Open Access Article
Open Access ArticleLarge-scale synthesis of carbon dots/TiO2 nanocomposites for the photocatalytic color switching system†
Aiwu
Wang‡
ab,
Xufen
Xiao‡
b,
Cangtao
Zhou
acd,
Fucong
Lyu
b,
Li
Fu
e,
Chundong
Wang
 *f and
Shuangchen
Ruan
ac
*f and
Shuangchen
Ruan
ac
aCenter for Advanced Material Diagnostic Technology, Shenzhen Technology University, Shenzhen 518118, People's Republic of China
bCenter of Super-Diamond and Advanced Films (COSDAF), City University of Hong Kong, Hong Kong, People's Republic of China
cCollege of Applied Technology, Shenzhen University, Shenzhen 518060, People's Republic of China
dCenter for Applied Physics and Technology, HEDPS, School of Physics, Peking University, Beijing 100871, People's Republic of China
eCollege of Materials and Environmental Engineering, Hangzhou Dianzi Univerisity, Hangzhou 310018, People's Republic of China
fSchool of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan 430074, People's Republic of China. E-mail: apcdwang@hust.edu.cn
First published on 28th February 2019
Abstract
In view of the easy control and contactless spatial nature of light, the photoreversible color switching system has attracted tremendous attention. Although some progress has been achieved in the past few years, the practical applications have been limited by the complicated preparation process, material toxicity and low reaction yield. Herein, we report a rapid, a one-pot large-scale synthesis approach for the preparation of carbon dots (CDs)/TiO2 nanocomposites via the thermal condensation at 160 °C, affording high photocatalytic color switching on/off performance. Under ambient conditions and with the introduction of some oxygen gas, MB rapidly changed from blue to colorless in one minute under UV-vis irradiation and recovered (again showed its original blue color) in twenty minutes. We anticipate that the designed low-cost and green carbon dots (CDs)/TiO2 nanocomposites have much potential in practical applications and represent a solid step toward color switching applications.
Introduction
Photoreversible color switching materials have attracted increasing interest because of their potential applications in developing displays, sensors and optoelectronic devices.1–4 Compared with other stimuli, such as heat, electric and magnetic fields, light has attracted the most attention because of its non-invasive manner and the precise control that can be achieved.5 The classical photoresponsive molecules, such as azobenzene, undergo cis–trans isomerizations (color change) with light irradiation.6 However, the incomplete reversibility of cis–trans isomerizations and side reactions can hardly make this a practical photo-switching application. Dithienylethene as photochromic organic compound has attracted interest due to its remarkable fatigue resistance.7 Nevertheless, its high-cost and complex preparation restricts its practical applications. Therefore, the development of low-cost, facile and large scale preparation methods for photochromic systems is extremely important.Methylene blue (tetramethylthionine chloride C16H18ClN3S, MB) is a heterocyclic, aromatic, and blue-coloured dye in an oxidizing environment with a λmax value of 660 nm. It is widely used in the different fields of biology, chemistry and in industry. The photochemistry of MB has been extensively studied and since the 1980s, MB remains a popular dye, used in inks and as a photo-sensitiser due to its long lifetime and high triplet oxidation potential.8 It can be photoreduced to leuco-methylene blue (LMB, colorless with a λmax values of 256 nm) in the presence of a sacrificial electron donor (SED), which is stable in de-aerated aqueous solutions.8 Upon the addition of oxygen, the LMB can be oxidised back to MB as a reversible photochromic system. Semiconductors with suitable band gaps, such as TiO2,9–13 Bi2WO6 (ref. 14) and SnO2, are usually used in these systems.15,16 Thus, a combination of TiO2, MB and a SED17 forms a photochromic system. Blue MB can be transformed to colorless LMB upon UV irradiation by an SED, where the SED, such as methanol, certain polymers or sodium citrate, is used to scavenge the photogenerated holes leaving the remaining photogenerated electrons to reduce the MB to LMB. The LMB would then transform back to MB in the presence of oxygen. Of note, the photochromic system requires the photobleaching of MB (MB to LMB) rather than its decomposition (which is irreversible). Yin et al. reported a titanium oxide-assisted photocatalytic reaction used for light-printable rewritable paper.18 The same group also used barium-doped TiO2,19 P123 TiO2,9 transition metal hexacyanometalate nanoparticles20 and SnO2 (ref. 16) in the photochromic systems. Nonetheless, these fabrication approaches involve the hydrolysis reactions under high temperatures, limiting the large-scale production of TiO2.
In this study, we report a low-cost, facile and very reproducible method for the large-scale synthesis of CDs/TiO2 nanocomposites as a photochromic system toward the reduction of MB and oxidation of LMB under different situations. It is the first time that the CDs/TiO2 nanocomposites have been used for photocatalytic color switching. CDs as a 0D materials (smaller than 10 nm) are usually composed of sp2/sp3 hybridized carbon atoms with various surface functional groups and excitation-dependent photoluminescence.21–27 In recent years, low cost, non-toxic, environmentally-friendly, large scale and easy to synthesize carbon dots (CDs) have greatly inspired their use in photocatalytic applications.28–30 The CDs act as an effective SED, avoiding the decomposition of MB and reducing the MB to LMB with the assistance of TiO2 (P25) under the UV-vis irradiation in one minute, while the colorless LMB can be switched back to blue MB through exposure to oxygen in twenty minutes. Moreover, the cycling performance could remain near 70% after six cycles.
Results and discussion
P25 is widely used commercial photocatalyst with a band gap of 3.8 eV. The size of P25 is around 20 nm, which is consistent with what is seen in the Fig. 1A, a TEM image shows that the CDs/TiO2 nanocomposites have irregular shape compared with that of the pure P25 particles. As shown in the TEM images, the size of the CDs/TiO2 nanocomposites were in the range of 10–30 nm, which is in agreement with the previous research. An HRTEM image was obtained to further confirm the coupling of CDs with P25 (Fig. 1B) with the interplanar spacings of 0.24 nm. As shown in Fig. 1C, the interplanar spacings of 0.350 nm and 0.24 nm agree well with the (101) plane of anatase TiO2 and the (001) plane of rutile TiO2, respectively (JCPDS card no. 21-1272), indicating the presence of P25. The interplanar spacing of 0.21 nm assigned to the CDs can also be observed in Fig. 1B, demonstrating the successful coupling of the CDs with P25. Moreover, Fig. 1C displays elemental dot maps of the CDs/TiO2 nanocomposites, with the distribution maps of the constituent elements, Ti, C, and N in the panels, respectively, showing that the materials consist of a relatively large amount of carbon and a small amount of nitrogen, indicating that the nitrogen-doped carbon dots have been decorated with P25. The nitrogen (N) element has been widely used for chemical doping of the CDs. We show that the N-CDs not only feature small-size particles with a rich nitrogen (18%) content but also can exhibit bright blue photoluminescence with a high quantum efficiency as well as excellent fluorescent stability in aqueous solution. These unique features, together with the advantages of non-toxicity and good biocompatibility allow the CDs to be suitable candidates as a new SED coupling with P25 in the photochromic system. Furthermore, XRD characterization was performed to confirm the formation of CDs/TiO2 nanocomposites. As shown in Fig. 1D, the broad peak at 25° is attributed to graphitic CDs,23 corresponding to the (002) crystal plane. The sample of CDs/TiO2 nanocomposites gives rise to the XRD peaks of both CDs and P25, indicating the existence of CDs in the composites. It should be noted that it is not easy to observe the peak of CDs in the composites when the content of CDs is low.31 Therefore, the content of CDs needs to be high in the composites to be beneficial to the photochromic system. The introduction of CDs to the P25 leads to a considerable broad light absorption in the visible range, as shown in Fig. 1E. Pure P25 mainly absorbs UV light with an absorption edge of 330 nm, originating from a band gap of 3.8 eV, in the case of P25, only UV light can be absorbed. CDs/TiO2 nanocomposites can absorb visible light due to the existence of CDs.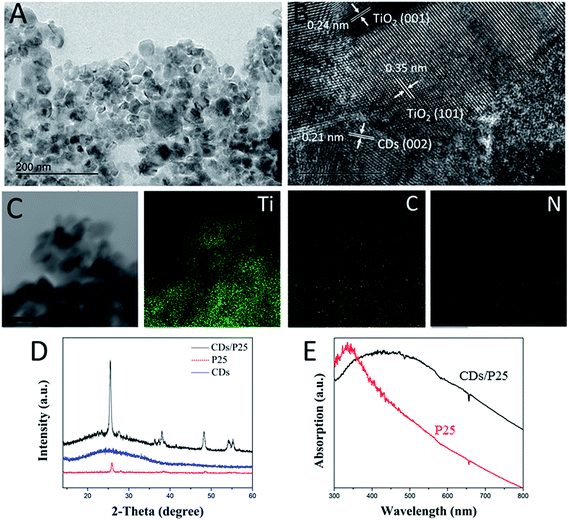 | ||
| Fig. 1 (A) TEM image, (B) HRTEM image and (C) element mapping of CDs/TiO2 nanocomposites, scale bar is 50 nm. (D) XRD patterns of P25, CDs and CDs/P25. (E) UV-vis spectrum of P25 and CDs/P25. | ||
In this one-pot synthesis, the precursors of carbon dots (citric acid as the carbon source and urea as the nitrogen source) and P25 powers were ground together and kept at 160 °C for several minutes, under the heating the carbon dots formed on the surface of P25. The process is very facile and can be carried out on a large scale under the low temperature.
To further confirm the interaction of the carbon dots with P25, XPS spectrum was obtained to study the surface properties and components of the CDs/TiO2 nanocomposites. The full survey spectrum in Fig. 2A indicates the existence of Ti (2p), C (1s), N (1s), O (1s) in the composites. The Ti 2p spectrum has two peaks at 458.4 eV and 464.2 eV, corresponding to the Ti 2p1/2 and Ti 2p3/2 of P25, respectively, while there is slight blue shift towards a higher binding energy compared with those in the spectrum of pure P25 (458.7 eV and 464.6 eV),32 indicating that the CDs could scavenge photo-generated holes and reduce the electron–hole recombination. The high-resolution C 1s spectrum in Fig. 2C has three peaks at 284.4 eV, 286.6 eV and 288.8 eV, which are contributed to the C–C bonds with sp2 orbital, C–N bonds with sp2 orbital and C–O bonds with oxygen-rich groups, respectively. No peak of C–Ti bond is observed in the high-resolution spectrum (only peaks for C 1s and Ti 2p), which illustrates that CDs couple on the surface on the P25. This behaviour is crucial in the photochromic system, since band-engineering, such as dopants in TiO2, usually lowers the band gap and greatly enhances the photocatalytic performance of TiO2. However, in such a case, photodegradation occurs more easily than the reduction of MB. Moreover, features at 398.7 eV (pyridinic) and 400.4 eV (amino) are observed in the N 1s spectrum, confirming the presence of nitrogen-doped CDs and –NH2 groups on the surface of CDs. No peak of Ti–N33(396 eV) is identified in the spectrum of N 1s, which reaffirms the presence of nitrogen-doped carbon dots on the surface of P25. Three features at 529.8 eV, 531.8 eV and 533.2 eV in the deconvoluted O 1s (Fig. S1†) are assigned to Ti–O, C–O and H–O,34 respectively. It suggests that the connection between CDs and P25 is due to the bond of Ti–O–C, indicating that the hydroxyl (–OH) groups should be on the surface of the CDs. Thus, chemical bonding of Ti–O–C should be formed between P25 and CDs, evidencing that CDs are not simple mixed with P25 physically. On the other hand, given that no peaks of Ti–C and Ti–N were discerned in the XPS spectrum, the introduced CDs are not working as the dopants because there is no evidence to suggest that visible light was excited from the CDs/TiO2 nanocomposites (see Fig. S2†).
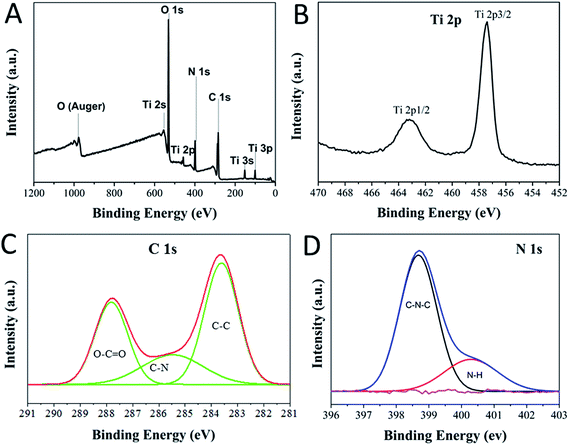 | ||
| Fig. 2 XPS spectra of CDs/TiO2 nanocomposites: (A) survey spectra, and (B) Ti 2p, (c) C 1s, and (d) N 1s core level spectra. | ||
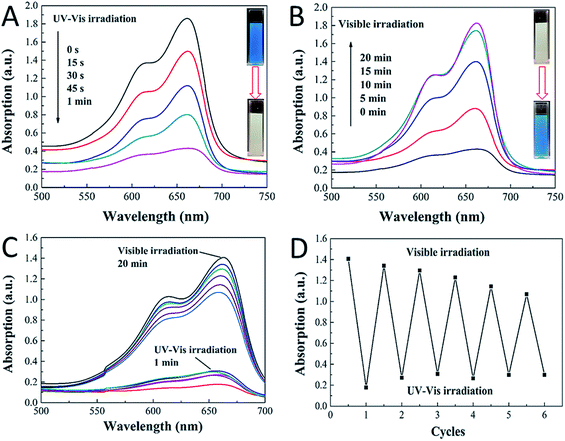
We then tested the photoreversible color switching systems with the obtained CDs/TiO2 nanocomposites mixed with MB dyes. The MB, which has an absorption band at 664 nm, can be photobleached by the composites in one minute upon UV-vis irradiation (152.6 mW cm−2). As shown in Fig. 3A, the blue color mixture turned to colorless due to the formation of LMB from MB. The blue color was then fully covered after 20 minutes under visible light (131.6 mW cm−2) (see Fig. 3B). The blue-colorless-blue process was repeated six times by irradiating the CDs/TiO2 nanocomposites in the MB solution with UV-vis light for 1 min, followed by exposure to visible light for 20 minutes, as shown in Fig. 3C. Thus, the rewritable paper could be fabricated with the help of hydroxyethyl cellulose, in which the light-printed information could be retained for over 20 minutes, being suitable for applied in the information security field. There are nearly 70% intensity after 6 cycles, as shown in Fig. 3D, but for practical applications, the photochromic system could only be used for no more than six times. The reduction of intensity and reversibility should be attributed to some irreversible photodecomposition of MB. Although it is unlikely that the cycling performance of the CDs/TiO2 nanocomposites will be able to improve on what was reported by the Yin et al.,9,16,18–20 the composites still have practical applications owing to their low-cost, facile and high-yield features. Compared with other photochromic systems, our CDs/TiO2 nanocomposites in the MB solution exhibit advantages of high reproducibility, superior switching rates and earth-abundant (the precursor of urea, citric acid and P25 are widely used in industries).
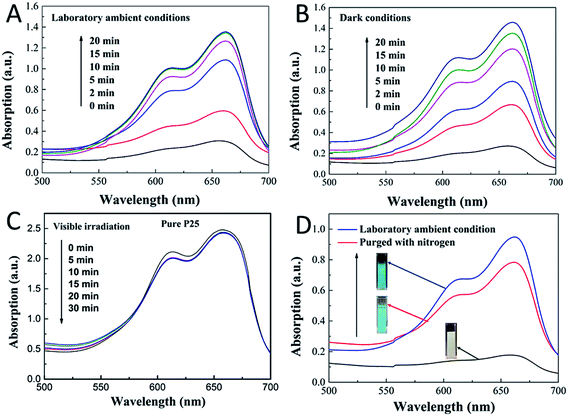
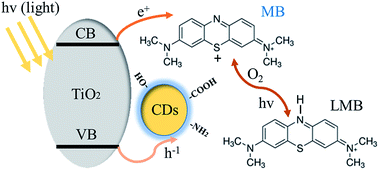
To further understand the mechanism of this novel photochromic system, we tried different conditions during the colorless and recoloration processes. We tested the photochromic systems under visible light by using a bandpass filter of 420 nm; however, no photobleaching of the MB was observed even after twenty minutes, as shown in Fig. S2.† This is because the introduction of CDs did not influence the band gap of P25; thus, the CDs-modified P25 still cannot be excited by visible light, which is same with the pure P25. This demonstrates that UV irradiation is a key to the process of photo-bleaching in this photochromic system. However in the recoloration process, the visible irradiation is dispensable. In a previous report,9 the visible light could promote the recoloration rate of the system, which is much faster than under other conditions, such as in a dark environment. In our system, whether under visible light, ambient laboratory conditions, or even under dark conditions, the recoloration occurred in the same time (20 minutes), as shown in Fig. 3B, 4A and B. Clearly the oxygen is the key in the recoloration process, which has been reported by many groups.8,10,13,14,17,35,36 There is nearly no decrease in twenty minutes under visible light and no photobleaching of MB, as shown in Fig. 4C, using the pure P25 nanoparticles as a control. Dissolved oxygen is essential for the conversion of LMB to MB, and as shown in Fig. 4D, under nitrogen exposure, the colorless LMB cannot be fully recovered to MB. Oxygen is in fact the only factor that influences the recoloration process. Even in the dark environment, the colorless material can turn back to blue color in twenty minutes, which is much faster than a previous report (240 minutes under dark conditions9). This conversion of LMB to MB is energy-saving and economic with good repeatability. The corresponding mechanism should be contributed to the carbon dots with rich oxygen-containing functional groups (–OH and –COOH), which is described in the above results. FTIR was further used to confirm the existence of function groups on the surface of CDs, as illustrated in Fig. S3.† The –OH and –COOH groups on the surface of the composites decrease the number of oxygen vacancies. As a result, the system could be regarded as an “oxidized” environment which is very helpful for the formation of MB. MB can then easily be reduced to LMB by ascorbic acid, tertiary amines, sulfonates, or glycerol.15,17 It is the first time that oxygen-rich carbon dots were used as a SED. This novel SED can not only react with the photo-generated holes rapidly, but could also work as an oxygen pump. Thus, carbon dots serving as a strong SED could omit the influence of visible light irradiation. As a strategy of killing two birds with one stone, the carbon dots not only work as a SED (prevent photodegradation of MB) but also conveniently serve as an oxygen donor. The scheme (Fig. 5) shows that the excited electrons are transformed from the conduction band of TiO2 to MB under UV-vis light irradiation, during which the CDs scavenge the photo-generated holes from the valence band of TiO2, leading to the formation of LMB. The novel photochromic system is UV-only absorbing and exhibits spontaneous recoloration. The MB could not be fully recovered due to the partially photodegradation of MB by TiO2 under UV-vis irradiation. As demonstrated in Fig. S4,† there is nearly 70% degradation of MB by pure P25 under UV-vis irradiation in thirty minutes, indicating that a small amount of MB has been photodegraded during the photobleaching time.
Conclusion and outlook
In summary, we have developed a large-scale, one-pot, low-cost, low-temperature and environmental friendly method via a solid state condensation reaction to prepare nitrogen rich carbon dots/TiO2 nanocomposites with small particles sizes as a photoreversible color switching system. In this system, the color change in MB is initiated by using citric acid as the carbon source, urea as the nitrogen source, and P25 as the commercial photocatalyst. More specifically, the interaction between the CDs and TiO2 occurs via the formation of the Ti–O–C bond. CDs with abundant functional groups operate as a SED in the photochromic system. The blue color MB could transform to a colorless LMB in one minute upon UV-vis light irradiation, before the colorless LMB was oxidized to blue MB in the presence of dissolved oxygen in twenty minutes. The carbon dots not only preclude the photodecomposition of MB, but also supply oxygen for this system. Compared with other systems, our system has the advantage of energy saving in the color-recovering process (LMB to MB). After six cycles of recoloration process, the system still maintains 70% of reversibility. This reversible photoreduction behavior can find wide applications in electro-optic devices, oxygen detection and rewritable papers. Considering that the synthetic conditions were quite facile, (low temperature at 160 °C temperature), we could realize the large-scale nitrogen rich CDs/TiO2 nanocomposites for commercial prospects, could be expected for industry activities.Experiment part
Materials and instruments
Citric acid, urea, methylene blue and P25 were purchased from Sigma-Aldrich. All other chemicals were of analytical grade reagents and were used without further purification. Milli-Q water (18.2 MΩ cm−1) was used throughout the experiments.XPS measurements of CDs/TiO2 nanocomposites were conducted on an ESCALAB-MKII 250 photoelectron spectrometer (Thermo, USA). The X-ray diffraction pattern of the N-CDs, P25 and CDs/TiO2 nanocomposites were achieved using a Bruker AXS D8 Quest CMOS diffractometer with Mo Kα radiation (λ = 0.71073 Å), operating at 40 kV and 15 mA. An X-ray diffractometer (D2 Phaser) with Cu-Kα radiation (λ = 1.5405 Å) was used to characterize the morphology of CDs/TiO2 nanocomposites with high-resolution (HR) TEM by JEM-2010 operated at 200 kV. UV-visible spectra were collected using a Varian Cary 50 UV-visible spectrophotometer. FTIR spectra of carbon dots were recorded on a Perkin-Elmer Spectrum 100 FTIR spectrometer.
Synthesis of nitrogen-doped carbon dots/P25 nanocomposites
A mixture containing citric acid (0.2 g) and urea (0.2 g) was ground for twenty minutes, the mixture was then mixed with P25 (0.2 g) and ground again for another twenty minutes. The resulting mixture was then kept at 160 °C for 10 min and then cooled to room temperature. The sample was washed by methanol three times and dried in an 80 °C oven overnight.Synthesis of nitrogen doped carbon dots
A mixture containing citric acid (0.2 g) and urea (0.2 g) was ground for twenty minutes. The resulting mixture was kept at 160 °C for 10 min and then cooled to room temperature. Finally, the products were centrifuged at 5000 rpm three times to remove the large particles in methanol, and the supernatant solution was collected.Photocatalytic test
A 300 W high-pressure Hg arc lamp was used as a light source. A glassfilter (>420 nm) was used to achieve visible light. Samples were analysed by recording the variations of the UV-vis absorption spectra of MB over time, which is at its characteristic wavelength of 664 nm by a UV-vis spectroscopy, periodically.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors appreciate the financial support from the National Key Research and Development Program of China (Grant No. 2017YFE0120500), the National Natural Science Foundation of China (NSFC Grants No. 51502099), and ‘the Fundamental Research Funds for the Central Universities’, HUST: 2018KFYYXJJ051. C. D. W. also acknowledges funding support from the Hubei “Chu-Tian Young Scholar” program. The authors appreciate the technical support from the Analytical and Testing Center of Huazhong University of Science and Technology.References
- Y. L. Rao, C. Hörl, H. Braunschweig and S. Wang, Angew. Chem., Int. Ed., 2014, 53, 9086–9089 CrossRef CAS PubMed.
- L. García-Fernández, C. Herbivo, V. S. M. Arranz, D. Warther, L. Donato, A. Specht and A. del Campo, Adv. Mater., 2014, 26, 5012–5017 CrossRef PubMed.
- J. Bu, K. Watanabe, H. Hayasaka and K. Akagi, Nat. Commun., 2014, 5, 3799 CrossRef CAS PubMed.
- K. Li, Y. Xiang, X. Wang, J. Li, R. Hu, A. Tong and B. Z. Tang, J. Am. Chem. Soc., 2014, 136, 1643–1649 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- M. Natali and S. Giordani, Chem. Soc. Rev., 2012, 41, 4010–4029 RSC.
- W. Zhu, Y. Yang, R. Métivier, Q. Zhang, R. Guillot, Y. Xie, H. Tian and K. Nakatani, Angew. Chem., Int. Ed., 2011, 50, 10986–10990 CrossRef CAS PubMed.
- A. Mills and J. Wang, J. Photochem. Photobiol., A, 1999, 127, 123–134 CrossRef CAS.
- W. Wang, M. Ye, L. He and Y. Yin, Nano Lett., 2014, 14, 1681–1686 CrossRef CAS PubMed.
- S.-K. Lee, M. Sheridan and A. Mills, Chem. Mater., 2005, 17, 2744–2751 CrossRef CAS.
- M. K. Nowotny, L. R. Sheppard, T. Bak and J. Nowotny, J. Phys. Chem. C, 2008, 112, 5275–5300 CrossRef CAS.
- X. Pan, M.-Q. Yang, X. Fu, N. Zhang and Y.-J. Xu, Nanoscale, 2013, 5, 3601–3614 RSC.
- E. J. Son, J. S. Lee, M. Lee, C. H. T. Vu, H. Lee, K. Won and C. B. Park, Sens. Actuators, B, 2015, 213, 322–328 CrossRef CAS.
- K. Lai, W. Wei, Y. Zhu, M. Guo, Y. Dai and B. Huang, J. Solid State Chem., 2012, 187, 103–108 CrossRef CAS.
- A. Mills and D. Hazafy, Sens. Actuators, B, 2009, 136, 344–349 CrossRef CAS.
- D. Han, B. Jiang, J. Feng, Y. Yin and W. Wang, Angew. Chem., Int. Ed., 2017, 56, 7792–7796 CrossRef CAS PubMed.
- Y. Galagan and W.-F. Su, J. Photochem. Photobiol., A, 2008, 195, 378–383 CrossRef CAS.
- W. Wang, N. Xie, L. He and Y. Yin, Nat. Commun., 2014, 5, 5459 CrossRef CAS PubMed.
- W. Wang, Y. Ye, J. Feng, M. Chi, J. Guo and Y. Yin, Angew. Chem., Int. Ed., 2015, 54, 1321–1326 CrossRef CAS PubMed.
- W. Wang, J. Feng, Y. Ye, F. Lyu, Y.-s. Liu, J. Guo and Y. Yin, Nano Lett., 2017, 17, 755–761 CrossRef CAS PubMed.
- H. Yu, R. Shi, Y. Zhao, G. I. Waterhouse, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2016, 28, 9454–9477 CrossRef CAS PubMed.
- A. W. Wang, C. D. Wang, L. Fu, W. N. Wong-Ng and Y. C. Lan, Nano-Micro Lett., 2017, 9, 47 CrossRef PubMed.
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- A. Wang, C. Lee, H. Bian, Z. Li, Y. Zhan, J. He, Y. Wang, J. Lu and Y. Y. Li, Part. Part. Syst. Charact., 2017, 34, 1600258 CrossRef.
- A. Wang, F. Kang, Z. Wang, Q. Shao, Z. Li, G. Zhu, J. Lu and Y. Y. Li, Adv. Sustainable Syst., 2018, 1800132 Search PubMed.
- L. Fu, A. Wang, G. Lai, C.-T. Lin, J. Yu, A. Yu, Z. Liu, K. Xie and W. Su, Microchim. Acta, 2018, 185, 87 CrossRef PubMed.
- A. Wang, Y. Hou, F. Kang, F. Lyu, Y. Xiong, W.-C. Chen, C.-S. Lee, Z. Xu, A. Rogach and J. Lu, J. Mater. Chem. C, 2019, 7, 2207–2211 RSC.
- Z. Zhang, T. Zheng, X. Li, J. Xu and H. Zeng, Part. Part. Syst. Charact., 2016, 33, 457–472 CrossRef.
- L. Fu, X. Xiao and A. Wang, J. Phys. Chem. Solids, 2018, 122, 104–108 CrossRef CAS.
- H. Bian, A. Wang, Z. Li, Z. Li, Y. Diao, J. Lu and Y. Y. Li, Part. Part. Syst. Charact., 2018, 35, 1800155 CrossRef.
- H. Yu, Y. Zhao, C. Zhou, L. Shang, Y. Peng, Y. Cao, L.-Z. Wu, C.-H. Tung and T. Zhang, J. Mater. Chem. A, 2014, 2, 3344–3351 RSC.
- F. Zheng, Z. Wang, J. Chen and S. Li, RSC Adv., 2014, 4, 30605–30609 RSC.
- S. Oktay, Z. Kahraman, M. Urgen and K. Kazmanli, Appl. Surf. Sci., 2015, 328, 255–261 CrossRef CAS.
- Y. Cong, X. Li, Y. Qin, Z. Dong, G. Yuan, Z. Cui and X. Lai, Appl. Catal., B, 2011, 107, 128–134 CrossRef CAS.
- S.-K. Lee, A. Mills and A. Lepre, Chem. Commun., 2004, 1912–1913 RSC.
- W. Wang, L. Liu, J. Feng and Y. Yin, Small Methods, 2018, 2, 1700273 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9na00054b |
| ‡ Dr Aiwu Wang and Xufen Xiao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |