Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
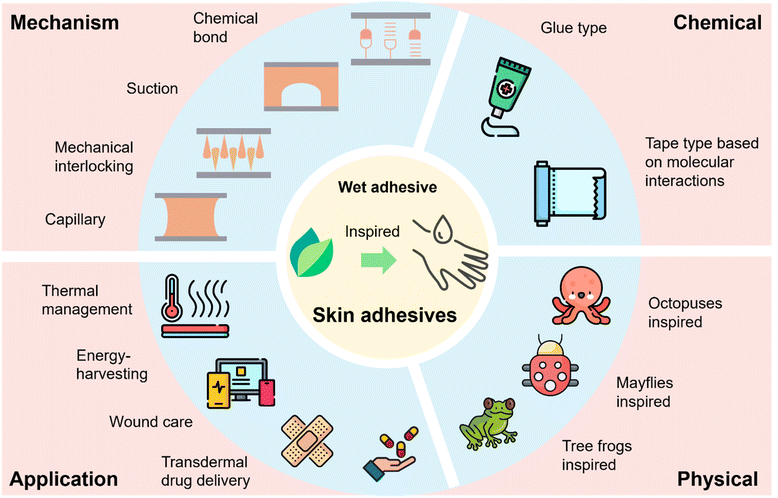
Role of chemistry in nature-inspired skin adhesives
Xiao
Yang†
ab,
Xiaonan
Liu†
 a,
Yeung Yeung
Chau†
a,
Xuezhi
Qin
a,
Yeung Yeung
Chau†
a,
Xuezhi
Qin
 a,
Hong
Zhu
a,
Liang
Peng
a,
Kannie W. Y.
Chan
b and
Zuankai
Wang
a,
Hong
Zhu
a,
Liang
Peng
a,
Kannie W. Y.
Chan
b and
Zuankai
Wang
 *ab
*ab
aDepartment of Mechanical Engineering, The Hong Kong Polytechnic University, Hong Kong 999077, China. E-mail: zk.wang@polyu.edu.hk
bHong Kong Centre for Cerebro-Cardiovascular Health Engineering (COCHE), Hong Kong 999077, China
First published on 30th May 2025
Abstract
As an essential component of wearable technology, skin adhesion plays a critical role in a wide range of wearable device applications. To maintain effectiveness and safety in daily use, skin adhesives must exhibit strong wet adhesion and high biocompatibility, particularly for devices that remain in contact with the skin for extended periods under humid and dynamic conditions. A comprehensive understanding of skin adhesion's chemical mechanisms is fundamental to advancing this technology. Nature offers valuable inspiration, as numerous organisms have evolved sophisticated chemical and physical adhesion strategies that enable strong and reversible bonding. This review begins by exploring the historical development of nature-inspired skin adhesives, followed by a detailed examination of their performance in moist environments. Particular emphasis is placed on the covalent and non-covalent interactions between adhesive materials and skin surface functional groups, considering both biocompatibility and wet adhesion properties. Additionally, we discuss strategies to mitigate hydration-related challenges alongside an overview of characterization techniques, including mechanical, chemical, and biological testing methods. The classification of nature-inspired skin adhesives into chemical and physical approaches is presented, highlighting their applications in thermal management, energy harvesting, wound care, and transdermal drug delivery. Finally, we identify current limitations and propose design strategies to guide the development of next-generation skin adhesives, providing a clear trajectory for future research.
1 Introduction
Skin adhesives are integral to the rapidly advancing field of wearable technologies and have become a focal point of research. These adhesives are indispensable for various applications, such as thermal management,1 energy harvesting,2 wound care,3 and transdermal drug delivery.4 Skin adhesives must exhibit excellent biocompatibility5 and strong wet adhesion6 properties to ensure their effectiveness and safety in daily use. Wet adhesion is particularly critical because many applications require prolonged contact between the adhesive and the skin, exposed to moist or dynamically changing environments. Achieving these desired properties necessitates a comprehensive understanding of the mechanisms that govern adhesive performance. This includes chemical interactions as well as physical processes. These insights are crucial for guiding the design of more efficient and reliable skin adhesives, addressing the increasing needs of both medical and consumer technology applications.7Nature provides diverse and efficient adhesion strategies, particularly effective on challenging surfaces such as wet or dynamic skin. Among them, octopuses, mayflies, and tree frogs represent three distinctive biological models that have profoundly inspired the development of synthetic skin adhesives.8,9 Octopus suction cups, capable of actively modulating internal pressure, enable reversible and robust negative-pressure adhesion even on wet and smooth substrates, informing the design of suction-based adhesive systems.10,11 The soft, mucus-coated tarsi of mayflies achieve stable adhesion to irregular surfaces through a combination of liquid bridging and mechanical interlocking, offering a blueprint for low-modulus, conformable adhesives.12 Tree frogs rely on toe pads with hexagonally arranged microstructures and mucus secretion channels to generate capillary forces and viscous fluid bridges, enabling reliable adhesion under wet and rough conditions.13 These organisms collectively employ a synergistic integration of chemical bonding, mechanical interlocking, capillary action, and suction mechanisms to overcome the challenges of wet adhesion, dynamic detachment, and substrate adaptability.
By systematically studying these natural systems, researchers have distilled core design principles that guide the development of synthetic skin adhesives. These nature-inspired adhesives have proven especially effective in diverse applications,14 where strong, conformal, and durable adhesion on moist or mobile skin is essential. The following sections will discuss how these biological concepts are translated into synthetic systems, emphasizing recent advances in adhesives based on chemical or physical approaches.15
This review begins by examining the development of nature-inspired skin adhesives and subsequently provides a detailed analysis of the performance of biomimetic adhesives in moist environments. The discussion emphasizes their covalent and non-covalent interactions with skin surface groups, considering both biocompatibility and wet adhesion. Critical strategies for overcoming the challenges associated with hydration are then highlighted to enhance adhesive efficacy. The review continues with an overview of characterization techniques relevant to nature-inspired skin adhesives, encompassing mechanical, chemical, and biological assessments. It further distinguishes between two primary formulation approaches, namely chemical and physical systems, and explores their respective applications in thermal management, energy harvesting, wound care, and transdermal drug delivery. Finally, the review identifies key technological challenges and proposes design strategies for next-generation skin adhesives, offering insights to guide future research efforts (Fig. 1).
2 Development of nature-inspired skin adhesives
As illustrated in Fig. 2, skin adhesives have evolved significantly since at least 600 BCE, when ancient civilizations utilized natural materials such as honey, tree sap, resin, and animal fats for wound closure and infection prevention.16 These early wound sealants relied on physical stickiness or simple chemical interactions, such as hydrogen bonding and van der Waals forces, to adhere to skin. The ancient Egyptians, for example, formulated mixtures of honey and animal fat to seal wounds. At the same time, sticky tree sap and plant resins served as early wound dressings by forming semi-permanent adhesive interfaces with skin.Between the 9th and 16th centuries, European innovations led to various glue-based medical adhesives, including starch-impregnated bandages that stabilized dressings and aided wound healing.17 Upon contact with moisture, starch granules swell and gelatinize, forming a sticky hydrogel-like paste that enhances adhesion between the bandage and the skin. In the 19th century, the introduction of rubber-based adhesives further improved flexibility and strength, ultimately leading to the commercialization of adhesive tapes for securing dressings.18
A breakthrough came in the 1950s with the development of cyanoacrylate adhesives (often called “superglue”). First discovered in 1942, cyanoacrylates polymerize rapidly upon exposure to trace amounts of water, including ambient moisture or tissue fluids. This anionic polymerization forms strong, durable polymer chains tightly binding to skin and other moist surfaces. Their ability to create robust adhesion within seconds in wet environments earned them the nickname “superglue.” During the Vietnam War, these adhesives were repurposed to control bleeding on the battlefield, demonstrating their potential in emergency wound closure.19
In modern times, a new generation of adhesives has drawn inspiration from marine organisms such as mussels and octopuses, demonstrating remarkable adhesion under wet and dynamic conditions. Mussel-inspired adhesives utilize catechol functional groups that form strong covalent and coordination bonds with wet surfaces, representing a chemical adhesion mechanism. In contrast, octopus-inspired designs often feature micro-suction structures that achieve adhesion through reversible physical interactions such as negative pressure and interfacial contact forces.20 These nature-inspired strategies highlight the complementary roles of chemical interactions, such as hydrogen bonding, covalent bonding, ionic interactions, and hydrophobic effects, and physical mechanisms in achieving robust and repeatable adhesion on skin.
Recent research also points to hydrogel-based adhesives with high water content and stimuli-responsive behavior (e.g., triggered by temperature,21 humidity, or pH22), which have shown great promise in diverse applications. Although their high water content typically limits adhesion on wet surfaces, this challenge is addressed by incorporating functional moieties such as catechol groups, aldehydes, or NHS esters that can form covalent or dynamic bonds with tissue surfaces. Additionally, phase-separated or energy-dissipating structures are often employed to enhance interfacial toughness, allowing these hydrogels to adhere firmly and conformably to moist, dynamic biological environments.
3 Design of nature-inspired skin adhesives
The development of nature-inspired skin adhesives requires a comprehensive understanding of how adhesion occurs at the skin interface and how it can be maintained under complex physiological conditions. Unlike traditional adhesives, those designed for skin must establish strong yet reversible attachment to a soft, irregular, and often moist surface, while ensuring safety and comfort. To meet these demands, researchers have focused on integrating multiple adhesion mechanisms, tuning material properties for biocompatibility, and overcoming the inherent challenges of wet environments. This section systematically explores the underlying design principles of such adhesives, beginning with the fundamental mechanisms of skin adhesion, followed by key considerations for biocompatibility and skin compatibility, culminating in strategies for achieving effective adhesion under wet or humid conditions.3.1 Mechanisms of nature-inspired skin adhesives
Adhesion at the skin interface arises from synergistic interaction of chemical bonding, suction forces, mechanical interlocking, and capillary effects, all modulated by the skin's surface characteristics and the adhesive.Chemical bonding involves covalent, ionic, or hydrogen bonds between the adhesive and skin biomolecules, particularly through interactions with the stratum corneum's amino, hydroxyl, or carboxyl groups. These interactions are primarily determined by the surface chemistry of the adhesive, including the type and density of functional groups it presents.23
Suction forces generated by negative pressure within confined microcavities at the adhesive and skin interface provide resistance against detachment. These forces are particularly effective on soft, compliant substrates such as skin, where a conformal seal enables the development of pressure differentials.24
Mechanical interlocking occurs when adhesives penetrate microscopic topographical features of the skin, such as pores or surface ridges. This interfacial anchoring is promoted by surface roughness, which increases contact area and enhances mechanical retention.25
Mediated by liquid bridges between the adhesive and skin, capillary effects promote close contact and interfacial cohesion, especially under humid or moist conditions. These effects depend on the wettability and geometry of the interface, which influence fluid distribution and retention.26
Significantly, the elastic modulus of the adhesive critically influences its ability to adapt to the skin's irregular and dynamic surface. Softer adhesives can deform in response to motion and topographical variation, maintaining intimate and continuous contact during movement or deformation.
We have also discussed how these mechanisms are regulated by three key surface characteristics: surface chemistry, surface roughness, and the elastic modulus of the adhesive materials.
3.2 Biocompatibility
Biocompatibility is paramount for the daily use of skin adhesives. Safety considerations mandate non-toxic formulations free from substances that can be absorbed through the skin and lead to systemic toxicity.27 Common allergens, such as latex and specific resin components, must be avoided to reduce the risk of allergic reactions, and thorough testing is required to confirm that the adhesives do not elicit adverse immune responses like contact dermatitis.28 Achieving balanced adhesion without skin irritation often requires maintaining a pH close to the skin's physiological range, approximately 4.5 to 5.5.29 Breathability is equally vital for preventing skin maceration; adhesives should allow moisture exchange while remaining intact on the skin.30 Biodegradable formulations are preferred whenever possible to reduce environmental impact and the accumulation of nondegradable waste.31Skin adhesives must also accommodate the needs of diverse skin types, including sensitive or compromised skin conditions. Some formulations integrate antimicrobial agents to deter infection risks.32,33 The adhesive must sustain its biocompatibility and bonding properties for longer-term applications without degrading the skin. Ease of removal is another critical factor to avoid discomfort or trauma.34,35 Finally, regulatory compliance and rigorous clinical evaluations ensure the adhesive's safety and performance across varied populations and environments.
3.3 Wet adhesion
Skin adhesives play a central role in ensuring secure and reliable attachment under physiological conditions. However, moisture from bodily fluids introduces substantial obstacles to wet adhesion by preventing the adhesive and the skin from forming intimate contact.36,37 Water molecules act as both physical and chemical barriers, segregating the adhesive layer from the skin's surface and impeding key bonding interactions. At the same time, sweat accumulating between the adhesive and the skin can lead to surface contamination or even outcompete reactive groups essential for bonding. Such interference can compromise adhesion strength, reducing immediate bond formation and long-term durability.38Researchers have concentrated on strategies that reinforce chemical interactions between the adhesive and the epidermis to address these issues.39 Achieving this goal requires a detailed understanding of skin surface chemistry and a deliberate matching of functional groups in the adhesive to those found on the skin. Covalent bonds, for instance, are formed through reactive functional groups such as N-hydroxysuccinimide esters, aldehydes, catechols, isocyanates, or aryl azides, which readily couple with skin amino or hydroxyl moieties. Adhesives that employ these chemistries maintain robust adhesion even in moisture. In addition to covalent crosslinking, non-covalent interactions—such as hydrogen bonding, van der Waals forces, electrostatic attractions, and hydrophobic interactions—contribute to the overall adhesion performance by providing reversible and humidity-tolerant bonding. These mechanisms are particularly relevant in skin adhesives, where pressure-sensitive adhesives often rely on van der Waals interactions combined with the viscoelastic deformation of the adhesive matrix to form effective contact under moist or dynamic skin conditions.
Recent technical advances have focused on mitigating the adverse effects of interfacial water through two primary lines of investigation. The first involves integrating hydrophilic elements, such as hygroscopic polymers, that can absorb or wick away water at the adhesive–skin interface, effectively reducing the thickness of the liquid layer. The second leverages phase-change materials or specialized coatings that modify surface energy in response to environmental stimuli, thereby repelling water or allowing it to disperse. These improvements are particularly beneficial in humid or constantly shifting environments, where maintaining a steady bond poses unique challenges.
Beyond direct chemical and materials engineering solutions, practical design considerations are vital in enhancing wet adhesion. Skin elasticity, for example, varies across different body regions and with user movement, so adhesives must accommodate stretching, bending, and dynamic loads without losing their hold.40,41 Individual anatomy further affects adhesion, as structures like folds or hair follicles can change the local surface texture. Adhesive formulations and architectures that account for these real-world variables can better maintain stable, reliable performance over a range of physiological conditions. By merging advanced chemistries with user-centric design, next-generation adhesives have the potential to achieve consistent, high-level adhesion despite the inherent difficulties posed by moisture in daily use.
An effective approach involves adding hygroscopic or amphiphilic components to the adhesive formulation. These materials can absorb or redistribute interfacial water, thereby thinning the hydration layer and enabling stronger, more direct interactions between adhesive functional groups and skin biomolecules. For instance, hydrogels containing polyethylene glycol segments or zwitterionic polymers can form structured hydration shells while supporting robust interfacial bonding.43
In parallel, hydrophobic groups or surface modifiers such as fluorinated moieties, silanes, and alkyl chains can be introduced to repel water and maintain effective contact by modulating surface energy, thereby enabling the adhesive to function under humid conditions.44
From an adhesion standpoint, reactive groups capable of forming covalent bonds under moist conditions, such as N-hydroxysuccinimide esters, cyanoacrylates, aldehydes, catechols, aromatic azides, and isocyanates, are particularly advantageous. These groups can displace interfacial water and react with amino, thiol, hydroxyl, and carboxyl residues on the stratum corneum to form stable linkages.45
Overall, these chemical regulation strategies not only mitigate the detrimental effects of water but also enable strong and durable adhesion under dynamic and high-moisture environments. Integrating such approaches is critical for advancing the performance and reliability of next-generation skin adhesives.
Materials with high surface energy or specific wettability can facilitate water displacement and increase real contact area. These mechanisms enable physical adhesives to maintain strong, reversible adhesion even on wet or mobile skin surfaces without relying on covalent or ionic bonding.
Physical adhesives are often implemented in tape-based formats and are particularly advantageous for applications requiring reversibility, gentle removal, and repeated use.
Because physical adhesion does not require molecular-level bonding, it is less affected by hydration, making it well-suited for applications where dynamic interfaces or reusability are prioritized.
3.4 Skin functional groups
Designing effective skin adhesives requires pinpointing functional groups such as amino, thiol, hydroxyl, and carboxyl in the skin's stratum corneum.46,47 Amino and thiol groups in keratin-containing proteins can form stable covalent bonds with complementary groups in adhesives (e.g., N-hydroxysuccinimide esters, aldehydes, catechols).48,49 Hydroxyl groups contribute to hydrogen bonding, while carboxyl groups participate in electrostatic and hydrogen-bond interactions.50,51 By leveraging these groups appropriately, adhesives can balance high bond strength with compatibility to avoid irritation or damage.52–54N-Hydroxysuccinimide esters are widely utilized in biochemical applications and adhesive formulations due to their high reactivity with primary amines under physiological to mildly alkaline conditions, forming stable amide bonds. Additionally, these esters can react with thiol groups to produce thioesters, broadening their reactivity and significantly reinforcing adhesive performance. This dual reactivity enables a robust and durable bonding mechanism for skin adhesion.
Aldehyde-functionalized adhesives promote strong and persistent adhesion by forming imine bonds with skin amines through Schiff base reactions. Simultaneously, aldehydes can interact with thiol groups to form hemithioacetals, introducing a reversible bonding mechanism that enhances adaptability in dynamic environments. This structural flexibility not only improves bonding durability but also expands the applicability of adhesives across varying environmental conditions.
Oxidized catechol derivatives, such as quinones, contribute to strong adhesion by forming covalent bonds with amine groups via Michael addition or Schiff base reactions. Additionally, catechol-based adhesives interact with skin proteins, including histamine, further strengthening adhesion. The thiol–catechol interaction, which involves Michael addition to aromatic rings, enhances bond stability, mimicking the exceptional wet adhesion observed in mussel-inspired adhesion mechanisms. This bioinspired approach significantly improves adhesive functionality in moist environments, offering enhanced performance for medical and everyday applications (Fig. 3A).
Isocyanates react with amine hydrogen to form stable urea bonds, ensuring strong and persistent adhesion under diverse conditions. Simultaneously, they engage in nucleophilic addition with hydroxyl groups, generating urethane bonds that enhance durability and flexibility. These properties make isocyanate-based adhesives particularly well-suited for long-term wear, as they maintain adhesion while accommodating skin movement (Fig. 3B).
Cyanoacrylates interact with amino groups through Michael addition, forming zwitterionic intermediates that polymerize other monomers. This polymerization process establishes robust covalent bonds, significantly reinforcing adhesive strength and ensuring stability under physiological conditions (Fig. 3C).
Aromatic azides generate highly reactive nitrene intermediates upon UV activation, which readily react with skin amines to form stable azo bonds. This photochemically induced bonding mechanism enables durable chemical adhesion, offering a versatile strategy for enhancing adhesive longevity and performance (Fig. 3D).
Carbodiimide chemistry facilitates the cross-linking of carboxyl groups with amino or thiol groups, improving adhesive strength and structural stability. Typically, this reaction employs 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide in conjunction with N-hydroxysuccinimide esters to activate carboxylic acids, increasing their reactivity toward amines or thiols. This cross-linking strategy is widely utilized to reinforce adhesive performance by stabilizing functional group interactions and ensuring long-lasting adhesion (Fig. 3E).
Hydrogen bonding occurs between functional groups such as amines, thiols, hydroxyls, and carboxyls, facilitating rapid adhesion between the skin and adhesive. Although hydrogen bonds are relatively weak and prone to dissociation in wet conditions, such as those involving sweat, their cumulative effect across multiple binding sites provides substantial adhesive strength. To improve stability, hydrogen bonding is often combined with additional adhesion mechanisms. However, adhesives that rely heavily on hydrogen bonds may swell due to their hydrophilic nature, impacting their long-term performance.58
van der Waals forces, arising from transient fluctuations in electron density between molecules, are individually weak but contribute significantly to adhesion when acting over large surface areas. These forces maintain higher stability in moist conditions than hydrogen bonds, making them particularly effective for adhesion in humid environments.59
Electrostatic interactions, driven by the attraction between oppositely charged molecules or functional groups, generate strong adhesion in dry or low-ion conditions. However, their effectiveness diminishes in aqueous environments due to ion interference. Despite this limitation, electrostatic forces can enhance overall adhesion when integrated with hydrogen bonding or covalent interactions.60
Hydrophobic interactions occur when non-polar molecules or regions aggregate to reduce exposure to water. These interactions are relatively strong, particularly in biological adhesives used in environments with sweat. While hydrophobic forces help maintain adhesion in wet conditions, they are most effective when combined with other bonding mechanisms such as hydrogen bonds or van der Waals forces.61 By integrating these non-covalent interactions with covalent bonding strategies, skin adhesives can achieve enhanced adhesion even in challenging, moist conditions, addressing critical requirements for both clinical and everyday applications.
Additionally, gentle mechanical pressure—on the order of 1 kPa—can enhance adhesion by improving the degree of contact between the adhesive and the skin.63 Applying pressure helps expel trapped fluids from the interface, promoting immediate bonding and reinforcing the hydrogels' moisture-absorbing capabilities. Consequently, hydrogel adhesives can achieve robust, secure attachment even in challenging conditions, such as sweaty skin or damp clinical environments.
However, absorbing interfacial water may lead to swelling in hydrogel-based adhesives, posing a risk to long-term performance.64 If the material significantly expands, its structural stability and mechanical properties can degrade, diminishing flexibility and adhesion strength. This concern underscores the need to optimize hydrogel composition and cross-link density carefully. Balancing strong water uptake for effective adhesion with minimized volume changes is crucial to maintaining durability.
Despite these potential drawbacks, hydrogels remain highly attractive for clinical and everyday applications on moist surfaces. Their ability to rapidly remove interfering water can be a game-changer for skin adhesives that must function under continuously humid or variable conditions. Ongoing research is focused on formulating hydrogel adhesives with controlled swelling and enhanced cross-linking strategies, aiming to preserve the benefits of water absorption without sacrificing mechanical integrity.65 By refining these approaches, future hydrogel-based adhesives may exhibit immediate bonding strength and consistent, long-term adherence in even the most demanding environments.
4 Characteristics of nature-inspired skin adhesives
Mechanical, chemical, and biological tests can evaluate skin adhesive properties. Mechanical tests measure adhesion strength and burst pressure, chemical tests assess degradation and swelling, and biological tests determine compatibility with cells and tissues and potential immune responses. By combining these methods, researchers can compare different adhesives, though caution is needed as performance often varies under various environmental conditions.664.1 Mechanical testing
The mechanical properties of a skin adhesive, including tensile strength, lap shear strength, peel resistance, and flat impact resistance, are crucial for its performance in daily applications. These properties ensure that the adhesive effectively bonds to the skin, remains flexible during movement, resists stretching or tearing, and retains its integrity over long-term use. Additionally, the adhesive must conform to the skin's texture and can be easily removed without causing discomfort or leaving a residue. Achieving the optimal balance among these characteristics ensures the adhesive's effectiveness, comfort, and durability in thermal management, energy harvesting, wound care, and transdermal drug delivery.The peel test is a widely used method for assessing the adhesive strength of skin adhesives (Fig. 4A). In this test, two substrates are bonded to the adhesive and then pulled vertically to the adhesive interface. The applied tensile force is monitored until the maximum peel force is reached, resulting in separation. In peel tests, the adhesive performance is typically characterized by the steady-state (plateau) peel force, which is normalized by the adhesive width to yield a force per unit width (N m−1). This metric reflects the energy required to propagate interfacial failure and is widely used for evaluating soft adhesives on skin or other compliant substrates. While the setup for this test is relatively simple, achieving accurate results requires careful alignment of the samples and precise application of force. This method helps avoid additional stresses, such as bending or twisting, ensuring uniform stress distribution across the sample.67 The peel test is also widely used for assessing the toughness of skin adhesives, quantifying the average peel force normalized to the width of the adhesive. This test is particularly suitable for soft adhesives, especially in applications with a risk of adhesive detachment from the substrate. While the preparation process is similar to that of shear tests, focusing on the adhesive's geometry, the primary goal of the peel test is to evaluate the toughness of the skin adhesive by detaching the adhesive from its surface.
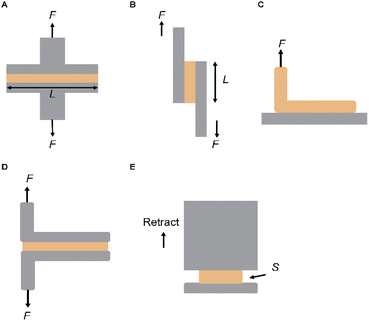 | ||
| Fig. 4 Mechanical testing of skin Adhesives. (A) Tensile test. (B) Lap shear test. (C) 90° peeling test. (D) 180° peeling test. (E) Flat punch test. | ||
In practical applications, skin adhesives are often subjected to shear stress, particularly when the underlying substrates are stretched. To evaluate this, a lap shear adhesion test has been developed, in which two surfaces overlap and are bonded in a defined region (Fig. 4B). Unlike the peel test, this test applies a force parallel to the adhesive interface. Separation occurs when the adhesive interface fails under maximum tensile force. The shear adhesion strength is then calculated by dividing the force by the bonded area. The lap shear test assesses the adhesive's resistance to in-plane stresses by applying a shear force on the bonded substrate. However, adhesive thickness and joint length can significantly influence the results, making geometric considerations essential for reliable evaluation.
During the test, one end of the adhesive is continuously pulled away from the surface at a fixed angle (y), typically at a constant speed. The angle y is usually chosen as either 90° or 180°, depending on the experimental requirements and the flexibility of the adhesive. Another variation is the T-peel test, in which both adhesive substrates are pulled simultaneously from one end, simulating a separate 90° peel operation. This setup is particularly advantageous when dealing with thin and flexible substrates (Fig. 4C and D).
The force–distance curve obtained from this test typically shows a phase where the force stabilizes, indicating the beginning of the stable peel process (F_peel). In both the 180° and T-peel tests, the energy required for crack propagation (interface toughness) is determined by multiplying the stable force by two and dividing by the adhesive width (measured in J m−2 or N m−1).
Additionally, it is essential to recognize that the interface adhesion performance of skin adhesives is influenced by both the mechanical and viscoelastic properties of the adhesive and the substrate. This means that the test results are closely related to the separation rate during the test. Skin adhesives are often used with stiffer backing materials to reduce sample stretching during peeling. This is crucial because the peeling process involves the bending of the backing material and the deformation of the adhesive, making the test highly sensitive to the mechanical and viscoelastic properties of the adhesive.
The indentation method is beneficial for studying skin adhesives, especially when analyzing the properties of surfaces with different surface chemistries or materials with heterogeneity. This technique involves applying pressure to a substrate using a probe of a specific shape until a predetermined displacement and/or force is achieved. Depending on the experimental goals, the applied pressure is maintained for a predetermined hold time, ranging from zero to several hours. After the hold period, the probe is withdrawn until completely detached from the substrate.
Researchers can gain insights into various aspects of adhesive performance by analyzing the force-displacement and force–time curves during the loading, holding, and unloading phases. For instance, the loading phase reveals mechanical properties, such as the elastic modulus of the skin adhesive. In contrast, the difference between the loading and unloading phases can indicate the system's hysteresis, where different unloading behaviors may arise from time-dependent physical or chemical interactions. Notably, the peel force (analogous to the maximum adhesion force in tensile and lap shear tests) is a key parameter in evaluating skin adhesive performance.
In indentation tests, researchers often adjust the shape of the probe (e.g., curved or flat, as shown in Fig. 4E). The primary advantage of using a flat punch for planar adhesion testing is that the contact area between the probe and the substrate remains constant and measurable. This is especially useful when evaluating the underwater adhesion performance of skin adhesives, as it eliminates the need for real-time monitoring of the contact area under complex operating conditions. Furthermore, the flat design simplifies the manufacturing process of the test equipment, making it particularly suitable when the adhesive is difficult to shape into a curved surface. Proper alignment between the flat probe and the substrate is crucial to ensure accurate results. It is important to note that high stresses at the edges of the contact area may lead to significant strains, resulting in additional viscoelastic effects.
Multiple mechanical testing strategies have been developed to evaluate the adhesion performance of skin adhesives under various physiological conditions. These methods assess different interfacial properties, including tensile, shear, and peeling strengths, and interfacial toughness under well-defined geometries and loading modes (Table 1). Such comprehensive characterization is essential for correlating adhesive mechanisms with performance outcomes in real-world applications.
| Testing methods | Geometry | Target parameters | Calculation formulas | Features |
|---|---|---|---|---|
| Tensile test | Width: W | Tensile strength (N m−2) | F max/WL | Evaluates adhesion strength in the normal direction by pulling apart the bonded surfaces |
| Length: L (adhesive) | ||||
| Lap shear test | Width: W | Shear strength (N m−2) | F max/WL | Assesses shear direction adhesion strength |
| Length: L (adhesive) | ||||
| 90° peeling test | Width: W (adhesive) | Interfacial toughness (J m−2) | F peel/W | Evaluates adhesion energy and average peel force at a 90° angle and fixed peel speed |
| 180° peeling test | Width: W (adhesive) | Interfacial toughness (J m−2) | 2Fpeel/W | Evaluates adhesion energy and average peel force at a 180° angle and fixed peel speed |
| Flat punch test | Contact area between flat probe and adhesive (S) | Planar adhesion strength (N m−2) | F max/S | Used for load–hold–unload adhesion tests on planar surfaces (contact area remains fixed) |
4.2 Chemical testing
Chemical testing provides comprehensive insights into skin adhesive composition, structural integrity, and potential degradation pathways. By revealing adhesive components' identity, purity, and stability, these analyses help researchers refine formulations to achieve safer and more effective materials. Among the most commonly employed techniques are nuclear magnetic resonance spectroscopy, Fourier transform infrared spectroscopy, and high-performance liquid chromatography.Nuclear magnetic resonance spectroscopy is particularly valuable for elucidating skin adhesives' composition and molecular architecture. It identifies various nuclei (1H and 13C) in the monomer or polymer backbone, clarifying side-chain substitutions, cross-linking density, and overall polymer conformation. In adhesives derived from dopamine-functionalized alginate, for example, NMR can precisely quantify the ratio of dopamine groups to alginate monomers, ensuring that the intended functionalization is achieved. Furthermore, by examining parameters such as chemical shifts and relaxation times, nuclear magnetic resonance offers insight into intermolecular interactions, such as hydrogen bonding, that can profoundly impact adhesive performance. While solution nuclear magnetic resonance is often used for materials dissolvable in specific solvents, solid-state nuclear magnetic resonance techniques enable the investigation of cross-linked or otherwise insoluble adhesives, furnishing a deeper understanding of polymer packing and network organization.
Fourier transform infrared spectroscopy, meanwhile, excels at identifying the functional groups present in skin adhesives and monitoring chemical changes throughout various stages of use or environmental exposure. By measuring infrared radiation absorption at characteristic wave numbers, Fourier transform infrared spectroscopy reveals the presence of essential groups like hydroxyl, carboxyl, or amine functionalities, key to wet adhesion and biocompatibility. This approach also detects newly formed bonds after polymerization or cross-linking and discerns bond scission during degradation. In situ Fourier transform infrared setups can observe chemical transitions in real time, assisting in process optimization and quality control. Moreover, an attenuated total reflectance variant of Fourier transform infrared probes the surface of the adhesive, which is especially relevant for studying skin–adhesive interfacial chemistry.
High-performance liquid chromatography offers a powerful means of separating and quantifying individual components in adhesive formulations. By evaluating solvent extracts or dissolved adhesive samples, high-performance liquid chromatography pinpoints residual monomers, cross-linkers, or plasticizers that might affect mechanical properties or biocompatibility. This analysis also facilitates the detection of leached substances or degradation by products over time, an important step in confirming the long-term safety of adhesives in direct contact with the skin. Confirming purity via high-performance liquid chromatography ensures the consistency of multi-component formulations, and any detected discrepancies can prompt further refinement before clinical or commercial application.
Beyond these three primary methods, researchers frequently employ complementary techniques such as thermogravimetric analysis and differential scanning calorimetry to assess thermal stability and transition points. X-ray photoelectron spectroscopy can determine surface elemental composition, while mass spectrometry identifies and characterizes low-molecular-weight species in the formulation or its degradation products. These chemical testing strategies deliver a thorough overview of the adhesive's composition, structural evolution, and potential interactions with the biological environment, enabling the design of high-performance skin adhesives that are safe and compliant with regulatory standards.
4.3 Biological tests
The biological performance of nature-inspired skin adhesives is typically assessed through a combination of in vitro and in vivo experiments to evaluate biocompatibility, inflammatory response, and tissue integration. In vitro assays commonly involve co-culturing the adhesive materials with fibroblasts, keratinocytes, or stem cells to assess cytotoxicity, often quantified through cell viability (e.g., MTT or live/dead assays)68 and proliferation metrics. These assessments follow standardized protocols, such as those outlined in ISO 10993, to support regulatory approval.In vivo studies are essential for capturing the host response in a physiologically relevant environment. Histological staining techniques such as hematoxylin and eosin and Masson's trichrome are used to examine tissue morphology, collagen deposition, and fibrotic responses. In addition, immunohistochemical staining for markers such as CD68 and CD11b enables the identification and quantification of macrophage infiltration, providing insight into immune activation and polarization. For example, a reduced CD68+ signal accompanied by increased CD206+ (M2 phenotype) expression is indicative of a more regenerative and less inflammatory microenvironment.69
Advanced techniques such as flow cytometry and proteomic profiling may be employed to characterize immune cell subpopulations and signaling pathways to gain deeper mechanistic insights. Together, these biological assessments are crucial for ensuring that nature-inspired adhesives are mechanically robust, safe, bioadaptive, and clinically translatable in the dynamic and sensitive environment of human skin.
5 Representative examples of nature-inspired skin adhesives
Advances in skin adhesive technologies have increasingly drawn inspiration from natural systems to address challenges such as wet adhesion, mechanical compliance, and biocompatibility. By emulating the key chemical interactions and physical architectures found in biological adhesion, researchers have developed innovative adhesives tailored to human skin's dynamic and irregular surface. This section explores representative examples of nature-inspired adhesives, categorizing them into two primary approaches: chemical and physical. Each approach leverages distinct mechanisms to achieve adhesion, with applications spanning from wound healing to wearable technologies.5.1 Adhesives based on chemical approaches
Chemical approaches to skin adhesion primarily rely on covalent, ionic, or hydrogen bonding to establish strong and durable interactions with biological tissues. These adhesives are designed with reactive functional groups capable of forming stable bonds with skin surfaces. Representative chemical adhesives include glue-type adhesives based on monomers, polymers, coacervates, and hydrogels, and tape-based adhesives based on molecular interactions (such as nanoscale coatings, films, and elastomers). These materials utilize distinct molecular interactions, including covalent cross-linking in polymer networks and electrostatic attraction in coacervates, to achieve robust adhesion under diverse physiological conditions.70Typically, after initial application, these adhesives require a curing period, ranging from minutes to several hours or even days, during which polymerization or cross-linking reactions occur. This curing process forms a stable polymer network, significantly enhancing the adhesive and skin bond. The final material properties, such as elasticity, toughness, and adhesive strength, depend highly on the polymer composition and cross-linking strategy.72 This versatility enables glue-type adhesives to be tailored for specific applications, offering reliable performance for a wide range of biomedical and wearable uses.
Monomer-based skin adhesives are polymer monomers or oligomers that polymerize or cross-link after application, forming a robust polymer network. This structure ensures excellent initial adhesion and facilitates uniform distribution on the skin surface, resulting in a strong and flexible bond. Polymerization can be initiated through chemical cross-linking (e.g., UV irradiation) or physical cross-linking (e.g., solvent evaporation or thermal curing). These adhesives perform exceptionally well in wearable skin devices, offering strong and flexible adhesion while maintaining skin comfort. Moreover, they are engineered for long-term stability and reliability, meeting the demands of diverse applications.73 Pal et al. reported a stable series of poly (α-lipoic acid) adhesives designed for closed-loop recycling, suitable for medical and non-medical applications. By making minor adjustments to the monomer composition, they created pressure-sensitive adhesives that are effective in both dry and wet conditions, with strengths comparable to traditional epoxy adhesives.74
Polymer-based skin adhesives can be divided into polymer solution-based and solvent-free polymer melt skin adhesives. Polymer solution-based skin adhesives are created by dissolving preformed high molecular weight polymers in a solvent.75 These adhesives capitalize on the inherent properties of the polymers to adhere firmly and flexibly to the skin's surface upon application. Unlike monomer-based adhesives, they eliminate the need for additional polymerization or cross-linking steps, enabling instant adhesion. Adhesion is achieved through physical interactions such as hydrogen bonding, van der Waals forces, electrostatic interactions, and hydrophobic interactions. As the solvent evaporates, the polymer solidifies into a durable, flexible film, ensuring a long-lasting bond.76 These adhesives are extensively utilized in wearable skin devices due to their ease of application, instant adhesion, flexibility, comfort, and biocompatibility.
Solvent-free polymer melt skin adhesives have attracted significant attention due to the potential health and environmental risks of organic solvents in traditional formulations.77 Achieving good spreadability typically requires low-viscosity polymer melts. However, lower viscosity often reduces cohesion, resulting in weaker adhesion performance.
To overcome this limitation, a widely adopted strategy is to introduce cross-linkable groups into polymers with low glass transition temperatures, thereby enhancing cohesion and adhesion. Furthermore, improving adhesion critically depends on effective surface dehydration, achieved through either of two primary mechanisms: increasing the polymer's hydrophobicity to disrupt the hydration layer or utilizing its hydrophilicity to absorb interfacial moisture.
Coacervate-based skin adhesives leverage the phenomenon of coacervation, where two or more polymers or biopolymers with complementary charges attract each other in solution to form a dense, gel-like coacervate phase. These adhesives are typically composed of natural or synthetic biopolymers, such as gelatin, collagen, chitosan, or alginate, which can form coacervates in the presence of appropriate counterions or other polymers.78 Electrostatic interactions between polymers primarily drive the adhesion of coacervate-based adhesives. Due to their gel-like structure, these adhesives demonstrate excellent adhesion in wet or dynamic environments, making them particularly suitable for moist surfaces like skin. Moreover, they offer good biocompatibility, non-toxicity, and high flexibility, ensuring long-lasting adhesion. As a result, coacervate-based skin adhesives are widely used in wearable skin devices and other biomedical applications.
For instance, Narayanan et al. developed a mussel foot protein-inspired, tropoelastin-like, bioabsorbable, nonionic self-coacervate polyester by leveraging the low critical solution temperature-driven coacervation phenomenon. This polyester enables the delivery of photo-crosslinked adhesives underwater, overcoming the challenges associated with adhesion in wet or submerged environments. The study demonstrated that these nonionic adhesives can coagulate stably across a broad range of pH and ionic strength conditions and form strong adhesion on underwater substrates in less than 300 seconds. This innovation highlights the potential of smart materials that mimic the self-coagulation properties and environmental stability of mussel foot protein, opening new avenues for the application of bioadhesives in environments with high water content, fluctuating salinity, and varying pH.79,80
Hydrogels are widely used as skin adhesives due to their soft, water-rich structure, excellent biocompatibility, flexibility, and strong adhesion on wet or moist surfaces. These materials can be classified into synthetic, protein, and polysaccharide types, forming three-dimensional network structures capable of retaining significant amounts of water. This hydration enhances comfort, reduces skin irritation, and promotes adhesion through molecular mechanisms such as hydrogen bonding, van der Waals forces, electrostatic interactions, and hydrophobic interactions. As a result, hydrogels outperform traditional adhesives, especially on sensitive, fragile, or moist skin surfaces, where they provide better adhesion. Their softness enables them to conform closely to irregular skin contours, ensuring continuous and stable adhesion. This makes hydrogels particularly valuable in applications such as wearable skin devices, where maintaining skin integrity and minimizing discomfort is essential.81
However, maintaining adhesion over extended use or under dynamic conditions remains challenging despite these advantages. The high water content in hydrogels can lead to a decline in their mechanical properties over time or cause them to lose adhesion under dehydrating conditions. Researchers have explored various strategies to improve hydrogel formulations to address these issues. For instance, incorporating biomimetic adhesive mechanisms (e.g., catechol) or dual cross-linking approaches has shown promise in enhancing hydrogels' mechanical strength, durability, and adhesion properties. Therefore, hydrogels represent a promising and versatile solution, especially in applications that require reliable yet gentle adhesion.82
Recent studies have introduced new directions for improving hydrogels. A pseudorotaxane hydrogel composed of polyacrylamide, β-cyclodextrin, and poly(2-(acryloyloxy) ethyltrimethylammonium chloride) bioionic liquid was developed through a supramolecular engineering strategy, resulting in a balanced combination of mechanical toughness (1.1 × 106 J m−3), conductivity (≈0.29 S m−1), and tissue adhesion (≈27 kPa), along with rapid self-healing and significant stretchability (approximately 3000%) (Fig. 5A).83 Additionally, a hydrogel tape was introduced that employs a time-dependent adhesion mechanism, combining immediate, strong wet adhesion with gradual covalent bond formation (Fig. 5B). Inspired by the catechol-based chemical reaction found in mussel foot proteins, electro-oxidation was used to convert catechol to catechol quinone, which then reacted with amines on tissue surfaces, significantly improving the tape's bioadhesion.84 Furthermore, a diatom-inspired biomimetic hydrophilic polysaccharide adhesive made from diatom biosilica and Bletilla striata polysaccharide exhibited enhanced bioadhesion and increased cross-linking density in wet environments, demonstrating the substantial potential for hemostasis and wound-sealing applications (Fig. 5C).85
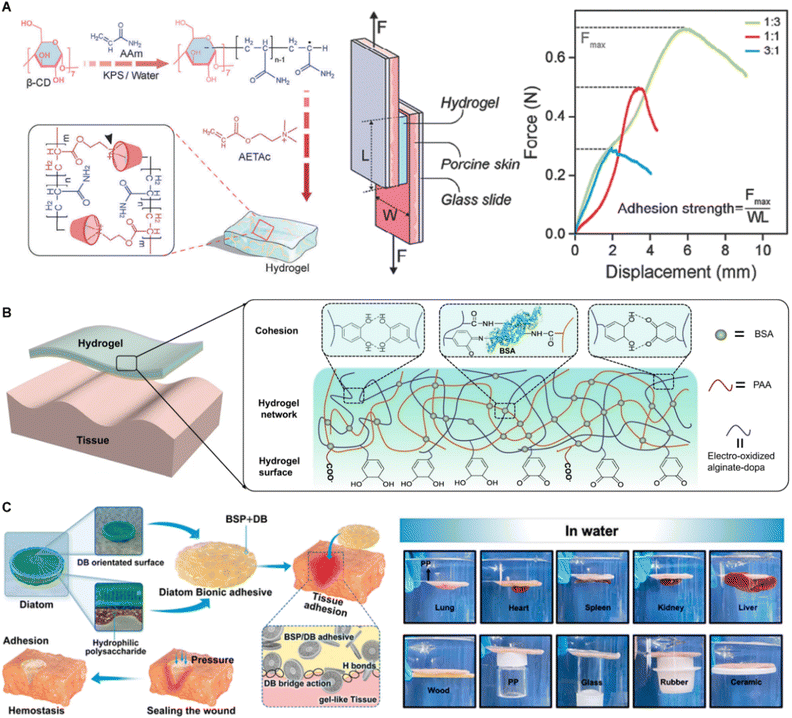 | ||
| Fig. 5 Skin adhesives based on hydrogel. (A) Synthetic hydrogel-based skin adhesives. Reproduced with permission.83 Copyright 2024, Wiley. (B) Protein hydrogel-based skin adhesives. Reproduced with permission.84 Copyright 2021, AAAS. (C) Polysaccharide hydrogel-based skin adhesives. Reproduced with permission.85 Copyright 2023, ACS. | ||
First, forming an interfacial hydration layer prevents direct contact between the adhesive and the skin. This water layer disrupts bonding, especially in bulk materials. In contrast, low-viscosity adhesives, such as molecular liquid glues, can better penetrate this layer and form stronger interfacial connections.87,88
Second, water droplets can become trapped between the adhesive and the skin, reducing the effective contact area and introducing defects. These issues weaken the overall bonding strength and compromise long-term durability.89
To improve wet adhesion, one promising strategy involves the use of adhesives that form molecular interactions with the skin. These adhesives depend on non-covalent forces such as hydrogen bonding, van der Waals interactions, electrostatic attraction, and hydrophobic effects. While generally easier to fabricate and more tunable, they often show lower adhesion strength, particularly in hydrophilic systems.90
Common examples of this category include nanoscale coatings, thin films, and elastomers. Their performance is influenced by both material properties and the specific interactions they establish with the skin.91,92
Nanoscale coatings are thin, flexible adhesive layers that enhance adhesion through molecular interactions. Materials commonly used include silicone, polyurethane, and acrylic-based polymers, which may also incorporate biomimetic molecules such as catechol to improve wet adhesion. The nanoscale thickness allows the coating to conform evenly to irregular skin surfaces, and its material properties can be tailored to achieve effective interactions with skin proteins, lipids, and moisture. This makes nanoscale coatings particularly advantageous for wearable skin devices. However, challenges remain in maintaining adhesion while avoiding skin irritation, especially when balancing strong adhesion with painless removal. Precise material engineering can provide strong adhesion and comfort, offering a multifunctional solution.93
Thin films and elastomers complement each other in molecular interaction-based skin adhesives, enhancing functionality through different material properties and molecular mechanisms.94 Thin films, made from polyethylene, polyurethane, or polydimethylsiloxane, are thin, flexible, and provide a smooth and breathable adhesion surface, balancing durability and comfort.95 In contrast, elastomers like silicone or styrene-based block copolymers have extremely high stretchability and elasticity, maintaining adhesion even during dynamic movements.96 These materials conform tightly to the skin, offering excellent recovery properties and ensuring adhesion under compression.
Molecular mechanisms like hydrogen bonding, van der Waals forces, electrostatic interactions, and hydrophobic interactions make them well-suited for wearable skin devices. However, challenges persist in optimizing adhesion to wet or irregular skin surfaces, ensuring biocompatibility, and balancing flexibility with ease of removal. For example, Zhang et al. developed a highly conductive, organic, self-adhesive, and stretchable dry electrode film made from a biocompatible blend of PEDOT: PSS, waterborne polyurethane, and D-sorbitol (Fig. 6A). This film exhibited excellent conductivity and adhesion on both wet and dry skin.97 Additionally, silicone adhesives are commonly used in wound care due to their mild adhesion and biocompatibility, minimizing skin irritation during removal. Jinkins et al. proposed a material strategy that wirelessly triggers a reduction in adhesion strength, minimizing skin damage during removal (Fig. 6B). This method involves a silicone composite containing a crystallizable oil, which undergoes a phase change when heated, significantly reducing adhesion at the skin interface.98
 | ||
| Fig. 6 Skin adhesives based on Films and Elastomers. (A) Film-based skin adhesives. Reproduced with permission.97 Copyright 2020, Springer Nature. (B) Elastomers-based skin adhesives. Reproduced with permission.98 Copyright 2022, AAAS. | ||
Table 2 provides a comparative overview based on their chemical adhesion mechanisms, environmental adaptability, mechanical properties, and application-specific advantages and limitations to further clarify the differences between glue-type and tape-type skin adhesives.
| Category | Glue type adhesives | Tape based adhesives based on molecular interactions |
|---|---|---|
| Representative forms | Monomers, polymers, coacervates, and hydrogels | Nano coatings, films, and elastomers |
| Adhesion mechanism | Primarily chemical bonding (e.g., covalent, hydrogen, ionic); often enhanced by curing-induced interfacial reactions | Mainly physical interactions (e.g., hydrogen bonding, van der Waals forces); occasional incorporation of chemical moieties for enhanced adhesion |
| Adhesion formation | Curing or phase transition post-application (e.g., cross-linking) | Instant adhesion without post-curing |
| Environmental adaptability | Excellent performance in wet/moist or irregular surfaces | Often limited by water layer interference and trapped moisture |
| Mechanical properties | Tunable via polymer composition; high flexibility; potential for self-healing | Generally fixed mechanical strength; less adaptable to dynamic motion |
| Advantages | Strong and stable adhesion on moist/curved surfaces; customizable properties | Ease of use; conformability; comfortable for dry conditions |
| Limitations | Requires curing time; may suffer from dehydration over long term | Poor wet adhesion; interfacial water reduces bonding |
| Applications | Wound dressing, transdermal delivery, bioelectronics, thermal control | Skin-mounted sensors, ECG/EMG patches, motion monitoring |
5.2 Adhesives based on physical approaches
In contrast to chemical adhesives, physical adhesives typically take the form of tapes and rely on non-covalent interactions such as suction, mechanical interlocking, and capillarity. These strategies, often inspired by biological systems such as octopuses, mayflies, and tree frogs, achieve adhesion through surface structure and physical contact rather than chemical bonding. Key mechanisms include suction, which generates vacuum-based adhesion as seen in octopus' suckers. Mechanical interlocking occurs when adhesives penetrate surface asperities to form interlocked interfaces, as observed in mayfly-inspired surface designs. Capillarity involves liquid adhesives filling micro- or nano-pores and solidifying to form bonds via surface tension, mimicking the attachment mechanisms of tree frogs. Owing to their tape-like construction, these physical adhesives are particularly suitable for applications requiring reversibility, gentle removal, and repeated use, especially in dry or mildly moist environments.99,100Additionally, skin adhesives inspired by octopuses have been developed into autonomous, self-healing, multi-layer adhesive patches (Fig. 7A). These patches demonstrate strong adhesion and self-healing properties under dry and underwater conditions. The self-healing structure of octopuses was replicated by developing a dynamic polymer reflow model for 3D patterning of self-healing elastomers. The multi-layer microstructures with varying moduli provide efficient self-healing, reversible adhesion, and stable mechanical deformation. These adhesives adhere strongly to rough skin surfaces and perform effectively in dry and wet conditions. By laminating a thin gold electrode layer onto this octopus-like adhesive structure, skin adhesives can be transformed into self-healing, skin-fitting electronic devices. These devices maintain excellent skin contact, minimize stimulation, and can be reapplied. Such adhesives reliably measure dynamic motion under dry, wet, and damaged conditions, demonstrating their potential for use in wearable technologies.102–105
 | ||
| Fig. 7 Skin adhesives based on suction. (A) Octopus-inspired skin adhesives. Reproduced with permission.102 Copyright 2024, Wiley. (B). Insect-inspired skin adhesives. Reproduced with permission.108 Copyright 2021, AAAS. | ||
Many practical applications, such as long-term aerial and underwater monitoring, require robots capable of seamless movement between air and water. Li et al. described a multifunctional air–water hitchhiking robot that can independently fly, swim, and adhere to surfaces in both environments. The robot's design incorporates a redundant, hydrostatic-enhanced hitchhiking device inspired by the morphology of the remora fish (Echeneis naucrates), which allows the device to adhere in a partially attached manner. This device can rapidly cross the air–water boundary in just 0.35 seconds and maintain long-term adhesion with minimal vibration.106
Skin adhesives inspired by insect systems enhance adhesion on wet or rough surfaces through oil-bearing spherical cavities and mushroom-shaped tips, optimizing suction effects. Furthermore, research shows that skin adhesives made from highly conformable polymers with multi-scale 3D structures can achieve stable adhesion on soft, wet, and non-flat surfaces, such as skin or organ tissues. Baik et al. developed skin adhesives that exhibit enhanced tensile strength and omnidirectional shear resistance, inspired by the hair-like structures of diving beetles (Fig. 7B). By adjusting the diameter of spherical cavities in micro-pillars and forming mushroom-shaped tips, these adhesives exhibit significant improvements in adhesion on both dry and wet skin and organ surfaces.107,108
 | ||
| Fig. 8 Skin adhesives based on capillarity force. (A) Tree frog-inspired skin adhesives. Reproduced with permission.110 Copyright 2024, Wiley-VCH. (B) Chinese cricket-inspired skin adhesives. Reproduced with permission.111 Copyright 2023, AAAS. | ||
Furthermore, skin adhesives based on nano-thin liquid bridges, similar to those found in tree frogs, have generated strong wet friction. However, the instability of the nanofluid properties in these bridges has presented challenges in improving wet friction. Researchers discovered that skin adhesives inspired by the Chinese cricket's micro-nano fiber pillars generate stronger wet friction—about 3.8 times higher than tree frogs' columns (Fig. 8B). By introducing a nanofiber pillar array coated with a thin film, skin adhesives enhance friction by reducing interface separation stress and ensuring more stable and larger nano-liquid bridges. These advances have been experimentally validated for use in wearable electronics.111
For example, a multifunctional skin adhesion interface platform integrates a water-excludable hexagonal array inspired by frog toe pads and an energy-dissipating matrix inspired by snail foot muscle. This design enables strong adhesion in both tensile and shear directions, even under sweating conditions. Microchannels between hexagonal arrays help expel liquid during sweating while enhancing conformal contact with the skin. This system also exhibits excellent anti-vibration performance across a wide range of frequencies (1–150 Hz) in both dry and wet environments, demonstrating its suitability for dynamic conditions.114
Another example features a biocompatible adhesive patch with a corrugated, mushroom-like structure, incorporating suction cup-like microcavities that enhance wet adhesion and facilitate biosignal monitoring. Inspired by the microstructure of male diving beetles' forelegs, this design significantly improves multi-directional adhesion on rough skin, both dry and wet, while maintaining high water/air permeability and minimizing skin irritation. A model was proposed to explain how the synergy between suction and capillary action generated by microcavities and micro-wrinkles enhances adhesion, especially in humid environments.115
To better understand the design trade-offs among existing skin adhesives, a quantitative comparison is summarized in Table 3. Each adhesive type varies significantly regarding peel strength, shear strength, toughness, and removability, reflecting different design inspirations and clinical applications. These comparisons underscore the need for next-generation adhesives that combine strong yet reversible adhesion, high toughness, and skin compatibility.
| Adhesive type | Mechanism/Inspiration | Peel strength (N m−1) | Shear strength (kPa) | Toughness (J m−2) | Removability | Application | Ref. |
|---|---|---|---|---|---|---|---|
| Monomer adhesives | Covalent crosslinking upon curing | 1000–2000 | 50–200 | Moderate-high | Often irreversible | Rapid wound closure, emergency use | 116 |
| Polymer adhesives | Long-chain entanglement, van der Waals forces | 300–700 | 20–80 | Moderate | Peelable or dissolvable | Medical tapes, wearable patches | 117 |
| Coacervate adhesives | Electrostatic complexation + hydrophobic aggregation | 400–1200 | 40–150 | High | Tunable | Wet skin, mucosal adhesion | 118 |
| Hydrogel adhesives | Hydrogen bonding, electrostatics, catechol | 300–1000 | 30–100 | >500 | Partial | Wet-tissue sealing, wearable sensors | 119 |
| Nano coatings | Nanoscale surface roughness + van der Waals forces | 200–500 | 15–50 | Low-moderate | Easy to detach | Conformal electronics, low-trauma removal | 120 |
| Film | Pressure-free adhesion via interfacial forces | 100–300 | 10–40 | Low-moderate | Easy peel | Bioelectronics, skin-conformal sensors | 121 |
| Elastomer adhesives | Viscoelasticity, conformal contact | 200–600 | 20–80 | ∼100 | Designed for easy removal | Long-term skin patches | 122 |
| Octopus-inspired adhesive | Microcup suction + capillarity | >1000 | >100 | Moderate | Pressure-switchable | Underwater or mobile skin attachment | 123 |
| Mayfly-inspired adhesive | Soft cuticular microstructure | ∼500 | ∼50 | Moderate | Gentle removal | Hairy or fragile skin regions | 109 |
| Tree frog-inspired adhesive | Toe pad microchannels, capillarity | 500–800 | 30–60 | Moderate | Passive, conformal | Moist, curved, or irregular surfaces | 124 |
6 Application of nature-inspired skin adhesives
Due to their versatile properties, nature-inspired skin adhesives have found a wide range of applications beyond traditional medical use. These materials offer innovative solutions in various fields by mimicking natural adhesives that are optimized for biological environments. This section explores the diverse applications of nature-inspired adhesives, focusing on four key areas: thermal management, energy harvesting, wound care, and transdermal drug delivery. These applications highlight the potential of bioinspired adhesives to enhance the functionality and performance of next-generation wearable technologies, medical devices, and other advanced systems.6.1 Thermal management
Skin adhesives play a pivotal enabling role in thermal management for wearable devices by ensuring stable integration of functional materials with the skin.125–127 While thermal regulation is primarily achieved through incorporated materials such as aerogels, hydrogels, or silver nanowires, the adhesive serves as a critical interface that provides durable skin attachment, mechanical compliance, and long-term user comfort. These properties are essential for the sustained performance of thermal management systems. Thermal management strategies typically fall into two categories: passive and active approaches.128,129In passive thermal management, skin adhesives incorporate thermally insulating materials, such as aerogels and layered hydrogels, to reduce heat transfer and enhance user protection in extreme environments. For instance, polymer aerogel fibers composed of crosslinked nanofibers demonstrate outstanding flame resistance (up to 650 °C) and low thermal conductivity, making them ideal for textile-based wearables in harsh settings (Fig. 9A).130 Additionally, porous hydrogel structures can maintain surface temperatures below ambient levels, significantly extending cooling duration under thermal stress (Fig. 9B).131
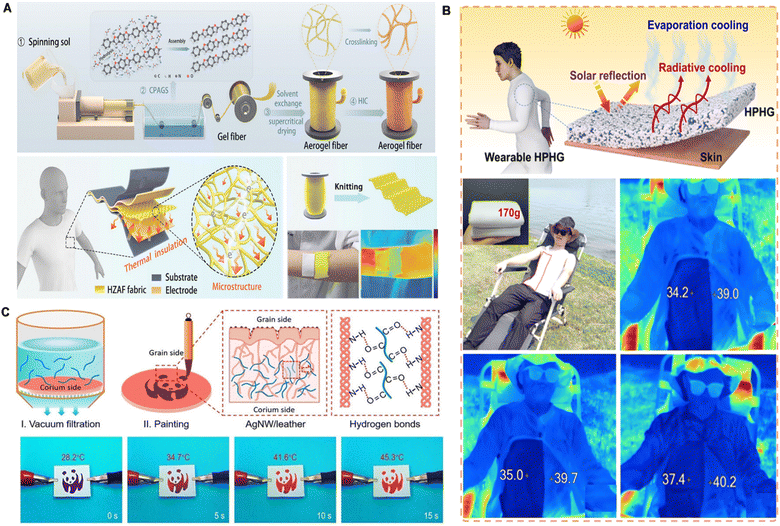 | ||
| Fig. 9 Skin adhesive for wearable thermal management devices. (A) Photos and device structures of polymer aerogel fibers for passive thermal management. Reproduced with permission.130 Copyright 2024, Elsevier. (B) Silver nanowire decorated leather with hierarchical structures for integrated visual Joule heating. Reproduced with permission.131 Copyright 2022, Wiley-VCH. (C) Schematic illustration of textiles based on zylon aerogel fibers for self-powered sensing in harsh environments. Reproduced with permission.132 Copyright 2024, Wiley-VCH. | ||
In active thermal management, adhesives can host phase-change materials that absorb or release heat in response to temperature changes. For example, flexible composite materials that integrate paraffin with olefin block copolymers and SEBS form a dual 3D cross-linked structure, offering improved thermal regulation, mechanical integrity, and leakage resistance—features essential for wearable thermal therapy.
Moreover, integrating multifunctional materials into the adhesive layer allows for additional capabilities, such as Joule heating, electromagnetic shielding, or piezoresistive sensing. While these functionalities arise from embedded components like silver nanowires or leather composites, the adhesive matrix ensures their stable performance on the skin by conforming to dynamic motions and maintaining intimate contact (Fig. 9C).132
Although the skin adhesive is not the primary functional material, its mechanical robustness, biocompatibility, and interface stability are vital for the effective application of thermal management systems in wearable electronics. As fabrication techniques advance, skin adhesives will continue to evolve as an adaptable platform for integrating thermal regulation into next-generation biomedical and AI-driven wearables.133
6.2 Energy-harvesting
Wearable electronics, such as smartwatches, fitness trackers, and health monitoring systems, have been widespread in medical and daily life. An untethered power supply to functional components guarantees sustainable operation for key elements of these devices.134,135 Flexible and stretchable energy storage devices are becoming optimal choices for next-generation wearable technology. Their ability to seamlessly integrate with wearable systems and conform closely to human skin makes them well-suited for enhancing functionality and user comfort.136–138Skin adhesives play a critical role in wearable energy storage devices by maintaining stable contact between the device and the skin, ensuring consistent functionality. A wide range of adhesive materials is used for this purpose, including commercial tapes, biomaterials such as hydrogels, and stretchable polymer materials. The selection of an appropriate adhesive depends on factors such as flexibility, durability, biocompatibility, and Influence on battery output, all of which contribute to optimizing the performance of wearable energy storage systems.
Commercial tapes are widely used in wearable devices due to their softness and strong adhesion to the skin. For instance, medical-grade adhesive films (3M medical tape 1524) have secured microfluidic systems for physiological signal monitoring in sweat-activated biocompatible battery devices.139 Stretchable polymers, such as polydimethylsiloxane, are commonly utilized as adhesive layers in wearable electronics. A notable approach involves the fabrication of micropillar arrays on polydimethylsiloxane surfaces, which enhance adhesion by mimicking the gecko-inspired adhesion mechanism. This biomimetic adhesive has been integrated with flexible micro-supercapacitors and strain sensors to form multifunctional wearable systems. When attached to the skin, these devices can detect biological signals, including arterial pulse, swallowing, and facial muscle movements, using the energy stored in the supercapacitor.140 Biomass-based materials also serve as effective adhesives in flexible wearable devices due to their degradability, permeability, biocompatibility, and adjustable adhesion properties. Silk fibroin hydrogel, for instance, exhibits both solid-like structural integrity and liquid-like adaptability while maintaining excellent ionic conductivity. A lateral design approach has been employed to directly integrate conductive polymer-based positive and zinc-based negative electrodes into a silk fibroin ion hydrogel membrane. This configuration supports the skin's natural breathability while ensuring stable electrical performance under mechanical stresses such as bending, stretching, and twisting, thereby preserving comfort and functionality during movement (Fig. 10A).141
 | ||
| Fig. 10 Skin adhesive for wearable power devices. (A) Photos and device structures of silk fibroin hydrogel based wearable stretchable battery. Reproduced with permission.141 Copyright 2024, Wiley-VCH. (B) Logic diagram of fingerprint-inspired energy-harvesting electronic skin. Reproduced with permission.149 Copyright 2019, Wiley-VCH. (C) Schematic illustration of a TCNQ/PVA blend film based flexible biomechanical energy harvester. Reproduced with permission.150 Copyright 2022, Wiley-VCH. | ||
Energy harvesting technology converts human-generated energy into electrical power as a complementary or alternative solution to traditional wearable energy storage systems. This advancement can reduce battery size or even eliminate the need for batteries.142 By extending device usage and minimizing the risks associated with battery replacement, such as infection and user inconvenience, this approach enhances both functionality and user experience. Wearable energy-harvesting devices have been extensively developed using various mechanisms, including piezoelectric,143 triboelectric,144 thermoelectric,145 and dielectric elastomer-based systems.146 These technologies effectively capture the thermal and mechanical energy produced by the human body, providing a sustainable power source for wearable electronics.
The adhesion layer in these devices plays a dual role by securing the device to the skin while facilitating energy collection and transmission. However, mechanical interference from the adhesive layer can create inconsistencies at the interface between the electronic device and the skin, potentially leading to motion artifacts in wearable bioelectronic devices and increasing the risk of inaccurate output.147 To mitigate these issues, the selection of adhesive materials is typically limited to stretchable polymers, often integrated with conductive components. Additionally, optimizing the structural design of the adhesive enhances both adhesion strength and energy-harvesting efficiency.
Integrating the bioanode and biocathode into a hydrogel substrate creates an “island-bridge” structure, where the “island” collects and stores energy from sweat. At the same time, a liquid metal component functions as a “bridge” connecting the system. This configuration ensures a secure fit against the skin while maintaining exceptional adaptability and stability during movement and stretching.148 Inspired by fingerprint patterns, polydimethylsiloxane wrinkles are the primary microstructure, with embedded silver nanowires forming secondary nanostructures to create conductive hierarchical wrinkles. This conductive layer adheres directly to the skin, acting as an energy collection interface within triboelectric nanogenerators. The design conforms closely to the skin surface, significantly improving the efficiency of mechanical energy harvesting and enabling precise self-powered pressure sensing (Fig. 10B).149 A hybrid film has also been developed by incorporating 7,7,8,8-tetracyano-p-xylidine into a polyvinyl alcohol matrix, allowing for a controlled transition between adhesive and non-adhesive states. During contact, the surface charge increases in proportion to adhesion strength. However, stronger adhesion increases interfacial binding energy, leading to more significant separation stress. This innovative switch balances the benefits of both adhesive and non-adhesive interactions, achieving a record peak power density of 20.5 W m2 Hz−1 at a low matching impedance of 1 MΩ (Fig. 10C).150
6.3 Wound care
Skin adhesives are crucial in managing wounds resulting from surgery, chronic diabetes, and burns, as scars can significantly impact appearance and psychosocial well-being. It is estimated that approximately 100 million people worldwide suffer from surgical or traumatic scars, which can range from fine linear scars to more severe forms, such as hypertrophic scars and keloids.151 Effective scar management aims to reduce the visibility of scars, minimize the adverse effects of abnormal scarring, and promote rapid healing. Skin adhesives have been demonstrated to help stop bleeding, stimulate tissue regeneration, and reduce allergic reactions. Natural materials like sodium,152 alginate,153 chitosan,154 gelatin,155 and hyaluronic acid156 are pivotal in wound care due to their biocompatibility and biodegradability.Wound healing typically progresses through four stages: hemostasis, inflammation, proliferation, and maturation.157 The smooth progression of each stage is crucial for optimal wound repair, and skin adhesives play an essential role in supporting these processes by enhancing hemostasis, reducing inflammation, and promoting tissue regeneration and angiogenesis.158
During the hemostasis phase, skin adhesives exhibit excellent hemostatic properties. For example, the collagen-starch hydrogel adhesive developed by Yang et al. demonstrates strong wound closure capabilities, efficient red blood cell blocking, and activation of hemostatic barrier membranes, outperforming traditional fibrin glue in hemostatic performance.156 Similarly, a modified hydrogel composed of chitosan, sodium alginate, and tannic acid, developed by Zou et al., demonstrated significantly improved hemostatic efficiency compared to commercial products, particularly in a rabbit liver injury model. This was due to the enhanced antibacterial and antioxidant properties of the hydrogel.159
In the inflammatory stage, skin adhesives are essential in inhibiting excessive inflammation and promoting tissue regeneration. These adhesives achieve this by reducing inflammation and accelerating tissue repair through their antibacterial and antioxidant functions. For instance, mussel-inspired biomimetic hydrogel adhesives have been shown to optimize mechanical properties and adhesion, effectively treating diabetic oral wounds by reversing reactive oxygen species-mediated immune disorders.160 Additionally, bioadhesives based on silk fibroin and silver nanoparticles further accelerate wound healing by exhibiting self-healing and antibacterial properties, as well as promoting tissue regeneration.161
The proliferation phase, characterized by fibroblast proliferation and angiogenesis, is crucial for wound repair. Skin adhesives support this phase by promoting angiogenesis, facilitating fibroblast migration, and stimulating collagen synthesis. For example, the snail mucus-derived bioadhesive developed by Deng et al. exhibited remarkable hemostatic activity, biocompatibility, and biodegradability.162 This adhesive accelerated skin wound healing in diabetic rats and promoted macrophage polarization toward an anti-inflammatory phenotype, significantly improving epithelial regeneration and angiogenesis.
In the final maturation stage, skin adhesives are critical for maintaining a moist environment and occlusion to prevent hypertrophic scar formation.163 Proper moisture retention and occlusion reduce excessive collagen deposition, minimizing scar hyperplasia.164 Mucosa-inspired hydrogels effectively maintain a moist environment and reduce scar formation. Furthermore, biomimetic membrane materials, which mimic the fetal extracellular matrix, help minimize inflammation and promote collagen reconstruction, further mitigating scar formation.165
By utilizing biomimetic and nature-inspired adhesives, wound healing can be significantly enhanced, and scar formation can be prevented. These adhesives improve tissue regeneration, provide anti-inflammatory and antibacterial benefits, and maintain a moist microenvironment, which is crucial for effective wound care. Therefore, adhesives derived from natural materials hold great promise for wound care applications.
6.4 Transdermal drug delivery
Skin adhesives play a crucial role in transdermal drug delivery (TDD), directly contacting human skin to facilitate the effective entry of drugs into systemic circulation. TDD, a non-invasive method of delivering drugs through the skin, offers several advantages over traditional oral and intravenous administration. It ensures the secure anchoring of drug patches, supports controlled release into the bloodstream, and bypasses the digestive system. This method is non-invasive and conducive to self-administration, also reduces gastrointestinal side effects, thereby improving patient compliance.166 Additionally, it eliminates the need for needles, lowering the risk of cross-infection and minimizing medical waste. The system can provide sustained, controlled drug release for up to one week. Due to their simple structure and ease of use, drug-adhesive patches have become widely adopted in TDD systems.167The skin adhesive TDD system significantly improves drug bioavailability, ensuring efficient pharmacokinetics with minimal invasiveness. However, variations in drug properties, such as molecule size, hydrophilicity, lipophilicity, and charge, often require chemical enhancers to increase skin barrier permeability.168 These enhancers offer additional driving forces to help drugs penetrate the skin while minimizing damage to deeper tissues. For example, magnesium microparticles have been successfully employed as micromotors in microneedle delivery platforms, facilitating deeper and faster intradermal drug delivery. These micromotors generate hydrogen bubbles rapidly, providing sufficient force to disrupt the dermal barrier and enhance the delivery of therapeutic payloads.169
A significant challenge for skin adhesives in TDD is achieving effective penetration of the skin surface barrier while maintaining strong adhesion, particularly in fluid environments. Inspired by marine blue mussel foot proteins, polydopamine has gained significant attention for its excellent adhesive properties. Rich in catechol groups, polydopamine forms covalent bonds with amino or thiol groups on tissue surfaces via Michael addition and Schiff base reactions, thereby enhancing adhesive strength.170 For instance, embedding mesoporous silica nanoparticles into polyacrylamide/polydopamine hydrogels has significantly improved both mechanical strength and adhesion, facilitating efficient transdermal drug delivery in in vitro studies.171
Nature-inspired nanostructures further enhance the adhesive performance of chemical TDD patches. For example, nanoporous hydrogel adhesive patches establish tight contact with the skin while releasing maltol. These patches, with asymmetric adhesive structures inspired by the surface properties of diving beetles, demonstrate significantly improved adhesion.172 Similarly, cup-shaped structures modeled after octopus suction cups achieve strong wet adhesion in liquid environments. These designs protect internal chemical bonds and leverage air pressure differentials to provide additional physical adhesion.173
Nanostructures significantly enhance the adhesive performance of transdermal patches by increasing surface contact, enabling mechanical interlocking, and incorporating functional modifications. These advancements pave the way for developing efficient and robust transdermal drug delivery systems.
7 Conclusion
Inspired by natural adhesion mechanisms, chemical innovations drive breakthroughs in biomimetic skin adhesives. Researchers have developed adhesives with strong adhesion, remarkable integration, and excellent environmental adaptability by replicating biological adhesion strategies. These adhesives have been widely applied in thermal management, energy harvesting, wound care, and transdermal drug delivery.Despite significant progress, biomimetic skin adhesives face challenges, particularly in practical applications in biomedicine and wearable devices. While existing strategies have made substantial advancements in optimizing adhesion, long-term stability, and performance in dynamic, humid environments, there is still room for improvement. Overcoming these key technical bottlenecks, such as improving adhesion strength under wet or dynamic conditions, enhancing material durability, and ensuring scalability for mass production, will drive broader applications in flexible electronics, biomedicine, and sustainable technologies.
Recent advancements in materials science are expanding the potential of biomimetic skin adhesives. Stimuli-responsive materials, phase-change compounds, and self-healing polymers provide dynamic adaptability, while conductive nanomaterials and thermosensitive compounds enhance comfort and responsiveness. These innovations drive their integration into healthcare, smart sensing, flexible electronics, and energy management. Additionally, advancements in manufacturing technologies allow for the precise fabrication of biomimetic structures, providing application-specific solutions that enhance both performance and versatility. The ability to engineer adhesives at the micro and nanoscale further refines their functionality in various domains.
Looking forward, interdisciplinary collaboration will be essential in optimizing biomimetic skin adhesives. Integrating biology, chemistry, materials science, and artificial intelligence will propel the development of next-generation intelligent adhesives, fostering innovations in medical devices, wearable electronics, and environmental applications. A deeper understanding of micro-scale regulatory mechanisms will establish the theoretical foundation for high-performance, sustainable adhesive materials, ultimately expanding their applications and enhancing human quality of life.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
X. Y., X. L., and Y. Y. C. have the same contribution. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the InnoHK Project on [Project 1.2 – Novel Drug Delivery Systems to Achieve Precision Medicine for Acute CVD Patients (a closed-loop CVD control system)] and the Science and Technology Planning Project of Guangdong Province (No. 2021A0505110002).Notes and references
- C. Wang, E. S. Sani, C. Shih, C. T. Lim, J. Wang, D. G. Armstrong and W. Gao, Nat. Rev. Mater., 2024, 9, 550–566 CrossRef.
- Q. Yang, Z. Hu and J. A. Rogers, Acc. Mater. Res., 2021, 2, 1010–1023 Search PubMed.
- Y. Zhao, S. Song, X. Ren, J. Zhang, Q. Lin and Y. Zhao, Chem. Rev., 2022, 122, 5604–5640 Search PubMed.
- A. B. Croll, N. Hosseini and M. D. Bartlett, Adv. Mater. Technol., 2019, 4, 1900193 CrossRef.
- W. Zhang, R. Wang, Z. Sun, X. Zhu, Q. Zhao, T. Zhang, A. Cholewinski, F. K. Yang, B. Zhao, R. Pinnaratip, P. K. Forooshani and B. P. Lee, Chem. Soc. Rev., 2020, 49, 433–464 RSC.
- Z. Ma, G. Bao and J. Li, Adv. Mater., 2021, 33, 2007663 CrossRef PubMed.
- H. Zhu, J. Wang, X. Yang, B. Zhang and Z. Wang, Adv. Mater., 2025, 2413112 Search PubMed.
- C. Zhang, B. Wu, Y. Zhou, F. Zhou, W. Liu and Z. Wang, Chem. Soc. Rev., 2020, 49, 3605 RSC.
- H. Yuk, J. Wu, T. L. Sarrafian, X. Mao, C. E. Varela, E. T. Roche, L. G. Griffiths, C. S. Nabzdyk and X. Zhao, Nat. Biomed. Eng., 2021, 5, 1131–1142 CrossRef CAS PubMed.
- E. Arzt, H. Quan, R. M. McMeeking and R. Hensel, Prog. Mater. Sci., 2021, 120, 100823 CrossRef.
- S. van Veggel, M. Wiertlewski, E. L. Doubrovski, A. Kooijman, E. Shahabi, B. Mazzolai and R. B. N. Scharff, Adv. Sci., 2024, 11, 2400806 CrossRef.
- Y. Zhang, X. Wan, X. Xu, P. Teng and S. Wang, Biosurf. Biotribol., 2022, 8, 279–289 CrossRef.
- P. Dwivedi, K. Singh, K. Chaudhary and R. Mangal, ACS Appl. Polym. Mater., 2022, 4, 4588–4608 CrossRef CAS.
- D. M. Fitzgerald, Y. L. Colson and M. W. Grinstaff, Prog. Polym. Sci., 2023, 142, 101692 CrossRef CAS PubMed.
- J. Zhang, W. Wang, Y. Zhang, Q. Wei, F. Han, S. Dong, D. Liu and S. Zhang, Nat. Commun., 2022, 13, 5214 CrossRef CAS PubMed.
- Z. Liu and F. Yan, Adv. Sci., 2022, 9, 2200264 CrossRef PubMed.
- R. Hu, X. Yang, W. Cui, L. Leng, X. Zhao, G. Ji, J. Zhao, Q. Zhu and J. Zheng, Adv. Mater., 2023, 35, 2303632 CrossRef CAS.
- S. H. D. Wong, G. R. Deen, J. S. Bates, C. Maiti, C. Y. K. Lam, A. Pachauri, R. AlAnsari, P. Bělský, J. Yoon and J. M. Dodda, Adv. Funct. Mater., 2023, 33, 2213560 CrossRef.
- A. Kumar and A. J. Domb, Macromol. Biosci., 2021, 21, 2100143 CrossRef PubMed.
- Y. Wang, G. Zheng, N. Jiang, G. Ying, Y. Li, X. Cai, J. Meng, L. Mai, M. Guo, Y. S. Zhang and X. Zhang, Nat. Rev. Methods Primers, 2023, 3, 68 CrossRef.
- X. Shi and P. Wu, Small, 2021, 17, 2101220 CrossRef.
- M. L. Picchio, M. S. Orellano, M. A. Motta, C. Huck-Iriart, D. Sánchez-deAlcázar, R. López-Domene, B. Martín-García, A. Larrañaga, A. Beloqui, D. Mecerreyes and M. Calderón, Adv. Funct. Mater., 2024, 34, 2313747 CrossRef.
- Z. Liu and F. Yan, Adv. Sci., 2022, 9, 2200264 CrossRef.
- M. Bruno, L. Portaluri, M. D. Vittorio, S. Gorb and M. Scaraggi, Small, 2025, 2410527 CrossRef.
- D. Lim, M. Song, M. Kim, H. K. Park, D. W. Kim and C. Pang, ACS Nano, 2025, 19(14), 13567–13590 CrossRef.
- B. Gouveia, Y. Kim, J. W. Shaevitz, S. Petry, H. A. Stone and C. P. Brangwynne, Nature, 2022, 609, 255–264 CrossRef.
- S. Shi, Y. Ming, H. Wu, C. Zhi, L. Yang, S. Meng, Y. Si, D. Wang, B. Fei and J. Hu, Adv. Mater., 2024, 36, 2306435 CrossRef.
- J. W. Yang, K. Song, J. Lee, S. Park, H. Huh, G. Choi, H. H. Shin and H. J. Cha, Adv. Mater., 2024, 36, 2310338 CrossRef.
- K. Gong, P. Sun, Y. Cai, X. Wang, Y. Pang, C. Liu, J. Guo and L. Fang, Acta Biomater., 2024, 179, 130–148 CrossRef PubMed.
- J. Kim, H. Keum, H. Albadawi, Z. Zhang, E. H. Graf, E. Cevik and R. Oklu, Adv. Mater., 2024, 2405805 CrossRef PubMed.
- Y. Zhang, C. Wang, X. Wang, M. Yin, K. Wang, D. Zhou, H. Zheng, S. Yu, S. Li, K. Chen and S. Qiao, Chem. Eng. J., 2024, 482, 148785 CrossRef.
- Y. Zheng, M. Wu, M. Duan, Q. Jin, S. Chen, X. Wang and D. Zhou, Chem. Eng. J., 2024, 488, 150459 CrossRef.
- H. Song, L. Wang, J. Wu, J. Liu, C. Liu, J. Guo and L. Fang, Acta Biomater., 2024, 181, 133–145 CrossRef PubMed.
- S. J. Wu and X. Zhao, Chem. Rev., 2023, 123, 14084–14118 CrossRef.
- S. Yang and X. Jiang, ACS Nano, 2024, 18(40), 27107–27125 CrossRef PubMed.
- C. Lee, H. Shi, J. Jung, B. Zheng, K. Wang, R. Tutika, R. Long, B. P. Lee, G. X. Gu and M. D. Bartlett, Cell Rep. Phys. Sci., 2023, 4(10), 101597 CrossRef.
- M. Kharaziha, T. Scheibel and S. Salehi, Prog. Polym. Sci., 2024, 150, 101792 CrossRef.
- A. Chetouani, M. Elkolli, H. Haffar, H. Chader, F. Riahi, T. Varacavoudin and D. L. Cerf, Int. J. Biol. Macromol., 2022, 212, 248–256 CrossRef PubMed.
- O. Hu, M. Lu, M. Cai, J. Liu, X. Qiu, C. F. Guo, C. Y. Zhang and Y. Qian, Adv. Mater., 2024, 36, 2407129 CrossRef CAS.
- Y. Zhang, S. Ma, B. Li, B. Yu, H. Lee, M. Cai, S. N. Gorb, F. Zhou and W. Liu, Chem. Mater., 2021, 33, 2785–2795 CrossRef CAS.
- S. Liu, Y. Rao, H. Jang, P. Tan and N. Lu, Matter, 2022, 5, 1104–1136 CrossRef CAS.
- K. Kwon, H. Wang, J. Lim, K. S. Chun, H. Jang, I. Yoo, D. Wu, A. J. Chen, C. G. Gu, L. Lipschultz, J. U. Kim, J. Kim, H. Jeong, H. Luan, Y. Park, C. Su, Y. Ishida, S. R. Madhvapathy, A. Ikoma, J. W. Kwak, D. S. Yang, A. Banks, S. Xu, Y. Huang, J. Chang and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2020398118 CrossRef CAS.
- Y. Cheng, X. Zhang, J. Zhang, Z. He, Y. Wang, J. Wang and J. Zhang, Chem. Eng. J., 2022, 441, 136016 CrossRef CAS.
- C. H. Luu, N. Nguyen and H. T. Ta, Adv. Healthcare Mater., 2024, 13, 2301039 CrossRef CAS.
- N. Jarach, R. Zuckerman, N. Naveh, H. Dodiuk and S. Kenig, Progress in Adhesion and Adhesives, 2021, vol. 6, pp. 587–619 Search PubMed.
- A. Kosik-Kozioł, P. Nakielski, D. Rybak, W. Frączek, C. Rinoldi, M. Lanzi, M. Grodzik and F. Pierini, ACS Appl. Mater. Interfaces, 2024, 16(25), 32128–32146 CrossRef.
- H. Guo, W. Zhang, Z. Jia, P. Wang, Q. Shao, H. Shen, J. Li, Q. Chen and B. Chi, Adv. Funct. Mater., 2024, 2401529 CrossRef CAS.
- Z. Hou, T. Wang, L. Wang, J. Wang, Y. Zhang, Q. Zhou, Z. Zhang, P. Li and W. Huang, Mater. Horiz., 2024, 11, 1997–2009 RSC.
- L. Zhang, L. Chen, S. Wang, S. Wang, D. Wang, L. Yu, X. Xu, H. Liu and C. Chen, Nat. Commun., 2024, 15, 3859 CrossRef CAS.
- M. Li, A. Mao, Q. Guan and E. Saiz, Chem. Soc. Rev., 2024, 53, 8240–8305 RSC.
- S. Nam and D. Mooney, Chem. Rev., 2021, 121, 11336–11384 CrossRef CAS.
- M. Chen, Y. Wu, B. Chen, A. M. Tucker, A. Jagota and S. Yang, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2203074119 CrossRef CAS.
- J. Chen, Q. Peng, X. Peng, H. Zhang and H. Zeng, Chem. Rev., 2022, 122, 14594–14678 CrossRef CAS.
- M. D. Bartlett, S. W. Case, A. J. Kinloch and D. A. Dillard, Prog. Mater. Sci., 2023, 137, 101086 CrossRef CAS.
- W. Song, Y. H. Choi, Y. G. Moon, C. Lee, M. N. Sundaram and N. S. Hwang, Bioact. Mater., 2024, 40, 582–596 CAS.
- J. Shi, Y. Zhang, Y. Fan, Y. Liu and M. Yang, Droplet, 2024, 3, e92 CrossRef.
- P. Tan, H. Wang, F. Xiao, X. Lu, W. Shang, X. Deng, H. Song, Z. Xu, J. Cao, T. Gan, B. Wang and X. Zhou, Nat. Commun., 2022, 13, 358 CrossRef CAS.
- J. Chen, W. Shi, Y. Ren, K. Zhao, Y. Liu, B. Jia, L. Zhao, M. Li, Y. Liu, J. Su, C. Ma, F. Wang, J. Sun, Y. Tian, J. Li, H. Zhang and K. Liu, Angew. Chem., Int. Ed., 2023, 62, e202304483 CrossRef CAS PubMed.
- S. Park, D. K. Kang, D. Lee, G. Choi, J. Kim, C. Lee, M. Seong, H. E. Jeong and M. D. Bartlett, Sci. Adv., 2024, 10, eadq3438 CrossRef CAS.
- C. McManamon, A. Cameron, J. P. de Silva, R. Daly, F. J. O'Brien and G. L. W. Cross, Acta Biomater., 2020, 111, 279–289 CrossRef CAS.
- P. J. M. Bouten, M. Zonjee, J. Bender, S. T. K. Yauw, H. van Goor, J. C. M. van Hest and R. Hoogenboom, Prog. Polym. Sci., 2014, 39, 1375–1405 CrossRef CAS.
- A. Shagan, W. Zhang, M. Mehta, S. Levi, D. S. Kohane and B. Mizrahi, Adv. Funct. Mater., 2020, 30, 1900998 CrossRef CAS.
- Q. Lyu, M. Li, L. Zhang and J. Zhu, Nat. Commun., 2024, 15, 8419 CrossRef CAS PubMed.
- H. Huang, J. Shen, S. Wan, L. Han, G. Dou and L. Sun, ACS Appl. Polym. Mater., 2020, 2, 1535–1542 CrossRef.
- W. Zhang, T. Ji, S. Lyon, M. Mehta, Y. Zheng, X. Deng, A. Liu, A. Shagan, B. Mizrahi and D. S. Kohane, ACS Appl. Mater. Interfaces, 2020, 12, 17314–17320 CrossRef CAS.
- A. Bal-Ozturk, B. Cecen, M. Avci-Adali, S. N. Topkaya, E. Alarcin, G. Yasayan, Y. E. Li, B. Bulkurcuoglu, A. Akpek, H. Avci, K. Shi, S. R. Shin and S. Hassan, Nano Today, 2021, 36, 101049 CrossRef CAS.
- B. Yang, J. Song, Y. Jiang, M. Li, J. Wei, J. Qin, W. Peng, F. L. Lasaosa, Y. He, H. Mao, J. Yang and Z. Gu, ACS Appl. Mater. Interfaces, 2020, 12, 57782–57797 CrossRef CAS.
- V. Rosa, N. Silikas, B. Yu, N. Dubey, G. Sriram, S. Zinelis, A. F. Lima, M. C. Bottino, J. N. Ferreira, G. Schmalz and D. C. Watts, Dent. Mater., 2024, 40(11), 1773–1785 CrossRef CAS PubMed.
- J. Li, X. Jiang, H. Li, M. Gelinsky and Z. Gu, Adv. Mater., 2021, 33, 2004172 CrossRef CAS PubMed.
- P. Sanjarnia, M. L. Picchio, A. N. P. Solis, K. Schuhladen, P. M. Fliss, N. Politakos, L. Metterhausen, M. Calderón and E. R. Osorio-Blanco, Adv. Drug Delivery Rev., 2024, 115217 CrossRef CAS.
- F. Chen, Q. Zhuang, Y. Ding, C. Zhang, X. Song, Z. Chen, Y. Zhang, Q. Mei, X. Zhao, Q. Huang and Z. Zheng, Adv. Mater., 2023, 35, 2305630 CrossRef CAS.
- H. Ren, Z. Zhang, X. Cheng, Z. Zou, X. Chen and C. He, Sci. Adv., 2023, 9, eadh4327 CrossRef CAS.
- C. Cui and W. Liu, Prog. Polym. Sci., 2021, 116, 101388 CrossRef CAS.
- S. Pal, J. Shin, K. DeFrates, M. Arslan, K. Dale, H. Chen, D. Ramirez and P. B. Messersmith, Science, 2024, 385, 877–883 CrossRef CAS.
- D. W. Kim, K. Song, D. Seong, Y. S. Lee, S. Baik, J. H. Song, H. J. Lee, D. Son and C. Pang, Adv. Mater., 2022, 34, 2105338 CrossRef CAS.
- W. Li, R. Zhou, Y. Ouyang, Q. Guan, Y. Shen, E. Saiz, M. Li and X. Hou, Small, 2024, 20, 2401859 CrossRef CAS PubMed.
- J. Sun, L. Xiao, B. Li, K. Zhao, Z. Wang, Y. Zhou, C. Ma, J. Li, H. Zhang, A. Herrmann and K. Liu, Angew. Chem., Int. Ed., 2021, 60, 23687–23694 CrossRef CAS.
- M. Vahdati, D. Hourdet and C. Creton, Prog. Polym. Sci., 2023, 139, 101649 CrossRef CAS.
- A. Narayanan, J. R. Menefee, Q. Liu, A. Dhinojwala and A. Joy, ACS Nano, 2020, 14, 8359–8367 CrossRef CAS PubMed.
- S. Kondaveeti, G. Choi, S. C. Veerla, S. Kim, J. Kim, H. J. Lee, U. Kuzhiumparambil, P. J. Ralph, J. Yeo and H. E. Jeong, Nano Convergence, 2024, 11, 12 CrossRef CAS PubMed.
- Y. Guo, Y. Wang, X. Zhao, X. Li, Q. Wang, W. Zhong, K. Mequanin, R. Zhan, M. Xing and G. Luo, Sci. Adv., 2021, 7, eabf9635 CrossRef CAS.
- J. A. Cintron-Cruz, B. R. Freedman, M. Lee, C. Johnson, H. Ijaz and D. J. Mooney, Adv. Mater., 2022, 34, 2205567 CrossRef CAS.
- A. Roy, S. Zenker, S. Jain, R. Afshari, Y. Oz, Y. Zheng and N. Annabi, Adv. Mater., 2024, 2404225 CrossRef CAS PubMed.
- B. Xue, J. Gu, L. Li, W. Yu, S. Yin, M. Qin, Q. Jiang, W. Wang and Y. Cao, Nat. Commun., 2021, 12, 7156 CrossRef CAS.
- X. Sun, N. Li, C. Su, Y. Mu, X. Cong, Z. Cao, X. Wang, X. Yang, X. Chen and C. Feng, ACS Nano, 2023, 17, 19121–19135 CrossRef CAS PubMed.
- D. Hwang, C. Lee, X. Yang, J. M. Pérez-González, J. Finnegan, B. Lee, E. J. Markvicka, R. Long and M. D. Bartlett, Nat. Mater., 2023, 22, 1030–1038 CrossRef CAS.
- Y. Chen, J. Meng, Z. Gu, X. Wan, L. Jiang and S. Wang, Adv. Funct. Mater., 2020, 30, 1905287 CrossRef CAS.
- Y. Liu, G. Guan, Y. Li, J. Tan, P. Cheng, M. Yang, B. Li, Q. Wang, W. Zhong, K. Mequanint, C. Zhu and M. Xing, Sci. Adv., 2022, 8, eabm9744 CrossRef CAS.
- H. Yuk, C. E. Varela, C. S. Nabzdyk, X. Mao, R. F. Padera, E. T. Roche and X. Zhao, Nature, 2019, 575, 169–174 CrossRef CAS.
- D. Hwang, K. Trent and M. D. Bartlett, ACS Appl. Mater. Interfaces, 2018, 10, 6747–6754 CrossRef CAS.
- J. Qi, S. Yang, Y. Jiang, J. Cheng, S. Wang, Q. Rao and X. Jiang, Chem. Rev., 2024, 124, 2081–2137 CrossRef CAS.
- Y. Jiang, X. Zhang, W. Zhang, M. Wang, L. Yan, K. Wang, L. Han and X. Lu, ACS Nano, 2022, 16, 8662–8676 CrossRef CAS.
- B. Rivkin, C. Becker, B. Singh, A. Aziz, F. Akbar, A. Egunov, D. D. Karnaushenko, R. Naumann, R. Schäfer, M. Medina-Sánchez, D. Karnaushenko and O. G. Schmidt, Sci. Adv., 2021, 7, eabl5408 CrossRef CAS PubMed.
- X. Wan, F. Zhang, K. Zhang, X. Liu, X. Xu, M. Liu, L. Jiang and S. Wang, Adv. Mater., 2023, 35, 2208413 CrossRef CAS.
- Q. Pen, T. Wang, D. Yang, X. Peng, H. Zhang and H. Zeng, Prog. Polym. Sci., 2024, 153, 101827 CrossRef.
- A. Narayanan, J. R. Menefee, Q. Liu, A. Dhinojwala and A. Joy, ACS Nano, 2020, 14, 8359–8367 CrossRef CAS.
- L. Zhang, K. S. Kumar, H. He, C. J. Cai, X. He, H. Gao, S. Yue, C. Li, R. C. Seet, H. Ren and J. Ouyang, Nat. Commun., 2020, 11, 4683 CrossRef CAS PubMed.
- K. R. Jinkins, S. Li, H. Arafa, H. Jeong, Y. J. Lee, C. Wu, E. Campisi, X. Ni, D. Cho, Y. Huang and J. A. Rogers, Sci. Adv., 2022, 8, eabo0537 CrossRef CAS.
- L. Pan, P. Cai, L. Mei, Y. Cheng, Y. Zeng, M. Wang, T. Wang, Y. Jiang, B. Ji, D. Li and X. Chen, Adv. Mater., 2020, 32, 2003723 CrossRef CAS.
- K. Mendez, W. Whyte, B. R. Freedman, Y. Fan, C. E. Varela, M. Singh, J. C. Cintron-Cruz, S. E. Rothenbücher, J. Li, D. J. Mooney and E. T. Roche, Adv. Mater., 2024, 36, 2303301 CrossRef CAS.
- S. T. Frey, A. B. M. T. Haque, R. Tutika, E. V. Krotz, C. Lee, C. B. Haverkamp, E. J. Markvicka and M. D. Bartlett, Sci. Adv., 2022, 8, eabq1905 CrossRef.
- C. B. Dayan, S. Chun, N. Krishna-Subbaiah, D. Drotlef, M. B. Akolpoglu and M. Sitti, Adv. Mater., 2022, 34, 2109325 CrossRef.
- D. Lim, M. W. Jeong, H. Min, Y. S. Lee, G. W. Hwang, S. H. Jeon, K. H. Jung, N. T. P. Vo, M. Kim, D. W. Kim, J. Y. Oh and C. Pang, InfoMat, 2024, 6, e12603 CrossRef CAS.
- C. Lee, A. C. Via, A. Heredia, D. A. Adjei and M. D. Bartlett, Adv. Sci., 2025, 12, 2407588 CrossRef CAS.
- S. Baik, J. Kim, H. J. Lee, T. H. Lee and C. Pang, Adv. Sci., 2018, 5, 1800100 CrossRef.
- L. Li, S. Wang, Y. Zhang, S. Song, C. Wang, S. Tan, W. Zhao, G. Wang, W. Sun, F. Yang, J. Liu, B. Chen, H. Xu, P. Nguyen, M. Kovac and L. Wen, Sci. Robot., 2022, 7, eabm6695 CrossRef PubMed.
- J. H. Song, S. Baik, D. W. Kim, T. Yang and C. Pang, Chem. Eng. J., 2021, 423, 130194 CrossRef CAS.
- S. Baik, J. Lee, E. J. Jeon, B. Park, D. W. Kim, J. H. Song, H. J. Lee, S. Y. Han, S. Cho and C. Pang, Sci. Adv., 2021, 7, eabf5695 CrossRef CAS PubMed.
- S. N. Gorb, R. G. Beutel, E. V. Gorb, Y. Jiao, V. Kastner, S. Niederegger, V. L. Popov, M. Scherge, U. Schwarz and W. Vötsch, Integr. Comp. Biol., 2002, 42, 1127–1139 CrossRef.
- T. Lan, H. Tian, X. Chen, X. Li, C. Wang, D. Wang, S. Li, G. Liu, X. Zhu and J. Shao, Adv. Mater., 2024, 36, e2404761 CrossRef.
- Y. Guo, L. Zhang, Y. Wang, J. Liang, X. Liu, Y. Jiang, L. Jiang and H. Chen, Sci. Adv., 2023, 9, eadi4843 CrossRef.
- A. Hassanpouryouzband, E. Joonaki, M. V. Farahani, S. Takeya, C. Ruppel, J. Yang, N. J. English, J. M. Schicks, K. Edlmann, H. Mehrabian, Z. M. Aman and B. Tohidi, Chem. Soc. Rev., 2020, 49, 5225–5309 RSC.
- H. Zheng, J. Li, Y. Zhou, C. Zhang, W. Xu, Y. Deng, J. Li, S. Feng, Z. Yi, X. Zhou, X. Ji, P. Shi and Z. Wang, Nat. Commun., 2022, 13, 4584 CrossRef CAS.
- J. Kim, G. W. Hwang, M. Song, D. Lim, J. Kim, J. Choi, Y. S. Lee, D. W. Kim, T. Yang and C. Pang, Adv. Funct. Mater., 2024, 34, 2311167 CrossRef CAS.
- H. Min, S. Baik, J. Lee, D. W. Kim, J. H. Song, K. H. Kim, M. Kim and C. Pang, Chem. Eng. J., 2022, 429, 132467 CrossRef CAS.
- M. A. Droesbeke, R. Aksakal, A. Simula, J. M. Asua and F. E. D. Prez, Prog. Polym. Sci., 2021, 117, 101396 CrossRef CAS.
- S. Chen, Z. Li, Y. Wu, N. Mahmood, F. Lortie, J. Bernard, W. H. Binder and J. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202203876 CrossRef CAS PubMed.
- A. N. Kwant, J. S. E. Sayed, M. Kamperman, J. K. Burgess, D. Slebos and S. D. Pouwels, Adv. Healthcare Mater., 2025, 14, 2402340 CrossRef CAS PubMed.
- X. Liu, H. Yu, L. Wang, Z. Huang, F. Haq, L. Teng, M. Jin and B. Ding, Adv. Mater. Interfaces, 2022, 9, 2101038 CrossRef CAS.
- R. M. Cherian, A. Tharayil, R. T. Varghese, T. Antony, H. Kargarzadeh, C. J. Chirayil and S. Thomas, Carbohydr. Polym., 2022, 282, 119123 CrossRef CAS PubMed.
- J. Chen, Z. Dong, M. Li, X. Li, K. Chen and P. Yin, Adv. Funct. Mater., 2022, 32, 2111892 CrossRef CAS.
- C. Li, W. Dong, L. Li, Z. Dou, Y. Li, L. Wei, Q. Zhang, Q. Fu and K. Wu, Mater. Horiz., 2023, 10, 4183–4191 RSC.
- S. Wang, H. Luo, C. Linghu and J. Song, Adv. Funct. Mater., 2021, 31, 2009217 CrossRef CAS.
- F. Meng, Q. Liu, Z. Shi, D. Tan, B. Yang, X. Wang, K. Shi, M. Kappl, Y. Lei, S. Liu and L. Xue, Adv. Mater. Interfaces, 2021, 8, 2100528 CrossRef.
- Y. Jung, M. Kim, T. Kim, J. Ahn, J. Lee and S. H. Ko, Nano-Micro Lett., 2023, 15, 160 CrossRef PubMed.
- Z. Yi, D. Wu, Y. Su, B. Yang, Y. Ma, N. Li, Y. Zhang, W. Zhang and Z. Wang, Device, 2024, 2, 100471 CrossRef.
- B. Zhang, W. Xu, L. Peng, W. Zhang, Y. Li and Z. Wang, Nat. Rev. Electr. Eng., 2024, 1, 218 CrossRef.
- H. Liu, Y. Zeng, C. Gong, X. Chen, P. Kijanka, J. Zhang, Y. Genyk, H. Tchelepi, C. Wang, Q. Zhou and X. Zhao, Sci. Adv., 2024, 10, eadk8426 CrossRef CAS.
- M. D. Bartlett, E. J. Markvicka and C. Majidi, Adv. Funct. Mater., 2016, 26, 8496–8504 CrossRef CAS.
- X. Hu, P. Hu, L. Liu, L. Zhao, S. Dou, W. Lv, Y. Long, J. Wang and Q. Li, Matter, 2024, 7(12), 4398–4409 CrossRef CAS.
- Z. Ma, X. Xiang, L. Shao, Y. Zhang and J. Gu, Angew. Chem., Int. Ed., 2022, 61, e202200705 CrossRef CAS PubMed.
- P. Hu, F. Wu, B. Ma, J. Luo, P. Zhang, Z. Tian, J. Wang and Z. Sun, Adv. Mater., 2024, 36, 2310023 CrossRef CAS.
- Y. Jing, Z. Zhao, X. Cao, Q. Sun, Y. Yuan and T. Li, Nat. Commun., 2023, 14, 8060 CrossRef CAS.
- R. Liu, Z. L. Wang, K. Fukuda and T. Someya, Nat. Rev. Mater., 2022, 7, 870–886 CrossRef.
- L. Li, X. Wang, W. Deng, J. Yin, X. Li and W. Guo, Droplet, 2023, 2, e77 CrossRef.
- D. G. Mackanic, M. Kao and Z. Bao, Adv. Energy Mater., 2020, 10, 2001424 CrossRef CAS.
- H. J. Lee, H. Kim, T. S. Kim, H. Y. Kim, J. Mun, G. Choi, H. E. Jeong and J. Yeo, Nano Energy, 2024, 121, 109274 CrossRef CAS.
- M. Gao, H. Wu, R. Plamthottam, Z. Xie, Y. Liu, J. Hu, S. Wu, L. Wu, X. He and Q. Pei, Matter, 2021, 4, 1962–1974 CrossRef CAS.
- A. J. Bandodkar, S. P. Lee, I. Huang, W. Li, S. Wang, C.-J. Su, W. J. Jeang, T. Hang, S. Mehta, N. Nyberg, P. Gutruf, J. Choi, J. Koo, J. T. Reeder, R. Tseng, R. Ghaffari and J. A. Rogers, Nat. Electron., 2020, 3, 554–562 CrossRef CAS.
- H. Park, C. Song, S. W. Jin, H. Lee, K. Keum, Y. H. Lee, G. Lee, Y. R. Jeong and J. S. Ha, Nano Energy, 2021, 83, 105837 CrossRef CAS.
- L. Gao, Z. Wang, Y. Zen, Y. Wang, Q. Ren, Z. Ding, K. Ni, J. Tang, C. Zhang, Y. Wang, Y. Zhou, J. Hui, Q. Lu, R. Liu and X. Zhang, Adv. Funct. Mater., 2024, 34, 2410140 CrossRef CAS.
- Z. Gao, Y. Zhou, J. Zhang, J. Foroughi, S. Peng, R. H. Baughman, Z. L. Wang and C. H. Wang, Adv. Mater., 2024, 36, 2404492 CrossRef CAS PubMed.
- Z. L. Wang and J. Song, Science, 2006, 312, 242 CrossRef CAS.
- C. Ning, R. Cheng, Y. Jiang, F. Sheng, J. Yi, S. Shen, Y. Zhang, X. Peng, K. Dong and Z. L. Wang, ACS Nano, 2022, 16, 2811 CrossRef CAS PubMed.
- J. Wang, Y. Song, F. Yu, Y. Zeng, C. Wu, X. Qin, L. Peng, Y. Li, Y. Zhou, R. Tao, H. Liu, H. Zhu, M. Sun, W. Xu, C. Zhang and Z. Wang, Nat. Commun., 2024, 15, 6704 CrossRef CAS.
- Y. Shi, E. Askounis, R. Plamthottam, T. Libby, Z. Peng, K. Youssef, J. Pu, R. Pelrine and Q. Pei, Science, 2022, 377, 228–232 CrossRef CAS.
- F. Ershad, A. Thukral, J. Yue, P. Comeaux, Y. Lu, H. Shim, K. Sim, N. Kim, Z. Rao, R. Guevara, L. Contreras, F. Pan, Y. Zhang, Y. Guan, P. Yang, X. Wang, P. Wang, X. Wu and C. Yu, Nat. Commun., 2020, 11, 3823 CrossRef CAS PubMed.
- S. Guan, Y. Yang, Y. Wang, X. Zhu, D. Ye, R. Chen and Q. Liao, Adv. Mater., 2024, 36, 2305854 CrossRef CAS.
- H. Kang, C. Zhao, J. Huang, D. H. Ho, Y. T. Megra, J. W. Suk, J. Sun, Z. L. Wang, Q. Sun and J. H. Cho, Adv. Funct. Mater., 2019, 29, 1903580 CrossRef CAS.
- L. Wang, Y. Wang, X. Bo, H. Wang, S. Yang, X. Tao, Y. Zi, W. W. Yu, W. J. Li and W. A. Daoud, Adv. Funct. Mater., 2022, 32, 2204304 CrossRef CAS.
- R. Basson and A. Bayat, Front. Med., 2022, 9, 942756 CrossRef.
- S. W. Kim, Arch. Plast. Surg., 2021, 48, 149–157 CrossRef PubMed.
- D. Li, J. Chen, X. Wang, M. Zhang, C. Li and J. Zhou, Front. Bioeng. Biotechnol., 2020, 8, 926 CrossRef.
- H. Ren, Z. Zhang, X. Chen and C. He, Macromol. Biosci., 2024, 24, 2300379 CrossRef CAS.
- J. L. Daristotle, M. Erdi, L. W. Lau, S. T. Zaki, P. Srinivasan, M. Balabhadrapatruni, O. B. Ayyub, A. D. Sandler and P. Kofinas, ACS Biomater. Sci. Eng., 2021, 7, 3908–3916 CrossRef CAS PubMed.
- A. Zhang, Y. Liu, D. Qin, M. Sun, T. Wang and X. Chen, Int. J. Biol. Macromol., 2020, 164, 2108–2123 CrossRef CAS PubMed.
- J. M. Reinke and H. Sorg, Eur. Surg. Res., 2012, 49, 35–43 CrossRef CAS.
- W. Yang, X. Kang, X. Gao, Y. Zhuang, C. Fan, H. Shen, Y. Chen and J. Dai, Adv. Funct. Mater., 2023, 33, 2211340 CrossRef CAS.
- C. Y. Zou, X. X. Lei, J. J. Hu, Y. L. Jiang, Q. J. Li, Y. T. Song, Q. Y. Zhang, J. L. Ling and H. Q. Xie, Bioact. Mater., 2022, 16, 388–402 CAS.
- J. Sun, T. Chen, B. Zhao, W. Fan, Y. Shen, H. Wei, M. Zhang, W. Zheng, J. Peng, J. Wang, Y. Wang, L. Fan, Y. Chu, L. Chen and C. Yang, ACS Appl. Mater. Interfaces, 2022, 15, 416–431 CrossRef PubMed.
- X. Ke, Z. Dong, S. Tang, W. Chu, X. Zheng, L. Zhen, X. Chen, C. Ding, J. Luo and J. Li, Biomater. Sci., 2020, 8, 4346–4357 RSC.
- T. Deng, D. Gao, X. Song, Z. Zhou, L. Zhou, M. Tao, Z. Jiang, L. Yang, L. Luo, A. Zhou, L. Hu, H. Qin and M. Wu, Nat. Commun., 2023, 14, 396 CrossRef CAS.
- Y. Sawada and K. Sone, Br. J. Plast. Surg., 1992, 45, 599–603 CrossRef CAS.
- X. Kong, J. Fu, K. Shao, L. Wang, X. Lan and J. Shi, Acta Biomater., 2019, 100, 255–269 CrossRef CAS.
- D. Zhang, G. Cai, S. Mukherjee, Y. Sun, C. Wang, B. Mai, K. Liu, C. Yang and Y. Chen, ACS Appl. Mater. Interfaces, 2020, 5542–5556 CrossRef CAS PubMed.
- D. Bird and N. M. Ravindra, Med. Devices Sens., 2020, 3, e10069 CrossRef.
- C. Pegoraro, S. MacNeil and G. Battaglia, Nanoscale, 2012, 4, 1881 RSC.
- M. R. Prausnitz and R. Langer, Nat. Biotechnol., 2008, 26, 1261–1268 CrossRef CAS.
- M. A. Lopez-Ramirez, F. Soto, C. Wang, R. Rueda, S. Shukla, C. Silva-Lopez, D. Kupor, D. A. McBride, J. K. Pokorski, A. Nourhani, N. F. Steinmetz, N. J. Shah and J. Wang, Adv. Mater., 2020, 32, 1905740 CrossRef CAS PubMed.
- L. Han, M. Wang, P. Li, D. Gan, L. Yan, J. Xu, K. Wang, L. Fang, C. W. Chan, H. Zhang, H. Yuan and X. Lu, ACS Appl. Mater. Interfaces, 2018, 10, 28015–28026 CrossRef CAS.
- H. Jung, M. K. Kim, J. Y. Lee, S. W. Choi and J. Kim, Adv. Funct. Mater., 2020, 30, 2004407 CrossRef CAS.
- J. Lee, B. S. Lee, S. Baik, D. W. Kim, N.-J. Park, J. W. Lee, S.-K. Bong, S. H. Lee, S.-N. Kim, J. H. Song, J. K. Kim, G.-R. Yi, K. H. Kim and C. Pang, Chem. Eng. J., 2022, 444, 136581 CrossRef CAS.
- Z. Zhu, J. Wang, X. Pei, J. Chen, X. Wei, Y. Liu, P. Xia, Q. Wan, Z. Gu and Y. He, Sci. Adv., 2023, 9, eadh2213 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |