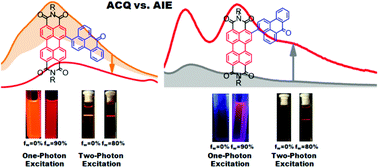
Two positional isomers (regioisomers) through changing the substituted position of perylenetetracarboxylic diimide and benzanthrone moieties were designed and synthesized. These two regioisomers exhibit totally different aggregation behaviors. The meta (bay)-substituted product shows the aggregation-caused quenching feature as the fluorescence quantum yield in tetrahydrofuran–water mixtures is reduced by 80% with an increase in the water fraction of the medium from 0% to 60%. Interestingly, an aggregation-induced emission is observed for the ortho-substituted product as the fluorescence quantum yield is increased by nearly one order of magnitude with an increase in the water fraction. This fluorescence enhancement effect for the ortho-substituted product is because of the restriction of the intramolecular rotation between the perylene core and the benzanthrone substituent in the aggregated state. The ortho-substituted product also exhibits excellent two-photon absorption performance because the ortho-substitution retains the planarity of the perylene core and improves the intramolecular charge-transfer among the molecules. Moreover, the ortho-substituted product shows two-photon excited aggregation-induced emission, which has potential application in organic two-photon excited fluorescence imaging agents.