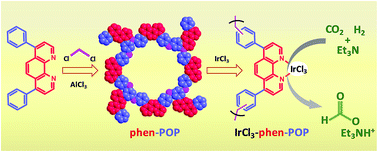
The phenanthroline unit represents one of the most significant ligand frameworks in coordination chemistry. Constructing phenanthroline-based porous organic polymers with nonreactive functional groups in the polymer skeleton is a very challenging goal in the field of heterogeneous catalysis, because it would offer a novel platform for doping molecular catalytic modules and generating single-site, stable, and porous heterogeneous catalytic entities for a wide range of catalytic applications. In this regard, a unique strategy to construct phenanthroline-based porous organic polymer (phen-POP) without other reactive/coordinating functional groups in the polymer skeleton has been designed for the first time, and synthesized via a solvent knitting Friedel–Crafts polymerization method with a high BET surface area of 560 m2 g−1. The well-defined and isolated phen sites of the phen-POP can serve as a platform for immobilizing transition metal catalysts through N–N coordination bonds. The post-synthetic metalation of phen-POP with iridium(III) chloride afforded the most active (initial turnover frequency of 40 000 h−1), simple and selective heterogeneous Ir catalyst for the hydrogenation of CO2 to formate. Thus, the work is significant in constructing novel POP-based heterogeneous catalysts for the development of industrially viable hydrogenation catalysts.