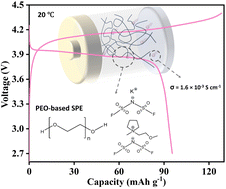
Exploring the next generation of batteries based on sustainable materials is crucial for transitioning to a post-lithium-technology era. Potassium-based technology is one of the candidates that could fulfil the sustainability criteria. The electrolyte plays a crucial role in battery performance, being responsible for ionic transport, cycling performance, working temperature, and safety. Polyethylene oxide (PEO)-based solid polymer electrolytes (SPEs) have been extensively studied. However, one of the drawbacks of SPEs is their poor ionic conductivity at room temperature (RT). Quasi-solid and solvent-free polymer electrolytes are a promising solution to this issue, combining the benefits of a liquid phase and SPEs. This work focuses on the development of cross-linked PEO, fluorinated K salt, and ionic liquid (IL)-based solvent-free SPEs for potassium batteries. The designed cross-linked ternary solvent-free SPEs are thoroughly characterized both physicochemically and electrochemically, achieving ionic conductivities of up to 1.6 × 10−3 S cm−1 at 20 °C. The solvent-free SPEs were tested in K cells at 20 °C, using a Prussian white (PW) cathode as a proof-of-concept. The effects that different fluorinated anions, such as bis(fluoromethanesulfonyl) imide (FSI−) and bis(trifluoromethanesulfonyl) imide (TFSI−), have on the electrochemical performance were analysed by investigating the solid electrolyte interphase (SEI) formed on the K metal surface through electrochemical impedance spectroscopy (EIS), X-ray photoelectron spectroscopy (XPS) and magic-angle spinning solid-state nuclear magnetic resonance spectroscopy (MAS-ssNMR).