Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Targeting cancer cells with folic acid–iminoboronate fluorescent conjugates†
Pedro M. S. D.
Cal
a,
Raquel F. M.
Frade
a,
Vijay
Chudasama
b,
Carlos
Cordeiro
c,
Stephen
Caddick
b and
Pedro M. P.
Gois
*a
aInstituto de Investigação do Medicamento (iMed.ULisboa), Faculdade de Farmácia, Universidade de Lisboa Av. Prof. Gama Pinto, 1649-003 Lisboa, Portugal. E-mail: pedrogois@ff.ul.pt; Fax: +351 217946470; Tel: +351 217946400
bDepartment of Chemistry, University College London, 20 Gordon St, London, UK
cCentro de Química e Bioquímica, Departamento de Química e Bioquímica, Faculdade de Ciências da Universidade de Lisboa, 1749-016 Lisboa, Portugal
First published on 5th November 2013
Abstract
Herein we present the synthesis of fluorescent 2-acetylbenzeneboronic acids that undergo B–N promoted conjugation with lysozyme and N-(2-aminoethyl) folic acid (EDA-FA), generating conjugates that are selectively recognized and internalized by cancer cells that over-express folic acid receptors.
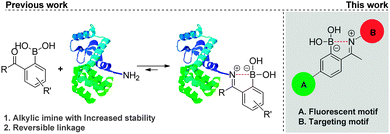
Boronic acids are well-defined neutral, planar trivalent Lewis acids that are readily converted into negatively charged tetravalent borates by forming reversible complexes with Lewis base donors such as amines.1 Appreciation of the unique B–N bond properties triggered a burgeoning interest for this bonding motif.2 B–N bonds have been extensively exploited to construct self-assembled molecularly defined nanostructures, polymeric materials and molecular sensors for the detection of carbohydrates.3 Recently, the isosterism between B–N and C–C bonds was also recognized as a powerful tool to tune the stereo electronic properties of organic molecules.4 In this context, we used the B–N bond to prepare natural product-like structures and heterocycles with inhibitory activity against human neutrophil elastase.5 More recently, we found that this bonding motif could be exploited to efficiently modify proteins at their N-terminal or at lysine's ε-amino group, via the formation of alkylic iminoboronates in aqueous media (Scheme 1).6 Despite their stability, these modifications were also shown to be reversible in the presence of fructose, dopamine and glutathione, as they presumably induce hydrolysis of the iminoboronate by disruption of the B–N bond.2 Based on this, we became very interested in understanding if the B–N bond could also be used to assemble constructs that are able to internalize into cells. If so, this simple, selective and reversible methodology to functionalize proteins may develop into an innovative strategy to design conjugates that may selectively target and deliver cargo to cancer cells.7
 | ||
| Scheme 1 Protein modification via the formation of iminoboronates. Proposed structure of N–B based cancer cell targeting fluorescent conjugates. | ||
In this communication, we address for the first time, the synthesis and evaluation of cancer cell targeting fluorescent conjugates, in which the recognition biomolecule is connected to the fluorescent motif via a B–N linkage (Scheme 1). In our previous study, 2-acetylbenzeneboronic acid afforded the most stable iminoboronate in buffer solutions (pH range 6–9).6 Consequently, this scaffold was selected to integrate the fluorescent motifs as outlined in Scheme 1.
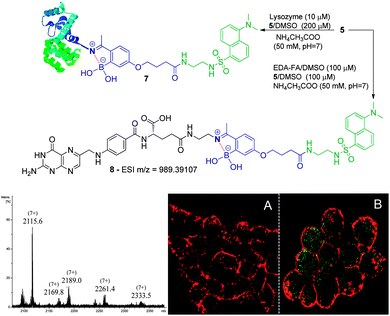
We initiated this study with the synthesis of fluorescent derivatives of 2-acetylbenzeneboronic acid. As shown in Scheme 2, 2,4-dihydroxyacetophenone was alkylated selectively with ethyl 4-bromobutyrate in a 5 g scale to yield compound 2 in 87% isolated yield. After this, phenol 2 was converted to a triflate, which then underwent a Miyaura borylation reaction with bis(pinacolato)diboron to afford 3 in 64% yield. Hydrolysis of the ester with TFA in aqueous media led to acid 4, which became the core structure for connecting fluorescent probes to targeting molecules. Dansyl and nitrobenzofurazan fluorescent motifs were modified with ethylenediamine and ethanolamine, respectively, and each subsequently reacted with 4. Removal of the pinacol group using a boronic acid resin under acidic conditions afforded the final fluorescent compounds 5 and 6 in moderate yields (Scheme 2). Once prepared, compounds 5 and 6 were immediately tested for their ability to conjugate with lysozyme. Gratifyingly, both compounds retained their ability to functionalize the protein, yielding the expected constructs in ammonium acetate buffer (50 mM, pH 7.0) at room temperature as confirmed by ESI-FTICR-MS (Schemes 3 and 4). Encouraged by these results, we next evaluated the possibility of designing a cancer cell targeting conjugate, in which the fluorescent motif is linked to the targeting molecule by a B–N bond.
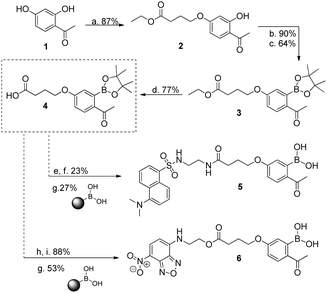 | ||
Scheme 2 (a) Ethyl 4-bromobutyrate, K2CO3, NaI, acetone, reflux, 20 h; (b) PhNTf2, TEA, DMF, rt, 2 h; (c) (Bpin)2, PdCl2(dppf), NaCH3CO2, dioxane, 95 °C, 4 h; (d) TFA, H2O, 90 °C, 3 h; (e) NHS-OH, DCC; THF, rt, 16 h; (f) 2-dansylaminoethylamine, THF, rt, 2 h; (g) CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl (1 M) (9 HCl (1 M) (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 20 h, rt, 20 h; (h) DMAP, DCC; DCM, 0 °C, 0.5 h; (i) 4-ethanolamine-7-nitrobenzofurazan, DCM, rt, 22 h. UV spectra: see ESI† for compounds 5 and 6. 1), 20 h, rt, 20 h; (h) DMAP, DCC; DCM, 0 °C, 0.5 h; (i) 4-ethanolamine-7-nitrobenzofurazan, DCM, rt, 22 h. UV spectra: see ESI† for compounds 5 and 6. | ||
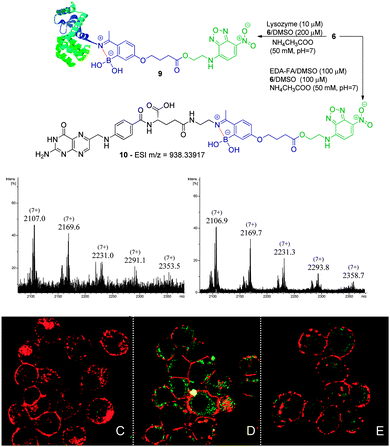 | ||
| Scheme 4 Reaction of lysozyme (10 μM) with 6 (200 μM) in acetate buffer (50 mM, pH 7) at room temperature. Zoom of the (7+) charge state of the ESI-FTICR-MS spectra after 30 min and 2 h of reaction. Reaction of EDA-FA (100 μM) with compound 6 (100 μM) in acetate buffer (50 mM, pH 7) to obtain 10 as confirmed by HRMS. NCI-H460 (C) and NCI-H460 without (D) and with pre-treatment with EDA-FA (E) were incubated with compound 10. The procedure is described in Scheme 3. | ||
Folic acid is essential for cell functioning, however, a considerable percentage of cancer cell lines over-express receptors for this small vitamin due to their fast cell division and growth.8 Consequently, folic acid has been extensively used as a recognition moiety in many conjugates.9 Based on this, the conjugation of 5 and 6 with N-(2-aminoethyl) folic acid (EDA-FA) was evaluated. As expected, upon addition of 100 μM of 5 or 6 in DMSO to EDA-FA in ammonium acetate buffer (50 mM, pH 7.0) at room temperature, their corresponding iminoboronates were formed readily, as characterized by high resolution mass spectrometry – HRMS (Schemes 3 and 4). Following this, the ability of the conjugates to differentiate between cancer and non-cancer cell lines was studied. To do this, conjugate 8 was generated prior to exposure to cells and tested against human non-small lung cancer cells (NCI-H460) and non-cancer human kidney embryonic cells (HEK 293). Interestingly, laser scanning confocal images of HEK 293 and NCI-H460 treated with 8 revealed that only the cancer cell line that over-expressed the FA receptor up took fluorescence, which suggests a selective folate-receptor mediated internalization of the B–N construct 8.
To further elucidate this mechanism, the cancer cell line NCI-H460 was treated with 6 without EDA-FA. In agreement with a folate-receptor mediated internalization mechanism, without the recognition moiety, no fluorescence was detected inside the cells. In contrast, when the same compound 6 was allowed to form a conjugate with EDA-FA, the previously generated B–N construct 10 smoothly underwent internalization as shown in Scheme 4. Finally, the NCI-H460 cells were treated with EDA-FA prior to the addition of the conjugate 10. In this case, the internalization of the conjugate 10 was considerably impaired due to the initial exposure to folic acid that reduced the cell's need for this vitamin. These results clearly stress the importance of the folate moiety to mediate the internalization process.
Finally, to determine the contribution of the B–N bond to the formation and stability of these conjugates, the fluorescent compound 14, featuring no boronic acid, was prepared via alkylation of 4-hydroxyacetophenone with ethyl 4-bromobutyrate, followed by hydrolysis and DMAP catalysed esterification with 4-ethanolamine-7-nitrobenzofurazan (NBDNH(CH2)2OH). Once prepared, 14 was solubilised in DMSO and combined with EDA-FA in an ammonium acetate buffer solution (50 mM, pH 7.0) at room temperature to form conjugate 16, as confirmed by HMRS. A mixture containing the conjugate was then used to treat the NCI-H460 cancer cells. This resulted in no fluorescence being detected inside the cells, presumably due to the poor stability of conjugate 16 under these conditions. Therefore, we studied the ability of compound 14 to modify lysozyme. As depicted in Scheme 5, the compound lacking the boronic acid functionality hardly formed the expected constructs with the protein after 30 min in ammonium acetate buffer (50 mM, pH 7.0). Even the ones that did form seemed to hydrolyse in only 2 h under these reaction conditions. In stark contrast, under the same conditions, boronated compound 6 readily afforded the constructs with lysozyme within 30 min and the modifications were persistent after 2 h in ammonium acetate buffer (50 mM, pH 7.0) at room temperature (Scheme 4). These results clearly highlight the contribution of boronic acid to imine stabilization and internalization of the conjugates.
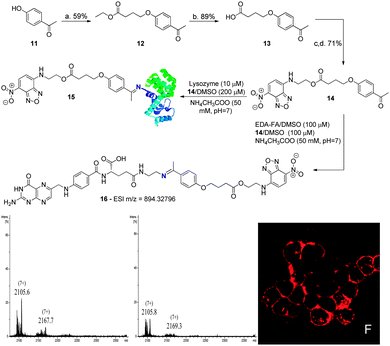 | ||
| Scheme 5 (a) Ethyl 4-bromobutyrate, K2CO3, NaI, acetone, reflux, 20 h; (b) TFA, H2O, 90 °C, 3 h; (c) DMAP, EDC; DCM, 0 °C, 1 h; (d). NBDNH(CH2)2OH, DCM, rt, 5 h. UV spectrum: see ESI† for compound 14. Reaction of lysozyme (10 μM) with 14 (200 μM) in acetate buffer (50 mM, pH 7) at room temperature. Zoom of the (7+) charge state of the ESI-FTICR-MS spectra after 30 min and 2 h of reaction. Image F shows NCI-H460 treated with compound 16. Incubation and imaging conditions are likewise described in Scheme 3. | ||
In this communication we show for the first time that the B–N dative bond may be used to synthesize conjugates that selectively target cancer cells. Fluorescent 2-acetylbenzeneboronic acid derivatives were successfully prepared and conjugated via a B–N linkage with lysozyme and N-(2-aminoethyl) folic acid, generating conjugates that were selectively recognized and internalized by NCI-H460 cancer cells, which over-express folic acid receptors. The ability of these iminoboronates to undergo a receptor mediated internalization, and their efficiency to promote the selective and reversible functionalization of proteins, highlights that these constructs have a promising future in the design of conjugates that selectively target and deliver cargo to cancer cells.
Fundação para a Ciência e Tecnologia (PTDC/QUI-QUI/118315/2010; Pest-OE/SAU/UI4013/2011; SFRH/BD/72376/2010: REDE/1501/REM/2005) is acknowledged for financial support.
Notes and references
- Boronic Acids – Preparation, Applications in Organic Synthesis and Medicine, ed. D. G. Hall, Wiley-VCH, Weinheim, Germany, 2011, ISBN 9783527325986 Search PubMed.
- (a) C. T. Hoang, I. Prokes, G. J. Clarkson, M. J. Rowland, J. H. R. Tucker, M. Shipman and T. R. Walsh, Chem. Commun., 2013, 49, 2509 RSC; (b) L. Zhu, S. H. Shabbir, M. Gray, V. M. Lynch, S. Sorey and E. V. Anslyn, J. Am. Chem. Soc., 2006, 128, 1222 CrossRef CAS PubMed; (c) B. E. Collins, S. Sorey, A. E. Horgrove, S. H. Shabbir, V. M. Lynch and E. V. Anslyn, J. Org. Chem., 2009, 74, 4055 CrossRef CAS PubMed; (d) J. D. Larkin, J. S. Fossey, T. D. James, B. R. Brooks and C. W. Bock, J. Phys. Chem. A, 2010, 114, 12531 CrossRef CAS PubMed.
- Selected examples: (a) N. Fujita, S. Shinkai and T. D. James, Chem.–Asian J., 2008, 3, 1076 CrossRef CAS PubMed; (b) R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2011, 47, 1124 RSC; (c) N. Christinat, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2008, 47, 1848 CrossRef CAS PubMed; (d) M. Mastalerz, Angew. Chem., Int. Ed., 2008, 47, 445 CrossRef CAS PubMed; (e) S. D. Bull, M. G. Davison, J. M. H. V. D. Elsen, J. S. Fossey, A. T. A. Jenkins, Y.-B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Acc. Chem. Res., 2013, 46, 312 CrossRef CAS PubMed.
- Selected examples: (a) M. J. D. Bosdet and W. E. Piers, Can. J. Chem., 2008, 86, 8 Search PubMed; (b) Z. Liu and T. B. Marder, Angew. Chem., Int. Ed., 2008, 47, 242 CrossRef CAS PubMed; (c) L. Liu, A. J. V. Marwitz, B. W. Matthews and S.-Y. Liu, Angew. Chem., Int. Ed., 2009, 48, 6817 CrossRef CAS PubMed; (d) P. G. Campbell, A. J. V. Marwitz and S.-Y. Liu, Angew. Chem., Int. Ed., 2012, 51, 6074 CrossRef CAS PubMed; (e) E. R. Abbey and S.-Y. Liu, Org. Biomol. Chem., 2013, 11, 2060 RSC.
- (a) F. Montalbano, N. R. Candeias, L. F. Veiros, V. André, M. T. Duarte, M. R. Bronze, R. Moreira and P. M. P. Gois, Org. Lett., 2012, 14, 988 CrossRef CAS PubMed; (b) F. Montalbano, P. M. S. D. Cal, M. A. B. R. Carvalho, L. M. Gonçalves, S. D. Lucas, R. C. Guedes, L. F. Veiros, R. Moreira and P. M. P. Gois, Org. Biomol. Chem., 2013, 11, 4465 RSC.
- P. M. S. D. Cal, J. B. Vicente, E. Pires, A. V. Coelho, L. F. Veiros, C. Cordeiro and P. M. P. Gois, J. Am. Chem. Soc., 2012, 134, 10299 CrossRef CAS PubMed.
- (a) M. E. B. Smith, F. F. Schumacher, C. P. Ryan, L. M. Tedadi, D. Papaioannou, G. Waksman, S. Caddick and J. R. Baker, J. Am. Chem. Soc., 2010, 132, 1960 CrossRef CAS PubMed; (b) R. L. Lundblad, in Chemical Reagents for Protein Modification, CRC Press, Boca Raton, London, New York, Washington, DC, 2005 Search PubMed; (c) D. Schrama, R. A. Reisfeld and J. C. Becker, Nat. Rev. Drug Discovery, 2006, 5, 147 CrossRef CAS PubMed.
- (a) L. E. Kelemen, Int. J. Cancer, 2006, 119, 243 CrossRef CAS PubMed; (b) J. Sudimack and R. J. Lee, Adv. Drug Delivery Rev., 2000, 41, 147 CrossRef CAS.
- (a) P. S. Low and S. A. Kularatne, Curr. Opin. Chem. Biol., 2009, 13, 256 CrossRef CAS PubMed; (b) Z. Yang, J. H. Lee, H. M. Jeon, J. H. Han, N. Park, Y. He, H. Lee, K. S. Hong, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2013, 135, 11657 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, full DFT reference list, atomic coordinates for the optimized species, and spectral data for all compounds. See DOI: 10.1039/c3cc47534d |
| This journal is © The Royal Society of Chemistry 2014 |