Synthesis, characterization and induction of ferroptosis of iridium(III) complexes against B16 melanoma cells†
Zhujun
Sheng
a,
Yuanzheng
Liu
a,
Li
Xu
*b,
Xin
Yao
a,
Ju
Chen
a,
Yunjun
Liu
 *a and
Xiuzhen
Wang
*a
*a and
Xiuzhen
Wang
*a
aSchool of Pharmacy, Guangdong Pharmaceutical University, Guangzhou, 510006, PR China. E-mail: lyjche@gdpu.edu.cn; wxzqq1234@gdpu.edu.cn
bSchool of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong 528458, PR China. E-mail: xuli@gdpu.edu.cn
First published on 19th May 2025
Abstract
The synthesis of the ligand 2-(2-methyl-4-hydroxyl)phenyl-1H-imidazo[4,5-f][1,10]phenanthroline (MHIP) and its corresponding new iridium(III) complexes [Ir(ppy)2(MHIP)]PF6 (ppy = 2-phenylpyridine, 9a), [Ir(bzq)2(MHIP)]PF6 (bzq = benzo[h]quinolone, 9b) and [Ir(piq)2(MHIP)]PF6 (piq = 1-phenylisoquinoline, 9c) was reported. The antiproliferative activity of compounds 9a–9c on HepG2, B16, and A549 cancer cells as well as on normal NIH 3T3 cells was tested using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. It was found that the three complexes showed moderate cytotoxicity in A549 and B16 cells. However, after further irradiation, the cytotoxicity was greatly enhanced; especially, 9a, 9b and 9c displayed significant cytotoxicity toward B16 cells with a low IC50 value of 3.1 ± 0.3 μM for 9a, 4.9 ± 0.8 μM for 9b, and 0.4 ± 0.1 μM for 9c. The effects of 9a–9c on the invasive ability of B16 cells were explored via colony formation and scratch experiments. Results demonstrated that the complexes could efficiently block cell proliferation and migration. The co-localization assay found that 9a–9c accumulated in the mitochondria and led to the apoptosis of B16 cells by decreasing mitochondrial membrane potential, altering the structure of microtubule proteins, damaging the structure of cellular DNA, and changing the expression of related proteins. The decrease in glutathione (GSH) concentration, the increase in malondialdehyde (MDA), the downregulation of GPX4, and C11-BODIPY staining results confirmed that 9a, 9b and 9c led to ferroptosis. In addition, we explored the relevant signaling pathways through an RNA sequencing assay and speculated on the possible anticancer mechanisms. Together, the results of this study indicate that the synthesized new iridium(III) complexes 9a–9c can induce cell death via ROS-mediated mitochondrial dysfunction, apoptosis and ferroptosis.
Introduction
According to relevant statistics, more than 10 million people die of cancer globally every year, making it the second leading cause of death worldwide.1 The current main treatment for cancer includes surgery, radiotherapy, and chemotherapy, while anti-cancer drugs are the mainstay of tumor containment.2 Cisplatin can exert anticancer activity by attacking multiple sites and inhibiting cell division, and it is widely used for treating various cancers.3–5 However, cisplatin has serious adverse effects such as nephrotoxicity, immunity loss and ototoxicity, which pose a challenge to cancer treatment.6 Photodynamic therapy (PDT) is an emerging and approved cancer treatment in clinical application. Unlike traditional treatments, PDT generates cytotoxic singlet oxygen through a chemical reaction, which leads to apoptosis by acting on cellular DNA and destroying mitochondrial function; it can also selectively act on cancer cells, which can improve therapeutic efficacy and reduce toxic side effects.7–10In PDT, the design of a photosensitizer (PS) is critical to its therapeutic efficacy, and iridium complexes represent an efficient PS. Tong et al. synthesized four novel iridium compounds and found that the compounds exhibited significant photodynamic activity against cancer cells in vitro under irradiation.11 Wang et al. found that the complex exhibits good biocompatibility, high oxygen generation capacity, and strong cytotoxicity against cancer cells.12 In our previous work, we observed that the iridium(III) complex [Ir(piq)2(DBDIP)]PF6 (piq: 1-phenylisoquinoline; DBDIP: 2-(2,3-dihydrobenzo[b]-[1,4]dioxin-6-yl)-1H-imidazo[4,5-f][1,10]phenanthroline) shows no cytotoxic activity, with an IC50 > 100 μM. However, upon irradiation, the complex exhibits a high inhibitory efficiency on cell proliferation in A549 cells.13 We also found that [Ir(ppy)2(MDIP)](PF6) (ppy = 2-phenylpyridine, MDIP = 2-(7-methoxybenzo[d][1,3]dioxol-5-yl)-1H-imidazo[4,5-f][1,10]phenanthroline) can open mitochondrial permeability transition pore (MPTP) channel, cause a decrease in membrane potential, the release of cytochrome C, and activation of caspase 3, and finally lead to apoptosis upon irradiation conditions.14 However, as one of the manners of cell death, ferroptosis has attracted great attention in recent years; several Ir(III) or Ru(II) complexes reduce glutathione (GSH), increase the amount of malondialdehyde (MDA), and downregulate the expression of GPX4, inducing ferroptosis.15–18 To gain more insight and further understand possible anticancer mechanisms of iridium compounds as photosensitizers, herein, we synthesized a new ligand MHIP (2-(2-methyl-4-hydroxylphenyl)-1H-imidazo[4,5-f][1,10]phenanthroline) and three new iridium(III) complexes [Ir(ppy)2(MHIP)](PF6) (9a), [Ir(bzq)2(MHIP)](PF6) (9b), and [Ir(piq)2(MHIP)](PF6) (9c), (ppy = deprotonated 2-phenylpyridine, bzq = deprotonated benzo[h]quinoline, piq = deprotonated 1-phenylisoquinoline). The characterization of complexes was carried out by HRMS, 1H NMR and 13C NMR spectra; the anticancer activities and possible mechanism toward B16 cells were explored in detail.
Results and discussion
Chemistry
MHIP was synthesized by reacting 1,10-phenanthroline-5,6-dione with 4-hydroxy-2-methylbenzaldehyde. 9a–9c were prepared by the reaction of MHIP with cis-[Ir(ppy)2Cl]2·H2O, cis-[Ir(bzq)2Cl]2·H2O or cis-[Ir(piq)2Cl]2·H2O in dichloromethane and methanol (Scheme 1). The obtained complexes 9a–9c were characterized by HRMS, 1H and 13C NMR spectra. In the HRMS spectra, the determined molecular weight of the complexes agreed with the calculated values. In the 1H NMR spectra of MHIP, the peak of 2.62 ppm is assigned the –CH3, and the peaks of 13.43 (s, 1H) and 9.81 (s, 1H) are attributed to the protons at the imidazole ring and –OH, while in the 1H NMR spectra of the complexes, the peaks of 2.67 ppm for 9a, 2.66 ppm for 9b and 2.67 ppm for 9c were attributed to the hydrogen atom on the methyl group. In addition, we did not observe the signal of the proton on the phenol hydroxyl group (–OH). Owing to a quick exchange between two nitrogen atoms in the imidazole ring, the signal of the proton in the imidazole ring was not observed. In the 13C NMR spectra, the peaks of 21.64 ppm for MHIP, 21.74 ppm for 9a, 21.93 ppm for 9b and 21.91 ppm for 9c are assigned to the carbon atom on the methyl group. The purity of 9a–9c was determined by HPLC (purity >95%) (Fig. S1, ESI†). Fig. S2a† shows UV-Vis spectra of complexes 9a–9c in PBS, and the maximum absorbance appeared at 275 nm (ε = 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450), 267 nm (ε = 29
450), 267 nm (ε = 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and 290 nm (ε = 14
000) and 290 nm (ε = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600). The complexes emit a weak fluorescence in PBS solution (Fig. S2b, ESI†), and the maximum peaks are located at 604 nm for 9a, 600 nm for 9b and 608 nm for 9c. The stability of 9a, 9b and 9c (10 μM) at 0, 24 and 48 h in PBS solution was determined, as shown in Fig. S2c (ESI†). No change in the peak shapes suggests that the complexes are stable in the PBS solution.
600). The complexes emit a weak fluorescence in PBS solution (Fig. S2b, ESI†), and the maximum peaks are located at 604 nm for 9a, 600 nm for 9b and 608 nm for 9c. The stability of 9a, 9b and 9c (10 μM) at 0, 24 and 48 h in PBS solution was determined, as shown in Fig. S2c (ESI†). No change in the peak shapes suggests that the complexes are stable in the PBS solution.
Cell uptake and cytotoxic activity in vitro studies
Before a compound can exert an anti-tumor effect, it first needs to enter cells. It is known that iridium(III) metal complexes can emit green fluorescence. To investigate whether iridium(III) complexes can enter the cells, B16 cells were exposed to twice IC50 concentrations of 9a, 9b and 9c for 6 h. As illustrated in Fig. 1a, we discovered that 9a, 9b and 9c can enter the cancer cells.The antiproliferative activity of 9a, 9b and 9c against HepG2, B16, A549 and normal NIH 3T3 was examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT).19 The IC50 values are listed in Table 1. In the dark, it can be found that 9a, 9b and 9c had moderate cytotoxicity on HepG2, A549 and B16 cells and no cytotoxicity toward NIH 3T3 cells. However, B16 cells were exposed to 9a–9c for 4 h, irradiated for 30 min, and continuously cultured for 48 h. The three complexes showed enhanced cytotoxicity to both cancer and normal cells, among which the cytotoxicity to B16 cells was the largest, with IC50 values of 3.1 ± 0.3 μM, 4.9 ± 0.8 μM, and 0.4 ± 0.1 μM for 9a, 9b and 9c, respectively; the cytotoxic activity of 9c toward A549 cells is comparable to that of [Ir(bzq)2(DBDIP)](PF6) (IC50 = 0.4 μM).13 Therefore, irradiation can significantly enhance the anticancer efficiency. In the subsequent cell experiments, except for cellular uptake and co-localization, B16 cells were treated with IC50 (or 2 × IC50) of 9a, 9b and 9c for 4 h, then irradiated for 30 min (white, LED lamp) and continuously cultured for 24 h.
| Complex | B16 | HepG2 | A549 | NIH 3T3 | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 dark | IC50 light | IC50 dark | IC50 light | IC50 dark | IC50 light | IC50 dark | IC50 light | |
| Data for cisplatin from ref. 18, nd: not determined. | ||||||||
| 9a | 28.8 ± 1.3 | 3.1 ± 0.3 | 30.0 ± 4.0 | 9.3 ± 1.5 | 23.4 ± 1.2 | 15.9 ± 2.8 | >100 | >100 |
| 9b | 26.1 ± 1.8 | 4.9 ± 0.8 | 19.2 ± 4.2 | 10.2 ± 3.1 | 23.8 ± 1.5 | 15.0 ± 2.0 | >100 | >100 |
| 9c | 30.8 ± 3.1 | 0.4 ± 0.1 | 46.7 ± 2.4 | 4.5 ± 1.4 | 24.9 ± 0.4 | 0.5 ± 0.3 | >100 | 21.7 ± 0.8 |
| Cisplatin | 9.1 ± 1.4 | 18.8 ± 1.2 | 6.6 ± 0.7 | nd | ||||
Single oxygen and superoxide anions are all excited state molecules from the ROS family, which can easily bind to DNA, proteins, and other biomolecules, causing damage to the cell and finally leading to cell death.20–24 1,3-Diphenylisobenofuran (DPBF) is a fluorescent probe with high specificity for singlet oxygen. After binding to singlet oxygen, DPBF undergoes irreversible oxidation, thereby leading to a decrease in the intensity of ultraviolet absorption.25 As depicted in Fig. 1b and c, the UV absorption value at 417 nm shows a decreasing trend, and changes in the absorbance of 9a, 9b and 9c reach 79.1, 80.8 and 87.5%, respectively, which indicates that the complexes 9a–9c produce singlet state oxygen during the light activation process. Additionally, we used Ru(bpy)32+ as the standard (methanol, Φ = 0.81)26 to determine the quantum yields based on the following equation:27
| Φsample = Φref × (Ksample/Kref) × (Fref/Fsample), |
Dihydrorhodamine (DHR123) is a non-fluorescent active oxygen indicator that emits fluorescence when oxidized to a cationic state. We use it to indirectly measure the generation of superoxide anions.28 As shown in Fig. 1d and e, the fluorescent intensity of DHR123 shows an increase of 3.42, 2.34 and 1.46 times for 9a, 9b and 9c, respectively, compared to the control during 60 s irradiation time. The quantum yields were calculated using the following equation:
| ΦS = ΦR × (FS/FR) × (AR/AS), |
The quantum yields Φ were determined to be 0.0298, 0.0125 and 0.0671 for 9a, 9b and 9c, respectively. These results suggest that 9a, 9b and 9c can effectively produce singlet oxygen and superoxide anions.
Cell scratch, cloning and cell cycle arrest studies
Cancer is a global problem that threatens human health. Tumor cells can proliferate and invade indefinitely, which has created a great challenge for treatment.29 To investigate the impact of 9a, 9b and 9c on the proliferation and migration of B16 cells, we carried out cell cloning and scratch experiments. The results of the scratch experiments are shown in Fig. 2a and b. The cells treated with 9a–9c show different degrees of migratory inhibition compared to the control. The cloning results are illustrated in Fig. 2c. Compared with the control group, the number of living cells in the complex-treated groups was greatly reduced, indicating that 9a, 9b and 9c had a greater antiproliferative effect on B16 cell proliferation. FAK has a promotional effect on the growth and metastasis of tumor cells. Increased FAK expression has been shown in many primary and metastatic cancers.30 Consequently, the change in FAK protein levels was investigated by western blot. Fig. 2d illustrates that the expression of FAK protein in the cells treated with 9a–9c shows a downward trend in contrast to the control group, which further shows that the three complexes possess an inhibitory effect on the proliferation of B16 cells.γ-H2AX is an important marker of DNA damage.31,32 To investigate the extent of DNA damage in B16 cells induced by metal complexes 9a–9c, we performed immunofluorescence staining of the cells using γ-H2AX dye. As shown in Fig. 2e, without the addition of the complex, the green fluorescence was very weak. However, after being treated with the complexes under irradiation conditions, the cells showed strong green fluorescence. It can be concluded that photoactivated metal complexes 9a–9c could induce cell death by causing DNA damage. In normal proliferating cells, a series of events, such as DNA replication and cell division, are in order.33 The anticancer effect can be produced by interfering with the cell cycle of tumor cells.34 We used flow cytometry to analyze the impact of 9a–9c on the cell cycle distribution, and the results are shown in Fig. 2f. Compared to the control group, the percentage of the cells in the G1/G0 and S phases decreased after treatment with 9a, 9b and 9c, and the percentage of the cells in the G2/M phase increased by about 9.69% for 9a, 8.12% for 9b and 7.76% for 9c. These results suggest that 9a–9c can inhibit cell proliferation in the G2/M phase. p53, p38 and γ-H2AX are closely related to the cell cycle. γ-H2AX is associated with DNA damage and can promote DNA repair.35 The protein kinase p38 can activate protein p53,36,37 which can regulate cell cycle progression.38 Consequently, we examined the expression of p38, p53 and γ-H2AX. As shown in Fig. 2g, the expression of p53, p38 and γ-H2AX increased after the treatment of B16 cells with IC50 concentration of 9a–9c upon irradiation. These results clearly demonstrate that 9a, 9b and 9c can cause DNA damage and block cell proliferation at the G2/M phase.
Localization, membrane potential and microtubule morphology
Mitochondria can provide energy in eukaryotic cells, and they are also critical organelles that mediate the onset of apoptosis and senescence.39 To investigate whether 9a–9c can enter mitochondria, we performed a localization assay. Mito tracker red can stain mitochondria in living cells. As depicted in Fig. 3a, the cells were treated with IC50 concentrations of 9a, 9b and 9c for 6 h, and the mitochondria were stained red. The overlap of red with green emitted by the complexes indicates that the complexes co-localize in the mitochondria. Mitochondrial membrane potential (MMP) is an important indicator of cellular normalcy and is lost in the early stages of apoptosis.40,41 JC-1 emits red fluorescence when the intracellular mitochondrial potential is sufficient, but the fluorescence changes to green when a decrease in MMP occurs.42 The results of the MMP assay are presented in Fig. 3b. After treatment with the complexes, the red fluorescence was significantly weakened. However, green fluorescence was significantly enhanced compared to the control, indicating that 9a–9c could trigger a decrease in MMP. Microtubules have a large impact on cellular functions, such as intracellular transport, cellular structure, and cell division.43–46 Hence, we further tested the changes in microtubule morphology after treatment with the complexes. Fig. 3c depicts that the microtubule structure in the control was neatly and tightly arranged, whereas the structure began to become loose and unordered after treatment with 9a–9c. The above results suggest that 9a, 9b and 9c disrupt microtubule structure and thus trigger cell death.Measurement of intracellular ROS generation and LDH release
Intracellular reactive oxygen species are highly active and produced within the mitochondria, which exert an influence on cellular metabolism by regulating signal transduction, inducing oxidative stress and causing DNA damage.47 2′,7′-Dichlorodihydro-fluorescein diacetate (DCHF-DA), as a non-fluorescent probe, is used to detect the level of intracellular ROS in cells.48 In the presence of ROS, DCHF-DA is converted into characteristic green fluorescence 2′,7′-dichlorofluorescein (DCF). As illustrated in Fig. 4a, in the control, a weak green fluorescence was observed, while in the complex-treated groups, a bright green fluorescence was observed. Hence, the green fluorescence was significantly increased, suggesting that 9a, 9b and 9c can elevate the intracellular ROS level. LDH is a cytoplasmic enzyme widely present in the cell, and when the cell is damaged or dies, LDH is released outside of the cell; therefore, we can validate the degree of cellular damage by quantitatively measuring the amount of released LDH.49–51Fig. 4b depicts that the cells treated with 9a–9c showed an increase in the release of LDH compared to the control, indicating that the LDH was released outside of the cells. These results suggest that 9a, 9b and 9c can promote B16 cell damage, which in turn induces cell death.RNA sequencing
We constructed heat maps and volcano maps using R software to investigate whether 9c can regulate gene expression. It can be observed from Fig. 5a and b that 1445 genes were regulated, among which 812 genes were upregulated and 633 genes were downregulated after B16 cells were treated with 9c for 24 h. Meanwhile, to further explore the signaling pathways, GO analysis and KEGG pathway analysis were performed. As shown in Fig. 5c and d, GO analysis provided the number of proteins in the cellular component (CC, red) and biological process (BP, blue), which helps understand the function, localization, and biological pathways of proteins. KEGG enrichment clearly showed the metabolic pathways, which include the PI3K/AKT signaling pathway and glutathione metabolism. Overall, these results suggest that 9c can cause a change in cellular gene expression and exert antitumor effects through relevant pathways.Apoptosis and mechanism
Apoptosis allows organisms to maintain homeostatic levels by regulating the balance between cell proliferation and death.52,53 However, unlimited proliferation of tumor cells and less apoptosis disrupts this balance.54 To explore the apoptotic efficiency of 9a, 9b and 9c on B16 cells, we used flow cytometry to examine the apoptotic percentage. Fig. 6a shows that the percentage of early (Q2) and late (Q3) apoptotic cells increased to 19.28% for 9a, 24.05% for 9b, and 18.45% for 9c compared to the control; meanwhile, the living cells (Q4) significantly decreased. These results suggest that 9a, 9b and 9c can efficiently promote the apoptosis of B16 cells. Tumor progression can be inhibited by the inhibition of PI3K expression.55–57 Therefore, we measured the expression of PI3K/AKT pathway-related proteins. It is well known that AKT activates Bax and inactivates Bcl-2 and caspase-9 proteins,58–60 while mTOR is a major downstream target of the PI3K/AKT pathway, and a critical regulator of cellular metabolism.61 As shown in Fig. 6b, the expression of PI3K, AKT, mTOR, and P-mTOR in B16 cells decreased compared to the control, which revealed that the PI3K/AKT signaling pathway was inhibited. In the caspase family, most proteins are associated with cell death or apoptosis,62,63 and when caspase 9 is activated, it induces apoptosis by activating caspase 3, while caspase 3 can inhibit DNA repair.64Fig. 6b displays that the expression of Bax increased and that of Bcl-2 decreased, while the expression of caspase 9 increased and that of caspase 3 decreased in the caspase family. Meanwhile, we observed the downregulation of PARP. Therefore, we speculate that 9a, 9b and 9c trigger apoptosis by ROS-mediated mitochondrial dysfunction and inhibit the PI3K/AKT signaling pathways.Induction of ferroptosis
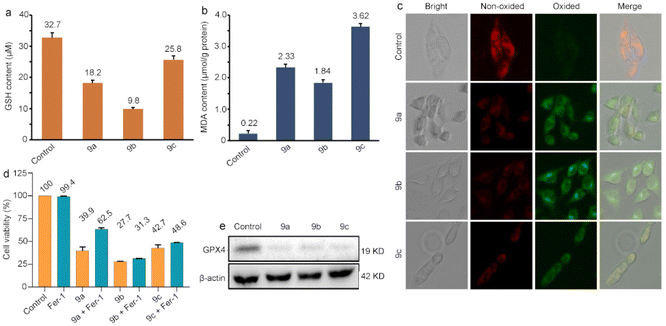
Ferroptosis is a mode of cell death regulated by oxidative and antioxidant systems, which produce lipid peroxides that lead to cell death.65 Glutathione (GSH) plays a great role in maintaining intracellular redox homeostasis,66 and the reduction of GSH levels triggers intracellular oxidative stress to induce ferroptosis.67 First, we measured the effect of 9a–9c on GSH content in B16 cells. The results can be observed in Fig. 7a. The contents of GSH in the cells treated with IC50 concentrations of 9a, 9b and 9c were greatly reduced, which is caused by the reactive oxygen species to oxide GSH into glutathione disulfide (GSSG), inducing an imbalance of the redox system. MDA is a lipid peroxidation product and biomarker that can reflect the degree of lipid peroxidation.68Fig. 7b shows that a large increase in intracellular malondialdehyde (MDA) content was found compared to the control, indicating that lipid peroxidation occurred. Additionally, we applied C11-BODIPY 581/591 fluorescent probes to examine lipid peroxidation. By redox reaction with intracellular reactive oxygen species, the fluorescence changes from red to green, thus reflecting the intracellular lipid peroxidation level.69,70 As shown in Fig. 7c, the significant reduction of red fluorescence and the enhancement of green fluorescence were observed after treatment of B16 cells with IC50 concentrations of 9a, 9b and 9c for 24 h, indicating an incidence of lipid peroxidation. Ferrostatin-1 (Fer-1) is a selective inhibitor of ferroptosis, exerting its anti-ferroptotic activity by eliminating alkoxyl radicals in lipid hydroperoxides in the presence of ferrous ions.71 Therefore, the Fer-1 inhibitor can be used to verify whether 9a, 9b, and 9c can disrupt the lipid peroxidation balance in tumor cells. Therefore, we examined cell viability in the presence of Fer-1. As shown in Fig. 7d, Fer-1 promotes the increment of cell viability compared to the complex alone. GPX4 is a key regulator in the ferroptosis process,72,73 and a decrease in its level makes cells more sensitive to ferroptosis. We used western blotting experiments to explore the expression of GPX4. Fig. 7e shows that the expression of GPX4 in the cells treated with 9a, 9b and 9c demonstrated a decreasing trend. All these results indicate that 9a–9c could reduce the content of GSH and downregulate the expression of GPX4, disrupt the balance of the intracellular redox system, promote the generation of cellular lipid peroxidation, and then induce the occurrence of ferroptosis.Conclusions
In this study, three complexes, 9a–9c, were successfully synthesized and characterized. In the MTT experiments, we found that the three complexes exhibited significant cytotoxicity to B16 cells after irradiation. Cellular uptake and co-localization assays found that the complexes can enter the cells and localize in the mitochondria. Increased expression of p38 and γ-H2AX indicated that DNA damage occurred in the B16 cells. Reduced glutathione (GSH) concentration, increased malondialdehyde (MDA) levels, down-regulation of GPX4 expression, and C11-BODIPY581/591 staining results demonstrate that the complexes induce ferroptosis. In conclusion, the present study demonstrates that newly synthesized iridium(III) complexes can induce cell death via apoptosis (a ROS-mediated mitochondrial dysfunction pathway) and ferroptosis. This work provides some insights into the anticancer activity of iridium(III) complexes and helps in the design and synthesis of new iridium(III) complexes as drug candidates.Experimental
Synthesis of ligands and complexes
Synthesis of complexes
The mixture of MHIP (0.326 g, 1 mmol) and cis-[Ir(ppy)2Cl]2·H2O74 (0.56 g, 0.5 mmol) or cis-[Ir(bzq)2Cl]2·H2O74 or cis-[Ir(piq)2Cl]2·H2O74 was weighed precisely and placed in a three-necked flask. CH2Cl2 (20 mL) and CH3OH (10 mL) were added as solvents. The reaction was carried out at 40 °C under argon. After 6 h, the reaction was added to an appropriate amount of NH4PF6 powder (about 1 g) to continue for 1 h. The yellow crude product was obtained after filtration. Then, the crude product was purified by column chromatography using dichloromethane and acetone as eluents.9a: yield, 82%. 1H NMR (DMSO-d6, 500 MHz, Fig. S3, ESI†): 9.28 (d, J = 6.0 Hz, 2H), 8.26 (d, J = 8.0 Hz, 2H), 8.11 (d, J = 6.0 Hz, 2H), 8.04 (dd, J = 8.0 Hz, 2H), 7.95 (d, J = 7.5 Hz, 2H), 7.88 (t, J = 7.5 Hz, 2H), 7.80 (d, J = 8.5 Hz, 1H), 7.51 (d, J = 5.5 Hz, 2H), 7.06 (t, J = 7.5 Hz, 2H), 6.99–6.93 (m, 4H), 6.83 (d, J = 9.0 Hz, 2H), 6.30 (d, J = 7.5 Hz, 2H), 2.67 (s, 3H). 13C NMR (DMSO-d6, 125 MHz, Fig. S4, ESI†): 167.35, 159.14, 151.04, 149.61, 148.36, 144.52, 144.23, 139.47, 139.10, 132.83, 131.84, 131.71, 130.70, 127.26, 125.52, 124.30, 122.78, 121.10, 120.41, 118.46, 113.56, 21.74. HRMS (CH3CN): calcd for C42H30IrN6OPF6: m/z = 827.2110 ([M − PF6])+, found: m/z = 827.2106 ([M − PF6])+.
9b: yield, 74%. 1H NMR (DMSO-d6, 500 MHz, Fig. S5, ESI†): 9.26 (d, J = 8.0 Hz, 2H), 8.51 (d, J = 7.5 Hz, 2H), 8.08 (d, J = 6.0 Hz, 2H), 7.98 (d, J = 8.5 Hz, 4H), 7.94–7.87 (m, 4H), 7.82 (d, J = 8.5 Hz, 1H), 7.57 (d, J = 8.0 Hz, 2H), 7.44 (dd, J = 8.0 Hz, 2H), 7.22 (t, J = 7.5 Hz, 2H), 6.82 (d, J = 9.0 Hz, 2H), 6.33 (d, J = 7 Hz, 2H), 2.66 (s, 3H). 13C NMR (DMSO-d6, 125 MHz, Fig. S6, ESI†): 159.03, 156.91, 149.31, 148.68, 147.91, 144.57, 140.87, 139.38, 137.96, 134.21, 132.83, 131.85, 130.18, 129.94, 129.04, 127.15, 124.69, 123.23, 120.76, 118.43, 113.52, 21.93. HRMS (CH3CN): calcd for C46H30IrN6OPF6: m/z = 875.2110 ([M − PF6])+, found: m/z = 875.2105 ([M − PF6])+.
9c: yield, 80%, 1H NMR (DMSO-d6, 500 MHz, Fig. S7, ESI†): 9.31 (d, J = 7.5 Hz, 2H), 9.01 (d, J = 7.5 Hz, 2H), 8.40 (d, J = 8.0 Hz, 2H), 8.03–7.94 (m, 6H), 7.89–7.85 (m, 5H), 7.43 (d, J = 6.5 Hz, 2H), 7.38 (d, J = 6.5 Hz, 2H), 7.16 (t, J = 8.5 Hz, 2H), 6.96 (t, J = 7.5 Hz, 2H), 6.81 (d, J = 9.0 Hz, 2H), 6.30 (d, J = 7.0 Hz, 2H), 2.67 (s, 3H). 13C NMR (DMSO-d6, 125 MHz, Fig. S8, ESI†): 168.31, 158.94, 154.57, 148.00, 145.85, 143.92, 141.22, 139.33, 136.94, 132.91, 132.46, 132.17, 131.90, 130.98, 129.81, 128.15, 127.09, 126.89, 126.00, 122.65, 118.40, 113.49, 21.91. HRMS (CH3CN): calcd for C50H34IrN6OPF6: m/z = 927.2423 ([M − PF6])+, found: m/z = 927.2476 ([M − PF6])+.
Single oxygen and superoxide anion detection
Single oxygen (1O2) is an essential substance in the process of killing tumor cells by photosensitizers. Similar to superoxide anions (O2˙−), they both belong to the ROS family and can directly or indirectly react with biological macromolecules to cause oxidative stress and damage, eventually triggering cell death. To investigate whether 9a, 9b and 9c produce single oxygen and superoxide anion in the treatment process, we conducted these experiments using 1,3-diphenylisobenofuran (DPBF) and dihydrorhodamine (DHR123) as quenching agents. The UV-Vis spectra of the mixed solution containing 10 μM of DPBF and 10 μM of complexes in methanol solution were recorded after irradiation at 0 s, 10 s, 20 s, 30 s, 40 s, 50 s, 60 s, 80 s, 120 s, 180 s, 240 s, and 300 s. To determine O2˙−, we recorded the fluorescence spectra of the mixed solution of DHR123 (1 mM) with complexes in PBS after 0 s, 5 s, 10 s, 15 s, 20 s, 25 s, 30 s, 40 s, 50 s, and 60 s of illumination.Cell apoptosis by flow cytometry
After the cells grew to a fusion degree of 80%–90% in 6-well plates, the cells were treated with 3.1 μM 9a, 4.9 μM 9b and 0.4 μM 9c for 4 h and irradiated for 30 min. The cells were continuously cultured for 24 h. Then, the cells were digested and washed twice with PBS. Finally, the cells were stained with 195 μL of Annexin-FITC conjugate, 10 μL of PI staining solution, and 5 μL of Annexin V in the dark, and the apoptotic percentage of the cells was examined with flow cytometry.Western blotting assay
When the cells grew to 80% in six-well plates, the cells were exposed to IC50 concentrations of 9a, 9b and 9c for 4 h and irradiated for 30 min. Then, the cells were continuously cultured for 24 h. The total protein was extracted and determined by applying the BCA method. The protein was diluted to the same concentration with a protein loading detection buffer and deionized water, and the protein was boiled for 10 min to facilitate the preservation of the sample. Subsequently, the various proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. The membranes were incubated overnight in primary antibody diluent, washed, and then incubated in secondary antibody diluent for 70 min. Protein bands were dyed in a chemiluminescence kit, and the results were analyzed as soon as possible using a FluorChem E instrument.RNA sequencing assay
B16 cells were treated with 9c (2 × IC50 concentration) for 4 h in six-well plates and illuminated for 30 min. The cells were continuously cultured for 24 h. The total RNA was extracted by adding Trizol reagent and quantitatively determined by applying a NanoDrop spectrophotometer.75 Measurements were completed and quantified using the AMpure XP system (Beckman Coulter, Beverly, CA, USA), a 2100 system (Agilent, Santa Clara, CA, USA), and finally sequenced using the Illumina NextSeq 500 sequencing platform.Note: The Materials and methods and the subsequent Experimental procedures can be found in the ESI.†
Cell culture, cytotoxicity assay, cellular uptake, cell colony assay and wound healing assay, cell cycle arrest analysis, mitochondrial localization and membrane potential measurement, cell microtubule morphology assay, DNA damage detection by γ-H2AX, determination of GSH and MDA content, C11-BODIPY 581/591 detection of ferroptosis, LDH release, intracellular ROS generation, and ferroptosis inhibitor.
Data analysis
Statistical significance was assessed with t-tests, and *P < 0.05 is significant.Data availability
Data will be made available upon request.Conflicts of interest
The authors declare no competing interest.Acknowledgements
We are grateful for the National Natural Science Foundation of China (no. 21877018) and Discipline Training Excellence, Innovation and Quality Improvement Engineering Team Project (no. 2024ZZ05).References
- F. J. Sulzmaier, C. Jean and D. D. Schlaepfer, FAK in cancer: mechanistic findings and clinical applications, Nat. Rev. Cancer, 2014, 14, 598–610 CrossRef CAS PubMed.
- E. Pérez-Herrero and A. Fernández-Medarde, Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy, Eur. J. Pharm. Biopharm., 2015, 93, 52–79 CrossRef PubMed.
- S. Ghosh, Cisplatin: The first metal based anticancer drug, Bioorg. Chem., 2019, 88, 102925 CrossRef CAS PubMed.
- A. M. P. Romani, Cisplatin in cancer treatment, Biochem. Pharmacol., 2022, 206, 115323 CrossRef CAS PubMed.
- X. Wang, Y. Zhou, D. Wang, Y. Wang, Z. Zhou, X. Ma, X. Liu and Y. Dong, Cisplatin-induced cytotoxicity: From signaling network to therapeutic targets, Biomed. Pharmacother., 2023, 157, 114045 CrossRef CAS PubMed.
- S. Dasari and P. B. Tchounwou, Cisplatin in cancer therapy: molecular mechanisms of action, Eur. J. Pharmacol., 2014, 740, 364–378 CrossRef CAS PubMed.
- B. Ji, M. Wei and B. Yang, Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy, Theranostics, 2022, 12, 434–458 CrossRef CAS PubMed.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Photodynamic therapy of cancer: an update, CA-Cancer J. Clin., 2011, 61, 250–281 CrossRef PubMed.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- B. M. Luby, C. D. Walsh and G. Zheng, Advanced photosensitizer activation strategies for smarter photodynamic therapy beacons, Angew. Chem., Int. Ed., 2019, 58, 2558–2569 CrossRef CAS PubMed.
- J. Tong, X. Yang, X. Song, J. Liang, S. Huang, H. Mao, M. Akhtar, A. Liu, G. G. Shan and G. Li, AIE-active Ir(III) complexes as type-I dominant photosensitizers for efficient photodynamic therapy, Dalton Trans., 2023, 52, 1105–1112 RSC.
- Z. Wang, L. Li, W. Wang, R. Wang, G. Li, H. Bian, D. Zhu and M. R. Bryce, Self-assembled nanoparticles based on cationic mono-/AIE tetra-nuclear Ir(III) complexes: long wavelength absorption/near-infrared emission photosensitizers for photodynamic therapy, Dalton Trans., 2023, 52, 1595–1601 RSC.
- L. Zhou, J. B. Li, J. Chen, X. Yao, X. D. Zeng, Y. J. Liu, Y. Wang and X. Z. Wang, Anticancer activity and mechanism studies of photoactivated iridium(III) complexes toward lung cancer A549, Dalton Trans., 2024, 53, 15176–15189 RSC.
- G. C. Li, J. Chen, Y. F. Xie, Y. Yang, Y. J. Niu, X. L. Chen, L. Zhou and Y. J. Liu, White light. increases anticancer effectiveness of iridium(III) complexes toward lung cancer A549 cells, J. Inorg. Biochem., 2024, 259, 112652 CrossRef CAS PubMed.
- S. Tian, H. X. Xu, X. Y. Wu, Y. Y. Ding, L. J. Liang, H. Yin, X. D. Zeng, Y. J. Liu and W. R. Zhu, Ruthenium(II) polypyridyl complexes inhibit tumor growth through stimulating immune system to increase CD8+ T Cell, Eur. J. Med. Chem., 2025, 290, 117470 CrossRef PubMed.
- H. Y. Hu, J. Chen, F. Zhang, Z. J. Sheng, Y. Yang, Y. F. Xie, L. Zhou and Y. J. Liu, Evaluation of efficiency of liposome-entrapped Iridium(III) complexes Inhibiting tumor growth in vitro and in vivo, J. Med. Chem., 2024, 67, 16195–16208 CrossRef CAS PubMed.
- H. W. Zhang, K. Wang, D. L. Ji, X. Liu, C. Wang, Y. Y. Jiang, Z. H. Jia, B. Xiong, Y. Ling and J. F. Miao, Novel tris-bipyridine based Ru(II) complexes as type-I/-II photosensitizers for antitumor photodynamic therapy through ferroptosis and immunogenic cell death, Eur. J. Med. Chem., 2024, 279, 116909 CrossRef PubMed.
- C. X. Huang, Y. H. Yuan, G. C. Li, S. Tian, H. Y. Hu, J. Chen, L. J. Liang, Y. Wang and Y. J. Liu, Mitochondria-targeted iridium(III) complexes encapsulated in liposome induce cell death through ferroptosis and gasdermin-mediated pyroptosis, Eur. J. Med. Chem., 2024, 266, 116112 Search PubMed.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
- X. Zheng and Q. Huang, Assessment of the antioxidant activities of representative optical and geometric isomers of astaxanthin against singlet oxygen in solution by a spectroscopic approach, Food Chem., 2022, 395, 133584 CrossRef CAS PubMed.
- A. Krieger-Liszkay, Singlet oxygen production in photosynthesis, J. Exp. Bot., 2005, 56, 337–346 CrossRef CAS PubMed.
- P. Di Mascio, G. R. Martinez, S. Miyamoto, G. E. Ronsein, M. H. G. Medeiros and J. Cadet, Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins, Chem. Rev., 2019, 119, 2043–2086 CrossRef CAS PubMed.
- W. Liu, S. He, X. Ma, C. Lv, H. Gu, J. Cao, J. Du, W. Sun, J. Fan and X. Peng, Near-infrared heptamethine cyanine photosensitizers with efficient singlet oxygen generation for anticancer photodynamic therapy, Angew. Chem., Int. Ed., 2024, 63, e202411802 CrossRef CAS PubMed.
- J. Zhang, Y. Wei, Y. Yue, H. Jiao, Y. Wu, W. Fu, K. M. Lin, C. Lu, S. Mou and Q. Zhong, IPK4 promotes oxidative stress and ferroptotic death through the downregulation of ACSM1, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2410628121 CrossRef CAS PubMed.
- X. Ouyang, X. Wang, H. B. Kraatz, S. Ahmadi, J. Gao, Y. Lv, X. Sun and Y. Huang, A Trojan horse biomimetic delivery strategy using mesenchymal stem cells for PDT/PTT therapy against lung melanoma metastasis, Biomater. Sci., 2020, 8, 1160–1170 RSC.
- K. Bhattacharyya and P. K. Das, Quantitative aspects of all-trans-retinol singlet and. triplet quenching by oxygen, Chem. Phys. Lett., 1985, 116, 326–331 CrossRef CAS.
- L. V. Lutkus, S. S. Rickenbach and T. M. Mccormick, Singlet oxygen quantum yields determined by oxygen consumption, J. Photochem. Photobiol., A, 2019, 378, 131–135 CrossRef CAS.
- Z. Wang, L. Wei, J. Lin, C. Huang, H. Chen, D. Fan, W. Hu, J. Liu, H. Huang, Z. Wang and X. Wang, Donor-acceptor-donor strategy rouses the photodynamic therapy anticancer activity of a bis-terpyridyl Ru(II) complex, J. Med. Chem., 2024, 67, 13435–13445 CrossRef CAS PubMed.
- M. A. Zaimy, N. Saffarzadeh, A. Mohammadi, H. Pourghadamyari, P. Izadi, A. Sarli, L. K. Moghaddam, S. R. Paschepari, H. Azizi, S. Torkamandi and J. Tavakkoly-Bazzaz, New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles, Cancer Gene Ther., 2017, 24, 233–243 CrossRef CAS PubMed.
- B. Y. Lee, P. Timpson, L. G. Horvath and R. J. Daly, FAK signaling in human cancer as a target for therapeutics, Pharmacol. Ther., 2015, 146, 132–149 CrossRef CAS PubMed.
- Q. Liu, Z. Lei, C. Gu, J. Guo, H. Yu, Z. Fatima, K. Zhou, M. A. B. Shabbir, M. K. Maan, Q. Wu, S. Xie, X. Wang and Z. Yuan, Mequindox induces apoptosis, DNA damage, and carcinogenicity in Wistar rats, Food Chem. Toxicol., 2019, 127, 270–279 CrossRef CAS PubMed.
- K. Khan, S. Tewari, N. P. Awasthi, S. P. Mishra, G. R. Agarwal, M. Rastogi and N. Husain, Flow cytometric detection of gamma-H2AX to evaluate DNA damage by low dose diagnostic irradiation, Med. Hypotheses, 2018, 115, 22–28 CrossRef CAS PubMed.
- L. Dewachter, N. Verstraeten, M. Fauvart and J. Michiels, An integrative view of cell cycle control in Escherichia coli, FEMS Microbiol. Rev., 2018, 42, 116–136 CrossRef CAS PubMed.
- J. Li and B. Z. Stanger, Cell cycle regulation meets tumor immunosuppression, Trends Immunol., 2020, 41, 859–863 CrossRef CAS PubMed.
- S. Riffle, R. N. Pandey, M. Albert and R. S. Hegde, Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids, BMC Cancer, 2017, 17, 338 CrossRef PubMed.
- X. Wang, E. R. Simpson and K. A. Brown, p53: Protection against tumor growth beyond effects on cell cycle and apoptosis, Cancer Res., 2015, 75, 5001–5007 CrossRef CAS PubMed.
- T. M. Thornton and M. Rincon, Non-classical p38 map kinase functions: cell cycle checkpoints and survival, Int. J. Biol. Sci., 2009, 5, 44–51 Search PubMed.
- R. H. Whitaker and J. G. Cook, Stress Relief Techniques: p38 MAPK Determines the Balance of Cell Cycle and Apoptosis Pathways, Biomolecules, 2021, 11, 1444 CrossRef CAS PubMed.
- S. C. H. Rehfeldt, S. Laufer and M. I. Goettert, A highly selective in vitro JNK3 inhibitor, FMU200, restores mitochondrial membrane potential and reduces oxidative stress and apoptosis in SH-SY5Y cells, Int. J. Mol. Sci., 2021, 22, 3701 CrossRef CAS PubMed.
- A. Cossarizza, C. Franceschi, D. Monti, S. Salvioli, E. Bellesia, R. Rivabene, L. Biondo, G. Rainaldi, A. Tinari and W. Malorni, Protective effect of N-acetylcysteine in tumor necrosis factor-alpha-induced apoptosis in U937 cells: the role of mitochondria, Exp. Cell Res., 1995, 220, 232–240 CrossRef CAS PubMed.
- A. Perelman, C. Wachtel, M. Cohen, S. Haupt, H. Shapiro and A. Tzur, JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry, Cell Death Dis., 2012, 3, e430 Search PubMed.
- K. Elefantova, B. Lakatos, J. Kubickova, Z. Sulova and A. Breier, Detection of the mitochondrial membrane potential by the cationic dye JC-1 in L1210 cells with massive overexpression of the plasma membrane ABCB1 drug transporter, Int. J. Mol. Sci., 2018, 19, 1985 CrossRef PubMed.
- P. Binarová and J. Tuszynski, Tubulin: structure, functions and roles in disease, Cells, 2019, 8, 1294 CrossRef PubMed.
- E. D. McKenna, S. L. Sarbanes, S. W. Cummings and A. Roll-Mecak, The tubulin code, from molecules to health and disease, Annu. Rev. Cell Dev. Biol., 2023, 39, 331–361 CrossRef CAS PubMed.
- M. O. Altonsy, A. Ganguly, M. Amrein, P. Surmanowicz, S. S. Li, G. J. Lauzon and P. R. Mydlarski, Beta3-tubulin is critical for microtubule dynamics, cell cycle regulation, and spontaneous release of microvesicles in human malignant melanoma cells (A375), Int. J. Mol. Sci., 2020, 21, 1656 CrossRef CAS PubMed.
- T. T. Miao, X. B. Tao, D. D. Li, H. Chen, X. Y. Jin, Y. Geng, S. F. Wang and W. Gu, Synthesis and biological evaluation of 2-aryl-benzimidazole derivatives of dehydroabietic acid as novel tubulin polymerization inhibitors, RSC Adv., 2018, 8, 17511–17526 RSC.
- Z. Zou, H. Chang, H. Li and S. Wang, Induction of reactive oxygen species: an emerging approach for cancer therapy, Apoptosis, 2017, 22, 1321–1335 CrossRef CAS PubMed.
- W. Xie, M. Peng, Y. Liu, B. Zhang, L. Yi and Y. Long, Simvastatin induces pyroptosis via ROS/caspase-1/GSDMD pathway in colon cancer, Cell Commun. Signaling, 2023, 21, 329 CrossRef CAS PubMed.
- N. Abutaha, Apoptotic potential and chemical composition of Jordanian propolis extract against different cancer cell lines, J. Microbiol. Biotechnol., 2019, 30, 893–902 CrossRef PubMed.
- C. Durchschein, A. Hufner, B. Rinner, A. Stallinger, A. Deutsch, B. Lohberger, R. Bauer and N. Kretschmer, Synthesis of novel shikonin derivatives and pharmacological effects of cyclopropylacetylshikonin on melanoma Cells, Molecules, 2018, 23, 2820 CrossRef PubMed.
- J. Li, S. H. Deng, J. Li, L. Li, F. Zhang, Y. Zou, D. M. Wu and Y. Xu, Obacunone alleviates ferroptosis during lipopolysaccharide-induced acute lung injury by upregulating Nrf2-dependent antioxidant responses, Cell. Mol. Biol. Lett., 2022, 27, 29 CrossRef CAS PubMed.
- C. Kim and B. Kim, Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A Review, Nutrients, 2018, 10, 1021 CrossRef PubMed.
- X. Xu, Y. Lai and Z. C. Hua, Apoptosis and apoptotic body: disease message and therapeutic target potentials, Biosci. Rep., 2019, 39, BSR20180992 CrossRef CAS PubMed.
- R. S. Wong, Apoptosis in cancer: from pathogenesis to treatment, J. Exp. Clin. Cancer Res., 2011, 30, 87 CrossRef CAS PubMed.
- D. Tewari, P. Patni, A. Bishayee, A. N. Sah and A. Bishayee, Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy, Semin. Cancer Biol., 2022, 80, 1–17 CrossRef PubMed.
- X. Peng, X. Huan, T. B. Lulu, W. Jia, S. Zhang, L. Cohen, S. Huang, J. Fan, X. Chen, S. Liu, Y. Wang, K. Wang, S. Isoyama, S. Dan, F. Wang, Z. Zhang, M. Elkabets and D. Kong, A novel pan-PI3K inhibitor KTC1101 synergizes with anti-PD-1 therapy by targeting tumor suppression and immune activation, Mol. Cancer, 2024, 23, 54 CrossRef CAS PubMed.
- J. Yang, J. Nie, X. Ma, Y. Wei, Y. Peng and X. Wei, Targeting PI3K in cancer: mechanisms and advances in clinical trials, Mol. Cancer, 2019, 18, 26 CrossRef PubMed.
- S. Revathidevi and A. K. Munirajan, Akt in cancer: Mediator and more, Semin. Cancer Biol., 2019, 59, 80–91 CrossRef CAS PubMed.
- L. Simonyan, T. T. Renault, M. J. Novais, M. J. Sousa, M. Côrte-Real, N. Camougrand, C. Gonzalez and S. Manon, Regulation of Bax/mitochondria interaction by AKT, FEBS Lett., 2016, 590, 13–21 CrossRef CAS PubMed.
- M. Shariati and F. Meric-Bernstam, Targeting AKT for cancer therapy, Expert Opin. Invest. Drugs, 2019, 28, 977–988 CrossRef CAS PubMed.
- D. Mossmann, S. Park and M. N. Hall, mTOR signaling and cellular metabolism are mutual determinants in cancer, Nat. Rev. Cancer, 2018, 18, 744–757 CrossRef CAS PubMed.
- H. Khan, A. Bangar, A. K. Grewal, P. Bansal and T. G. Singh, Caspase-mediated regulation of the distinct signaling pathways and mechanisms in neuronal survival, Int. Immunopharmacol., 2022, 110, 108951 CrossRef CAS PubMed.
- M. I. Avrutsky and C. M. Troy, Caspase-9: A multimodal therapeutic target with diverse cellular expression in human disease, Front. Pharmacol., 2021, 12, 701301 CrossRef CAS PubMed.
- A. Unnisa, N. H. Greig and M. A. Kamal, Inhibition of Caspase 3 and Caspase 9 Mediated Apoptosis: A Multimodal Therapeutic Target in Traumatic Brain Injury, Curr. Neuropharmacol., 2023, 21, 1001–1012 CrossRef CAS PubMed.
- X. Chen, J. Li, R. Kang, D. J. Klionsky and D. Tang, Ferroptosis: machinery and regulation, Autophagy, 2021, 17, 2054–2081 CrossRef CAS PubMed.
- S. K. Georgiou-Siafis and A. S. Tsiftsoglou, The key role of GSH in keeping the redox balance in mammalian cells: mechanisms and significance of GSH in detoxification via formation of conjugates, Antioxidants, 2023, 12, 1953 CrossRef CAS PubMed.
- B. Niu, K. Liao, Y. Zhou, T. Wen, G. Quan, X. Pan and C. Wu, Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy, Biomaterials, 2021, 277, 121110 CrossRef CAS PubMed.
- Y. Wu, J. Wang, T. Zhao, J. Chen, L. Kang, Y. Wei, L. Han, L. Shen, C. Long, S. Wu and G. Wei, Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes, J. Hazard. Mater., 2022, 426, 127807 CrossRef CAS PubMed.
- G. P. Drummen, B. M. Gadella, J. A. Post and J. F. Brouwers, Mass spectrometric characterization of the oxidation of the fluorescent lipid peroxidation reporter molecule C11-BODIPY (581/591), Free Radical Biol. Med., 2004, 36, 1635–1644 CrossRef CAS PubMed.
- C. Chen, W. Chen, X. Zhou, Y. Li, X. Pan and X. Chen, Hyperbaric oxygen protects HT22 cells and PC12 cells from damage caused by oxygen-glucose deprivation/reperfusion via the inhibition of Nrf2/System Xc-/GPX4 axis-mediated ferroptosis, PLoS One, 2022, 17, e0276083 CrossRef CAS PubMed.
- G. Miotto, M. Rossetto, M. L. Di, L. Orian, R. Venerando, A. Roveri, A. M. Vučković, V. Bosello, M. Zaccarin, L. Zennaro, M. Maiorino, S. Toppo, F. Ursini and G. Cozza, Insight into the mechanism of ferroptosis inhibition by ferrostatin-1, Redox Biol., 2020, 28, 101328 CrossRef CAS PubMed.
- T. M. Seibt, B. Proneth and M. Conrad, Role of GPX4 in ferroptosis and its pharmacological implication, Free Radical Biol. Med., 2019, 133, 144–152 CrossRef CAS PubMed.
- G. C. Forcina and S. J. Dixon, GPX4 at the crossroads of lipid homeostasis and ferroptosis, Proteomics, 2019, 19, e1800311 CrossRef PubMed.
- S. Sprouse, K. A. King, P. J. Spellane and R. J. Watts, Photophysical effects of metal-carbon σ bonds in ortho-metalated complexes of iridium(III) and rhodium(III), J. Am. Chem. Soc., 1985, 106, 6647–6653 CrossRef.
- Y. D. Sarathkumara, D. J. Browne, A. M. Kelly, D. J. Pattinson, C. M. Rush, J. Warner, C. Proietti and D. L. Doolan, The Effect of tropical temperatures on the quality of RNA extracted from stabilized whole-blood samples, Int. J. Mol. Sci., 2022, 23, 10609 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5dt00899a |
| This journal is © The Royal Society of Chemistry 2025 |