Continuous green biocatalytic processes using ionic liquids and supercritical carbon dioxide
Pedro
Lozano
a,
Teresa de
Diego
a,
Daniel
Carrié
b,
Michel
Vaultier
b and
José L.
Iborra
*a
aDepartamento de Bioquímica y Biología Molecular B e Inmunología. Facultad de Química, Universidad de Murcia, P.O. Box 4021, E-30100, Murcia, España. E-mail: jliborra@um.es; Fax: 34.968.36.41.48; Tel: 34.968.36.73.98
bUniversité de Rennes1, Institut de Chimie, UMR CNRS 6510, Campus de Beaulieu. Av. Général Leclerc, 35042, Rennes, France. E-mail: Michel.Vaultier@univ-rennes1.fr; Fax: 33.2 99 28 69 55; Tel: 33 2 99 28 62 74
First published on 4th March 2002
Abstract
Soluble Candida antarctica lipase B dissolved in ionic liquids showed good synthetic activity, enantioselectivity and operational stability in supercritical carbon dioxide for both butyl butyrate synthesis and the kinetic resolution of 1-phenylethanol processes by transesterification.
The use of enzymes in organic solvents rather than in aqueous media greatly enhances their technological applications.1 However, volatile organic solvents (vos) have a detrimental impact on the environment and human health, and it is necessary to develop green and clean reaction media before scaling-up biocatalytic processes of industrial interest. Neoteric solvents, e.g. ionic liquids and supercritical fluids, seem to be promising alternative because of their physical and chemical characteristics.2 Room temperature ionic liquids (ILs), have been shown to be good solvents for many chemical3–5 and biochemical6 processes, and to have an exceptional ability to stabilize enzymes during continuous operation.7 Supercritical CO2 (scCO2) has been widely described as an excellent solvent for hydrophobic compounds, and is billed as clean technology for a range of industrial extractive processes.8 However, scCO2 has an adverse effect for enzymatic catalysis, because the enzymes exhibit important deactivation phenomena probably due to local pH changes caused by the CO2, or conformational changes produced during the pressurization/depressurisation steps.9
In this paper, we propose a new concept for continuous biphasic biocatalysis, where a homogeneous enzyme solution is immobilized in one liquid phase (working phase) and substrates and products reside largely in a supercritical phase (extractive phase). This concept is illustrated as follows: an aqueous solution of Candida antarctica lipase B (CALB) dissolved in pure 1-ethyl-3-methylimidazolium triflimide ([EMIM][Tf2N]) or 1-butyl-3-methylimidazolium triflimide ([BMIM][Tf2N]), was used as biocatalyst. Both the synthesis of butyl butyrate from vinyl butyrate and butan-1-ol, and the kinetic resolution of rac-1-phenylethanol by transesterification with vinyl propionate were selected as reaction models. In all cases, the synthetic activity of each enzyme-IL system was continuously tested by operation/storage cycles. During the operation period (4 h d−1) an ISCO 220SX high pressure extraction apparatus was used as a continuous packed bed reactor in the selected conditions (Fig. 1). The packed bed reactor was stored (20 h d−1) under dry conditions over P2O5 in a dessicator at room temperature.†
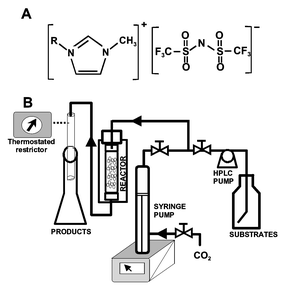 | ||
| Fig. 1 A. Structure of 1-alkyl-3-methylimidazolium triflimide, obtained according to Lozano et al.7B. Experimental set-up. | ||
Table 1 summarizes the activity and stability parameters of the CALB–[BMIM][Tf2N] system for butyl butyrate synthesis under different scCO2 conditions. As can be seen, lipase was able to catalyse the transesterification reaction in all the assayed conditions, the specific activity increasing with temperature; the high selectivity (>95%) observed was due to the low water content (< 4% v/v) used, giving a conversion degree higher than 50%. No activity was observed in the absence of enzyme. The increased selectivity with temperature could be explained by a reduction in water solubility in the ionic liquid fraction. However, the previously mentioned adverse effect of CO2 was also observed because the specific activity of the CALB–[BMIM][Tf2N] system with scCO2 was 10-fold lower than that observed without scCO2.7 The increase in synthetic activity with increasing temperature could also be related to a reduction in scCO2 density. Almeida et al.9 also reported enhanced activity of immobilized CALB (Novozyme) with decreasing scCO2 densities, which they attributed to a reduction in the adverse effects produced by the enzyme solvation with CO2 molecules. Blanchard et al.6 reported that scCO2 can dissolve (up 0.6 mole fraction for [BMIM][PF6]) in the IL phase, and so a decrease in scCO2 density could improve the transfer-rate of substrates to the enzyme microenvironment, thus favouring the enzyme action. Fig. 2 depicts activity loss profiles obtained when the CALB–[BMIM][Tf2N] system was reused in the different supercritical conditions. As can be seen, activity decay was enhanced by the increase in temperature. In all cases, enzyme deactivation followed first-order kinetics. Table 1 shows the half-life time of the enzyme. The protective effect of the IL against thermal and solvent denaturation was clearly observed because the enzyme showed practically the same half-life time with and without scCO2. Additionally, the enzyme exhibited interesting activity and stability levels at 100 °C, a clearly denaturative condition in free scCO2 reaction medium.7‡
| Temp./°C | Pressure/MPa | CO2 density/g mL−1 | Specific activity (U/ mg Enz | Selectivitya (%) | Half-life time (cycles)b |
|---|---|---|---|---|---|
| a Ratio between synthetic and acyl-donor consumption rates. b Cycles: operation (4 h) followed by storage (20 h, 25 °C). | |||||
| 40 | 15.0 | 0.792 | 44 ± 2.1 | 96 ± 0.8 | 284 |
| 50 | 12.5 | 0.618 | 62 ± 3.8 | 98 ± 0.5 | 101 |
| 100 | 15.0 | 0.336 | 71 ± 3.9 | 99 ± 0.9 | 12 |
![Deactivation profiles of C. antarctica lipase B in [BMIM][Tf2N] for continuous butyl butyrate synthesis in scCO2 under different conditions (●, 40 °C and 15.0 MPa; ■, 50 °C and 12.5 MPa; ▲, 100 °C, 15.0 MPa).](/image/article/2002/CC/b200055e/b200055e-f2.gif) | ||
| Fig. 2 Deactivation profiles of C. antarctica lipase B in [BMIM][Tf2N] for continuous butyl butyrate synthesis in scCO2 under different conditions (●, 40 °C and 15.0 MPa; ■, 50 °C and 12.5 MPa; ▲, 100 °C, 15.0 MPa). | ||
The synthetic activity of two CALB-IL ([EMIM][Tf2N] and [BMIM][Tf2N]) systems in scCO2 was also tested for an enantioselective reaction (see Table 2). As can be seen, all the assayed conditions were able to catalyse the racemic resolution of rac-1-phenylethanol. However, the synthetic activity of the enzyme and selectivity of this reaction were lower than in the case of butyl butyrate synthesis. These results were the consequence of a drop in nucleophilic power from butan-1-ol to rac-1-phenyltheanol due to the primary position of the hydroxy group in the former and the secondary position in the latter. Furthermore, the suitability of this reaction system operating in a continuous way was demonstrated by the high enantioselectivity exhibited by the enzyme, because the (S)-1-phenylethyl propionate isomer was never detected, and the (R)-1-phenylethyl propionate isomer was always obtained at a conversion degree higher than 35%. Additionally, the half-life times of these CALB-ILs systems for continuous reuse are shown in Table 2, where it can be seen that they are lower than those observed for butyl butyrate synthesis. Furthermore, it seems that the increase in polarity of IL caused by shortening the alkyl chain on the N3 of the imidazolium ring slightly enhances all the activity and operational stability parameters, probably due to a more adequate microenvironment for the enzyme. Once again, the protective effect of ILs towards enzyme deactivation by temperature and/or CO2 was demonstrated by the observed decrease in activity and stability of CALB, when it was assayed in the absence of IL.
| Ionic liquid | Temp.°C | Specific activity (U/mg Enz.) | Selectivity (%) | Ee (%) | Half-life time (cycles) |
|---|---|---|---|---|---|
| a Lyophilised powder of aqueous enzyme solution adsorbed on Celite. | |||||
| [EMIM][Tf2N] | 50 | 1.6 ± 0.3 | 86.3 ± 1.3 | >99.9 | 24 |
| 100 | 1.1 ± 0.1 | 95.2 ± 1.5 | >99.9 | 16 | |
| [BMIM][Tf2N] | 50 | 1.7 ± 0.2 | 84.8 ± 3.2 | >99.9 | 22 |
| 100 | 0.6 ± 0.1 | 88.1 ± 4.6 | >99.9 | 8 | |
| Nonea | 50 | 0.2 ± 0.02 | 81.5 ± 2.9 | >99.9 | 10 |
In conclusion, this work clearly demonstrates the exciting potential of combining ILs with scCO2 for carrying out synthetic biocatalytic processes in anhydrous conditions. ILs provide the enzyme with an adequate microenvironment, allowing high activity, enantioselectivity and stability, together with their possible continuous reuse. Supercritical CO2 is seen as a suitable solvent for the efficient transport of substrates and easy product recovery. This paper shows how adequately designing the enzyme microenvironment, green and clean enzymatic biotransformations of industrial interest can be carried out.
This work was partially supported by CICYT grant BIO99-0492-C02-01. We thank Ms C. Sáez for technical assistance and Novo España, S.A. for the gift of Novozym 525 solution.
Notes and references
- A. M. Klibanov, Nature, 2001, 409, 241 CrossRef CAS.
- M. Freemantle, Chem. Eng. News, 1998, 76, 32 Search PubMed; M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS; J. F. Brennecke and E. J. Maginn, AIChE J., 2001, 47, 2384 Search PubMed.
- J. G. Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765 RSC.
- L. A. Blanchard, D. Hancu, E. J. Beckman and J. F. Brennecke, Nature, 1999, 399, 28 CrossRef; F. Liu, M. B. Abrams, R. T. Baker and W. Tumas, Chem. Commun., 2001, 433 RSC.
- M. Erbeldinger, A. J. Mesiano and A. J. Russel, Biotechnol. Prog., 2000, 16, 1129 CrossRef CAS; R. Madeira-Lau, F. van Rantwijk, K. R. Seddon and R. A. Sheldon, Org. Lett., 2000, 2, 4189 CrossRef CAS; S. H. Schöfer, N. Kaftzik, P. Wasserscheid and U. Kragl, Chem. Commun., 2001, 425 RSC.
- P. Lozano, T. De Diego, J. P. Guegan, M. Vaultier and J. L. Iborra, Biotechnol. Bioeng., 2001, 75, 563 CrossRef CAS; P. Lozano, T. De Diego, D. Carrié, M. Vaultier and J. L. Iborra, Biotechnol. Lett., 2001, 23, 1529 CrossRef CAS.
- M. Perrut and E. Revenchon, in Proc. 7th Meeting on Supercritical Fluids. Natural products processing, Vol. 2, ed. M. Perrut and E. Revenchon, Vandeouvre, I.N.P.L., 2000 Search PubMed.
- S. Kamat, J. Barera, . J. Beckman and A. J. Russel, Biotechnol. Bioeng., 1995, 40, 158 CrossRef CAS; P. Lozano, A. Avellaneda, R. Pascual and J. L. Iborra, Biotechnol. Lett., 1996, 18, 1345 CrossRef CAS; M. C. Almeida, R. Ruivo, C. Maia, L. Freire, T. Corrêa de Sampaio and S. Barreiros, Enzyme Microb. Technol., 1998, 22, 494 CrossRef CAS.
Footnotes |
| † Typical experimental procedure for butyl butyrate synthesis: the soluble enzyme (0.6 mg/65 μL water) was dissolved in 2 mL [BMIM][Tf2N], and then introduced into the 10 mL cartridge of an ISCO 220SX high pressure extraction apparatus, containing 3 g of glass wool. Reactions were carried out by continuously pumping substrate solution (0.38 M vinyl butyrate and 0.76 M butan-1-ol in hexane) at 0.1 mL min−1, which was mixed with the scCO2 flow of the system. Substrates and products were fully soluble in scCO2, and the reaction mixture was recovered by depressurising through a calibrated heated restrictor (1 mL min−1, 70 °C) every 30 min. Samples were analysed by GC.7 |
| ‡ Typical experimental procedure for kinetic resolution of rac-1-phenylethanol. The soluble enzyme (1.3 mg/150 μL water) was dissolved in 4 mL ionic liquid, and then introduced into the 10 mL cartridge of a ISCO 220SX high pressure extraction apparatus, containing 3 g Celite. The enzyme without ionic liquid was prepared by adsorption of the aqueous enzyme solution (1.3 mg/4 mL) in the same amount of Celite, and then lyophilising. Reactions were carried out by continuously pumping substrate solution (50 mM vinyl propionate and 100 mM rac-1-phenylethanol in hexane) at 0.1 mL min−1, and mixed with the scCO2 flow of the system. Samples, containing 10 mM butyl butyrate as internal standard, were analysed by GC using a capillary Beta DEX-120 column (30 m × 0.25 mm × 0.25 μm, Supelco) and a FID, under the following conditions: carrier gas (He) at 1 MPa (205 ml min−1 total flow); temperature programme: 60 °C, 10 °C min−1, 130 °C; split ratio, 100∶1; detector, 300 °C. Other details are included in the above protocol.One unit of activity was defined as the amount of enzyme that produced one micromole of product per min. |
| This journal is © The Royal Society of Chemistry 2002 |
