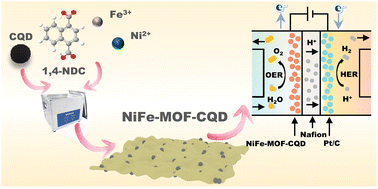
The widespread utilization of noble metal-based catalysts for the oxygen evolution reaction (OER) is hindered by their rarity and substantial expense, posing significant challenges for large-scale applications. Therefore, developing an efficient OER electrocatalyst for proton exchange membrane (PEM) water electrolyzers remains a significant challenge. Here, we present a bottom-up synthesis strategy utilizing ultrasound-assisted exfoliation to design nickel–iron bimetallic organic framework (NiFe-MOF) nanosheets with high electrooxidation activity, in situ induced by carbon quantum dots (CQDs). This approach eliminates the reliance on intricate and inefficient exfoliation techniques, producing NiFe-MOF nanosheets with a regulated thickness of just 10 nm. This enhanced electron transport induced by CQDs plays a pivotal role in improving the OER performance of NiFe-MOF, achieving a current density of 10 mA cm−2 with an overpotential of only 280 mV, with a Tafel slope of 71.98 mV dec−1, lower Rct, and larger ECSA. In situ FTIR spectroscopy suggests that the OER mechanism in NiFe-MOF-CQD mainly follows the adsorbate evolution mechanism. The NiFe-MOF-CQD catalyst demonstrates remarkable durability and resilience during PEM water electrolysis, reaching industrially relevant current densities of 2 A cm−2 at 2 V. This research's results not only promote green and low-carbon development but also inject new vitality into the development of hydrogen energy technologies.