Surface reinforcement of perovskite films with heteroatom-modulated carbon nanosheets for heat-resistant solar cells†
Lele
Qiu
 *ab,
Wanyu
Tian
a,
Ming
Xu
a,
Jian
Xiao
ab,
Jing
Liang
*ab,
Wanyu
Tian
a,
Ming
Xu
a,
Jian
Xiao
ab,
Jing
Liang
 a,
Fangjing
Liu
ab and
Yunpeng
Zhao
a,
Fangjing
Liu
ab and
Yunpeng
Zhao
 *ab
*ab
aJiangsu Province Engineering Research Center of Fine Utilization of Carbon Resources, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
bKey Laboratory of Coal Processing and Efficient Utilization, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China. E-mail: qiull@cumt.edu.cn; zhaoyp@cumt.edu.cn
First published on 17th April 2025
Abstract
The stability issue induced by surface defects in perovskite films remains one of the key constraints in facilitating the commercial adoption of perovskite solar cells (PSCs). Developing reliable passivation strategies based on inexpensive multifunctional passivation materials is expected to solve the above problems. Here, gelatin-derived carbon nanosheets (G-DC) prepared by a self-doping template method are developed to strengthen the surface of perovskite films, thus enhancing the heat resistance of PSCs. This two-dimensional passivation material has an ultra-thin layered structure and contains abundant heteroatoms such as N and O, which can effectively reduce surface defects and mitigate the impact of residual lead iodide in perovskite films. The excellent interfacial compatibility of G-DC promotes more efficient carrier extraction at the rear interface of PSCs, greatly reducing non-radiative recombination. Thanks to these, the optimal device with G-DC modification achieves a power conversion efficiency of up to 21.65%, which is higher than the 20.32% of the control device. Furthermore, thermally induced organic component loss and ion migration of the modified PSCs are significantly suppressed due to the interactions between G-DC and the perovskite. Finally, the G-DC-modified devices retain 87% of the initial efficiency after aging under an inert atmosphere at 85 °C for 720 h.
1. Introduction
After more than ten years of rapid development, the power conversion efficiency (PCE) of metal halide perovskite solar cells (PSCs) has advanced by leaps and bounds, which makes them highly competitive devices in the energy conversion industry.1,2 The interface between perovskite films and hole transport layers (HTLs), as an important region for carrier extraction and transport in regular n-i-p PSCs, plays an important role in enhancing the moisture and heat resistance of perovskite films and inhibiting ion migration.3,4 However, the ionic nature of perovskite materials and the commonly used low-temperature solution preparation methods for perovskite films result in multitudinous defects at the surface and interfaces of the perovskite in devices. These sites have undoubtedly become non-radiative charge recombination centres and affect the device performance, and on the other hand, they provide a pathway for the invasion of water and oxygen into the perovskite layers, impairing the long-term stability of the device.5–7 In addition, non-stoichiometric PbI2 (usually excessive) in perovskite precursor solutions has proven to be a feasible method to improve the quality of perovskite films; however, excess PbI2 remaining on the surface of the perovskite decomposes into metallic lead and iodine vapor under continuous light and heat conditions, accelerating the decomposition of perovskite films.8,9 In response to these problems, interfacial engineering has been developed and has proven to be an effective strategy to improve the performance and stability of PSCs by passivating interfacial defects, modulating lead ions, and acting as a protective layer for perovskite films.10–13In recent years, organic ammonium salts,14 Lewis acid–base molecules,15 multifunctional polymers,16 ionic liquids,17 and two-dimensional (2D) carbon materials12 have been used as interfacial materials, which achieve defect passivation and interfacial modification through the interactions between functional groups contained in themselves and defect sites. However, many small molecules are prone to decomposition under the conditions of device preparation and operation or cannot sustain the passivation effect for a long time. Compared to these materials, 2D carbon materials are characterized by high thermal conductivity, chemical inertness and exceptional electrical conductivity, which make them excellent candidate for interfacial materials. Unfortunately, the current application of 2D carbon materials in PSCs is mainly based on natural crystalline graphite,18,19 which requires complex processing to achieve the functionalization of the passivation materials. In addition, the high preparation cost is also not conducive to the commercial development of these devices. Biomass-derived carbon materials effectively compensate for the aforementioned shortcomings and can achieve functionalization through simple self-doping.20 These advantages have enabled them to be well applied as electrodes for PSCs,21,22 not only improving device stability but also simplifying the device structure.23,24 However, due to difficulties in morphology and dimension control, there are few reports on their application in other functional or interfacial layers. Therefore, obtaining interfacially compatible multifunctional 2D carbon materials is of great significance for expanding the scope of use of biomass-based carbon materials. Meanwhile, it is expected to yield highly efficient and stable PSCs based on biomass-derived interfacial passivation materials.
In this study, based on the long-range ordered self-assembly strategy of renewable biomass on the surface of 2D layered crystal templates, gelatin-derived 2D carbon materials (G-DC) were successfully obtained. The thickness of G-DC was only 5 nm, and the self-doping ratio of N and O heteroatoms reached 9.16% and 13.73%, respectively, which ensures the interaction and compatibility between the interfaces in devices. Interface engineering based on G-DC could regulate excessive PbI2 on the surface of perovskite films, thus improving the crystal quality and defect density of the photoactive layer. The interfacial connection strengthened by G-DC modification effectively improved the extraction and transport of carriers at the interface and suppressed the occurrence of recombination behaviours. The PSCs with G-DC modification achieve a champion PCE of 21.65%, higher than that of the control device (20.32%). The interfacial modification based on G-DC has also significantly improved the humidity and thermal stability of the devices.
2. Results and discussion
To control the morphology of carbon materials and achieve interfacial compatibility in devices, the self-doping template method was chosen to prepare biomass-derived carbon. As shown in Fig. 1a and b, G-DC was composed of regular flakes, and the overall 2D lamellar structure was presented in the scanning electron microscope (SEM) images. Meanwhile, the thickness of G-DC was further characterized using an atomic force microscope (AFM, Fig. 1c and f), which showed that the apparent thickness of G-DC was about 5 nm. The regular morphology and ultra-thin thickness of G-DC are conducive to its use as an interfacial material to achieve good interfacial contact when modifying the interfaces of perovskite/HTLs. Under high-magnification transmission electron microscopy (Fig. S1†), G-DC exhibited a complete layered structure and distinct lattice fringes. The Raman spectrum characterization result of G-DC shows two bands located at about 1350 cm−1 and 1580 cm−1, corresponding to the D and G bands, respectively (Fig. S2†). The D band is usually associated with the vibration of defective carbon, while the G band represents the sp2 vibration of graphitic carbon. The relatively low intensity ratio of D and G bands (ID/IG = 0.94) indicated that G-DC had a high degree of graphitization, which is beneficial for charge transport.25–27 The elemental composition of G-DC and the bonding state between heteroatoms were analysed by X-ray photoelectron spectroscopy (XPS). Fig. S3† shows that G-DC contained B, C, N, and O elements, and the proportion of N and O elements reached 9.16% and 13.73%, respectively. The high content of heteroatoms comes from biomass self-doping, without the need for additional doping reagents. A small amount of B element was introduced by the template agent during the preparation process, which can be removed by simple water reflux and can be economically reused.28 A split-peak fitting of the C 1s spectrum (Fig. 1d) revealed that the oxygen-containing functional groups in G-DC were the considerable component. Since C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups are prone to interact with the perovskite through hydrogen bonding and lead-oxygen coordination bonding, G-DC is expected to enhance the interactions between the perovskite and carbon materials when used as a modifier of perovskite/HTL interfaces, thus improving the interfacial quality.29 In addition, a split-peak fitting of the N 1s spectrum (Fig. 1e) of G-DC yielded characteristic peaks attributed to pyridinic nitrogen, C–N–B, pyrrolic nitrogen, graphitic nitrogen, and nitrogen oxide. Both pyridinic nitrogen and pyrrolic nitrogen contain lone pairs of electrons, which are capable of providing unbonded electrons to the uncoordinated Pb2+ for defect passivation. In brief, this ultra-thin carbon nanosheet rich in heteroatoms exhibits excellent potential as an interfacial passivation material.
O groups are prone to interact with the perovskite through hydrogen bonding and lead-oxygen coordination bonding, G-DC is expected to enhance the interactions between the perovskite and carbon materials when used as a modifier of perovskite/HTL interfaces, thus improving the interfacial quality.29 In addition, a split-peak fitting of the N 1s spectrum (Fig. 1e) of G-DC yielded characteristic peaks attributed to pyridinic nitrogen, C–N–B, pyrrolic nitrogen, graphitic nitrogen, and nitrogen oxide. Both pyridinic nitrogen and pyrrolic nitrogen contain lone pairs of electrons, which are capable of providing unbonded electrons to the uncoordinated Pb2+ for defect passivation. In brief, this ultra-thin carbon nanosheet rich in heteroatoms exhibits excellent potential as an interfacial passivation material.
 | ||
| Fig. 1 (a and b) SEM images of G-DC. (c) AFM image of G-DC and (f) related height distribution map. (d and e) High-resolution XPS spectra of C 1s and N 1s of G-DC. | ||
The presence of PbI2 and dangling bonds on the surface of perovskite films has adverse effects on the stable operation of PSCs. To improve the quality of perovskite films and inhibit interfacial recombination, an isopropanol solution of G-DC with a concentration of 0.1 mg mL−1 was spin-coated on the surface of perovskite films, and the surface defects were expected to be eliminated by thermal annealing-induced chemical reactions. Fig. 2a and b show the SEM images of the control and the perovskite films treated with G-DC. Although both the perovskite films showed typical perovskite crystal features, it was noteworthy that the perovskite films modified with G-DC showed a significant reduction in bright patchy spots (indexed to PbI2) at the grain boundaries9 and a slight increase in the perovskite grain size, which may be attributed to the Ostwald ripening effect.17 Furthermore, with the help of interactions between heteroatoms in G-DC and the perovskite, the grains would obtain more robust termination.30 The effects of G-DC modification on the crystallinity of the perovskite films were further evaluated by X-ray diffraction (XRD). As shown in Fig. 2c, the diffraction peak at 12.7° attributed to PbI2 was reduced for the G-DC modified perovskite film compared with that of the control, which is in accordance with the results of SEM images. Meanwhile, the (001) and (002) diffraction peak intensities of the G-DC-treated perovskite film were enhanced, and the full width at half maximum (FWHM) was smaller (Fig. S4†), indicating that G-DC improved the crystalline quality of the perovskite film. The modified film showed a slight increase in the light absorption intensity, while having almost no effect on the optical bandgap before and after G-DC modification (Fig. 2d). These characterization results indicate that G-DC modification can optimize the surface of perovskite films, potentially reducing surface defects without affecting optical absorption and film crystallinity.
 | ||
| Fig. 2 SEM images of (a) the control and (b) modified perovskite films. (c) XRD patterns and (d) Tauc plots of the perovskite films with and without G-DC modification. | ||
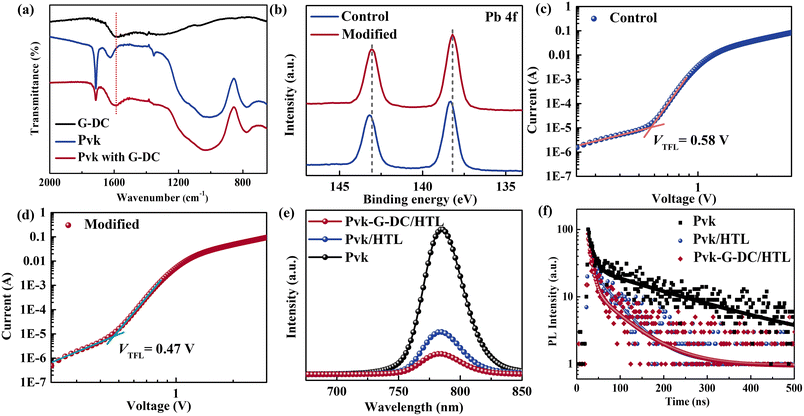
Considering that SEM characterization cannot visually observe the modified layer, we further characterized the perovskite films before and after G-DC modification using Fourier Transform infrared (FTIR) spectroscopy. As shown in Fig. 3a, the perovskite with G-DC modification exhibited a unique aromatic ring stretching vibration peak in carbon materials, which proved the existence of the G-DC modification layer on the surface of perovskite films. The oxygen-containing functional groups and other heteroatom groups containing lone pair electrons in G-DC have a potential role with Lewis acid-type uncoordinated Pb2+ defects at the surface of the perovskite. Therefore, the interactions between G-DC and lead ions on the surface of the perovskite films were investigated using XPS (Fig. 3b). The Pb 4f characteristic peaks of the perovskite films were slightly shifted after modification by G-DC, proving the existence of the interaction, which contributes to the passivation of the surface defects. The trap state density was further evaluated using the space charge limited current (SCLC) method.31 Hole-only devices with the structure of FTO/PEDOT:PSS/perovskite/Spiro-OMeTAD/Au were fabricated to collect the current–voltage curve of the devices in the dark. The trap filling limit voltages (VTFL) of control and modified devices were 0.58 V and 0.47 V, respectively, and the trap state densities were calculated to be 1.18 × 1016 cm−3 and 9.57 × 1015 cm−3 (Fig. 3c and d). This shows that G-DC can effectively reduce the defect state density at the interface of perovskite/HTLs, which is conducive to suppressing the occurrence of non-radiative recombination and enhancing the carrier utilization. To further investigate the effect of G-DC on the carrier recombination behaviors at the interface of perovskite/HTLs, steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL) measurements were performed. As can be observed from Fig. 3e, after coating with the HTL, the perovskite film exhibited obvious fluorescence quenching behavior. Compared with the control perovskite film, the G-DC-modified perovskite film could more effectively quench PL. The fluorescence quenching behavior was attributed to the rapid extraction of photogenerated holes by the HTLs under the irradiation of excitation light. The fluorescence lifetime of the modified perovskite film covered with a hole transport layer was 58.98 ns, which was lower than the 64.51 ns of the reference film. The above results indicate that the G-DC-modified perovskite films could promote the rapid extraction and transport of photogenerated charges and inhibit defect-induced nonradiative recombination, which is conducive to the realization of highly efficient PSCs.
In order to investigate the effects of G-DC on the photovoltaic performance of PSCs, devices based on the FTO/SnO2/PCBM/perovskite/G-DC/HTLs/Ag structure were prepared and evaluated for their photovoltaic performance. Fig. 4a and b show the structural schematic diagram and cross-sectional SEM image of the device with and without G-DC modification. The tight connection of the functional layers indicated that the introduction of the G-DC interfacial layer did not affect the interfacial contact between the perovskite and HTLs. Devices based on different concentrations of G-DC modification were prepared and the PCE statistical distribution analysis was performed to determine the optimal concentration of the G-DC-modified devices (Fig. S5†). The results showed that the devices presented optimal efficiency when the concentration was 0.1 mg mL−1, and the J–V curves are presented in Fig. 4c. High-concentration modification is prone to causing G-DC curling and aggregation on the surface of perovskite films, which is not conducive to good contact between interfaces and thus suppresses the transport of charge carriers (Fig. S6 and S7†). The champion device modified with G-DC gave rise to a PCE of 21.65%, with an open-circuit voltage (VOC) of 1.161 V, a high fill factor (FF) of 79.4%, and a short-circuit photocurrent density (JSC) of 23.49 mA cm−2. In contrast, the control PSCs only exhibited an inferior PCE of 20.32%, with a VOC of 1.128 V, an FF of 77.3%, and a JSC of 23.31 mA cm−2 (Fig. 4c and Table S1†). The enhancement of device photovoltaic performance by the introduction of G-DC is mainly attributed to its modulation of excess lead iodide on the surface of perovskite films and the passivation of defects, which improves the crystallinity of the perovskite film and reduces the non-radiative recombination at the interfaces. To further evaluate the charge recombination behaviors, dark J–V curves were plotted. As shown in Fig. S8,† the ideality factor (m) of the PSCs with G-DC modification is smaller than that of the control device, indicating suppressed nonradiative recombination.32–34 To evaluate the reproducibility of the G-DC modification strategy, 20 control devices and 20 modified devices with the optimal concentration of G-DC were prepared, respectively. Fig. S9† shows that the G-DC modified devices exhibited superior reproducibility and the average PCE of the modified devices increased from 19.96% to 21.18% compared to the control devices. The improvement of device performance mainly comes from the increase of VOC and FF, benefiting from interfacial optimization. Stabilized output efficiency and IPCE tests were performed on the devices before and after modification to verify the accuracy of the J–V curves and to examine the utilization of light throughout the visible region of the devices (Fig. 4d and e). The devices can operate stably under AM 1.5G simulated sunlight before and after G-DC modification. The integrated current values obtained from the IPCE curves coincided with the results of the J–V curves, but the G-DC modified device showed better photoresponse in the long-wavelength region. Defect sites present in the device could hinder carrier extraction and lead to the accumulation of carriers at the interfaces, which results in carrier extraction imbalance and severe photocurrent hysteresis.35Fig. 4f presents the hysteresis phenomenon of the devices before and after the G-DC modification, and the device photovoltaic parameters obtained from the devices under forward and reverse scanning conditions are listed in Table S2.† The hysteresis factors (HI) of devices were calculated according to the equation: HI = (PCEreverse − PCEforward)/PCEreverse. It was found that the HI of the G-DC modified device was 1.65%, compared to 3.43% of the control device. The decrease in the hysteresis factor of the device was mainly attributed to the passivation of the defects at the interface of the perovskite/HTLs, which reduced the trap states of the captured carriers and facilitated the extraction and transport of the carriers. To further investigate the carrier dynamics of the device before and after modification, open-circuit voltage decay (OCVD) measurements were performed (Fig. 4g). The results revealed that the modified device had a longer electron life. The electrochemical impedance spectroscopy (EIS) measurement also supported this result. Fig. 4h shows that the charge transfer impedance (Rtr) of the modified device is 1584 Ω and the charge recombination resistance (Rrec) is 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 Ω. In contrast, the two values of the control device are 2274 Ω and 43
410 Ω. In contrast, the two values of the control device are 2274 Ω and 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 Ω, respectively. Combined with the increase in Rrec of the EIS, these results indicate that the non-radiative charge recombination is suppressed, which is mainly due to the improved carrier utilization through the G-DC modification.
710 Ω, respectively. Combined with the increase in Rrec of the EIS, these results indicate that the non-radiative charge recombination is suppressed, which is mainly due to the improved carrier utilization through the G-DC modification.
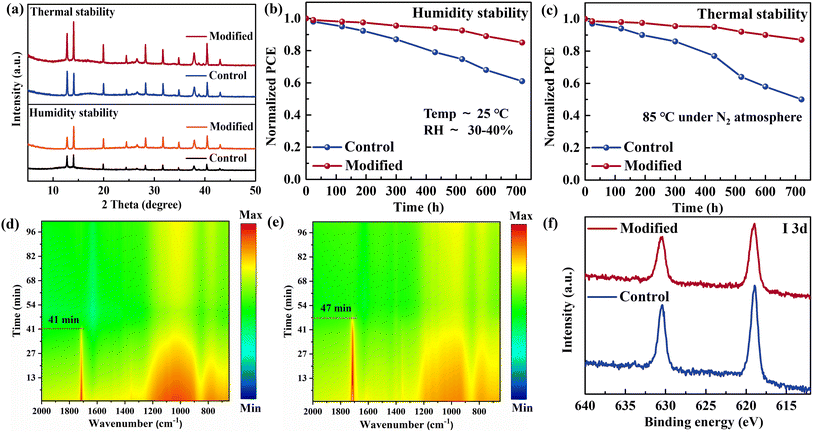
To investigate the effects of G-DC modification on device stability, we first analyzed the aging of perovskite films under different conditions. After G-DC modification, the water contact angle of the perovskite film slightly increased, which also contributed to the improvement of the film's humidity stability (Fig. 5a and S10†). After aging for 720 h in the surrounding environment (25 °C, RH = 30–40%), the film with G-DC modification could better maintain the crystal structure of the perovskite and suppress the decomposition of the film. The surface strengthening of the perovskite film is also beneficial for improving the thermal stability of the film. Meanwhile, it is worth noting that the quality of perovskite films aged at 85 °C for 720 h under a nitrogen atmosphere was superior to those aged under humid conditions, regardless of whether they had been modified with G-DC. In order to further investigate the stability of PSCs before and after G-DC modification, the devices were subjected to aging treatment under the same conditions as the perovskite films to monitor the evolution of device efficiency over time. As shown in Fig. 5b and c, the modified PSCs showed significant improvement in device performance after humidity or thermal aging, but the performance improvement of thermally aged devices was even higher. The presence of HTLs and metal electrodes plays a role in protecting the perovskite films, but the degradation process related to thermal aging is not limited to the decomposition of the perovskite. Therefore, in situ FTIR technology was first used to investigate the effect of G-DC on the perovskite during thermal aging (Fig. 5d and e). The perovskite powders scraped off from the substrates were detected under a nitrogen flow, while being heated from room temperature to 300 °C and then maintained at this temperature. The results showed that the organic components related to FA+ were continuously lost during the heating process and eventually stabilized.36,37 Given that the decomposition temperature of the perovskite is generally greater than 300 °C, the organic components lost mainly come from the surface of the grains and residual FAI in the film. The modified perovskite powder showed a longer organic component loss time, indicating that the surface strengthening strategy of G-DC can improve the structural stability of the perovskite. Furthermore, the migration of iodine ions during the aging process was evaluated by XPS measurements. Fig. 5f shows that the number of iodide ions migrating to the Ag electrode was significantly reduced after G-DC modification, indicating that the introduction of G-DC was also beneficial for suppressing thermally induced ion migration. As a result, the interfacial modification strategy based on G-DC significantly improved the humidity and thermal stability of PSCs by strengthening the surface of the perovskite.
3. Conclusions
In summary, biomass-derived ultra-thin carbon nanosheets prepared by a self-doping template method have been proven to strengthen the surface of perovskite films, thus enhancing the efficiency and stability of PSCs. The N and O heteroatoms containing lone-pair electrons in G-DC could form Lewis acid–base adducts by providing unbonded electrons to uncoordinated Pb2+, which effectively reduces defects and improves carrier utilization. In addition, the modulation of residual PbI2 in the films using G-DC can improve the crystalline quality of perovskite films, while suppressing the loss of FA-based organic components during thermal aging. Thanks to the surface strengthening of perovskite films and interfacial modification by G-DC, the optimized devices obtained a PCE of 21.65% with improved thermal stability.4. Experimental section
4.1. Materials
The materials and solvents used in the experiments were purchased from commercial sources and used without further purification. Tin oxide (SnO2, 15% in H2O colloidal dispersion), lead iodide (PbI2, 99.99%), lead bromide (PbBr2, 99.9%), cesium iodide (CsI, 99.999%), methylammonium bromide (MABr, 99.9%), 4-tert-butylpyridine (t-BP, 99%), 2,2′,7,7′-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene (Spiro-OMeTAD, 99.86%) and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM, 99.9%) were purchased from Advanced Election Technology Co., Ltd. Formamidinium iodide (FAI, 99.9%) and lithium bis(trifluoromethyl sulfonyl) imide salt (Li-TFSI, 99%) were purchased from Xi'an Yuri Solar Co., Ltd. Boric acid (≥99.5%) and gelatin were purchased from Aladdin. Ammonium hydroxide solution (NH3·H2O, 28–30% NH3 basis), N,N-dimethylformamide (DMF, 99.8%), dimethyl sulfoxide (DMSO, 99.9%), 2-propanol (IPA, 99.5%) and chlorobenzene (CB, 99.8%) were purchased from Sigma-Aldrich.4.2. Synthesis of G-DC
Gelatin-derived carbon materials were prepared based on the long-term ordered self-assembly strategy of renewable biomass molecules on a two-dimensional template.28 The specific preparation process is as follows: 4 g of boric acid was dissolved in 50 mL of deionized water at 80 °C. 0.4 g of gelatin was added under stirring until the water was almost completely evaporated. The mixed solid was dried for 12 h in an oven at 80 °C, followed by annealing at 900 °C for 1 h with a ramp rate of 5 °C min−1 under a nitrogen atmosphere, yielding a black, shaggy product. After refluxing for 1.5 h, the boric acid template was removed by filtration and could be reused. Finally, G-DC was obtained by drying.4.3. Fabrication of PSCs
The etched FTO glass substrates were ultrasonically cleaned for about 20 min using deionized water, acetone, and isopropanol sequentially. Pre-treatment of FTO substrates with UV ozone equipment was performed before spin-coating SnO2 electron transport layers. SnO2–NH3·H2O solution was obtained by diluting 1 mL of tin oxide colloid with 1 mL of deionized water and 1 mL of NH3·H2O and stirring for 2 h at room temperature.38 Then, the SnO2–NH3·H2O solution was spin-coated on the FTO substrates at 3000 rpm for 30 s and annealed at 180 °C for 30 min in air. The resulting substrates were then treated with UV ozone for 15 min and transferred into an N2 glove box. PCBM (5 mg mL−1 in chlorobenzene) was spin-coated on the SnO2/FTO substrates at 3000 rpm for 30 s and annealed at 70 °C for 10 min. Subsequently, FAI (0.1719 g), MABr (0.0223 g), PbBr2 (0.0734 g), and PbI2 (0.5071 g) were dissolved in 1 mL of mixed solvent (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO = 4
DMSO = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and then 56 μL of CsI solution (1.5 M in DMSO) was added to prepare the perovskite precursor solution. 50 μL of the perovskite precursor solution was dripped onto the SnO2 film with a two-step spin-coating procedure (1000 rpm and 6000 rpm for 10 s and 20 s, respectively). Chlorobenzene as an anti-solvent was quickly dripped onto the surface 5 s before the end of the step. The obtained perovskite intermediate films were annealed at 100 °C for 60 min. The isopropanol solution of G-DC with a concentration of 0.1 mg mL−1 was spin-coated on the perovskite for partial devices at 4000 rpm for 30 s and annealed at 70 °C for 5 min. Then, 72.3 mg of Spiro-OMeTAD, 17.5 μL of Li-TFSI solution (520 mg in 1 mL acetonitrile), and 28.8 μL of t-BP were dissolved in 1 mL of chlorobenzene to form the hole transport solution. 20 μL of Spiro-OMeTAD solution was spin-coated on the perovskite film at 4000 rpm for 20 s to form the hole transport layer. Finally, a 100 nm Ag electrode was deposited by thermal evaporation under vacuum as the back contact.
1) and then 56 μL of CsI solution (1.5 M in DMSO) was added to prepare the perovskite precursor solution. 50 μL of the perovskite precursor solution was dripped onto the SnO2 film with a two-step spin-coating procedure (1000 rpm and 6000 rpm for 10 s and 20 s, respectively). Chlorobenzene as an anti-solvent was quickly dripped onto the surface 5 s before the end of the step. The obtained perovskite intermediate films were annealed at 100 °C for 60 min. The isopropanol solution of G-DC with a concentration of 0.1 mg mL−1 was spin-coated on the perovskite for partial devices at 4000 rpm for 30 s and annealed at 70 °C for 5 min. Then, 72.3 mg of Spiro-OMeTAD, 17.5 μL of Li-TFSI solution (520 mg in 1 mL acetonitrile), and 28.8 μL of t-BP were dissolved in 1 mL of chlorobenzene to form the hole transport solution. 20 μL of Spiro-OMeTAD solution was spin-coated on the perovskite film at 4000 rpm for 20 s to form the hole transport layer. Finally, a 100 nm Ag electrode was deposited by thermal evaporation under vacuum as the back contact.
4.4. Characterization
Scanning electron microscopy (SEM, ZEISS Sigma 300) and transmission electron microscopy (TEM, Tecnai G2 F20) were used to characterize the morphology of all samples. The thickness of G-DC was analyzed by atomic force microscopy (AFM, Dimension ICON). Raman spectroscopy (Bruker VERTEX 80v) and X-ray diffraction (XRD, Bruker D8 Advance) with Cu Kα radiation were used to study the composition and structure of the samples. The surface elements, valence states, and chemical bond information of the samples were examined by X-ray photoelectron spectroscopy (XPS, Thermo Fisher ESCALAB 250Xi). Ultraviolet-visible absorption spectroscopy (UV-vis) was performed using a UV spectrophotometer (UV-1800CPC). An Edinburgh Instrument FLS1000 was used to measure the steady-state photoluminescence (PL) and time-resolved photoluminescence (TRPL). Fourier transform infrared spectroscopy (FTIR) was conducted using a Thermo Fisher Nicolet iS10. Under the conditions of a simulated light intensity of 100 mW cm−2 and a scan rate of 100 mV s−1, the current density–voltage (J–V) curves of PSCs were recorded using a Keithley 2400 electrochemical workstation, and an effective area of 0.06 cm2 was determined using an aperture mask. In the reverse dark J–V curves, the ideality factor (m) is obtained according to the slope extracted from the diffusion-dominated current region. The equation is described as follows: . The incident photon-to-electron conversion efficiency (IPCE) was determined using a Newport IPCE measurement system.
. The incident photon-to-electron conversion efficiency (IPCE) was determined using a Newport IPCE measurement system.
Author contributions
L. Qiu: conceptualization, funding acquisition, and writing – original draft. W. Tian: data curation, investigation, and writing – review & editing. M. Xu: investigation and writing – original draft. J. Xiao: formal analysis and methodology. J. Liang: investigation and writing – review & editing. F. Liu: supervision and project administration. Y. Zhao: conceptualization, project administration, supervision and writing – review & editing.Data availability
The data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was funded by the Basic Research Program of Jiangsu (Grant BK20221114) and the Fundamental Research Funds for the Central Universities (2022QN1067). We also thank the Open Sharing Fund for the Large-Scale Instruments and Equipment of China University of Mining and Technology (CUMT) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- S. S. Li, Y. Z. Jiang, J. Xu, D. Wang, Z. J. Ding, T. Zhu, B. Chen, Y. G. Yang, M. Y. Wei, R. J. Guo, Y. Hou, Y. Chen, C. J. Sun, K. Y. Wei, S. M. H. Qaid, H. Z. Lu, H. R. Tan, D. W. Di, J. Chen, M. Grätzel, E. H. Sargent and M. J. Yuan, High-efficiency and thermally stable FACsPbI3 perovskite photovoltaics, Nature, 2024, 635, 82–88 CrossRef CAS PubMed.
- M. Ravi, M. D. Zhai, C. Chen, H. X. Wang, Z. Y. Xia, Y. Tian, D. Kumar, G. Sathiyan and M. Cheng, Tuning pyrrolo[3,2-b] pyrrole core-based hole transport materials properties via addition of fluorine for highly efficient and stable planar perovskite solar cells, Appl. Surf. Sci., 2025, 680, 161312 CrossRef CAS.
- X. F. Li, W. H. Wang, K. Wei, J. D. Deng, P. Y. Huang, P. Y. Dong, X. Y. Cai, L. Yang, W. H. Tang and J. B. Zhang, Conjugated phosphonic acids enable robust hole transport layers for efficient and intrinsically stable perovskite solar cells, Adv. Mater., 2024, 36, 2308969 CrossRef CAS PubMed.
- R. H. Chen, Y. K. Wang, S. Q. Nie, H. Shen, Y. Hui, J. Peng, B. H. Wu, J. Yin, J. Li and N. F. Zheng, Sulfonate-assisted surface iodide management for high-performance perovskite solar cells and modules, J. Am. Chem. Soc., 2021, 143, 10624–10632 CrossRef CAS PubMed.
- H. P. Jiang, C. Y. Wei, J. Wang, H. Dong, X. M. Fu, L. Zhang, F. Y. Wang, L. Fan, M. B. Wei, H. L. Liu, L. L. Yang and Y. S. Yan, Molecular design engineering regulates the ground-state passivation ability of benzophenone derivatives in perovskite solar cells, ACS Appl. Mater. Interfaces, 2024, 16, 52583–52594 CrossRef CAS PubMed.
- X. F. Ji, K. Feng, S. X. Ma, J. W. Wang, Q. G. Liao, Z. J. Wang, B. L. Li, J. C. Huang, H. L. Sun, K. Wang and X. G. Guo, Interfacial passivation engineering for highly efficient perovskite solar cells with a fill factor over 83%, ACS Nano, 2022, 16, 11902–11911 CrossRef CAS PubMed.
- H. M. Li, Y. L. Tang, S. Q. Liu, M. Li, H. Luo, Z. Zhang, H. H. Li, Y. H. Wei, B. An, X. C. Liu and H. Y. Wang, Dipoles with donor-π-acceptor structure inhibit ion migration to improve the efficiency and stability of inverted perovskite solar cells, Appl. Surf. Sci., 2024, 665, 160257 CrossRef CAS.
- Y. Gao, H. Raza, Z. P. Zhang, W. Chen and Z. H. Liu, Rethinking the role of excess/residual lead iodide in perovskite solar cells, Adv. Funct. Mater., 2023, 33, 2215171 CrossRef CAS.
- L. C. Zhao, Q. Y. Li, C. H. Hou, S. D. Li, X. Y. Yang, J. Wu, S. Y. Zhang, Q. Hu, Y. J. Wang, Y. Z. Zhang, Y. F. Jiang, S. Jia, J. J. Shyue, T. P. Russell, Q. H. Gong, X. Y. Hu and R. Zhu, Chemical polishing of perovskite surface enhances photovoltaic performances, J. Am. Chem. Soc., 2022, 144, 1700–1708 CrossRef CAS PubMed.
- C. R. Lu, Y. X. Wu, F. Gao, Y. R. Li, B. A. Shi, X. D. Cai, J. Q. Zhang, F. Yang and S. F. Liu, 3-ethoxy-4-hydroxybenzadehyde surface passivation of perovskite films enables exceeding 24% efficiency in solar cells, ACS Appl. Energy Mater., 2023, 6, 6981–6992 CrossRef CAS.
- H. D. Guo, Y. Y. Fang, H. B. Cheng, J. P. Wu, Y. Lei, S. M. Wang, X. R. Li, Y. H. Dai, W. C. Xiang, D. J. Xue, Y. Lin and A. Hagfeldt, Robust self-assembled molecular passivation for high-performance perovskite solar cells, Angew. Chem., Int. Ed., 2022, 61, e202204148 CrossRef CAS PubMed.
- G. X. Li, Y. L. Hu, M. Li, Y. Tang, Z. H. Zhang, A. Musiienko, Q. Cao, F. Akhundova, J. Z. Li, K. Prashanthan, F. J. Yang, P. Janasik, A. N. S. Appiah, S. Trofimov, N. Livakas, S. N. Zuo, L. Y. Wu, L. Y. Wang, Y. Q. Yang, B. Agyei-Tuffour, R. W. MacQueen, B. Naydenov, T. Unold, E. Unger, E. Aktas, S. Eigler and A. Abate, Managing excess lead iodide with functionalized oxo-graphene nanosheets for stable perovskite solar cells, Angew. Chem., Int. Ed., 2023, 62, e202307395 CrossRef CAS PubMed.
- L. A. Shen, P. Q. Song, L. F. Zheng, L. P. Wang, X. G. Zhang, K. K. Liu, Y. M. Liang, W. J. Tian, Y. J. Luo, J. H. Qiu, C. B. Tian, L. Q. Xie and Z. H. Wei, Ion-diffusion management enables all-interface defect passivation of perovskite solar cells, Adv. Mater., 2023, 35, 2301624 CrossRef CAS PubMed.
- Y. Yu, M. X. Xu, R. Liu, H. R. Ren and J. Z. Chen, Molecular cation passivation and bromine vacancy supplement strategy for efficient wide-bandgap perovskite solar cells, Chem. Eng. J., 2025, 507, 160339 CrossRef CAS.
- L. Han, D. D. Luo, W. Huang, F. Wang, C. Jia, X. H. Li and Y. Q. Chen, Multifunctional chemical anchors achieve a boosted fill factor and mitigate ion migration of high-stability perovskite solar cells, Dalton Trans., 2024, 53, 8356 RSC.
- M. H. Wang, Y. P. Zhao, X. Q. Jiang, Y. F. Yin, I. Yavuz, P. C. Zhu, A. N. Zhang, G. S. Han, H. S. Jung, Y. F. Zhou, W. X. Yang, J. M. Bian, S. Y. Jin, J. W. Lee and Y. Yang, Rational selection of the polymeric structure for interface engineering of perovskite solar cells, Joule, 2022, 6, 1032–1048 CrossRef CAS.
- H. K. Zhang, W. Yu, J. X. Guo, C. Xu, Z. W. Ren, K. Liu, G. Yang, M. C. Qin, J. M. Huang, Z. L. Chen, Q. Liang, D. Shen, Z. H. Wu, Y. K. Zhang, H. T. Chandran, J. H. Hao, Y. Zhu, C. S. Lee, X. H. Lu, Z. J. Zheng, J. S. Huang and G. Li, Excess PbI2 management via multimode supramolecular complex engineering enables high-performance perovskite solar cells, Adv. Energy Mater., 2022, 12, 2201663 CrossRef CAS.
- Z. Niazi, A. Hagfeldt and E. K. Goharshadi, Recent progress on the use of graphene-based nanomaterials in perovskite solar cells, J. Mater. Chem. A, 2023, 11, 6659–6687 RSC.
- Y. B. Wang, T. H. Wu, J. Barbaud, W. Y. Kong, D. Y. Cui, H. Chen, X. D. Yang and L. Y. Han, Stabilizing heterostructures of soft perovskite semiconductors, Science, 2019, 365, 687–691 CrossRef CAS PubMed.
- L. L. Qiu, M. Xu, W. Y. Tian, J. Wei, Y. X. Chen, J. Xiao, J. Liang, F. J. Liu and Y. P. Zhao, Biomass derived self-doped carbon nanosheets enable robust hole transport layers with ion buffer for perovskite solar cells, ChemSusChem, 2024, 18, e202400510 CrossRef PubMed.
- L. G. Gao, Y. Zhou, F. N. Meng, Y. Li, A. M. Liu, Y. Q. Li, C. Zhang, M. Q. Fan, G. Y. Wei and T. L. Ma, Several economical and eco-friendly bio-carbon electrodes for highly efficient perovskite solar cells, Carbon, 2020, 162, 267–272 CrossRef CAS.
- Y. Q. Gan, J. Sun, P. C. Guo, H. D. Jiang, J. K. Li, H. Zhu, X. Y. Fan, L. Q. Huang and Y. X. Huang, Advances in the research of carbon electrodes for perovskite solar cells, Dalton Trans., 2023, 52, 16558 RSC.
- X. N. Huo, J. Q. Lv, K. X. Wang, W. W. Sun, W. F. Liu, R. Yin, Y. S. Sun, Y. K. Gao, T. T. You and P. G. Yin, Surface sulfidation constructing gradient heterojunctions for high-efficiency (approaching 18%) HTL-free carbon-based inorganic perovskite solar cells, Carbon Energy, 2024, 6, e586 CrossRef CAS.
- Y. T. Cheng, Q. B. Wei, N. N. Wang, Z. W. Ye, Y. B. Zhao, Q. Y. Wang, D. P. Chu, L. X. Zan, F. Fu and Y. C. Liu, Antioxidant induced bulk passivation for efficient and stable hole transport layer-free carbon electrode perovskite solar cells, Chin. Chem. Lett., 2023, 34, 107933 CrossRef CAS.
- G. B. Gu, X. T. Meng, Y. Y. Xie, W. L. Wang, J. L. Wang, C. Liu, J. W. Li and J. S. Qiu, Phenolic resin-templating synthesis of coal pitch-based porous carbon electrodes for high-performance double-layer supercapacitors, Chem. Eng. Sci., 2025, 308, 121370 CrossRef CAS.
- Y. D. Wang, W. R. Li, Y. F. Yin, M. H. Wang, W. X. Cai, Y. Y. Shi, J. Y. Guo, W. Z. Shang, C. Y. Zhang, Q. S. Dong, H. R. Ma, J. Liu, W. M. Tian, S. Y. Jin, J. M. Bian and Y. T. Shi, Defective MWCNT enabled dual interface coupling for carbon-based perovskite solar cells with efficiency exceeding 22%, Adv. Funct. Mater., 2022, 32, 2204831 CrossRef CAS.
- M. Kim, J. F. S. Fernando, Z. B. Li, A. Alowasheeir, A. Ashok, R. J. Xin, D. Martin, A. K. Nanjundan, D. V. Golberg, Y. Yamauchi, N. Amiralian and J. L. Li, Ultra-stable sodium ion storage of biomass porous carbon derived from sugarcane, Chem. Eng. J., 2022, 445, 136344 CrossRef CAS.
- Z. Ling, Z. Y. Wang, M. D. Zhang, C. Yu, G. Wang, Y. F. Dong, S. H. Liu, Y. W. Wang and J. S. Qiu, Sustainable synthesis and assembly of biomass-derived B/N co-doped carbon nanosheets with ultrahigh aspect ratio for high-performance supercapacitors, Adv. Funct. Mater., 2016, 26, 111–119 CrossRef CAS.
- Q. Cao, Y. X. Zhang, X. Y. Pu, J. S. Zhao, T. Wang, K. Zhang, H. Chen, X. L. He, J. B. Yang, C. Zhang and X. H. Li, Surface passivation by multifunctional carbon dots toward highly efficient and stable inverted perovskite solar cells, J. Energy Chem., 2023, 86, 9–15 CrossRef CAS.
- L. L. Qiu, K. Xing, J. Zhang, Y. L. Yang, W. Cao, X. S. Zhou, K. Zhu, D. B. Xia and R. Q. Fan, Two-dimensional metal-organic frameworks-based grain termination strategy enables high-efficiency perovskite photovoltaics with enhanced moisture and thermal stability, Adv. Funct. Mater., 2021, 31, 2010368 CrossRef CAS.
- Y. L. Wang, Y. F. Yang, N. Li, M. Hu, S. R. Raga, Y. Jiang, C. Wang, X. L. Zhang, M. Lira-Cantu, F. Z. Huang, Y. B. Cheng and J. F. Lu, Ionic liquid stabilized perovskite solar modules with power conversion efficiency exceeding 20%, Adv. Funct. Mater., 2022, 32, 2204396 CrossRef CAS.
- Q. Zhou, C. S. Cai, Q. Xiong, Z. L. Zhang, J. B. Xu, L. S. Liang, S. B. Wang, W. H. Sun, Z. Y. Yuan and P. Gao, Surface polarity regulation by relieving fermi-level pinning with naphthalocyanine tetraimides toward efficient perovskite solar cells with improved photostability, Adv. Energy Mater., 2022, 12, 2201243 CrossRef CAS.
- S. B. Xiong, Z. Y. Hou, S. J. Zou, X. S. Lu, J. M. Yang, T. Y. Hao, Z. H. Zhou, J. H. Xu, Y. H. Zeng, W. Xiao, W. Dong, D. Q. Li, X. Wang, Z. G. Hu, L. Sun, Y. N. Wu, X. J. Liu, L. M. Ding, Z. R. Sun, M. Fahlman and Q. Y. Bao, Direct observation on p- to n-Type transformation of perovskite surface region during defect passivation driving high photovoltaic efficiency, Joule, 2021, 5, 467–480 CrossRef CAS.
- Y. Gao, W. Gong, Z. Zhang, J. Guo, J. Ma, X. Li, Y. Zeng and M. Wu, Aminomethyl phosphonic acid as highly effective multifunctional additive for modification of electron transport layer and perovskite in photovoltaic solar cells, Angew. Chem., Int. Ed., 2025, e202424479 Search PubMed.
- E. H. Balaguera and J. Bisquert, Accelerating the assessment of hysteresis in perovskite solar cells, ACS Energy Lett., 2024, 9, 478–486 CrossRef PubMed.
- E. Ugur, A. A. Said, P. Dally, S. S. Zhang, C. E. Petoukhoff, D. Rosas-Villalva, S. Zhumagali, B. K. Yildirim, A. Razzaq, S. Sarwade, A. Yazmaciyan, D. Baran, F. Laquai, C. Deger, I. Yavuz, T. G. Allen, E. Aydin and S. De Wolf, Enhanced cation interaction in perovskites for efficient tandem solar cells with silicon, Science, 2024, 385, 533–538 CrossRef CAS PubMed.
- L. Ma, D. Q. Guo, M. T. Li, C. Wang, Z. L. Zhou, X. Zhao, F. T. Zhang, Z. M. Ao and Z. G. Nie, Temperature-dependent thermal decomposition pathway of organic-inorganic halide perovskite materials, Chem. Mater., 2019, 31, 8515–8522 CrossRef CAS.
- J. B. Chen, H. Dong, J. R. Li, X. Y. Zhu, J. Xu, F. Pan, R. Y. Xu, J. Xi, B. Jiao, X. Hou, K. W. Ng, S. P. Wang and Z. X. Wu, Solar cell efficiency exceeding 25% through Rb-based perovskitoid scaffold stabilizing the buried perovskite surface, ACS Energy Lett., 2022, 7, 3685–3694 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00652j |
| This journal is © The Royal Society of Chemistry 2025 |