Catalytic regioselective 3,4-difunctionalization of 3-iodo-o-carborane via Pd migration†
Xianren
Jiang
ab,
Chang-Hua
Ding
 a,
Zaozao
Qiu
a,
Zaozao
Qiu
 *ab and
Zuowei
Xie
*ab and
Zuowei
Xie
 bc
bc
aInnovation Institute of Carbon Neutrality, International Joint Laboratory of Catalytic Chemistry, Department of Chemistry, College of Sciences, Shanghai University, Shanghai 200444, China
bShanghai-Hong Kong Joint Laboratory in Chemical Synthesis, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200032, China. E-mail: qiuzz@sioc.ac.cn
cShenzhen Grubbs Institute and Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China
First published on 7th May 2025
Abstract
A Pd-catalyzed one-pot regioselective difunctionalization of 3-iodo-o-carborane has been achieved for the synthesis of a wide variety of 3-alkyl-4-Nu-o-carboranes (Nu = aryl, alkyl, amino, or thio groups) in moderate to excellent yields. This protocol combines the sequential activation of cage B(3)–I and B(4)–H bonds via Pd 1,4-migration.
Carboranes represent a unique class of polyhedral boron hydrides in which one or more BH vertices are replaced by CH units.1 These molecules exhibit distinctive structural characteristics, including nearly spherical geometry and extensive three-dimensional electron delocalization, rendering them valuable building blocks for applications ranging from advanced materials to pharmaceuticals.2,3 Although transition metal-catalyzed regioselective B–H functionalization of carboranes has witnessed remarkable progress, enabling access to structurally diverse derivatives that cannot be generated by other conventional methods,4 there remains significant interest in developing efficient, versatile methodologies for the synthesis of difunctionalized carboranes bearing different substituents.5 The metal migration strategy is a promising approach, which enables B–H functionalization at cage positions distinct from the initial site of bond activation.6,7 In this context, the 1,2-metal migration strategy was first used in 2017 by the Spokoyny group, who reported sequential cage-walking during Pd-catalyzed B–Br/B–H activation of 9-Br-m-carborane, affording functionalization at B(2), B(4), B(5), and B(9) positions.8 Our group subsequently disclosed an acylamino-directed Pd cage-walking process from B(4) to B(8) in o-carboranes, enabling selective B(8)-arylation.9 On the other hand, B–I functionalization followed by 1,4-Pd migration can offer difunctionalized o-carborane in a one-pot reaction. We reported a Pd-catalyzed alkenylation-iodine migration cascade reaction of 3-iodo-o-carboranes with alkynes, which combines the sequential activation of cage B–I and B–H bonds via Pd 1,4-migration with excellent regioselectivity (Scheme 1).10 This prompted us to investigate whether alkenes could be employed to achieve regioselective B(3)-alkylation and B(4)-functionalization via a similar Pd 1,4-migration pathway (Scheme 1).11 The results of our investigation are presented in this Communication.
We first evaluated the feasibility of B(3)-alkylation and iodine migration in the reaction of 3-iodo-o-carborane (1) with alkenes. Norbornene (2) was selected as the model substrate to suppress β-hydride elimination during the alkylation process. In the presence of 10 mol% Pd(dba)2 and 10 mol% PPh3, treatment of 1 with 5.0 equivalents of 2 in toluene at 80 °C for 3 days afforded 3-(2-norbornyl)-4-iodo-o-carborane (3) in 46% yield (Table 1, entry 1). Other monodentate phosphine ligands such as PCy3 and PPh2Cy failed to produce 3 (Table 1, entries 2 and 3), while PPh2Py led to a slightly improved yield of 53% (Table 1, entry 4). Bidentate ligands DPPB and DPPF afforded low yields (Table 1, entries 5 and 6), and bisphosphine L1 deactivated the catalyst entirely (Table 1, entry 7). The use of PhDavePhos (L2) gave a 16% yield (Table 1, entry 8), whereas the phosphine-amide ligand L3 significantly enhanced reaction efficiency (Table 1, entry 9). In addition, bisphosphine-amide ligand L4 enabled the formation of 3 in 92% yield with excellent regioselectivity (Table 1, entry 10). Screening of other Pd(0) catalysts did not yield better results, while Pd(II) was not suitable for this reaction (Table 1, entries 11, 12, 14 and 15). In the case of Pd(OAc)2, the reaction can be initiated by reactive Pd(0) species generated in situ through reduction of Pd(II) by the phosphine ligand (Table 1, entry 13).12 Reducing the catalyst or ligand loading or decreasing the amount of 2 led to diminished yields (Table 1, entries 16–18). Notably, no reactivity was observed with other alkenes, such as cyclohexene, 1-hexene, styrene, methyl acrylate, or 2-vinylpyridine, likely due to the unique ring strain and high reactivity of norbornene.
| Entry | L | [Pd] | Yieldb (%) |
|---|---|---|---|
| a Reactions were conducted on a 0.2 mmol scale of 1 in 2.0 mL of toluene; DPPB = 1,4-bis(diphenylphosphino)butane; DPPF = 1,1′-bis(diphenylphosphino)ferrocene. b Yield determined by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard. c 5 mol% Pd2(dba)3. d 5 mol% Pd(dba)2. e 5 mol% L4. f 4 equiv. of 2. | |||
| 1 | PPh3 | Pd(dba)2 | 46 |
| 2 | PCy3 | Pd(dba)2 | Messy |
| 3 | PPh2Cy | Pd(dba)2 | Messy |
| 4 | PPh2Py | Pd(dba)2 | 53 |
| 5 | DPPB | Pd(dba)2 | 27 |
| 6 | DPPF | Pd(dba)2 | 13 |
| 7 | L1 | Pd(dba)2 | N.R. |
| 8 | L2 | Pd(dba)2 | 16 |
| 9 | L3 | Pd(dba)2 | 72 |
| 10 | L4 | Pd(dba)2 | 92 |
| 11 | L4 | Pd2(dba)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
69 |
| 12 | L4 | Pd(PPh3)4 | 53 |
| 13 | L4 | Pd(OAc)2 | 56 |
| 14 | L4 | PdCl2(PPh3)2 | N.R. |
| 15 | L4 | [Pd(Allyl)2Cl]2 | N.R. |
| 16d | L4 | Pd(dba)2 | 80 |
| 17e | L4 | Pd(dba)2 | 42 |
| 18f | L4 | Pd(dba)2 | 72 |
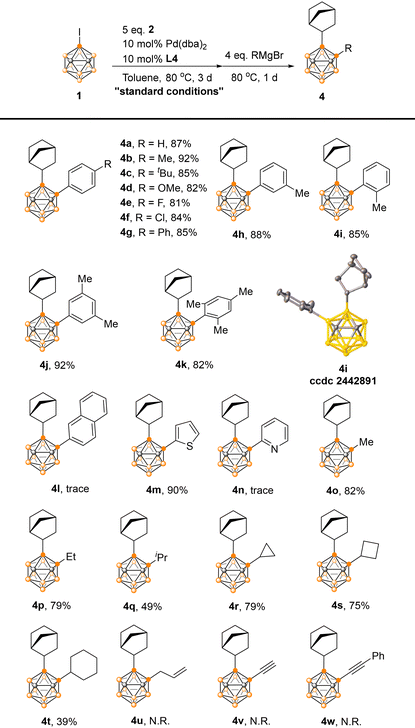
Subsequently, the scope of the one-pot difunctionalization process was explored under the optimized conditions through Pd-catalyzed cage B–C coupling reactions of 3-iodo-o-carborane with Grignard reagents (Table 2).13 It was found that this method was quite general and a range of aryl Grignard reagents delivered the desired products 4a–4k in 81–92% yields, regardless of their electronic nature. The sterically demanding 2,6-dimethylphenyl Grignard reagent furnished the corresponding 4k in 82% yield, though 2-naphthyl Grignard reagents were incompatible. For substrates bearing heteroaryls, 2-thienyl successfully afforded 4m in 90% yield, while no coupling reaction occurred with 2-pyridyl substrates, likely due to nitrogen coordination inhibiting Pd catalysis. A variety of alkyl Grignard reagents also reacted to afford 4o–4t in 39–82% yields. Lower yields for 4q and 4t were attributed to β-H elimination, generating 3-(2-norbornyl)-o-carborane as a byproduct. Allyl and alkynyl Grignard reagents failed to react under the optimized conditions due to lower nucleophilicity.
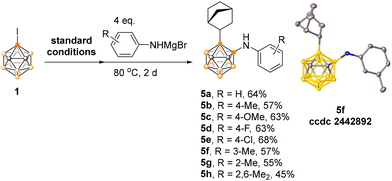
Given the utility of iodinated carboranes as key intermediates for boron vertex derivatization with the efficient construction of B–heteroatom bonds,14 we next examined the use of arylamino Grignard reagents for constructing B–N bonds (Scheme 2). One-pot reactions with phenylamino Grignard reagents afforded the difunctionalized product 5a in 64% yield. A series of substituted arylamino magnesium bromides bearing either electron-donating or -withdrawing groups were well tolerated, affording the corresponding 5b–g in 55%–68% isolated yields. Increasing steric hindrance on the aryl ring led to a reduced yield of 5h. Attempts to use alkylamino Grignard reagents resulted in complex mixtures.
After examining the one-pot B(3)-alkylation and B(4) cross-coupling reaction of 3-iodo-o-carborane and norbornene with Grignard reagents, we then evaluated the reactivity of sodium mercaptides as nucleophiles (Scheme 3). Reactions with these sulfur-based nucleophiles yielded B(4)-alkylthio derivatives 6a–6c in moderate to good yields. Sodium thiophenolate afforded 6d in 74% yield, while substrates bearing electron-donating (–OMe) or electron-withdrawing (–F) groups on the aryl ring also proved compatible, affording 6e and 6f in 35% and 47% yields, respectively.
All products 3, 4, 5 and 6 were fully characterized by 1H, 13C, and 11B NMR spectroscopy as well as high-resolution mass spectrometry. The molecular structures of 4i, 5f and 6b were further confirmed by single-crystal X-ray analyses.
Based on our previous studies10 and current observations, a plausible reaction mechanism is proposed in Scheme 4. The catalysis begins with oxidative addition of the B(3)–I bond in 3-iodo-o-carborane onto Pd(0), followed by norbornene insertion into the Pd–B bond to form a Pd(II) intermediate B. The subsequent 1,4-Pd migration from the alkyl carbon to the B(4) vertex furnishes intermediate C, which undergoes reductive elimination to deliver product 3 and regenerate Pd(0). Many attempts for the direct detection or characterization of the Pd–B intermediates failed.15 In the presence of nucleophiles, transmetallation and a second reductive elimination afford the difunctionalized products 4, 5, or 6, completing the catalytic cycle.
Conclusions
In summary, we have developed a Pd-catalyzed one-pot regioselective difunctionalization of 3-iodo-o-carborane via 1,4-Pd migration. This protocol enables the construction of a wide variety of 3-alkyl-4-Nu-o-carboranes (Nu = aryl, alkyl, amino, or thio groups) in moderate to excellent yields. The strategy integrates sequential B(3)–I and B(4)–H activation, followed by Pd-catalyzed B(4)–Nu bond formation, offering a powerful platform for diversifying o-carborane scaffolds.Author contributions
Z. Q. and Z. X. directed and conceived this project. X. J. conducted the experiments. All authors discussed the results and wrote the manuscript.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 4i, 5f and 6b have been deposited at the CCDC under deposition numbers 2442891 (4i), 2442892 (5f), and 2442893 (6b) and can be obtained from the CCDC viahttps://www.ccdc.cam.ac.uk/structures/.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Project No. 22371290 to Z. Q.; Project No. 22331005 to Z. X.), the Shenzhen Science and Technology Program (Project No. KQTD20221101093558015 to Z. X.), the Shanghai-Hong Kong Joint Laboratory in Chemical Synthesis, CAS, and the Hong Kong Research Grants Council (Project No. JLFS/P-404/24).References
- (a) R. N. Grimes, Carboranes, Academic Press, Amsterdam, The Netherlands, 3rd edn, 2016 Search PubMed; (b) N. S. Hosmane and R. D. Eagling, Handbook of Boron Science, World Scientific, 2018, vol. 2, pp. 1–155 Search PubMed; (c) J. Poater, C. Viñas, M. Sola and F. Teixidor, Nat. Commun., 2022, 13, 3844 CrossRef CAS PubMed; (d) F. Sun, S. Tan, H.-J. Cao, C.-S. Lu, D. Tu, J. Poater, M. Solà and H. Yan, J. Am. Chem. Soc., 2023, 145, 3577–3587 CrossRef CAS PubMed.
- (a) R. Núñez, M. Tarrés, A. Ferrer-Ugalde, F. F. de Biani and F. Teixidor, Chem. Rev., 2016, 116, 14307 CrossRef PubMed; (b) S. Mukherjee and P. Thilagar, Chem. Commun., 2016, 52, 1070–1093 RSC; (c) X. Li, H. Yan and Q. Zhao, Chem. – Eur. J., 2016, 22, 1888–1898 CrossRef CAS PubMed; (d) R. Núñez, I. Romero, F. Teixidor and C. Viñas, Chem. Soc. Rev., 2016, 45, 5147–5173 RSC; (e) D. Jung, L. M. A. Saleh, Z. J. Berkson, M. F. El-Kady, J. Y. Hwang, N. Mohamed, A. I. Wixtrom, E. Titarenko, Y. Shao, K. McCarthy, J. Guo, I. B. Martini, S. Kraemer, E. C. Wegener, P. Saint-Cricq, B. Ruehle, R. R. Langeslay, M. Delferro, J. L. Brosmer, C. H. Hendon, M. Gallagher-Jones, J. Rodriguez, K. W. Chapman, J. T. Miller, X. Duan, R. B. Kaner, J. I. Zink, B. F. Chmelka and A. M. Spokoyny, Nat. Mater., 2018, 17, 341–348 CrossRef CAS PubMed; (f) S. P. Fisher, A. W. Tomich, S. O. Lovera, J. F. Kleinsasser, J. Guo, M. J. Asay, H. M. Nelson and V. Lavallo, Chem. Rev., 2019, 119, 8262–8290 CrossRef CAS PubMed; (g) X. Wei, M.-J. Zhu, Z. Cheng, M. Lee, H. Yan, C. Lu and J.-J. Xu, Angew. Chem., Int. Ed., 2019, 58, 3162–3166 CrossRef CAS PubMed; (h) M. Keener, C. Hunt, T. G. Carroll, V. Kampel, R. Dobrovetsky, T. W. Hayton and G. Ménard, Nature, 2020, 577, 652–655 CrossRef CAS PubMed; (i) A. Saha, E. Oleshkevich, C. Viñas and F. Teixidor, Adv. Mater., 2017, 29, 1704238 CrossRef PubMed; (j) J. Tong, Y. Cao, Y. W. Zhang, P. Wang, P. Wang, X. J. Liao, W. Zhang, Y. Wang, Y. X. Zheng, J. J. Zhu and Y. Pan, Angew. Chem., Int. Ed., 2022, 61, e202209438 CrossRef CAS PubMed; (k) M. Wang, S. Zhang, Y. Gong, W. Zhang, Y. Wang, Y. Chen, Q. Zheng, Z. Liu and C. Tang, Angew. Chem., Int. Ed., 2024, 63, e202409283 CrossRef CAS PubMed.
- (a) M. F. Hawthorne and A. Maderna, Chem. Rev., 1999, 99, 3421–3434 CrossRef CAS PubMed; (b) Z. J. Leśnikowski, J. Med. Chem., 2016, 59, 7738–7758 CrossRef PubMed; (c) P. Stockmann, M. Gozzi, R. Kuhnert, M. B. Sárosi and E. Hey-Hawkins, Chem. Soc. Rev., 2019, 48, 3497–3512 RSC; (d) K. Fink and M. Uchman, Coord. Chem. Rev., 2021, 431, 213684 CrossRef CAS; (e) M. Couto, M. F. García, C. Alamón, M. Cabrera, P. Cabral, A. Merlino, F. Teixidor, H. Cerecetto and C. Viñas, Chem. – Eur. J., 2018, 24, 3122–3126 CrossRef CAS PubMed.
- (a) Y. Quan, Z. Qiu and Z. Xie, Chem. – Eur. J., 2018, 24, 2795–2805 CrossRef CAS PubMed; (b) Y. Quan and Z. Xie, Chem. Soc. Rev., 2019, 48, 3660–3673 RSC; (c) Y. K. Au and Z. Xie, Bull. Chem. Soc. Jpn., 2021, 94, 879–899 CrossRef CAS; (d) Z. Qiu and Z. Xie, Acc. Chem. Res., 2021, 54, 4065–4079 CrossRef CAS PubMed; (e) W.-B. Yu, P.-F. Cui, W.-X. Gao and G.-X. Jin, Coord. Chem. Rev., 2017, 350, 300–319 CrossRef CAS; (f) I. B. Sivaev, Russ. J. Inorg. Chem., 2021, 66, 1289–1342 CrossRef CAS; (g) H. Jia and Z. Qiu, Chin. J. Org. Chem., 2023, 43, 1045–1068 CrossRef CAS; (h) J. Zhang and Z. Xie, Synthesis, 2025, 57, 495–521 CrossRef CAS.
- (a) Y. K. Au, H. Lyu, Y. Quan and Z. Xie, J. Am. Chem. Soc., 2019, 141, 12855–12862 CrossRef CAS PubMed; (b) T. T. Xu, K. Cao, C. Y. Zhang, J. Wu, L. F. Ding and J. Yang, Org. Lett., 2019, 21, 9276–9279 CrossRef CAS PubMed; (c) R. Cheng, Z. Qiu and Z. Xie, Chem. – Eur. J., 2020, 26, 7212–7218 CrossRef CAS PubMed; (d) H.-J. Cao, M. Chen, F. Sun, Y. Zhao, C. Lu, X. Zhang, Z. Shi and H. Yan, ACS Catal., 2021, 11, 14047–14057 CrossRef CAS; (e) Z. Yang, Y. Wu, Y. Fu, J. Yang, J. Lu and J. Y. Lu, Chem. Commun., 2021, 57, 1655–1658 RSC; (f) Y. Baek, K. Cheong, D. Kim and P. H. Lee, Org. Lett., 2021, 23, 1188–1193 CrossRef CAS PubMed; (g) B. Cheng, Y. Chen, P. Zhou and Z. Xie, Chem. Commun., 2022, 58, 629–632 RSC; (h) C. Y. Zhang, K. Cao, D. Liu, H. B. Yang, C. C. Teng, B. Li and J. Yang, Dalton Trans., 2023, 52, 2933–2936 RSC.
- (a) A. Rahim, J. Feng and Z. Gu, Chin. J. Chem., 2019, 37, 929–945 CrossRef CAS; (b) X. Dong, H. Wang, H. Liu and F. Wang, Org. Chem. Front., 2020, 7, 3530–3556 RSC; (c) M. Y. Li, D. Wei, C. G. Feng and G. Q. Lin, Chem. – Asian J., 2022, 17, e202200456 CrossRef CAS PubMed.
- (a) D. Liu, L. Dang, Y. Sun, H.-S. Chan, Z. Lin and Z. Xie, J. Am. Chem. Soc., 2008, 130, 16103–16110 CrossRef CAS PubMed; (b) B. J. Eleazer, M. D. Smith, A. A. Popov and D. V. Peryshkov, Chem. Sci., 2017, 8, 5399–5407 RSC; (c) C. Guo, Z. Qiu and Z. Xie, ACS Catal., 2021, 11, 2134–2140 CrossRef CAS.
- (a) R. M. Dziedzic, J. L. Martin, J. C. Axtell, L. M. A. Saleh, T.-C. Ong, Y.-F. Yang, M. S. Messina, A. L. Rheingold, K. N. Houk and A. M. Spokoyny, J. Am. Chem. Soc., 2017, 139, 7729–7732 CrossRef CAS PubMed; (b) R. M. Dziedzic, J. C. Axtell, A. L. Rheingold and A. M. Spokoyny, Org. Process Res. Dev., 2019, 23, 1638–1645 CrossRef CAS PubMed.
- H. Lyu, J. Zhang, J. Yang, Y. Quan and Z. Xie, J. Am. Chem. Soc., 2019, 141, 4219–4224 CrossRef CAS PubMed.
- Y. Ge, J. Zhang, Z. Qiu and Z. Xie, Angew. Chem., Int. Ed., 2020, 59, 4851–4855 CrossRef CAS PubMed.
- (a) Y. Ge, Z. Qiu and Z. Xie, Chem. Commun., 2021, 57, 8071–8074 RSC; (b) Y. Ge, Z. Qiu and Z. Xie, Acta Chim. Sin., 2022, 80, 432–437 CrossRef CAS.
- (a) C. Amatore, M. Azzabi and A. Jutand, J. Am. Chem. Soc., 1991, 113, 8375–8384 CrossRef CAS; (b) Z. Csákai, R. Skoda-Földes and L. Kollár, Inorg. Chim. Acta, 1999, 286, 93–97 CrossRef; (c) C. S. Wei, G. H. M. Davies, O. Soltani, J. Albrecht, Q. Gao, C. Pathirana, Y. Hsiao, S. Tummala and M. D. Eastgate, Angew. Chem., Int. Ed., 2013, 52, 5822–5826 CrossRef CAS PubMed.
- (a) J. Li, C. F. Logan and M. Jones, Inorg. Chem., 1991, 30, 4866–4868 CrossRef CAS; (b) W. Jiang, C. B. Knobler, C. E. Curtis, M. D. Mortimer and M. F. Hawthorne, Inorg. Chem., 1995, 34, 3491–3498 CrossRef CAS; (c) Z. Zheng, W. Jiang, A. A. Zinn, C. B. Knobler and M. F. Hawthorne, Inorg. Chem., 1995, 34, 2095–2100 CrossRef CAS; (d) G. Harakas, T. Vu, C. B. Knobler and M. F. Hawthorne, J. Am. Chem. Soc., 1998, 120, 6405–6406 CrossRef CAS; (e) L. Crăciun and R. Custelcean, Inorg. Chem., 1999, 38, 4916–4919 CrossRef PubMed; (f) C. Viñas, G. Barberà, J. M. Oliva, F. Teixidor, A. J. Welch and G. M. Rosair, Inorg. Chem., 2001, 40, 6555–6562 CrossRef PubMed; (g) F. Teixidor, G. Barberà, A. Vaca, R. Kivekäs, R. Sillanpää, J. Oliva and C. Viñas, J. Am. Chem. Soc., 2005, 127, 10158–10159 CrossRef CAS PubMed; (h) G. Barberà, A. Vaca, F. Teixidor, R. Sillanpää, R. Kivekäs and C. Viñas, Inorg. Chem., 2008, 47, 7309–7316 CrossRef PubMed.
- (a) Y. Sevryugina, R. L. Julius and M. F. Hawthorne, Inorg. Chem., 2010, 49, 10627–10634 CrossRef CAS PubMed; (b) R. M. Dziedzic, L. M. A. Saleh, J. C. Axtell, J. L. Martin, S. L. Stevens, A. T. Royappa, A. L. Rheingold and A. M. Spokoyny, J. Am. Chem. Soc., 2016, 138, 9081–9084 CrossRef CAS PubMed; (c) K. Z. Kabytaev, T. A. Everett, A. V. Safronov, Y. V. Sevryugina, S. S. Jalisatgi and M. F. Hawthorne, Eur. J. Inorg. Chem., 2013, 2013, 2488–2491 CrossRef CAS.
- (a) F. Sun, S. Tan, H.-J. Cao, J. Xu, V. I. Bregadze, D. Tu, C. Lu and H. Yan, Angew. Chem., Int. Ed., 2022, 61, e202207125 CrossRef CAS PubMed; (b) Z. Sun, J. Zong, H. Ren, C. Lu, D. Tu, J. Poater, M. Solàand, Z. Shi and H. Yan, Nat. Commun., 2024, 15, 7934 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterization data of compounds. CCDC 2442891–2442893. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5dt00981b |
| This journal is © The Royal Society of Chemistry 2025 |