Open Access Article
Open Access ArticleTo be or not to be degraded: in defense of persistence assessment of chemicals
Andreas
Schäffer
 *abc,
Kathrin
Fenner
*abc,
Kathrin
Fenner
 d,
Zhanyun
Wang
ef and
Martin
Scheringer
d,
Zhanyun
Wang
ef and
Martin
Scheringer
 *gh
*gh
aRWTH Aachen University, Institute for Environmental Research, Worringerweg 1, 52074 Aachen, Germany. E-mail: andreas.schaeffer@bio5.rwth-aachen.de
bState Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, 163 Xianling Road, 210023 Nanjing, China
cKey Laboratory of the Three Gorges Reservoir Region's Eco-Environment, Chongqing University, 174 Shazheng Street Shapingba, 400045, Chongqing, China
dUniversity of Zürich, Department of Chemistry, Winterthurerstrasse 190, 8057 Zurich, Switzerland
eETH Zürich, Institute of Environmental Engineering and National Centre of Competence in Research (NCCR) Catalysis, Stefano-Franscini-Platz 5, 8093 Zürich, Switzerland
fEmpa−Swiss Federal Laboratories for Materials Science and Technology, Technology and Society Laboratory, CH-9014 St. Gallen, Switzerland
gETH Zürich, Institute of Biogeochemistry and Pollutant Dynamics, Universitätstrasse 16, 8092 Zürich, Switzerland. E-mail: scheringer@usys.ethz.ch
hRECETOX, Faculty of Science, Masaryk University, Kotlarska 2, 611 37 Brno, Czech Republic
First published on 7th July 2022
Abstract
Characterizing the degradation behavior of chemicals in the environment is a key component of chemical hazard and risk assessment. Persistence has been successfully characterized for readily and for slowly degradable chemicals using standardized tests, but for the third group of chemicals with intermediate degradability (“middle group”), the assessment is less straightforward. Whether chemicals of this group behave as persistent or not in a given environment depends on environmental factors such as the presence of sorbents that can limit the bioavailability of chemicals. Uncertainties associated with current persistence assessments of chemicals in the middle group do not imply that persistence assessment is generally inconsistent, too ambiguous for regulatory use, and not useful in chemical hazard and risk assessment. Given the complexity of the environmental factors influencing chemical degradation, and the diversity of commercial chemicals, it has to be accepted though that for chemicals in the middle group even improved testing methods will not remove all of the immanent heterogeneity in their persistence data. For cases with widely different but technically valid persistence data, a weight-of-evidence approach is necessary and the “benefit of the doubt” should follow the precautionary principle in order to protect human and ecosystem health. We maintain that technically valid persistence data, although they might be considered dissatisfying from a scientific point of view because of high variability or even inconclusiveness, can well be sufficient for regulatory purposes. As with anything, also in persistence assessment, the scientific logic aims for a mechanistic description of the processes involved, low uncertainty, and a comprehensive understanding derived from a broad empirical basis. If the scientific logic is used as a benchmark in the regulatory context, this may easily lead to “paralysis by analysis”. While regulatory decisions should be based on sound science, discrepancies between scientific goals and regulatory needs and, consequently, different levels of requirements (must-have versus nice-to-have) for degradation studies need to be recognized and appreciated. We further advocate for enhancing consistency between regulatory persistence assessments (“one substance–one assessment”), which is currently not the case.
Environmental significancePersistence (P), even on its own, is an essential component of assessing the worldwide risk of chemicals to environmental and human health. Identifying existing uncertainties in the P assessment of chemicals and proposing steps to deal with these uncertainties in an assessment context is therefore environmentally significant. Regulatory P assessments have been successfully performed as part of many regulatory schemes to identify highly persistent chemicals for regulatory measures. Uncertainties and inconsistencies in the persistence assessment of chemicals seemingly complicate the classification of those chemicals that are not either clearly readily degradable or (highly) persistent. Reasons for the difficulties in classification of the intermediate group are, for instance, how to consider the bioavailability and the variability of environmental conditions which can lead to wide variations in empirical observations of degradation half-lives. Here we offer suggestions for improvement, including how to incorporate recent scientific findings and how to handle immanent and irreducible uncertainties. |
1. Importance of persistence assessment
It has been reported that the planetary boundary for novel entities, including synthetic chemicals, has already been exceeded, since the ever increasing production and release of synthetic chemicals into the environment are beyond the capacity of regulatory bodies for assessing their risk.1,2 This calls for elevated efforts on chemicals management. As a major element of chemicals management, many regulations and guidelines across the world have been established, often including requirements for assessing the persistence (P), bioaccumulation (B) and toxicity (T) of chemicals. All three characteristics are important elements of the hazard and risk of chemicals, but it has been argued that even high persistence alone is a sufficient criterion for triggering risk management to mitigate potentially significant risks posed by a given chemical.3 This is because later observations of harmful effects of highly persistent chemicals in use can lead to environmental damage for long periods of time, even long after a ban.3 The EU REACH guidance document R.114 explicitly states that high persistence (in combination with bioaccumulation and toxicity) leads to unacceptably high uncertainties of risk assessment results with the imperative that emissions of highly persistent chemicals must be reduced as far as possible. A detailed discussion of the reasons why P assessments are not overly precautionary but provide valuable information for chemical hazard and risk assessment is provided by Cousins et al. (2019).3The persistence of chemicals can be defined as the time span (in units of time) that characterizes how long-lived a chemical is after its release to the environment. In regulatory persistence assessment (here: P assessment), all abiotic and microbial transformation processes have to be considered in order to draw a conclusion. Transformation and degradation of chemicals depend both on their intrinsic properties, as well as on environmental conditions and processes5–8 that may act as confounding factors causing various uncertainties associated with the scientific basis of P assessment.
On the regulatory side, a different type of uncertainty arises from the fact that regulatory decision-making relies primarily on single cut-off values for degradation half-lives in single media. These cut-off values are, because they are historically grown, to some extent arbitrary and, therefore, can vary across regulations due to different rationales. In addition, some regulations consider the persistence of transformation products as part of the persistence of the parent compound, whereas others do not. For example, etoxazole is assessed by the European Food Safety Authority (EFSA) as a non-persistent pesticide. However, it forms significant amounts (up to 23% of the applied amount) of a metabolite (R-13) exhibiting medium to high persistence.9 If this chemical had to be assessed by the European Chemicals Agency (ECHA) as an industrial chemical, the presence of the persistent metabolite would trigger the assessment of etoxazole as being persistent, as defined by the EU REACH guidance document R11: “also substances concluded as fulfilling PBT or vPvB criteria because their constituents, impurities, additives or degradation/transformation products fulfil the PBT or vPvB criteria must be subjected to emission characterisation and minimisation of releases for their whole life-cycle”.4 Further discrepancies between different regulatory authorities are related to the assessment of non-extractable residues (NER). While, for pesticides, EFSA10 considers NER as a degraded fraction, ECHA,4 in the assessment of industrial chemicals, considers them in part as remobilizable and relevant for persistence assessment. Similar inconsistencies between regulatory schemes were also reported in ecotoxicology, which has led to the demand for more congruence of regulatory assessment in the sense of “one chemical–one assessment".11
Both aspects, scientific and technical questions, as well as uncertainties in relation to regulatory schemes, are interwoven, which makes the situation complex, impeding a clear and effective discussion on future development of persistence assessment in a regulatory context. Here, we attempt to disentangle the two aspects, with a focus on matters learned on the scientific side and recommend a way forward for the overall discussion on P assessment.
2. What has worked in P assessment?
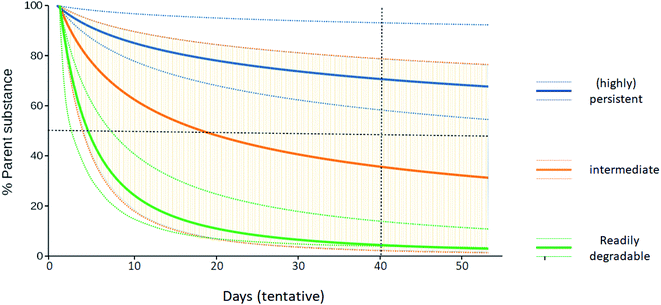
Over 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 chemicals and chemical mixtures have been registered for production and use globally2 and production is still increasing.1 Regarding persistence, chemicals can be assigned to three groups: readily degradable substances such as mono- and oligosaccharides, highly persistent substances such as perfluorooctanoic acid (PFOA) and other perfluoroalkyl acids, and a third group of substances that lie between these two extremes (“middle group”). P assessments typically provide unequivocal outcomes for the first two groups and have therefore been successfully performed as part of many regulatory schemes, including the EU REACH regulation and the Stockholm Convention on Persistent Organic Pollutants, to identify highly persistent chemicals for regulatory measures. Persistence can of course also be a desired property of chemicals, such as pigments in automotive paint, and there are numerous natural polymers such as lignin that are highly persistent. In this article, however, we are concerned with environmentally relevant non-polymeric organic substances whose persistence assessment is fraught with uncertainty.3 In that case, P assessment is particularly challenging for those substances in the middle group whose classification as either readily degradable or persistent may not be clear-cut (Fig. 1). For example, degradation half-lives of the biocides triclosan and triclocarban in soil strongly vary depending on the soil's sorption potential. This is largely due to the reduced bioavailability of the sorbed chemicals with increasing soil organic matter contents, which may be modulated, for instance, by amendment of biosolids used in agriculture as fertilizers.12
000 chemicals and chemical mixtures have been registered for production and use globally2 and production is still increasing.1 Regarding persistence, chemicals can be assigned to three groups: readily degradable substances such as mono- and oligosaccharides, highly persistent substances such as perfluorooctanoic acid (PFOA) and other perfluoroalkyl acids, and a third group of substances that lie between these two extremes (“middle group”). P assessments typically provide unequivocal outcomes for the first two groups and have therefore been successfully performed as part of many regulatory schemes, including the EU REACH regulation and the Stockholm Convention on Persistent Organic Pollutants, to identify highly persistent chemicals for regulatory measures. Persistence can of course also be a desired property of chemicals, such as pigments in automotive paint, and there are numerous natural polymers such as lignin that are highly persistent. In this article, however, we are concerned with environmentally relevant non-polymeric organic substances whose persistence assessment is fraught with uncertainty.3 In that case, P assessment is particularly challenging for those substances in the middle group whose classification as either readily degradable or persistent may not be clear-cut (Fig. 1). For example, degradation half-lives of the biocides triclosan and triclocarban in soil strongly vary depending on the soil's sorption potential. This is largely due to the reduced bioavailability of the sorbed chemicals with increasing soil organic matter contents, which may be modulated, for instance, by amendment of biosolids used in agriculture as fertilizers.12
3. Confounding factors and how they affect P assessments of chemicals of intermediate degradability
Many factors can contribute to the uncertainty of P assessment outcomes for substances belonging to the group with intermediate degradability. There are factors related to the testing conditions, including the environmental conditions such as temperature, pH, exposure to light, the presence of further contaminants13–15 as well as the state of the environmental matrix such as its nutrient status, microbial activity, and potential adaptation due to pre-exposure to the pollutant or structurally related chemicals.8,16Apart from these natural variabilities, another particular factor that renders P assessments difficult for this group of chemicals is bioavailability limitations due to the interaction of substances with solid environmental media such as soils, sediments, and particulate matter, which leads to sorption and covalent binding, with the latter particularly resulting in the formation of non-extractable residues (NER).
Yet, bioavailability is a pre-requisite for microbial degradation. Micropollutants in the pore water of soils are taken up by microorganisms and degraded, if chemical structures are fit for direct catabolism, to products that can be used for anabolic biomass formation and energy gain. Alternatively, degradation may occur co-metabolically if readily degradable alternative substrates are available. Although sorbed chemicals can also be metabolized,17–19 microbial degradation of dissolved substrates is more rapid and efficient. Examples for the challenges in P assessment in this context are the above-mentioned triclosan and triclocarban, as well as phenanthrene, for which Hughes et al. (2020) provided an extensive literature review of environmental fate and degradation tests.20 The authors concluded that phenanthrene was non-persistent because the ready-biodegradability tests demonstrated that the corresponding cut-off value of mineralization was met, i.e., 60% of the theoretical CO2 produced in a 10-day time window following the attainment of 10% biodegradation within the 28-day period of the test. However, in soil, and even more so in soils containing strongly sorbing components such as black carbon21,22 or sewage-sludge amendment,23 a significant fraction of phenanthrene is not bioavailable for microbes due to strong sorption. Consequently, much longer degradation half-lives of phenanthrene in soil have been observed under such conditions.
One may argue that strongly sorbed chemicals and NER will not result in environmental and human exposure and thus can be neglected. However, this is not true. The herbicide atrazine according to the data of Koskinen and Clay (1997) has a degradation half-life of weeks to months24 in dependence on environmental conditions.5 Still, after the ban of atrazine some thirty years ago in the EU, atrazine residues at low concentrations are detected in aquatic media25 and soil,26 likely due to sequestration in the pores of the soil matrix and continuous release from this reservoir if the concentration in the soil pore water decreases upon degradation. Also, other pesticides remain in agricultural soils for long periods of time27,28 and, therefore, often mixtures of various substances are detected29 because they have been accumulating for years from previous applications. Such accumulated mixtures harm exposed organisms in soil.30 Therefore, these examples point to a critical discrepancy between the limited range of conditions that can be covered by regulatory P assessment, and the conditions of actual degradation in the environment. Finding ways to accommodate this discrepancy is a key task for regulatory persistence assessment.
4. Way forward for regulatory P assessment
A first important point for persistence-related regulatory decision-making is that the uncertainties associated with P assessments of chemicals in the middle group between those classified as readily degradable and highly persistent do not imply that persistence assessment schemes are generally inconsistent, too uncertain for regulatory use, and hence not useful in chemical hazard and risk assessment. Chemicals with (very) high and (very) low persistence can be reliably identified, and the uncertainties in the middle group do not negate the importance of persistence as a key dimension in chemical hazard and risk assessment.3For the middle group, two points are important: first, as far as technically and conceptually possible, the assessment methods need to be improved, particularly for substances that strongly adsorb onto soil, sediment, and other particulate matter. As a way forward to evaluate whether bioavailability limitation of chemicals is a reason to consider them as persistent, we propose remobilization tests under environmentally relevant conditions for exposure assessment. As an example for remobilization under natural conditions, a soil containing strongly sorbed chemicals or NER could be treated physically (simulation of heavy rain events, freezing/thawing, wet/dry cycles), chemically (change in pH), and biologically (solubilizing enzymes, treatment with soil feeding organisms, growth of plants).31 If the parent chemical or relevant metabolites are released and can be identified and quantified, the amounts of such residues should be considered in the P assessment, just as the sequestered fraction of NER.32 If residues remain immobilized in the matrix and are not released by such treatment, they may be considered as too strongly bound to be of environmental relevance as it is the case for the covalently bound fraction of NER. This proposal, which is based on scientific evidence on sequestration and potential release mechanisms, could be taken up by regulatory bodies, i.e., they could request such additional information from the applicants in the registration process if there are large discrepancies in P assessment outcomes and if the data point towards a strong influence of bioavailability on those P outcomes.
Second, given the multitude and complexity of environmental factors influencing chemical degradation, and the variability and diversity of the reactivity and behavior of many chemicals, it has to be accepted that for chemicals in the middle group even improved testing methods will not remove all of the often considerable heterogeneity in the persistence data. A lot of this heterogeneity is irreducible, as many years of research into persistence assessments have shown.33 This implies that a certain extent of heterogeneity has to be processed as such by the regulatory system.
For cases with widely different persistence data, as long as the diverging results are technically valid, a weight-of-evidence approach is necessary in order to determine the meaning and relative importance of the results. Generally, the “benefit of the doubt” should be used in favor of human and ecosystem health. In practice, this would mean, for example, that technically valid half-life data above a certain persistence threshold should be prioritized and considered as a strong indication for the chemical to be classified as persistent rather than to dilute these findings by merging them with shorter half-lives and using the average. It is the regulators' task and responsibility to check the validity of data to protect human and environmental health, which is the foundation of long-term economic and societal prosperity, in particular in light of the mounting evidence that current schemes of chemical risk assessment are underprotective.34–37
It is often stated that the “bright-line” criteria of fixed cut-off values are not suitable for assessments that involve so much uncertainty. In response to this, it should be noted that it is not necessarily uncertainty that is reflected by diverging persistence data, but real heterogeneity of how rapidly or slowly the same chemical may be degraded. In such a situation, the cut-off criteria are still useful as a basis for decision making, but the task is to decide on which side of the line a chemical should be placed (Fig. 1), given the available persistence data. This requires a careful analysis of the individual data so that their relative importance can be determined, but the decision-making task is still well-defined, and the situation does not imply that cut-off criteria are inappropriate.
A key point that illustrates why and how a stricter differentiation between scientific and regulatory aspects of persistence assessments is helpful is the following: scientifically, the outcomes of persistence studies may be considered dissatisfying (meaning that the results are highly noisy or even inconclusive although all data considered are technically valid), and improved assessment methods may be asked for. However, a scientifically dissatisfying result may well be sufficient for regulatory purposes. The scientific logic aims for a mechanistic description of the processes involved, low uncertainty, and a comprehensive understanding derived from a broad empirical basis. If this logic is used as a benchmark in the regulatory context, this may easily lead to “paralysis by analysis”. This does not at all imply that regulatory decisions should not be based on sound science, but the point is that scientific goals and regulatory needs are different and lead to different requirements regarding, e.g., the level of detail in the results obtained, the extent of mechanistic understanding, and the type and extent of uncertainty that can be accommodated.
Uncertainties similar to those in the P assessment of the middle group apply in principle also to the other two important elements of hazard assessment, the bioaccumulation potential and toxicity of chemicals. For example, in toxicity, non-monotonic dose–response relationships lead to uncertainties of assessment; for bioaccumulation, uncertainties may arise from comparison of in vitro and in silico predictions, but these aspects need to be considered in further work. Also, difficulties in the persistence assessment for other types of chemicals, such as substances of unknown or variable composition, complex reaction products or biological materials (UVCBs) as well as polymers, do exist, but treatment of these topics would go beyond the scope of the present work.38–40
5. Conclusion
In conclusion, to strengthen the regulatory assessment of chemical persistence, improvements on both the scientific and the regulatory side are desirable. Scientifically, it is important to better understand confounding factors, particularly those that can cause significant underestimation of persistence in the standardized testing, and to exploit the potential of data-driven analyses of persistence data. On the regulatory side, consistency between regulations should be achieved (“one substance–one assessment”), for instance as to whether the formation of NER is considered as degradation or not (in the EU, currently the way in which NER are treated by different agencies is not consistent). Most important, however, may be that the inherent and irreducible heterogeneity of persistence data needs to be appreciated, and that weight-of-evidence schemes used in regulatory decision-making accommodate this heterogeneity as a challenging but unavoidable feature of persistence assessment.41Conflicts of interest
All authors confirm that they have no conflict of interest.Acknowledgements
The manuscript has been solely prepared by the authors, which of course profited from numerous discussions with colleagues in this research field, such as Dr R. Ottermanns and Dr B. Daniels, RWTH Aachen University, Dr D. Claßen, German Environment Agency, Dr S. Trapp, Technical University of Denmark, and Dr M. Kaestner, Helmholtz Center for Environmental Research. Z. W. gratefully acknowledges financial support by the European Union under the Horizon 2020 Research and Innovation Programme (grant agreement number 101036756), and his work at ETH Zürich as part of the NCCR Catalysis (grant number 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. M. S. acknowledges funding by the RECETOX research infrastructure (the Czech Ministry of Education, Youth and Sports: LM2018121), the CETOCOEN PLUS project (CZ.02.1.01/0.0/0.0/15_003/0000469), and the CETOCOEN EXCELLENCE Teaming 2 project supported by the Czech Ministry of Education, Youth and Sports (No. CZ.02.1.01/0.0/0.0/17_043/0009632).References
- L. Persson, C. Almroth, C. D. Collins, S. Cornell, C. A. de Wit and M. L. Diamond,
et al., Outside the safe operating space of the planetary boundary for novel entities, Environ. Sci. Technol., 2022, 56(3), 1510–1521 CrossRef PubMed
.
- Z. Y. Wang, G. W. Walker, D. C. G. Muir and K. Nagatani-Yoshida, Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories, Environ. Sci. Technol., 2020, 54(5), 2575–2584 CrossRef CAS PubMed
.
- I. T. Cousins, C. A. Ng, Z. Y. Wang and M. Scheringer, Why is high persistence alone a major cause of concern?, Environ. Sci.: Processes Impacts, 2019, 21(5), 781–792 RSC
.
-
ECHA_2017_R.11. Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.11, Endpoint Specific Guidance (PBT/vPvB Assessment), Version 3.0, June 2017 Search PubMed
.
- K. Fenner, V. A. Lanz, M. Scheringer and M. E. Borsuk, Relating atrazine degradation rate in soil to environmental conditions: Implications for global fate modeling, Environ. Sci. Technol., 2007, 41(8), 2840–2846 CrossRef CAS PubMed
.
- M. Matthies and S. Beulke, Considerations of Temperature in the Context of the Persistence Classification in the EU, Environ. Sci. Eur., 2017, 29 Search PubMed
.
- G. Whale, J. Parsons, K. van Ginkel, R. Davenport, E. Vaiopoulou and K. Fenner,
et al., Improving our understanding of the environmental persistence of chemicals, Integr. Environ. Assess. Manage., 2021, 17(6), 1123–1135 CrossRef CAS PubMed
.
- R. Davenport, P. Curtis-Jackson, P. Dalkmann, J. Davies, K. Fenner and L. Hand,
et al., Scientific concepts and methods for moving persistence assessments into the 21st century, Integr. Environ. Assess. Manage., 2022, 1–34, DOI:10.1002/ieam.4575
.
- M. Arena, D. Auteri, S. Barmaz, G. Bellisai, A. Brancato and D. Brocca,
et al., Peer review of the pesticide risk assessment of the active substance etoxazole, EFSA J., 2017, 15(10), 1–27 Search PubMed
.
- C. Ockleford, A. F. Hernandez-Jerez, S. H. Bennekou, M. Klein, T. P. Adriaanse and P. Berny,
et al., Scientific Opinion about the Guidance of the Chemical Regulation Directorate (UK) on how aged sorption studies for pesticides should be conducted, analysed and used in regulatory assessments, EFSA J., 2018, 16(8), 1–86 Search PubMed
.
- J. van Dijk, M. Gustavsson, S. C. Dekker and A. P. van Wezel, Towards 'one substance - one assessment': An analysis of EU chemical registration and aquatic risk assessment frameworks, J. Environ. Manage., 2021, 280 Search PubMed
.
- Q. G. Fu, E. Sanganyado, Q. F. Ye and J. Gan, Meta-analysis of biosolid effects on persistence of triclosan and triclocarban in soil, Environ. Pollut., 2016, 210, 137–144 CrossRef CAS PubMed
.
- C. Wijntjes, Y. Weber, S. Hoger, H. Hollert and A. Schaffer, Effects of algae and fungicides on the fate of a sulfonylurea herbicide in a water-sediment system, Chemosphere, 2022, 290, 133234 CrossRef CAS PubMed
.
- X. Yi, Y. Wei, W. Zhai, P. Wang, D. Liu and Z. Zhou, Effects of three surfactants on the degradation and environmental risk of metolachlor in aquatic environment, Chemosphere, 2022, 300(134295) CAS
.
- M. S. Rodriguez-Cruz, M. J. Sanchez-Martin and M. Sanchez-Camazano, Surfactant-enhanced desorption of atrazine and linuron residues as affected by aging of herbicides in soil, Arch. Environ. Contam. Toxicol., 2006, 50(1), 128–137 CrossRef CAS PubMed
.
- B. A. J. Poursat, R. J. M. van Spanning, P. de Voogt and J. R. Parsons, Implications of microbial adaptation for the assessment of environmental persistence of chemicals, Crit. Rev. Environ. Sci. Technol., 2019, 49(23), 2220–2255 CrossRef
.
- A. Katayama, R. Bhula, G. R. Burns, E. Carazo, A. Felsot and D. Hamilton,
et al., Bioavailability of Xenobiotics in the Soil Environment, Rev. Environ. Contam. Toxicol., 2010, 203, 1–86, DOI:10.1007/978-1-4419-1352-4_1
.
- Y. Yang, L. Shu, X. L. Wang, B. S. Xing and S. Tao, Mechanisms regulating bioavailability of phenanthrene sorbed on a peat soil-origin humic substance, Environ. Toxicol. Chem., 2012, 31(7), 1431–1437 CrossRef CAS PubMed
.
- A. R. Johnsen and U. Karlson, Evaluation of bacterial strategies to promote the bioavailability of polycyclic aromatic hydrocarbons, Appl. Microbiol. Biotechnol., 2004, 63(4), 452–459 CrossRef CAS PubMed
.
- C. B. Hughes, D. M. Brown, L. Camenzuli, A. D. Redman, J. S. Arey and D. Vione,
et al., Can a chemical be both readily biodegradable AND very persistent (vP)? Weight-of-evidence determination demonstrates that phenanthrene is not persistent in the environment, Environ. Sci. Eur., 2020, 32, 148 CrossRef CAS
.
- F. Lian and B. S. Xing, Black Carbon (Biochar) In Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk, Environ. Sci. Technol., 2017, 51(23), 13517–13532 CrossRef CAS PubMed
.
- F. J. Qi, S. Kuppusamy, R. Naidu, N. S. Bolan, Y. S. Ok and D. Lamb,
et al., Pyrogenic carbon and its role in contaminant immobilization in soils, Crit. Rev. Environ. Sci. Technol., 2017, 47(10), 795–876 CrossRef CAS
.
- S. R. Wild, M. L. Berrow and K. C. Jones, The persistence of polynuclear aromatic-hydrocarbons (PAHs) in sewage-sludge amended agricultural soils, Environ. Pollut., 1991, 72(2), 141–157 CrossRef CAS PubMed
.
- W. C. Koskinen and S. A. Clay, Factors Affecting Atrazine Fate in North Central U.S. Soils, Rev. Environ. Contam. Toxicol., 1997, 151, 117–165 CAS
.
- A. Ghirardelli, S. Otto, R. Masin, C. Bano, L. Altissimo and S. Russo,
et al., Thirty-year Monitoring of S-Triazine Herbicide Contamination in the Aquifer North of Vicenza (North-east Italy), Sci. Total Environ., 2021, 752 Search PubMed
.
- N. D. Jablonowski, A. Schaffer and P. Burauel, Still present after all these years: persistence plus potential toxicity raise questions about the use of atrazine, Environ. Sci. Pollut. Res., 2011, 18(2), 328–331 CrossRef CAS PubMed
.
- A. C. Chiaia-Hernandez, A. Keller, D. Wachter, C. Steinlin, L. Camenzuli and J. Hollender,
et al., Long-Term Persistence of Pesticides and TPs in Archived Agricultural Soil Samples and Comparison with Pesticide Application, Environ. Sci. Technol., 2017, 51(18), 10642–10651 CrossRef CAS PubMed
.
- J. Riedo, F. E. Wettstein, A. Rösch, C. Herzog, S. Banerjee and L. Büchi,
et al., Widespread occurrence of pesticides in organically managed agricultural soils - the ghost of a conventional agricultural past?, Environ. Sci. Technol., 2021, 55(5), 2919–2928, DOI:10.1021/acs.est.0c06405
.
- V. Silva, H. G. J. Mol, P. Zomer, M. Tienstra, C. J. Ritsema and V. Geissen, Pesticide residues in European agricultural soils - A hidden reality unfolded, Sci. Total Environ., 2019, 653, 1532–1545 CrossRef CAS PubMed
.
- A. Sybertz, R. Ottermanns, A. Schaffer, B. Scholz-Starke, B. Daniels and T. Frische,
et al., Simulating spray series of pesticides in agricultural practice reveals evidence for accumulation of environmental risk in soil, Sci. Total Environ., 2020, 710, 135004 CrossRef CAS PubMed
.
- M. Kaestner, K. M. Nowak, A. Miltner, S. Trapp and A. Schaeffer, Classification and Modelling of Nonextractable Residue (NER) Formation of Xenobiotics in Soil - A Synthesis, Crit. Rev. Environ. Sci. Technol., 2014, 44(19), 2107–2171 CrossRef CAS
.
- A. Schaeffer, M. Kaestner and S. Trapp, A unified approach for including non-extractable residues (NER) of chemicals and pesticides in the assessment of persistence, Environ. Sci. Eur., 2018, 30, 51 CrossRef PubMed
.
- K. Fenner, M. Elsner, T. Lueders, M. S. McLachlan, L. P. Wackett and M. Zimmermann,
et al., Methodological Advances to Study Contaminant Biotransformation: New Prospects for Understanding and Reducing Environmental Persistence?, ACS ES&T
Water, 2021, 1(7), 1541–1554 Search PubMed
.
- R. Schulz, S. Bub, L. L. Petschick, S. Stehle and J. Wolfram, Applied pesticide toxicity shifts toward plants and invertebrates, even in GM crops, Science, 2021, 372(6537), 81–84 CrossRef CAS PubMed
.
- R. B. Schäfer, M. Liess, R. Altenburger, J. Filser, H. Hollert and M. Roß-Nickoll,
et al., Future pesticide risk assessment: narrowing the gap between intention and reality, Environ. Sci. Eur., 2019, 31(1), 21 CrossRef
.
- S. Persson and U. Magnusson, Environmental pollutants and alterations in the reproductive system in wild male mink (Neovison vison) from Sweden, Chemosphere, 2015, 120, 237–245 CrossRef CAS PubMed
.
- H. Q. Li, S. Hammarstrand, B. Midberg, Y. Y. Xu, Y. Li and D. S. Olsson,
et al., Cancer incidence in a Swedish cohort with high exposure to perfluoroalkyl substances in drinking water, Environ. Res., 2022, 204 Search PubMed
.
- P. N. H. Wassenaar and E. M. J. Verbruggen, Persistence, bioaccumulation and toxicity-assessment of petroleum UVCBs: A case study on alkylated three-ring PAHs, Chemosphere, 2021, 276 Search PubMed
.
- F. P. La Mantia, M. Morreale, L. Botta, M. C. Mistretta, M. Ceraulo and R. Scaffaro, Degradation of polymer blends: A brief review, Polym. Degrad. Stab., 2017, 145, 79–92 CrossRef CAS
.
- L. C. Liu, M. J. Xu, Y. H. Ye and B. Zhang, On the Degradation of (Micro)plastics: Degradation Methods, Influencing Factors, Environmental Impacts, Sci. Total Environ., 2022, 806 Search PubMed
.
- A. D. Redman, J. Bietz, J. W. Davis, D. Lyon, E. Maloney and A. Ott,
et al., Moving Persistence Assessments into the 21st Century: A Role for Weight-Of-Evidence and Overall Persistence, Integr. Environ. Assess. Manage., 2022, 18(4), 868–887 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2022 |