Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advancements in ammonia-based pretreatment: key benefits and industry applications
Venkatesh Balan
 *a,
Maedeh Mohammadi
a and
Bruce E. Dale
*a,
Maedeh Mohammadi
a and
Bruce E. Dale
 b
b
aDepartment of Engineering Technology, Biotechnology Program, Cullen College of Engineering, University of Houston, Sugar Land, TX 77479, USA. E-mail: vbalan@uh.edu
bDepartment of Chemical Engineering and Materials Science, Department of Energy (DOE) Great Lakes Bioenergy Research Center (GLBRC), Michigan State University, East Lansing, Michigan 48824, USA
First published on 26th May 2025
Abstract
The development of sustainable pretreatment technologies is essential for improving biomass conversion efficiency in second generation biorefineries. This review provides a comprehensive analysis of ammonia-based pretreatment methods, tracing their evolution from early advancements to recent innovations. It highlights advanced technologies such as Ammonia Fiber Expansion (AFEX), Extractive Ammonia (EA), and Compacted Biomass with Recycled Ammonia (COBRA), alongside other approaches, including dilute ammonia, gaseous, and aqueous methods. These pretreatment methods vary in their effectiveness, particularly in lignin removal and lignin carbohydrate complex modification, with most ammonia preserving cellulose while their impact on hemicellulose and lignin range from minimal alterations to extensive removal. The review also explores the integration of ammonia-based pretreatment with biomass densification strategies, emphasizing their role in improving feedstock logistics while maintaining conversion efficiency. Recent innovations in sustainable ammonia production such as electrochemical synthesis and biomass-based processes are discussed, showcasing opportunities to develop environmentally friendly pretreatment solutions. Additionally, the applications of ammonia-pretreated biomass are examined across three key sectors: biofuel production, leveraging enzymatic hydrolysis and fermentation; animal feed, with enhanced digestibility and nutritional benefits; and biomaterial development, including lipid and protein extraction for value-added products. This review offers a comprehensive understanding of ammonia-based pretreatment technologies and their expanding potential in sustainable biorefining applications.
Sustainability spotlightThis review advances sustainable biorefinery through ammonia-based pretreatment technologies that convert agricultural residues into renewable fuels and value-added products. The technology enables efficient processing of biomass while reusing ammonia catalysts, minimizing chemical waste and environmental impact. By establishing integrated biorefinery systems that produce biofuels, animal feed and biomaterials from waste biomass, this work directly supports several UN Sustainable Development Goals: SDG 7 (Affordable and Clean Energy) through the production of renewable fuels, SDG 12 (Responsible Consumption and Production) through circular processing approaches, and SDG 13 (Climate Action) through the reduction of emissions in the transportation sector. These sustainable pretreatment methods are crucial for the development of economically viable biorefineries that meet global energy needs while mitigating climate change. |
1. Growing population and fuel security for sustainable living
There is a strong linear relationship between per capita energy consumption and GDP: more energy enables more work, which in turn drives economic growth. Human well-being, as measured by the Human Development Index (HDI), also improves with increasing energy, especially at lower consumption levels, but this benefit plateaus at around 4 kilowatts (kW) per capita. Beyond that point, additional energy yields diminishing returns in human development. If all 8 billion people consumed energy at this saturation level, global energy demand would rise to approximately 32 terawatts (TW) nearly double today's demand of about 19 TW, most of which still comes from fossil fuels.High energy use has historically fueled prosperity in developed nations. More recently, emerging economies like China and India have increased their energy consumption to improve wealth, education, and health outcomes. However, the global challenge is to expand energy access equitably and sustainably, without locking future development into fossil-fuel dependence.
Despite growing energy use, developing nations still consume far less power per capita and exhibit lower HDI scores than wealthier countries. As developed countries reduce their fossil fuel consumption, any resulting supply surpluses will likely be absorbed by developing nations striving to raise living standards. This continued reliance on fossil energy risks accelerating climate change and worsening environmental degradation. Two major conclusions arise from this situation:
(1) In the absence of scalable renewable alternatives, fossil fuels will be depleted more rapidly as global demand rises.
(2) Fossil fuels are fundamentally inadequate for sustaining long-term human well-being, as their environmental impacts increasingly undermine health, biodiversity, and economic stability.
The fossil-fuel-based economy is inherently unsustainable. Conventional oil production peaked around 2004–2005 at approximately 76 million barrels per day and has since entered a period of irreversible decline—despite trillions of dollars invested to boost supply. Recent production growth relies on unconventional sources such as tight shale oil, deep-water drilling, and tar sands, which are more expensive and environmentally damaging than conventional oil.
For the past century, economic growth has been closely tied to rising oil consumption. Today, the plateau or decline in oil availability presents serious risks to global economic, social, and political stability. Without a fundamental transition to renewable energy sources—such as wind, solar, geothermal, and hydro—these disruptions will likely intensify (Fig. 1). These renewables are critical for delivering the core energy services that sustain modern societies: heat, electricity, and mobility.1
While renewable technologies can increasingly meet heat and electricity demands, providing sustainable mobility—especially for aviation, shipping, and heavy freight—remains a much greater challenge. Even for light-duty vehicles, studies show that electricity alone is unlikely to meet long-term energy security and greenhouse gas (GHG) targets. By 2050, around 80% of global transportation fuels will still need to come from energy-dense liquid fuels, even with aggressive electrification of the light-duty fleet.
Decarbonizing light-duty transport will require significant shifts: limited travel growth, near-complete electrification of vehicles by 2050, and a largely decarbonized electricity grid. Even if these conditions are met, they may still fall short of achieving an 80% GHG reduction unless low-carbon, lignocellulosic biofuels are integrated into the transportation energy mix. Therefore, relying solely on renewable electricity for mobility is unlikely to succeed without the complementary use of sustainable biofuels.
2. Lignocellulosic biomass and its composition
Lignocellulosic biomass, derived from a variety of plant sources that are abundantly available around the world, holds great potential as a sustainable feedstock for producing liquid transportation fuels. Recent studies have shown that about 1 billion tons of lignocellulosic material are available annually in the U.S. alone.2 Under other assumptions (e.g., massive inputs of nuclear heat and hydrogen to eliminate biomass consumption for heat or electricity in the biorefinery) the amount of available biomass increases several folds. Availability of cellulosic biomass is therefore unlikely to limit the production of biofuels. These plant materials can be classified into two main categories: Gymnosperms and Angiosperms. Gymnosperms include species that bear cones, such as softwoods like pine, spruce, fir, and cedar, while Angiosperms consist of species that bear flowers (Fig. 1). Angiosperms are categorized into monocots and dicots. Monocots include grasses such as corn, rice, wheat, sugarcane, switchgrass, orchard grass, miscanthus, and sorghum. Dicots, on the other hand, consist of hardwood trees like willow and poplar, as well as herbaceous flowering plants such as soybean, alfalfa, and tobacco.3While all bioenergy plants contain cellulose (30–50%), hemicellulose (15–30%), lignin (13–28%), and ash (3–15%), the relative amounts of these components vary significantly among species. For instance, the secondary cell wall of dicots typically consists of Type I primary cell walls, primarily composed of xylan with minimal arabinoxylan and a small proportion of mannan. In contrast, monocots feature Type II primary cell walls that are predominantly made of glucuronoarabinoxylan, which are hydrogen-bonded to cellulose microfibrils.4 Lignin, a complex phenolic polymer, is primarily made up of three monolignols: syringyl (S), guaiacyl (G), and p-hydroxyphenyl (H). Hardwood species, which are herbaceous dicots, predominantly consist of S- and G-units with minimal H-units. In contrast, grasses and other monocots contain similar levels of S- and G-units but exhibit considerably higher concentrations of H-units compared to dicots or gymnosperms.5 Grasses also have ester linkages, such as feruloyl or arabinose residues, between the lignin polymer and hemicellulose, while hemicelluloses in some species are connected by di-ferulate ester linkages.6
The varying structural components and linkages in plant cells mean that different species respond to pretreatment processes in distinct ways. In general, grasses with glucuronoarabinoxylan ester linkages tend to respond favorably to ammonia pretreatment.7 However, not all grass species behave in the same manner. For example, corn stover, which contains a higher concentration of glucuronoarabinoxylan, exhibits greater sugar conversion during enzymatic hydrolysis after ammonia pretreatment, while miscanthus, which has a lower amount of glucuronoarabinoxylan in its cell walls, results in relatively lower conversion rates.8 These variations highlight the importance of considering species-specific characteristics when selecting plant feedstocks for biofuel production, as different pretreatment methods will yield different efficiencies based on the plant's inherent structural makeup.
3. Petrochemical versus bioderived products
Commerce has long been recognized as a key driver of trade and economic development, leading to greater wealth and opportunities for human advancement. Currently, commerce is almost entirely reliant on high-energy-density liquid fuels, which are predominantly derived from petroleum. As a result, much of human wealth and development opportunities are tied to petroleum consumption. Securing renewable, sustainable liquid fuels is therefore a critical priority in the transition to renewable energy. Among the available sources of renewable liquid fuels, non-food plant matter, or cellulosic biomass, stands out as the largest and most cost-effective raw material.Plant biomass offers several significant advantages over other renewable sources, such as solar, wind, and hydro, for storing solar energy. First, plants serve not only as collectors of solar energy but also as energy storage systems. In contrast, storing electricity generated from solar and wind energy is both expensive and challenging. Second, most of the solar energy captured by plants is stored in carbon–carbon and carbon–hydrogen bonds, which are the very chemical bonds that form the basis of current liquid fuels. Third, when harnessed correctly, plant biomass can provide a host of important environmental and social benefits, such as improved water quality, enhanced biodiversity, carbon capture and sequestration in soils, and rural economic development.
Unfortunately, these potential environmental services provided by plant biomass are often overlooked by the public, policymakers, academics, and non-government organizations (NGOs). This aspect, however, warrants further exploration in another article. Therefore, the conversion of cellulosic biomass into liquid fuels is a key area of focus for research, development, and deployment.
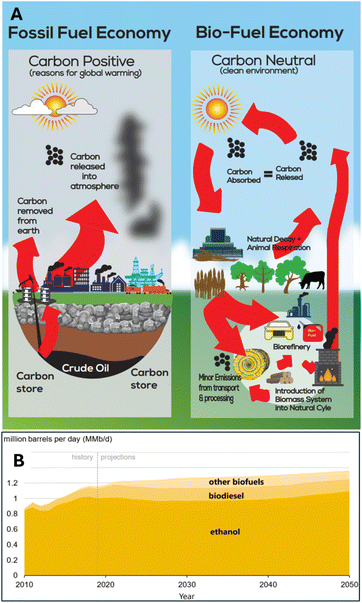
In a fossil fuel-based economy, natural resources rich in carbon, such as crude oil, coal, and natural gas, are extracted from the earth, processed, and used in various applications. The combustion of these fossil fuels releases substantial amounts of carbon dioxide (CO2), a GHG responsible for global warming and the resulting rise in global temperatures. This temperature increase leads to the melting of glaciers in the Arctic, raising sea levels and potentially inundating vast areas of habitable land. In contrast, when biofuels derived from plants are used, the carbon dioxide emitted during combustion is reabsorbed by plants, which then use it for subsequent biofuel production.
There are two primary pathways for producing liquid fuels from cellulosic biomass. The thermochemical platform, like oil refining, relies primarily on heat and chemical catalysts to convert biomass into fuels. The second pathway is the biochemical or sugar platform, which focuses on extracting sugars from biomass. These sugars are then converted into fuels using microbial and/or chemical catalysts. Hybrid approaches that combine both thermochemical and biochemical methods are also being actively developed. This paper focuses on the sugar platform, with detailed explanations of biofuel production from sugar plants provided in the following section.
Ethanol continues to dominate U.S. biofuel production, primarily derived from corn. According to the EIA's Annual Energy Outlook 2020, ethanol production is projected to remain relatively stable through 2050, comprising most of the total biofuel output at roughly 0.8–1.0 million barrels per day. While biodiesel and other biofuels are expected to see modest growth, their combined production volumes remain significantly smaller than ethanol throughout the projection period. The graph shows a historical increase in biofuel production from 2010 to 2020, followed by a relatively flat trajectory through 2050, suggesting that the rapid growth seen in the early 2000s has largely stabilized. The total U.S. biofuel production is projected to reach approximately 1.3–1.4 million barrels per day by 2050, with ethanol maintaining its position as the predominant biofuel (Fig. 2B).9 These targets reflect ongoing efforts to diversify biofuel sources, reduce reliance on corn ethanol, and expand the role of renewable fuels like cellulosic ethanol and renewable natural gas.
Beyond biofuels, there is a growing interest in the use of fractionated biomass components for thermochemical conversions to produce a wide range of chemicals and materials. Effective fractionation of biomass is critical to maximize the value of the individual components—cellulose, hemicellulose and lignin. It is particularly important to maintain the structural integrity of lignin during fractionation, as this significantly affects its potential for subsequent utilization. In contrast to acidic pretreatments, where lignin can be degraded by condensation reactions,10 ammonia-based processes often preserve the native bonds of lignin, making it easier to depolymerize into valuable aromatic compounds and platform chemicals.
The lignin obtained from ammonia-based fractionation processes, in particular pretreatment with Extractive Ammonia (EA), can be further processed into high-value products such as aromatic monomers (e.g. vanillin, syringaldehyde), polymeric materials, carbon fibers and specialty chemicals. This represents a significant advance over conventional biorefinery concepts, where the lignin is often underutilized for low-value applications such as combustion for heat and power generation. By developing integrated biorefinery concepts that utilize all biomass components, the economic and environmental sustainability of these processes can be significantly improved.
The evolution from petroleum refineries to integrated biorefineries represents a major shift in manufacturing. While current commercial applications are primarily focused on ethanol, ammonia-based pretreatment technologies enable effective biomass fractionation for multiple product streams. This approach, extracting fuels from carbohydrates and value-added chemicals from lignin, improves economic viability while reducing environmental impact, thus meeting both the principles of the circular economy and the growing demand for sustainable alternatives to petrochemical products.
4. Bio-refinery concept
Like chemical refineries, which process crude oil obtained from fossil resources into various fuels (such as petroleum and diesel), chemicals (like benzene, toluene, and naphthalene used in materials production), and tar (a byproduct left after refining crude oil into these products), a biorefinery uses bio-based materials, such as agricultural, forest, and municipal solid waste and also purpose-grown crops (“energy crops”), to produce fuels and chemicals.11 These bio-based materials are abundant and renewable, offering a reliable feedstock for a sustainable energy future. The concept of the first-generation biorefinery emerged a few decades ago, focusing on processing food-grade materials into fuels such as ethanol and byproducts like molasses and distillers' grains.12 These refineries are particularly prevalent in countries like Brazil (using sugar cane), the United States (using corn), the European Union (using wheat and sugar beet), and Southeast Asia (using cassava, sorghum, and sweet potatoes). However, the use of food-grade materials for fuel and chemical production has sparked significant controversy,13 especially given that many people in underdeveloped and developing countries suffer from hunger and lack access to sufficient food.This controversy has prompted a shift in focus toward inedible feedstocks—such as corn stover, wheat straw, and other agricultural residues—as alternatives for fuel and chemical production. In response, various government agencies have increased funding for research projects aimed at developing second-generation biorefinery technologies that can produce fuels, chemicals, animal feed, and biomaterials from these non-food resources.14 Bio-based technologies offer a pathway to sustainable development, defined by the Brundtland Commission as development that meets the needs of the present generation without compromising the ability of future generations to meet their own need.15 In comparison to fossil fuel-based technologies, sustainable development encompasses not only environmental protection but also economic and social development, which can benefit society at large. Certain agricultural products contain free sugars—like sucrose in sugar beets and sugarcane—that require minimal processing to produce fuels and chemicals. Other feedstocks,16 such as corn, wheat, cassava, and sweet potatoes, contain starch, a combination of the linear polysaccharide amylose and the branched polysaccharide amylopectin. These starches consist of 4000–8000 glucose monomers linked by α-1,4-glycosidic bonds and must be hydrolyzed by industrial enzymes (α-amylase and gluco-amylase) to release free sugars that can be fermented by microbes, such as bacteria or yeast, into fuels and chemicals.17
In contrast, lignocellulosic biomass, which comprises a complex network of cellulose, hemicellulose, and lignin, poses additional challenges. Cellulose is a linear polysaccharide composed of 7000–15,000 glucose monomers linked by β-1,4-glycosidic bonds, and it is typically found in agricultural residues. Hemicellulose, a branched polysaccharide, contains 500–3000 sugar monomers such as xylose, mannose, galactose, rhamnose, and arabinose.18 Lignin is an aromatic polymer with over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units, primarily made from phenylpropanoid building blocks such as p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol. To convert cellulose into fermentable sugars, industrial enzymes like cellulase (a mixture of cellobiohydrolase I, cellobiohydrolase II, and β-glucosidase) are employed. Hemicellulose is broken down using enzyme mixtures like hemicellulase, which includes xylanases, β-mannanases, α-arabinofuranosidases, α-glucuronidases, β-xylosidases, and acetyl xylan esterases.19
000 units, primarily made from phenylpropanoid building blocks such as p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol. To convert cellulose into fermentable sugars, industrial enzymes like cellulase (a mixture of cellobiohydrolase I, cellobiohydrolase II, and β-glucosidase) are employed. Hemicellulose is broken down using enzyme mixtures like hemicellulase, which includes xylanases, β-mannanases, α-arabinofuranosidases, α-glucuronidases, β-xylosidases, and acetyl xylan esterases.19
Lignocellulosic biomass is highly recalcitrant, meaning it resists degradation to simple sugars. As such, chemical pretreatment is necessary to break open the plant cell wall, making cellulose and hemicellulose more accessible to biomass-degrading enzymes. This pretreatment enhances the breakdown of these complex polymers into monomeric sugars, which can then be fermented into fuels and chemicals. The process flow diagrams for both first-generation and second-generation biorefineries, showing how fuels and chemicals are produced from these feedstocks, are presented in Fig. 3.20
5. Biomass pretreatment
The sugar platform, as a key component of biofuel production, relies on the biological deconstruction of plant biomass using enzymes followed by fermentation using different native and genetically modified organisms. While such deconstruction occurs naturally, the rate and sugar yields in nature are far below what is required for commercial viability. This highlights the importance of preprocessing cellulosic biomass to enhance both the rate and yield of sugars. This preprocessing step, known as “pretreatment”, has been a focal point of research for over a century. Numerous pretreatment approaches have been explored, although only a few have scaled beyond laboratory settings.To compete with the highly efficient petroleum fuel system, biofuels must achieve cost-effectiveness while providing long-term prosperity and contributing to environmental services such as a stable climate. The question arises: What can we learn from the history of petroleum refining to guide biofuel development? Petroleum refining primarily faces three major cost centers: (1) feedstock or raw material costs (typically accounting for 60–70% of the total cost of producing fuels and commodity chemicals), (2) capital equipment costs (upfront investment), and (3) operating costs, including utilities, chemicals, and other supplies. Feedstock costs are the dominant factor in the cost structure of refining and, similarly, will play a major role in the cost of biofuel production.
Pretreatment significantly influences sugar yield, concentration, and rate. The impacts of these factors on feedstock costs, capital costs, and operational costs are critical in the development of a biofuel production system. The key parameters to consider in pretreatment are: (i) yield, defined as the amount of sugars (both monomeric and oligomeric) obtained per kilogram of dry, untreated biomass at a specific catalyst loading; (ii) concentration, which refers to the quantity of sugars per liter of the hydrolysate solution; and (iii) rate, representing the speed at which sugars are produced per liter of hydrolysate per hour. These parameters are crucial for determining how efficiently biofuels can be produced from biomass, as they directly affect overall costs and efficiency.
Sugar yield, particularly from untreated biomass, is considered the most important measure of pretreatment effectiveness. Unfortunately, sugar yield is often either unreported or ambiguously measured. Maximizing sugar yield minimizes feedstock costs (more product per unit of biomass), but it also reduces capital and operational costs. High yields mean less equipment is required to process the biomass and less energy is needed during the conversion process. In addition to yielding sugar concentration (the amount of sugar per unit volume of hydrolysate) plays a critical role. High sugar concentrations enhance the efficiency of downstream processes, including the separation of sugars and the conversion of sugars into fuels. This, in turn, reduces utility costs and the need for large reactors, making concentration or sugar titer a vital focus of pretreatment research.
The plant cell wall's natural recalcitrance—its resistance to degradation—has evolved as a defense mechanism against pathogens. However, this same recalcitrance becomes a significant barrier when we attempt to convert plant biomass into valuable biofuels. Overcoming this barrier is the central challenge in biomass pretreatment. Numerous studies have explored methods to break down this recalcitrance, with many reviews summarizing advances in this area.21–23 Researchers have categorized pretreatment methods into three broad types: (i) physical, (ii) chemical, and (iii) biological.
Physical pretreatments involve mechanical processes aimed at reducing biomass particle size, which can be energy intensive. Techniques such as disk milling, chipping, ball milling, hammer milling, and biomass extrusion are commonly used. Though these methods alone may not suffice, they are often used in conjunction with chemical pretreatments to enhance biomass processability. Chemical pretreatments use various chemicals to disrupt the cell wall. These include acidic, neutral, and alkaline conditions. Acidic pretreatment typically involves the use of mineral acids (e.g., H2SO4, HCl) or organic acids (e.g., acetic, oxalic) to solubilize hemicellulose into monomeric sugars such as xylose, arabinose, and galactose, while leaving cellulose largely intact for enzymatic hydrolysis. The major disadvantage of acidic pretreatment, especially mineral acids, is the formation of degradation products like 5-hydroxymethylfurfural (HMF), furfural, and phenolic lignin, which can inhibit subsequent processes such as enzymatic hydrolysis and fermentation. Expensive detoxification methods are required to remove these toxic byproducts from the sugar streams. Neutral pretreatment processes, such as ionic liquids, ozonolysis, wet oxidation, and organosolv, have shown promise.24 However, these methods often face challenges, including high costs associated with the chemicals used (e.g., ionic liquids) and the difficulty of recovering solvents that are miscible with water.25
Alkaline pretreatment offers several advantages: it operates under milder conditions, selectively removes lignin, and creates a more porous biomass, thus increasing the surface area available for enzymatic action. Common alkaline catalysts include ammonia (dilute, gaseous, and liquid forms), NaOH, KOH, and H2O2. This method enhances the efficiency of subsequent enzymatic hydrolysis by breaking down lignin without significantly degrading carbohydrates. Biological pretreatment employs white rot or brown rot fungi, which decompose the biomass in a mild, low-temperature process compared to chemical pretreatments. While this approach is less energy-intensive, it is time-consuming, often taking a week to 10-days to achieve meaningful biomass modification, which adds capital costs to the processing. The progress of pretreatment technologies is crucial for advancing biofuel production from biomass, and the appropriate choice of pretreatment strategy depends on the specific feedstock, cost considerations, and the desired product. Advances in pretreatment technologies will significantly enhance the efficiency, sustainability, and scalability of biofuel production systems.
5.1 Advantages of alkaline over acidic pretreatment
The acidic pretreatment process is widely recognized for its ability to hydrolyze most of the hemicellulose fraction in lignocellulosic biomass, and under certain conditions, it can also hydrolyze a portion of cellulose into monomeric sugars. This results in the production of two distinct sugar streams—one rich in monomeric sugars derived from hemicellulose and another with sugars derived from cellulose. However, the acidic conditions during pretreatment also lead to the unavoidable formation of degradation products such as HMF, furfural, and formic acid. These by-products are known to be inhibitory to downstream processes, including enzymatic hydrolysis and microbial fermentation. On the other hand, alkali pretreatment processes differ significantly in their impact on the biomass structure and the resulting products. In contrast to acid pretreatment, alkali processes primarily serve to remove lignin from the biomass while leaving the cellulose and hemicellulose fractions largely intact.26Alkali treatment works through the cleavage of ester linkages that connect phenolic and aliphatic acids, a process that occurs via nucleophilic acyl substitution of ester bonds. This results in the formation of carboxylic salts and alcohols, which further helps in reducing the lignin content of the biomass. At elevated temperatures and in the presence of strong alkali agents such as sodium hydroxide (NaOH), the lignin matrix, which is composed of ether bonds connecting lignol units, undergoes catalytic cleavage. This reaction plays a critical role in disrupting the biomass structure, making it more amenable to subsequent processing steps. The alkali pretreatment also causes the lignocellulosic material to swell, which leads to an increase in internal surface area, a reduction in the degree of polymerization, a decrease in crystallinity, and a disruption of the structural linkages between lignin and carbohydrates.27
The removal of lignin and partial removal of xylan from the biomass through alkali pretreatment have significant advantages in enhancing the digestibility of cellulose during enzymatic hydrolysis. However, some soluble lignin fractions, which contain phenolic acids, aldehydes, catechol, and vanillin, are released during the process. These soluble compounds can inhibit the efficiency of enzymes during hydrolysis and inhibit microorganisms during fermentation.28 While the removal of soluble lignin improves the digestibility of cellulose, it also results in the loss of some soluble hemicellulose sugars. This sugar loss can be minimized when using volatile alkalis, such as ammonia, which are less likely to lead to the loss of valuable hemicellulose sugars during liquid stream separation.
5.2 Unique properties of ammonia as pretreatment catalyst
Almost all catalysts used in the pretreatment process are water-miscible and tend to become embedded within the biomass. While some catalysts are relatively inexpensive and do not require recovery, they necessitate neutralization with either acid or base post-pretreatment to bring the biomass to a pH of 5, which is optimal for enzyme hydrolysis. This neutralization step results in the formation of salts, which must be removed when reusing water in subsequent operations within the biorefinery (Fig. 4). In certain pulping processes that involve high concentrations of caustic substances, most of the catalyst is recovered by an expensive high-temperature kiln, in compliance with environmental regulations. However, the use of ammonia as a catalyst presents several advantages and can address many of these challenges.Ammonia, a volatile alkali, can be recovered and reused after pretreatment, which helps to mitigate issues related to salt formation and the presence of residual catalysts. The recovery process involves the use of compressors and condensers, with approximately 97% of the ammonia being recoverable and reusable. Less than 3% of the ammonia reacts with biomass during pretreatment, incorporating into the biomass as amides. Although the ammonia recovery process incurs additional costs, it improves the efficiency of downstream processes such as enzyme hydrolysis and microbial fermentation, compared to other pretreatment methods where the catalyst remains with the biomass29,30 (Fig. 4B). In addition to catalyst recovery, ammonia pretreatment offers several other notable advantages:
• Exothermic reaction: ammonia reacts exothermically with moist biomass, generating heat that can instantly elevate the biomass temperature during pretreatment. For instance, when biomass with 40% moisture content is exposed to ammonia at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 (ammonia to biomass loading), the temperature can reach 140 °C within minutes. This rapid heat generation is advantageous because biomass is typically a poor conductor of heat, and this reaction helps to avoid the need for expensive heating systems in the pretreatment reactor.31
0.6 (ammonia to biomass loading), the temperature can reach 140 °C within minutes. This rapid heat generation is advantageous because biomass is typically a poor conductor of heat, and this reaction helps to avoid the need for expensive heating systems in the pretreatment reactor.31
• Flexibility in ammonia forms: ammonia can be used in various forms—dilute ammonia in water, concentrated gaseous ammonia, or liquid anhydrous ammonia—depending on the specific pretreatment requirements. The concentration of ammonia and the conditions of the process significantly influence the digestibility of the biomass.32 This flexibility also allows for the effective removal of solubilized lignin, enhancing the overall pretreatment efficiency.
• Modification of cellulose crystallinity: ammonia has the unique ability to alter the crystallinity of cellulose from its native form (cellulose I) to a more digestible allomorph form (cellulose III). This change in crystallinity occurs depending on the concentration of ammonia used during pretreatment.33 Cellulose III is hydrophilic and exhibits reduced binding capacity with biomass-degrading enzymes compared to native cellulose I, thus improving enzyme accessibility and increasing biomass digestibility.34
The ability of ammonia to modify cellulose crystallinity distinguishes it from other chemicals, such as NaOH and ionic liquids, which can convert cellulose I to cellulose II, and phosphoric acid, which can convert cellulose I to amorphous cellulose (Fig. 5A).35 Fig. 5B shows the cellulose conversion efficiency, where AC (amorphous cellulose) shows the highest conversion rate, followed by cellulose III, while cellulose I shows the lowest conversion efficiency. Fig. 5C demonstrates enzyme binding patterns, where cellulose I exhibits higher enzyme binding compared to cellulose III, explaining cellulose III's enhanced digestibility as less enzyme becomes bound to the surface. Fig. 5D presents X-ray diffraction patterns for four cellulose allomorphs (Iβ, II, IIII, and IIIII), with their distinct peak patterns revealing the unique crystalline structures that directly influence their physical properties and digestibility characteristics. These structural modifications are crucial for applications in biofuel production and biomass conversion processes.
5.3 Producing ammonia using renewable resources
Fritz Haber revolutionized the production of ammonia in 1908 by developing a process that involved reacting hydrogen and nitrogen at temperatures between 400–450 °C and pressures of 200 atm (approximately 3000 psi) in the presence of an iron catalyst. This reaction produced gaseous ammonia, which was then cooled and condensed into liquid ammonia. The high temperature was necessary to ensure fast reaction kinetics, while the high pressure was required to achieve high conversion rates. In recognition of this groundbreaking invention, Haber was awarded the Nobel Prize in Chemistry in 1918 (Scheme 1). | ||
| Scheme 1 Ammonia synthesis using Haber process and other environmentally friendly method of producing ammonia or bio-ammonia. | ||
Subsequently, BASF acquired the patent for this process, and Carl Bosch successfully scaled it up, earning the Nobel Prize in 1931 for his contributions. The ammonia synthesis process was eventually named the Haber–Bosch process.36
Today, approximately 88% of the ammonia produced through this process is used to produce nitrogen fertilizers, including urea, ammonium sulfate, ammonium nitrate, and ammonium carbonate. This innovation played a crucial role in increasing agricultural productivity, contributing to the global population's ability to thrive and dominate the planet. The widespread availability of nitrogen fertilizers has significantly transformed agriculture and food production. Presently, around 110 million tons of nitrogen fertilizer are produced annually to meet global demands.37 Beyond fertilizers, ammonia is an essential industrial chemical used in the production of various compounds and products. These include hydrazine (used in the Olin Raschig process and the peroxide process), hydroxylamine, ammonium carbonate, amino acids (via the Strecker amino-acid synthesis), acrylonitrile (produced through the Sohio process), and applications in cleaning, microbial fermentation (as a nitrogen source), and antimicrobial agents for food products. Ammonia also plays a vital role in refrigeration, the scrubbing of sulfur dioxide (SO2) from fossil fuel combustion, as well as in fuel for internal combustion engines, textiles (such as mercerization), woodworking (where it reacts with tannins in wood to cause color changes), and lifting gases in industrial processes.
Historically, the production of ammonia required hydrogen derived from fossil resources like crude oil, natural gas, or coal, and nitrogen was extracted from the air, which consists of 78% nitrogen. As a result, the Haber–Bosch process was not considered a renewable or sustainable method for ammonia production. However, in recent years, several novel technologies have been developed to produce “green” ammonia with electrochemical synthesis and biomass-based production being the primary approaches.38
• NH3 Canada (http://www.nh3canada.com/): this company produces ammonia from air and water using energy derived from wind power. They have developed a modular system, the NH3 500 Standalone Fuel Synthesizer, which has a production rate of 20 L h−1.
• Monolith (https://monolith-corp.com/): this is a U.S.-based clean energy company, produces ammonia using its innovative methane pyrolysis process. This method splits natural gas into solid carbon and hydrogen without emitting carbon dioxide. Hydrogen is then used to produce ammonia through a traditional Haber–Bosch process. Monolith's facility has a production capacity of approximately 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of ammonia annually.
000 metric tons of ammonia annually.
• Yara International (https://www.yara.com/): this company produces green ammonia using renewable energy-powered electrolysis to extract hydrogen from water, which is then combined with nitrogen through the Haber–Bosch process. Yara operates Europe's largest green ammonia facility in Porsgrunn, Norway, and has plans to scale up production to over 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons annually to support sustainable agriculture and shipping fuels.
000 metric tons annually to support sustainable agriculture and shipping fuels.
• TalusAg (https://www.talusag.com/): this company focuses on producing green ammonia locally using renewable energy in regions like sub-Saharan Africa. The company employs a process that uses renewable energy-powered electrolysis to produce hydrogen from water, which is combined with nitrogen from the air via the Haber–Bosch process. TalusAg's pilot system in Kenya has demonstrated its feasibility, with the capacity to reduce fertilizer costs by 20–30% while addressing food security challenges. The company aims to scale up its production capabilities to meet the growing demand for sustainable fertilizers in the region.
Other companies such as Starfire Energy, AmmPower, Siemen Energy and Topsoe are pioneering renewable ammonia production companies using green hydrogen with project in Europe and Australia. These companies are paving the way for more localized, sustainable ammonia production methods, which could reduce the reliance on fossil fuels and contribute to a greener, more sustainable future for ammonia and fertilizer production.
5.4 Different ammonia-based pretreatment approaches
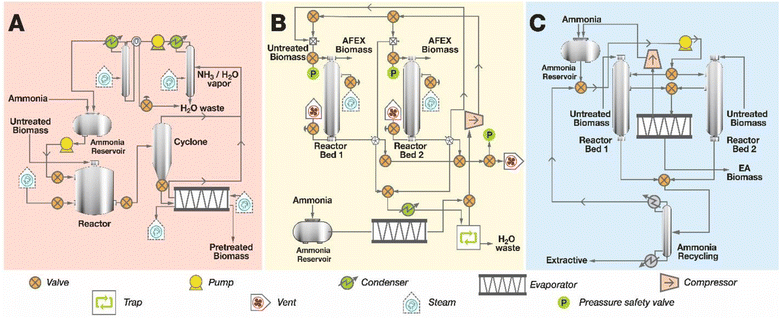
Due to the reversible nature of ammonia's interaction with moist biomass, ammonia catalysts can be recovered and reused. This characteristic has led various researchers to explore ammonia as a viable pretreatment for biomass, offering a potential edge over other thermochemical methods that use non-recyclable catalysts. For several decades, farmers in countries like China have utilized ammonia to treat alfalfa or orchard grass hay, enhancing its fiber digestibility for animal feed. The process involves stacking bales of hay in a pit, injecting 3–4 g of ammonia per 100 g of dry biomass, and covering them with tarpaulin. The hay is allowed to stand at ambient temperature for 6–8 weeks. This process can increase fiber digestibility by 10–35%, eliminate mold spoilage, and increase the nitrogen content of the biomass by 5–10%. However, the method has several drawbacks, including a slow treatment process, inability to recover from ammonia, and the resultant ammoniated biomass being inferior to traditional high-quality animal feeds. Below is a detailed exploration of different ammonia-based pretreatment methods.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2–5.0 w/w) and operates at ambient temperature and pressure for an extended period (4–12 weeks). While ammonia usage is lower compared to ARP and SAA methods, sugar conversion rates are relatively low, with only 73% glucan and xylan conversion from untreated material. This process's long duration still limits its commercial viability.46
0.2–5.0 w/w) and operates at ambient temperature and pressure for an extended period (4–12 weeks). While ammonia usage is lower compared to ARP and SAA methods, sugar conversion rates are relatively low, with only 73% glucan and xylan conversion from untreated material. This process's long duration still limits its commercial viability.46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, pretreatment at 80 °C for 84 hours. This process allows for efficient pretreatment but requires significant energy for ammonia recovery.47
0.1, pretreatment at 80 °C for 84 hours. This process allows for efficient pretreatment but requires significant energy for ammonia recovery.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and moisture content (30–80%). The treatment occurs at temperatures between 80–140 °C for 10–60 minutes (Fig. 6A). After pretreatment, pressure is released, and the biomass is transferred to a tray to remove residual ammonia. In an industrial setting, the released ammonia can be compressed and reused, although the process requires additional energy for ammonia condensation, which increases operational costs.49
1) and moisture content (30–80%). The treatment occurs at temperatures between 80–140 °C for 10–60 minutes (Fig. 6A). After pretreatment, pressure is released, and the biomass is transferred to a tray to remove residual ammonia. In an industrial setting, the released ammonia can be compressed and reused, although the process requires additional energy for ammonia condensation, which increases operational costs.49
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An exothermic reaction occurs when ammonia reacts with water, generating instant heat and maintaining the reaction temperature for 30 to 60 minutes (Fig. 6B). Once the reaction is complete, the pressure in the first reactor is released, allowing ammonia to flow into the second reactor, which is also filled with biomass. As approximately 2–3% of the ammonia is consumed during pretreatment, additional ammonia is added to the second reactor to maintain the desired ratio. Residual ammonia adsorbed to the biomass in the first reactor is stripped with steam, recovered via a condenser and compression system, and reused in subsequent cycles.51
1. An exothermic reaction occurs when ammonia reacts with water, generating instant heat and maintaining the reaction temperature for 30 to 60 minutes (Fig. 6B). Once the reaction is complete, the pressure in the first reactor is released, allowing ammonia to flow into the second reactor, which is also filled with biomass. As approximately 2–3% of the ammonia is consumed during pretreatment, additional ammonia is added to the second reactor to maintain the desired ratio. Residual ammonia adsorbed to the biomass in the first reactor is stripped with steam, recovered via a condenser and compression system, and reused in subsequent cycles.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which also raises the liquid-to-solid ratio. This process typically operates at temperatures between 90–140 °C, with a very low biomass moisture content (5–10%).31 The solubilized lignin is separated as a liquid stream and can be recovered by evaporating the ammonia into its gaseous form. This gas is then condensed and compressed back into liquid ammonia for reuse in future pretreatment cycles. The extracted lignin can be fractionated into several streams, potentially providing additional revenue for the biorefinery when this technology is commercialized.
1, which also raises the liquid-to-solid ratio. This process typically operates at temperatures between 90–140 °C, with a very low biomass moisture content (5–10%).31 The solubilized lignin is separated as a liquid stream and can be recovered by evaporating the ammonia into its gaseous form. This gas is then condensed and compressed back into liquid ammonia for reuse in future pretreatment cycles. The extracted lignin can be fractionated into several streams, potentially providing additional revenue for the biorefinery when this technology is commercialized.The lignin recovered from EA pretreatment has distinct structural characteristics that influence its potential for valorization. In contrast to lignin from acidic pretreatments, which often undergoes condensation reactions leading to recalcitrant C–C bonds, EA-extracted lignin retains largely native β-O-4 linkages that are more susceptible to depolymerization. Studies have shown that this preserved structure enables more efficient catalytic conversion to aromatic monomers such as phenol, guaiacol and syringol. According to 2D NMR analysis, the extracts from the EA process contain most of the native lignin functionalities typically found in native biomass. The β-aryl ether bonds remain intact after EA pretreatment, without the degradation, condensation or polymerization reactions that typically occur during steam explosion or pretreatment with acids.31 These structural features make the EA-extracted lignin particularly suitable for applications requiring higher molecular weight lignin fragments and for downstream catalytic upgrading to high-value aromatic compounds.
Biomass treated via EA appears lighter in color due to the removal of lignin and is found to be more digestible when treated with commercial enzymes, compared to AFEX-treated biomass. Additionally, the higher ammonia concentrations used in EA promote the formation of cellulose III, which is twice as reactive as the native cellulose I. However, one major drawback of this process is the need for higher ammonia-to-biomass ratios and operating pressures, which range from 900 to 1200 psi.33 Ongoing research aims to optimize the process by reducing operating pressures to levels comparable to those of the AFEX process, which would improve the economic feasibility of EA pretreatment.33
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) biomass ratio (0.8
biomass ratio (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) compared to EA (6
1) compared to EA (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), while still effectively converting native cellulose I to cellulose III allomorph. Unlike AFEX, which uses high moisture content preventing cellulose III formation, or EA, which requires high pressure (1300 psi) and temperature (120 °C), COBRA achieves similar biomass digestibility under milder conditions. The process demonstrates comparable sugar yields to EA without requiring extensive lignin extraction steps. While COBRA requires longer residence times (5.5 h), its improved reactor utilization and reduced ammonia consumption offer significant advantages for industrial-scale operations.52 The use of densified feedstocks also enhances downstream logistics and enables better integration with decentralized biomass processing depots. These features, combined with the elimination of washing steps and reduced operational severity, position COBRA as a promising technology for large-scale biorefinery applications, particularly in regions with established biomass densification infrastructure.45 Further developments led to COBRA-LE (COBRA with lignin extraction), which can selectively extract up to 26% of the lignin present while maintaining high carbohydrate conversion. When tested on sugarcane bagasse, COBRA-LE achieved >80% conversion of carbohydrates to monomeric and oligomeric sugars under industrially relevant conditions (6% glucan loading). The extracted lignin from COBRA-LE could potentially serve as a feedstock for valuable biobased chemicals.45
1), while still effectively converting native cellulose I to cellulose III allomorph. Unlike AFEX, which uses high moisture content preventing cellulose III formation, or EA, which requires high pressure (1300 psi) and temperature (120 °C), COBRA achieves similar biomass digestibility under milder conditions. The process demonstrates comparable sugar yields to EA without requiring extensive lignin extraction steps. While COBRA requires longer residence times (5.5 h), its improved reactor utilization and reduced ammonia consumption offer significant advantages for industrial-scale operations.52 The use of densified feedstocks also enhances downstream logistics and enables better integration with decentralized biomass processing depots. These features, combined with the elimination of washing steps and reduced operational severity, position COBRA as a promising technology for large-scale biorefinery applications, particularly in regions with established biomass densification infrastructure.45 Further developments led to COBRA-LE (COBRA with lignin extraction), which can selectively extract up to 26% of the lignin present while maintaining high carbohydrate conversion. When tested on sugarcane bagasse, COBRA-LE achieved >80% conversion of carbohydrates to monomeric and oligomeric sugars under industrially relevant conditions (6% glucan loading). The extracted lignin from COBRA-LE could potentially serve as a feedstock for valuable biobased chemicals.455.5 Insight into the mechanisms of chemical reactions during ammonia pretreatment
The mechanisms of chemical reactions occurring during ammonia pretreatment can be better understood by analyzing the type and concentration of degradation products formed under varying conditions of ammonia loading, residence time, and temperature. Degradation products are typically extracted using organic solvents and analyzed using various advanced spectroscopy techniques, including Liquid Chromatography coupled with Mass Spectrometry (LC-MS), Gas Chromatography coupled with Mass Spectrometry (GC-MS), Electron Spectroscopy for Chemical Analysis (ESCA), and Nuclear Magnetic Resonance Spectroscopy (NMR).53,54 These analytical tools provide valuable insights into the structural changes and chemical interactions occurring during pretreatment.In addition to spectroscopy, imaging techniques such as Scanning Electron Microscopy (SEM), Atomic Force Microscopy (AFM), Transmission Electron Microscopy (TEM), Confocal Microscopy (CFM), and Laser Scanning Confocal Fluorescence Microscopy (LSCM) have been employed to gain further understanding of the physical alterations that take place in biomass during ammonia pretreatment.53 From these studies, a series of key events have been identified as taking place during the pretreatment process (Fig. 7). Series of events during ammonia pretreatment are given below.
• Initial reactions: when moist biomass is exposed to high pressure and elevated temperature in a reactor, both hydrolysis and ammonolysis reactions occur. Hydroxyl ions from water and ammonium ions from ammonia react with acetyl, feruloyl, and coumaroyl ester linkages between lignin and hemicellulose, breaking them down into respective acids or amides. The relative ratio of hydrolysis and ammonolysis products depends on the moisture content in the biomass. For example, several di-ferulate isomers have been isolated from AFEX-pretreated corn stover, including cyclic and non-cyclic 8–8- and 8–5-diferulates, as well as di-amide, acid-amide, and di-acid forms.55
• Formation of nano-pores: following the cleavage of lignin–carbohydrate complex (LCC) linkages, ammonia further solubilizes the cleavage products, creating nano-pores in the middle lamella and secondary cell wall. These nano-pores typically range in size from 10 to 100 nm. This structural modification facilitates the access of enzymes and microorganisms to the cell wall components, which is critical for subsequent hydrolysis steps.
• Cellulose structure modification: the crystalline structure of cellulose is modified during pretreatment, converting from cellulose I to cellulose III. This transformation is influenced by the concentration of ammonia and water during the pretreatment process. Specifically, lower moisture content and higher ammonia concentrations favor the formation of cellulose III allomorph, which is more reactive than cellulose I.53 This change in structure is vital for improving the digestibility of the biomass and enhancing the effectiveness of enzymatic hydrolysis.
• Transport of solubilized products: during the latter stages of the pretreatment process, solubilized degradation products, lignin, minerals, and oligosaccharides (mainly glucose and xylose oligosaccharides with degrees of polymerization between 2 and 6, and some >10) are transported to the outer periphery of the biomass cell wall. This occurs when the reactor valve is opened at the end of the pretreatment cycle, leading to rapid decompression. The ammonia vapor is released, carrying the solubilized components with it.56
It is important to note that during ammonia pretreatment, only ester linkages are cleaved. In contrast, stronger bases such as NaOH or KOH cleave both ether and ester linkages. As a result, the solubilized lignin produced by ammonia pretreatment retains a structure that is more like native lignin compared to that solubilized by NaOH or KOH. This distinction is significant because the presence of phenolic monomers in pretreated biomass can be inhibitory to downstream biofuel processing. Research indicates that about 2 to 3% of the ammonia is consumed during the AFEX pretreatment process, forming soluble nitrogenous products, including acetamide, phenolic amides, and Maillard reaction products such as methyl imidazole and pyrazine derivatives.57 These compounds account for approximately 50% of the total ammonia consumed during pretreatment.
The remaining 50% of the ammonia that reacts with the biomass may form insoluble adduct products, such as nitrogenous phenolic compounds and aminated tricine derivatives. This unreacted ammonia plays a critical role in modifying the biomass structure and facilitating the formation of lignin solubilization products, which have significant implications for subsequent biofuel production. It is also worth noting that AFEX pretreatment is conducted at lower temperatures (<120 °C), which results in relatively minimal decomposition of carbohydrates into organic acids (e.g., lactic acid, succinic acid) compared to dilute acid pretreatment, which operates at higher temperatures (>160 °C). The milder conditions used in AFEX pretreatment allow for a more selective cleavage of lignin–carbohydrate linkages, without the extensive degradation of sugars that can occur in other pretreatment methods.
5.6 Ammonia pretreatment and biomass densification
Lignocellulosic biomass typically has a low bulk density, (less than 50 kg m−3) and is difficult to move, posing challenges for its storage and transportation from the field to processing facilities. To address this, various technologies are being developed to increase the bulk density of biomass, including (a) pellet mills, (b) balers, (c) briquette presses, (d) screw presses, and (e) agglomerates. Enhancing the bulk density of biomass provides several benefits, such as improved feedstock handling, more efficient transportation, better conveyance, and enhanced composition quality and uniformity during loading and unloading.58The densification processes, such as pelleting and briquetting, are influenced by multiple factors, including the biomass's chemical composition (e.g., protein, fat, cellulose, hemicellulose, and lignin content), particle size (with smaller particles offering a higher surface area for better binding), pre-conditioning temperature (using steam before the material is sent to the extruder), and the die rotation speed.59 Detailed evaluations of physical properties—such as water absorption index, water solubility index, thermal properties, durability, and bulk density—have been conducted on densified feedstocks like corn stover, switchgrass, and prairie cord grass.60
External binders, such as starch, protein, or fat, are often used to facilitate the binding process during pelleting or briquetting. In the case of ammonia treatment, some of the solubilized lignin is relocated to the surface of the biomass, acting as a natural binder during pelleting. Studies have shown that the bulk density of AFEX-treated biomass pellets ranges from 300–575 kg m−3, which is closer to the bulk density of corn grain (760 kg m−3).61 The durability of these pellets is also similar (Fig. 8), making them suitable for use in existing infrastructure designed for storing and transporting corn. Densified biomass also shows slight improvements in sugar conversion compared to loosen biomass. This enhancement is likely due to the thermal softening or plasticization of lignin, as well as physical disruption, compression, and extrusion during the densification process.62 Additionally, enzyme hydrolysis of pelleted AFEX-treated biomass at high solid loading has shown benefits over loose AFEX-treated biomass, suggesting that the rheological properties of the pelleted biomass play a significant role in these improvements.
Although pelleting biomass requires additional resources and adds cost to the feedstock, it offers numerous advantages in terms of storage, transportation, and handling during subsequent processing stages. Biomass densification is particularly beneficial for decentralized large-scale biorefineries, where biomass is first processed at regional depots before being transported to a central biorefinery. These regional depots can handle feed stocks ranging from 5000 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per day63 In contrast, centralized biorefineries, where biomass is transported directly from the field to the facility, are more sustainable for smaller-scale operations, capable of handling up to 2000 tons per day.
000 tons per day63 In contrast, centralized biorefineries, where biomass is transported directly from the field to the facility, are more sustainable for smaller-scale operations, capable of handling up to 2000 tons per day.
6. Biomass processing depot and how it can help to increase the feedstock supply
It is estimated that lignocellulosic biorefineries will process feedstocks ranging from 2000 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per day. The demand for such large volumes of feedstock presents significant logistical challenges, especially when transporting less dense biomass (such as agricultural residues and perennial grasses) from the field to the biorefinery. These challenges are particularly pronounced when biomass is transported in the form of square or round bales, which can be bulky and inefficient to handle over long distances. Two different operational models for biorefineries are considered: centralized biorefineries and decentralized biorefineries. In a centralized biorefinery model, all four major unit operations—pretreatment, hydrolysis, fermentation, and distillation—are carried out at a single location. This approach allows for the processing of large quantities of biomass in a centralized facility, but it can face significant transportation issues due to the high volume of biomass that needs to be transported to the site. In contrast, a decentralized biorefinery model involves conducting pretreatment closer to the source of biomass, typically at regional depots or processing hubs near the fields. The remaining unit operations—such as hydrolysis, fermentation, and distillation—are then carried out at a central location. The decentralized model offers several advantages over the centralized approach, including:
000 tons per day. The demand for such large volumes of feedstock presents significant logistical challenges, especially when transporting less dense biomass (such as agricultural residues and perennial grasses) from the field to the biorefinery. These challenges are particularly pronounced when biomass is transported in the form of square or round bales, which can be bulky and inefficient to handle over long distances. Two different operational models for biorefineries are considered: centralized biorefineries and decentralized biorefineries. In a centralized biorefinery model, all four major unit operations—pretreatment, hydrolysis, fermentation, and distillation—are carried out at a single location. This approach allows for the processing of large quantities of biomass in a centralized facility, but it can face significant transportation issues due to the high volume of biomass that needs to be transported to the site. In contrast, a decentralized biorefinery model involves conducting pretreatment closer to the source of biomass, typically at regional depots or processing hubs near the fields. The remaining unit operations—such as hydrolysis, fermentation, and distillation—are then carried out at a central location. The decentralized model offers several advantages over the centralized approach, including:
• Pretreatment for biomass densification: by performing pretreatment closer to the biomass source, it is possible to densify the biomass at the regional depots. This densification process improves the bulk density of the biomass, making it easier and more cost-effective to transport large quantities over long distances. As previously mentioned, densified biomass, such as pellets or briquettes, offer enhanced transport efficiency and reduces handling costs.
• Reduced transportation challenges: densified biomass is far easier to handle, and transport compared to lose, low-density materials. With pretreatment occurring at regional depots, the biomass can be processed into a more compact form before transportation, significantly improving logistical efficiency. This results in reduced transportation costs and ensures that the biomass reaches the central biorefinery in a more manageable form, thus optimizing overall supply chain operations.
• Localized pretreatment: decentralizing the pretreatment process allows for greater flexibility in how biomass is managed and processed. Biomass can be preprocessed closer to the source, reducing the need for extensive transportation infrastructure and ensuring that the biomass remains fresh for processing. Additionally, localized pretreatment could offer a more sustainable approach by minimizing the carbon footprint associated with transporting raw biomass to a centralized facility.
Fig. 9 presents a detailed illustration of the utilization of corn stover and grain utilization in the U.S., emphasizing the role of regional biomass processing depots within an integrated bioeconomy. The diagram highlights how harvested corn is allocated across multiple sectors: 38% is used for animal feed, 29% for biofuel production, 12% for food and industrial products, 8% for export, 8% for distillers, and 5% reserved for future use. A notable aspect of the diagram is the management of corn stover, which is transported to regional biomass processing depots for pretreatment and densification. This processed biomass follows two primary pathways: it can be utilized as enhanced animal products or serve as feedstock for biorefineries. Biorefineries transform the stover into biofuels and valuable co-products, such as lignin and yeast meal. This integrated system optimizes resource utilization, minimizes transportation costs through localized preprocessing, and enhances the sustainability of bioeconomy.
7. Ammonia pretreated biomass to produce fuels and chemicals
7.1 Enzymatic hydrolysis
Enzymatic hydrolysis is a critical step in the bioconversion of AFEX-pretreated lignocellulosic biomass into fermentable sugars for biofuel production. Unlike acidic pretreatments, which remove significant portions of hemicellulose, AFEX preserves the native carbohydrate composition while altering the biomass structure to improve enzyme accessibility. This preservation necessitates the use of a synergistic enzyme cocktail including cellulases, hemicellulases, pectinases, and accessory enzymes to achieve efficient sugar release.64,65 Although AFEX-pretreatment biomass requires a more complex enzyme mixture compared to other pretreatment methods, it typically has higher sugar recovery with minimal formation of inhibitory compounds that could hinder downstream fermentation. Research into enzyme–substrate interactions highlights several mechanisms by which AFEX enhances enzyme accessibility. First, the pretreatment modifies lignin structure, reducing non-productive enzyme binding and making more enzymes available for cellulose hydrolysis.66 Second, the ammoniating lignin's methoxy groups during AFEX mitigates inhibitory enzyme–lignin interactions, further improving enzyme efficiency.67 These structural modifications contribute to high conversion rates, with studies reporting over 90% conversion of both cellulose and hemicellulose to fermentable sugars across diverse biomass types.68 Additionally, AFEX-induced ultrastructural changes, such as increased porosity and surface area, significantly enhance enzyme accessibility. For instance, AFEX-treated corn stover develops pores exceeding 10 nm in diameter, an optimal size for cellulase enzymes (approximately 5–12 nm), facilitating effective substrate access.69An industrial advantage of AFEX hydrolysates is their high sugar content with minimal inhibitory compounds, eliminating the need for expensive detoxification steps.69 This enables high-solids processing (>18% w/w), which is essential for achieving ethanol titers exceeding 40 g L−1, thereby reducing operational costs.70 Moreover, AFEX-treated biomass retains nutrients such as proteins, amino acids, and minerals, resulting in an enriched hydrolysate that supports robust microbial growth without requiring costly supplementation. Table 1 summarizes key studies on the enzymatic hydrolysis of AFEX-pretreated biomass, underscoring its potential for efficient and sustainable biofuel production.
| Feedstock | AFEX conditions | Enzyme cocktail & loading | Sugar conversion/consumption | Ref. |
|---|---|---|---|---|
| Agave tequilana bagasse | NH3/biomass: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, moisture: 0.4 g g−1, 120 °C, 38 min 1, moisture: 0.4 g g−1, 120 °C, 38 min |
CTec2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HTec2 (78 HTec2 (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22%), 20 mg per g glucan 22%), 20 mg per g glucan |
Glucose cons.: 250.7 kg, xylose cons.: 58.8 kg | 73 |
| Corn stover | NH3/biomass: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, moisture: 60%, 140 °C, 15 min 1, moisture: 60%, 140 °C, 15 min |
CTec2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HTec2 HTec2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pectinase (67 Pectinase (67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17%), 30 mg per g glucan 17%), 30 mg per g glucan |
Glucose conv.: 99%, xylose conv.: 84% | 74 |
| Corn stover | NH3/biomass: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, moisture: 60%, 100 °C, 30 min 1, moisture: 60%, 100 °C, 30 min |
CTec3 (13 mg per g glucan) and HTec3 (11.8 mg per g glucan) | Glucose conv.: 95%, xylose conv.: 83% | 72 |
| Corn stover | NH3/biomass: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, moisture: 0.67 g g−1, T: 100 ± 10 °C, t: 30 min 1, moisture: 0.67 g g−1, T: 100 ± 10 °C, t: 30 min |
CTec2 (32 mg protein per g glucan) and HTec2 (9 mg protein per g glucan) | Glucose cons.: 63.9 g L−1, xylose cons.: 23.7 g L−1 | 75 |
| Corn stover | NH3/biomass: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, moisture: 0.6 g g−1, T: 140 °C, t: 15 min 1, moisture: 0.6 g g−1, T: 140 °C, t: 15 min |
Accellerase 1500 (24 mg per g glucan), accellerase XY (6 mg per g glucan), and multifect pectinase (6 mg per g glucan) | Glucose conv.: 77%, xylose conv.: 47.5% | 76 |
| Sugarcane bagasse | NH3/biomass: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, moisture: 0.6 g g−1, 140 °C, 60 min 1, moisture: 0.6 g g−1, 140 °C, 60 min |
CTec3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HTec3 HTec3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pectinex (68 Pectinex (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%), 15 mg per g glucan 10%), 15 mg per g glucan |
Glucose conv.: 100%, xylose conv.: 96% | 77 |
| Whole corn plant | NH3/biomass: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, moisture: 60%, T: 90 °C, t: 30 min 1, moisture: 60%, T: 90 °C, t: 30 min |
Cellulase: spezyme CP (88 mg ml−1) and accellerase 1000 (84 mg ml−1), amylase: novozyme 188 (149 mg ml−1) and stargen 001 (62 mg ml−1), hemicellulase: multifect xylanase (35 mg ml−1) | Glucose conv.: 100%, xylose conv.: 80–82% | 78 |
7.2 Microbial fermentation to biofuel
The nutrient-preserved, low-inhibitor nature of AFEX-treated biomass provides an optimal fermentation medium, supporting robust microbial growth without fermentation medium such as yeast extract or corn steep liquor supporting robust microbial growth without the need of supplementation. Engineered microbial strains such as Saccharomyces cerevisiae, Escherichia coli, and Zymomonas mobilis have demonstrated high sugar conversion efficiencies in these hydrolysates.71,72 Notably, studies have reported nutrient-rich profiles in AFEX-treated corn stover hydrolysate, including approximately 750 mg per L ammonia and 1231 mg per L amino acids in a 6% hydrolysate solution.79Comparative analyses consistently highlight the superior performance of AFEX over alternative pretreatments in terms of ethanol yield. For instance, AFEX-treated corn stover achieved a 98% metabolic ethanol yield, outperforming ionic liquid and dilute acid pretreatments, which reached yields of 90–93%.74 Similarly, for sugarcane bagasse, AFEX pretreatment resulted in bioethanol yields of 316–325 L per metric ton of raw biomass, significantly surpassing the 205–257 L per metric ton achieved with steam explosion. This enhanced performance is attributed to better preservation of carbohydrates and minimal inhibitor formation during AFEX pretreatment.77
Innovative fermentation strategies have further boosted process efficiency. One such approach, Rapid Bioconversion with Integrated Recycling Technology (RaBIT), has shown significant enzyme savings and productivity gains. By recycling adsorbed enzymes between fermentation cycles, RaBIT achieved over a 35% reduction in enzyme loading and a 2–3-fold increase in ethanol productivity compared to conventional methods.80 Another major advancement, the EA process, combines cellulose modification with lignin removal. EA pretreatment transforms native cellulose into its more digestible cellulose III form while extracting approximately 45% of lignin. This dual action enables a 60% reduction in enzyme loading compared to traditional processes. Under industrial conditions with 7.5 mg protein per g glucan and 8% glucan loading, EA-treated corn stover yields 18.2 kg of ethanol per 100 kg of untreated biomass, demonstrating its remarkable efficiency and industrial applicability.31
Recent advancements have introduced more efficient ammonia-based pretreatment techniques, such as the COBRA process. Operating under milder conditions (67 °C) and using a lower ammonia-to-biomass ratio (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), COBRA achieves significant ethanol yields with reduced energy and ammonia requirements. When paired with an optimized enzyme cocktail (CTec2-HTec2-Pectinex), fermentation of COBRA-pretreated corn stover using Saccharomyces cerevisiae 424A produced 38.3 g per L ethanol at an 88% metabolic yield, showcasing its potential for industrial scalability.52
1), COBRA achieves significant ethanol yields with reduced energy and ammonia requirements. When paired with an optimized enzyme cocktail (CTec2-HTec2-Pectinex), fermentation of COBRA-pretreated corn stover using Saccharomyces cerevisiae 424A produced 38.3 g per L ethanol at an 88% metabolic yield, showcasing its potential for industrial scalability.52
Commercial-scale studies of AFEX technology have also yielded encouraging results. In pilot-scale fermentations, Zymomonas mobilis 8b was used to process AFEX-treated and pelletized corn stover in 2500 L reactors. The process demonstrated feasibility at scale, although ethanol yields (61.7 g L−1) were slightly lower than laboratory-scale results, primarily due to mixing limitations in larger vessels.81 Despite this, consistent performance was observed across multiple batches of AFEX-treated agricultural residues.82 These findings underscore the potential for industrial implementation of AFEX technology, while highlighting challenges such as process integration with biomass densification strategies. Table 2 provides a comprehensive summary of fermentation studies utilizing AFEX-pretreated biomass hydrolysates for biofuel production.
| Feedstock | Fermentation conditions | Microorganism | Product | Production/yield | Ref. |
|---|---|---|---|---|---|
| Corn stover | Continuous SSF, 6% glucan | S. cerevisiae 424A | Ethanol | 36.5 g L−1 | 76 |
| Corn stover | CBP, 0.5% glucan | C. phytofermentans | Ethanol | 2.8 g L−1 | 89 |
| Corn stover | SHF, 6% glucan | Z. mobilis | Ethanol | 39.1 g L−1 (82% yield) | 75 |
| Sugarcane bagasse | SHF, 1% glucan + CSL | S. cerevisiae 424A | Ethanol | 44.17 g L−1 (92% yield) | 77 |
| Sugarcane bagasse (SB) and cane leaf (CL) | SHF, 6% glucan | S. cerevisiae 424A | Ethanol | SB: 33.7 g L−1, CL: 36.4 g L−1 (91.6% yield) | 90 |
| Rice straw | CBP | Anaerobic microflora | H2 | 67.8% higher yield vs. untreated | 91 |
| Oil palm empty fruit bunch | SHF | Enterobacter sp. KBH6958 | H2 | 50.4 mmol L−1 after 72h | 34 |
Corn stover + dairy manure (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Anaerobic co-digestion | Mixed anaerobic consortia | Biogas | 175 L per kg VS (22% higher vs. untreated) | 83 |
| Sugarcane bagasse + dairy manure | Anaerobic co-digestion | Mixed anaerobic consortia | Methane | 292–299 L CH4 per kg VS (57–59% v/v) | 84 |
Beyond bioethanol production, ammonia-pretreated biomass has shown significant potential for biogas generation through anaerobic digestion processes. Co-digestion of ammonia-pretreated agricultural residues with dairy manure has demonstrated enhanced biogas yields compared to untreated biomass.83,84 This improvement can be attributed to the structural modifications caused by ammonia pretreatment, which makes the biomass more accessible to anaerobic microorganisms. The presence of residual ammonia also helps maintain an optimal carbon-to-nitrogen ratio for stable digestion conditions, while the degradation of products from pretreatment can serve as additional substrates for methane-producing microorganisms.83 The integration of anaerobic digestion with ammonia pretreatment thus offers an alternative pathway for renewable energy production from lignocellulosic biomass, particularly in biorefinery settings where multiple value streams are desired.
8. Ammonia pretreated biomass as animal feed
In many low- and middle-income countries, over 70% of livestock feed comes from crop residues, with urban and peri-urban dairy systems relying on these materials for more than half of their feed requirements.85 However, these residues typically exhibit low total digestible nutrient content (40–46%) and inadequate levels of nitrogen, vitamins, and minerals, resulting in reduced voluntary intake by livestock.86AFEX pretreatment has been shown to significantly enhance the nutritional quality of these crop residues. For instance, a comparison between AFEX and steam explosion pretreatments applied to sugarcane residues demonstrated that AFEX-treated material achieved a 69% improvement in vitro rumen digestibility and a 26% increase in metabolizable energy. Notably, AFEX pretreatment increased nitrogen content by 230% compared to untreated biomass, whereas steam explosion showed no significant effect on total nitrogen.87
Practical feeding trials using Karan-Fries cattle and Murrah buffalo further demonstrated the benefits of AFEX-treated feed. Cattle consuming AFEX-treated wheat straw exhibited an 18% increase in milk energy and a 42% rise in dry matter intake compared to those on traditional feed. Additionally, buffalo maintained their body weight (−0.07 kg per day) on AFEX-treated diets, whereas those fed conventional diets experienced an average weight loss of 1.0 kg per day.88 These improvements in feed digestibility were attributed to enhanced enzyme accessibility and increased surface area for microbial attachment, enabling better nutrient utilization.
A key consideration in AFEX-treated feeds is the formation of acetamide, which occurs during pretreatment as ammonia reacts with acetyl groups in the biomass through ammonolysis reactions. Studies have shown that acetamide, naturally present in commercial meat and milk samples at levels of 0.27–0.67 mg kg−1, increases significantly in animals consuming AFEX diets. Research has documented 16–23-fold increases in acetamide levels in cattle milk and 19–28-fold increases in buffalo milk compared to baseline levels.92 To address these concerns, ongoing research is exploring modified processing techniques, such as pre-treatment alkali washing steps, which effectively neutralize acetamide precursors and reduce their formation during the AFEX process.93 These advancements hold promises for improving the safety and nutritional efficacy of AFEX-treated feeds, ensuring their suitability for widespread adoption in livestock production systems.
9. Ammonia pretreated biomass for biomaterial applications
AFEX-treated biomass has demonstrated significant potential for producing value-added biomaterials, particularly lipids, proteins, and fungal-based products. The nutrient-preserved composition and enhanced accessibility of AFEX-treated substrates create optimal conditions for diverse bioconversion processes. In lipid production AFEX-treated corn stover has proven to be an effective substrate for oleaginous microorganisms. Using Lipomyces tetrasporus NRRL Y-11562, researchers achieved 36.7 g of lipids per kg of pretreated biomass at a titer of 8.4 g L−1.94 Further screening identified Cryptococcus humicola UCDFST 10–1004 as an exceptional performer, accumulating 15.5 g per L lipids, which represented 40% of its cell dry weight. A broader evaluation of multiple yeast strains demonstrated lipid accumulation up to 65% of cell biomass, with yields of 25–30 g L−1 from undetoxified hydrolysates.95Ammonia pretreatment also facilitates efficient protein extraction from biomass. Research has shown successful protein recovery from AFEX-treated switchgrass using an ammonia-based extraction method. Optimized with a 3% aqueous ammonium hydroxide solution at pH 10, this process enables protein extraction while maintaining the biomass's suitability for subsequent enzymatic hydrolysis and biofuel production.96 The integrated approach allows for ammonia recycling between AFEX pretreatment and protein extraction steps, with residual ammonia enhancing the nutritional value of the biomass for ruminant feed applications.97
Additionally, AFEX pretreatment benefits fungal cultivation. When paired with the growth of white rot fungi, AFEX-treated substrates exhibited enhanced enzyme production and accelerated mycelial development. This combined process improved mushroom yields by 44.6%, leveraging the structural modifications and enriched nitrogen content introduced during pretreatment.98 These diverse applications underscore the versatility of AFEX pretreatment in generating various value-added bioproducts beyond traditional biofuel production, further advancing its role in sustainable bioprocessing.
10. Comparative economics of AFEX and other biomass pretreatment technologies
Techno-economic analyses show that the costs of the various biomass pretreatment methods differ. A comparative study of corn stover processing found sugar production costs of $0.43 per kg for steam explosion, $0.42 per kg for dilute sulfuric acid, $0.65 per kg for AFEX, and $1.41 per kg for biological pretreatment.99 Another study found that pretreatment of corn stover with ionic liquids resulted in significantly higher sugar production costs of $2.7 per kg.100 The moderate cost of AFEX results from the need for specialized equipment, including high-pressure reactors, ammonia recovery systems, and safety infrastructure. However, AFEX has superior feedstock efficiency, requiring only 666![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 805 dry tons per year compared to steam explosion (818
805 dry tons per year compared to steam explosion (818![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826), dilute acid (746
826), dilute acid (746![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 797), and biological methods (1
797), and biological methods (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 675
675![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743) for a 113.5 million liter per year butanol production plant.99
743) for a 113.5 million liter per year butanol production plant.99
AFEX technology offers significant operational advantages that partially offset the higher capital costs compared to acid-based or steam explosion processes, while being significantly more economical than ionic liquid pretreatment. The process enables the recovery and reuse of approximately 97% of the ammonia, which significantly reduces long-term operating costs. Unlike acid-based pretreatment processes, AFEX-treated biomass does not need to be detoxified prior to fermentation, eliminating these processing costs. In addition, AFEX-treated biomass has proven to be a valuable animal feed, opening additional revenue streams.
11. Concluding remarks and future directions
The evolution of ammonia-based pretreatment technologies, from early pulping applications to advanced processes like AFEX, EA, and COBRA, demonstrates the versatility and potential of ammonia as a pretreatment catalyst. These technologies have found applications across multiple sectors including biofuel production, animal feed enhancement, and biomaterial development. However, several critical challenges must be addressed for broader implementation of ammonia-based technologies: (a) sustainability of ammonia production itself represents a fundamental challenge. The transition from conventional Haber–Bosch process to green ammonia production through electrochemical synthesis and biomass-based approaches is crucial for improving the environmental footprint of these pretreatment technologies; (b) establishing regional biomass processing depots show promise for addressing logistical challenges, the economic viability of decentralized processing requires further validation and (c) new reactor design and process safety considerations at industrial scales remain significant hurdles, particularly for high-pressure processes.Looking ahead, several opportunities exist for advancing ammonia-based pretreatment technologies: (1) process optimization: developing methods that operate under milder conditions with reduced ammonia requirements to enhance efficiency and sustainability; (2) integrated approaches: combining multiple pretreatment strategies, such as alkali-ammonia treatments, to maximize effectiveness while minimizing drawbacks; (3) ammonia recovery and recycling: improving recovery and recycling systems to enhance economic feasibility and reduce environmental impact and (4) expanding applications: exploring novel uses, including protein extraction and biomaterial production, to broaden the technology's impact. As the biorefinery concept continues to evolve, the demand for more efficient and sustainable pretreatment methods grows. Future research should focus on process intensification, cost reduction, and flexible systems capable of handling diverse feedstocks. Additionally, gaining deeper insights into the molecular mechanisms of ammonia pretreatment could enable more targeted and efficient process improvements. Progress in these areas will be essential for positioning ammonia-based pretreatments as a key technology in the sustainable bioeconomy.
Data availability
This review article is exclusively based on analysis of previously published literature and publicly available data. No original research data, experimental results, or computational analyses were generated during the preparation of this manuscript.Author contributions
Venkatesh Balan: conceptualization, investigation, visualization, writing – original draft, writing: review & editing. Maedeh Mohammadi: investigation, writing – original draft, writing: review & editing. Bruce E. Dale: resources, funding acquisition, writing –review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This review article was possible with funding support from DOE Great Lakes Bioenergy Research Center (https://www.greatlakesbioenergy.org), funded by the U.S. Department of Energy through Cooperative Agreement DE-FC02-07ER64494, as well as Michigan State University AgBioResearch and the National Institute for Food and Agriculture of the U.S. Department of Agriculture #2021-67022-34889. Dr Balan would like to thank the University of Houston and the state of Texas for his startup funds.References
- H. Shao and Y. Wang, Am. J. Econ. Sociol., 2025, 84(3), 449–465 CrossRef.
- M. H. Langholtz, 2023 Billion-Ton Report: An Assessment of U.S. Renewable Carbon Resources, US Department of Energy, 2024, DOI:10.23720/BT2023/2316165.
- V. Balan, Int. Scholarly Res. Not., 2014, 2014, 463074 Search PubMed.
- N. C. Carpita and M. C. McCann, J. Biol. Chem., 2020, 295, 15144–15157 CrossRef.
- J. C. Del Río, J. Rencoret, A. Gutiérrez, T. Elder, H. Kim and J. Ralph, ACS Sustain. Chem. Eng., 2020, 8, 4997–5012 Search PubMed.
- M. M. De and O. Buanafina, Mol. Plant, 2009, 2, 861–872 Search PubMed.
- K. Sakuragi, K. Igarashi and M. Samejima, Polym. Degrad. Stab., 2018, 148, 19–25 Search PubMed.
- V. Balan, L. da Costa Sousa, S. P. S. Chundawat, J. Humpula and B. E. Dale, Dynamic Biochemistry Process Biotechnology and Molecular Biology, 2012 Search PubMed.
- EIA projects U.S. biofuel production to slowly increase through 2050 – U.S. Energy Information Administration (EIA), https://www.eia.gov/todayinenergy/detail.php?id=43096, accessed 28 January 2025.
- J. Hao, H. Chen, C. Zhang, H. Li, X. Chen, Z. Xiong, C. Wang, H. Guo, H. Zhang, L. Xiong, S. Yu and X. Chen, Chem. Eng. J., 2025, 511, 162012 CrossRef.
- A. Arias, G. Feijoo and M. T. Moreira, J. Clean. Prod., 2023, 418, 137925 CrossRef.
- M. Tena, L. S. Buller, W. G. Sganzerla, M. Berni, T. Forster-Carneiro, R. Solera and M. Pérez, Fuel, 2022, 309, 122171 Search PubMed.
- D. Biofuels, K. Malik, S. C. Capareda, B. Raj Kamboj, S. Malik, K. Singh, S. Arya and D. Kumar Bishnoi, Fuels, 2024, 5, 157–175 CrossRef.
- M. K. Shahid, A. Batool, A. Kashif, M. H. Nawaz, M. Aslam, N. Iqbal and Y. Choi, J. Environ. Manage., 2021, 297, 113268 CrossRef PubMed.
- T. Kuhlman and J. Farrington, Sustainability, 2010, 2, 3436–3448 CrossRef.
- J. Tomaszewska, D. Bieliński, M. Binczarski, J. Berlowska, P. Dziugan, J. Piotrowski, A. Stanishevsky and I. A. Witońska, RSC Adv., 2018, 8, 3161–3177 Search PubMed.
- A. Lovegrove, C. H. Edwards, I. De Noni, H. Patel, S. N. El, T. Grassby, C. Zielke, M. Ulmius, L. Nilsson, P. J. Butterworth, P. R. Ellis and P. R. Shewry, Crit. Rev. Food Sci. Nutr., 2017, 57, 237–253 CrossRef PubMed.
- K. E. Achyuthan, A. M. Achyuthan, P. D. Adams, S. M. Dirk, J. C. Harper, B. A. Simmons and A. K. Singh, Molecules, 2010, 15, 8641–8688 CrossRef.
- M. Shrivastava, P. D. Maibam, A. Aishwarya and A. Goyal, Handbook of Biorefinery Research and Technology: Biomass Logistics to Saccharification, 2024, pp. 731–753 Search PubMed.
- B. E. Dale, in AGRO, vol. 27, 2007 Search PubMed.
- I. Alawad and H. Ibrahim, Biomass Convers. Biorefin., 2022, 14(5), 6155–6183 CrossRef.
- M. Jayakumar, G. T. Gindaba, K. B. Gebeyehu, S. Periyasamy, A. Jabesa, G. Baskar, B. I. John and A. Pugazhendhi, Sci. Total Environ., 2023, 879, 163158 CrossRef PubMed.
- A. Shukla, D. Kumar, M. Girdhar, A. Kumar, A. Goyal, T. Malik and A. Mohan, Biotechnol. Biofuels Bioprod., 2023, 16(1), 1–33 CrossRef.
- S. Jain and S. Kumar, Sustain. Chem. Clim. Action, 2024, 5, 100053 CrossRef.
- B. Pan, L. R. Karadaghi, R. L. Brutchey and N. Malmstadt, Ind. Eng. Chem. Res., 2024, 63, 489–497 CrossRef.
- L. Xu, S. J. Zhang, C. Zhong, B. Z. Li and Y. J. Yuan, Ind. Eng. Chem. Res., 2020, 59, 16923–16938 CrossRef.
- R. Roy, M. S. Rahman and D. E. Raynie, Curr. Res. Green Sustainable Chem., 2020, 3, 100035 CrossRef.
- R. H. Narron, H. Kim, H. M. Chang, H. Jameel and S. Park, Curr. Opin. Biotechnol., 2016, 38, 39–46 Search PubMed.
- P. M. Abdul, J. M. Jahim, S. Harun, M. Markom, N. A. Lutpi, O. Hassan, V. Balan, B. E. Dale and M. T. Mohd Nor, Bioresour. Technol., 2016, 211, 200–208 CrossRef PubMed.
- V. Balan, B. Dale, S. Chundawat and L. Sousa, Methods for pretreating biomass, US Pat., US8968515B2, 2017, https://patents.google.com/patent/US8968515B2/en Search PubMed.
- L. Da Costa Sousa, M. Jin, S. P. S. Chundawat, V. Bokade, X. Tang, A. Azarpira, F. Lu, U. Avci, J. Humpula, N. Uppugundla, C. Gunawan, S. Pattathil, A. M. Cheh, N. Kothari, R. Kumar, J. Ralph, M. G. Hahn, C. E. Wyman, S. Singh, B. A. Simmons, B. E. Dale and V. Balan, Energy Environ. Sci., 2016, 9, 1215–1223 RSC.
- M. Mohammadi, M. Alian, B. Dale, B. Ubanwa and V. Balan, Biotechnol. Adv., 2024, 72, 108341 CrossRef CAS.
- U. Avci, X. Zhou, S. Pattathil, L. da Costa Sousa, M. G. Hahn, B. Dale, Y. Xu and V. Balan, Front. Energy Res., 2019, 7, 477734 Search PubMed.
- R. S. Abolore, S. Jaiswal and A. K. Jaiswal, Carbohydr. Polym. Technol. Appl., 2024, 7, 100396 CAS.
- D. Ciolacu and V. I. Popa, Environ. Eng. Manage. J., 2011, 10, 467–468 Search PubMed.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1(10), 636–639 Search PubMed.
- B. Hu, W. Wang, J. Chen, Y. Liu and C. Chu, Mol. Plant, 2023, 16, 64–74 Search PubMed.
- N. Bora, A. Kumar Singh, P. Pal, U. Kumar Sahoo, D. Seth, D. Rathore, S. Bhadra, S. Sevda, V. Venkatramanan, S. Prasad, A. Singh, R. Kataki and P. Kumar Sarangi, Fuel, 2024, 369, 131808 CrossRef CAS.
- P. K. Sarangi, R. K. Srivastava, J. Gitanjali, G. Sathiyan, G. Venkatesan and S. Kandasamy, Fuel, 2024, 371, 131863 CrossRef CAS.
- R. C. Peterson and R. W. Strauss, J. Polym. Sci., 1971, 36, 241–250 Search PubMed.
- V. Balan, B. Bals, L. Da Costa Sousa, R. Garlock and B. E. Dale, RSC Energy Environ. Ser., 2011, 89–114 CAS.
- Y.-C. T. Chou, Supercritical ammonia treatment of lignocellulosic materials, US Pat., US4644060A, 1987, https://patents.google.com/patent/US4644060A/en Search PubMed.
- T. H. Kim and Y. Y. Lee, Appl. Biochem. Biotechnol., 2007, 81–92 Search PubMed.
- C. Zhao, Q. Shao and S. P. S. Chundawat, Bioresour. Technol., 2020, 298, 122446 CrossRef CAS.
- A. R. C. Morais, J. Zhang, H. Dong, W. G. Otto, T. Mokomele, D. Hodge, V. Balan, B. E. Dale, R. M. Lukasik and L. da Costa Sousa, Green Chem., 2022, 24, 4443–4462 RSC.
- X. Li and T. H. Kim, Bioresour. Technol., 2011, 102, 4779–4786 CrossRef CAS.
- C. G. Yoo, N. P. Nghiem, K. B. Hicks and T. H. Kim, Bioresour. Technol., 2011, 102, 10028–10034 CrossRef CAS PubMed.
- Treatment of biomass to obtain fermentable sugars (Patent)|OSTI.GOV, https://www.osti.gov/biblio/1018212, accessed 25 January 2025.
- S. P. S. Chundawat, R. K. Pal, C. Zhao, T. Campbell, F. Teymouri, J. Videto, C. Nielson, B. Wieferich, L. Sousa, B. E. Dale, V. Balan, S. Chipkar, J. Aguado, E. Burke and R. G. Ong, J. Vis. Exp., 2020, 2020, e57488 Search PubMed.
- F. Teymouri, Technical Report: Process Improvements to Biomass Pretreatment of Fuels and Chemicals, U.S. Department of Energy, 2015, DOI:10.2172/1235580.
- V. Balan, B. Ubanwa and B. Dale, Densification Impact on Raw, Chemically and Thermally Pretreated Biomass: Physical Properties and Biofuels Production, 2023, pp. 265–289 Search PubMed.
- J. Zhang, M. Mohammadi, H. Gong, D. B. Hodge, J. Tumuluru, L. da Costa Sousa, B. Dale and V. Balan, Chem. Eng. J., 2025, 505, 159731 Search PubMed.
- S. P. S. Chundawat, G. Bellesia, N. Uppugundla, L. Da Costa Sousa, D. Gao, A. M. Cheh, U. P. Agarwal, C. M. Bianchetti, G. N. Phillips, P. Langan, V. Balan, S. Gnanakaran and B. E. Dale, J. Am. Chem. Soc., 2011, 133, 11163–11174 Search PubMed.
- S. P. S. Chundawat, B. S. Donohoe, L. Da Costa Sousa, T. Elder, U. P. Agarwal, F. Lu, J. Ralph, M. E. Himmel, V. Balan and B. E. Dale, Energy Environ. Sci., 2011, 4, 973–984 RSC.
- R. Vismeh, F. Lu, S. P. S. Chundawat, J. F. Humpula, A. Azarpira, V. Balan, B. E. Dale, J. Ralph and A. D. Jones, Analyst, 2013, 138, 6683–6692 Search PubMed.
- R. Vismeh, J. F. Humpula, S. P. S. Chundawat, V. Balan, B. E. Dale and A. D. Jones, Carbohydr. Polym., 2013, 94, 791–799 CrossRef PubMed.
- S. P. S. Chundawat, R. Vismeh, L. N. Sharma, J. F. Humpula, L. da Costa Sousa, C. K. Chambliss, A. D. Jones, V. Balan and B. E. Dale, Bioresour. Technol., 2010, 101, 8429–8438 Search PubMed.
- J. S. Tumuluru, C. Igathinathane, D. Archer and R. McCulloch, Front. Energy Res., 2024, 12, 1347581 CrossRef.
- A. N. Hoover, J. S. Tumuluru, F. Teymouri, J. Moore and G. Gresham, Bioresour. Technol., 2014, 164, 128–135 CrossRef PubMed.
- B. Karki, K. Muthukumarappan, Y. Wang, B. Dale, V. Balan, W. R. Gibbons and C. Karunanithy, Biomass Bioenergy, 2015, 78, 164–174 CrossRef.
- B. D. Bals, C. Gunawan, J. Moore, F. Teymouri and B. E. Dale, Biotechnol. Bioeng., 2014, 111, 264–271 CrossRef.
- V. Sundaram and K. Muthukumarappan, Ind. Crops Prod., 2016, 83, 537–544 CrossRef.
- S. Kim, B. E. Dale, M. Jin, K. D. Thelen, X. Zhang, P. Meier, A. D. Reddy, C. D. Jones, R. Cesar Izaurralde, V. Balan, T. Runge and M. Sharara, GCB Bioenergy, 2019, 11, 871–882 CrossRef.
- C. Gunawan, S. Xue, S. Pattathil, L. Da Costa Sousa, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2017, 10, 1–14 Search PubMed.
- Q. Shao and C. Zhao, Energy Fuels, 2016, 30, 9517–9523 CrossRef.
- D. Gao, S. P. S. Chundawat, N. Uppugundla, V. Balan and B. E. Dale, Biotechnol. Bioeng., 2011, 108, 1788–1800 CrossRef PubMed.
- C. Li, G. Cheng, V. Balan, M. S. Kent, M. Ong, S. P. S. Chundawat, L. da C. Sousa, Y. B. Melnichenko, B. E. Dale, B. A. Simmons and S. Singh, Bioresour. Technol., 2011, 102, 6928–6936 CrossRef PubMed.
- X. Shao, M. Jin, A. Guseva, C. Liu, V. Balan, D. Hogsett, B. E. Dale and L. Lynd, Bioresour. Technol., 2011, 102, 8040–8045 CrossRef PubMed.
- A. K. Mathew, B. Parameshwaran, R. K. Sukumaran and A. Pandey, Bioresour. Technol., 2016, 199, 13–20 CrossRef CAS PubMed.
- M. Jin and B. E. Dale, Handbook of Biorefinery Research and Technology, 2019, pp. 1–16 Search PubMed.
- M. W. Lau, C. Gunawan, V. Balan and B. E. Dale, Biotechnol. Biofuels, 2010, 3, 1–10 CrossRef PubMed.
- C. Sarks, A. Higbee, J. Piotrowski, S. Xue, J. J. Coon, T. K. Sato, M. Jin, V. Balan and B. E. Dale, Bioresour. Technol., 2016, 205, 24–33 CrossRef CAS.
- C. A. Flores-Gómez, E. M. Escamilla Silva, C. Zhong, B. E. Dale, L. Da Costa Sousa and V. Balan, Biotechnol. Biofuels, 2018, 11, 1–18 CrossRef.
- N. Uppugundla, L. Da Costa Sousa, S. P. S. Chundawat, X. Yu, B. Simmons, S. Singh, X. Gao, R. Kumar, C. E. Wyman, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2014, 7, 1–14 CrossRef PubMed.
- J. Serate, D. Xie, E. Pohlmann, C. Donald, M. Shabani, L. Hinchman, A. Higbee, M. McGee, A. La Reau, G. E. Klinger, S. Li, C. L. Myers, C. Boone, D. M. Bates, D. Cavalier, D. Eilert, L. G. Oates, G. Sanford, T. K. Sato, B. Dale, R. Landick, J. Piotrowski, R. G. Ong and Y. Zhang, Biotechnol. Biofuels, 2015, 8, 1–17 CrossRef PubMed.
- M. Jin, C. Gunawan, V. Balan, X. Yu and B. E. Dale, Biotechnol. Bioeng., 2013, 110, 1302–1311 CrossRef CAS.
- T. Mokomele, L. Da Costa Sousa, V. Balan, E. Van Rensburg, B. E. Dale and J. F. Görgens, Biotechnol. Biofuels, 2018, 11, 1–21 CrossRef.
- Q. Shao, S. P. S. Chundawat, C. Krishnan, B. Bals, L. Da Costa Sousa, K. D. Thelen, B. E. Dale and V. Balan, Biotechnol. Biofuels, 2010, 3, 1–10 CrossRef PubMed.
- M. W. Lau, B. D. Bals, S. P. S. Chundawat, M. Jin, C. Gunawan, V. Balan, A. D. Jones and B. E. Dale, Energy Environ. Sci., 2012, 5, 7100–7110 RSC.
- C. Sarks, M. Jin, V. Balan and B. E. Dale, J. Ind. Microbiol. Biotechnol., 2017, 44, 1261–1272 CrossRef PubMed.
- C. Sarks, B. D. Bals, J. Wynn, F. Teymouri, S. Schwegmann, K. Sanders, M. Jin, V. Balan and B. E. Dale, Biofuels, 2016, 7, 253–262 CrossRef.
- T. Campbell, B. Bals, F. Teymouri, J. Glassbrook, C. Nielson, J. Videto, A. Rinard, J. Moore, A. Julian and V. Bringi, Biotechnol. Bioeng., 2020, 117, 1241–1246 CrossRef.
- J. P. Rojas-Sossa, Y. Zhong, F. Valenti, J. Blackhurst, T. Marsh, D. Kirk, D. Fang, B. Dale and W. Liao, Biomass Bioenergy, 2019, 127, 105263 CrossRef.
- T. Mokomele, L. da Costa Sousa, V. Balan, E. van Rensburg, B. E. Dale and J. F. Görgens, Bioresour. Technol., 2019, 272, 326–336 CrossRef PubMed.
- M. Blümmel, F. Teymouri, J. Moore, C. Nielson, J. Videto, P. Kodukula, S. Pothu, R. Devulapalli and P. Varijakshapanicker, Anim. Feed Sci. Technol., 2018, 236, 178–186 CrossRef.
- P. Mor, B. Bals, S. Kumar, N. Tyagi, J. K. Reen, B. Tyagi, P. K. Choudhury and A. K. Tyagi, Small Rumin. Res., 2019, 170, 109–115 CrossRef.
- T. Mokomele, L. da Costa Sousa, B. Bals, V. Balan, N. Goosen, B. E. Dale and J. F. Görgens, Biofuel Bioprod. Biorefining, 2018, 12, 978–996 CrossRef.
- P. Mor, B. Bals, A. K. Tyagi, F. Teymouri, N. Tyagi, S. Kumar, V. Bringi and M. VandeHaar, J. Dairy Sci., 2018, 101, 7990–8003 CrossRef.
- M. Jin, V. Balan, C. Gunawan and B. E. Dale, Biotechnol. Bioeng., 2011, 108, 1290–1297 CrossRef.
- C. Krishnan, L. da Costa Sousa, M. Jin, L. Chang, B. E. Dale and V. Balan, Biotechnol. Bioeng., 2010, 107, 441–450 CrossRef PubMed.
- G. L. Cao, X. F. Xia, L. Zhao, Z. Y. Wang, X. Li and Q. Yang, Int. J. Hydrogen Energy, 2013, 38, 15653–15659 CrossRef.
- B. Bals, F. Teymouri, D. Haddad, W. A. Julian, R. Vismeh, A. D. Jones, P. Mor, B. Van Soest, A. Tyagi, M. Vandehaar and V. Bringi, J. Agric. Food Chem., 2019, 67, 10756–10763 CrossRef.
- H. Dong, L. da C. Sousa, B. Ubanwa, A. D. Jones and V. Balan, Front. Chem., 2022, 9, 826625 CrossRef.
- Y. P. Xue, M. Jin, A. Orjuela, P. J. Slininger, B. S. Dien, B. E. Dale and V. Balan, RSC Adv., 2015, 5, 28725–28734 RSC.
- P. J. Slininger, B. S. Dien, C. P. Kurtzman, B. R. Moser, E. L. Bakota, S. R. Thompson, P. J. O'Bryan, M. A. Cotta, V. Balan, M. Jin, L. D. C. Sousa and B. E. Dale, Biotechnol. Bioeng., 2016, 113, 1676–1690 CrossRef PubMed.
- B. Bals, L. Teachworth, B. Dale and V. Balan, Appl. Biochem. Biotechnol., 2007, 143, 187–198 CrossRef PubMed.
- V. Balan, E. D. Bruce and B. Bals, Separation of proteins from grasses integrated with ammonia fiber explosion (afex) pretreatment and cellulose hydrolysis, WO2008020901A2, 2007, https://patents.google.com/patent/WO2008020901A2/en.
- M. Hu, L. Yuan, Z. Cai, J. Zhang, D. Ji and L. Zang, ACS Omega, 2021, 6, 31689–31698 CrossRef.
- N. R. Baral and A. Shah, Bioresour. Technol., 2017, 232, 331–343 CrossRef PubMed.
- N. R. Baral and A. Shah, Biofuel Bioprod. Biorefining, 2016, 10, 70–88 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |