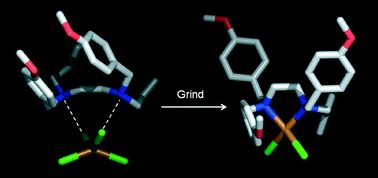
We report the solid state structural transformation of a hydrogen bonded complex salt into a metal complex via dehydrochlorination using mechanochemistry. A crystalline salt containing a large and flexible bidentate dication hydrogen bonded to a tetrachlorometalate (II) anion has been ground in the presence of KOH. Substitution of charge-assisted hydrogen bonding interactions by coordination bonds via chelation has been demonstrated by single-crystal and powder X-ray diffraction analysis. By-product water molecules are included in the structure, playing an important role establishing electrostatic interactions. The irreversibility property of the transformation of the coordination complex into a hydrogen bonded complex salt was determined experimentally. Density functional calculations were used to attempt a rationalisation of the structural results into the mechanochemical reactions.