Room-temperature growth of covalent organic frameworks as the stationary phase for open-tubular capillary electrochromatography†
Qiaoyan
Li‡
ab,
Zhentao
Li‡
ab,
Yuanyuan
Fu
a,
Igor
Clarot
c,
Ariane
Boudier
 c and
Zilin
Chen
c and
Zilin
Chen
 *ab
*ab
aKey Laboratory of Combinatorial Biosynthesis and Drug Discovery, Ministry of Education, Hubei Province Engineering and Technology Research Center for Fluorinated Pharmaceuticals, and Wuhan University School of Pharmaceutical Sciences, Wuhan, 430071, China. E-mail: chenzl@whu.edu.cn
bState Key Laboratory of Transducer Technology, Chinese Academy of Sciences, Beijing 100080, China
cUniversité de Lorraine, CITHEFOR, F-54000 Nancy, France
First published on 6th September 2021
Abstract
Covalent organic frameworks (COFs) are a class of porous materials with high surface area, high porosity, good stability and tunable structure that have been widely used in the separation area. In this work, we have proposed the in situ synthesis of a novel COF composed of 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline (Tz) and 1,4-dihydroxyterephthalaldehyde (Da) onto the capillary inner surface for electrochromatographic separation. Fourier transform infrared (FT-IR) spectroscopy, elemental analysis (EA) and scanning electron microscopy (SEM) have facilitated the characterization of the prepared capillary columns. The COF (TzDa) modified OT-CEC column exhibited satisfactory separation selectivity towards neutral compounds (such as chlorobenzenes and alkylbenzenes), acidic and basic compounds (such as phenols and anilines), food additives (vanillin and its analogues) and small biomolecules (such as amino acids and polypeptides). Furthermore, the TzDa modified capillary was quite stable and reproducible. The relative standard deviations for retention times of the test analytes (alkylbenzenes) were as follows: for intra-day (n = 3) runs (≤1.74%), inter-day (n = 3) runs (≤2.25%) and between columns (n = 3) (≤4.83%). This new type of COF-based stationary phase has tremendous potential in separation science.
Introduction
Capillary electrochromatography (CEC)1–3 endowed with both the merits of high performance liquid chromatography (HPLC) (high selectivity)4–6 and capillary electrophoresis (CE) (high efficiency)7,8 is a well-known microcolumn separation method. Hence, it has been considered as a powerful chromatographic analysis tool and has attracted great attention in the last few decades. It should be noted that column technology is the key to the development of the CEC field in which an open-tubular column is a prominent CEC column format used in CEC.9 Superior to the other two forms (packed columns and monolithic columns), an open-tubular column has advantages such as easy preparation, facile instrument operation, absence of bubble formation and satisfactory permeability. Even so, the existing drawbacks of low phase ratio, sample capacity and poor separation efficiency of an open-tubular column have limited its potential application and these shortcomings still need to be solved. Hence, various novel material-based open-tubular columns have been explored and utilized in electrochromatographic separation, such as metal–organic frameworks (MOFs),10–13 covalent-organic frameworks (COFs),14–18 layered double hydroxides (LDHs)19 and metal–organic cages (MOCs).20,21COFs are composed of a variety of organic linkers. They are new kinds of crystalline porous materials having attractive structural features including low density, outstanding stability, good porosity and specific surface area,16 which allow them to attract more and more attention in different fields including gas storage,22,23 drug delivery,24–26 separation27–29 and catalysis.30–32 For the past few years, COFs have attracted more interest in the analytical chemistry field, especially in separation areas. To date, various COFs have been explored as stationary phases for chromatographic separation. For example, Kong et al. reported that COF-LZU1 was grown in situ on the capillary inner surface for OT-CEC separation.15 Niu et al. prepared a 2D COF-LZU1 coated OT column and used it for chromatographic separation of small organic molecules including alkylbenzenes, PAHs, and anilines. This work provided a new method for chromatographic separation.18 Bao et al. reported the polydopamine-assisted immobilization of the COF-5 on the capillary inner wall for OT-CEC separation of neutral, acidic and basic analytes. The success of the above work indicated that boron COFs can be grown on versatile substrates by the polydopamine-supported method, which paves the way for application of boron COFs to other analytical fields.17 The COF TzDa is a representative COF and consists of 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline (Tz) and 2,5-dihydroxyterephthalaldehyde (Da) at room temperature. TzDa has excellent stability, high specific surface areas and abundant interaction sites. The unique properties of TzDa make it an ideal candidate as a new stationary phase for CEC.
Here, we proposed the application of the COF TzDa as a new stationary phase for electrochromatographic separation. The successful growth of TzDa was confirmed by SEM, EA and FT-IR. The separation performance of the TzDa modified column was evaluated through the satisfactory separation performances towards chlorobenzenes, alkylbenzenes, phenols, anilines, vanillin and its analogues, amino acids and polypeptides obtained on the TzDa@capillary. In addition, the prepared columns were quite stable and reproducible. Our work broadens the utilization of TzDa in the electrochromatographic separation field.
Experimental
Chemicals and materials
Methanol and acetonitrile were obtained from Tedia (OH, USA) and were of HPLC grade. Four tested neutral alkylbenzenes (methylbenzene, ethylbenzene, n-propylbenzene, n-butylbenzene), tyrosine, tryptophan, phenylalanine, vanillin, isovanillin, and eugenol and three tested neutral chlorobenzenes (chlorobenzene, 1,2-dichlorobenzene, 1,2,4-trichlorobenzene), acetic acid, 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)trianiline (Tz), 2,5-dihydroxyterephthalaldehyde (Da) and 3-aminopropyltriethoxysilane (APTES) were purchased from Shanghai Aladdin Co., Ltd. Pyrocatechol, resorcinol, hydroquinone, aniline, 2-phenylethylamine, N,N-dimethylaniline, hydrochloric acid (HCl), thiourea, absolute ethyl alcohol, sodium hydroxide (NaOH), glutaraldehyde solution (25% v/v in H2O), sodium phosphate dibasic dodecahydrate (Na2HPO4·12H2O), tetrahydrofuran (THF) and dichloromethane (DCM) were obtained from the Sinopharm Group Chemical Reagent Co., Ltd (Shanghai, China). Untreated fused-silica capillary columns (50 μm i.d. and 375 μm o.d.) were obtained from Ruifeng Chromatographic Devices (Yongnian, Hebei, China). Ultrapure water used in this experiment was purified using an ultrapure water system (Milli-Q, MA, USA).Instrumentation
OT-CEC separation operation was implemented using Agilent 7100 CE apparatus (Waldbronn, Germany) possessing a temperature-controlled column compartment, UV detector (λ = 190–400 nm). The experiment results were recorded on the online workstation software and handled on offline workstation software. A field emission scanning electron microscope (Carl Zeiss, Germany) was used to collect scanning electron microscopy (SEM) images of the prepared capillary columns. The prepared capillary columns were smashed and further characterized by Fourier transform infrared (FT-IR) spectroscopy and elemental analysis (EA). The results were recorded on a Thermo Nicolet 5700 FT-IR spectrometer (MA, USA) and an elemental analyzer (Vario EL III, Germany), respectively. All solutions were pumped through capillaries using a mechanical pump obtained from Longer Pump Company (Baoding, China). The pH values of running buffers were regulated via a Mettler-Toledo pH meter (Shanghai, China).Buffer and standard solutions
The standard solutions of chlorobenzenes and alkylbenzenes (4.0 mg mL−1) were prepared using methanol as the solvent, respectively. The standard solutions of tryptophan, tyrosine and phenylalanine (2.0 mg mL−1) were prepared by dissolving in HCl aqueous solution (0.1 M), respectively. Other standard solutions were prepared in methanol (3.0 mg mL−1), individually. The thiourea was chosen as an EOF marker and its solution was prepared by dissolving in methanol (3.0 mg mL−1). All running buffers (phosphate buffers) were prepared by dissolving gradient concentrations of Na2HPO4·12H2O in H2O and their pH values were regulated by sodium hydroxide or phosphoric acid. The above solutions and buffers were treated with 0.22 μm membrane filters and stocked in a refrigerator (4 °C) before use.Fabrication of the TzDa coated capillary column
COF-TzDa was prepared by referring to the method reported by Liu et al.16 Tz (16 mg, 0.045 mM), Da (14 mg, 0.084 mM) and acetic acid solution (0.5 mL, 6 M) were mixed in 10 mL of 1,2-dichlorobenzene/ethanol solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = v/v). Subsequently, the above dispersion was sonicated for 5 min, and the reaction proceeded at ambient temperature standing still for 3 days. The synthesized dark red precipitates were obtained by centrifugation (10
1 = v/v). Subsequently, the above dispersion was sonicated for 5 min, and the reaction proceeded at ambient temperature standing still for 3 days. The synthesized dark red precipitates were obtained by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 5 min. Finally, the TzDa dark red powders were washed with THF and DCM and dried under vacuum at 60 °C overnight.
000 rpm) for 5 min. Finally, the TzDa dark red powders were washed with THF and DCM and dried under vacuum at 60 °C overnight.
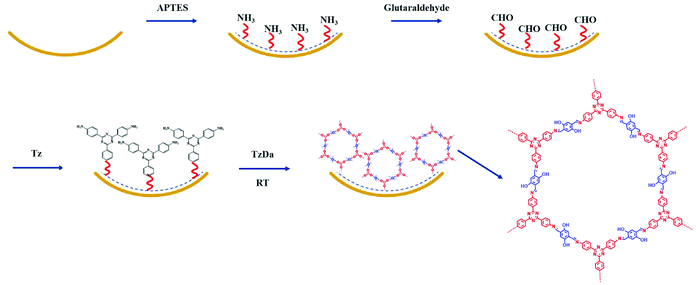
The fabrication process of the TzDa coated capillary column is demonstrated in Fig. 1. The capillary columns need to be pretreated before modification. The newly untreated capillary columns were flushed sequentially using 1.0 M NaOH (1 h), H2O (0.5 h), 1.0 M HCl (1 h), H2O (0.5 h), and methanol (0.5 h) and then dried under a nitrogen stream. Then, the APTES solution (10%, v/v, H2O) was pumped into the above capillary columns and stored in a 95 °C water bath for 1 h with both ends sealed. This modification process was repeated once more. Subsequently, the capillaries were washed with H2O and dried with N2 to achieve the modification of amino groups on the capillary inner wall. The glutaraldehyde solution (2%, v/v in H2O) was continually pumped through the APTES modified capillary at room temperature (60 min). This modification process was repeated once more and then the capillary was flushed using H2O and dried under a N2 stream to achieve the modification of aldehyde groups on the inner wall of the capillary. Subsequently, the Tz monomer was dissolved in dioxane with a concentration of 2 mg mL−1. Then, the aldehyde group modified capillary was filled with the Tz solution and reacted at 150 °C for 1 h. The above step was repeated once more. Finally, the capillary coated with Tz groups was washed with methanol. The Tz modified capillary was filled with the above prepared COF precursor solution16 and placed at room temperature for 3 days. After the reaction was completed, the capillary was pumped into methanol to remove unreacted solutions and monomers and dried using N2 to achieve the modification of TzDa onto the capillary inner wall.
Equations
The separation parameters including column efficiency (N) and separation resolution (Rs): N = 16 × (t/W)2 and Rs = 2(t2 − t1)/W1 + W2, where t is the retention time of the analyte and W is the peak width of the analytes. 1 and 2 represent the first and second peaks of the analytes, individually. The electroosmotic flow (EOF) mobilities were obtained by the equation:33μeof = LeL/V/t0, in which t0 is the migration time of thiourea (EOF marker), L is the total length (0.45 m) and Le represents the effective length (0.365 m), V is the voltage applied in the experiments. The retention factor (k) was calculated by the formula: k = tr − t0/t0, tr is the retention time of four alkylbenzenes.Results and discussion
Characterization of the prepared columns
The morphology and formation of the TzDa and TzDa@capillary were confirmed by SEM, FT-IR and EA experiments. It can be seen that the structures of the as-synthesized TzDa were in accordance with the previous report (Fig. S1†).16 The SEM images of the bare capillary and TzDa@capillary are shown in Fig. 2. The inner surface of the uncoated capillary is obviously smooth. In the TzDa@capillary, plenty of TzDa crystals were uniformly immobilized on the inner surface of the capillary, which greatly changed the morphology features. Furthermore, the prepared columns were smashed and confirmed according to FT-IR spectra and EA. It can be seen from Fig. S2.† The obtained TzDa showed the coincident feature absorption peaks with the previous report. After the bare column was modified with TzDa, the characteristic absorption peaks at 1624.9 cm−1 and 2924.5 cm−1 attributed to the stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and N–H in the TzDa structure appeared. The FT-IR result confirmed that the TzDa was successfully coated on the capillary inner wall. Besides, from the EA experiment results (Table S1†), we can see that the increase of the C and N elements on the TzDa@capillary indicated the successful immobilization of the TzDa on the inner wall of the capillary. The above results of SEM, FT-IR and EA confirmed that the TzDa was successfully synthesized and modified onto the capillary inner surface.
N and N–H in the TzDa structure appeared. The FT-IR result confirmed that the TzDa was successfully coated on the capillary inner wall. Besides, from the EA experiment results (Table S1†), we can see that the increase of the C and N elements on the TzDa@capillary indicated the successful immobilization of the TzDa on the inner wall of the capillary. The above results of SEM, FT-IR and EA confirmed that the TzDa was successfully synthesized and modified onto the capillary inner surface.
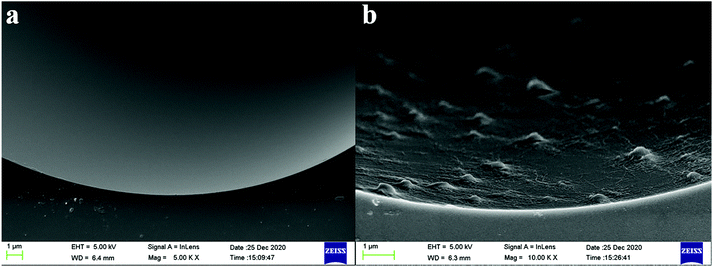 | ||
Fig. 2 SEM images of the inner wall of the bare capillary (a, 5000×) and the TzDa@capillary (b, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). 000×). | ||
Electroosmotic flow (EOF)
In CEC experiments, the EOF was regarded as the driving force of the mobile phase. In this work, the influence of pH values of buffer on the EOF mobilities of the prepared column was confirmed. As shown in Fig. S3,† the cathode EOF mobilities of two kinds of columns were enhanced gradually with the increase of pH values ranging from 5 to 10, which is due to the increasing ionization degree of silicon hydroxyl groups on the inner wall of the capillaries. However, the EOF mobility of the TzDa@capillary was significantly lower than that of the bare column. This is mainly because the silica hydroxyl groups on the capillary inner surface wall were partly covered by the COF coating layers.Influence of acetonitrile content on the separation performance
The influence of acetonitrile content of the mobile phase on the electrochromatographic separation performance of the TzDa@capillary was studied, and four alkylbenzenes were used as test analytes. As shown in Fig. S4a,† baseline separation of four alkylbenzenes was obtained with 25% acetonitrile in 10 mM phosphate buffer, and the retention factors of four alkylbenzenes decreased with the increase of acetonitrile content (Fig. S4b†). This is mainly due to the increase in acetonitrile content leading to the increased elution ability, resulting in the reduction of separation performance, indicating a typical reversed-phase retention ability of the TzDa modified column.Separation of neutral compounds
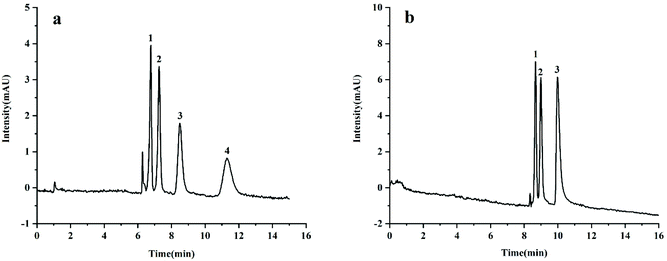
The reversed separation performance of the TzDa@capillary was studied by separating two groups of neutral compounds including chlorobenzenes (chlorobenzene (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 2.84), 1,2-dichlorobenzene (log
P 2.84), 1,2-dichlorobenzene (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 3.44), and 1,2,4-trichlorobenzene (log
P 3.44), and 1,2,4-trichlorobenzene (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.10)) and alkylbenzenes (methylbenzene (log
P 4.10)) and alkylbenzenes (methylbenzene (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 2.72), ethylbenzene (log
P 2.72), ethylbenzene (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 3.23), n-propylbenzene (log
P 3.23), n-propylbenzene (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 3.74), and n-butylbenzene (log
P 3.74), and n-butylbenzene (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.25)). The result is shown in Fig. 3, and the TzDa@capillary showed good separation performance towards the above test compounds. The peak order of analytes was consistent with the increase in log
P 4.25)). The result is shown in Fig. 3, and the TzDa@capillary showed good separation performance towards the above test compounds. The peak order of analytes was consistent with the increase in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, indicating that the hydrophobic interaction and π-interaction were mainly separation mechanisms.
P values, indicating that the hydrophobic interaction and π-interaction were mainly separation mechanisms.
Separation of phenolic compounds and food additives
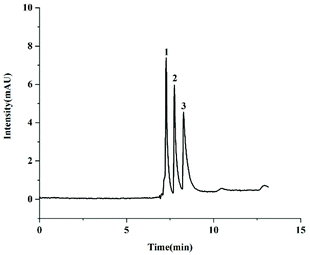
The separation performance of the TzDa@capillary was also evaluated by separating three phenolic compounds. Here, three diphenol isomers including catechol, resorcinol and hydroquinone were chosen as test compounds. The pKa of catechol, resorcinol and hydroquinone is 9.50, 9.45 and 10.33, respectively. As illustrated in Fig. 4, good separation performance of the three diphenol isomers was obtained on the TzDa@capillary. The peak order of catechol isomers was identical to the order of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values from low to high.
P values from low to high.
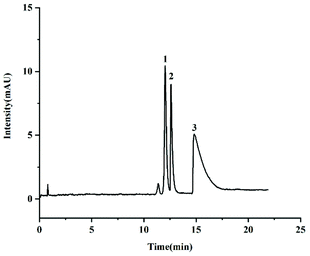
Vanillin and its analogues (isovanillin and eugenol) are widely used food additives in the food industry. Furthermore, vanillin and its analogues are the crucial indexes of characterizing the quality of vanilla extract.14 Therefore, the determination of vanillin, isovanillin and eugenol is of great significance in the food industry. As shown in Fig. 5, the TzDa@capillary exhibited good separation towards the above three compounds. It confirmed that the hydrophobic interaction between the test analytes and the TzDa modification layer plays a vital role.
Separation of basic compounds
The TzDa@capillary was used for the electrochromatographic separation of three anilines including phenylamine, 2-phenylethylamine and N,N-dimethylbenzenamine. As shown in Fig. S5,† the TzDa@capillary exhibited satisfactory separation performance towards these three anilines. It should be noted that the pKa of phenylamine and N,N-dimethylbenzenamine was 4.6 and 5.1, respectively. Under this experimental condition, they were not charged, while the 2-phenylethylamine (pKa = 9.9) was dissociated and possessed a positive charge. Hence, the differences in hydrophobicity and electrophoretic mobility contributed to the successful separation of these compounds.Separation of small biomolecules
Amino acids and polypeptides are important biomolecules in human metabolism. The separation and determination of these small biomolecules are not only beneficial for life health administration but also helpful in the food industry. The result is shown in Fig. 6. Good separation performance of three amino acids (tyrosine, tryptophan, and phenylalanine) with high resolution and satisfactory efficiency was achieved on the TzDa modified column. In addition, the TzDa@capillary exhibited good separation performance towards the slightly larger biomolecules (polypeptides) (Fig. S6†). The above results indicated that the TzDa@capillary was promising for the separation of small biomolecules.Reproducibility and stability
For the newly developed OT-CEC columns, repeatability and stability are the crucial evaluation parameters. The RSDs of analysis time of tested neutral compounds were utilized to confirm the repeatability of the TzDa coated capillary column. The result is shown in Table 1. The RSDs of intra-day (n = 3), inter-day (n = 3) and column-to-column (n = 3) were below 1.74%, 2.25%, and 4.83%, respectively. The results indicated that the TzDa coated capillary columns possessed satisfactory reproducibility. In addition, no considerable decrease in the separation performance was observed after continuously injecting 60 runs on the TzDa modified open-tubular column (Fig. S7†). The above data indicated that the TzDa coated columns were quite stable and repeatable.| Analytes | Intra-day RSD (%) (n = 3) | Inter-day RSD (%) (n = 3) | Between columns (%) (n = 3) |
|---|---|---|---|
| Methybenzene | 1.74 | 1.71 | 4.76 |
| Ethylbenzene | 1.73 | 1.78 | 4.83 |
| Propylbenzene | 1.57 | 1.94 | 3.96 |
| Butylbenzene | 1.52 | 2.25 | 2.58 |
Comparison with other MOF or COF-based OT-CEC columns
A series of previously reported MOF or COF-based stationary phases have been successfully applied for OT-CEC separation. The TzDa coated column was compared with previously reported research in order to confirm the superiority of this OT-CEC column. As shown in Table S3,† the obtained maximum column efficiency of the presented TzDa coated column was significantly higher than those of all other OT columns. In addition, the prepared column showed outstanding reproducibility and stability. This novel kind of COF-based column showed greater application potential for the chromatographic separation field.Conclusions
In summary, we have successfully prepared the COF-TzDa modified CEC OT-column. The inner wall of the bare capillary was first modified with APTES to create surface amino sites and then treated with glutaraldehyde and Tz monomers in sequence, to achieve the modification of Tz on the capillary inner wall. Finally, COF-TzDa can be successfully grown on the inner wall of the capillary by covalent bonding with Tz monomers on the inner wall. The obtained results indicated that the TzDa was successfully synthesized and immobilized on the capillary inner wall. The TzDa modified OT-CEC column possessed outstanding separation performance towards different compounds. Furthermore, the TzDa coated capillary possessed excellent repeatability and stability. This novel COF-based OT-CEC capillary column showed promising application in chromatographic separation science.Author contributions
Qiaoyan Li: investigation, conceptualization, methodology, paper draft writing, and data analysis. Zhentao Li: investigation, conceptualization, methodology, paper draft, and writing. Yuanyuan Fu: validation. Igor Clarot: validation. Ariane Boudier: validation. Zilin Chen: supervision, funding acquisition, project administration, conceptualization, and manuscript revising.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 82073808, 81872828 and 81573384).Notes and references
- P. X. Tang and Z. L. Chen, Talanta, 2018, 178, 650–655 CrossRef CAS PubMed.
- J. Zhang, W. P. Zhang, T. Bao and Z. L. Chen, Analyst, 2014, 139, 242–250 RSC.
- J. Zhang, W. P. Zhang, T. Bao and Z. L. Chen, J. Chromatogr. A, 2014, 1339, 192–199 CrossRef CAS PubMed.
- J. Y. Sun, S. M. Ma, B. B. Liu, J. Yu and X. J. Guo, Talanta, 2019, 204, 817–825 CrossRef CAS PubMed.
- L. Li, B. P. Cheng, R. D. Zhou, Z. G. Cao, C. Zeng and L. S. Li, Talanta, 2017, 174, 179–191 CrossRef CAS PubMed.
- X. Hang, J. J. Huang, C. Yuan, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140, 892–895 CrossRef PubMed.
- X. Liu, X. L. Liu, M. Li, L. P. Guo and L. Yang, J. Chromatogr. A, 2013, 1277, 93–97 CrossRef CAS PubMed.
- J. Zhang, H. W. Huang and K. K. Phoon, J. Geotech. Geoenviron. Eng., 2013, 139, 651–655 CrossRef.
- Z. T. Li, Z. K. Mao, W. Zhou and Z. L. Chen, Talanta, 2020, 218, 121160 CrossRef CAS PubMed.
- B. D. McCarthy, T. Liseev, A. M. Beiler, K. L. Materna and S. Ott, ACS Appl. Mater. Interfaces, 2019, 11, 38294–38302 CrossRef CAS PubMed.
- C. J. Pan, W. F. Wang, H. G. Zhang, L. F. Xu and X. G. Chen, J. Chromatogr. A, 2015, 1388, 207–216 CrossRef CAS PubMed.
- T. Bao, J. Zhang, W. P. Zhang and Z. L. Chen, J. Chromatogr. A, 2015, 1381, 239–246 CrossRef CAS PubMed.
- T. Bao, P. X. Tang, Z. K. Mao and Z. L. Chen, Talanta, 2016, 154, 360–366 CrossRef CAS PubMed.
- D. Y. Kong and Z. L. Chen, Electrophoresis, 2018, 39, 2912–2918 CrossRef CAS PubMed.
- D. Y. Kong, T. Bao and Z. L. Chen, Microchim. Acta, 2017, 184, 1169–1176 CrossRef CAS.
- F. Liu, H. L. Qian, C. Yang and X. P. Yan, RSC Adv., 2020, 10, 15383–15386 RSC.
- T. Bao, P. X. Tang, D. Y. Kong, Z. K. Mao and Z. L. Chen, J. Chromatogr. A, 2016, 1445, 140–148 CrossRef CAS PubMed.
- X. Y. Niu, S. Y. Ding, W. F. Wang, Y. L. Xu, Y. Y. Xu, H. L. Chen and X. G. Chen, J. Chromatogr. A, 2016, 1436, 109–117 CrossRef CAS PubMed.
- X. H. Yu, W. Zhou and Z. L. Chen, J. Chromatogr. A, 2017, 1530, 219–225 CrossRef CAS PubMed.
- Z. T. Li, Z. K. Mao and Z. L. Chen, Microchim. Acta, 2019, 186, 53 CrossRef PubMed.
- S. M. Xie, N. Fu, L. Li, B. Y. Yuan, J. H. Zhang, Y. X. Li and L. M. Yuan, Anal. Chem., 2018, 90, 9182–9188 CrossRef CAS PubMed.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed.
- G. O. Aksu, H. Daglar, C. Altintas and S. Keskin, J. Phys. Chem. C, 2020, 124, 22577–22590 CrossRef CAS PubMed.
- M. Li, Y. Peng, F. Yan, C. G. Li, Y. Q. He, Y. Lou, D. X. Ma, Y. Li, Z. Shi and S. H. Feng, New J. Chem., 2021, 45, 3343–3348 RSC.
- K. Zhao, P. W. Gong, J. Huang, Y. Huang, D. D. Wang, J. Y. Peng, D. Y. Shen, X. F. Zheng, J. M. You and Z. Liu, Microporous Mesoporous Mater., 2021, 311, 110713 CrossRef CAS.
- G. Y. Zhang, X. L. Li, Q. B. Liao, Y. F. Liu, K. Xi, W. Y. Huang and X. D. Jia, Nat. Commun., 2018, 9, 2785 CrossRef PubMed.
- F. Yuan, Z. F. Yang, X. Y. Zhang, C. Y. Tong, G. Gahungu, W. L. Li and J. P. Zhang, J. Comput. Chem., 2021, 42, 888–896 CrossRef CAS PubMed.
- Z. F. Wang, S. N. Zhang, Y. Chen, Z. J. Zhang and S. Q. Ma, Chem. Soc. Rev., 2020, 49, 708–735 RSC.
- C. Zhang, B. H. Wu, M. Q. Ma, Z. K. Wang and Z. K. Xu, Chem. Soc. Rev., 2019, 48, 3811–3841 RSC.
- S. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. B. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Science, 2015, 349, 1208–1213 CrossRef CAS PubMed.
- X. W. Hu, Y. Long, M. Y. Fan, M. Yuan, H. Zhao, J. T. Ma and Z. P. Dong, Appl. Catal., B, 2019, 244, 25–35 CrossRef CAS.
- S. L. Lu, Y. M. Hu, S. Wan, R. McCaffrey, Y. H. Jin, H. W. Gu and W. Zhang, J. Am. Chem. Soc., 2017, 139, 17082–17088 CrossRef CAS PubMed.
- Z. T. Li, Z. K. Mao, C. J. Hu, Q. Y. Li and Z. L. Chen, J. Chromatogr. A, 2020, 1625, 461269 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1an01402a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |