Assessment methods for identifying suitable charge carriers for non-aqueous redox flow batteries†
Mamta
Dagar
 * and
Ellen M.
Matson
* and
Ellen M.
Matson
 *
*
Department of Chemistry, University of Rochester, Rochester, New York 14627, USA. E-mail: mdagar2@ur.rochester.edu; matson@chem.rochester.edu
First published on 9th June 2025
Abstract
Redox flow batteries (RFBs) are emerging as a promising battery technology for grid-scale energy storage. The utilization of non-aqueous solvents expands the repertoire of existing electrolytes toward wider electrochemical windows, which is critical for achieving high energy densities. Successful implementation of non-aqueous RFBs on a large scale necessitates identification of suitable charge carriers through the thorough evaluation of key physicochemical properties, such as redox potential, solubility, solution resistance, transport, and electrokinetic properties. These characteristics further inform the performance metrics of the resulting batteries. To date, there is a lack of systematic guidelines and protocols that direct synthetic chemists with consistent procedures to screen electrolytes for practical applications. This is especially true for researchers interested in studying redox-active inorganic molecules as charge carriers for these applications. In this tutorial-review, we discuss the design criteria, testing methods, and H-cell experimental design for inorganic candidates for emergent non-aqueous redox flow battery technologies. We also present a general framework and recommendations on testing procedures that are suitable in different scenarios based on the relevant chemical information that is desired on a given electrolyte. Finally, we conclude the discussion on our envisioned strategies to enable predictive design strategies for next-generation non-aqueous redox flow batteries.
Key learning pointsThe readers of this review will learn about: (1) Prevalent methodologies for assessing the physicochemical properties of inorganic electrolytes in non-aqueous redox flow batteries (RFBs). (2) Key design considerations for improving the efficiency of the battery electrolyte. (3) A systematic framework encompassing initial screening criteria and advanced characterization techniques for selecting suitable electrolyte candidates for RFBs. (4) A step-by-step guide for data collection, interpretation, and benchmarking of transport, electrokinetic, and electrochemical properties of electrolyte systems. |
Introduction
The development of grid-scale energy storage technologies is crucial for a reliable and sustainable energy future.1,2 An emergent technology uniquely suited for the flexible operation and requirements of grid-scale energy storage are redox flow batteries (RFBs).3,4 RFBs are attractive alternatives for grid-scale energy storage due to their scalability, lifetimes, durability, low cost, and efficiency.5 These secondary batteries are stationary electrochemical energy storage devices that store energy in the form of charged redox-active molecules in either all-liquid or hybrid-phase systems. The active species are stored in tanks outside the cell stack in two separate chambers. One tank stores the charge carrier that undergoes an oxidation reaction and is known as the positive electrolyte or posolyte. The second chamber stores the species that undergoes a reduction reaction, which is referred to as the negative electrolyte or negolyte. Often, terminologies such as catholyte (electrolyte associated with the cathode) and anolyte (electrolyte associated with the anode) are also used to refer to the electrolyte solutions. In the cell stack, each of these half-cell reactions are separated by a membrane or other style of separator.The operational distinction between conventional batteries and RFBs lie in the fact that the electrodes in the latter do not directly undergo any faradaic reactions themselves. This leads to decoupling of energy storage capacity from power capacity, as the electroactive redox couples are stored in external reservoirs and brought together in the reactor during operation.6 Decoupling of energy and power is difficult to implement in rechargeable metal-ion batteries because of their enclosed nature, where energy is stored in solid-state electrode materials.7 RFBs are also considered more versatile than their solid-state counterparts owing to wider operable temperature range and require low maintenance due to stable chemistries of the electrolyte.8–10 As such, RFBs hold promise in meeting the ever-growing energy demands of society by providing stable strategies for the integration of renewable resources into the electrical grid.
In considering the construction of a flowable electrochemical energy storage device, one can dissolve redox mediators in either aqueous or non-aqueous solution. The identity of the solvent differentiates between two major classifications of RFBs, aqueous and non-aqueous. Non-aqueous RFBs, the focus of this tutorial, offer a wider operational potential window and temperatures, which are important factors in considering the variable design needs for energy storage (for example, 1.4 V versus 2.2 V for aqueous and non-aqueous RFBs, respectively).11,12 The use of organic solvents also expands the library of organic and inorganic complexes as candidates for charge carriers, including molecular entities that might have reduced stability or compatibility with water. Despite these advantages, key challenges remain in the development of energy carriers for non-aqueous flowable energy storage, including persistent limitations in both cost and performance that stem in large part from solubility and cell voltage (note that these obstacles remain prevalent in even the most mature RFB technologies based on aqueous transition metal compounds, like vanadium). However, in our opinion, continued investment in non-aqueous RFB technologies is necessary to avoid premature technology “lock-in”.
The advancement of non-aqueous RFB technologies hinges on the identification and optimization of charge carriers that balance high energy density, robust stability, and favorable electron transfer kinetics. Several studies have summarized the fundamental concepts,13,14 performance trends,10,15 or focused on specific key physicochemical properties relevant to RFB operation.16–18 There is also a comprehensive review that provides a detailed account of device engineering, flow visualization, and diagnostic techniques for the electrolyte.19 However, there is a lack of reports that delve into the intricacies of evaluating transport, stability, and electrochemical properties of electrolyte systems. One notable exception is the work by Odom and Minteer,13 which has been instrumental in establishing methodology-driven investigations of non-aqueous RFBs, particularly for organic charge carriers. However, comprehensive evaluation strategies tailored to inorganic redox-active compounds are less developed.
Inorganic charge carriers refer to electroactive species based on metal centers or non-carbon redox-active elements that undergo reversible redox reactions to store and transfer charge within the electrolyte system (Fig. 1). These include simple ions (typically metal ions in aqueous solutions; for e.g., V2+/V3+), inorganic complexes (e.g., M(acac)3 and M(bpy)3; M = metal; acac = acetylacetonate; bpy = 2,2′-bipyridine), and organometallic compounds (e.g., ferrocene). Some of these metal-based charge carriers exhibit multiple, stable oxidation states that enable reversible redox reactions, and often exhibit improved thermal and chemical stability across a range of charge states in comparison to their organic counterparts. Moreover, multimetallic systems (such as polyoxometalates) can prevent crossover through common ion-exchange membranes due to their tunable size and charge.
The goal of this tutorial review is to complement the work on organic active materials by providing a detailed framework specifically for inorganic charge carriers, encompassing both initial screening criteria and advanced characterization techniques. We subsequently present a detailed analysis of uncompensated resistance, kinetic assessment, and lay out the distinctions between different cycling strategies. Finally, the discussion addresses the critical link between H-cell testing and flow cell performance. This work aims to bridge the methodological deficiencies and guide synthetic inorganic chemists with recommended best practices in advancing non-aqueous RFB technology.
Identifying suitable inorganic charge carriers
The identification of suitable inorganic charge carriers for non-aqueous RFB technologies requires a systematic approach that integrates well-defined selection criteria with robust screening techniques. These general initial screening criteria for the redox mediator include redox potential, compatibility with the electrolyte system's stability window, solubility, and prolonged durability under operational conditions (Fig. 2). The motivation behind assessing these specific characteristics stems from the need to maximize the energy density of the battery electrolyte. The energy density (E) of a redox flow battery can be estimated by integrating the cell voltage versus capacity and dividing by volume or weight.20 Capacity is a measure of the total amount of electricity generated due to the electrochemical reactions that the battery can store (typically expressed in Ah) or deliver (typically expressed in Wh). Since the theoretical capacity is related to the number of electrons transferred by each charge carrier and the total number of charge carriers, a simple relationship emerges if the cell voltage (Vcell) is treated as a constant, typically the value at a 50% state-of-charge (SoC). In the latter case, the theoretical energy density can be expressed as shown in eqn (1).| E = Vcell × capacity | (1) |
When the charge carriers are dissolved or suspended in a solution, the capacity in coulombs is related to the number of electrons stored or released per mole of the charge carrier (n), the concentration (Cactive), and volume (Volactive) of either the negative or positive electrolyte, and Faraday's constant (F) as shown in eqn (2).
| Capacity = n × F × Cactive × Volactive | (2) |
The theoretical energy corresponding to the charge carrier can be evaluated using eqn (3), which is derived by combining eqn (1) and (2).
| E = n × F × Vcell × Cactive × Volactive | (3) |
Dividing the above expression by the total device volume (Voltotal) gives the means to calculate the volumetric energy density (Evol) of a given battery electrolyte (eqn (4)).
| Evol = (n × F × Vcell × Cactive × Volactive)/Voltotal | (4) |
The derivation of eqn (4) demonstrates how key molecular parameters correlate to the theoretical energy density of an RFB – for example, redox potential of the charge carrier is equivalent to Vcell, solubility of the compound is correlated to Cactive, and the charge accessed by the active species during the redox reaction affects n. However, there remain molecular traits that are absent from eqn (4), but are important when evaluating a charge carrier's suitability for implementation. The redox mediator (and electrolyte system) must exhibit extended stability over the entire voltage window accessed during battery (dis)charging schematics, as this translates directly to the lifetime and associated costs of the system. Additionally, the molecular charge carriers should be able to facilitate rapid electron transfer at the electrode–electrolyte interface, as this is a limiting factor in the overall energy efficiency in flow-based technologies.
There are multiple approaches to measure the solubilities of redox mediators, including electronic absorption spectroscopy and gravimetric analysis. These measurements should be performed in the corresponding electrolyte system (i.e., in the presence of supporting electrolyte and desired solvent) to mirror the battery's operating conditions. Such considerations become pertinent as the presence of ten-fold concentration of supporting electrolyte will impact the solubility of the charge carrier in a given amount of solvent. Electronic absorption spectroscopy can determine saturation limits by monitoring changes in absorbance as a function of solute concentrations, revealing the point at which no further solute dissolves. On the other hand, gravimetric analysis involves dissolving a known excess of the charge carrier in the solvent, filtering out undissolved material, and weighing the solution residue after solvent evaporation to quantify the maximum solubility. Our recommendation is to use electronic absorption spectroscopy for solubility measurements, as it not only yields quantitative information about the maximum concentration of the analyte but also provides insight into the electronic structure and properties of a charge carrier across various redox states, which is unavailable through gravimetric analysis. This technique is especially useful for inorganic charge carriers as compared to their organic counterparts as transition metal-based complexes tend to yield simpler, sharper spectra with well-defined, diagnostic peaks. We recommend that researchers should at a minimum assess the solubilities of the least soluble charge state, but it is preferable to synthesize all disparate charge states involved in the battery cycling and measure their corresponding solubility for accuracy. A step-by-step guide on determining solubility values for a molecular complex using electronic absorption spectroscopy is included in the ESI.†
A caveat to high solubilities of the charge carriers is the trade-off between ionic conductivity and solution viscosity, which typically results in a hyperbolic trend for conductivity as a function of redox species concentration. While higher concentration improves charge storage capacity and ionic strength, it often leads to elevated viscosity of the solution, which translates to reduced mass transport and impaired cell performance.27 Several strategies have emerged to manage this balance, including solvent optimization using low-viscosity, high-permittivity media, molecular tuning of redox-active species to include solubilizing side chains or ionic groups, and co-solvent systems that maintain solubility while reducing viscosity. In parallel, symmetric or mixed-electrolyte designs (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 posolyte/negolyte) can be adopted to address crossover through membranes with limited selectivity, mitigating net concentration gradients and preserving cell efficiency. As such, electrolyte formulations must be assessed to evaluate transport properties (diffusion coefficients, conductivity, viscosity) to elicit design trade-offs and better understand structure–function relationships.
1 posolyte/negolyte) can be adopted to address crossover through membranes with limited selectivity, mitigating net concentration gradients and preserving cell efficiency. As such, electrolyte formulations must be assessed to evaluate transport properties (diffusion coefficients, conductivity, viscosity) to elicit design trade-offs and better understand structure–function relationships.
Electron paramagnetic resonance (EPR) is another spectroscopic handle that can be valuable in determining the durability of the electrolyte if the redox-active complex is EPR active (i.e., it contains unpaired electrons in the relevant oxidation states). EPR parameters such as g-value, hyperfine splitting, linewidth, and signal intensities are fingerprints of specific oxidation states, coordination environments, and unpaired electron densities. As such, periodic monitoring of the EPR signal intensities (with appropriate spin quantification techniques) can reveal information about decomposition of the paramagnetic species through either change in oxidation state or ligand loss. The appearance of any new signatures can aid in further deducing the identity of the degradation products. Additionally, kinetic degradation profiles of the electrolyte system can be mapped by plotting EPR signal intensity versus time to define a threshold value (for example, 50% EPR signal loss) on calendar life.
Computational methods, such as Density Functional Theory (DFT), can complement experimental efforts by predicting redox potentials, solubility trends, and possible degradation pathways of molecular charge carriers (Fig. 3). For instance, DFT calculations can simulate solvent interactions to estimate solubility limits or assess the stability of coordination complexes under varying electrochemical conditions.28 Indeed, computational insights serve as a useful tool to narrow down the pool of candidate materials before experimental validation and streamlining the discovery process.
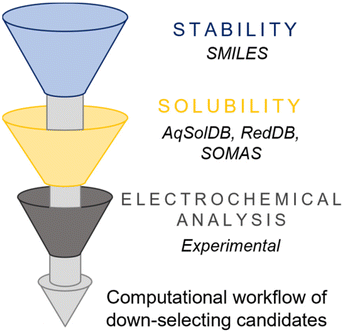 | ||
| Fig. 3 Available computational databases to perform initial screening of solubility (for e.g., AqSolDB, RedDB, and SOMAS) and stability (such as SMILES) for prospective charge carriers. | ||
It should be noted that the discovery of high-performing materials for applications in RFBs relies on an understanding that these criteria serve as an initial screening step for the identification of good candidates for redox mediators. The following section details experimental methodologies that can be adopted to garner deeper insights into the physicochemical properties of the electrolyte system.
Evaluating physicochemical properties
A polarization curve illustrates the relationship between the voltage output as a function of current flowing through an electrode. It is a standard method for characterizing the performance of electrochemical devices, such as batteries. In the context of RFBs, the voltage efficiency (i.e., ratio of discharge voltage to the charge voltage) is dictated by the kinetic losses, resistive losses, and concentration polarization in the system. As shown in Fig. 4, the losses in the cell are primarily attributed to high kinetic overpotential at low current densities. These overpotentials arise due to the sluggish charge transfer kinetics at the electrode–electrolyte interface. At intermediate current density values, the polarization curve exhibits a linear relationship between current and voltage. The slope of this straight line is proportional to the internal resistance within the cell, which is often governed by the ionic resistance of the membrane. Lastly, concentration losses or mass transport limitations occur at the highest current densities. In such cases, the charge carrier's diffusion to and from the electrode surface limits cell operation. This prevents the current from increasing despite increasing overpotential in the cell. As such, the estimation of physicochemical properties such as diffusivity and electrochemical kinetics is crucial prior to RFB testing as these parameters directly influence charge transport, reaction rates, and overall battery performance.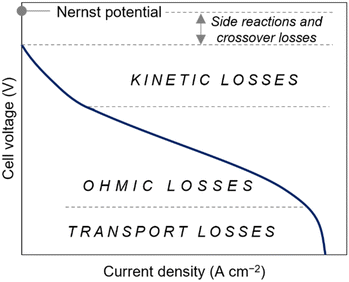 | ||
| Fig. 4 General depiction of a polarization curve for an electrochemical system and the corresponding dominant losses affecting the cell voltage at lower and higher current density regimes. | ||
Pulsed field gradient NMR spectroscopy. Pulsed Field Gradient (PFG) NMR spectroscopy directly measures the diffusion coefficients of charge carriers in a given solvent, providing insights into their transport properties. A PFG-NMR spectrum tracks the displacement of molecules by acquiring a set of data after varying either the field gradient strength (g) or the length of the gradient pulse (δ). During the application of these pulsed magnetic field gradients, the other parameters are held constant and the plot of the intensity of the echo versus g or δ allows for the determination of diffusivity with high precision.29 Diffusion ordered spectroscopy (DOSY) combines principles from conventional NMR spectroscopy with PFG-NMR spectroscopy to separate similarly diffusing species in a mixture based on their resonance frequency.30 A detailed description of using DOSY for estimating diffusion coefficients is included in the ESI.† This method is particularly valuable for assessing how electrolyte composition and solvent viscosity influence charge transport, revealing the impact of solvation structure and ionic interactions on charge carrier mobility. For a detailed reading on the fundamentals of PFG techniques specifically for battery research, the readers are referred to the review published by Han and Mueller.31
Rotating disk voltammetry. The D0 values can also be calculated electrochemically by utilizing rotating disk electrodes (RDEs). RDE voltammetry is a technique used to determine the diffusion coefficients of charge carriers by measuring steady state limiting currents (iL) as a function of rotation speed (ω). The slope of the iLversus ω1/2 plot, known as the Levich plot can be used to extract the associated D0 values.
| iL = 0.620 × n × F × A × D2/30 × ω1/2×ϑ−1/6×C | (5) |
In eqn (5), ϑ is the kinematic viscosity and A is the electrode area.
RDE studies have been employed to compare the diffusivity of different metal-based coordination complexes, revealing how ligand modifications influence solubility and charge transport.32 While this approach has been invoked to establish the diffusivity of inorganic charge carriers, the major limitation of Levich method arises when applied to systems with lower Schmidt numbers (Sc < 1000).33 The Sc value is a dimensionless quantity that relates the viscosity of a fluid to its diffusion coefficient. Viscous solutions, such as polymeric electrolytes, metal-based eutectic systems, and non-aqueous solutions (solvents such as propylene carbonate and dimethylformamide), possess low Sc values. Consequently, the Levich equation cannot be used to predict the diffusion coefficients accurately in such media due to edge effects.33,34 Other electrochemical protocols, such as the Randles–Ševčík method, can be a better method for electroanalysis in such scenarios.
Randles–Ševčík cyclic voltammetry method. Randles–Ševčík analysis is one of the most straightforward and popular methods for estimating diffusion coefficients.35,36 It involves recording peak currents (ip) at variable scan rates (ν) using cyclic voltammetry. The linear relationship between ip and ν1/2 yields a slope proportional to the D0 of the charge carrier. At room temperature, the Randles–Ševčík equation for an electrochemical reversible redox process can be expressed as eqn (6).
| ip = 2.69 × 105 × n3/2 × A × C × D1/20 × ν1/2 | (6) |
This method is particularly useful when conducting initial screening experiments, as it requires minimal sample preparation and provides estimates of diffusivity of relevance to battery cycling (i.e., under electrochemical conditions). In cyclic voltammetry measurements obtained to estimate D0, glassy carbon is often employed as the working electrode owing to its non-catalytic nature and a Pt plate or wire can serve as the counter electrode. For accurate recording of potential-current curves, it is important to employ appropriate reference electrodes; Ag/AgNO3 should be employed in non-aqueous conditions.37 The variations in mathematical relations for Randles–Ševčík analysis of electrochemically irreversible and quasi-reversible redox processes are provided in the ESI.†
R u should be thoroughly accounted for prior to performing kinetic analysis to ensure reliable extraction of rate constants and reproducibility across experiments. To begin with, the experimental conditions should be kept such that the solution resistance can be minimized. This includes ensuring sufficient conductivity of the electrolyte by employing an excess of the supporting electrolyte. Additionally, the working electrode must be kept as close to the reference electrode as possible without touching to avoid short-circuiting during the measurement. It has been demonstrated that Ru declines exponentially as this distance is decreased. Caution must also be placed towards the geometry and placement of the counter electrode. The counter electrode must be much larger in area than the working electrode and a large interelectrode distance should be adopted. Ru can still persist after these practical considerations have been addressed in the cell setup. Some recommended techniques for measuring Ru include positive feedback compensation, iR-free extrapolation, electrochemical impedance spectroscopy (EIS), and four-electrode configuration.
Four-electrode configuration. Modern electrochemical potentiostats are four-probe instruments that can be used to carry out a variety of measurements with either two electrode, three electrode, or four electrode setups. The four-electrode configuration uses two separate electrode pairs – the working sense is decoupled from the working electrode and current is passed through one pair (working and counter) while the potential drop is measured across a separate pair (sense and reference). The latter allows for accurate estimation of Ru. While it is a useful technique for measuring the impedance of an electrochemical system, or studying potential drops across a specific region (for instance, between the working and reference electrodes), a four-electrode setup must be extremely robust when applied for highly resistive non-aqueous media. Particularly, high impedance of the reference and sensing electrodes in organic solvents can introduce artifacts in the measurements. Thus, careful analysis of different contributions, such as sample resistance, positioning, and geometry of the electrodes, becomes necessary in organic media.39
Electrochemical impedance spectroscopy. EIS is a suitable method for quantifying the ohmic drop of an electrolyte regardless of the kinetics of the system. Ru is estimated by analyzing the response of an electrochemical cell to a small amplitude of alternating current signal across a wide range of frequencies. This method gives information about the resistance of the electrolyte solution between the working electrode and the reference electrode, which is not automatically compensated by the instrument itself. To primarily isolate Ru, the high-frequency region of the EIS spectrum is analyzed, as this part is primarily dominated by the solution resistance with minimal contributions from other electrochemical processes. EIS is widely used in battery research to evaluate the internal resistance of the cell. However, analyzing impedance data is not straightforward; it is subject to correct fitting of the data to appropriate equivalent electrical circuit models and subsequently using specialized software to extract the uncompensated resistance value based on the circuit model chosen for a given system. Incorrect choice of equivalent circuit models to fit the data can lead to inaccurate estimations of solution resistance and thus leaves more room for error via misinterpretation, making it sometimes challenging to get reliable data.
Positive feedback compensation. The positive feedback iR compensation is a technique that corrects for the voltage drop caused by solution resistance between the working and reference electrodes by feeding back a portion of the measured current to the potentiostat. This feedback process involves multiplying the observed current value with the estimated Ru and adding the resulting voltage value back to the applied potential, effectively compensating for the voltage loss in the solution. The accuracy of the compensation relies on knowing the precise value of the solution resistance and feeding it into the software. However, if the solution resistance changes during the experiment due to external factors such as transient changes in temperature, electrodeposition, or material decomposition, the compensation may become inaccurate. Moreover, over-compensation can lead to oscillations in the system due to the positive feedback loop. To avoid these unfavorable scenarios, iR-free extrapolation is a better choice.
iR-free extrapolation. The electronics in a potentiostat is inclined to oscillate when fully compensated for the ohmic drop. This can have a deleterious impact on the internal circuitry as well as the attached electrodes. Thus, rather than fully accounting for the Ru value as sometimes done using positive feedback, we recommend using the iR-free extrapolation method. Moreover, full Ru compensation might lead to artificial overcompensation, resulting in peak separation values which are less than the theoretical peak potential separation of 59 mV. The suggested technique involves correcting a percentage of the recorded solution resistance using the potentiostat and then accounting for the remaining Ru manually to correct for the observed potential.40 This extrapolates the results in a way that results in 100% compensation without introducing the complications associated with instrumental oscillations to the system. To illustrate, the default correction value in most potentiostats is 85% for solution resistance. However, partial instrumental iR correction can be performed between 85% and 95%. The first step involves monitoring the cyclic voltammograms at a given correction value for all employed scan rates. In cases where the voltammograms look distorted, we recommend decreasing this value. For multimetallic inorganic compounds, such as polyoxovanadate-alkoxide clusters and octadecavanadate assemblies, 95% compensation via the instrument works well in the scan range of 10–10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mV s−1. Moving towards higher values, say 98%, warps the cyclic voltammogram profile. The remaining amount can be accounted for manually to achieve full compensation as shown in eqn (7).
000 mV s−1. Moving towards higher values, say 98%, warps the cyclic voltammogram profile. The remaining amount can be accounted for manually to achieve full compensation as shown in eqn (7).| Etrue = Eapp − UI | (7) |
In eqn (7), Eapp is the potential after the software's internal correction, U is the percent uncompensated resistance left after the instrumental iR correction, and I is the current response. While it might seem feasible to stick with the default value and do the remaining 15% manually using eqn (7) to achieve 100% compensation, this can lead to erroneous calculations of properties based on the data as well as lead to larger errors on the obtained values (see ESI† for a detailed example). We recommend choosing a suitable value in between 85% to 95% range and account for the remaining percentage manually. The readers are also advised to ensure that the employed iR correction value does not result in peak separations less than the theoretical value of 59 mV.
While the foregoing estimation of uncompensated resistance is focused on determining the charge transfer resistance and electrode kinetics in a three-electrode setup, the resistance of a two-electrode cell (as observed in a battery setup) includes additional components such as those introduced by the membrane. Thus, the experimentally determined impedance values can significantly differ in a cyclic voltammetry analysis versus a battery experiment. In a two-electrode system, it is difficult to deconvolute the behavior of individual components (charge transfer resistance, double-layer capacitance, and diffusion resistance) as the electrochemical measurements generally yield the total cell resistance. In such scenarios, pre-screening using cyclic voltammetry or EIS analysis can come in handy to assign the source of resistance more precisely. For example, if the charge transfer resistance of a given redox process is high at low potentials in a three-electrode measurement, one can expect to observe sluggish kinetics at low voltage end of (dis)charge in a full cell. It is quite likely that the corresponding reaction will become a bottleneck in the overall performance of a battery, which can be prevented by either modifying the underlying electrode surface or employing a different electrolyte system. The increased internal resistance in the battery leads to premature cutoffs if not properly compensated, thus affecting overall performance (Fig. 5b). Thus, prior screening of electrodes and redox-active materials in half-cell conditions can help identify low-resistance, high-rate candidates before integrating them into a full battery.
Koutecký–Levich method. The Koutecký–Levich method employs RDE voltammetry to deduce the standard rate constant (k0) and the symmetry factor (α). This method distinguishes between the diffusion-controlled and kinetically controlled processes and allows for the accurate determination of k0 (eqn (8)).41 However, it should be noted that the Koutecký–Levich analysis is only valid for sluggish reactions (k0 ≤ 10−2 cm s−1). We recommend employing other strategies for deducing k0 values for cluster-based charge carriers where the electron transfer can be inherently fast as a virtue of charge delocalization.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ik = log(nFCAk0) + (nFαη/2.303RT) ik = log(nFCAk0) + (nFαη/2.303RT) | (8) |
In eqn (8), R is the gas constant, T is temperature, and η is the overpotential.
Gileadi method. The Gileadi method for evaluation of heterogeneous rate constants relies on the identification of a critical scan rate (νc) at which the nature of the electrode reaction changes from reversible to irreversible. The latter is determined by plotting the E1/2 values against the logarithm of ν ranging from low to high scan rates.42 The working principle lies in separately fitting the two linear curves obtained from the lower and higher ranges of scan rates, extrapolating them, and finding the precise scan rate at which the two curves intersect. This scan rate is the νc of the system. Subsequent fitting of the data to eqn (9) yields the corresponding k0.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k0 = −0.48α + 0.52 + log[nFανcD0/2.303RT] k0 = −0.48α + 0.52 + log[nFανcD0/2.303RT] | (9) |
The Gileadi method is attractive as it allows for direct rate constant determination and does not rely on peak separation between the anodic and cathodic waves of the corresponding cyclic voltammograms. However, this approach often necessitates employment of extremely high scan rates to determine νc (scan rate >10 V s−1). This can be problematic in cases where the cyclic voltammograms become distorted (i.e., lose the classical “duck” shape) at higher scan rates. Moreover, the method also requires precise information about α for accurate k0 determination, which can vary with slight changes in ligand composition in inorganic active materials.
Kochi–Klinger method. The method proposed by Kochi and Klinger correlates the separation of peak potentials to the rate constant for heterogeneous electron transfer (eqn (10)). This method is applicable to electrochemically irreversible systems (nΔEp > 150 mV) and thus is particularly useful for inorganic redox mediators, as they can often exhibit borderline electrochemical irreversible behavior if accurate values of the transfer coefficient are not accounted.43 The Kochi–Klinger strategy incorporates correction for electrode reaction mechanisms, which can arise because of electronic and structural differences in the charge carrier. Thus, this method for k0 evaluation is useful for non-aqueous redox systems where modulation of the inorganic complex may impact the sterics and electronics of the resulting charge carrier. However, its applicability is limited as the Kochi-Klinger method is specifically suited for entirely electrochemically reversible systems, and a majority of the inorganic redox couples are quasi-reversible in nature.
| k0 = 2.18 × (αnFD0ν/RT)−1/2 × exp[−α2nF(ΔEp)/RT] | (10) |
Nicholson method. The Nicholson method for estimating rate constants at the electrode–electrolyte interface is the most popular voltammetry technique to evaluate electrochemical kinetics. It is a peak separation method that is applicable to electrochemically quasi-reversible systems (60 < nΔEp < 220 mV).44 The mathematical relation associates k0 with a dimensionless parameter Ψ, which is usually evaluated graphically. While this method is ideal for the assessment of rate constants owing to the relatively simple electroanalytical procedure, skepticism toward the adoption of this method germinates from the poor understanding of calculating Ψ. Seminal texts like Bard and Faulkner state the values of Ψ for certain specific values of ΔEp.45 As such, interpreting Ψ for other peak potential separation values is not always straightforward. We recommend using Nicholson's working curve (Fig. S5†) to discern the specific Ψ values for the corresponding ΔEp obtained after appropriate iR compensation from the variable scan rate voltammetry data. Moreover, the employed scan rates should be as high as possible to get an upper bound of the rates being measured.
| k0 = Ψ(πnFD0ν/RT)1/2 | (11) |
The slope of the Ψ versus the square root of scan rate yields the value of k0 (eqn (11)). A detailed example of using this strategy to evaluate the heterogeneous rates of charge transfer for a polyoxovanadate-based charge carrier is included in the ESI.†
Battery testing in static H-cells
While flow testing serves as a pivotal performance indicator in realizing the potential of an electrolyte system for large-scale implementation, the resulting metrics are an outcome of not only the redox chemistries of the electrolyte components but also several engineering constraints (such as cell and stack design, flow dispersion, fluid flow rate, etc.).46 To understand the behavior of the underlying chemistries, H-cell testing serves as an important first step in evaluating electrolyte systems for RFB applications. Such cells provide a controlled environment to assess the intrinsic electrochemical properties of candidate materials without the complexities introduced by full flow systems. For instance, by isolating variables such as mass transport and flow dynamics, H-cells allow to decouple fundamental kinetic and stability parameters from engineering considerations. These insights form a foundation for transitioning promising materials to flow configurations with greater confidence, ensuring efficient resource utilization and accelerating efforts towards deployment.The H-cell is usually a glass container with two compartments separated by either a semi-permeable membrane or a glass frit of appropriate porosity (Fig. 6). The separator material must be resistant to the organic solvents used during testing, lower ionic resistance to enable operation at higher current densities, and stabilize net electrolyte transport to circumvent capacity imbalance. The compatibility of the separator can be assessed by soaking the H-cell with the desired electrolyte for at least 24 h and assessing for any precipitation or disintegration of the membrane. The employed electrodes must exhibit high surface area to minimize resistance in the electrochemical cell.
Lastly, voltammograms must be recorded of both the positive and negative electrolytes prior to their introduction to the H-cell. Cyclic voltammetry analyses establish baseline redox behavior of the electrolyte immediately before testing and can serve as a reference (especially for the associated current) for post-cycling analytical protocols.
An important consideration in choosing cycling parameters, irrespective of the charge–discharge strategy, is choosing the current densities and voltage limits. For example, the ohmic resistance of an operating cell can be different than the ones used in the initial screening stages. This resistance can be measured using EIS by calculating the area specific resistance (ASR). Generally, non-aqueous RFBs exhibit high ASRs owing to meager ionic conductivities, which limits the cell operation to lower current densities to achieve decent efficiency. To illustrate, a thin porous separator (∼175 μm) with 2.5 mm thick graphite-felt electrodes exhibit ASR values in the range of 3 to 18 Ω cm2, which can allow to operate at higher current densities (up to 600 mA m−2).48 Thus, the operating current densities and the voltage limits should be carefully selected during electrolyte screening by accounting for all overpotentials (ohmic, transport, kinetic).
Galvanostatic cycling. Galvanostatic charge–discharge is the commonly used methodology to gain insights into the long-term battery performance and capacity retention. It involves charging the electrolyte at a specific current (or charge) value until an upper cut-off voltage is reached. Subsequently, either the same or different constant current (or charge) value is used to discharge the battery until the lower cut-off voltage is achieved. This process is repeated for the desired number of cycles/time duration. The observed capacity reflects the overall effect of the cell resistance, geometry, temperature fluctuations, and decomposition of the electrolyte. As such, it becomes difficult to deconvolute the different sources of capacity fade. Moreover, galvanostatic cycling is not a true representation of the overall capacity as it is greatly affected by the polarization resistance that changes the state of charge at which the voltage limits are reached.
Potentiostatic cycling. Potentiostatic charge–discharge is the appropriate method if the purpose of the cycling experiment is to characterize the true capacity of the electrolyte. It involves using a constant voltage for the charge and discharge processes until a steady state background current is achieved. While this approach limits the occurrence of side reactions due to strict monitoring of the applied potential, it is however a time-consuming approach as the process becomes slower with cycling time due to the lower concentration of the active species. This limitation makes it a non-ideal approach for scenarios where the purpose of the experiment is to quickly screen robust electrolytes and identify molecular decomposition using H-cell cycling.
Galvanostatic-potentiostatic cycling. To overcome the individual limitations associated with galvanostatic only and potentiostatic only (dis)charge modes, galvanostatic cycling with potential holds is a neat approach which combines the advantages of both methods. It involves charging the battery first at a constant current until the set cut-off voltage limit is reached and subsequently holding this voltage until the desired state-of-charge is achieved. The battery is then allowed to discharge in a similar manner. Essentially, the major difference between this protocol and a purely potentiostatic mode of charge–discharge is the time distribution of the applied potential. Moreover, the total capacity accessed during a galvanostatic cycling with potential hold remains unaffected by any temperature fluctuations arising within the battery electrolyte during the redox reactions.49 This method of battery cycling is particularly useful for organic and organometallic charge carriers where capacity fade is independent of the number of charge–discharge cycles imposed and the molecular decomposition is a function of time, which may be state-of-charge dependent.
Fig. 7 depicts the differences in charge–discharge curves obtained from a purely galvanostatic and galvanostatic-potentiostatic charge–discharge experiment. In Fig. 7a, a polyoxovanadate-alkoxide charge carrier was subjected to galvanostatic cycling at a constant current of 0.2 mA between the potential limits of 1.9 V and 1.4 V. The corresponding potential versus time trace shows an increase in the potential during the charging mode until the upper cutoff of 1.9 V is reached followed by the subsequent drop in potential in the discharge mode until the lower cutoff of 1.4 V is achieved. One cycle (i.e., cycle number 2) under this mode takes approximately 3 h 45 minutes. However, the cycling duration increases to approximately 5 h (for cycle number 2) when the same electrolyte formulation is subjected to galvanostatic cycling followed by a potentiostatic hold, resulting in enhanced capacity of the system (Fig. 7b). The differences in these capacities are a reflection of polarization resistance occurring in the cell that changes the state of charge at which the voltage limits are reached. As such, galvanostatic-potentiostatic cycling represents the true capacity of the system.
Polarity-reversal cycling. Polarity-reversal refers to the process during symmetric charge–discharge where the positive and negative sides are completely switched so that the respective oxidation and redox reactions occur at the opposite electrode. It can be used in scenarios where the electroactive species needs to be regenerated, the state-of-charge has to be rebalanced in the electrochemical cell, or testing the performance of symmetrical cells using different cycling protocols. This technique is not ideal for solid-state and aqueous batteries, as the heat generated by the battery's reverse polarity may produce flammable hydrogen gas and destroy the ensemble. However, polarity reversal cycling experiments can be particularly useful for symmetric non-aqueous systems to limit capacity fade arising due to decomposition. For example, if only one half-cell reaction is prone to instability, polarity-reversal cycling can distribute the degradation over both electrolyte sides so that the capacity improves without significant decomposition of one electrolyte. Several studies have alluded to the formation of solid electrolyte interphase (SEI) in semi-solid RFBs, analogous to their solid-state counterparts.50,51 Polarity reversal cycling can be a powerful strategy in such scenarios where a more uniform electrode surface can be maintained by alternating the current direction. Fig. 8 depicts an illustration of a galvanostatic polarity cycling methodology and the corresponding charge–discharge curve.
 | ||
| Fig. 8 An illustrative example of sequential inputs to achieve a galvanostatic polarity-reversal charge–discharge in a redox flow battery (top) and the corresponding cycling curves (bottom) retrieved after the experiment (reproduced with permission from ref. 52). | ||
Our team has demonstrated that the practical capacity of polyoxovanadate-based charge carrier remains higher (20% average capacity fade as compared to 62% with other techniques for the same number of cycles) when degradation is distributed over both of the symmetric battery electrolytes.52 We also observed the least amount of formation of the decomposition product using polarity reversal (as compared to galvanostatic-potentiostatic and compositionally imbalanced cycling) and the results obtained from this cycling strategy were further instrumental in assigning a purely chemical decomposition mechanism as the source of capacity fade. As such, our recommendation is that all symmetric RFB systems should be assessed with polarity reversals and conventional cycling to compare capacity fades to get a deeper understanding of the role of intrinsic chemistries of the active species on battery performance.
Compositionally imbalanced cycling. Compositionally imbalanced cycling, either volumetric or concentration, is a useful charge–discharge protocol to gain a thorough understanding of the prevalent molecular decay mechanisms in RFBs, such as electrolyte side reactions and self-decomposition of the charge carrier. Both volumetric or concentration imbalanced cycling operate on the principle that creating a capacity limiting side by the virtue of different compositions can limit decomposition, improve state-of-charge and ultimately improve the observed capacity. Our team and others have tested the efficacy of this unbalanced compositionally-symmetric cell methodology in revealing and quantifying different mechanisms for capacity fade and minimizing them as compared to other charge–discharge techniques.49,52
The primary features to look for in post-cycling analysis include crossover and decomposition of the active material. These two phenomena account for the major capacity fade during battery operation. Crossover can be detected by monitoring the resulting electrolyte solutions (posolyte and negolyte) using electronic absorption spectroscopy and/or cyclic voltammtery. During electronic absorption analysis, any changes in absorption features of each electrolyte should be evaluated before and after charge–discharge and then compared to the corresponding change in the other electrolyte. For example, if the positive electrolyte shows a single absorption feature after chemical oxidation but other features emerge after cycling that match with the absorption profile of the negative electrolyte, then it is quite likely that the species have crossed over during the experiment. However, if the additional features do not match with any charge state of the negative electrolyte, then it is probable that the positive electrolyte has undergone decomposition during the course of the experiment. Similar pre- and post-analysis can also be done using cyclic voltammetry.
In addition to electrolyte characterization, thorough investigations of the electrodes pre- and post-cycling can yield information about the interfacial chemistries specifically in cases where capacity losses are attributable to degradation. Surface characterization techniques, such as scanning electron microscopy (SEM) and atomic force microscopy (AFM), are valuable to visualize changes in surface morphology as a result of structural degradation such as roughening, cracking, or material deposition. X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and energy-dispersive X-ray spectroscopy (EDS) can detect compositional changes, revealing electrode corrosion, passivation layers, or unwanted side reactions that contribute to performance fade. Additionally, techniques such as electrochemical quartz crystal microbalance (EQCM) offers a dynamic method to track real-time mass fluctuations at the electrode surface, shedding light on material dissolution, film formation, or ion adsorption during redox cycling. Collectively, these analyses give a comprehensive understanding of how capacity loss, increased resistance, and efficiency decline occur over time, guiding the development of more stable and durable RFB materials.
Conclusions
The evaluation of inorganic charge carriers for non-aqueous redox flow batteries requires a systematic approach that integrates fundamental electrochemical analysis, kinetic and transport properties assessment, and rigorous battery testing. We discussed the utility of a myriad of experimental, spectroscopic, and electroanalytical tools to understand the physicochemical properties and robustness of charge carriers to identify the most promising candidates for efficient and stable energy storage. Notably, synthetic chemists can use the foregoing discussion to adopt the most viable methods for their desired charge carrier to guide material optimization and electrolyte formulation. Future work should focus on establishing clear correlations between H-cell experiments and full flow battery performance to ensure lab-scale findings translate effectively into practical systems. We envision that in situ characterization techniques and refining existing kinetic models will help to bridge this gap and enable predictive design strategies for next-generation redox flow batteries.Data availability
All data for this project has been deposited in the main text or ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Dr. Tessa Baker for helpful advice on collecting and interpreting DOSY NMR spectra. M. D. and E. M. M. acknowledge support from the National Science Foundation Division of Chemical, Bioengineering, Environmental & Transport Systems through award 2350223. The authors also acknowledge the use of JEOL NMR spectrometers acquired with support from the NSF (MRI-2215973).References
- M. Amir, R. G. Deshmukh, H. M. Khalid, Z. Said, A. Raza, S. M. Muyeen, A.-S. Nizami, R. M. Elavarasan, R. Saidur and K. Sopian, J. Energy Storage, 2023, 72, 108694 CrossRef.
- B. Obama, Science, 2017, 355, 126–129 CrossRef CAS PubMed.
- A. Z. Weber, M. M. Mench, J. P. Meyers, P. N. Ross, J. T. Gostick and Q. H. Liu, J. Appl. Electrochem., 2011, 41, 1137–1164 CrossRef CAS.
- L. Y. Zhang, R. Z. Feng, W. Wang and G. H. Yip, Nat. Rev. Chem., 2022, 6, 524–543 CrossRef PubMed.
- J. Winsberg, T. Hagemann, T. Janoschka, M. D. Hager and U. S. Schubert, Angew. Chem., Int. Ed., 2017, 56, 686–711 CrossRef CAS PubMed.
- G. L. Soloveichik, Chem. Rev., 2015, 115, 11533–11558 CrossRef CAS PubMed.
- L. Trahey, F. R. Brushett, N. P. Balsara, G. Ceder, L. Cheng, Y. M. Chiang, N. T. Hahn, B. J. Ingram, S. D. Minteer, J. S. Moore, K. T. Mueller, L. F. Nazar, K. A. Persson, D. J. Siegel, K. Xu, K. R. Zavadil, V. Srinivasan and G. W. Crabtree, Proc. Natl. Sci. Acad., 2020, 117, 12550–12557 CrossRef CAS PubMed.
- Z. Liang, R. K. Jha, T. M. Suduwella, N. H. Attanayake, Y. Wang, W. Zhang, C. Cao, A. P. Kaur, J. Landon and S. A. Odom, J. Mater. Chem. A, 2022, 10, 24685–24693 RSC.
- J. Luo, B. Hu, M. Hu, Y. Zhao and T. L. Liu, ACS Energy Lett., 2019, 4, 2220–2240 CrossRef CAS.
- K. Gong, Q. R. Fang, S. Gu, S. F. Y. Li and Y. S. Yan, Energy Environ. Sci., 2015, 8, 3515–3530 RSC.
- M. Skyllas-Kazacos and F. Grossmith, J. Electrochem. Soc., 1987, 134, 2950–2953 CrossRef CAS.
- Q. H. Liu, A. E. S. Sleightholme, A. A. Shinkle, Y. D. Li and L. T. Thompson, Electrochem. Commun., 2009, 11, 2312–2315 CrossRef CAS.
- M. Li, S. A. Odom, A. R. Pancoast, L. A. Robertson, T. P. Vaid, G. Agarwal, H. A. Doan, Y. L. Wang, T. M. Suduwella, S. R. Bheemireddy, R. H. Ewoldt, R. S. Assary, L. Zhang, M. S. Sigman and S. D. Minteer, ACS Energy Lett., 2021, 6, 3932–3943 CrossRef CAS.
- A. C. Lazanas and M. I. Prodromidis, ACS Meas. Sci. Au, 2023, 3, 162–193 CrossRef CAS PubMed.
- T. C. Palmer, A. Beamer, T. Pitt, I. A. Popov, C. X. Cammack, H. D. Pratt, T. M. Anderson, E. R. Batista, P. Yang and B. L. Davis, ChemSusChem, 2021, 14, 1214–1228 CrossRef CAS PubMed.
- H. Wang, S. Y. Sayed, E. J. Luber, B. C. Olsen, S. M. Shirurkar, S. Venkatakrishnan, U. M. Tefashe, A. K. Farquhar, E. S. Smotkin, R. L. McCreery and J. M. Buriak, ACS Nano, 2020, 14, 2575–2584 CrossRef CAS PubMed.
- D. O. Wipf, E. W. Kristensen, M. R. Deakin and R. M. Wightman, Anal. Chem., 1988, 60, 306–310 CrossRef CAS.
- O. Nolte, I. A. Volodin, C. Stolze, M. D. Hager and U. S. Schubert, Mater. Horiz., 2021, 8, 1866–1925 RSC.
- Y. A. Gandomi, D. S. Aaron, J. R. Houser, M. C. Daugherty, J. T. Clement, A. M. Pezeshki, T. Y. Ertugrul, D. P. Moseley and M. M. Mench, J. Electrochem. Soc., 2018, 165, A970–A1010 CrossRef CAS.
- Q. Huang and Q. Wang, ChemPlusChem, 2015, 80, 312–322 CrossRef CAS.
- M. A. Raihan and C. A. Dyker, J. Energy Chem., 2025, 100, 125–143 CrossRef CAS.
- R. A. Potash, J. R. McKone, S. Conte and H. D. Abruna, J. Electrochem. Soc., 2016, 163, A338–A344 CrossRef CAS.
- A. F. Barton, Chem. Rev., 1975, 75, 731–753 CrossRef CAS.
- M. Li, Z. Rhodes, J. R. Cabrera-Pardo and S. D. Minteer, Sustainable Energy Fuels, 2020, 4, 4370–4389 RSC.
- J. D. Milshtein, A. P. Kaur, M. D. Casselman, J. A. Kowalski, S. Modekrutti, P. L. Zhang, N. Harsha Attanayake, C. F. Elliott, S. R. Parkin, C. Risko, F. R. Brushett and S. A. Odom, Energy Environ. Sci., 2016, 9, 3531–3543 RSC.
- L. Su, M. Ferrandon, J. A. Kowalski, J. T. Vaughey and F. R. Brushett, J. Electrochem. Soc., 2014, 161, A1905–A1914 CrossRef.
- A. M. Fenton, R. K. Jha, B. J. Neyhouse, A. P. Kaur, D. A. Dailey, S. A. Odom and F. R. Brushett, J. Mater. Chem. A, 2022, 10, 17988–17999 RSC.
- L. Cheng, R. S. Assary, X. Qu, A. Jain, S. P. Ong, N. N. Rajput, K. Persson and L. A. Curtiss, J. Phys. Chem. Lett., 2015, 6, 283–291 CrossRef CAS PubMed.
- G. Pagès, V. Gilard, R. Martino and M. Malet-Martino, Analyst, 2017, 142, 3771–3796 RSC.
- K. F. Morris and C. S. Johnson Jr, J. Am. Chem. Soc., 1992, 114, 3139–3141 CrossRef CAS.
- K. S. Han, J. D. Bazak, Y. Chen, T. R. Graham, N. M. Washton, J. Z. Hu, V. Murugesan and K. T. Mueller, Chem. Mater., 2021, 33, 8562–8590 CrossRef CAS.
- A. M. Kosswattaarachchi, L. E. VanGelder, O. Nachtigall, J. P. Hazelnis, W. W. Brennessel, E. M. Matson and T. R. Cook, J. Electrochem. Soc., 2019, 166, A464–A472 CrossRef CAS.
- H. Chen, E. Kätelhön and R. G. Compton, Anal. Chem., 2023, 95, 12826–12834 CrossRef CAS PubMed.
- J. Legrand, E. Dumont, J. Comiti and F. Fayolle, Electrochim. Acta, 2000, 45, 1791–1803 CrossRef CAS.
- J. E. B. Randles, Trans. Faraday Soc., 1948, 44, 327–338 RSC.
- A. Ševčík, Collect. Czech. Chem. Commun., 1948, 13, 349–377 CrossRef.
- N. Elgrishi, K. J. Rountree, B. D. McCarthy, E. S. Rountree, T. T. Eisenhart and J. L. Dempsey, J. Chem. Educ., 2018, 95, 197–206 CrossRef CAS.
- W. Zheng, ACS Energy Lett., 2023, 8, 1952–1958 CrossRef CAS.
- V. F. Lvovich and M. F. Smiechowski, ECS Trans., 2010, 25, 1–25 CrossRef CAS.
- J. C. Myland and K. B. Oldham, Anal. Chem., 2000, 72, 3972–3980 CrossRef CAS PubMed.
- S. Treimer, A. Tang and D. C. Johnson, Electroanalysis, 2002, 14, 165–171 CrossRef CAS.
- H. Muhammad, I. A. Tahiri, M. Muhammad, Z. Masood, M. A. Versiani, O. Khaliq, M. Latif and M. Hanif, J. Electroanal. Chem., 2016, 775, 157–162 CrossRef CAS.
- R. J. Klingler and J. K. Kochi, J. Phys. Chem., 1981, 85, 1731–1741 CrossRef CAS.
- R. S. Nicholson, Anal. Chem., 1965, 37, 1351–1355 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2nd edn, 2000 Search PubMed.
- L. F. Arenas, C. Ponce de León and F. C. Walsh, J. Energy Storage, 2017, 11, 119–153 CrossRef.
- D. R. Burfield and R. H. Smithers, J. Org. Chem., 1978, 43, 3966–3968 CrossRef CAS PubMed.
- X. L. Wei, W. T. Duan, J. H. Huang, L. Zhang, B. Li, D. Reed, W. Xu, V. Sprenkle and W. Wang, ACS Energy Lett., 2016, 1, 705–711 CrossRef CAS.
- M. A. Goulet and M. J. Aziz, J. Electrochem. Soc., 2018, 165, A1466–A1477 CrossRef CAS.
- E. Ventosa, G. Zampardi, C. Flox, F. La Mantia, W. Schuhmann and J. R. Morante, Chem. Commun., 2015, 51, 14973–14976 RSC.
- A. Narayanan, D. Wijnperlé, F. Mugele, D. Buchholz, C. Vaalma, X. Dou, S. Passerini and M. H. G. Duits, Electrochim. Acta, 2017, 251, 388–395 CrossRef CAS.
- M. Dagar, D. Dissanyake, D. N. Kesler, M. Corr, J. D. McPherson, W. W. Brennessel, J. R. McKone and E. M. Matson, Dalton Trans., 2023, 53, 93–104 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00830a |
| This journal is © The Royal Society of Chemistry 2025 |