Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymer colour converter with very high modulation bandwidth for visible light communications†
D. A.
Vithanage
a,
A. L.
Kanibolotsky
bc,
S.
Rajbhandari
de,
P. P.
Manousiadis
a,
M. T.
Sajjad
 a,
H.
Chun
d,
G. E.
Faulkner
d,
D. C.
O’Brien
d,
P. J.
Skabara
a,
H.
Chun
d,
G. E.
Faulkner
d,
D. C.
O’Brien
d,
P. J.
Skabara
 *b,
I. D. W.
Samuel
*b,
I. D. W.
Samuel
 *a and
G. A.
Turnbull
*a and
G. A.
Turnbull
 *a
*a
aOrganic Semiconductor Centre, SUPA, School of Physics and Astronomy, University of St Andrews, St. Andrews, KY16 9SS, UK. E-mail: gat@st-andrews.ac.uk; idws@st-andrews.ac.uk
bWestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, Glasgow, G1 1XL, UK
cInstitute of Physical-Organic Chemistry and Coal Chemistry, 02160 Kyiv, Ukraine
dDepartment of Engineering Science, University of Oxford, Oxford, OX1 3PJ, UK
eCentre for Mobility & Transport, School of Computing, Electronics and Mathematics, Coventry University, Coventry, CV1 2JH, UK
First published on 5th September 2017
Abstract
For white light data communications, broadband light emitting materials are required, whose emission can be rapidly modulated in intensity. We report the synthesis, photophysics and application of a novel semiconducting polymer for use as a high bandwidth colour converter, to replace commercial phosphors in white LEDs. The high modulation bandwidth (470 MHz) is 140 times higher than that measured using a conventional LED phosphor.
Introduction
The increasing demand for high speed wireless communication has driven research into alternative technologies to existing radio frequency (RF) systems. In particular, visible light communications (VLC)1,2 offer great promise as a new wireless communications approach, based on the advances in semiconductor lighting. VLC systems use a modulated light emitting diode (LED) source to transmit data at rates up to Gigabits per second (Gbps). White light for solid-state lighting can be produced in two ways: using blue LEDs with phosphor colour converters or with red-green-blue (RGB) LED modules. The former is more widespread as it is simpler to implement whilst achieving a high colour rendering index (CRI). However, for VLC the light source also requires to be modulated at high speed, which in turn needs an efficient colour conversion material with fast response (short photoluminescence (PL) lifetime). Commercial inorganic phosphors have lifetimes in the order of microseconds to milliseconds, which limit the communication bandwidth to a few MHz.2Organic colour converters3–5 have the potential to overcome this limit, as they have a shorter emission lifetime (in the order of nanoseconds), and consequently higher communication bandwidths can be obtained.6–8 Semiconducting polymers are low cost, solution-processable materials that can be integrated on a wide range of substrates. They can have high photoluminescence quantum yields (PLQY) and broadband emission that can be tuned by changing the molecular structure, both of which, along with short lifetime, are required in colour converters for VLC. In one example, light from a blue LED was combined with photoluminescence from the commercial polymer Super Yellow (in a concentrated solution with modulation bandwidth of >90 MHz).6 The source achieved white light data transmission at 1.8 Gbps using advanced modulation techniques, but with a modest colour rendering index (CRI) of 53. BODIPY cored materials (in solution) have been used to extend VLC conversion wavelengths to the red, as optical transmitters with 39 MHz bandwidth and data rates (with simple on–off keying) of 98 Mbit per s.8 To produce a material with a short lifetime and improved colour rendering, a solid blend of two organic materials was made using the green emitter, poly[2,5-bis(2′,5′-bis(2′′-ethylhexyloxy)phenyl)–p-phenylenevinylene] (BBEHP–PPV) and the red emitter, poly[2-methoxy-5-(2′-ethyl-hexyloxy)–p-phenylene-vinylene] (MEH–PPV).7 The blend gave a broad emission spectrum with modulation bandwidth of 200 MHz, which had a high CRI value of 76 when mixed with blue LED light, but was limited in efficiency by the 17% PLQY of MEH–PPV. A key materials challenge for high performance VLC sources is therefore to develop new orange-red emitters that can combine fast modulation with high PLQY.
We present here the design of a novel, fast, orange-emitting polymer which combines efficient emission with an exceptionally high modulation bandwidth. The material is a poly(phenylenevinylene) (PPV) derivative, poly[(2,5-bis((2′,5′-bis((2′′-ethylhexyl)oxy)benzyl)oxy)–p-phenylene)vinylene] (BBEHBO–PPV) and is the first example of a conjugated polymer that has been custom designed for application in VLC. The extended conjugation of semiconducting polymers offers the fastest radiative rates, and we show here that the polymer structure can be designed to combine high solid-state PLQY and fast orange light emission. Bulky side groups separate the conjugated polymer backbones9,12 to achieve high PLQY in thin films, while their alkoxy bridges allow the emission wavelength to be tuned and give a high radiative rate.
The molecular design was inspired by the high bandwidth achieved using the blend of BBEHP–PPV and MEH–PPV.7 BBEHP–PPV9 and MEH–PPV are well known green- and red-emitting polymers, which have been researched for OLEDs,10 lasers and optical amplifiers,9,11–13 sensors for explosives9,14 and solar cells.15,16 BBEHBO–PPV (Scheme 1 and Fig. S1 in ESI†) is designed to have a poly(2,5-dialkoxy-p-phenylene vinylene) backbone with bulky substituents similar to those of BBEHP–PPV, but with an alkoxy bridge within the side groups, as applied in MEH–PPV. BBEHBO–PPV thereby combining advantages of the two materials: the high PLQY of BBEHP–PPV with the fast orange PL lifetime of MEH–PPV. This allows us to achieve a modulation bandwidth of 470 MHz for a fluorescent film of the material. This is 140 times higher than that in commercially available phosphor-coated light emitting diodes (LEDs) and currently the highest recorded bandwidth for an organic colour converter.
Results and discussion
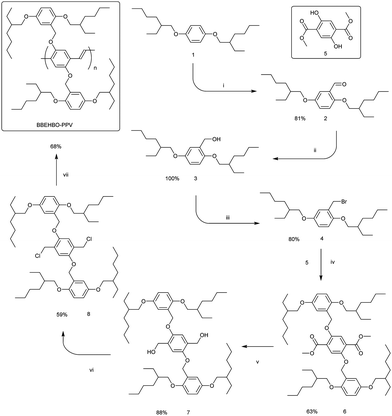
BBEHP–PPV was synthesised following the method in ref. 12 and MEH–PPV was purchased from ADS Dyes. The synthesis of the polymer BBEHBO–PPV was accomplished in seven steps (Scheme 1), starting from 1,4-bis((2-ethylhexyl)oxy)benzene (1).17,18 The lithiation of compound 1 with BuLi in hexane in the presence of TMEDA and subsequent reaction with DMF afforded aldehyde 2 in a very good yield. The aldehyde 2 was reduced to carbinol 3 with NaBH4 in quantitative yield, and the compound 3 after work up was pure enough to be used in the next step without any further purification. Conversion of alcohol 3 into bromomethyl derivative 4 was performed using Appel conditions in a good yield. Compound 4 was used for alkylation of 2,5-dihydroxyterephthalate (5)19 in DMF in the presence of K2CO3, which afforded diester 6 in a fair 63% yield. The latter was reduced to diol 7 with LiAlH4 in a very good 88% yield. It was found that the best conditions for converting diol 7 to the monomer 8 are provided by the microwave-assisted Appel reaction in freshly distilled (over CaH2) carbon tetrachloride. The yield of the monomer 8 was 59%. The final polymerisation step was carried out in THF with tert-BuOK as a base following the Gilch protocol.The target polymer BBEHBO–PPV was obtained as a red resin-like (due to low Tg, vide infra) solid in a fair yield of 68%. GPC analysis revealed the molecular weight Mn = 499 kDa with PDI = 2.67 (Fig. S8, ESI†).
BBEHBO–PPV turned out to be fairly thermally stable. Thermogravimetric analysis (Fig. S9, ESI† solid line) revealed a decomposition temperature of 290 °C (5% weight loss). Due to the bulky substituents and high internal volume of the polymer repeat unit the target compound BBEHBO–PPV exhibited a low glass transition temperature (Tg = −17 °C) in a differential scanning calorimetry experiment (Fig. S9, ESI† dashed line), which is responsible for the resin-like appearance of this polymer at ambient temperature.
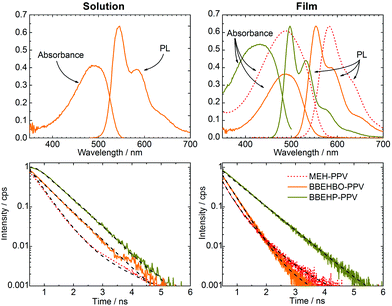
The PL lifetime, absorption and PL spectra for BBEHBO–PPV, BBEHP–PPV, MEH–PPV are shown in Fig. 1. In solution and in film, BBEHBO–PPV has absorption peaks at 490 and 488 nm, respectively and covers the blue LED emission region. Transient absorption measurements show an excited state absorption band at 1100 nm (Fig. S2, ESI†). The emission peaks (0–0) are at 544 and 555 nm with a shoulder (0–1) at 585 and 586 nm in solution and film, respectively. PL lifetime measurements were conducted by exciting at 393 nm and detecting at 550 nm. The lifetimes were fitted using a multi exponential decay. The fit values are given in Table 1 and Table S1 (ESI†). In solution, the decay was single exponential with a lifetime of 0.7 ns. In the film, there was a double exponential decay with the dominant component at 0.37 ns. The PLQY was measured to be 67% in solution, which dropped to 45% in films. The CIE coordinates of BBEHBO–PPV lie between BBEHP–PPV and MEH–PPV, and are red-shifted from Super Yellow.7 The blue absorption band, short lifetime and high PLQY values show that the design of the material, using a PPV derivative, successfully meets the requirements in colour converters for VLC.
| BBEHBO–PPV (solution) | BBEHBO–PPV (film) | BBEHP–PPV (film) | MEH–PPV (film) | |
|---|---|---|---|---|
| PLQY (%) | 67 | 45 | 85 | 17 |
| τ 1 (ns) | 0.65 | 0.371 | 0.722 | 0.295 |
| A 1 | 1 | 0.996 | 1 | 0.885 |
| τ 2 (ns) | — | 1.63 | — | 0.658 |
| A 2 | — | 0.004 | — | 0.116 |
| τ 3 (ns) | — | — | — | 7.34 |
| A 3 | — | — | — | 0.001 |
| CIE coordinates | 0.46, 0.53 | 0.46, 0.53 | 0.25, 0.57 | 0.57, 0.43 |
In comparison, the absorption spectrum of BBEHBO–PPV closely resembles that of MEH–PPV at λ > 375 nm with similar emission peaks. The emission of BBEHBO–PPV is red-shifted compared to that of BBEHP–PPV which has a peak at 535 nm, overlapping the PL spectra of both BBEHP–PPV and MEH–PPV over a range of 300 nm. The PLQY of BBEHBO–PPV is lower than BBEHP–PPV which is highly luminescent at 75% (as a film) but an improvement from the <20% quantum yield in MEH–PPV. To compare with BBEHBO–PPV, PL lifetime measurements were conducted on BBEHP–PPV and MEH–PPV, exciting at 393 and 470 nm, respectively. The resulting emission was detected at 530 and 595 nm for BBEHP–PPV, and MEH–PPV, respectively. In solution, the lifetime of BBEHBO–PPV is very similar to that of BBEHP–PPV, with both materials showing a lifetime of ∼0.7 ns for the same concentration of 0.05 mg ml−1 (Table S1 in ESI†). This is longer than MEH–PPV which has a lifetime of 0.4 ns. In the film however, BBEHBO–PPV has a shorter lifetime which is similar to MEH–PPV films (see Fig. 1). The radiative rates were 2.6 × 109, 1.4 × 109 and 2.94 × 109 s−1 for BBEHBO–PPV, BBEHP–PPV and MEH–PPV, respectively. BBEHBO–PPV has a faster rate (due to the short lifetime) than BBEHP–PPV, much closer to that of MEH–PPV, indicating the effect of the oxymethylene bridges between the benzene ring and bulky substituents, which may enhance the optical transition from an excited state to the ground state.
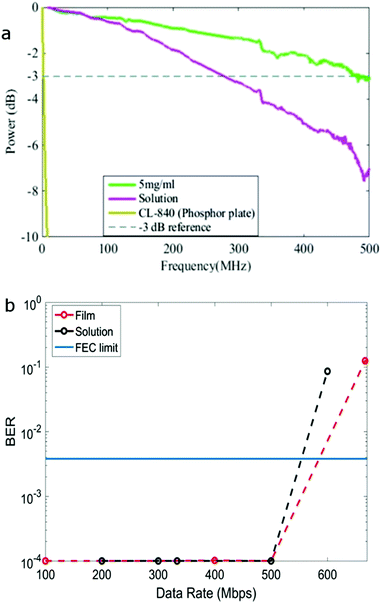
The modulation bandwidth of the colour converter depends on the PL lifetime and BBEHBO–PPV has the shortest life time among a number of colour converters such as BBEHP–PPV7 and BODIPY cored oligo-fluorenes.8 A solution with concentration of 0.025 mg ml−1 and solid state film spin-coated from a solution of concentration 5 mg ml−1 were tested. Fig. 2a shows the measured electrical power attenuation against the frequency of BBEHBO–PPV in film and solution. A reference −3 dB power point (which refers to the electrical bandwidth of the system) is also shown. The BBEHBO–PPV film has a bandwidth of ∼470 MHz independent of the concentration of solutions used for spin-coating (see ESI†). This is significantly higher than that of the phosphor which is measured at 4.4 MHz7 and other organic semiconductors, such as BBEHP–PPV, Super Yellow and BODIPY Y3, which exhibited bandwidths of 130, 90 and 39 MHz, respectively.6–8 In solution, it has a lower bandwidth of 280 MHz, which is consistent with the longer PL lifetime.
In this paper, we used Pulse Amplitude Modulation (PAM) schemes with 2 and 4 levels (see ESI† for latter) to undertake data transmission. The study of higher multilevel modulation schemes is not presented as they offered inferior performance in comparison to 2 and 4-PAM. The measured bit error rate is shown as a function of transmitted data rate in Fig. 2b. Eye-diagrams at different data rates are shown in ESI.† A clear eye-opening is demonstrated at 500 Mbps (Fig. S6 and S7, ESI†) indicating error free performance at this rate. Considering a forward error correction (FEC) error floor of 3.8 × 10−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (shown as solid line in Fig. 2b), the achievable data rate for PAM-2 and PAM-4 is >550 Mbps. The performance in the current set up is limited by the APD receiver which has bandwidth of less than 100 MHz. By adopting a complex modulation scheme, such as orthogonal frequency division multiplexing and/or equalisation, a significantly higher data rate could be achieved.21
20 (shown as solid line in Fig. 2b), the achievable data rate for PAM-2 and PAM-4 is >550 Mbps. The performance in the current set up is limited by the APD receiver which has bandwidth of less than 100 MHz. By adopting a complex modulation scheme, such as orthogonal frequency division multiplexing and/or equalisation, a significantly higher data rate could be achieved.21
Conclusion
In conclusion, we present a new organic colour converter material that has a red-shifted emission with a PLQY ≥40% in both solution and film states. It has been designed on the basis of two materials, exhibiting the advantages of both high PLQY and a fast decay rate. It also achieves an exceptionally high bandwidth of 470 MHz in film and allows a data transmission rate of 550 Mbps with multi-level data encoding. This bandwidth is the highest reported for any colour converter material. The data rate is 55 times more than that measured with commercially available phosphor colour converters.7 Such colour converters pave the way for high bandwidth materials that can suitably replace phosphors.Experimental methods
Material synthesis
The general experimental methods for the synthesis and characterisations of the new compounds as well as the exact procedures for the syntheses are presented in ESI.†Photophysical measurements
Solutions of BBEHBO–PPV were made by mixing 0.025 mg in 1 ml of toluene for solution measurements and 5 mg in 1 ml for film measurements. Films were made by spin coating the solution at 1500 rpm for 60 s in a nitrogen glovebox. For communications measurements, samples were encapsulated using two layers of glass and sealed using UV cured epoxy. Absorption and photoluminescence measurements were conducted using a Cary 300 UV-vis spectrophotometer and an Edinburgh Photonics Instrument FLS980, respectively. PL lifetime measurements were conducted using time correlated single photon counting (FLS980). The samples were excited with a PicoQuant, pico-second laser at 393 and 470 nm. Transient absorption measurements were conducted using a fs PHAROS laser system and excited at 515 nm. Transient absorption measurements were conducted using a fs PHAROS laser system. The sample was excited at 515 nm delayed against a white light continuum probe. PLQY was measured with a Hamamatsu integrating sphere C9920-02 luminescence measurement system using 450 nm excitation.Frequency response
In order to measure the intrinsic bandwidth of the material, a blue laser excited the material and PL emission was captured at the receiver (see Fig. S4, ESI†). Optical filters were used to ensure that there is no residual blue emission at the receiver. In order to measure the frequency response of PL emission from BBEHBO–PPV, the system was first calibrated following the experimental procedure reported in ref. 7. First the receiver was directly illuminated with the laser diode, and then the frequency response of the BBEHBO–PPV was established by deconvolution of the response of the other components in the system.Communication test
In order to test the communication capability of the BBEHBO–PPV, a free space link of 15 cm was created and the polymer excited by an intensity modulated, blue laser diode. As with measuring the case of the frequency response, optical filters were placed in front of the APD to cut off the residual blue light. The simplest form of modulation for VLC system is baseband binary pulse amplitude modulation (PAM-2), also known as on–off Keying (OOK). In PAM-2, a binary ‘one’ is represented by an optical pulse of bit duration and a binary ‘zero’ by absence of the optical pulse. At the receiver, a fixed threshold can be applied to detect the signal i.e. if the received signal is above the threshold level, it is assumed to be one, and otherwise it is zero. A multilevel modulation which can effectively utilise the bandwidth can also be adopted.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank EPSRC for financial support from the UP-VLC Project Grant (EP/K00042X/1) and grant EP/009908/1. I. D. W. S. and P. J. S. are Royal Society Wolfson Research Merit Award holders. The research data supporting this publication can be accessed at http://dx.doi.org/10.15129/d8fbd4d0-a6f1-493c-abae-95ff2b54fede.Notes and references
- O. Ergul, E. Dinc and O. B. Akan, Phys. Commun., 2015, 17, 72–85 CrossRef.
- H. Chun, S. Rajbhandari, G. Faulkner and D. O'Brien, Optical Wireless Communications (IWOW), 2014 Search PubMed.
- F. Hide, P. Kozodoy, S. P. DenBaars and A. J. Heeger, Appl. Phys. Lett., 1997, 70, 2664–2666 CrossRef CAS.
- D. DiMartino, L. Beverina, M. Sassi, S. Brovelli, R. Tubino and F. Meinardi, Sci. Rep., 2014, 4, 4400 CrossRef PubMed.
- N. J. Findlay, J. Bruckbauer, A. R. Inigo, B. Breig, S. Arumugam, D. J. Wallis, R. W. Martin and P. J. Skabara, Adv. Mater., 2014, 26, 7290–7294 CrossRef CAS PubMed.
- H. Chun, P. Manousiadis, S. Rajbhandari, D. A. Vithanage, G. Faulkner, D. Tsonev, J. J. D. McKendry, S. Videv, E. Y. Xie, E. D. Gu, M. D. Dawson, H. Haas, G. A. Turnbull, I. D. W. Samuel and D. C. O'Brien, IEEE Photonics Technol. Lett., 2014, 26, 2035–2038 CrossRef CAS.
- M. T. Sajjad, P. P. Manousiadis, H. Chun, D. A. Vithanage, S. Rajbhandari, A. L. Kanibolotsky, G. Faulkner, D. O'Brien, P. J. Skabara, I. D. W. Samuel and G. A. Turnbull, ACS Photonics, 2015, 2, 194–199 CrossRef CAS.
- M. T. Sajjad, P. P. Manousiadis, C. Orofino, D. Cortizo-Lacalle, A. L. Kanibolotsky, S. Rajbhandari, D. Amarasinghe, H. Chun, G. Faulkner, D. C. O'Brien, P. J. Skabara, G. A. Turnbull and I. D. W. Samuel, Adv. Opt. Mater., 2015, 3, 536–540 CrossRef CAS.
- A. Rose, Z. Zhu, C. F. Madigan, T. M. Swager and V. Bulović, Nature, 2005, 434, 876–879 CrossRef CAS PubMed.
- D. Braun and A. J. Heeger, Appl. Phys. Lett., 1991, 58, 1982–1984 CrossRef CAS.
- F. Hide, M. A. DiazGarcia, B. J. Schwartz, M. R. Andersson, Q. B. Pei and A. J. Heeger, Science, 1996, 273, 1833–1836 CrossRef CAS.
- G. Tsiminis, Y. Wang, A. L. Kanibolotsky, A. R. Inigo, P. J. Skabara, I. D. W. Samuel and G. A. Turnbull, Adv. Mater., 2013, 25, 2826–2830 CrossRef CAS PubMed.
- D. Amarasinghe, A. Ruseckas, A. E. Vasdekis, M. Goossens, G. A. Turnbull and I. D. W. Samuel, Appl. Phys. Lett., 2006, 89, 201119 CrossRef.
- Y. Wang, P. O. Morawska, A. L. Kanibolotsky, P. J. Skabara, G. A. Turnbull and I. D. W. Samuel, Laser Photonics Rev., 2013, 7, L71–L76 CrossRef CAS PubMed.
- B. C. Thompson, Y. G. Kim, J. R. Reynolds and J. R. Spectral, Macromolecules, 2005, 38, 5359–5362 CrossRef CAS.
- T.-W. Zeng, Y. Lin, H.-H. Lo, C.-W. Chen, C.-H. Chen, S.-C. Liou, H.-Y. Huang and W.-F. Su, Nanotechnology, 2006, 17, 5387 CrossRef CAS.
- C. Weder and M. S. Wrighton, Macromolecules, 1996, 29, 5157–5165 CrossRef CAS.
- D. A. M. Egbe, C. P. Roll, E. Birckner, U.-W. Grummt, R. Stockmann and E. Klemm, Macromolecules, 2002, 35, 3825–3837 CrossRef CAS.
- L. Hintermann and K. Suzuki, Synthesis, 2008, 2303–2306 CAS.
- ITU, Forward referror correction for high bit-rate DWDM submarine systems ITU, 2004.
- D. Tsonev, H. Chun, S. Rajbhandari, J. McKendry, S. Videv, E. Gu, M. Haji, S. Watson, A. Kelly, G. Faulkner, M. Dawson, H. Haas and D. O'Brien, IEEE Photonics Technol. Lett., 2014, 26, 637–640 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7tc03787b |
| This journal is © The Royal Society of Chemistry 2017 |