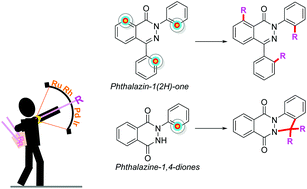
Phthalazinones and their higher congeners are commonly prevalent structural motifs that occur in natural products, bioactive molecules, and pharmaceuticals. In the past few decades, transition-metal-catalyzed reactions have received an overwhelming response from organic chemists as challenging organics and heterocycles could be built with ease. Currently, the synthesis of phthalazinones largely depends on transition-metal catalysis, especially by palladium-catalyzed carbonylation. Further, the dominance of transition-metal catalysts was realized from the phthalazinones viewpoint, as nitrogen and oxygen atoms endowed upon them act as directing groups to facilitate diverse C–H activation/functionalization/annulation reactions. This highlight describes the various synthetic methods used to access phthalazinones and functionalize them by reacting with various coupling partners via chelation assistance strategy involving C(sp2)–H/N–H bond activation in the presence of transition-metal (Rh, Ru, Pd, and Ir) catalysts. The mechanisms of sulfonylation, halogenation, acylmethylation, alkylation, and annulation reactions are discussed.