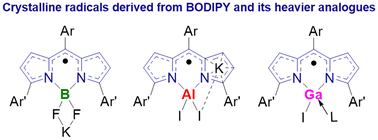
Boron-dipyrromethene (BODIPY) compounds have been the subject of intense scrutiny over the past few decades owing to their unique properties and various applications; however, structurally characterized BODIPY-centered radicals are still limited and isolable radicals derived from heavier analogues of BODIPY have never been described. Herein, we report the facile isolation and structural characterization of the first series of radicals [K(THF)2][TripDPMBF2] (4), [K(η6-toluene)][TripDPMAlI2] (5), and [TripDPMGaI(iPrNHC)] (6) derived from boron-dipyrromethene TripDPMBF2 (1) and its heavier analogues TripDPMAlI2 (2) and TripDPMGaI2 (3). Spectroscopy analysis, X-ray crystallography, and theoretical calculations reveal that one-electron reduction results in increasing delocalization over the dipyrromethene scaffold and the unpaired electrons in 4–6 are mainly delocalized over the dipyrromethene backbone with almost negligible spin density on the group 13 element centers.