 696, 16–36 CrossRef CAS.
- S. Teng and J. S. Zhou, Chem. Soc. Rev., 2022, 51, 1592–1607 RSC.
- S. Ma and J. F. Hartwig, Acc. Chem. Res., 2023, 56, 1565–1577 CrossRef CAS PubMed.
- J. Guo, Z. Cheng, J. Chen, X. Chen and Z. Lu, Acc. Chem. Res., 2021, 54, 2701–2716 CrossRef CAS PubMed.
- S. Biswas, M. M. Parsutkar, S. M. Jing, V. V. Pagar, J. H. Herbort and T. V. RajanBabu, Acc. Chem. Res., 2021, 54, 4545–4564 CrossRef CAS PubMed.
- D. S. Belov, G. Tejeda and K. V. Bukhryakov, ChemPlusChem, 2021, 86, 924–937 CrossRef CAS PubMed.
- H. Albright, A. J. Davis, J. L. Gomez-Lopez, H. L. Vonesh, P. K. Quach, T. H. Lambert and C. S. Schindler, Chem. Rev., 2021, 121, 9359–9406 CrossRef CAS PubMed.
- O. M. Ogba, N. C. Warner, D. J. O'Leary and R. H. Grubbs, Chem. Soc. Rev., 2018, 47, 4510–4544 RSC.
- A. Fürstner, Angew. Chem., Int. Ed., 2000, 39, 3012–3043 CrossRef.
- A. H. Hoveyda and A. R. Zhugralin, Nature, 2007, 450, 243–251 CrossRef CAS PubMed.
- M. Schuster and S. Blechert, Angew. Chem., Int. Ed. Engl., 1997, 36, 2036–2056 CrossRef.
- C. Copéret, Z. J. Berkson, K. W. Chan, J. de Jesus Silva, C. P. Gordon, M. Pucino and P. A. Zhizhko, Chem. Sci., 2021, 12, 3092–3115 RSC.
- H. Ehrhorn and M. Tamm, Chem. – Eur. J., 2019, 25, 3190–3208 CrossRef CAS PubMed.
- F.-P. Wu, Y. Yang, D. P. Fuentes and X.-F. Wu, Chem, 2022, 8, 1982–1992 CAS.
- H. Makio, H. Terao, A. Iwashita and T. Fujita, Chem. Rev., 2011, 111, 2363–2449 CrossRef CAS PubMed.
- V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283–316 CrossRef CAS PubMed.
- M. Delferro and T. J. Marks, Chem. Rev., 2011, 111, 2450–2485 CrossRef CAS PubMed.
- C. Chen, Nat. Rev. Chem., 2018, 2, 6–14 CrossRef CAS.
- T. Matsugi and T. Fujita, Chem. Soc. Rev., 2008, 37, 1264–1277 RSC.
- S. Kumar, B. Z. Dholakiya and R. Jangir, J. Organomet. Chem., 2021, 953, 122066 CrossRef CAS.
- D. W. Stephan, J. Am. Chem. Soc., 2015, 137, 10018–10032 CrossRef CAS PubMed.
- A. R. Jupp and D. W. Stephan, Trends Chem., 2019, 1, 35–48 CrossRef CAS.
- F.-G. Fontaine and D. W. Stephan, Philos. Trans. R. Soc., A., 2017, 375, 20170004 CrossRef PubMed.
- D. W. Stephan, Acc. Chem. Res., 2015, 48, 306–316 CrossRef CAS PubMed.
- D. J. Scott, M. J. Fuchter and A. E. Ashley, Chem. Soc. Rev., 2017, 46, 5689–5700 RSC.
- D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2010, 49, 46–76 CrossRef CAS PubMed.
- D. W. Stephan, J. Am. Chem. Soc., 2021, 143, 20002–20014 CrossRef CAS PubMed.
- G. C. Welch, R. R. S. Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124–1126 CrossRef CAS PubMed.
- M.-A. Légaré, M.-A. Courtemanche, É. Rochette and F.-G. Fontaine, Science, 2015, 349, 513–516 CrossRef PubMed.
- J. Légaré Lavergne, A. Jayaraman, L. C. Misal Castro, É. Rochette and F.-G. Fontaine, J. Am. Chem. Soc., 2017, 139, 14714–14723 CrossRef PubMed.
- M.-A. Légaré, É. Rochette, J. L. Lavergne, N. Bouchard and F.-G. Fontaine, Chem. Commun., 2016, 52, 5387–5390 RSC.
- F.-G. Fontaine and V. Desrosiers, Synthesis, 2021, 4599–4613 CrossRef CAS.
- J. Guo, M. Yan and D. W. Stephan, Org. Chem. Front., 2024, 11, 2375–2396 RSC.
- D. W. Stephan, Science, 2016, 354, aaf7229 CrossRef PubMed.
- K. Chernichenko, Á. Madarász, I. Pápai, M. Nieger, M. Leskelä and T. Repo, Nat. Chem., 2013, 5, 718–723 CrossRef CAS PubMed.
- S. Das, R. C. Turnell-Ritson, P. J. Dyson and C. Corminboeuf, Angew. Chem., Int. Ed., 2022, 61, e202208987 CrossRef CAS PubMed.
- C. Jiang, O. Blacque and H. Berke, Organometallics, 2010, 29, 125–133 CrossRef CAS.
- V. Fasano, L. D. Curless, J. E. Radcliffe and M. J. Ingleson, Angew. Chem., Int. Ed., 2017, 56, 9202–9206 CrossRef CAS PubMed.
- D. Wu, L. Kong, Y. Li, R. Ganguly and R. Kinjo, Nat. Commun., 2015, 6, 7340 CrossRef CAS PubMed.
- B. Wang, Y. Li, R. Ganguly, H. Hirao and R. Kinjo, Nat. Commun., 2016, 7, 11871 CrossRef CAS PubMed.
- Y. Su, D. C. Huan Do, Y. Li and R. Kinjo, J. Am. Chem. Soc., 2019, 141, 13729–13733 CrossRef CAS PubMed.
- Y. Su, Y. Li, R. Ganguly and R. Kinjo, Angew. Chem., Int. Ed., 2018, 57, 7846–7849 CrossRef CAS PubMed.
- S. E. Prey and M. Wagner, Adv. Synth. Catal., 2021, 363, 2290–2309 CrossRef CAS.
- A. Lorbach, M. Bolte, H.-W. Lerner and M. Wagner, Organometallics, 2010, 29, 5762–5765 CrossRef CAS.
- Y. Su and R. Kinjo, Chem. Soc. Rev., 2019, 48, 3613–3659 RSC.
- J. E. Barker, A. D. Obi, D. A. Dickie and R. J. Gilliard Jr., J. Am. Chem. Soc., 2023, 145, 2028–2034 CrossRef CAS PubMed.
- D. Wu, R. Wang, Y. Li, R. Ganguly, H. Hirao and R. Kinjo, Chem, 2017, 3, 134–151 CAS.
- T. K. Wood, W. E. Piers, B. A. Keay and M. Parvez, Org. Lett., 2006, 8, 2875–2878 CrossRef CAS PubMed.
- D. A. Hoic, J. R. Wolf, W. M. Davis and G. C. Fu, Organometallics, 1996, 15, 1315–1318 CrossRef CAS.
- T. K. Wood, W. E. Piers, B. A. Keay and M. Parvez, Angew. Chem., Int. Ed., 2009, 48, 4009–4012 CrossRef CAS PubMed.
- R. J. Burford, B. Li, M. Vasiliu, D. A. Dixon and S.-Y. Liu, Angew. Chem., Int. Ed., 2015, 54, 7823–7827 CrossRef CAS PubMed.
- B. Su, Y. Li, R. Ganguly, J. Lim and R. Kinjo, J. Am. Chem. Soc., 2015, 137, 11274–11277 CrossRef CAS PubMed.
- J. Li, C. G. Daniliuc, G. Kehr and G. Erker, Angew. Chem., Int. Ed., 2021, 60, 27053–27061 CrossRef CAS PubMed.
- K. Huang and C. D. Martin, Inorg. Chem., 2015, 54, 1869–1875 CrossRef CAS PubMed.
- J. H. Barnard, S. Yruegas, K. Huang and C. D. Martin, Chem. Commun., 2016, 52, 9985–9991 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2431184, 2431182, 2431179, 2431181, 2431190, 2431186 and 2431178. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00642b |
| This journal is © The Royal Society of Chemistry 2025 |