Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cordycepin: a dual-function molecular element for aptamer engineering with enhanced anticancer activity†
Fei Gao‡
 a,
Li Na‡
a,
Li Na‡ a,
Shuyue Fu‡a,
Jinsong Penga,
Shipeng He
a,
Shuyue Fu‡a,
Jinsong Penga,
Shipeng He *a,
Ruowen Wang*b and
Weihong Tan
*a,
Ruowen Wang*b and
Weihong Tan *b
*b
aInstitute of Translation Medicine, School of Life Science, Shanghai University, Shanghai, 200444, China. E-mail: heshipeng@shu.edu.cn
bInstitute of Molecular Medicine (IMM), Renji Hospital, State Key Laboratory of Systems Medicine for Cancer, Shanghai Jiao Tong University School of Medicine, College of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: wangwrw@sjtu.edu.cn; tan@him.cas.cn
First published on 30th June 2025
Abstract
Cordycepin (3′-deoxyadenosine, 3′-dA), derived from the fungus Cordyceps sinensis, has shown significant bioactivity as an inhibitor of enzymes related to 2′-deoxyadenosine (dA). However, its therapeutic efficacy is insufficient for clinical use, which may be addressed through targeted delivery systems. In this study, we designed and synthesized a 3′-dA phosphoramidite to incorporate cordycepin into the well-known cancer-targeting Sgc8c aptamer, where it functions both as a structural modulator and as a bioactive drug element for constructing aptamer–drug conjugates. Its structural similarity to dA makes cordycepin a unique molecular tool for probing the structure–activity relationship of aptamers. Additionally, cordycepin can be seamlessly integrated into aptamers, replacing dA. This led to the generation of a series of cordycepin-modified aptamers, among which Sgc8-23A demonstrated enhanced antitumor activity against HCT116 human colon cancer cells. Compared to free cordycepin, Sgc8-23A exhibited superior bioactivity and stability. In a zebrafish patient-derived xenograft (PDX) model, Sgc8-23A significantly inhibited tumor growth, highlighting its potential as an effective aptamer–drug conjugate for targeted cancer therapy. These findings emphasize the dual functional potential of cordycepin as both a structural element for aptamer optimization and a therapeutic drug component, paving the way for the development of more efficient aptamer-based drug delivery systems.
Introduction
Cordycepin, a naturally occurring nucleoside derived from the fungus Cordyceps sinensis, has been a staple of traditional Chinese medicine for centuries.1,2 Recent studies have highlighted the therapeutic potential of 3′-dA across multiple biological targets.3 Notably, a derivative of 3′-dA has been developed as its prodrug and is currently undergoing phase I clinical trials.4,5 Despite their promising attributes, the relatively mild efficacy of natural products such as 3′-dA can often be optimized through targeted delivery systems. Previous work has demonstrated that aptamers serve as an effective tool for achieving such targeted delivery.6,7Aptamers are functional nucleic acids with intricate secondary and tertiary structures capable of binding a wide range of targets, including small molecules, proteins, and even whole cells, with remarkable affinity and specificity.8,9 Over the past few years, numerous aptamers have been explored for diagnostic and therapeutic applications, addressing conditions such as Alzheimer's disease and cancer.10,11 Owing to their synthesis via automated solid-phase methods, aptamers are often referred to as “chemical antibodies”. Aptamers, selected through SELEX (Systematic Evolution of Ligands by Exponential Enrichment) technology, do not always require chemical modifications, but such modifications can be beneficial for overcoming in vivo challenges associated with oligonucleotide therapeutics. For example, the FDA-approved aptamer-based therapeutics Pegaptanib for treating wet age-related macular degeneration, or wet AMD, and avacincaptad pegol (brand name: Izervay) for treating geographic atrophy secondary to AMD both incorporate polyethylene glycol (PEG) modifications to enhance biostability and prolong circulation times.12,13 Structural optimization of lead compounds, guided by the crystal structures of target proteins, is a common strategy in drug discovery and has led to the development of “perfect molecules” for therapeutic purposes. However, obtaining detailed structural information on oligonucleotides poses significant challenges. Recent advances in structural biology have begun to address these limitations, facilitating the study of aptamer structures.14
Cordycepin, a 3′-dA structurally similar to the DNA nucleoside dA, introduces subtle, yet impactful, differences when substituted into aptamer sequences.3,15 Thus, this modification may serve as an invaluable model within the field of nucleic acids for improving the antitumor ability and stability of aptamers. Such replacements lead to analogs with delicate changes in secondary structures and distinct binding affinities.16–19 This unique property makes 3′-dA a valuable dual-function molecular element for aptamer optimization, enabling the exploration of aptamer structure–activity relationships. Additionally, cordycepin-modified aptamers offer dual functionality. That is, the targeted delivery of aptamers ensures cellular internalization, while the subsequent release of cordycepin provides therapeutic benefits. This dual-function nature positions cordycepin as a powerful tool for both aptamer structural refinement and therapeutic application.
Given this duality, we propose a novel strategy to design cordycepin as a dual-function molecular element and incorporate it into the nucleotide sequence of aptamer Sgc8c via solid-phase synthesis to replace deoxyadenosine (Fig. 1). This prodrug-like approach aims to enhance the bioavailability and therapeutic efficacy of aptamer Sgc8c. By substituting adenine nucleotides with cordycepin, we created a series of modified Sgc8c aptamers, allowing us to investigate the resulting changes in their properties. Remarkably, cordycepin-modified Sgc8-23A not only retained its superior targeting ability towards HCT116 human colon cancer cells but also exhibited significantly enhanced stability and antitumor activity. The advantages of the cordycepin-modified Sgc8c (Sgc8-23A) were further highlighted by its excellent in vivo tumor-targeting ability and potent antitumor efficacy. Therefore, the cordycepin-modified Sgc8c strategy may offer a valuable tool for the clinical diagnosis and targeted treatment of tumors by enhancing the antitumor efficacy and stability of Sgc8c.
Results and discussion
Rational design of compounds
Cordycepin (3′-dA), a nucleoside antibiotic structurally similar to adenosine, has been under consideration as an anticancer drug based on its extensive biological and pharmacological effects, which include inhibiting cell proliferation, inducing apoptosis, preventing metastasis, and activating the immune system.20–22 Research suggests that 3′-dA is phosphorylated by certain phosphate kinases to 3′-deoxyadenosine triphosphate (3′-dATP) after entering cells.23 Owing to its structural resemblance to ATP, 3′-dATP can terminate RNA elongation by incorporating into RNA and may compete with ATP for the EGFR binding site, thereby inhibiting EGFR phosphorylation and disrupting caspase and GSK-3β pathways.15,24–26 Additionally, 3′-dA has been suggested to mediate apoptotic signaling in tumor cells through death receptors (DRs) or adenosine receptors (ADORAs).27 However, the efficacy of 3′-dA was limited by its rapid metabolism into an inactive metabolite by adenosine deaminase (ADA). Therefore, given the chemical similarity between cordycepin and deoxyadenosine, substituting deoxyadenosine with cordycepin in the Sgc8c aptamer is anticipated to enhance cordycepin's bioavailability. Furthermore, research substantiated that 2′–5′ phosphodiester bonds demonstrated significantly enhanced resistance to nuclease degradation compared to the traditional 3′–5′ bonds. Thus, this modification may serve as an invaluable model within the field of nucleic acids for improving the antitumor ability and stability of aptamer Sgc8c.The synthesis of aromatic polycarbonates (aPCs) is outlined in Scheme 1. The pharmaceutical element, cordycepin phosphoramidite 3, was prepared in three steps starting from commercially available cordycepin. First, trimethylsilyl chloride (TMSCl) was employed to protect the free amine, affording the intermediate 1 with a yield of 41%. This protection step is essential in solid-phase synthesis to prevent undesired side reactions. Intermediate 1 was then converted to the corresponding phosphoramidite 3 using standard protocols. With the phosphoramidite 3 in hand, we proceeded to synthesize cordycepin-modified Sgc8c to explore how the incorporation of cordycepin affects the overall properties of the aptamer. Based on previous studies,7 the A23-T30 region and the terminal sequences of Sgc8c are modifiable sites, and modifications at these positions still preserve the Sgc8c's strong binding affinity. As a result, a series of cordycepin-modified aptamers were successfully prepared (Table S1†).
 | ||
| Scheme 1 The synthesis of cordycepin phosphoramidite. (1) TMSCl, pyridine, BzCl, NaHCO3, 0 °C, 41%; (2) DMTrCl, pyridine, 51%; (3) chlorophosphoramidite, DIEA, DCM, 91%. | ||
Structural integrity and stability of cordycepin-modified Sgc8c
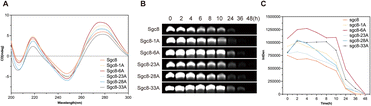
Subsequently, we evaluated the secondary structure and stability of cordycepin-modified Sgc8c. As reported in the literature, replacing the 3′–5′ phosphodiester bond linkage in oligonucleotides with a 2′–5′ linkage can lead to structural alterations.28,29 To investigate these effects, we examined the secondary structure of cordycepin-modified Sgc8c using circular dichroism (CD) spectroscopy. As shown in Fig. 2A, cordycepin-modified Sgc8c, similar to aptamer Sgc8c, displays distinct absorption peaks at 255 nm and 280 nm. Among the different modifications, Sgc8-23A exhibited the smallest change in absorbance, indicating minimal structural alteration, while Sgc8-6A showed the largest change, suggesting the most significant structural modification. These results demonstrated that modifications at different sites had varying effects on the properties of aptamer Sgc8c. Subsequently, nuclease resistance of the cordycepin-modified aptamers was assessed via PAGE analysis after incubation in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) at 37 °C. The results indicated that the cordycepin-modified aptamers demonstrated greater stability in biological environments compared to aptamer Sgc8c (Fig. 2B).Binding affinity and cellular internalization of cordycepin-modified Sgc8c
Aptamer Sgc8c is well known for its specific recognition of the PTK 7 receptor overexpressed in various types of tumors.30 Studies indicate that the aptamer's binding affinity is closely related to its structural stability because increased binding affinity is correlated with increased stability.31 To explore how cordycepin substitution at different positions affects the binding and internalization abilities of Sgc8c, we used flow cytometry to assess changes in binding specificity and laser confocal microscopy to evaluate internalization efficiency. As shown in Fig. 3A, the fluorescence of HCT116 cells incubated with Sgc8-1A, Sgc8-23A, Sgc8-28A, or Sgc8-33A was comparable to that of Sgc8c and significantly higher than that of the negative control group (Lib, a random sequence). Notably, Sgc8-6A led to weaker fluorescence. In Ramos cells, cordycepin-modified aptamers showed fluorescence levels as low as those in the Lib group. The results demonstrated that these modified aptamers maintain exceptional specificity for tumor cells. To gain deeper insight into the changes in binding affinity between cordycepin-modified aptamers and the receptor, we also used flow cytometry to measure the Kd value of the nucleic acid aptamers. The Kd value of Sgc8-23A (6.30 ± 0.95 nM) was closest to that of Sgc8c (5.92 ± 0.84 nM), while Sgc8-6A displayed a nonspecific linear trend, indicating a lack of specific binding to HCT116 cells (Fig. 3B). Confocal microscopy was employed to assess cellular internalization of the cordycepin-modified aptamers. As displayed in Fig. 3C, HCT116 cells treated with Sgc8-1A, Sgc8-23A, Sgc8-28A, or Sgc8-33A displayed significantly stronger fluorescence compared to the Lib group. In contrast, the fluorescence of HCT116 cells incubated with Sgc8-6A was comparable to that of the Lib sequence, further suggesting that Sgc8-6A lacks substantial specific binding affinity for HCT116 cells. Furthermore, molecular docking analyses were performed to explore the binding interactions of both Sgc8c and Sgc8-23A with proteins. As shown in Fig. 4, the predicted binding mode of Sgc8-23A (Fig. 4B) closely mirrors the orientation observed for Sgc8c (Fig. 4A). This similarity in binding patterns between the parent aptamer and its modified variant provides further support for the potential functional equivalence of Sgc8-23A. | ||
| Fig. 4 Molecular docking. (A) Molecular docking of Sgc8c with the PTK 7 protein. (B) Molecular docking of Sgc8-23A with the PTK 7 protein. | ||
Tumor inhibitory activity of cordycepin-modified Sgc8c on HCT116 cells
After confirming that Sgc8c modified with cordycepin retains strong targeting capability, we next examined its potential antitumor activity. Extensive studies indicated that Sgc8c exhibited minimal cytotoxicity toward tumor cells. By introducing cordycepin, we aimed to develop an Sgc8c derivative with both precise targeting and enhanced antitumor efficacy. We further evaluated the cytotoxic effects of Sgc8-23A on HCT116 cells using the CCK-8 assay, given that it had a Kd value closest to that of Sgc8c. As shown in Fig. 5A, Sgc8-23A exhibited significantly greater efficacy in inhibiting the growth of HCT116 cells compared to free 3′-dA across various concentrations. Additionally, the 48 h cytotoxicity test results, as presented in Fig. 5B, were used to compare 3′-dA, Sgc8c, Sgc8-23A and Sgc8-Cor. Notably, Sgc8-23A achieved an IC50 value of 22.65 μM, markedly lower than that of 3′-dA (IC50 = 131.4 μM), but comparable to that of Sgc8-Cor (IC50 = 22.19 μM). No substantial inhibition was observed in HCT116 cells treated with 100 μM Sgc8c, and this result was consistently validated across multiple experiments.In vivo antitumor evaluation of Sgc8-23A in a zebrafish PDX model
The zebrafish xenograft model, known for its ease of manipulation and rapid experimental turnaround, provided an ideal platform for our investigation of tumor progression.32 To assess the in vivo antitumor potential of Sgc8-23A, we established an optimized PDX model of colorectal cancer in zebrafish (Danio rerio). Cy5-labeled Sgc8-23A was administered intravenously at a dose of 10 ng per fish, along with a positive control (aptamer Sgc8c) and a negative control (Lib, random sequences). After injection, the zebrafish were incubated at 35 °C for 24 hours. Seven zebrafish were randomly chosen from each group (n = 7), and fluorescence microscopy was employed to assess the distribution of the samples. As illustrated in Fig. 6A, Sgc8-23A, like Sgc8c, exhibited preferential accumulation in the intestines, blood vessels, and tumor cells, underscoring its targeted affinity for tumor cells. Conversely, Lib was predominantly localized in the intestines and blood vessels. Then, to evaluate the antitumor potential of Sgc8-23A, we randomly divided the zebrafish into four groups, including control, Sgc8c, 3′-dA, and Sgc8-23A. Following 48 h of treatment, zebrafish from the experimental groups were randomly chosen for fluorescence microscopy analysis. As displayed in Fig. 6B, red fluorescence intensity in the Sgc8-23A group was significantly lower than that of the control group after exposure to the various compounds. Quantitative analysis using ImageJ software revealed a marked reduction in the overall tumor cell density after Sgc8-23A treatment. Notably, Sgc8-23A demonstrated significantly enhanced antitumor effects compared to 3′-dA. These findings underscore the promising therapeutic potential of Sgc8-23A.Conclusions
In summary, aptamer Sgc8c represents a promising tool for targeted cancer therapy and diagnostics. However, it faces several limitations, including insufficient antitumor activity, and inadequate stability and circulation time in vivo. In our work, dual-function cordycepin has been used to improve the antitumor efficacy and stability of Sgc8c through the substitution of adenine nucleotides. We have shown that cordycepin-modified Sgc8c, or Sgc8-23A, exhibits remarkable antitumor activity and specific targeting capabilities, opening new avenues in the field of targeted drug delivery. Our research has laid the groundwork for more efficient and adaptable strategies to enhance aptamer performance. We anticipate that this strategy will further evolve, providing innovative solutions for advancing targeted cancer treatment and diagnostic methods.Ethical statement
All zebrafish experiments were conducted in strict accordance with the guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of Hangzhou Hunter Biotechnology Co., Ltd (IACUC approval number: IACUC-2024-9368-01).Data availability
The datasets supporting this article have been uploaded as part of the ESI† material.Author contributions
Fei Gao conducted the majority of the biological and cell-based assays. Na Li synthesized the target compounds, with additional contributions from Shuyue Fu in their synthesis. Jinsong Peng assisted with data analysis. Shipeng He, Ruowen Wang, and Weihong Tan conceptualized the project, provided overall guidance, contributed to data analysis, and participated in the writing, review, and editing of the manuscript. All authors have read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and Technology of China (grant 2021YFA0909400 to R. W.) and Fujian Provincial Natural Science Foundation of China (grant 2024J01223 to F. G.).Notes and references
- K. G. Cunningham, W. Manson, F. S. Spring and S. A. Hutchinson, Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link, Nature, 1950, 166, 949 CrossRef CAS PubMed.
- J. Y. Ling, Y. J. Sun, H. Zhang, P. Lv and C. K. Zhang, Measurement of cordycepin and adenosine in stroma of Cordyceps sp. by capillary zone electrophoresis (CZE), J. Biosci. Bioeng., 2002, 94, 371–374 CrossRef CAS PubMed.
- P. Qin, X. Li, H. Yang, Z. Y. Wang and D. Lu, Therapeutic potential and biological applications of cordycepin and metabolic mechanisms in cordycepin-producing fungi, Molecules, 2019, 24, 2231 CrossRef CAS PubMed.
- M. Serpi, V. Ferrari, C. McGuigan, E. Ghazaly and C. Pepper, Synthesis and characterization of NUC-7738, an aryloxy phosphoramidate of 3'-deoxyadenosine, as a potential anticancer agent, J. Med. Chem., 2022, 65, 15789–15804 CrossRef CAS PubMed.
- H. Schwenzer, E. De Zan, M. Elshani, R. van Stiphout, M. Kudsy, J. Morris, V. Ferrari, I. H. Um, J. Chettle, F. Kazmi, L. Campo, A. Easton, S. Nijman, M. Serpi, S. Symeonides, R. Plummer, D. J. Harrison, G. Bond and S. P. Blagden, The novel nucleoside analogue proTide NUC-7738 overcomes cancer resistance mechanisms in vitro and in a first-in-human phase I clinical trial, Clin. Cancer Res., 2021, 27, 6500–6513 CrossRef CAS PubMed.
- R. Wang, G. Zhu, L. Mei, Y. Xie, H. Ma, M. Ye, F.-L. Qing and W. Tan, Automated modular synthesis of aptamer-drug conjugates for targeted drug delivery, J. Am. Chem. Soc., 2014, 136, 2731–2734 CrossRef CAS PubMed.
- D. Shangguan, Z. Tang, P. Mallikaratchy, Z. Xiao and W. Tan, Optimization and modifications of aptamers selected from live cancer cell lines, ChemBioChem, 2007, 8, 603–606 CrossRef CAS PubMed.
- S. M. Nimjee, R. R. White, R. C. Becker and B. A. Sullenger, Aptamers as therapeutics, Annu. Rev. Pharmacol. Toxicol., 2017, 57, 61–79 CrossRef CAS PubMed.
- D. Shangguan, Y. Li, Z. Tang, Z. C. Cao, H. W. Chen, P. Mallikaratchy, K. Sefah, C. J. Yang and W. Tan, Aptamers evolved from live cells as effective molecular probes for cancer study, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11838–11843 CrossRef CAS PubMed.
- A. D. Keefe, S. Pai and A. Ellington, Aptamers as therapeutics, Nat. Rev. Drug Discovery, 2010, 9, 537–550 CrossRef CAS PubMed.
- C. R. Ireson and L. R. Kelland, Discovery and development of anticancer aptamers, Mol. Cancer Ther., 2006, 5, 2957–2962 CrossRef CAS PubMed.
- E. W. Ng, D. T. Shima, P. Calias, E. T. Cunningham Jr, D. R. Guyer and A. P. Adamis, Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease, Nat. Rev. Drug Discovery, 2006, 5, 123–132 CrossRef CAS PubMed.
- D. G. Vavvas, A. B. Daniels, Z. G. Kapsala, J. W. Goldfarb, E. Ganotakis, J. I. Loewenstein, L. H. Young, E. S. Gragoudas, D. Eliott, I. K. Kim, M. K. Tsilimbaris and J. W. Miller, Regression of some high-risk features of age-related macular degeneration (AMD) in patients receiving intensive statin treatment, EBioMedicine, 2016, 5, 198–203 CrossRef PubMed.
- D. Ji, H. Feng, S. W. Liew and C. K. Kwok, Modified nucleic acid aptamers: development, characterization, and biological applications, Trends Biotechnol., 2023, 41, 1360–1384 CrossRef CAS PubMed.
- B. Li, Y. Hou, M. Zhu, H. Bao, J. Nie, G. Y. Zhang, L. Shan, Y. Yao, K. Du, H. Yang, M. Li, B. Zheng, X. Xu, C. Xiao and J. Du, 3'-Deoxyadenosine (Cordycepin) produces a rapid and robust antidepressant effect via enhancing prefrontal AMPA receptor signaling pathway, Int. J. Neuropsychopharmacol., 2016, 19, pyv112 CrossRef PubMed.
- H. Aurup, T. Tuschl, F. Benseler, J. Ludwig and F. Eckstein, Oligonucleotide duplexes containing 2'-amino-2'-deoxycytidines: thermal stability and chemical reactivity, Nucleic Acids Res., 1994, 22, 20–24 CrossRef CAS PubMed.
- C. M. M. Benckendorff, P. Sunde-Brown, A. Ni Cheallaigh and G. J. Miller, Synthesis of Nucleoside Analogs Containing Sulfur or Selenium Replacements of the Ribose Ring Oxygen or Carbon, J. Org. Chem., 2024, 89, 16977–16989 CrossRef CAS PubMed.
- T. Angelov, H. A. Dahlmann and S. J. Sturla, Oligonucleotide probes containing pyrimidine analogs reveal diminished hydrogen bonding capacity of the DNA adduct O(6)-methyl-G in DNA duplexes, Bioorg. Med. Chem., 2013, 21, 6212–6216 CrossRef CAS PubMed.
- K. C. Schneider and S. A. Benner, Oligonucleotides containing flexible nucleoside analogs, J. Am. Chem. Soc., 1990, 112, 453–455 CrossRef CAS.
- C. R. Dass, E. G. Saravolac, Y. Li and L. Q. Sun, Cellular uptake, distribution, and stability of 10–23 deoxyribozymes, Antisense Nucleic Acid Drug Dev., 2002, 12, 289–299 CrossRef CAS PubMed.
- X. Tian, Y. Li, Y. Shen, Q. Li, Q. Wang and L. Feng, Apoptosis and inhibition of proliferation of cancer cells induced by cordycepin, Oncol. Lett., 2015, 10, 595–599 CrossRef CAS PubMed.
- K. Yue, M. Ye, Z. Zhou, W. Sun and X. Lin, The genus Cordyceps: a chemical and pharmacological review, J. Pharm. Pharmacol., 2013, 65, 474–493 CrossRef CAS PubMed.
- L. C. Griffin, G. F. Tidmarsh, L. C. Bock, J. J. Toole and L. Leung, In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits, Blood, 1993, 81, 3271–3276 CrossRef CAS PubMed.
- D. V. Morrissey, K. Blanchard, L. Shaw, K. Jensen, J. A. Lockridge, B. Dickinson, J. A. McSwiggen, C. Vargeese, K. Bowman and C. S. Shaffer, Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication, Hepatology, 2005, 41, 1349–1356 CrossRef CAS PubMed.
- Z. Wang, X. Wu, Y.-N. Liang, L. Wang, Z.-X. Song, J.-L. Liu and Z.-S. Tang, Cordycepin induces apoptosis and inhibits proliferation of human lung cancer cell line H1975 via inhibiting the phosphorylation of EGFR, Molecules, 2016, 21, 1267 CrossRef PubMed.
- B.-S. Ko, Y.-J. Lu, W.-L. Yao, T.-A. Liu, S.-S. Tzean, T.-L. Shen and J.-Y. Liou, Cordycepin regulates GSK-3β/β-Catenin signaling in human leukemia cells, PLoS One, 2013, 8, e76320 CrossRef CAS PubMed.
- S. Y. Yoon, S. J. Park and Y. J. Park, The anticancer properties of cordycepin and their underlying mechanisms, Int. J. Mol. Sci., 2018, 19, 3027 CrossRef PubMed.
- S. Verma and F. Eckstein, Modified oligonucleotides: synthesis and strategy for users, Annu. Rev. Biochem., 1998, 67, 99–134 CrossRef CAS PubMed.
- M. D. Matteucci and M. H. Caruthers, Synthesis of deoxyoligonucleotides on a polymer support, J. Am.Chem. Soc., 1981, 103, 3185–3191 CrossRef CAS.
- D. Shangguan, Z. Cao, L. Meng, P. Mallikaratchy, K. Sefah, H. Wang, Y. Li and W. Tan, Cell-specific aptamer probes for membrane protein elucidation in cancer cells, J. Proteome Res., 2008, 7, 2133–2139 CrossRef CAS PubMed.
- J. P. Elskens, J. M. Elskens and A. Madder, Chemical modification of aptamers for increased binding affinity in diagnostic applications: current status and future prospects, Int. J. Mol. Sci., 2020, 21, 4522 CrossRef CAS PubMed.
- J. T. Gamble, D. J. Elson, J. A. Greenwood, R. L. Tanguay and S. K. Kolluri, The zebrafish xenograft models for investigating cancer and cancer therapeutics, Biology, 2021, 10, 252 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02571k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |