Ryukichi Takagi and Duyen Thi Duong
Org. Biomol. Chem., 2021,19, 8806-8811
DOI:
10.1039/D1OB01672E,
Paper
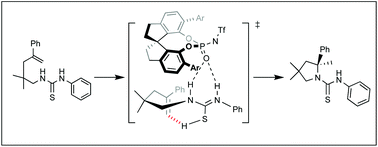
The mechanism of the enantioselective intramolecular hydroamination of alkenyl thiourea catalyzed by chiral binaphthol N-triflylphosphoramide (NPTA) was investigated using density functional theory calculations. This study reveals the details of the hydrogen bonding mode between NPTA and the substrate and indicates the importance of the dual hydrogen binding properties of the thiourea moiety for the reactivity and stereoselectivity of the hydroamination.