Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
ESIPT-based fluorescence probe for the rapid detection of peroxynitrite ‘AND’ biological thiols†
Luling
Wu‡
 a,
Hai-Hao
Han‡
a,
Hai-Hao
Han‡
 b,
Liyuan
Liu
b,
Liyuan
Liu
 a,
Jordan E.
Gardiner
a,
Jordan E.
Gardiner
 a,
Adam C.
Sedgwick
a,
Adam C.
Sedgwick
 ac,
Chusen
Huang
ac,
Chusen
Huang
 *d,
Steven D.
Bull
*d,
Steven D.
Bull
 *a,
Xiao-Peng
He
*a,
Xiao-Peng
He
 *b and
Tony D.
James
*b and
Tony D.
James
 *a
*a
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; s.d.bull@bath.ac.uk
bKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Rd., Shanghai 200237, China. E-mail: xphe@ecust.edu.cn
cDepartment of Chemistry, University of Texas at Austin, 105 E 24th Street A5300, Austin, TX 78712-1224, USA
dThe Education Ministry Key Laboratory of Resource Chemistry, Shanghai Key Laboratory of Rare Earth Functional Materials, and Shanghai Municipal Education Committee Key Laboratory of Molecular Imaging Probes and Sensors, Department of Chemistry, Shanghai Normal University, 100 Guilin Road, Shanghai 200234, China. E-mail: huangcs@shnu.edu.cn
First published on 11th September 2018
Abstract
An ESIPT-based ‘AND’ logic fluorescence probe (GSH-ABAH) was developed for the simultaneous detection of ONOO− and biological thiols. GSH-ABAH was shown to have good cell permeability and with the addition of just SIN-1 (ONOO− donor) or GSH, no fluorescence response was observed in live cells. However, in the presence of both analytes GSH-ABAH could be used to image exogenous ONOO− ‘AND’ GSH added to RAW264.7 cells.
Peroxynitrite (ONOO−) is a highly reactive nitrogen species1 with an incredibly short biological half-life (<10 ms).2 ONOO− is known for its deleterious effects, causing irreversible damage to a range of biological targets such as lipids, proteins and nucleic acids.3 As a result, abnormal concentrations of ONOO− are thought to be associated with inflammation, cancer, atherosclerosis and neurodegenerative diseases.4–7 In addition, biological thiols such as glutathione (GSH) and cysteine (Cys) are essential in maintaining biological redox homeostasis.8–10
GSH is a natural tripeptide (γ-L-glutamyl-cysteinyl-glycine) that exists in the thiol reduced form (GSH) and disulphide-oxidised (GSSG) form.11 GSH is the predominant form, which exists in mammalian and eukaryotic cells where it functions as an antioxidant.12–14 More importantly, GSH serves as an ONOO− scavenger through its direct oxidation by ONOO−.15 Therefore, it is common to find elevated levels of GSH when cells are undergoing oxidative stress. Therefore, the susceptibility of a cell towards ONOO− largely depends on the concentration of intracellular GSH.7,16,17
Within our research groups, we are interested in developing small molecule fluorescent probes for the detection of biological reactive oxygen species as well as biological thiols.18–21 While many literature reported fluorescent probes have been used to understand the roles of single chemical species, which include metal ions22 and reactive oxygen species23,24 in biological systems.25 Relatively, few probes have been developed to report on the role of two or more analytes in a biological system. In parallel to the development of fluorescent probes, the field of molecular logic gates has developed.26,27
Molecular logic gates are molecules that have the ability to bind to multiple analytes and transform the multiple binding events to a measurable output. Recently, we have developed dual activated fluorescent probes. Where, the ‘AND’ logic operation requires two analytes to produce a positive output signal. These ‘AND’ logic systems have the ability to detect two different analytes within the same biological sample and hence provide a simple approach for monitoring complex bimolecular events, where two species may be intimately responsible for a particular disease.28
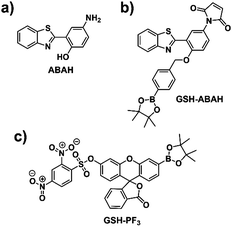
Dual fluorescence based probes for monitoring the relationship between ONOO− and GSH are uncommon,29,30 despite numerous fluorescence based probes being developed for the sensing of these analytes separately.31,32 Recently, we have developed a fluorescein-based ‘AND’ logic gate, which was capable of detecting ONOO− ‘AND’ GSH in cells (Fig. 1c).33 ‘AND’ logic based fluorescence probes for ONOO− ‘AND’ GSH are of particular interest as they could potentially be used to evaluate the therapeutic efficacy of a particular treatment towards Alzheimer's disease.34
In this work, we set out to improve on our earlier system by developing an excited state intramolecular proton transfer (ESIPT) ‘AND’ logic gate for the simultaneous detection of ONOO− ‘AND’ GSH. Owing to the attractive characteristics of ESIPT fluorophores, which include: ratiometric sensing, large stokes shift and environmental sensitivity. Essentially, if a ratiometric system could be developed then this would be a significant advance, potentially allowing for calibration free monitoring.35–37
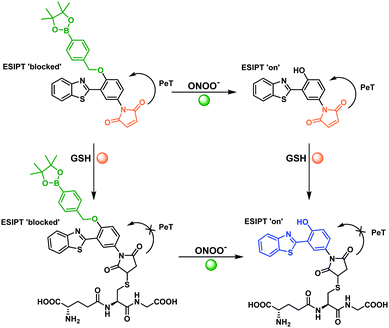
4-Amino-2-(benzo[d]thiazol-2-yl)phenol (ABAH) was regarded as an ideal ESIPT fluorophore for the development of an ‘AND’ based fluorescence probe due to having a free phenol and amino group, which can be independently derivatized (Fig. 1 and Scheme S1, ESI†).36,38–41 We believed the functionalization of the free phenolic unit of ABAH with a benzyl boronic ester would block the ESIPT process and serve as the reactive unit for ONOO−. Due to aromatic boronates having a greater reactivity towards ONOO− over HClO/ClO− and H2O2.42 Previously, the functionalization of the amino group of ABAH with the thiol-reactive maleimide group resulted in the quenching of the fluorescence intensity due to a PET process. However, in the presence of biological thiols the fluorescence intensity was rapidly restored.43 Therefore, we thought that the combination of these two reactive units with ABAH would result in an effective PET+ESIPT ‘AND’-logic probe for the detection of ONOO− ‘AND’ biological thiols (Fig. 1 and Scheme 1).
To test this hypothesis, we synthesized probe GSH-ABAH over three steps (Scheme S2 – see ESI†). ABAH was first synthesized in excellent yield (73%) by heating 2-aminothiophenol and 5-aminosalicylic acid in polyphosphoric acid (PPA) at 180 °C. With ABAH in hand, maleic anhydride was then added to a solution of ABAH in glacial acetic acid. This condensation reaction was performed under reflux for 4 hours to afford the desired intermediate 2 as a yellow solid. 2 was then alkylated using (4-bromomethylphenyl)boronic acid pinacol ester and K2CO3 in DMF to afford GSH-ABAH in 27% yield (Scheme S2, ESI†). The chemical structure of GSH-ABAH was fully characterized by 1H NMR, 13C NMR and high resolution mass spectrometry (HRMS).
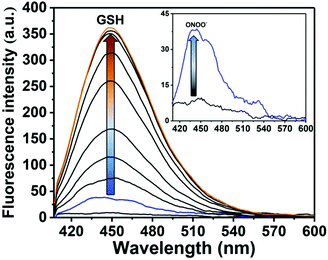
We then evaluated the changes in the UV-Vis absorption of GSH-ABAH in the presence of both GSH and ONOO−. The maximum absorption of GSH-ABAH at 326 nm shifted to 370 nm with the addition of ONOO− while the absorption peak does not change with addition of GSH, which is consistent with the PET process (Fig. S1 and S2, ESI†). Fluorescence experiments with ONOO− were then carried out. As shown in Fig. 2 and Fig. S3 (ESI†), GSH-ABAH was initially non-fluorescent, however upon the addition of ONOO− (4 μM), a small fluorescence increase was observed. However, a large increase in fluorescence intensity (>10-fold, see Fig. 2 and Fig. S4, ESI†) was then observed following the subsequent addition of GSH (0–2 μM). This observation demonstrated the requirement of both ONOO− ‘AND’ GSH to obtain a significant turn “on” fluorescence response.
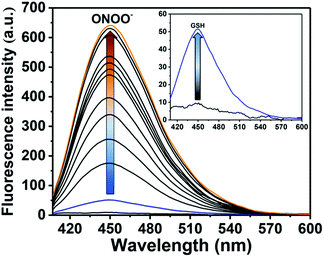
The addition of both analytes was then carried out in reverse order. Similarly, the addition of GSH (5 μM) only resulted in a small increase in fluorescence intensity (Fig. 3 and Fig. S5, ESI†). However, as expected a large fluorescence increase was observed after the subsequent addition of ONOO− (0–14 μM) (Fig. 3 and Fig. S6, ESI†).
Next, we evaluated the selectivity of probe GSH-ABAH towards a number of biologically relevant amino acids including serine, lysine and methionine (Fig. S7, ESI†). The amino acids without a thiol (S–H) group led to no change in fluorescence intensity of GSH-ABAH. However, as predicted, thiol (S–H) containing biological analytes (glutathione, cystine and homocystine) induced an enhancement in fluorescence intensity. While GSH-ABAH demonstrated an excellent selectivity for ONOO− over reactive oxygen/nitrogen species including H2O2 (Fig. S8, ESI†).
We then carried out kinetic studies for GSH-ABAH with both ONOO− and GSH (Fig. S9 and S10, ESI†). After initial addition of GSH or ONOO−, followed by the subsequent addition of the second analyte a significant increase in fluorescence within 30 s was observed. HRMS experiments were performed, in order to confirm the reaction mechanism. When 2 eq. of ONOO− (in water) was added to a solution of GSH-ABAH (HRMS in acetonitrile Fig. S11, ESI†) the mass spectra was consistent with deprotection of the phenol (Fig. S12 (ESI†) and Scheme 1). Subsequently, 1 eq. GSH (in water) was added a mass peak at 630.1354 was observed confirming the reaction of GSH with the maleic anhydride group via electrophilic addition (Fig. S13 (ESI†) and Scheme 1). These results clearly demonstrate the ability of GSH-ABAH to perform ‘AND’ logic with ONOO ‘AND’ GSH.
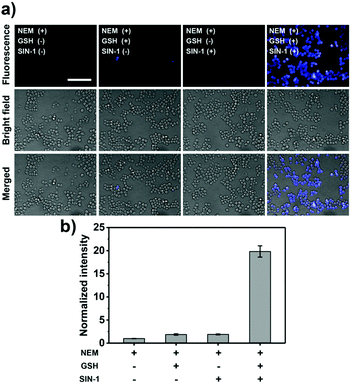
Due to these results, GSH-ABAH was then evaluated for cellular imaging of GSH and ONOO−. RAW264.7 cells were pre-treated with N-ethylmaleimide (NEM, GSH scavenger) before incubation with GSH-ABAH. Subsequently, GSH or SIN-1 (a peroxynitrite donor)15 were added to produce intracellular GSH or ONOO−. As shown in Fig. 4 and Fig. S14 (ESI†), the addition of GSH or ONOO− led to no fluorescence response in cells. However, treatment with both GSH and SIN-1 resulted in a significant increase in the fluorescence intensity enabling the visualisation of both species in living cells.
In summary, we have developed an ESIPT-based ‘AND’ logic fluorescence probe (GSH-ABAH) for the detection of ONOO− and biological thiols. GSH-ABAH was shown to have high sensitivity and selectivity towards ONOO− and biothiols. More importantly, GSH-ABAH was able to visualise exogenous ONOO− and GSH in RAW264.7 cells. This simple novel ‘AND’ logic-based system provides a scaffold for the further development of a multi-analyte probes. We are now turning our attention to the development of longer wavelength ESIPT-based probes for multi-analyte in vivo imaging.
LW wishes to thank China Scholarship Council and the University of Bath for supporting his PhD work in the UK. We would like to thank the EPSRC and the University of Bath for funding. ACS and JEG thank the EPSRC for a studentship. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award. X.-P. He thanks the National Natural Science Foundation of China (21722801 and 21572058), the Programme of Introducing Talents of Discipline to Universities (B16017) and the Shanghai Rising-Star Program (16QA1401400). CH would like to thank the National Natural Science Foundation of China (Grants 21672150, and 213021125), Shanghai “Chenguang” Program (Grant 14CG42), and Program for Changjiang Scholars and Innovative (IRT_16R49). NMR characterisation facilities were provided through the Chemical Characterisation and Analysis Facility (CCAF) at the University of Bath (http://www.bath.ac.uk/ccaf). The EPSRC UK National Mass Spectrometry Facility at Swansea University is thanked for analyses. All data supporting this study are provided as supplementary information accompanying this paper (ESI†).
Conflicts of interest
No conflicts of interest.Notes and references
- C. Szabó, H. Ischiropoulos and R. Radi, Nat. Rev. Drug Discovery, 2007, 6, 662 CrossRef PubMed.
- G. Ferrer-Sueta and R. Radi, ACS Chem. Biol., 2009, 4, 161–177 CrossRef PubMed.
- P. Ascenzi, A. di Masi, C. Sciorati and E. Clementi, BioFactors, 2010, 36, 264–273 CrossRef PubMed.
- H. Ischiropoulos and J. S. Beckman, J. Clin. Invest., 2003, 111, 163–169 CrossRef PubMed.
- P. Sarchielli, F. Galli, A. Floridi, A. Floridi and V. Gallai, Amino Acids, 2003, 25, 427–436 CrossRef PubMed.
- D. A. Wink, Y. Vodovotz, J. Laval, F. Laval, M. W. Dewhirst and J. B. Mitchell, Carcinogenesis, 1998, 19, 711–721 CrossRef PubMed.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef PubMed.
- J. S. Stamler and A. Slivka, Nutr. Rev., 1996, 54, 1–30 Search PubMed.
- S. Iwata, T. Hori, N. Sato, Y. Ueda-Taniguchi, T. Yamabe, H. Nakamura, H. Masutani and J. Yodoi, J. Immunol., 1994, 152, 5633–5642 Search PubMed.
- H. Nakamura, K. Nakamura and J. Yodoi, Annu. Rev. Immunol., 1997, 15, 351–369 CrossRef PubMed.
- G. Wu, Y.-Z. Fang, S. Yang, J. R. Lupton and N. D. Turner, J. Nutr., 2004, 134, 489–492 CrossRef PubMed.
- S. C. Lu, Mol. Aspects Med., 2009, 30, 42–59 CrossRef PubMed.
- S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'agostino, P. W. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476–483 CrossRef PubMed.
- D. M. Townsend, K. D. Tew and H. Tapiero, Biomed. Pharmacother., 2003, 57, 145–155 CrossRef.
- M. Balazy, P. M. Kaminski, K. Mao, J. Tan and M. S. Wolin, J. Biol. Chem., 1998, 273, 32009–32015 CrossRef PubMed.
- K.-A. Marshall, M. Reist, P. Jenner and B. Halliwell, Free Radical Biol. Med., 1999, 27, 515–520 CrossRef PubMed.
- J. P. Bolaños, S. J. Heales, J. M. Land and J. B. Clark, J. Neurochem., 1995, 64, 1965–1972 CrossRef.
- A. C. Sedgwick, J. E. Gardiner, G. Kim, M. Yevglevskis, M. D. Lloyd, A. T. A. Jenkins, S. D. Bull, J. Yoon and T. D. James, Chem. Commun., 2018, 54, 4786–4789 RSC.
- M. L. Odyniec, A. C. Sedgwick, A. H. Swan, M. Weber, T. S. Tang, J. E. Gardiner, M. Zhang, Y.-B. Jiang, G. Kociok-Kohn, R. B. Elmes, S. D. Bull, X.-P. He and T. D. James, Chem. Commun., 2018, 54, 8466–8469 RSC.
- L. Wu, Q. Yang, L. Liu, A. C. Sedgwick, A. J. Cresswell, S. D. Bull, C. Huang and T. D. James, Chem. Commun., 2018, 54, 8522–8525 RSC.
- L. Wu, Y. Wang, M. Weber, L. Liu, A. C. Sedgwick, S. D. Bull, C. Huang and T. D. James, Chem. Commun., 2018, 54, 9953–9956 RSC.
- D. Wu, L. Chen, W. Lee, G. Ko, J. Yin and J. Yoon, Coord. Chem. Rev., 2018, 354, 74–97 CrossRef.
- N. Soh, Anal. Bioanal. Chem., 2006, 386, 532–543 CrossRef PubMed.
- X. Jiao, Y. Li, J. Niu, X. Xie, X. Wang and B. Tang, Anal. Chem., 2017, 90, 533–555 CrossRef PubMed.
- (a) D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC; (b) X.-P. He and H. Tian, Chem, 2018, 4, 246–268 CrossRef; (c) Y. Fu, H.-H. Han, J. Zhang, X.-P. He, B. L. Feringa and H. Tian, J. Am. Chem. Soc., 2018, 140, 8671–8674 CrossRef PubMed; (d) J. Zhang, Y. Fu, H.-H. Han, Y. Zang, J. Li, X.-P. He, B. L. Feringa and H. Tian, Nat. Commun., 2017, 8, 987 CrossRef PubMed.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- J. L. Kolanowski, F. Liu and E. J. New, Chem. Soc. Rev., 2018, 47, 195–208 RSC.
- (a) A. Romieu, Org. Biomol. Chem., 2015, 13, 1294–1306 RSC; (b) X.-P. He, X.-L. Hu, T. D. James, J. Yoon and H. Tian, Chem. Soc. Rev., 2017, 46, 6687–6696 RSC.
- F. Yu, P. Li, G. Li, G. Zhao, T. Chu and K. Han, J. Am. Chem. Soc., 2011, 133, 11030–11033 CrossRef PubMed.
- F. Yu, P. Li, B. Wang and K. Han, J. Am. Chem. Soc., 2013, 135, 7674–7680 CrossRef PubMed.
- S. Wang, L. Chen, P. Jangili, A. Sharma, W. Li, J.-T. Hou, C. Qin, J. Yoon and J. S. Kim, Coord. Chem. Rev., 2018, 374, 36–54 CrossRef.
- H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019–6031 RSC.
- A. C. Sedgwick, H.-H. Han, J. E. Gardiner, S. D. Bull, X.-P. He and T. D. James, Chem. Sci., 2018, 9, 3672–3676 RSC.
- C. B. Pocernich and D. A. Butterfield, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822, 625–630 CrossRef PubMed.
- V. V. Shynkar, A. S. Klymchenko, C. Kunzelmann, G. Duportail, C. D. Muller, A. P. Demchenko, J. M. Freyssinet and Y. Mely, J. Am. Chem. Soc., 2007, 129, 2187–2193 CrossRef PubMed.
- M. Santra, B. Roy and K. H. Ahn, Org. Lett., 2011, 13, 3422–3425 CrossRef PubMed.
- J. E. Kwon and S. Y. Park, Adv. Mater., 2011, 23, 3615–3642 CrossRef PubMed.
- K. S. Hwang, K. Y. Park, D. B. Kim and S.-K. Chang, Dyes Pigm., 2017, 147, 413–419 CrossRef.
- Y. Zhao, Y. Xue, H. Li, R. Zhu, Y. Ren, Q. Shi, S. Wang and W. Guo, Spectrochim. Acta, Part A, 2017, 175, 215–221 CrossRef PubMed.
- H. Yao and T. Funada, Chem. Commun., 2014, 50, 2748–2750 RSC.
- L. Cui, W. Zhu, Y. Xu and X. Qian, Anal. Chim. Acta, 2013, 786, 139–145 CrossRef PubMed.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radical Biol. Med., 2009, 47, 1401–1407 CrossRef PubMed.
- T. Matsumoto, Y. Urano, T. Shoda, H. Kojima and T. Nagano, Org. Lett., 2007, 9, 3375–3377 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8cc06917d |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2018 |