Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemistry of silylated rare earths and heavier f-elements: ready to enter the next stage
Christoph
Marschner

Institute of Inorganic Chemistry, Graz University of Technology, 8010 Graz, Austria
First published on 28th May 2025
Abstract
The last few years have witnessed exciting development in the chemistry of silylated rare earths and heavier f-elements. First examples of silylated lanthanum (La) and praseodymium (Pr) complexes have been reported so that currently out of all lanthanides only for the radioactive promethium (Pm) silylated complexes are unknown. Previously unknown U(III) silyl complexes have also been described. Spectacular examples of the elusive trisilylated complexes of lanthanides and actinides have been presented. These show unusual magnetic properties, making them susceptible to facile 29Si NMR spectroscopic characterization, which could be rather difficult for compounds with diminished silylation degree. Novel silylene complexes were shown to catalyze hydrosilylation reactions. The novel compound class of silole coordinated f-element complexes was introduced by La and erbium (Er) complexes with the latter exhibiting single molecule magnetic properties.
Introduction
Despite the first compound containing a transition metal-silicon σ-bond being reported by Wilkinson and co-workers as early as in 1956,1 systematic investigations of transition metal-silyls started only in the 1980s.2 In a similar way, first compounds containing silicon-lanthanide bonds were reported by Schumann and co-workers in the 1980s,3–5 followed by a first silyl uranium compound described by Porchia et al. shortly therafter.6An excellent and detailed compilation of the chemistry of silylated f-element compounds until 2020 was published recently by Réant, Liddle, and Mills.7 Following their summary, it is possible to come to a number of conclusions. For instance, at 2020, of the 15 lanthanides, there were no known examples for silylated complexes of La, Pr, and Pm and only one or two examples for Ce, Nd, Tb, Dy, Er, and Lu, each. At that time, the by far best studied element was Yb with 15 silylated examples followed by Sm with 11 examples and Eu and Tm with 5 examples, each. All reported silylated Eu and most of the Yb complexes featured the metal in the oxidation state +2. While most of the divalent complexes were disilylated, much less disilylated trivalent complexes had been prepared, left alone examples of trisilylated complexes. In 2020, the number of silylated actinide complexes amounted to 3 Th and 5 U complexes, all of them monosilylated.7 All of the uranium complexes featured U(IV).
A reason for the long-lasting neglect of silyl f-element chemistry may to be that many of these compounds are paramagnetic. Typically, strong paramagnetism causes problems with NMR spectroscopic characterization. Even if meaningful spectra can be obtained, their diagnostic value is often hard to assess. This is especially true for hetero-nuclei with diminished receptivity such as 29Si.
The present account attempts to provide an overview over the work on silylated rare earths and heavier f-elements, since the mentioned review by Réant, Liddle, and Mills.7
The solvent dissociation problem
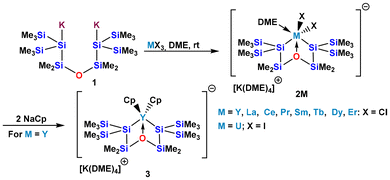
A common issue of f-element chemistry, associated not only with silylated but also with alkylated complexes, is that of facile dissociation of solvent molecules. Owing to the rather large ionic radii of the f-elements, coordination numbers of six and higher are rather common. As the number of covalently bound (X-type) ligands, is typically two or three for these complexes, at least three or more coordinating entities, frequently Lewis basic solvent molecules, can be expected. The extent of attractive interaction between f-element ions and coordinated Lewis bases is not very high and thus dissociation can be a facile process. Complexes with diminished coordination number are, however, prone to decomposition or aggregation processes.8 Therefore, the stability of isolated solvent adducts toward conditions facilitating dissociation is not very high. Information about bond strengths between silicon (or heavy group 14 elements in general) and f-element atoms is scarce. An early contribution by Marks and King reports a bond dissociation energy of 37 kcal mol−1 for the U–Si bond of Cp3USi(SiMe3)3.9To address problems arising from ligand-dissociation induced decomposition, our research group has sought to suppress such processes by use of multidentate ligands such as oligosilanylene diides and dimethoxyethane (DME) as solvent. In addition, the attachment of additional Lewis basic sites to the ligand, which was successfully applied to trialkyl complexes such as Ln(CH2C6H4-o-NMe2)3,10 was attempted.8 Utilizing the 1,5-oligosilanylene diide 1 containing a disiloxane unit, it was possible to isolate first examples of silylated La and Pr complexes as well as a first example of a structurally characterized Si–Dy complex along with complexes of Y, Ce, Sm, Tb, and Er (2M) (Scheme 1).11
Although a fair number of silylated rare earths and f-element complexes have been synthesized, the reactivity of these complexes has been barely addressed. In particular, reactions under the preservation of the silyl–metal bond are rare. The reaction of the yttrium complex 2Y with NaCp is thus extremely promising as it highlights clean replacement of chlorides for cyclopentadienyl ligands to give 3 (Scheme 1).
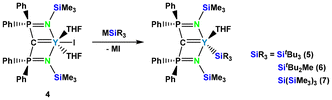
Similarly, Liddle, Mills and co-workers used a substituted methane diide ligand system, which also features additional donor sites. Reaction of yttrium iodide complex 4 with alkali silanides gave the Y complexes 5, 6, and 7 with attached SitBu3, SitBu2Me, and Si(SiMe3)3 groups, respectively (Scheme 2).12
Uranium(III) and Th(IV) complexes
The same group reported synthesis of Cp′′2Th(Cl)Si(SiMe3)3 (8Th) (Cp′′ = C5H3(SiMe3)2-1,3)13 and the first example of a U(III)–silyl complex: Cp′′2USi(SiMe3)3 (9U).14 The compounds were prepared by reaction of Cp′′2ThCl2 and (Cp′′2UI)2 with KSi(SiMe3)3 or alternatively, reaction of Cp′′2UCl2 with 2 equiv. KSi(SiMe3)3, resembling reaction of Cp2TiCl2 with 2 equiv. KSi(SiMe3)3, where the elimination of a (Me3Si)3Si dimer caused formal reduction to Ti(III).15 La, Ce, and Nd complexes (9Ln) isostructural to 9U of were also reported in this work.14Shortly after this, Bart and co-workers demonstrated that the mentioned 1,5-oligosilanylene diide ligand 1 can also be used in actinide chemistry. They were able to prepare the first disilylated uranium(III) complex (2U) by reacting 1 with UI3 (Scheme 1).16 Later, they reported on the reactivity of 2U with aryl diazenes, which results in the formal insertion of aryl imido units into the Si–U bonds.17
New complexes of La and Ce
Fang and co-workers reported a number of silylated La and also Ce ate-complexes of the type M[Cp3LnSiR3] (M = Li, Na, K; Ln = La, Ce; R3Si = Si(H)Mes2 (10Ln), SiMePh2 (11Ln), SiPh3 (12Ln) and SiPh2SiPh3 (13Ln)).18 This was followed by the synthesis of related monosilylated ate-complexes of other trivalent lanthanides (Ln = Sm, Tb, Dy, Yb) of the type [Li(DME)3] [Cp3LnSiPh3] (12Ln) and an example of a disilylated Lu ate-complex: [Li(DME)3][Cp2Lu(SiPh3)2] (14Lu).19 Fang and co-workers showed also that their synthetic strategy can be extended to triphenylgermyl and triphenylstannyl groups, yielding the respective La and Ce complexes: [Li(DME)3][Cp3LnEPh3] (Ln = La, Ce; E = Ge, Sn).20Di- and trisilylated f-element complexes
Extending the series of disilylated Yb, Eu and Sm complexes Kaltsoyannis, Mills, Liddle and co-workers prepared Yb, Eu and Sm complexes with SitBu3 and SitBu2Me ligands: (tBu3Si)2Ln(THF)(2 or 3) (15) and (tBu2MeSi)2Ln(THF)3 (16) (Ln = Yb, Eu, Sm).21Reviewing the organometallic chemistry of the lanthanides, it becomes evident that the number of homoleptic neutral trialkylated complexes is rather small.22 Although examples of Ln{CH(SiMe3)2}3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23–25 and Ln(CH2Ph)3(THF)3
23–25 and Ln(CH2Ph)3(THF)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26,27 are known, also these can suffer from ligand-dissociation caused instability. For this reason, it seemed not surprising that there were also no examples of homoleptic neutral trisilylated complexes known. However, recently Réant et al. were able to present first examples of this previously elusive class of compounds.
26,27 are known, also these can suffer from ligand-dissociation caused instability. For this reason, it seemed not surprising that there were also no examples of homoleptic neutral trisilylated complexes known. However, recently Réant et al. were able to present first examples of this previously elusive class of compounds.
Already in 2014 Sgro and Piers had reported the reactions of the THF adducts of YI3 and GdI3 with (Me3Si)3SiK to proceed to (Me3Si)3SiYI2(THF)3 and (Me3Si)3SiGdI2(THF)3, respectively.28 Attempts to obtain di- or even trisilylated complexes were not mentioned in the study. Nevertheless, utilizing a similar approach, formation of the trisilylated complexes of La, Ce, Pr, Nd, and U (17M) by reacting three equivalents of (Me3Si)3SiK with the respective triodides was reported (Scheme 3).29
These compounds are quite unique. They constitute the first ever known examples of trisilylated f-element complexes and show most unusual properties with respect to their receptivity to NMR spectroscopic characterization. As mentioned, the detection of NMR signals of silylated f-element complexes is frequently hampered by their paramagnetism. However, for compounds 17M29Si NMR chemical shift values for the metal bound silicon atoms could be detected for all (i.e. also the paramagnetic Ce, Pr, Nd, and U) complexes. An alignment of the most magnetic axis along the three-fold rotation axis occupied by the coordinated THF molecules seems responsible for this unexpected behavior. The metal-bound silicon atoms and also the trimethylsilyl groups are thus not much affected (see Fig. 1 for calculated magnetic axes of complex 17Ce). The chemical shift value of diamagnetic [(Me3Si)3Si]3La(THF)3 (17La) in solution (C6D6/C4D8O: 9/1) was found to be −82.3 ppm. The fact that the respective values of paramagnetic [(Me3Si)3Si]3M(THF)2 (M = Ce, Pr, Nd, and U) were all observed within a small spectral window between −65.5 and −79.4 ppm, indicates how effective the suppression of the paramagnetic influence for this particular geometry is. CASSCF-SO-calculations were used to obtain a suitable model of the magnetic susceptibility tensor and to model the pNMR shifts of the paramagnetic 17M complexes for 0, 1, and 2 coordinated THF molecules.29
 | ||
| Fig. 1 CASSCF-SO-calculated magnetic axes for 17Ce (blue: g1, most magnetic; green: g2, intermediate; red: g3, least magnetic) for complexes. Metal, silicon, carbon, and hydrogen atoms shown as metallic green, orange, gray, and light gray, respectively (figure reproduced from ref. 29). | ||
New silylene complexes of di- and trivalent rare earth metals
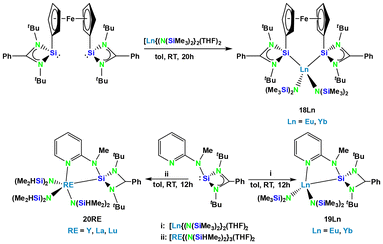
Continuing work on silylene coordinated divalent f-elements,30 P. Roesky and co-workers have introduced new ligand systems.31 A ferrocene based bis(silylene) ligand provided thermally stable complexes 18Ln (Ln = Eu, Yb) of Eu and Yb (Scheme 4). The other ligand is a silylene with an attached 2-pyridine unit which gave the respective complexes of divalent Eu and Yb (19Ln) (M = Eu, Yb) as well as trivalent Y, La, and Lu complexes (20RE) (M = Y, La, Lu) (Scheme 4). Complexes 18Yb, 20Y, 20La, and 20Lu were shown to be effective catalysts for the hydrosilylation of terminal alkenes with PhSiH3.31Silole complexes
P. Roesky and co-workers also reported the first example of a rare earths silole complex.32 Reaction of the cyclooctatetra-dienyl (COT) lanthanum iodide complex [La(η8-COT)I(THF)3]33 with a 2,5-bis(trimethylsilyl)silole dianion34 gave the anionic silole COT La complex (21La) which in the solid state was found to exist as a dimer, with a silole–Si lanthanum (La–Si′) interaction providing the attractive force for the dimerization (Scheme 5).32Layfield and co-workers reported the first erbium COT silole and stannole complexes.35 Reacting a COT [BH4] [THF]2 erbium complex with the same 2,5-bis(trimethylsilyl)silole dianion used by Roesky in 1,4-dioxane gave the anionic complex 22Er, which is monomeric in the solid state (Scheme 5).35 Investigations of the magnetic properties of the respective silole, stannole35 and germole36 erbium complexes revealed them as single-molecule magnets.
Si–M distances and 29Si NMR shifts of the presented compounds
Table 1 provides a summary of reported metal–Si distances and 29Si NMR shifts of the discussed compounds.| Complex | Si–M (Å) | δ Si (Si–M) (ppm) | Complex | Si–M (Å) | δ Si (Si–M) (ppm) |
|---|---|---|---|---|---|
| a Solid state CP/MAS 29Si NMR data. b Tentatively assigned. | |||||
2Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3.064(2)/3.057(1) | −161.6 |
12Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
2.9389(13) | n.d. |
2Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3.144(2)/3.159(2) | n.d. |
13La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1860(15) | −21.1a |
2Pr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3.119(2)–3.167(2) | n.d. |
13Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1705(9) | n.d. |
2Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3.089(2)/3.099(2) | n.d. |
14Lu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
2.982(2)/2.9162(17) | n.d. |
2Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3.073(2)/3.081(2) | n.d. |
15Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
3.060(3) | 54.2 |
2Dy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3.077(3)/3.077(3) | n.d |
15Eu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
3.258(4) | n.d. |
3Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
3.1315(9)/3.1459(9) | −153.4 |
15Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
3.261(3) | n.d. |
2U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
3.1149(6)–3.1713(14) | n.d. |
16Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
3.0678(14) | 29.2 |
5Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
3.062(2) | 33.5 |
16Eu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
3.2075(9) | n.d. |
6Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
3.017(10) | 12.9 |
16Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
3.2196(10) | n.d. |
7Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
3.0126(7) | −148.5 |
17La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
3.197(3) | −82.3 |
8Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
3.1053(13) | −66.3 |
17Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
3.172(2) | −79.4 |
9U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
3.116(2) | n.d. |
17Pr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
3.161(3) | −65.5 |
9La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
3.178(2) | −130.3b |
17Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
3.131(2) | −71.6 |
9Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
3.153(2) | n.d. |
17U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
3.114(2) | −70.5 |
9Nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
3.112(2) | n.d. |
18Eu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3.3276(10)/3.3294(9) | n.d. |
10La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1897(10) | −36.0a |
18Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3.2431(5)/3.1881(6) | 64.6 |
10Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1446(16) | n.d. |
19Eu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3.2309(6) | n.d. |
11La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1846(6) | −1.7a |
19Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3.0766(7) | 14.3 |
11Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1446(16) | n.d. |
20Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3.0134(5) | 9.4 |
12La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1733(4) | 7.0a |
20La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
3.1868(8) | 14.7 |
12Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.1415(6) | n.d. |
20Lu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
2.9380(7) | 27.9 |
12Sm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
3.0729(7) | n.d. |
21La![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
3.0888(5) Si/3.2908(6) Si′ | 187.9 |
12Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
3.0277(7) | n.d. |
22Er![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
2.882(2) | n.d. |
12Dy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
3.0092(7) | n.d. | |||
Conclusions
The chemistry of silylated rare earth and heavier f-elements has started as a lab curiosity some 40 years ago. Less than 70 papers dealing with these substances have been published. Approximately half of these derive from the last decade. Within these years, substantial progress has been made filling a number of obvious gaps in the field. The chemistry seems ready to enter the next phase, from exploratory discoveries to systematic studies of reactivity and properties and further on to applications in catalysis and material science.Data availability
Since this is a review type article, the data used for the composition of the article consist only of the cited references.Conflicts of interest
There are no conflicts to declare.Acknowledgements
I am most grateful to my wife Dr Judith Baumgartner, who has been the (unstoppable) driving force behind all our chemistry involving silylated rare earth and f-element compounds.References
- T. S. Piper, D. Lemal and G. Wilkinson, Naturwissenschaften, 1956, 43, 129 CrossRef CAS.
- M. S. Eisen, in The Chemistry of Organic Silicon Compounds, ed. Z. Rappoport and Y. Apeloig, John Wiley & Sons, Chichester, UK, 1998, vol. 2, pp. 2037–2128 Search PubMed.
- H. Schumann, S. Nickel, E. Hahn and M. J. Heeg, Organometallics, 1985, 4, 800–801 CrossRef CAS.
- H. Schumann, S. Nickel, J. Loebel and J. Pickardt, Organometallics, 1988, 7, 2004–2009 CrossRef CAS.
- H. Schumann, J. A. Meese-Marktscheffel and F. E. Hahn, J. Organomet. Chem., 1990, 390, 301–308 CrossRef CAS.
- M. Porchia, N. Brianese, U. Casellato, F. Ossola, G. Rossetto, P. Zanella and R. Graziani, J. Chem. Soc., Dalton Trans., 1989, 677–681 RSC.
- B. L. L. Réant, S. T. Liddle and D. P. Mills, Chem. Sci., 2020, 11, 10871–10886 RSC.
- R. Zitz, J. Hlina, M. Aghazadeh Meshgi, H. Krenn, C. Marschner, T. Szilvási and J. Baumgartner, Inorg. Chem., 2017, 56, 5328–5341 CrossRef CAS PubMed.
- W. A. King and T. J. Marks, Inorg. Chim. Acta, 1995, 229, 343–354 CrossRef CAS.
- H. Xie, C. Wu, D. Cui and Y. Wang, J. Organomet. Chem., 2018, 875, 5–10 CrossRef CAS.
- A. Pöcheim, C. Marschner and J. Baumgartner, Inorg. Chem., 2021, 60, 8218–8226 CrossRef PubMed.
- B. L. L. Réant, A. J. Wooles, S. T. Liddle and D. P. Mills, Inorg. Chem., 2022, 62, 137–146 CrossRef PubMed.
- B. L. L. Réant, D. D. A. Jayasinghe, A. J. Wooles, S. T. Liddle and D. P. Mills, Dalton Trans., 2023, 52, 7635–7645 RSC.
- G. K. Gransbury, B. L. L. Réant, A. J. Wooles, J. Emerson-King, N. F. Chilton, S. T. Liddle and D. P. Mills, Chem. Sci., 2023, 14, 621–634 RSC.
- H. Arp, M. Zirngast, C. Marschner, J. Baumgartner, K. Rasmussen, P. Zark and T. Müller, Organometallics, 2012, 31, 4309–4319 CrossRef CAS PubMed.
- N. J. Lin, M. Zeller and S. C. Bart, Chem. Commun., 2024, 60, 3954–3957 RSC.
- N. J. Lin, K. L. Gullett, U. K. Muna, R. Galloway, M. Zeller and S. C. Bart, Organometallics, 2025, 44, 289–299 CrossRef CAS.
- X. Pan, C. Wu, H. Fang and C. Yan, Inorg. Chem., 2022, 61, 14288–14296 CrossRef CAS PubMed.
- X. Pan, C. Wu, H. Fang and C. Yan, Chin. J. Chem., 2023, 41, 2995–3002 CrossRef CAS.
- X. Pan, C. Wu, H. Fang and C. Yan, Inorg. Chem., 2023, 62, 5660–5668 CrossRef CAS PubMed.
- B. L. L. Réant, V. E. J. Berryman, A. R. Basford, L. E. Nodaraki, A. J. Wooles, F. Tuna, N. Kaltsoyannis, D. P. Mills and S. T. Liddle, J. Am. Chem. Soc., 2021, 143, 9813–9824 CrossRef PubMed.
- K. C. Boteju, A. Ellern and A. D. Sadow, Chem. Commun., 2017, 53, 716–719 RSC.
- P. B. Hitchcock, M. F. Lappert, R. G. Smith, R. A. Bartlett and P. P. Power, J. Chem. Soc., Chem. Commun., 1988, 1007–1009 RSC.
- A. G. Avent, C. F. Caro, P. B. Hitchcock, M. F. Lappert, Z. Li and X.-H. Wei, Dalton Trans., 2004, 1567–1577 RSC.
- Y.-Y. Chu, A. García Alejo, S. L. Bud'ko, K. Boteju, S. Patnaik, A. Ellern, M. Pérez García and A. D. Sadow, Inorg. Chem., 2023, 62, 11751–11760 CrossRef CAS PubMed.
- A. J. Wooles, D. P. Mills, W. Lewis, A. J. Blake and S. T. Liddle, Dalton Trans., 2010, 39, 500–510 RSC.
- S. Bambirra, A. Meetsma and B. Hessen, Organometallics, 2006, 25, 3454–3462 CrossRef CAS.
- M. J. Sgro and W. E. Piers, Inorg. Chim. Acta, 2014, 422, 243–250 CrossRef CAS.
- B. L. L. Réant, F. J. Mackintosh, G. K. Gransbury, C. A. Mattei, B. Alnami, B. E. Atkinson, K. L. Bonham, J. Baldwin, A. J. Wooles, I. J. Vitorica-Yrezabal, D. Lee, N. F. Chilton, S. T. Liddle and D. P. Mills, JACS Au, 2024, 4, 2695–2711 CrossRef PubMed.
- X. Sun, T. Simler, K. Reiter, F. Weigend and P. W. Roesky, Chem. – Eur. J., 2020, 26, 14888–14895 CrossRef CAS PubMed.
- X. Sun, T. Simler, F. Kraetschmer and P. W. Roesky, Organometallics, 2021, 40, 2100–2107 CrossRef CAS.
- X. Sun, L. Münzfeld, D. Jin, A. Hauser and P. W. Roesky, Chem. Commun., 2022, 58, 7976–7979 RSC.
- L. Münzfeld, C. Schoo, S. Bestgen, E. Moreno-Pineda, R. Köppe, M. Ruben and P. W. Roesky, Nat. Commun., 2019, 10, 3135 CrossRef PubMed.
- Z. Dong, C. R. W. Reinhold, M. Schmidtmann and T. Müller, Organometallics, 2018, 37, 4736–4743 CrossRef CAS.
- S. De, A. Mondal, Y.-C. Chen, M.-L. Tong and R. A. Layfield, Chem. – Eur. J., 2025, 31, e202500011 CrossRef CAS PubMed.
- S. De, A. Mondal, Z.-Y. Ruan, M.-L. Tong and R. Layfield, Chem. – Eur. J., 2023, 29, e202300567 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |