Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Review of the molluscicide metaldehyde in the environment
G. D.
Castle
a,
G. A.
Mills
b,
A.
Gravell
c,
L.
Jones
d,
I.
Townsend
d,
D. G.
Cameron
e and
G. R.
Fones
 *a
*a
aSchool of Earth and Environmental Sciences, University of Portsmouth, Burnaby Building, Burnaby Road, Portsmouth, PO1 3QL, UK. E-mail: gary.fones@port.ac.uk
bSchool of Pharmacy and Biomedical Sciences, University of Portsmouth, St Michael's Building, White Swan Road, Portsmouth, PO1 2DT, UK
cNatural Resources Wales, NRW Analytical Services at Swansea University, Swansea University, Faraday Building, Singleton Campus, Swansea, SA2 8PP, UK
dSouth West Water Ltd., Bridge Road, Countess Wear, Exeter, EX27AA, UK
eDe Sangosse Ltd., Hillside Mill, Swaffham Bulbeck, Cambridgeshire, CB25 0LU, UK
First published on 12th April 2017
Abstract
Metaldehyde is the active ingredient in most slug pellets used to protect crops. This molluscicide is considered an emerging pollutant and is frequently detected in surface water bodies above the EU statutory drinking water limit of 0.1 μg L−1 for a pesticide. This presents a challenge for providers of drinking water. Understanding the sources, transport and environmental fate of this compound is therefore important. This critical review discusses these aspects including monitoring and analytical techniques used for the detection of metaldehyde in environmental matrices. Novel techniques used for the removal of metaldehyde from drinking water are presented together with potential catchment management strategies and initiatives useful for the mitigation of this molluscicide in the environment.
Water impactMetaldehyde is a potent molluscicide and is applied to land as baited-pellets. Due to its physicochemical properties, metaldehyde runs off readily from fields and enters surface water bodies where often it can be present at drinking water capitation sites at elevated concentrations. Understanding the occurrence, fate and mitigation of this pesticide in the aquatic environment is now a major concern. |
Introduction
Metaldehyde is the active ingredient, typically at 1.5, 3.0 or 4.0% by weight, in 80% of slug pellets used globally. It has been used as a molluscicide since the early 1940's. Metaldehyde is manufactured by Lonza as Meta® Metaldehyde. This active ingredient is then formulated by a number of suppliers (e.g. Certis or De Sangosse)1–3 into granular bait pellets available under a range of trade names (e.g. Cekumeta®, Deadline®, Hardy®, Metarex® and Metason®).4 In Europe, metaldehyde slug pellets are manufactured/formulated at three plants in the UK, four in France, two in Italy and Germany, one in Spain and Switzerland. Metaldehyde is classified as a ‘moderately hazardous' pesticide (class II) by the World Health Organization5 and a ‘restricted use pesticide’ by the United States Environmental Protection Agency.6Metaldehyde is not phytotoxic and is used by arable farmers to protect crops such as cereals, oilseed rape and potatoes. The effective control of molluscs is a serious concern, as without the use of such a pesticide, there would be high losses of valuable crop products, together with associated economic consequences.7 In the UK it has been estimated that a lack of effective slug control products could cost up to £100 million a year in lost production.7
Approximately 1640 t of metaldehyde were used in Great Britain between 2008 and 2014.2 Metaldehyde is generally applied to land in the autumn and winter months when molluscs thrive in the wet weather conditions.8
Due to the physico-chemical properties of metaldehyde, it is highly mobile in soil, and hence once applied, it can run-off under wet conditions into field drains, gullies and surface waters. There is no designated substance specific concentration limit set for metaldehyde in surface or drinking waters. Concentrations of metaldehyde in water bodies in the UK have frequently exceeded the European Union's regulatory drinking water standard for an individual pesticide (0.1 μg L−1 and 0.5 μg L−1 for total pesticides present) during periods when slug pellets are applied.9 This has become a major issue for water companies in the UK and elsewhere when such surface waters are used subsequently as potable supplies. Furthermore, the high polarity of metaldehyde makes it difficult to remove using conventional (e.g. granular activated carbon (GAC)) drinking water treatment processes.10 Consequently, metaldehyde is now considered an emerging pollutant of concern11 and there is interest in understanding its source and fate in the environment. This is evidenced by the increase in the number of scientific publications related to metaldehyde, with sixty papers published since 2013 (Fig. 1).
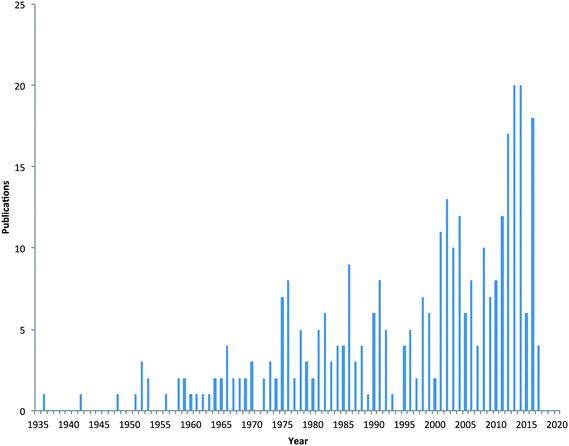 | ||
| Fig. 1 Number of scientific papers published on metaldehyde from 1935 to early 2017 (source of data Scopus). | ||
This review assimilates the current knowledge on metaldehyde with particular emphasis on the effects of this pollutant in the aquatic environment. It addresses the properties, fate and concentrations of metaldehyde in water bodies, monitoring and analytical techniques, and methods for its removal from potable waters. Finally, future strategies for mitigating the effects of metaldehyde and areas for future research are discussed.
Properties and toxicity of metaldehyde
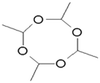
Metaldehyde is a solid, synthetic, non-chiral aldehyde with the chemical formula of C8H16O4 and was first discovered by von Liebig in 1835.3 Metaldehyde is a dry alcohol, obtained via the process of treating acetaldehyde with an acid catalyst, such as hydrogen bromide. It is a cyclic tetramer of acetaldehyde and is classified as a highly polar organic compound. It degrades to acetaldehyde, and thereafter into water and carbon dioxide. Metaldehyde is soluble and relatively stable in water (Table 1).| Structure | |
|---|---|
| Molar mass1 | 176.21 g mol−1 |
| CAS number1 | 108-62-3 |
| IUPAC name1 | 2,4,6,8-Tetramethyl-1,3,5,7-tetraoxocane |
| Boiling point2 | 112 to 115 °C |
| Water solubility1 | 0.188 g L−1 at 20 °C |
| Vapour pressure3 | 0.66 mmHg at 25 °C |
| Flash point2 | 36 to 40 °C |
| Density1 | 1.27 g cm−3 |
| log octanol/water partition coefficient (Kow)1 | 0.12 at 20 °C |
| log organic-carbon/water partition coefficient (Koc)4 | 0.18–0.37 |
The mode of action of metaldehyde is as follows – once ingested it is rapidly hydrolysed to acetaldehyde, this causes the mollusc to produce excess mucus, dehydrate and ultimately die.15 Metaldehyde is a poison to most organisms that ingest it, either directly or from consuming poisoned prey. In mammals, metaldehyde is an irritant to the skin, eyes, mucous membranes, throat and respiratory tract.16 Acute exposure generally results in excitation or depression of the central nervous system and is associated with symptoms such as an inability to stand, changes in respiratory rate, excessive sweating, salivation, blindness, seizures or death.17 Following exposure, cellular changes in the liver and kidneys have also been reported.17 These symptoms of poisoning are thought to be caused by the metaldehyde molecule itself rather than its break down product, acetaldehyde.18 The oral LD50 of metaldehyde is species dependent, as discussed below.
Cases of metaldehyde poisoning in humans are not common.18,19 Although metaldehyde has a mild toxicity, in rare cases the clinical course of metaldehyde poisoning can be rapidly deteriorating health leading to death.20 Ellenhorn and Barceloux21 reported that for minor effects to be observed several mg kg−1 of compound must be ingested and serious impacts are observed above 100 mg kg−1, with death likely above 400 mg kg−1 concentrations. Human deaths arising from metaldehyde poisoning in England and Wales have been documented by Thompson et al.22
Metaldehyde poisoning is commoner in other mammals23–27 and is the second most common cause of poisoning in canines after chocolate.28 This is thought to be due to the formulation of slug pellets which contain baits to attract molluscs; unfortunately these baits also have a tendency to attract domestic pets.29,30 In companion animals, ingestion of metaldehyde causes seizures and convulsions in combination with a fever, which has given rise to the name ‘shake and bake syndrome’.31 Toxicity of metaldehyde in cats and dogs has been reported32–34 with LD50 estimates of 207 and 500 mg kg−1 respectively.17 Poisoning by metaldehyde can occur in cattle35,36 and horses37,38 with LD50 values of 400–500 and 300–400 mg kg−1 respectively.17
The effects of metaldehyde have also been studied in other animal species. As earthworms share the same habitat as molluscs they are often the most exposed soil dwelling organism during application of metaldehyde. Studies by Edwards et al.39 and Langan et al.40 showed that exposure of Lumbricus terrestris L. in microcosms to high concentrations of metaldehyde had no effect on mortality, growth or feeding rate. Hallett et al.41 investigated in the laboratory the impact of metaldehyde on embryo development in the pond snail Lymnaea stagnalis. Chronic effects from metaldehyde exposure (high mg L−1 range) on embryo development were observed, however, the authors' suggest that at typical environmental exposures (μg L−1) there is a low risk to the early developmental stages of this gastropod mollusc. Furthermore, recent work by Moreau et al.42 found that short term in vivo exposure to metaldehyde (0.1 μg L−1 in sea water) had a negative effect on the immune system of Pacific oysters, thereby increasing their susceptibility to various infectious agents present in the aquatic environment. Poisoning by metaldehyde has been reported in some bird species.43
Environmental behaviour and fate of metaldehyde
In the UK, the agricultural use of metaldehyde as a molluscicide has risen sharply since 1990, peaking over 2008 to 2009.2 This may, in part, be as a consequence of the banning of stubble burning in 1993.44 Stubble burning was traditionally used to kill snails and slugs and other pests and weeds once crops were harvested. Additionally, other factors such as changes to the crops being grown e.g. an increase in coverage of oil seed rape and changes in weather patterns have also contributed to increased usage. Typically metaldehyde containing pellets are applied to land using a spinning disc applicator. Application is dependent on the percentage of molluscicide present in the pellet. Guidelines for the loading per hectare in the UK are available from the Metaldehyde Stewardship Group (a consortium comprising Certis Europe, Chiltern Farm Chemicals Ltd., De Sangosse Ltd., Doff Portland Ltd., Frunol Delicia GmbH, Lonza AG, Makhteshim-Agan (UK) Ltd., SBM Development).45 The maximum application rate is set as 210 g metaldehyde as active substance (a.s.) per ha. However, for the additional protection of water courses, a reduced rate of 160 g a.s. per ha is recommended with a maximum of 700 g metaldehyde applied per ha per year. Alternatively, if applying 4%, 3% or 1.5% metaldehyde pellets to crops, the MSG Guidelines suggest the spread should be 5, 7 and 7.5 kg ha−1 respectively and 4, 5 and 7.5 kg ha−1 near water courses.45,46When applied to land, metaldehyde degrades in soil to acetaldehyde and then CO2 and water, with a reported half-life varying between 3.17–223 days depending on environmental conditions.9,47 Due to its low organic-carbon/water partition coefficient (Koc) (Table 1) metaldehyde moves in soil easily and hence is found frequently in the aquatic environment. Its movement in soil was studied by Zhang et al.,48 they showed that following spiking with metaldehyde (1 mg kg−1), adsorption to soil was highest 4 days after application reducing slowly thereafter. After 21 days the concentration of metaldehyde was reduced significantly ∼0.04 mg kg−1. In addition to movement through soils and subsequently entering field drainage systems, metaldehyde can also enter water bodies directly by inadvertent spreading of pellets into watercourses. This includes point sources such as spills onto hard surfaces that are eventually washed into drains and surface run-off from fields following heavy periods of rainfall. Research by the MSG has shown that metaldehyde pellets are readily washed into surface waters after storm events.9 Once in water, metaldehyde becomes more persistent as degradation is slowed, hence the compound has some semi-persistence in the aquatic environment.3
Plants can take up metaldehyde, although a study showed in cabbages (exposed to concentrations of 17.4–68.6 mg kg−1) it was rapidly degraded to undetectable concentrations within 12 days.4 Simms et al.49 investigated factors leading to uptake of metaldehyde into oilseed rape and wheat. Results showed that once seedlings had emerged metaldehyde was taken up by roots and transported within the plant tissue.
Environmental concentrations of metaldehyde
Recent improvements in analytical techniques have allowed metaldehyde to be detected readily in river catchments.50 Metaldehyde was first detected in surface water in the UK in 2007 and subsequently has been identified intermittently in some rivers (particularly those that run through intensively farmed arable land) and reservoirs at concentrations that exceeded the EU Drinking Water Directive value of 0.1 μg L−1 for any pesticide51,52 (Table 2). A major concern is where such surface water bodies are used as supplies for potable water. In order to mitigate this problem of deteriorating water quality, field level scale pesticide risk maps (1 km grid resolution as digital image files) have been produced for the UK. These are used to identify areas at a high risk.53 This information has been used to identify and establish Drinking Water Protected Areas (DrWPAs). In England (2014), over one hundred DrWPAs were identified as being ‘at risk’ due to pesticide contamination exceeding the EU Drinking Water Directive limit in raw water.54 Metaldehyde is the most significant active substance, causing a compliance risk in 102 (21%) of these DrWPAs. At these sites deemed to be ‘at risk’ of being polluted, safeguard zones (together with associated action plans) in the upstream parts of the river catchment are then established. There are 118 safeguard zones in place in England due to pesticide pressures. Of these, there are 96 in place for surface water and 22 for groundwater.55 Similar action plans are in place for other parts of the UK. Although the agricultural usage of metaldehyde in continental Europe is generally higher than in the UK, most of these countries rely on the use of ground rather than surface water for their potable supplies.| Water company | Total number of tests sites | Tests exceeding limit | Supply points failing DWD limit |
|---|---|---|---|
| Affinity Water | 252 | 4 | 2 |
| Albion Water | 4 | 0 | 0 |
| Anglian Water (leaving bulk supply) | 268 | 32 | 10 |
| Anglian Water (at consumer taps) | 8 | 1 | 1 |
| Bournemouth Water | 99 | 0 | 0 |
| Bristol Water | 210 | 0 | 0 |
| Cambridge Water (leaving bulk supply) | 32 | 0 | 0 |
| Cambridge Water (at consumer taps) | 1 | 0 | 0 |
| Dee Valley Water | 48 | 0 | 0 |
| Dŵr Cymru Welsh Water (leaving bulk supply) | 8 | 0 | 0 |
| Dŵr Cymru Welsh Water (at consumer taps) | 73 | 0 | 0 |
| Essex and Suffolk Water (leaving bulk supply) | 76 | 0 | 0 |
| Essex and Suffolk Water (at consumer taps) | 134 | 0 | 0 |
| Hartlepool Water | 12 | 0 | 0 |
| Independent Water Networks Ltd. | 20 | 0 | 0 |
| Northumbrian Water | 282 | 1 | 1 |
| Portsmouth Water | 118 | 0 | 0 |
| Severn Trent Water (leaving bulk supply) | 545 | 2 | 1 |
| Severn Trent Water (at consumer taps) | 130 | 1 | 1 |
| South East Water (leaving bulk supply) | 303 | 2 | 2 |
| South East Water (at consumer taps) | 124 | 3 | 3 |
| South Staffordshire Water | 124 | 0 | 0 |
| Southern Water | 593 | 5 | 4 |
| SSE Water (formerly Scottish & Southern Energy) | 80 | 1 | 1 |
| Sutton and East Surrey Water | 23 | 0 | 0 |
| Thames Water (leaving bulk supply) | 706 | 2 | 1 |
| Thames Water (at consumer taps) | 9 | 2 | 1 |
| United Utilities Water | 56 | 0 | 0 |
| Wessex Water | 36 | 0 | 0 |
| Yorkshire Water (leaving bulk supply) | 136 | 2 | 1 |
| Yorkshire Water (at consumer taps) | 420 | 7 | 7 |
These issues led to pressure from regulators and water companies, on the manufacturers of such molluscicides, to identify ways of reducing their overall environmental impact. The industry-led MSG, based in the UK, started a campaign called ‘Get Pelletwise’ (http://www.getpelletwise.co.uk/). This educational initiative was directed at large-scale users of slug pellets and aimed to try and prevent or minimise the movement of metaldehyde to water sources. The ‘Get Pelletwise’ campaign developed the best practice guidelines when using metaldehyde, covering issues including dosage rates per hectare, maximum application rates, no application within 6 m of a watercourse, no application when heavy rain or winds were forecast and no application if there was flow in field drains.45 Other factors relating to field topography (e.g. slope, soil type and drainage) affecting the movement of metaldehyde through the soil profile also need to be considered. MSG in association with the Environment Agency (England and Wales) also developed an information tool for farmers called ‘What's in your backyard?’ (http://apps.environment-agency.gov.uk/wiyby). This is a geographical mapping application showing areas in the UK at high-risk of potential contamination by metaldehyde as well as other agricultural pollutants. It was hoped by use of this tool a reduction in overall usage of metaldehyde could be achieved.45
Despite these various campaigns, metaldehyde continues to be found in surface and potable waters. For example in the River Thames, concentrations of metaldehyde as high as 8.0 μg L−1 were found during late August–October, 2012; months often associated with heavy rainfall.56 The maximum concentration of metaldehyde found in treated drinking water was ∼1.03 μg L−1; detected in the UK in November 2007 and December 2008.57 However, this concentration does not present an immediate human health risk as is below the acceptable daily intake (0.02 mg metaldehyde per kg body weight).57 More recently concentrations of metaldehyde found in the environment have tended to be lower as the introduction of the MSG guidelines has reduced its overall input to water. However, metaldehyde can still be detected regularly above 0.1 μg L−1 (Table 2).
A study undertaken by Kay & Grayson9 assessed UK water quality data over a two and a half year period (2008–2011) for concentrations of metaldehyde in surface and potable water. Importantly their study showed that pollution by metaldehyde was not correlated with soil type, slope or crops grown. Furthermore, the measured concentration of metaldehyde in water sampled downstream of the water treatment works showed no significant difference with the concentrations found entering the works, thus highlighting the ineffectiveness of conventional techniques for the removal of metaldehyde from water. Due to its semi-persistence in the aquatic environment, metaldehyde remains a concern with respect to drinking water quality, adding to the pressure on water companies and environmental agencies to monitor this pesticide effectively in river catchments and at point sources.
Analytical techniques for measuring metaldehyde
With the regular detection of metaldehyde in surface and potable waters there has been a focused effort to develop suitable monitoring and sensitive, quantitative analytical techniques for measuring this pesticide at low concentrations in a range of environmental matrices.52,59 Typically, instrumental techniques, such as gas chromatography (GC) coupled with mass spectrometry (MS) and liquid chromatography (LC) coupled with a triple quadrupole mass spectrometry (MS/MS) are used. These are often used in conjunction with isolation and pre-concentration techniques such as solid-phase extraction (SPE). Some newer methods permit low limits of quantification (<1.0 ng L−1) for metaldehyde.4,60Gas chromatography methods
Early work used GC to detect metaldehyde in a range of matrices after its conversion to acetaldehyde61 or by using derivatisation techniques.62,63 More recently, GC/MS has proved popular for the analysis of metaldehyde, being a robust and relatively simple methodology.64,65 Most workers use similar GC conditions with a non-polar column such as DB5-MS.66 Typically, metaldehyde has a short retention time (typically ≲8 min) on this stationary phase. High oven temperatures of up to 300 °C are needed to elute all the analytes that can be present, as dimers of metaldehyde and acetaldehyde can be formed in analysis and these bond more strongly to the non-polar stationary phase.66Table 3 summarises some GC methods that have been used to measure metaldehyde in water. Highly specific, gas chromatography triple quadrupole mass spectrometry (GC/MS/MS) instruments have been used to analyse metaldehyde. ALS Environmental compared the results obtained from GC/MS and GC/MS/MS techniques for the analysis of metaldehyde in water. Generally measurements obtained using the two approaches were in good agreement, however, GC/MS/MS showed a higher degree of compound specificity.67| GC column | Carrier gas | Injection temp | Injection volume | Purge flow | Purge time | Retention time | Limit of detection |
|---|---|---|---|---|---|---|---|
| HP-5MS, 30 m × 0.25 mm diameter, 0.25 μm film thickness1 | Helium, 30 mL s−1 | 300 °C | 1 μL | 50 mL min−1 | 1 min | 6.3 min | 0.005 μg L−1 |
| DB5-MS, 30 m × 0.25 mm diameter, 0.25 μm film thickness1 | Helium, 2 mL min−1 | 250 °C | 2 μL | 50 mL min−1 | 2 min | 6 min | 0.003 μg L−1 |
| DB5-MS, 30 m × 0.25 mm diameter, 0.25 μm film thickness1 | Helium, 1.5 mL min−1 | 250 °C | 1 μL | 50 mL min−1 | 2 min | 6 min | 0.006 μg L−1 |
| HP-5MS, 30 m × 0.25 mm diameter, 0.25 μm film thickness1 | Helium, 1 mL min−1 | 63 °C | 1 μL | _ | _ | 7 min | 0.004 μg L−1 |
| DB5-MS, 30 m × 0.25 mm diameter, 0.25 μm film thickness1 | Helium, 2 mL min−1 | 270 °C | 2 μL | 50 mL min−1 | 2 min | 6.5 min | 0.004 μg L−1 |
| SPB™-5, 30 m × 0.53 mm diameter, 0.5 μm film thickness2 | Helium 5 mL min−1 | 100 °C | _ | 30 mL min−1 | _ | _ | _ |
| HP-5MS, 30 m × 0.25 mm diameter, 0.25 μm film thickness3 | 1 mL min−1 | 35 °C | 1 μL | _ | _ | _ | _ |
As water cannot be injected directly into a GC column, aqueous samples require pre-concentration prior to instrumental analysis. This adds extra cost and is time consuming. However, pre-treatment steps allow lower limits of detection to be reached since 250–1000 mL water samples can be extracted into a few mL of elution solvent. Often techniques such as liquid/liquid extraction or SPE with different sorbents are used for this purpose; some examples for the pre-concentration of metaldehyde in water are given in Table 4.66
| SPE cartridge | Conditioning solvent | Sample volume | Elution solvent | Evaporation step | Internal standard |
|---|---|---|---|---|---|
| BakerBond™ SDB1 200 mg (3 mL) | 10 mL methanol | 250 mL | 2 × 1 mL dichloromethane | To 0.5 mL | 5 μL 1,4-dichlorobenzene-d4 |
| Strata-X 200 mg (3 mL) | 2 × 2 mL methanol | 250 mL | 0.4 mL ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone 50/50% (v/v), then add 1 mL iso-octane acetone 50/50% (v/v), then add 1 mL iso-octane |
N/A | 50 μL 1,4-dichlorobenzene-d4 |
| Isolute® ENV+ 200 mg (3 mL) | 3 mL methanol + 3 mL buffer solution (5.420 g potassium dihydrogen phosphate + 7.772 g disodium hydrogen phosphate in 2 L water) | 1 L + 25 mL buffer solution | 2 × 1 mL ethyl acetate | N/A | 100 μL 1,4-dichlorobenzene |
| Strata-X 200 mg (3 mL) | 3 mL 2,2,4-trimethylpentane + 3 mL acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 50/50% (v/v) ethyl acetate 50/50% (v/v) |
100 mL | 3 mL acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate 50/50% (v/v) (soak for 1 min before elution), then add 3 mL 2,2,4 trimethylpentane (allow to soak for 1 min before elution) ethyl acetate 50/50% (v/v) (soak for 1 min before elution), then add 3 mL 2,2,4 trimethylpentane (allow to soak for 1 min before elution) |
Post internal standard to 200 μL | 50 μL 1,3,5-trichlorobenzene-d3 |
| Strata-X 200 mg (6 mL) | 5 mL dichloromethane | 500 mL | 2 × 2 mL dichloromethane each left for 5 min before eluted | Post internal standard to 0.5 mL using nitrogen | 50 μL 1,4-dichlorobenzene-d4 |
Liquid chromatography methods
Use of LC/MS methods can overcome many of the drawbacks associated with GC and LC/MS is now the method of choice for most end-users. Although LC/MS instruments are more expensive to purchase initially, they can measure simultaneously a wide range (>100 compounds) of polar pesticides in water and other environmental matrices at low limits of detection.70Table 5 shows some of the LC/MS methods used to measure metaldehyde in environmental matrices. Most methods use triple quadrupole (LC/MS/MS) detection systems or more recently time of flight mass spectrometry71 to allow for greater analyte specificity. Generally the separation methods rely on reverse-phase interactions using a non-polar stationary phase in combination with a polar mobile phase e.g. aqueous mixtures of acetonitrile or methanol.72 Usually the mobile phase contains a buffer such as ammonium acetate/formic acid,73 but this can lead to the formation of multiple adduct ions (e.g. [M + H]+, [M + Na]+, [M + NH4]+, [M + K]+), thereby decreasing analytical sensitivity. Schumacher et al.74 overcame this problem by using an alkyl-ammonium buffer (methylamine) as the mobile phase additive, thus suppressing the formation of unwanted alkali adducts and dimers of metaldehyde leading to an improved detection limit. Modern instruments are now highly sensitive (∼low ng L−1 detection limits) and enable metaldehyde to be quantified by direct injection of environmental water samples.| Matrix | LC conditions | Column flow rate | Detector | LoD | LoQ | Reference |
|---|---|---|---|---|---|---|
| Water | Agilent1260 Infinity system. Atlantis T3 C18 column. Mobile phase: water, methylamine, acetic acid, acetonitrile. | 0.3 mL min−1 | Agilent 6460 MS/MS | 2.0 ng L−1(tap) 9.0 ng L−1 (river) |
4.0 ng L−1 (tap)20.0 ng L−1 (river) | Schumacher et al., (2016)74 |
| Water | Waters 2695 system. Mobile phase: water, ammonium acetate, formic acid, acetonitrile. | 0.2 mL min−1 | Waters Quattro Premier Xe MS/MS | 0.5 μg L−1 | — | Jefferson et al., (2016)75 |
| Water | Agilent system. Phenomenex Kinetex phenyl-hexyl column. Mobile phase: water, formic acid, ammonia, acetonitrile. | 0.35 mL min−1 | Agilent 6410 MS/MS | — | — | Semitsoglou-Tsiapou et al., (2016)76 |
| Water | Agilent 1100 system. Ascentis express fused core C18 column. Mobile phase: water, methanol. | 0.25 mL min−1 | Bruker Daltonik HCT Esquire ion trap | 0.05 μg L−1 | — | Busquets et al., (2014)10 |
| Water | Waters 2695 system. Mobile phase: water, ammonium acetate, formic acid, acetonitrile. | 0.2 mL min−1 | Waters Quattro Premier Xe MS/MS | — | — | Autin et al., (2013a)73 |
| Water | Waters Acquity system. Acquity BHE C18 column. Mobile phase: water, ammonium acetate, methanol. | 0.2 mL min−1 | Waters Xevo TQ MS/MS | 3.0 ng L−1 | 10.0 ng L−1 | Li et al., (2010)4 |
| Vegetables | Agilent 1200 system. Zorbax C18 column. Mobile phase: water, acetonitrile. | 0.3 mL min−1 | Agilent 6410 MS/MS | 100.0 ng L−1 | 200.0 ng L−1 | Zhang et al. (2011a and 2011b)60,72 |
| Animal stomach/intestinal contents | Spherisorb ODS-2 column. Mobile phase: water, methanol. | 0.8 mL min−1 | Shimadzu RF-535 fluorimeter. Excitation λ = 380 nm, emission λ = 450 nm | Brown et al., (1996)77 |
Monitoring of metaldehyde
Monitoring strategies
The prescribed concentration value (PCV) for metaldehyde at drinking water capitation sites is often exceeded in the UK, and is now a major problem for water supply companies (Table 2). This led to the establishment of river catchment monitoring programmes for this pollutant, and to initiatives to convince the major users of this pesticide to reduce its use in their agricultural practises. These include guidance in The Voluntary Initiative – Promoting responsible pesticide use,78 Catchment Sensitive Farming79 and the MSG45 whom have promoted best practice in the application of metaldehyde. However, the degree of voluntary action available to the agricultural sector is constrained by both practical factors and financial considerations. These aspects together with their impacts on European regulatory considerations are discussed further by Dolan et al.80,81Metaldehyde is usually monitored in water by the use of low volume (<5 L) spot (bottle or grab) sampling with subsequent analysis in the laboratory by GC/MS or LC/MS methods. As the concentration of metaldehyde in surface water can fluctuate over time the use of infrequent spot sampling (typically collected weekly or monthly) is often an ineffective monitoring technique. This has been demonstrated by Rabiet et al.,82 who showed that infrequent spot sampling largely underestimated pesticide concentrations and fluxes during storm events in a catchment. This finding agrees with the earlier work of Louchart et al.,83 and Chen et al.,84 investigating loads and fluxes of pesticides during high flow events.
To overcome these issues associated with diffuse pollution events and to further understand the fate and movement of chemicals in water, more complex, high frequency, monitoring tools are required. Automated water collection devices (e.g. ISCO – http://www.teledyneisco.com) capable of collecting a series of water samples at prescribed time intervals (e.g. hourly or daily) can be used for this purpose. Such samplers can be triggered remotely to collect water during a high flow storm-type event. This approach to monitoring is expensive in terms of the capital cost of the equipment.52 In addition the number of samples generated during a monitoring campaign can add to laboratory operating costs. An alternative is the use of on-line or in situ methods.
On-line and in situ methods
GC or LC instruments coupled to various detectors have been used at surface water sites so as to provide a rapid means for the analysis of pesticides.85,86 Here instruments are connected on-line and may be used in combination with directly coupled sample preparation techniques. Additionally, the data generated can be transmitted telemetrically to a remote control centre to facilitate management decisions, e.g. for stopping the abstraction of water into a treatment works. Such an on-line GC/MS system is currently being trialled by Affinity Water, UK for monitoring metaldehyde.87Recently a novel reactive paper spray mass spectrometry method has been used to measure metaldehyde in water with a limit of detection of 0.1 μg L−1 without any pre-concentration or separation steps.88 The technique has potential in the future to be coupled with a miniature mass spectrometer to allow the on-site monitoring of metaldehyde and other pesticides of concern.
An alternative on-line approach for measuring metaldehyde in water has been described. The Lonestar™ portable analyser,89 uses a Field Asymmetric Ion Mobility Spectrometry (FAIMS) as the detection system (limit of detection, 0.1 μg L−1). This device is used for on-site detection in locations such as water reservoirs. A spot sample of water is taken directly in the field and mixed with nitric acid to break down the metaldehyde tetramer into four molecules of acetaldehyde, which are subsequently measured. Analysis time is typically 15 min. Data can be visually presented as a series of trigger or alarm values depending on the concentration of metaldehyde measured (<0.1 μg L−1 = green, 0.1 > 0.5 μg L−1 = amber and >0.5 μg L−1 = red). To our knowledge there have been no published field studies using the Lonestar™ analyser. This portable system has potential in the future for use as an on-line continuous monitoring system that could transmit an alarm if concentration of metaldehyde exceeds a specified threshold value. Alternatives for future application could be the development of simple, rapid dip or stick tests to rapidly semi-quantify metaldehyde in water.
Passive sampling devices are an in situ monitoring method. These devices have been used extensively to measure a wide range of pollutants in the aquatic environment.90 Samplers can be deployed in the field for extended periods (from days to months) where they continually sequester compounds. Once calibrated, in the laboratory or in the field, they permit the estimation of time-weighted average concentrations of substances over the deployment period.91 Several different designs of sampler are available for monitoring polar pollutants such as metaldehyde, including: Polar Organic Chemical Integrative Sampler (POCIS),92,93 Chemcatcher®94 and Diffusive Gradients in Thin-films (DGT).95 A variant of the Chemcatcher® using an HLB (Horizon Atlantic 47 mm disk) receiving phase overlaid with a polyethersulfone diffusion limiting membrane has been shown to sequester metaldehyde in surface waters. Uptake of metaldehyde (expressed as volume of water cleared per unit time, ∼16 mL per day) was found to be linear for over 14 days both in laboratory and in-field calibration experiments. The performance of this device for investigating the sources and fluxes of metaldehyde is presently being evaluated alongside routine spot sampling procedures at a number of river catchments in the UK.96
Remediation strategies for metaldehyde
Removal techniques
Due to its high polarity metaldehyde dissolves readily in water (solubility of ∼200 mg L−1 at 17 °C) and is considered semi-persistent in the aquatic environment.97 This property makes metaldehyde recalcitrant to removal using conventional drinking water treatment processes that are based on adsorption of substances to GAC or powdered activated carbon (PAC) materials. Both materials require differing carbon bed volumes and contact times and have different break through capacities. Using GAC to remove metaldehyde is problematic; firstly, due to competitive adsorptive binding by dissolved organic carbon and with other polar pesticides that may also be present in the environmental waters and secondly, due to desorption under certain operational conditions, such as a significant decrease in its influent concentration. Furthermore, the percentage removed is also a function of the concentration of metaldehyde present. Tests showed that water bodies with higher concentrations of metaldehyde can give relative removal efficiencies of up to 90% using GAC. This, however, decreases at lower values e.g. 30–50% removal at 0.5 μg L−1 and significantly less removal at lower concentrations ∼0.2 μg L−1.59 Data from Kay & Grayson9 suggested, that at typical environmental concentrations, such conventional water treatment processes have marginal or no effect on reducing metaldehyde found at the inlet and outlet of the works. Removing metaldehyde from water using GAC techniques also shortens the functional lifetime of the bed, which then requires expensive regeneration or disposal. Due to the increased need to remove metaldehyde from potable supplies in many areas of the UK, this in the short-term, has placed significant demands on the limited operational capacity of the available regeneration plants. Increasing this capacity over the longer term will be expensive, with new facilities expected to cost ∼£30–44 million.59 Continual removal of used material and its replacement with freshly activated carbon is an alternative process, but this very expensive to operate.These issues have led to the development alternative clean-up techniques. These include ‘designer’ materials such as tailored phenolic-based carbons10 or nano-sized zinc composites,12 biologically active sand filters98 and more sophisticated clean-up methods (e.g. ultra-violet (UV) oxidation combined with hydrogen peroxide (H2O2) treatment99 for the effective removal of metaldehyde (Table 6). Although standalone catalytic and electrochemical approaches are useful, the most widely used removal methods are based on UV advanced oxidation, often combined with another technique. Several different types of oxidation processes have been tested, such as UV/H2O2, UV/TiO2 and UV emitting diodes,73,99,100 all with different performance characteristics. In pilot scale studies high removal efficiencies in excess of 90% are now achievable for metaldehyde. A recent study by Semitsoglou-Tsiapou et al.,76 investigated the degradation kinetics for metaldehyde and the reaction products formed when using low pressure UV/H2O2 advanced oxidation processes. Their results showed that metaldehyde is effectively degraded, undergoing hydroxylation to ultimately yield, relatively benign, acetic acid as the major reaction end product. However, all these processes have high energy demands, predicted to be typically fifty times that of water disinfection processes. Use of light emitting diodes, however, can reduce energy consumption. In the future there maybe the possibility to use genetically modified bacteria that target single contaminant as a clean-up method.
| Removal technique | Adsorption capacity for metaldehyde | Removal | Analysis technique | Reference | |
|---|---|---|---|---|---|
| Conc. before | % removed | ||||
| Photocatalytic reactions using nano-sized zinc oxide composites | 500 μg L−1 | 56 | GC/MS | Doria et al., (2013)12 | |
| Tailored phenolic carbon | 76 mg metaldehyde/g carbon | — | — | LC/MS | Busquets et al., (2014)10 |
| Modified graphene | Up to mg L−1 | >92 | LC/MS | Nguyen et al. (2017)105 | |
| Biologically active sand filters in pesticide degraders | 10 μg L−1 | 70 | GC/MS | Rolph et al., (2014)98 | |
| Novel coupled adsorption and electrochemical destruction technique | 250 μg L−1 | 45 | LC/MS, GC/MS | Nabeerasool et al., (2015)69 | |
| 2000 μg L−1 | 25 | ||||
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1 000 μg L−1 |
12 | ||||
| 8000 μg L−1 | >90 | ||||
| Heterogeneous catalytic degradation using Macronets, followed by acetaldehyde removal using amine functionalised ion-exchange resin | 200 mg g−1 Macronet | GC, LC | Tao & Fletcher, (2016)101 | ||
| UV/H2O2 advanced oxidation process | 10 μg L−1 | >90 | LC/MS/MS | Autin et al., (2012)99 | |
| UV/TiO2 advanced oxidation process | 10 μg L−1 | <50 | LC/MS/MS | Autin et al., (2013b)100 | |
| UV light emitting diodes advanced oxidation process | 0.25 μg L−1 | 40 to 0.1 μg L−1 | LC/MS/MS | Autin et al., (2013a)73 | |
| UV/H2O2 advanced oxidation process incorporating micro-filtration and reverse osmosis | 0.2 μg L−1 | >90 | LC/MS/MS | James et al., (2014)102 | |
| Adsorption and photocatalytic degradation using nano-sized ZnO/LAPONITE® composite under UV irradiation | 0.1 mg L−1 | 95 | GC/MS | Kim & Campos, (2015)103 | |
| 2 mg L−1 | 55 | ||||
| 0.5 mg L−1 | 92 | ||||
| 1 mg L−1 | 69 | ||||
| Low pressure UV/H2O2 degradation at UV fluence of 1000 mJ cm−2 and 15 mg L−1 H2O2 | 5 mg L−1 | 97.7 | LC/MS/MS | Semitsoglou-Tsiapou et al., (2016)76 | |
| High UV dose and alkaline UV/H2O2 degradation | 10 μg L−1 | 8 mM | LC/MS/MS | Jefferson et al., (2016)75 | |
| Oxidative degradation using macrocyclic ligand catalysis, TAML/H2O2 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg g−1 TAML 000 mg g−1 TAML |
1H–NMR | Tang et al., (2016)104 | ||
Use of these advanced ‘end-of-pipe’ water treatment processes is expensive. In 2015, Anglian Water, the major drinking water supplier in the East of England (an agricultural region with high use of molluscicides) began operating a metaldehyde removal plant at a treatment works in Lincolnshire that abstracts water from the River Trent. The ‘low-energy’ plant uses membrane filters followed by UV/H2O2 oxidation then GAC water polishing adsorbers to give high removal rates for metaldehyde and other pesticides that may also be present.106 Based on operational costs at this site, Anglian Water predicted that to introduce similar systems across their supply region (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 km2) would cost an additional £17 million per year to operate. This would lead to an increase of 21% to their customer's utility bill.107 It was highlighted, therefore, that such a technology approach was not practicable and unlikely to be acceptable. More viable alternatives, such as a cross-sector collaboration in the management of river catchments and an increased awareness of water quality issues within the agricultural community were suggested as possible solutions to the problem.
500 km2) would cost an additional £17 million per year to operate. This would lead to an increase of 21% to their customer's utility bill.107 It was highlighted, therefore, that such a technology approach was not practicable and unlikely to be acceptable. More viable alternatives, such as a cross-sector collaboration in the management of river catchments and an increased awareness of water quality issues within the agricultural community were suggested as possible solutions to the problem.
Catchment initiatives
In the arable farming industry there have been many attempts to prevent applied pesticides entering the aquatic environment. One of the most common methods is the construction of swales; channels lined with grass that control the velocity of run-off from fields and can also remove some pollutants through the filtration of water through vegetation.108 There has been recent interest in using this approach for the capitation of metaldehyde arising from diffuse run-off from fields during excessive rainfall events. Swales are often used in combination with other catchment sensitive farming initiatives. These include rural sustainable drainage systems that slow down or prevent the transport of pollutants to watercourses by breaking the delivery pathway between the pollutant source and the receptor109 and better pesticide storage and management. Training (e.g. on calibration of pellet applicators) and advice for farmers are also available in collaborative practices to mitigate the environmental impact of pesticides.110 Several water companies, rivers trusts and non-governmental agencies in the UK are also involved in these projects concerned with metaldehyde. A recent example is the ‘Slug It Out’ campaign111 funded by Anglian Water in the UK with the aim of protecting a number of reservoirs. Results to date are promising with major decreases, up to 60%, in the concentration of metaldehyde being found in the associated river tributaries.112 Other water companies in the UK (e.g. Affinity, Severn Trent, Southern Water and Thames Water) have on-going catchment-based initiatives, including payments for non-usage, designed to reduce inputs of metaldehyde.Alternative molluscicides
Metaldehyde is the most widely used molluscicide and is dominant in the marketplace (Fig. 2). Since 1990 its use gradually increased, peaking between the years 2008–2009. After this time period, application rates have dropped significantly, due largely to the campaign ‘Get pellet wise’ that started in 2010 which established improved guidelines for the safe use of products containing the active ingredient metaldehyde. Other substances such as Methiocarb and ferric phosphate can also be used as molluscicides.2 Some of the recent decreases in the number of drinking water PCV exceedances found for metaldehyde in the UK, is due in part, to the increased take up of ferric phosphate by the agricultural sector.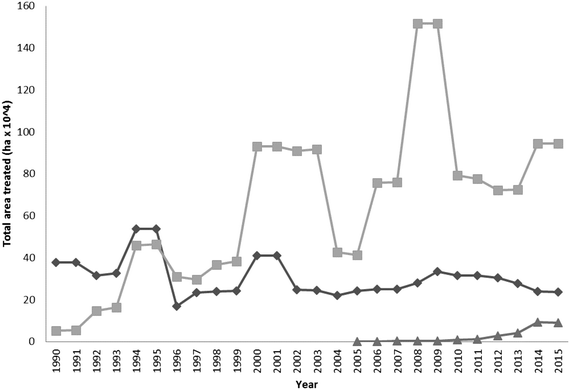 | ||
| Fig. 2 Comparison of the use (expressed as area of land treated) of metaldehyde (square), methiocarb (diamond) and ferric phosphate (triangle) molluscicides in the UK between the years 1990–2015 (source: FERA, 2016).2 | ||
Methiocarb (C11H15NO2S, also known as mercaptodimethur) is a carbamate pesticide and has been available since the early 1960s.1 It has a number of agricultural applications including being a powerful molluscicide (e.g. in products such Draza Forte and Decoy Wetex manufacturer by Bayer CropScience). Once ingested, Methiocarb exhibits neurotoxic effects on molluscs and has a potency higher (∼10 times) than that of metaldehyde.113,114 Methiocarb is less polar (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow = 3.18 at 20 °C) and less water soluble (0.027 g L−1 at 20 °C) than metaldehyde.1 The formulated pellets degrade slowly and hence are effective even in wet conditions and are less repellent to slugs compared with metaldehyde, allowing more active ingredient to be consumed before termination of feeding.115 Typically they were used on high value crops such as potatoes. However, due to recent concerns over its toxicity, particularly towards seed eating birds, such as sparrows and finches, use of this pesticide in formulated pellets was banned across the European Union in late 2014.116 All farm stocks had to be used by September, 2015. In the short term this ban is likely to increase the use of metaldehyde. However, Methiocarb can still be used in seed treatments products such a Mesurol.
Kow = 3.18 at 20 °C) and less water soluble (0.027 g L−1 at 20 °C) than metaldehyde.1 The formulated pellets degrade slowly and hence are effective even in wet conditions and are less repellent to slugs compared with metaldehyde, allowing more active ingredient to be consumed before termination of feeding.115 Typically they were used on high value crops such as potatoes. However, due to recent concerns over its toxicity, particularly towards seed eating birds, such as sparrows and finches, use of this pesticide in formulated pellets was banned across the European Union in late 2014.116 All farm stocks had to be used by September, 2015. In the short term this ban is likely to increase the use of metaldehyde. However, Methiocarb can still be used in seed treatments products such a Mesurol.
Currently, the only alternative for metaldehyde is ferric phosphate (FePO4).117 This compound is highly insoluble in water.1 It is generally formulated in pasta type pellets, and several varieties of these are available commercially (e.g. Derrex®, Ferramol®, Ironmax Pro®, Sluggo®, or Sluxx®) containing differing amounts of ferric phosphate. Neudorff GmbH, based in Germany, is the largest producer. Ferric phosphate can be formulated with ethylene diamine tetracetic acid (EDTA) or ethylene diamine succinic acid (EDDS) so as to increase the solubility of the iron, and hence toxicity towards the mollusc.39 Application rates are typically 3–7 kg ha−1 of a 3% formulation (90–210 g ha−1 as ferric phosphate). Once ingested, this chemical causes pathological changes to a mollusc's digestive system, particularly calcium metabolism, quickly causing it to stop feeding and become less mobile. Death usually occurs within 3–6 days. There is evidence, however, that some ferric phosphate formulations have adverse effects on earthworms and other soil-inhabiting invertebrates such as beetles, millipedes and woodlice.39
The use of ferric phosphate has gradually increased since its introduction to the market in 2005 (Fig. 2). Often metaldehyde is still preferred, due to its long and widespread use and also as it is perceived to cost less than ferric phosphate on a per kg basis. Some water companies as part of their catchment initiatives offer a financial incentive to use ferric phosphate (e.g. Southern Water offers a £1 kg−1 used incentive). Additionally, metaldehyde causes slugs to remain above the soil upon death and this allows the user to see that the product is working. Ferric phosphate causes the slugs to bury themselves in the soil where they subsequently die, this does not show the user that the molluscicide has been effective. Instead growers have to be guided by the degree of crop damage after the application of the chemical.118 The overall impact, particularly on the aquatic environment, of ferric phosphate is significantly less than that of metaldehyde. For example, restricting the use of ferric phosphate baits near watercourses and wet weather conditions are unnecessary. Although it is difficult to predict with certainty, it is expected that the use of ferric phosphate formulations will grow in the future, as pressure increases from within the regulatory sector and water suppliers on limiting the agricultural use of metaldehyde.
Conclusions
Metaldehyde is a potent tool in fighting pests that damage crops. Even though it has been used for over 75 years, until recently little research has been directed to the occurrence, persistence and environmental fate of this pesticide. There have been sparse efforts directed towards its widespread monitoring in surface waters, even though during the autumn and winter months in the UK, peak concentrations of metaldehyde often exceed the European Union regulatory limit (0.1 μg L−1) for any pesticide. Alternative approaches to monitoring and better defined campaigns are now needed, as the use of infrequent spot sampling, does not give the required temporal and spatial resolution needed to develop effective management plans for reducing the environmental impact of this pollutant.Even though based on the available toxicity data for typical environmental concentration of metaldehyde, there are no perceived health risks; use of surface water contaminated with metaldehyde to produce potable supplies is a growing concern for water companies. The problem is particularly difficult to address because the physico-chemical properties of this compound make it resistant to removal by conventional drinking water treatment processes. Recent collaborative river catchment initiatives have helped reduce concentrations of metaldehyde in several regions of the UK, but some water companies who are impacted by this issue, are now also assessing expensive end-of-pipe treatment processes. Once functioning, these advanced treatment works will have significant operating costs and over the short-to-medium term lead to increased utility bills for their customers.
Metaldehyde still remains the molluscicide of choice within the agricultural community. Recently controlled release formulations have been developed so as to slow the input of the active ingredient into the environment.119 Pilot trials for these preparations are on going. Alternatively there are encouraging results that the new ferric phosphate-based formulations can be used as a direct, cost effective replacement for metaldehyde on all crop types. The long-term future use of metaldehyde is difficult to predict with certainty. One solution might be to amend the blanket European Union Drinking Water Directive limit to take into consideration compound specific toxicological and ecological effects. This would potentially permit a higher concentration of metaldehyde to be present in surface water capitation sites. If this is not the case, then there is a realistic chance that the use of metaldehyde in poison-baited pellets could be restricted in the UK, with some predictions by as early as 2020.
Acknowledgements
This work was part funded by the Natural Environment Research Council (NERC) as an iCASE award (NE/L009145/1) to GC.References
- PPDB: Pesticide Properties DataBase, http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm, Accessed September, 2016 Search PubMed.
- FERA, http://pusstats.fera.defra.gov.uk/myindex.cfm, Accessed September, 2016 Search PubMed.
- M. Bieri, in Slugs & Snails: Agricultural, Veterinary & Environmental Perspectives, ed. G. B. J. Dussart, British Crop Protection Council, Farnham, 2003, pp. 255–260 Search PubMed.
- H. Y. Zhang, C. Wang, H. Z. Lu, W. B. Guan and Y. Q. Ma, Ecotoxicol. Environ. Saf., 2011, 74, 1653–1658 CrossRef CAS PubMed.
- WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2009, World Health Organization, Stuttgart, Germany, 2010 Search PubMed.
- Reregistration Eligibility Decision for Metaldehyde, U.S. Environmental Protection Agency Web Archive [Online], July 27, 2006, http://archive.epa.gov/pesticides/reregistration/web/pdf/metaldehyde_red.pdf Search PubMed.
- C. J. Nicholls, 2014 HGCA Research Review 79, https://cereals.ahdb.org.uk/publications/2014/march/10/implications-of-not-controlling-slugs-in-oilseed-rape-and-wheat-in-the-uk.aspx Search PubMed.
- D. B. Green, Brighton Crop Protection Conference: Pests & Diseases − 1996, vol. 1–3, 1996 Search PubMed.
- P. Kay and R. Grayson, Water Environ. J., 2014, 28, 410–417 CAS.
- R. Busquets, O. P. Kozynchenko, R. L. D. Whitby, S. R. Tennison and A. B. Cundy, Water Res., 2014, 61, 46–56 CrossRef CAS PubMed.
- M. Stuart, D. Lapworth, E. Crane and A. Hart, Sci. Total Environ., 2012, 416, 1–21 CrossRef CAS PubMed.
- F. C. Doria, A. C. Borges, J. K. Kim, A. Nathan, J. C. Joo and L. C. Campos, Water, Air, Soil Pollut., 2013, 224(2), 1434 CrossRef.
- IPCS, International Programme on Chemical Safety Poisons Information Monograph 332 (Metaldehyde), http://www.inchem.org/documents/pims/chemical/pim332.htm Search PubMed.
- S. E. Kegley, B. R. Hill, S. Orme and A. H. Choi, PAN Pesticides Database, http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC32878 Search PubMed.
- R. Triebskorn, K. Christensen and I. Heim, J. Mollus. Stud., 1998, 64, 467–487 CrossRef.
- EXTOXNET: Extension Toxicology Network, 1993, http://pmep.cce.cornell.edu/profiles/extoxnet/haloxyfop-methylparathion/metaldehyde-ext.html Search PubMed.
- R. C. Gupta, in Veterinary toxicology: basic and clinical principles, ed. R. C. Gupta, Academic press, 2nd edn, 2012, pp. 624–628 Search PubMed.
- T. F. Booze and F. W. Oehme, Vet. Hum. Toxicol., 1985, 27, 11–19 CAS.
- W. A. Watson, T. L. Litovitz, G. C. Rodgers, W. Klein-Schwartz, J. Youniss, S. R. Rose, D. Borys and M. E. May, Am. J. Emerg. Med., 2003, 21, 353–421 CrossRef PubMed.
- C. C. Shih, S. S. Chang, Y. L. Chan, J. C. Chen, M. W. Chang, M. S. Tung, J. F. Deng and C. C. Yang, Vet. Hum. Toxicol., 2004, 46, 140–143 Search PubMed.
- M. J. Ellenhorn and D. G. Barceloux, in Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning, ed. M. Ellenhorn, S. Schonwald, G. Ordog, J. Wasserberger and S. Ellenhorn, Williams and Wilkins, Philadelphia, 1997, pp. 609–610 Search PubMed.
- J. P. Thompson, P. B. Casey and J. A. Vale, Hum. Exp. Toxicol., 1995, 14, 437–445 CAS.
- A. Campbell, Vet. Rec., 2008, 163, 343 CrossRef PubMed.
- N. J. Mills, Vet. Rec., 2008, 163, 310 CrossRef PubMed.
- N. Bates, N. Sutton and A. Campbell, Vet. Rec., 2012, 171, 324 CrossRef CAS PubMed.
- S. N. Tawde, B. Puschner, T. Albin, S. Stump and R. H. Poppenga, J. Med. Toxicol., 2012, 8, 436–440 CrossRef CAS PubMed.
- F. Caloni, C. Cortinovis, M. Rivolta and F. Davanzo, Sci. Total Environ., 2016, 539, 331–336 CrossRef CAS PubMed.
- R. B. Cope, K. S. White, E. More, K. Holmes, A. Nair, P. Chauvin and A. Oncken, J. Vet. Pharmacol. Ther., 2006, 29, 233–236 CrossRef CAS PubMed.
- L. K. Dolder, Vet. Med., 2003, 98, 213–215 Search PubMed.
- K. J. Buhl, F. W. Berman and D. L. Stone, J. Am. Vet. Med. Assoc., 2013, 242, 1244–1248 CrossRef CAS PubMed.
- I. Nolte, Prakt. Tierarzt, 2012, 93, 886–893 Search PubMed.
- J. A. Richardson, S. L. Welch, S. M. Gwaltney-Brant, J. D. Huffman and M. E. Rosendale, Compendium on Continuing Education for the Practising Veterinarian - North American Edition, 2003, vol. 25, pp. 376–380 Search PubMed.
- A. Chilla, S. Scheulen, J. Borchert, W. Danner and S. Neumann, Prakt. Tierarzt, 2010, 91, 948–959 Search PubMed.
- R. Zimmermann, T. A. Steinberg, K. Raith, V. Hülsmeyer and A. Fischer, Tierärztliche Praxis Kleintiere, 2010, 38, 285–294 CAS.
- D. P. Stubbings, A. B. Edington, D. G. Lyon, J. B. Spence and M. H. Clark, Vet. Rec., 1976, 98, 356–357 CAS.
- B. A. Valentine, W. K. Rumbeiha, T. S. Hensley and R. R. Halse, J. Vet. Diagn. Invest., 2007, 19, 212–215 CrossRef PubMed.
- K. H. Plumlee, Vet. Clin. North Am. Equine Pract., 2001, 17, 491–500 CAS.
- M. Sperling, A. Schönfelder, K. Köhler, H. Desel and L. F. Litzke, Wien. Tieraerztl. Monatsschr., 2010, 97, 290–293 Search PubMed.
- C. A. Edwards, N. Q. Arancon, M. Vasko-Bennett, B. Little and A. Askar, Crop Prot., 2009, 28, 289–294 CrossRef CAS.
- A. M. Langan and E. M. Shaw, Appl. Soil Ecol., 2006, 34, 184–189 CrossRef.
- K. C. Hallett, A. Atfield, S. Comber and T. H. Hutchinson, Sci. Total Environ., 2016, 543, 37–43 CrossRef CAS PubMed.
- P. Moreau, T. Burgeot and T. Renault, Environ. Sci. Pollut. Res., 2015, 22, 8003–8009 CrossRef CAS PubMed.
- R. L. Reece, P. C. Scott, W. N. Forsyth, J. A. Gould and D. A. Barr, Vet. Rec., 1985, 117, 525–527 CAS.
- Department for Environment, Air pollution from farming: preventing and minimising, https://www.gov.uk/guidance/reducing-air-pollution-on-farms, 2016 Search PubMed.
- Metaldehyde Stewardship Group, MSG guidelines, http://www.getpelletwise.co.uk/msg-guidelines/, 2016 Search PubMed.
- L. A. Fogg, Catchment study to determine the relative significance of the different pathways by which metaldehyde may impact on surface water, Cambridge Environmental Assessments Research Report Number CEA.481, Metaldehyde Stewardship Group, 2009 Search PubMed.
- Y. Ma, X. Wu, Z. Zheng, Y. Yang, C. Wang, H. Zhang and L. Meng, in Renewable and Sustainable Energy, Pts 1–7, ed. W. Pan, J. X. Ren and Y. G. Li, 2012, vol. 347–353, pp. 1987–1993 Search PubMed.
- X. Y. Zhang and X. F. Dai, Nongyaoxue Xuebao, 2006, 8, 344–348 CAS.
- L. C. Simms, J. J. Dawson, G. I. Paton and M. J. Wilson, J. Agric. Food Chem., 2006, 54, 3646–3650 CrossRef CAS PubMed.
- S. Gillman, P. Brown, D. Burgess, B. Bickle, A. Zyndul and C. Chapman, Proceedings of Crop Protection in Northern Britain, 2012 Search PubMed.
- Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, Official Journal of the European Communities L 330, 1998, pp. 32–54 Search PubMed.
- A. Davey, T. Hall, J. Horn, J. Jönsson and R. Keirle, Evidence Review of Catchment Strategies for Managing Metaldehyde Report Ref. No. 13/DW/14/7, UK Water Industry Research Limited, 2014 Search PubMed.
- S. G. Anthony, N. Brettell, K. Hewson-Fisher, B. Hockridge, G. O. Hughes, H. L. Lyons, T. J. Pepper, S. Twining and L. Wilson, Pesticide Risk Mapping and Catchment Interventions–Phase 1 Report Ref. No. 15/DW/14/11, UK Water Industry Research Limited, 2015 Search PubMed.
- H. Martineau, N. Shamier and R. Smithers, Appraisal of Policy Options to Manage Pesticides Ricardo-AEA/R/ED59190/Issue Number 6 Final Report, Ricardo-AEA Ltd, 2014 Search PubMed.
- The Pesticides Forum, Pesticides in the UK: The 2015 report on the impacts and sustainable use of pesticides, Health and Safety Executive, 2015 Search PubMed.
- D. Henehan, Metaldehyde and PES in the Ampney Brook catchment, http://www.slideshare.net/CCRI/pes-farmerworkshop-henehan, 2016 Search PubMed.
- Water UK, Briefing paper on metaldehyde, http://www.water.org.uk/publications/policy-briefings/metaldehyde, 2016 Search PubMed.
- Drinking Water Inspectorate, Annual Report Drinking Water 2015, Drinking Water Inspectorate, 2016 Search PubMed.
- G. Dillon, T. Hall, J. Jönsson, K. Murrell, D. Shepherd, J. Song and M. Stanger, Treatment for New and Emerging Pesticides Report Ref. No. 11/DW/14/4, UK Water Industry Research Limited, 2011 Search PubMed.
- C. Li, Y.-L. Wu, T. Yang and Y. Zhang, Chromatographia, 2010, 72, 987–991 CAS.
- M. Paolo and B. Renzo, Bull. Environ. Contam. Toxicol., 1983, 30, 479–484 CrossRef CAS PubMed.
- S. Selim and J. N. Seiber, J. Agric. Food Chem., 1973, 21, 430–433 CrossRef CAS PubMed.
- Y. Iwata, G. E. Carman, T. M. Dinoff and F. A. Gunther, J. Agric. Food Chem., 1982, 30, 606–608 CrossRef CAS PubMed.
- A. Jones, A. Charlton and J. Agric, Food Chem., 1999, 47, 4675–4677 CrossRef CAS.
- T. Saito, S. Morita, M. Motojyuku, K. Akieda and H. Otsuka, J. Chromatogr., B, 2008, 875, 573–576 CrossRef CAS PubMed.
- Environment Agency, in Methods for the Examination of Waters and Associated Materials, Environment Agency, 2009, Blue Book 226, p. 52 Search PubMed.
- ALS Environmental, Method Statement-Metaldehyde, https://www.alsenvironmental.co.uk/media-uk/method_statements/wakefield/drinking-water/organics/wpc62_metaldehyde_tof_method-statement.pdf Search PubMed.
- B. Tao and A. J. Fletcher, J. Hazard. Mater., 2013, 244, 240–250 CrossRef PubMed.
- M. A. Nabeerasool, A. K. Campen, D. A. Polya, N. W. Brown and B. E. van Dongen, Water, 2015, 7, 3057–3071 CrossRef.
- L. Alder, K. Greulich, G. Kempe and B. Vieth, Mass Spectrom. Rev., 2006, 25, 838–865 CrossRef CAS PubMed.
- ALS Environmental, Method Statement-Metaldehyde, https://www.alsenvironmental.co.uk/media-uk/method_statements/wakefield/drinking-water/organics/wpc31_metaldehyde_method-statement.pdf Search PubMed.
- H. Y. Zhang, C. Wang, P. J. Xu and Y. Q. Ma, Food Addit. Contam., Part A, 2011, 28, 1034–1040 CrossRef CAS PubMed.
- O. Autin, C. Romelot, L. Rust, J. Hart, P. Jarvis, J. MacAdam and B. Jefferson, Chemosphere, 2013, 92, 745–751 CrossRef CAS PubMed.
- M. Schumacher, G. Castle, A. Gravell, G. A. Mills and G. R. Fones, MethodsX, 2016, 3, 188–194 CrossRef PubMed.
- B. Jefferson, P. Jarvis, G. K. Bhagianathan, H. Smith, O. Autin, E. H. Goslan and I. Carra, Chem. Eng. J., 2016, 288, 359–367 CrossRef CAS.
- S. Semitsoglou-Tsiapou, M. R. Templeton, N. J. Graham, L. H. Leal, B. J. Martijn, A. Royce and J. C. Kruithof, Water Res., 2016, 91, 284–295 CrossRef PubMed.
- P. Brown, A. Charlton, M. Cuthbert, L. Barnett, L. Ross, M. Green and M. Fletcher, J. Chromatogr. A, 1996, 754, 463–478 CrossRef CAS PubMed.
- The Voluntary Initiative, Promoting Responsible Pesticide Use, http://www.voluntaryinitiative.org.uk/ Search PubMed.
- Natural England, Catchment Sensitive Farming: reduce agricultural water pollution, https://www.gov.uk/guidance/catchment-sensitive-farming-reduce-agricultural-water-pollution Search PubMed.
- T. Dolan, D. J. Parsons, P. Howsam, M. J. Whelan and L. Varga, Water Resour. Manag., 2014, 28, 511–526 CrossRef.
- T. Dolan, P. Howsam, D. J. Parsons and M. J. Whelan, Water Policy, 2014b, 16, 280–297 Search PubMed.
- M. Rabiet, C. Margoum, V. Gouy, N. Carluer and M. Coquery, Environ. Pollut., 2010, 158, 737–748 CrossRef CAS PubMed.
- X. Louchart, M. Voltz, P. Andrieux and R. Moussa, J. Environ. Qual., 2001, 30, 982–991 CrossRef CAS PubMed.
- B. Chen, G. Huang, Y.-f. Li, B. Zhang and Y. Huang, Water Int., 2005, 30, 88–98 CrossRef CAS.
- P. J. M. van Hout and U. A. Th. Brinkman, TrAC, Trends Anal. Chem., 1994, 13, 382–388 CrossRef CAS.
- S. Lacorte, J. J. Vreuls, J. S. Salau, F. Ventura and D. Barcelo, J. Chromatogr. A, 1998, 795, 71–82 CrossRef CAS.
- Water & Wastewater Treatment, Promising results for metaldehyde detection trial, http://wwtonline.co.uk/news/promising-results-for-metaldehyde-detection-trial#.WIsb8n1y1dd Search PubMed.
- S. Maher, F. P. M. Jjunju, D. E. Damon, H. Gorton, Y. S. Maher, S. U. Syed, R. M. A. Heeren, I. S. Young, S. Taylor and A. K. Badu-Tawiah, Sci. Rep., 2016, 6, 35643 CrossRef CAS PubMed.
- Owlstone, Detection of Metaldehyde in Water Using Lonestar, http://www.owlstonenanotech.com/lonestar/apps/detect-metaldehyde-water Search PubMed.
- R. Greenwood, G. Mills and B. Vrana, Passive sampling techniques in environmental monitoring, Elsevier, 2007 Search PubMed.
- G. A. Mills, A. Gravell, B. Vrana, C. Harman, H. Budzinski, N. Mazzella and T. Ocelka, Environ. Sci.: Processes Impacts, 2014, 16, 369–373 CAS.
- D. A. Alvarez, J. D. Petty, J. N. Huckins, T. L. Jones-Lepp, D. T. Getting, J. P. Goddard and S. E. Manahan, Environ. Toxicol. Chem., 2004, 23, 1640–1648 CrossRef CAS PubMed.
- P. C. Van Metre, D. A. Alvarez, B. J. Mahler, L. Nowell, M. Sandstrom and P. Moran, Environ. Pollut., 2017, 220, 431–440 CrossRef CAS PubMed.
- J. K. Kingston, R. Greenwood, G. A. Mills, G. M. Morrison and L. B. Persson, J. Environ. Monit., 2000, 2, 487–495 RSC.
- J. K. Challis, M. L. Hanson and C. S. Wong, Anal. Chem., 2016, 88, 10583–10591 CrossRef CAS PubMed.
- G. Castle, IPSW 2016, Prague, Czech Republic, 2016, http://ipsw.eu/2016/detailed-programme/ Search PubMed.
- WHO and FAO United Nations, WHO/FAO data sheet on pesticides. no. 93, Metaldehyde, World Health Organization, Geneva, 1996, http://apps.who.int/iris/handle/10665/63291 Search PubMed.
- Switching on pesticide degraders in biological filters used in drinking water production, ed. C. A. Rolph, B. Jefferson and R. Villa, 2014 Search PubMed.
- O. Autin, J. Hart, P. Jarvis, J. MacAdam, S. A. Parsons and B. Jefferson, Water Res., 2012, 46, 5655–5662 CrossRef CAS PubMed.
- O. Autin, J. Hart, P. Jarvis, J. MacAdam, S. A. Parsons and B. Jefferson, Appl. Catal., B, 2013, 138, 268–275 CrossRef.
- B. Tao and A. Fletcher, Chem. Eng. J., 2016, 284, 741–749 CrossRef CAS.
- C. P. James, E. Germain and S. Judd, Sep. Purif. Technol., 2014, 127, 77–83 CrossRef CAS.
- J. K. Kim and L. C. Campos, Water Sci. Technol., 2015, 15, 533–540 CAS.
- L. L. Tang, M. A. DeNardo, C. Gayathri, R. R. Gil, R. Kanda and T. J. Collins, Environ. Sci. Technol., 2016, 50, 5261–5268 CrossRef CAS PubMed.
- L. V. Nguyen, R. Busquets, S. Ray and A. B. Cundy, Chem. Eng. J., 2017, 307, 159–167 CrossRef CAS.
- http://wwtonline.co.uk/features/project-focus-hall-claims-uk-first-in-water-treatment#.WAoBy03fP1J .
- http://www.anglianwater.co.uk/news/got-a-spare-600million.aspx .
- http://adlib.everysite.co.uk/adlib/defra/content.aspx?doc=266470&id=276556 .
- https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/291508/scho0612buwh-e-e.pdf .
- http://publications.naturalengland.org.uk/file/6538023361576960 .
- www.anglianwater.co.uk/slugitout .
- http://utilityweek.co.uk/news/anglian-water-farming-trial-sees-60-per-cent-drop-in-pesticide-levels/1240172#.WAnyFU3fP1I .
- L. C. Simms, M. J. Wilson, D. M. Glen and D. B. Green, The BCPC Conference: Pests and diseases, Proceedings of an international conference held at the Brighton Hilton Metropole Hotel, Brighton, UK, 18–21 November, 2002, vol. 1 and 2, pp. 679–684 Search PubMed.
- B. Speiser, Bulletin OEPP/EPPO Bulletin, 1997, vol. 27, pp. 235–242 Search PubMed.
- N. B. Bourne, G. W. Jones and I. D. Bowen, J. Molluscan Stud., 1988, 54, 327–338 CrossRef.
- http://www.fwi.co.uk/arable/eu-votes-to-ban-methiocarb-slug-pellets.htm .
- B. Speiser and C. Kistler, Crop Prot., 2002, 21, 389–394 CrossRef CAS.
- https://cereals.ahdb.org.uk/media/246568/ahdb-is04-integrated-slug-control.pdf .
- http://www.farmersguide.co.uk/2016/01/metaldehyde-controlled-release-possibility/ .
| This journal is © The Royal Society of Chemistry 2017 |