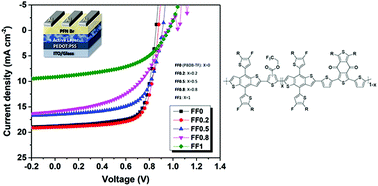
Fluorination of polymer donors is a widely adopted approach to make efficient organic solar cells (OSCs). Herein, four new polymer donors were synthesized by introducing a trifluoromethyl-ester group-substituted thiophene (TEST) unit with different ratios into a typical polymer donor PBDB-TF which resulted in downshifting the highest occupied molecular orbital (HOMO) energy-level to −5.8 eV, thereby improving the open-circuit voltage (Voc) of the corresponding OSCs from 0.86 to 0.96 V. Besides, trifluoromethylation improved the intermolecular aggregation, and consequently, PBDB-TF with a TEST unit content of 20% exhibited superior light absorption, effective charge transport, and favorable morphology, leading to the formation of efficient OSCs with a high Voc of 0.88 V, a large fill factor (FF) of 72.70% and a best power conversion efficiency (PCE) of 12.02%. This work suggests a promising method to design efficient photoactive materials with well-optimized optoelectronic characteristics for high-performance OSCs via facile introduction of an electron-withdrawing group into polymer backbones.