Open Access Article
Open Access ArticleTransition metal quantum dots for the electrocatalytic hydrogen evolution reaction: recent progresses and challenges
Brahmari
Honnappa
a,
Sathya
Mohan
b,
Mariyappan
Shanmugam
 b,
Ashil
Augustin
b,
Ashil
Augustin
 b,
Prince J. J.
Sagayaraj
b,
Chitiphon
Chuaicham
c,
Saravanan
Rajendran
b,
Prince J. J.
Sagayaraj
b,
Chitiphon
Chuaicham
c,
Saravanan
Rajendran
 d,
Tuan K. A.
Hoang
d,
Tuan K. A.
Hoang
 e,
Keiko
Sasaki
e,
Keiko
Sasaki
 c and
Karthikeyan
Sekar
c and
Karthikeyan
Sekar
 *b
*b
aDepartment of Physics and Nanotechnology, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu 603203, India
bSustainable Energy and Environmental Research Laboratory, Department of Chemistry, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu 603203, India. E-mail: karthiks13@srmist.edu.in; kart@mine.kyushu-u.ac.jp
cDepartment of Earth Resources Engineering, Kyushu University, Fukuoka 819-0395, Japan
dDepartamento de Ingeniería Mecánica, Facultad de Ingeniería, Universidad de Tarapacá, Avda. General Velásquez, 1775, Arica, Chile
eeInstitut de Recherche d’Hydro-Québec 1806, boul. Lionel-Boulet, Varennes, Québec J3X 1S1, Canada
First published on 29th September 2022
Abstract
Hydrogen, a renewable, green, clean, and energy source since long, has been attracting the interest of many researchers as hydrogen is produced alongside oxygen via water splitting using a simple electrochemical method. To realizehydrogen economy, sustainable hydrogen generation is a major expedient. Several reports have surfaced on water splitting using various catalysts with simultaneous hydrogen and oxygen evolution, among which transition metal quantum dots (TMQDs) are intriguingly great candidates for industrialization due to their extraordinary properties such as optical, electrical, and fluorescence. This study summarizes in-depth the basic concepts of the hydrogen evolution reaction and reviews the most recent advances in TMQDs as electrocatalysts, where a deeper evaluation of the synthesis, characteristics, and properties of TMQDs as well as insights into the catalyst's activity, morphology, composition, and other factors, are correlatively addressed. There are well-developed strategies to date for effectively modifying the activity and increasing the active sites, among which, quantum confinement is studied and emphasized. The prospects and difficulties in electrochemical water splitting are then analysed.
1. Introduction
The world is growing faster as a result of increased energy access for inhabitants, cleaner fuel, and the growing penetration of renewables. Constructing a sustainable and cleaner energy resource to concurrently meet the current as well as future demands is one of the most pressing challenges. Towards this, initiatives are being taken globally to boost the progress as well as the application of renewable energies, which comprise the solar energy, wind energy, and hydel energy.1 Correspondingly, as proposed by Bockris et al. in 1970,2 the term “hydrogen economy” is deemed as among the cleanest and enticing utilisation techniques with the output being nothing more than water. We can produce clean hydrogen by splitting water using renewable energy sources, which are then collected, stored, and used for a variety of other purposes, such as the production of ammonia, fuel cell electric vehicles, coke/iron production, refining processes, and human needs.3–5 However, electrochemical methods are rather discovered to be the most straightforward, practical, and successful strategies for hydrogen evolution as compared to the conventional reformation of steam or gasification with coal. Based on reactions between fossil fuels and steam or other renewable methods, one of the most captivating and non-carbon-emitting methods for producing hydrogen, is water electrolysis.6–8 The goal of obtaining a significant amount of H2 on a large scale remains an obstacle, and thus this solution is still incomplete.9–11 Hydrogen fuel cells based on membrane technologies, which are considered the state-of-the-art technology for H2 methodologies and studies, are another way of harvesting clean energy at a low temperature,12–14 nevertheless, electrolysis is one of the easiest harvesting ways to extract clean and green fuel, which is efficient and effective for future technologies;15 thus, the target is drawn back with the fabrication of an electrocatalyst, which is efficient and cost-effective due to lacklustre kinetic parameters to accelerate the reaction.16 The research community has been striving for a solution to design an electrocatalyst that is reliable, environmentally friendly, and cost-effective. There are basically two ways to increase the catalytic activity to tackle this issue.17–19 Either to increase the superficial activity of the total electrode via morphological variation or/and to improve the activity at every active site of the electrode by engineering the composition.16,20–22 To meet these two goals and to formulate a profound electrocatalyst for HER and due to their numerous surface sites that remain active and also because of quantum confinement, quantum dots are one such material, which when designed into a composite, makes for dependable future electrocatalysts. Our review focuses on the transition metal-based quantum dot composites as proficient electrocatalysts for electrochemical HER. This review provides considerate and comprehensive insights that cover the HER, focusing in particular on the acidic and alkaline medium electrolytes used in the reaction and the underlying mechanistic pathway. Moreover, the recent development of TMQDs as an effective electrocatalyst is discussed, as well as the comprehension of their activity due to their composition, structure, and active sites available for the reaction to proceed, along with future perspectives and challenges.2. The reaction of hydrogen evolution (acidic and alkaline medium)
Electrochemical water splitting involves the concurrent usage of water in order to produce hydrogen and oxygen. It looks like a simple solution for transforming electrical energy into chemical energy.8,23,24 In an electrocatalytic HER, H2 is obtained after the reduction of the proton (H+) or H2O at the interface of an electrode and electrolyte (redox reaction), which entails a series of simple steps that are solely based on electrolyte pH. The overall HER reaction is a simple process of water being split into hydrogen and oxygen but the entire electrochemical process is not that simple, involving a multi-step process on the electrode surface. This is because, at higher current density, there can be significant ohmic losses due to ineffective ion transportation in the electrolytic solution. To control this effect, it is necessary to use highly conducting electrolytes. Thus, the two most conductive ions H3O+ and OH− are to be utilized in the electrolysis processes either in highly acidic or highly basic medium (Fig. 1).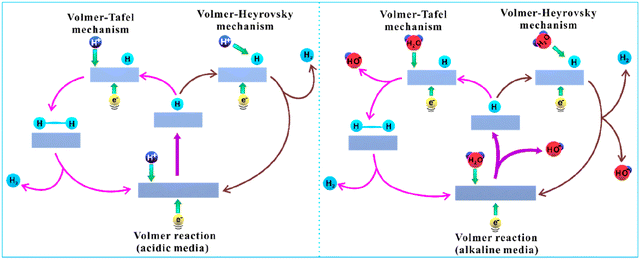 | ||
| Fig. 1 Generalized mechanism of HER in acidic and alkaline medium. Reproduced from ref. 25 (Copyright 2019, American Chemical Society). | ||
Though commercial applications work in any of the mediums, the usual outlook has been to focus on the acidic medium because it will have greater potential and have higher conducting nature.26 The kinetics of the reaction depends on the material used in the electrode, for example, platinum is the renowned material for the hydrogen evolution reaction, whereas a mercury electrode has slower kinetics. Other parameters include electrode orientations (e.g., amorphous surface, polycrystalline, single crystal) and the nature of the electrolyte. Table 1 gives the overall idea about the HER reaction mechanism occurring in acidic and alkaline mediums. To put the entire mechanism in a nutshell, the hydrogen evolution begins on the surface of the catalyst by adsorbing the H atom, which is the Volmer reaction. Further, based on the nature of electrocatalysts, the reaction can proceed in two ways, which are with two adsorbed H, the Tafel reaction, or single adsorbed H, the Heyrovsky reaction; thus, facilitating the liberation of the H2 molecule. Hence, it is important to have a good choice of electrocatalyst for the reaction, which can give good adsorption of H.
| Acid | Alkaline | Tafel slope | |
|---|---|---|---|
| Volmer | M + H* + e− → M − H | M + H2O + e− → M − H + OH− | b = 2.303RT/(∝F) ≈ 120 mV dec−1 |
| Heyrovsky | M + H+ + e− + M − H → H2 + M | M + H2O + e− + M − H → H2 + OH− + M | b = 2.303RT/((1 + ∝)F) ≈ 40 mV dec−1 |
| Tafel | 2M − H → H2 + 2M | 2M − H → H2 + 2M | b = 2.303RT/(∝F) ≈ 30 mV dec−1 |
| Overall | M + 2H + 2e− → H2 | M + H2O + 2e− → H2 + 2OH− |
2.1. Understanding the mechanistic approach of acidic HER
Water electrolysis in an acidic medium is the cathodic reaction where the hydronium ions get reduced to hydrogen gas. Considering thermodynamics, the reaction occurs at a potential that is equal to that of the reference hydrogen electrode (RHE) reaction occurring, initially with proton reduction at the active catalyst site – Volmer step, followed by the molecular hydrogen evolution, which is through the second electron/proton transfer – Heyrovsky step and if the recombination of adsorbed protons occur that is the Tafel step (eqn (1)–(3)).| H3O+ + e− + * → H* + H2O | (1) |
| H* + H3O+ + e− → H2 + H2O | (2) |
| 2H* → H2 | (3) |
In electrochemical hydrogen evolution, many reports focus on the surface of the Pt in an acidic medium for the mechanism of the reaction (eqn (4)),
| H+ + e− + Pt* → Pt − HUPD | (4) |
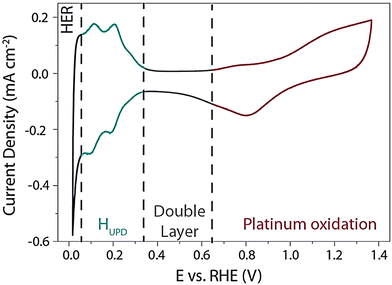 | ||
| Fig. 2 Cyclic voltammogram of Pt(pc) electrode at scan rate of 50 mV s−1 in an electrolyte solution of 0.5 M H2SO4 solution which was degassed in argon. Reproduced from ref. 28 (Copyright 2019, The Royal Society). | ||
The second region is the potential in the range of 0.35 V and 0.65 V vs. RHE, the region of no faradaic process occurrence referred to as a “double layer region.” The third one is the hydrogen underpotential deposition (HUPD) region in the potential 0.05 V and 0.35 V vs. RHE. The proton discharge from the surface of Pt occurs in the final region at the higher negative potential, and hydrogen gas is evolved.29–31 The HUPD region is distinguished by a single broad envelope and a couple of redox peaks at around 0.2 V vs. RHE, followed by the peaks at around 0.1 V vs. RHE.28
Nørskov et al. gathered the experimental data on the HER in 2004 for various metals, by utilization of Density Functional Theory (DFT) that corresponds to adsorption energies by presenting the modern volcano plots.32 The findings explain the Pt's superior activity in HER as well as provide an insightful explanation of HER's activity and its dependence on hydrogen binding energy.
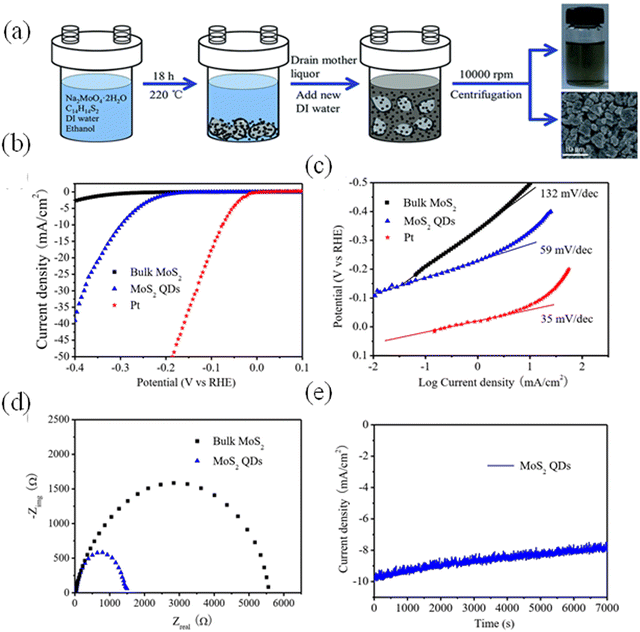
In Fig. 3, the volcano plot shows an overall idea of the choice of electrocatalysts. Basically, Pt is the current benchmark material, but considering the TMQDs, the widely studied material is MoS2 QDs, the reason lies with the bases on a close-to-neutral 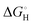 of MoS2 edges, which has later been established with experiments. The edge sites of MoS2 could have high HER activity as a successful pattern.36–38 Further, more research has been conducted and advanced in HER catalyst, emphasizing the theoretical and practical progression for the same.39
of MoS2 edges, which has later been established with experiments. The edge sites of MoS2 could have high HER activity as a successful pattern.36–38 Further, more research has been conducted and advanced in HER catalyst, emphasizing the theoretical and practical progression for the same.39
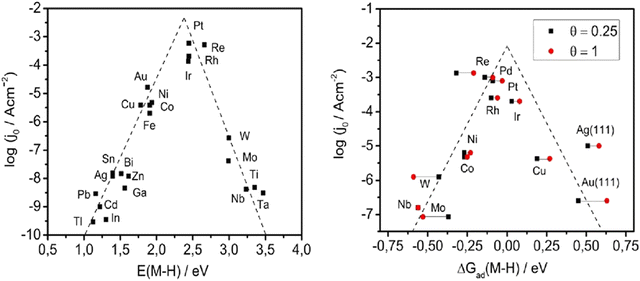 | ||
| Fig. 3 (a and b) The “volcano” plot by Trasatti33 from experimental and the “volcano” plot derived from DFT by Norskov et al.34 Reproduced from ref. 35 (Copyright 2016, John Wiley & Sons). | ||
2.2. Understanding the mechanistic approach for alkaline medium
HER in alkaline medium is critical and important as it is a popular technology within the industrial sector, and can perform an important role in treating discharged alkaline water as alkali and chlor-alkali electrolyzers.40 The reports to date have no alternative to Pt when studied under an alkaline medium on efficiency grounds.41 Thus, the pathway of the reaction must be understood and validated using the basic laws of electrode kinetics, paving the way for the development of efficient electrocatalysts.42 It is also crucial to have HER activity as inclusion with the viewpoint of designing a catalyst and to improve the rate-determining step mechanistically, for the reduction–reaction potential with improved HER activity.43,44 As a well-established fact, there is water adsorption, hydrogen binding energy, water dissociation and OH− adsorption, which influence the HER activity. Nonetheless, the reaction mechanism is well accepted, and computational chemistry has largely been neglecting the theory-based negotiations on the topic of energetics and kinetics of the HER in the alkaline medium that constantly remains in debate among chemists.45 Though many electrochemical reports report distinct aspects of the HER mechanism, the metal–Had bond energy33,46–48 for the reaction, mostly used for the description is insufficient to elaborate the lower activity in basic media. From the mechanism of HER, for the whole catalytic activity, the dependence of pH of the catalyst underpins that it remains a debate.49 Conway et al. explored the characteristics of UPD and OPD (under and over potential deposited) on H over the Pt surface based on the energy of the bond and exchange current density corresponding to the chemisorbed hydrogen atoms over the metal or stand Gibbs energies of the chemisorption of 2H from H2, i.e., the volcano relation.51,52 It was found that kinetically important intermediate is being adsorbed on the Pt or other metals being utilized in HER activity is due to the weakly bound H of OPD rather than strongly bound H of UPD, which means higher the OPD H, the activity is also eventually higher. Under basic conditions, the coverage of hydrogen atoms of OPD is fairly lower in comparison with the acidic medium, hence resulting in the slower reaction rate.53 The reaction mechanism for alkaline HER underlies from the point that at the potential of −1000 mV, HER is observed,as shown in Fig. 4. H2 desorption and desorption from the surface of the electrocatalyst lies between the potential of −500 mV to 0 mV. The OH adsorption or the Pt oxidation lies in the potential of 0 V–750 mV and a further increase in the positive potential leads to the oxygen evolution reaction (OER). The cyclic voltammogram clearly indicates that the reaction occurring on the polycrystalline Pt electrode surface is hydrogen evolution with clear peaks indicating the H adsorption and desorption at a particular potential. Moving towards the computational aspect, the studies for alkaline HER are still very limited due to inadequate data about the electrode potential and the pH that are dependent on the kinetics of water dissociation and thermodynamic hydrogen adsorption processes.54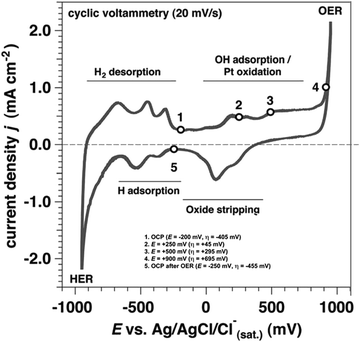 | ||
| Fig. 4 Cyclic Voltammogram of polycrystalline Pt electrode with a rate of scanning equivalent to 20 mV s−1 in an electrolyte solution of 1 M KOH solution. Reproduced from ref. 50 (Copyright 2017, The Royal Society). | ||
3. Choice of transition metal quantum dots as electrocatalyst
The performance of the electrochemical hydrogen evolution reaction depends on various factors starting from the electrocatalyst, environmental conditions, electrolyte solution, which is pH dependent, and so on. Amongst the mentioned factors, the choice of electrocatalyst plays a prominent role in determining the efficiency of the material, which in turn determines the hydrogen evolution. To achieve, higher hydrogen evolution along with cost efficiency, an electrocatalyst should have good compatibility, abundant in nature, and should have efficient working in a wide pH range.The benchmark catalysts such as Pt or Ru also have drawbacks, such as cost and availability. Numerous earth-abundant catalysts with good activity have been reported to date. In consideration of HER, transition metals have been extensively studied as transition metal dichalcogenides, transition metal carbides, transition metal nitrides, phosphides, and so on. Recently, a variety of materials are studied as active catalysts for electrocatalyst properties.55,56 Amongst all of these materials, the transition metal quantum dots have enhanced properties such as increased surface area, a higher surface-to-volume ratio, fluorescence properties, and conductivity, together contributing to an exemplary activity of these materials in the electrochemical hydrogen evolution reaction.57–59
3.1. Transition metal quantum dots: synthesis and their application in HER
Quantum dots (QDs), in general, nano-sized semiconductor crystals, have found fascinating applications in numerous scientific fields. In 1980, Russian Physicist, Alexei Ekimov first discovered QDs.60 Essentially, Bohr's radius of the group II–VI or III–V-based materials is larger than their physical dimensions.61 After more than two decades of its introduction into research, its usability has increased.62,63 Quantum confinement effect and controllable fluorescence emission properties of QDs have been extensively studied initially, which was applicable in the field of biosensing and bioimaging.64,65Quantum dots do have a broad range of unique optical, electrical, physical, and chemical properties, with their applications in illumination, energy harvesting, biology, medicine, sensors, cameras, displays, and communication and information technology. QDs are often used to build displays, effective lasers, solar harvesting devices, and biotags that are now on the market, and they continue to gain attraction in sensing, quantum information, and photovoltaics. Anisotropic geometry and confinement effects in QDs are utilized in a wide variety of applications (Fig. 5) such as photochemical reagents,66 light emitting diodes,67 catalysis,68 solar cells,69 due to their properties such as photochemical,70–72 magnetic,73–76 optoelectrical,77–79 piezoelectrical.80–83 QDs with enhancing properties exhibited by the semiconductors are proved to be effective electrocatalysts in HER in the current research. Predominantly, due to a reduction in size, the metallic materials will have a wider surface area, less mechanical fracture, and larger active sites, amidst the above attractive characteristics, metallic QDs lack good electrical conductivity and have a higher tendency to agglomerate,84,85 thus making them inefficient as a single-component electrocatalyst, but QDs effectively work when they are engineered in composites. These composites have been widely manipulated in research as efficient electrocatalysts.86
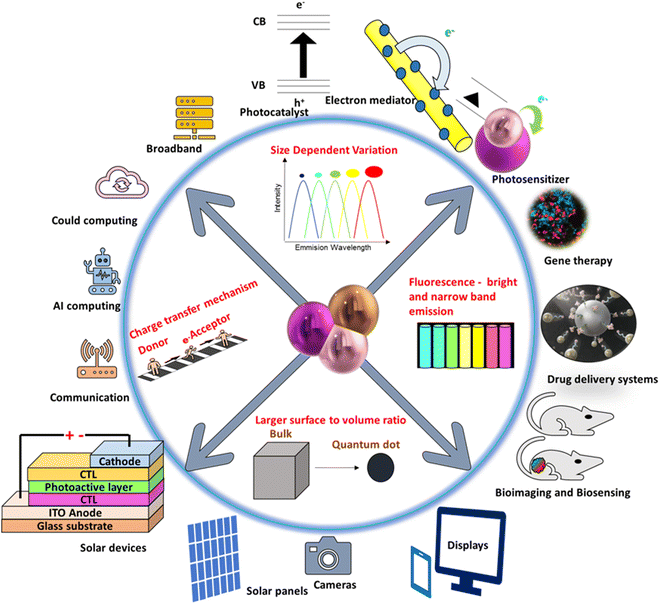 | ||
| Fig. 5 Progress in quantum dots for a wide range of applications; there are still opportunities for the configuration of QD-enabled new device architectures, a few of which are listed here. | ||
3.2. Synthesis and properties of TMQDs
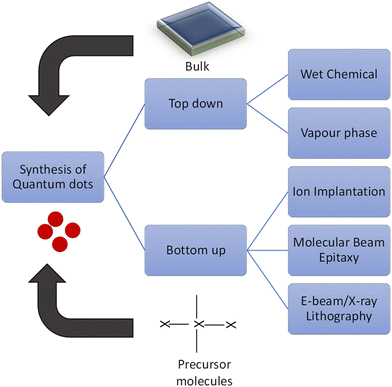
Among the many methods proposed for the synthesis of QDs, the broad classification includes “Top-down” and “Bottom-up” approaches (Fig. 6), and the properties are modified with the preparation methods or post-synthesis treatments.87 Top-Down approaches include molecular beam epitaxy (MBE) for thin film deposition, X-ray, and electron beam lithography or ion implantation techniques. The other bottom-up methods include chemical reduction with self-assembly in the solution.88–90 The top-down approach to the synthesis of QDs involves nothing but a process in which a bulk semiconductor is bought down to a diameter of approximately 30 nm range by systematically controlling the reaction atmosphere, thus achieving materials with a quantum confinement effect. Such materials have a controlled size and shape with exceptional packing geometries.There are similarly other methods for synthesis of QDs, but the major disadvantage is the incorporation of impurities and structural imperfections that arise during the process.91
Lasek et al. have successfully synthesized transition metal tellurides in an ultra-high vacuum MBE chamber by co-deposition of the transition metal and tellurium with the highly oriented pyrolytic graphite (HOPG-) or MoS2 substrates (van der Waals substrate).92 Bouravleuv et al. utilized the MBE route on the semi-insulating and n-type GaAs(001) substrates synthesizing AlxGayAsz quantum dots with selective doping of Mn.93 Thevuthasan et al. synthesized Au nanocrystals with ion beam implantation.94 Ueda et al. synthesized InAs QDs by sequential implantation as in a-SiO2.95 Weiwei Chen et al.96 successfully synthesized CsPbCl3 quantum dots by understanding the Palazon's group's synthesis of CsPbX3 QDs surface via the X-ray lithography route, resulting in enhanced stability of the synthetic QDs.97
To date, the most pronounced synthesis of QDs follows the bottom-up method, which is broadly divided into vapour-phase and wet-chemical methods.91 In the vapour-phase method, the QDs are built from atomic processes. Basically, no pattern is observed in the self-assembled molecules.98–101 In the self-assembly process the MBE, sputtering, or aggregation of monomers, are also categorised.
Forleo et al. synthesized ZnO quantum dots by using the zinc acetate as a precursor, which was performed at room temperature, making the possibility of attaining uniform-sized QDs with enhancement from the application point of view.102 Hui Zhang et al. successfully confirmed the synthesis of CdS quantum dots doped over Mn, Cu, and Ni with bimetallic clusters for gram-scale synthesis under mild reaction conditions.103 Currently, the wet chemical method is applied widely to control various factors of the changes in the properties of the materials.
A typical quantum dot is one in which the structural orientation has a metalloid crystalline core and this core material is known to be encapsulated or engulfed within a thin shell form. This is usually performed to enhance the electronic and optical properties of the quantum dots themselves. Examples of such metalloid crystalline cores include CdS, CdSe, or CdTe and the thin shell is composed of transition metals such as ZnS. In general, the quantum dots possess properties that prominently depend on the particle size and chemical composition of quantum dots.104 It is evident from an MQD system that a non-metallic atom with enhanced electronegativity is preferably selected to passivate the MQD active sites efficiently.105 Mei et al. in 2019 discussed the enriched optical properties that are colour tuned, made of cadmium-free transition metal ions that are doped with InP/ZnS quantum dots. This work inculcated a nucleation doping method via an organic synthetic pathway wherein a series of transition metals co-doped with InP/ZnS quantum dots with variable colour-tuned optical properties were synthesized. From the resultant PL spectra of the transition metal-co-doped quantum dots, it is known that all the QDs possess color-tunable dual emissions and indicates that the dopant emission is predominantly due to the doping of Cu whilst that of Mn.106 To be more precise, MQD has enhanced physical, chemical, and various other mechanical properties in comparison with those of the 2D materials that include sulphides and selenides of metals. This enhancement of properties is basically due to their larger surface area, edge-effect, and quantum confinement effect being lower. At the near Fermi level, the MXQD possesses increased metallic conductivity and have more electron density, which is attributed to the small size of the material itself. This property of electrical conductivity can be furthermore increased by doping of any heteroatom or by the synthesis of carbonitrides or nitrides of quantum dots. One preferable advantage of enhancing the electrical conductivity of MQD is that they tend to uplift the processing of electron transfer, which in turn attributes to the creation of more active sites.107 TMQDs including carbides, oxides, and nitrides are generating wide attention due to their vast range of properties. These include conductivity, high active sites, cost-effectiveness, good dispersion ability, biocompatibility, tuneable framework, remarkable optical properties, and functionalization with different groups. The use of MQDs for the heterostructure composite is a strategy to tune the band gap and improve the catalytic efficiency.105 Mo2C QDs and WC QDs incorporated with N-doped graphene nanosheets have been reported for HER. The catalyst shows good durability and high exchange current density. Ti3C3 Mxene QDs show more grain boundaries and edge defects than the 2D Mxene perfect crystal. The use of OsOx quantum dots with graphydine has been recently reported as an electrocatalyst for HER under visible light irradiation. The progress in the field of MQDs is in the growing stage in the field of HER. TMQDs and their surface modifications for improved catalysis. Furthermore, it is important to understand the clear mechanism from experiments, followed by theoretical data to enhance development in this field.
3.3. TMQDs in electrochemical HER as an efficient electrocatalyst
Transition metal quantum dots have gained increasing attention recently due to their tuneable characteristics in both optical and electronic stands.In comparison with the semiconductors which are used traditionally over the years, the layered transition metal dichalcogenides show significantly better properties of fluorescence when they are confined into quantum dots.
In the field of bio-sensing and bio-imaging, TMQDs are proving themselves as efficient candidates primarily due to their high dispersibility and extremely low toxicity.108
It is known that when the bulk layered transition metal dichalcogenides are reduced to quantum sizes, they tend to show enhanced properties, which is due to the quantum confinement effect and the prominently grown-up edges. Research studies reveal that the metal quantum dots of zero dimensions are known to have increased the surface-to-volume ratio, which means that the exposed edges are larger in proportion and the chemical and physical properties are largely tuneable possessing profound solubility when dissolved in aqueous solutions.110
Shao et al. in 2020 emphasized the synthesis and application prospectus of 2-dimensional transition–metal-based MXenes that are driven out into quantum dots via fragmentation into ultra-small layers by utilising the technique of sonication (Fig. 7).109
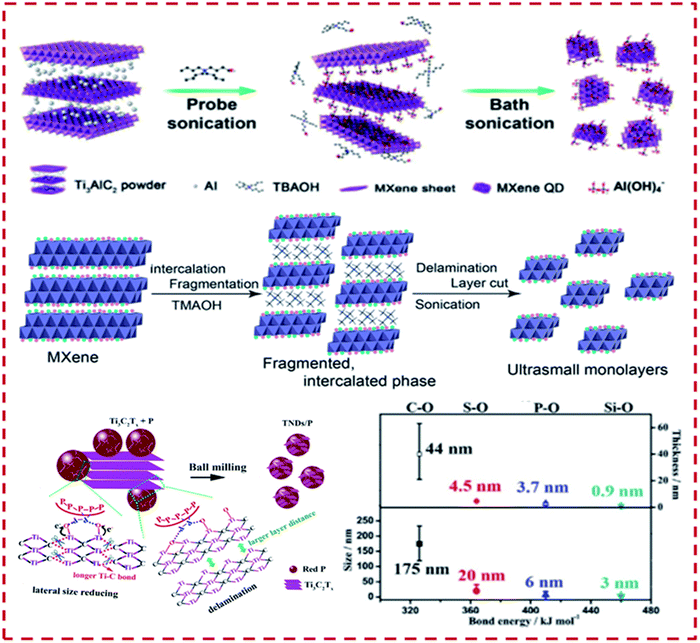 | ||
| Fig. 7 Schematic representation of the synthetic methodology of Ti3C2 QDs involving a double step ultrasound method. Reproduced from ref. 109 (Copyright 2020, The Royal Society). | ||
Huang et al. observed the controlled construction of heterostructure with ultrafine MoS2 QDs, which were decorated on the Ti3C2Tx MXene nanosheets (MQDs/Ti3C2Tx) (Fig. 8).111 With the optimisation study, the lowest onset potential of 66 mV was obtained with a Tafel slope of 74 mV dec−1, which is small with increased life showing contrast behaviour of individual Mxene or QDs.
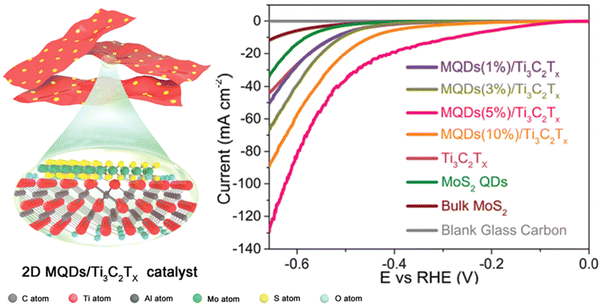 | ||
| Fig. 8 The overall graphical representation of HER with MoS2 QDs with MXene composite. Reproduced from ref. 111 (Copyright 2022, The Royal Society). | ||
Liu et al. reported the introduction of transition metals onto the Ru metal-based carbon quantum dots. In this method, transition metals such as Ni, Mn, and Cu are doped over the ruthenium lattice and the electrochemical production of hydrogen is studied.112 To be precise, the nickel doped ruthenium carbon quantum dots show low overpotential of about 13, 58, and 18 mV that achieves a current density of about 10 mA cm−2 in solutions of 1 M KOH, 0.5 M H2SO4, as well as 1 M PBS. (Fig. 9a–e).
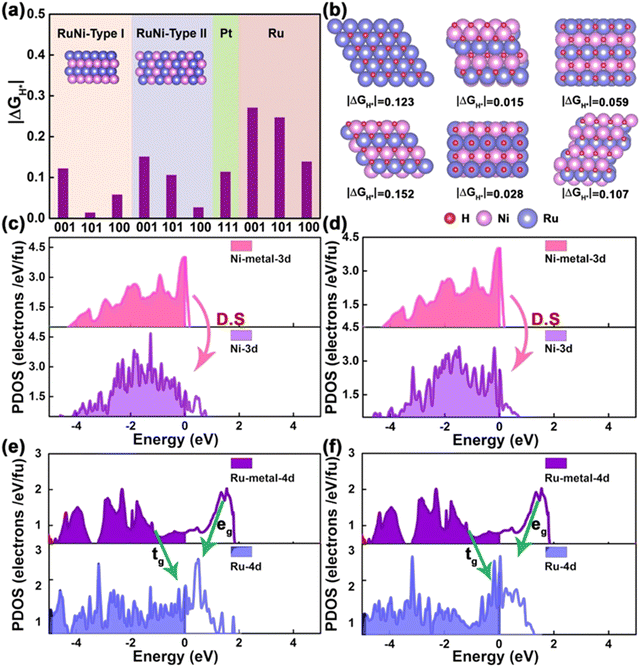 | ||
| Fig. 9 Enhanced activity of the attributed to the energies of the adsorbed energies (a), crystal structure (b), partial density of states (c–f). Reproduced from ref. 112 (Copyright 2020, John Wiley & Sons). | ||
Experimental reports suggest that the excellent activity of the Ni-based catalyst is largely due to its selectivity towards crystalline surface of the hcp RuNi particles and its variation in the electronic structure after the doping of transition metal. Ou et al. formulated a facile and a generally-extremely fast laser ablation technique in order to synthesize MoS2 quantum dots possessing a higher number of active sites along with upskilled conductivity of electrocatalytic HER.113 The electrochemical analysis of the prepared sample MoS2 was compared with that of the pristine MoS2.
The results are in accordance with the improved active efficiency of the MoS2 catalyst. After ultrafast ablation treatment at a 100 mA cm−2 current rate, it was evident that the overpotential was decreased from 450 mV to 187 mV. From the above results, the team found that the overpotential values of pristine MoS2, MoS2, and the commercially available Pt/C were known to be 345, 108, and 38 mV, respectively (Fig. 10a–e). This material tends to show an overpotential value that is very much closer to the commercially available Pt/C. This high performance of the MoS2 quantum dots has been acknowledged, thanks to the improved specific surface area, as well as enlarged active sites in addition to good conductivity and high hydrophilicity. Xianpei et al. designed a novel method of increasing the efficiency of the electrocatalyst for hydrogen evolution by synthesizing MoS2 nano-catalyst, which is of ultra-small size and has few layers, that can enhance the activity of the catalyst (Fig. 11a).114
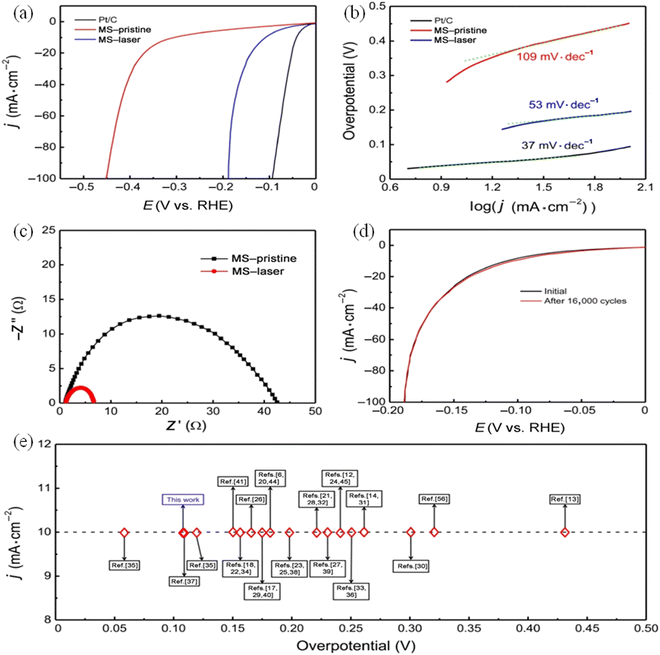 | ||
| Fig. 10 Electrochemical studies as polarisation curves before (b) and after (d), Tafel slope (b), Nyquist plot (c) and stability (e) of MS-QDs compared with bulk MoS2. Reproduced from ref. 113 (Copyrights (2022, Springer Nature)). | ||
 | ||
| Fig. 11 (a) Synthesis methodology for the preparation of monolayer MoS2. Electrochemical polarisation curve (b), Tafel slope (c), Nyquist plot (d) and stability study (e) of the monolayer MoS2. Reproduced from ref. 114 (Copyrights 2022, Royal Society of Chemistry). | ||
This preparatory method could be facilitated by a hydrothermal synthesis route. Comparative electrochemical studies of the MoS2 catalyst with that of the bulk MoS2 and the commercial Pt electrode reveal that the overpotential value of the MoS2 electrode is about 160 mV, and that of the Pt is near zero, while the overpotential value of the bulk MoS2 was much higher than the stated values.
The Tafel slope of the Pt catalyst is ∼35 mV dec−1 and for molybdenum quantum dots it is about ∼59 mV dec−1 which are comparatively lower value than that of the bulk sample with the value of ∼132 mV dec−1 (Fig. 11b and c).
This work idealizes MoS2 quantum dots and their layering to increase the efficiency of this cost-efficient electrocatalyst and lower them into ultra-small sizes for profound results of hydrogen evolution (Fig. 11d and e).
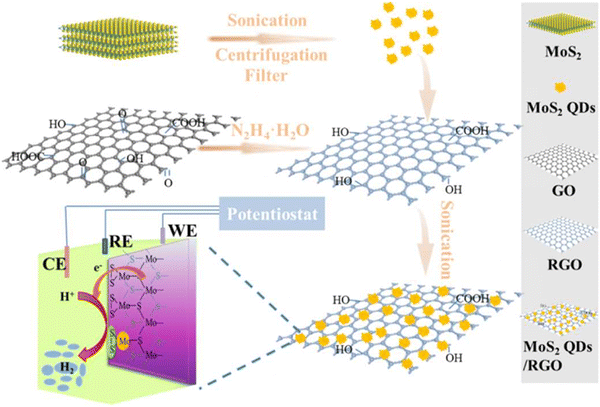 | ||
| Fig. 12 Graphical depiction of the design and development of MoS2 quantum dots on reduced graphene oxide catalyst. Reproduced from ref. 116 (Copyrights 2022, Royal Society of Chemistry). | ||
The synthesized catalyst is known to have electro-conductive RGO, which can create freestanding, thin van der Waals heterostructures automatically. The synthetic nanocomposite showed enhanced HER activity with an onset potential of about −8 mV, a current density of 0.669 mA cm−2, and a Tafel plot value measuring 63 mV dec−1, showing better stability as well.
The composite is known to be highly active, durable, and considered a promising alternative material in place of noble metal catalysts used for HER.
Zhang et al. tailored an efficient route for the synthesis of cobalt phosphide (Co2P) quantum dots incorporated with nitrogen, carbon over a carbon cloth was doped dually with phosphorous, and the reaction is carried out in situ by carbonization of cobalt ions, which is carefully induced to polyaniline and phytic acid macromolecule precursors.117 Highly active electrochemical sites and charge transport are enabled with quite low resistance, which is primarily due to the cumulative effects of N, P dual-doped carbon frameworks and Co2P quantum dots. Electrochemical studies reveal that the as-prepared catalyst is known to exhibit good electrochemical activity for efficient hydrogen production in both acidic and alkaline media. The acidic medium was found to be superior in activity to the alkaline one. The overpotential of the catalyst at a current density of 10 mA cm−2 is experimentally known to be 116 mV in the acidic medium and 129 mV in the alkaline medium, respectively. In comparison to the Tafel slope values for the catalyst, a smaller Tafel slope value of about 93 mV dec−1 was calculated for acidic electrolytes. Thus, the obtained catalyst Co2P@NPC-800 works well in both acidic and alkaline electrolytic conditions and concluded that the catalytic performance is enhanced in an acidic medium than in an alkaline medium. Zonghua Pu et al., in 2016, illustrated a one-step simple methodology for the synthesis of MoP Nps@NC.118 The synthesized catalyst is known to show good HER efficacy with an overpotential value of about 136 mV and 80 mV in 0.5 M H2SO4 and 1.0 M phosphate buffer solutions, respectively, when the current density is about 10 mA cm−2 (Fig. 13a–e).
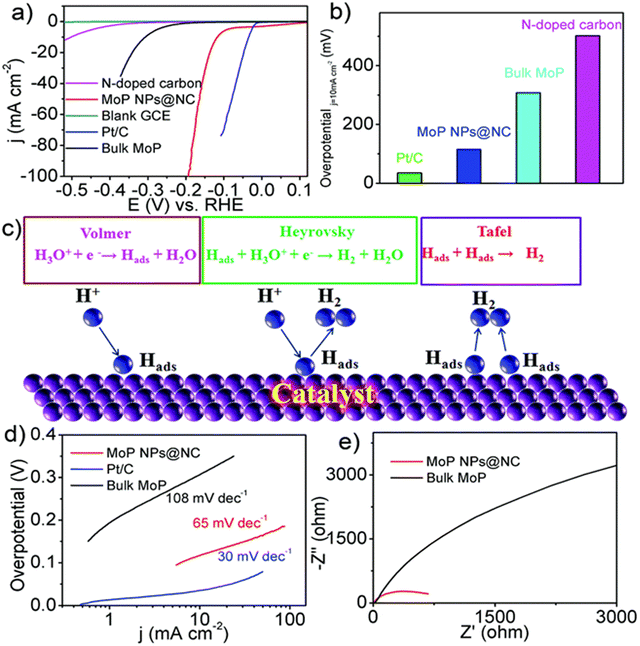 | ||
| Fig. 13 Polarisation curve (a), the overpotential values at 10 mA cm−2 (b), the HER (c), Tafel plot (d) and Nyquist plot (e). Reproduced from ref. 118 (Copyrights 2022, Royal Society of Chemistry). | ||
MoS2/WS2 quantum dots could be prepared under simple experimental conditions such as ultra-sonication and solvothermal processes under mild temperature from the bulk MoS2/WS2. To measure the hydrogen evolution capacity of this material, a standard three-electrode was set up by employing 0.5 M sulphuric acid as the electrolyte, with a scan rate of about 5 mV s−1. As it is known that bulk MoS2 is associated with lower catalytic activity, as well as MoS2/WS2 when compiled to form a composite, the decrease in the catalytic activity was anticipated. After the solvothermal process, the as-prepared composite of MoS2/WS2 tends to show a lower onset overpotential ≈120 mV for the hydrogen evolution reaction.
This enhanced catalytic activity is specifically due to the MoS2 quantum dots that are interspersed in MoS2 nanosheets, which are believed to have enriched defects. As such, the materials have more active edge sites for hydrogen evolution. The random layering of the nanosheets onto the surface of the glassy carbon electrode is used as a key for the efficient electron transfer between the underlying electrode and the active edge sites. The work reported that the combination of sonication and the solvothermal process can be a perspective synthesis method for preparing effective TMD material for HER since the as-prepared composite is said to possess better catalytic activity than the individual WS2 and MoS2.
Yu et al.,120 fabricated a facile synthesis method for the self-assembly of Au/MoS2, which is a core-satellite hybrid material, efficiently utilized as a catalyst for electro-catalytic Hydrogen Evolution Reaction (HER). In this work, the performance of the catalyst was in comparison with that of Au nanoparticles, 20% Pt/C and MoS2 quantum dots. The presence of a higher number of edge sites on MoS2 and the aggregated high conductance of the Au nanoparticles was attributed to the better HER performance of the hybrid material. On the basis of the experiments, it was studied that the hybrid Au/MoS2 quantum dots will require overpotential values of about 206, 146, and 112 mV to drive current density values of 50, 20, and 10 mA cm−1, respectively (Fig. 14a–d).
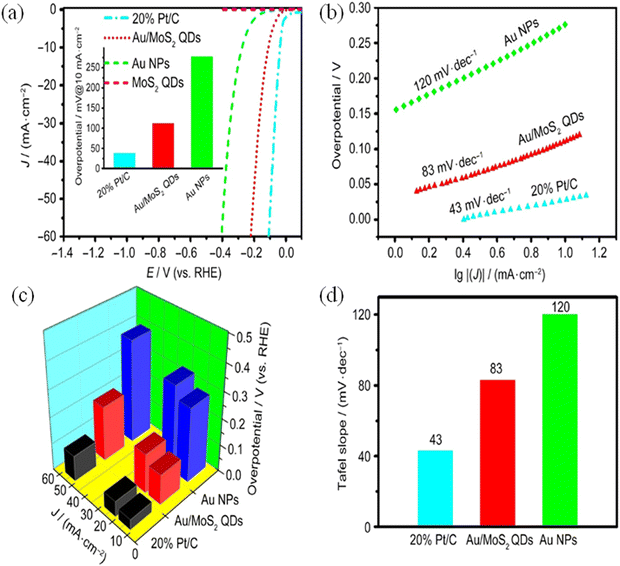 | ||
| Fig. 14 Electrocatalytic performance with polarisation curve (a), Tafel plot (b), overpotential at different current densities and composite (c) and histogram for Tafel slope (d). Reproduced from ref. 120 (Copyrights 2022, Springer Nature). | ||
Li et al.,121 developed a new novel ruthenium nanoparticle that is loaded with carbon quantum dots used to be a competent electrocatalyst in the HER. The electrochemical studies of this material showed that under extreme conditions of alkalinity, the as-synthesized material showed up outstanding catalytic effects with an overpotential of about 10 mV and has good durability. The value of the Tafel slope for this material was 47 mV dec−1 (Fig. 15). Dang et al.,122 reported a novel work by obtaining Rh–Si composites, wherein the Rh nanocrystals are made to grow on the surface of the silicon quantum dots which is aided by the Si–H bonds (Fig. 16).
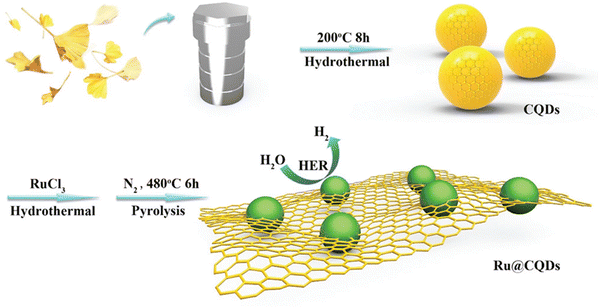 | ||
| Fig. 15 Pictorial depiction of the synthesis and HER activity of Ru@CQDs480 electrocatalyst. Reproduced from ref. 121 (Copyrights 2018, John Wiley & Sons). | ||
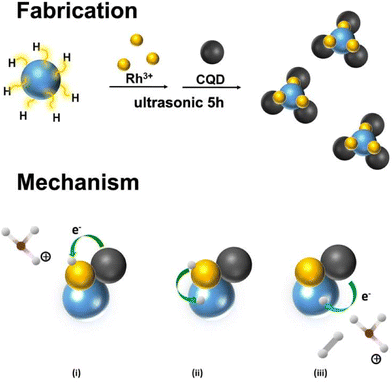 | ||
| Fig. 16 Fabrication of Rh/SiQD/CQD nanocomposites and mechanism of reaction. Reproduced from ref. 122 (Copyrights 2022, Elsevier B.V.). | ||
Based on electrochemical studies at optimal conditions, the as-prepared material shows a low Tafel slope value of 26 mV dec−1 (Fig. 17a–d), as it is found to be stable for a longer time. The efficiency provided by this material is shown in spite of lower amounts of noble metal usage than usually employed in 20 wt% Pt/C, substantiating novelty.
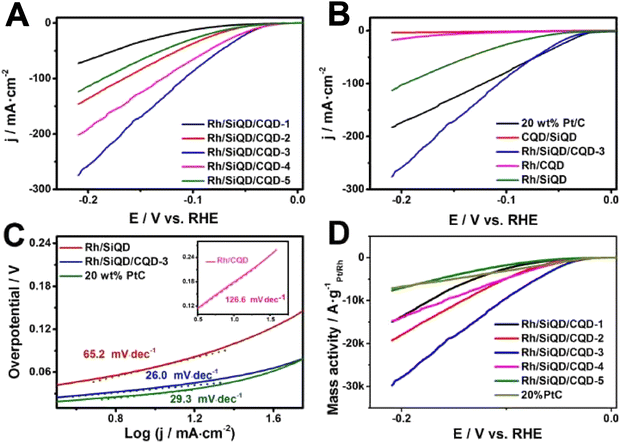 | ||
| Fig. 17 LSV curves (a and b), Tafel plot (c) and mass activity (d) of the nanocomposite. Reproduced from ref. 122 (Copyrights 2022, Elsevier B.V.). | ||
Thus, this curtails the pathway available for electron transfer. Such results are possible only when the material is in nanoscale and not in bulk scales.123 Either way of enhancing the efficiency of electrocatalyst is to electronically modify the structural properties of the electrocatalyst to increase the activity of each active site.124 An additional method to overcome this issue is to make available higher surface area in materials by proper nanoscale designing, for example, mesopores, nanoparticles, nanosheets and nanowires.125
Zhang et al. tailored an efficient synthesis method for the preparation of cobalt-doped Mo, Z-ZIF derived Co9S8 quantum dots wherein the MoS2 was embarked in the nanoflake arrays of carbon, which was doped with 3D nitrogen, and the system was supported on carbon nanofibers (Co9S8-MoS2/N-CNAs@CNFs).126 This material is said to have a unique 3D structure owing to its trifunctionality for OER and HER applications. Electrocatalytic studies revealed that Co9S8-MoS2/N-CNAs@CNFs are known to have a current density with the value of 10 mA cm−2 and possess an overpotential of 163 mV for hydrogen evolution reactions. This scale value is typically an efficient outcome when compared with the traditional MOF-derived transition metal sulphide catalysts (Fig. 18a and b). Huo et al. synthesized a novel well-defined heterojunction of molybdenum carbide-tungsten carbide quantum dots, which were 0-dimensional and 2-dimensional decorated over N-doped graphene nanosheets through a nano-casting method using a standard template KIT 6/graphene.127 Under acidic conditions, (Mo2C)0.24-(WC)0.52-QDs/NG, (Mo2C)0.41-(WC)0.18-QDs/NG, and (Mo2C)0.34-(WC)0.32-QDs/NG needed an overpotential value of about 100, 100, 119, 146, and 175 mV, respectively (Fig. 19a and b).
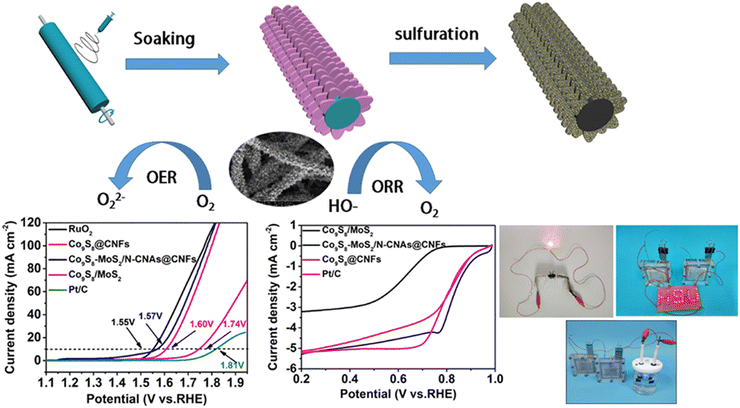 | ||
| Fig. 18 Schematic representation of the preparatory scheme of Co9S8-MoS2/NCNAs@CNFs (a). Polarisation curve with the pictorial representation of the circuit (b). Reproduced from ref. 126 (Copyright 2022, American Chemical Society). | ||
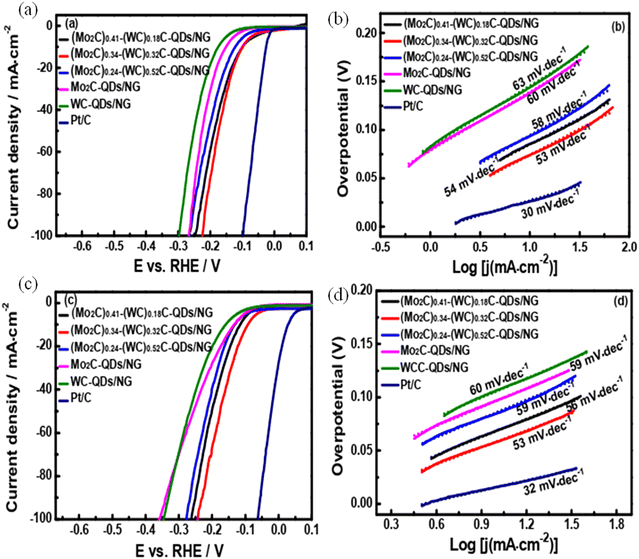 | ||
| Fig. 19 The polarisation curves (a and c) and the Tafel slope (b and d) for the studied samples. Reproduced from ref. 127 (Copyrights 2017, Royal Society of Chemistry). | ||
These overpotential values are lower than that of WC-QDs/NG (175 mV and 204 mV) and Mo2C-QDs/NG (146 and 181 mV). The greater efficiency of this catalyst could be attributed to the incorporation of MoC-WC, which has led to the redistribution of electrons in the valence bands and boosted conductivity as observed with the Tafel values (Fig. 19c and d). With all this effectiveness, this material has proved to be an excellent catalyst to produce hydrogen from water.
Ye et al. reported Pt(III) quantum dots on flower-like α-Fe2O3 thin film nanosheets. The material showed excellent water splitting activity due to the bifunctional catalytic activity, which is attributed to the quantum size effect and many active sites.128 The active sites could offer greater efficiency with a lower overpotential of 90 mV and a Tafel slope of 49.6 mV dec−1 and the overall water splitting was obtained at a low voltage of 1.51 V in alkaline KOH conditions, which is remarkable.
The close contact of Pt QDs with the nanosheets reinforced the quantum size effect, thus leading to the synergetic effect and the enhancement of the electron transfer rate. Das et al. incorporated N,N′-dibenzoyl-L-cystine (DBC) hydrogels into the polymer matrix of polyaniline(PANI) synthesizing an aerogel DBC-MoS2-PANI with 2 different composition ratios and acquired the lowest overpotential of 196 mV and a Tafel slope of 58 mV dec−1 (Fig. 20).129 The surface modification results in higher efficiency along with the photo-response property of the material. Liu et al.130 in 2022 studied the evolution of surface-active groups of MXene Quantum Dots composed of Ti2CTx, wherein Cl – groups are continuously replaced by O-terminated groups, while the reaction takes place in the cathode. The material was found to have good electrocatalytic efficiency towards HER with a lower overpotential value of about 175 mV owning to a current density of 10 mA cm−1 in an electrolytic solution of 1 M aq. KOH. For this value of current density, the electrocatalyst was found to be highly stable over a period of 165 h.
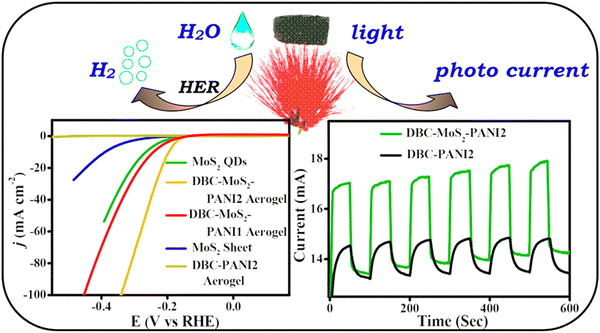 | ||
| Fig. 20 The overall representation of the hydrogen evolution along with the polarisation curve and photo response property of DBC-MoS2-PANI and its composites. Reproduced from ref. 129 (Copyright 2018, American Chemical Society). | ||
Dinda et al., amorphized MoS2 QDs via a simple chemical reaction, obtaining a QD of ∼4 nm.131 The onset potential and superior electrocatalytic activity of the material are ascribed to the unsaturated sulphur ligands and a higher number of active edge sites to an overpotential of 65 mV and the Tafel slope value of 73.9 mV decade. The amorphization could clearly be observed in the SAED pattern and blue shift in the peaks of the Raman analysis. The unsaturated sulphur ligands were confirmed with the XPS analysis (Fig. 21).
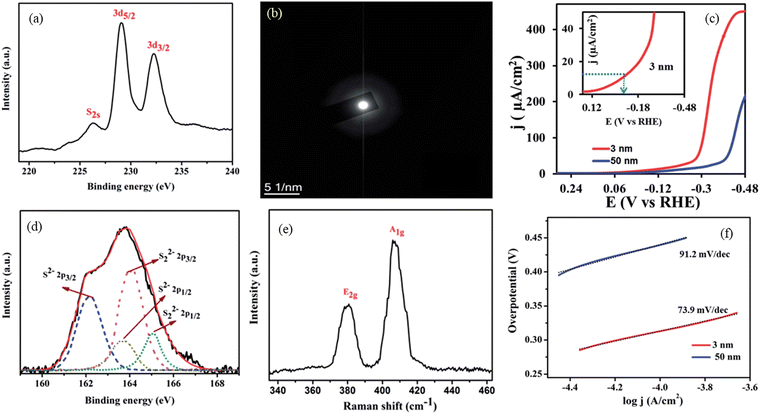 | ||
| Fig. 21 S 3d and S 2s XPS (a), SAED pattern (c) for the catalyst with polarisation curves (d) S 2p XPS, (e) Raman spectra (f) the Tafel slope for the studied samples. Reproduced from ref. 131 (Copyrights 2017, Royal Society of Chemistry). | ||
This material showed a pathway as an efficient electrocatalyst for energy production due to its quantum confinement and more active unsaturated sulphur ligands. Currently, there are several reports with quantum dots as electrocatalysts for efficient HER, and the current review comprises the study of the recent advances, efficiency, as well as advantages of these TMQDs composites as electrocatalysts for HER; Table 2 represents the few recent reports of QDs based electrocatalysts utilized in HER.
| Sl. no. | Material | Synthesis methods | Electrolyte | Mechanistic pathway followed | Overpotential at 10 mA cm−2 | Tafel slope (mV dec−1) | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | MoS2 quantum dots decorated RGO | Facile sonication | 0.5 M H2SO4 | Heyrovsky-Volmer | 64 mV | 63 | 132 |
| 2. | Monolayer MoS2 quantum dots | Facile hydrothermal | 0.5 M H2SO4 | — | 160 mV | 59 | 133 |
| 3. | Solution-processed hybrid graphene flake/2H-MoS2 quantum dot heterostructures | One-step solvothermal | 0.5 M H2SO4 | Heyrovsky–Volmer | 136 mV | ∼82 | 134 |
| 4. | Two-dimensional dual-carbon-coupled defective nickel quantum dots | one-pot thermal treatment | 1 M KOH | Heyrovsky–Volmer | 133 mV | 65 | 135 |
| 5. | SnS2 quantum dots growth on MoS2 | Facile hydrothermal | 0.5 M H2SO4 | Heyrovsky–Volmer | 240 mV | 65 | 136 |
| 6. | MoS2 quantum dots and pore-rich monolayer MoS2 | Gas phase etching | 0.5 M H2SO4 | Volmer | 241 mV | 163 | 137 |
| 7. | VSe2 quantum dots on carbon cloth | Tip sonication | 0.5 M H2SO4 | — | 0.40 V | 80 | 138 |
| 8. | Pt QDs@Nb2CTx nanowire | Solution-based | 1 M H2SO4 | Heyrovsky–Volmer | 33.3 mV | 29 | 139 |
| 9. | Pt QDs@Nb2CTx nanowire | Solution-based | 1 M KOH | Tafel–Volmer | 61.5 mV | 58 | 139 |
| 10. | Quasi zero-dimensional MoS2 quantum dots decorated 2D Ti3C2Tx MXene | Facile hydrothermal | 0.5 M H2SO4 | Heyrovsky–Volmer | 220 mV | 72 | 140 |
| 11. | Two-dimensional PtS2 quantum dots/TiC | Thermal treatment | 0.5 M H2SO4 | Heyrovsky–Volmer | 55 mV | 60 | 141 |
| 12. | Ni3Sn2S2@Ni3S2 NF | Chemical vapor deposition | 1.0 M KOH | Heyrovsky–Volmer | 53.2 mV | 73.2 | 142 |
| 13. | Ruthenium quantum dots supported on carbon nanofibers | Simple adsorption and pyrolysis | 1.0 M KOH | — | 20 mV | 31 | 143 |
| 14. | MoS2 quantum dots@TiO2 nanotube arrays | Electrochemical exfoliation with electrophoretic deposition (EFED) | 0.5 M H2SO4 | — | 76 mV | 93 | 144 |
| 15. | CoSe2 QDs with MoSe2 nanosheets heterostructures | Hydrothermal | 1.0 M KOH | Heyrovsky–Volmer | 218 mV | 76 | 145 |
| 16. | PbTe quantum dots with amorphous MoSx/TiO2 nanotube arrays | Successive ionic layer adsorption and reaction (SILAR) and electrodeposition | 0.5 M H2SO4 | Heyrovsky–Volmer | ∼300 mV | 42 | 146 |
| 17. | Mo2C quantum dots embedded chitosan-derived nitrogen-doped carbon | Scalable solid-state reaction | 1.0 M KOH | Heyrovsky–Volmer | 160 mV | 55 | 147 |
| 18. | α-MoC1−x quantum dots encapsulated in nitrogen-doped carbon | Solidoid nitridation reduction | 1.0 M KOH | Heyrovsky–Volmer | 118 mV | 84 | 148 |
| 19. | MoS2 QDs@Graphene | lyophilization | 0.5 M H2SO4 | Heyrovsky–Volmer | 140 mV | 68 | 149 |
| 20. | Three-dimensional (3D) MoS2 quantum dots (MoS2QD) aaerogel | Hydrothermal | 0.5 M H2SO4 | Heyrovsk–Volmer | 53 mV | 41 | 150 |
3.4. Major challenges and impact of TMQDs as electrocatalysts
Electrocatalysts are indispensable for lowering the kinetic barrier for HER. The specific challenge faced is that utilisation of QDs as a single-component catalyst is not practically stable and can only be used as an efficient catalyst when designed as a composite. The stability of the electrocatalyst and agglomeration of the material are the major challenges with QDs. Thus, it is important to design and develop QDs, which can stand alone and act as an electrocatalyst that possesses greater stability. The issue of agglomeration can be addressed by storing it in a conducive atmosphere, such as storing it at low temperatures, etc.3.5. Future perspective
Even though breakthroughs are frequently based on a rationale that includes the results of numerous studies in the field, the basic attributes of favourable electrocatalysts can be explained simply as in the electrochemical reactions. With reference to the work done earlier, there are reports that showed hybrid structures’ effect of the electrocatalyst with positive outcomes. It can be assumed that there will be fewer obstacles in scaling up the electrocatalyst for HER application if we lower the cost of the catalyst production by lowering the economic scales. when the HER application is synergistically utilized with industry, the burden on fossil fuel consumption will be reduced, thereby reducing the carbon footprint, with an efficient and stable electrocatalyst. Along with the experimental studies, a better understanding of the mechanism, and designing a catalyst, there is a great need for computational studies to provide the direction of the reaction pathway. With this, there is a greater need for future research to focus on finding, predicting, and screening the TMQDs for high-performance output, which can be further combined with machine learning and can be utilized effectively for scale-up. Standardization of measurements, such as electrode surface area, electrode configuration, catalyst activity, mass loading, and other calculation methods, is essential to accurately and fairly understand the performances of the reported works. Furthermore, in order to bridge the gap between the research lab and the market, the researchers should aim to achieve good experiment results that are practically applicable in real time. As a result, electrochemical measurements are highly recommended in the current situation to further enhance hydrogen evolution.4. Conclusions
The electrochemical HER is growing as one of the prominent replacements for energy generation in sustainable energy goals and making the hydrogen economy the reality for everyday use. For this, a cost-effective catalyst, efficient, and has higher activity, stability, and durability for the long term is essential to realise hydrogen generation economically. In this review, we briefed the current upliftment of the vision of QDs as an electrocatalyst for HER application, which emphasizes the electrochemical performance along with the mechanism related to its activity. To generalize, the good catalytic performance of the QDs in HER application is accredited to the greater intrinsic activity, increased number of active sites, a higher number of atoms exposed, or increased charge transfer and mass transfer process. All together were finely spindled to the tuning of the surface, the collaborative effect between the materials, structure modulation, and many more factors. Despite all these achievements in the QDs as electrocatalysts in HER, hydrogen production is a few steps away from practical application. The electrocatalyst having a greater surface area and a large number of active atoms is efficient and effective for hydrogen production. Along with this, a few basic and real-world issues should be addressed completely to equip the QDs as electrocatalysts. Still, there is a need to understand the fundamentals and mechanisms of HER. Along with shifting from the expensive Pt-electrocatalysts with lower stability until it is made of a composite with carbon for support, which increases the operation cost higher, to less expensive materials with higher stability are rather mandatory than left out as a choice. The prospects depend on finding a solution to the pressing issues, which range from catalyst design to reaction kinetics and mechanism.Importantly, the vast bulk TMQDs can demonstrate excellent HER in the acidic medium and their performance in alkaline or neutral electrolytes are generally poor or has not been fully reviewed. Improving the electrocatalytic activity of TMQDs and expounding their reaction mechanisms in different electrolytes are essential for the widespread application of these materials. Attempts should be made to implement strategies that reduce the energy barriers for water dissociation on catalyst surfaces in alkaline media. For instance, in recent years, with the incorporation of the first row of transition metals (Ni, Co, and Fe) or the doping of rare metals (Ru), TMQDs have shown superb HER performances in the alkaline environment.
Author contributions
Brahmari Honnappa: investigation, writing – review and editing, writing – original draft. Sathya Mohan: writing – review and editing. Ashil Augustin: writing – review and editing. Mariyappan Shanmugam: writing – review and editing. Prince J. J. Sagayaraj: writing – editing. Chitiphon Chuaicham: writing – review and editing. Saravanan Rajendran-review and editing. Tuan K. A. Hoang – review and editing. Keiko Sasaki: writing – review and editing. Karthikeyan Sekar: writing – review and editing, investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. K. acknowledges the Royal Society-Newton International Fellowship Alumni follow-on funding support AL/211016, UK and Department of Chemistry at the SRM Institute of Science and Technology, India.References
- E. Guilyardi, L. Lescarmontier, R. Matthews, S. P. Point, A. B. Rumjaun, J. Schlüpmann and D. Wilgenbus, 2018.
- B. JO'M, Int. J. Hydrogen Energy, 2002, 27, 731–740 CrossRef.
- G. Marbán and T. Valdés-Solís, Int. J. Hydrogen Energy, 2007, 32, 1625–1637 CrossRef.
- L. Barreto, A. Makihira and K. Riahi, Int. J. Hydrogen Energy, 2003, 28, 267–284 CrossRef CAS.
- Z.-Y. Yu, Y. Duan, X.-Y. Feng, X. Yu, M.-R. Gao and S.-H. Yu, Adv. Mater., 2021, 33, 2007100 CrossRef CAS PubMed.
- B. Ma, H. Zhao, T. Li, Q. Liu, Y. Luo, C. Li, S. Lu, A. M. Asiri, D. Ma and X. Sun, Nano Res., 2021, 14, 555–569 CrossRef CAS.
- Y. Wang, B. Liu, Y. Liu, C. Song, W. Wang, W. Li, Q. Feng and Y. Lei, Chem. Commun., 2020, 56, 14019–14022 RSC.
- Y. Jiao, Y. Zheng, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2015, 44, 2060–2086 RSC.
- L. Wang, Y. Zhu, Y. Wen, S. Li, C. Cui, F. Ni, Y. Liu, H. Lin, Y. Li and H. Peng, Angew. Chem., 2021, 133, 10671–10676 CrossRef.
- I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem., 2017, 1, 1–13 CrossRef.
- J. Liu, T. He, Q. Wang, Z. Zhou, Y. Zhang, H. Wu, Q. Li, J. Zheng, Z. Sun and Y. Lei, J. Mater. Chem. A, 2019, 7, 12451–12456 RSC.
- E. Liu, J. Li, L. Jiao, H. T. T. Doan, Z. Liu, Z. Zhao, Y. Huang, K. Abraham, S. Mukerjee and Q. Jia, J. Am. Chem. Soc., 2019, 141, 3232–3239 CrossRef CAS PubMed.
- G. Zhao, J. Chen, W. Sun and H. Pan, Adv. Funct. Mater., 2021, 31, 2010633 CrossRef CAS.
- Y. Zhou, Z. Xie, J. Jiang, J. Wang, X. Song, Q. He, W. Ding and Z. Wei, Nat. Catal., 2020, 3, 454–462 CrossRef CAS.
- X. X. Wang, M. T. Swihart and G. Wu, Nat. Catal., 2019, 2, 578–589 CrossRef CAS.
- M. Yang, C. H. Zhang, N. W. Li, D. Luan, L. Yu and X. W. Lou, Adv. Sci., 2022, 9, 2105135 CrossRef CAS PubMed.
- G. Zhan, P. Li and H. C. Zeng, Adv. Mater., 2018, 30, 1802094 CrossRef PubMed.
- Y. Yu, Y. Shi and B. Zhang, Acc. Chem. Res., 2018, 51, 1711–1721 CrossRef CAS PubMed.
- J. Liu, D. Zhu, C. Guo, A. Vasileff and S. Z. Qiao, Adv. Energy Mater., 2017, 7, 1700518 CrossRef.
- J. Lee, S. M. Kim and I. S. Lee, Nano Today, 2014, 9, 631–667 CrossRef CAS.
- J. Park, T. Kwon, J. Kim, H. Jin, H. Y. Kim, B. Kim, S. H. Joo and K. Lee, Chem. Soc. Rev., 2018, 47, 8173–8202 RSC.
- B. W. Xue, C. H. Zhang, Y. Z. Wang, W. W. Xie, N.-W. Li and L. Yu, Nanoscale Adv., 2020, 2, 5555–5566 RSC.
- R. E. Blankenship, D. M. Tiede, J. Barber, G. W. Brudvig, G. Fleming, M. Ghirardi, M. Gunner, W. Junge, D. M. Kramer and A. Melis, Science, 2011, 332, 805–809 CrossRef CAS PubMed.
- H. Schafer, D. Chevrier, K. Kuepper, P. Zhang, J. Wollschlaeger, D. Daum and M. Steinhart, Energy Environ. Sci., 2016, 9, 2609–2622 RSC.
- J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K.-Y. Wong, Chem. Rev., 2020, 120, 851–918 CrossRef CAS PubMed.
- P. C. K. Vesborg, B. Seger and I. Chorkendorff, J. Phys. Chem. Lett., 2015, 6, 951–957 CrossRef CAS PubMed.
- W. Vielstich, A. Lamm and H. Gasteiger, 2003.
- N. Dubouis and A. Grimaud, Chem. Sci., 2019, 10, 9165–9181 RSC.
- M. J. T. C. van der Niet, N. Garcia-Araez, J. Hernández, J. M. Feliu and M. T. M. Koper, Catal. Today, 2013, 202, 105–113 CrossRef CAS.
- I. T. McCrum and M. J. Janik, J. Phys. Chem. C, 2016, 120, 457–471 CrossRef CAS.
- X. Chen, I. T. McCrum, K. A. Schwarz, M. J. Janik and M. T. M. Koper, Angew. Chem., Int. Ed., 2017, 56, 15025–15029 CrossRef CAS PubMed.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 CrossRef.
- S. Trasatti, J. Electroanal. Chem. Interfacial Electrochem., 1972, 39, 163–184 CrossRef CAS.
- J. K. Nørskov, T. Bligaard, A. Logadottir, J. R. Kitchin, J. G. Chen, S. Pandelov and U. Stimming, J. Electrochem. Soc., 2005, 152, J23 CrossRef.
- A. R. Zeradjanin, J.-P. Grote, G. Polymeros and K. J. J. Mayrhofer, Electroanalysis, 2016, 28, 2256–2269 CrossRef CAS.
- T. F. Jaramillo, K. P. Jørgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100–102 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- L. Liao, J. Zhu, X. Bian, L. Zhu, M. D. Scanlon, H. H. Girault and B. Liu, Adv. Funct. Mater., 2013, 23, 5326–5333 CrossRef CAS.
- E. Skúlason, V. Tripkovic, M. E. Björketun, S. Gudmundsdóttir, G. Karlberg, J. Rossmeisl, T. Bligaard, H. Jónsson and J. K. Nørskov, J. Phys. Chem. C, 2010, 114, 18182–18197 CrossRef.
- D. M. Santos, C. A. Sequeira and J. L. Figueiredo, Química Nova, 2013, 36, 1176–1193 CrossRef CAS.
- T. J. Schmidt, V. Stamenkovic, J. P. N. Ross and N. M. Markovic, Phys. Chem. Chem. Phys., 2003, 5, 400–406 RSC.
- N. Mahmood, Y. Yao, J.-W. Zhang, L. Pan, X. Zhang and J.-J. Zou, Adv. Sci., 2018, 5, 1700464 CrossRef PubMed.
- M. Lao, K. Rui, G. Zhao, P. Cui, X. Zheng, S. X. Dou and W. Sun, Angew. Chem., Int. Ed., 2019, 58, 5432–5437 CrossRef CAS PubMed.
- Q. Xu, Y. Liu, H. Jiang, Y. Hu, H. Liu and C. Li, Adv. Energy Mater., 2019, 9, 1802553 CrossRef.
- D. Strmcnik, P. P. Lopes, B. Genorio, V. R. Stamenkovic and N. M. Markovic, Nano Energy, 2016, 29, 29–36 CrossRef CAS.
- M. M. Jakšić, Int. J. Hydrogen Energy, 1987, 12, 727–752 CrossRef.
- B. E. Conway and G. Jerkiewicz, Solid State Ionics, 2002, 150, 93–103 CrossRef CAS.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. B. Chorkendorff and J. K. Nørskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS PubMed.
- Z. Zeng, K.-C. Chang, J. Kubal, N. M. Markovic and J. Greeley, Nat. Energy, 2017, 2, 17070 CrossRef CAS.
- M. Favaro, C. Valero-Vidal, J. Eichhorn, F. M. Toma, P. N. Ross, J. Yano, Z. Liu and E. J. Crumlin, J. Mater. Chem. A, 2017, 5, 11634–11643 RSC.
- B. E. Conway and L. Bai, J. Electroanal. Chem. Interfacial Electrochem., 1986, 198, 149–175 CrossRef CAS.
- B. E. Conway and G. Jerkiewicz, Electrochim. Acta, 2000, 45, 4075–4083 CrossRef CAS.
- J. Wei, M. Zhou, A. Long, Y. Xue, H. Liao, C. Wei and Z. J. Xu, Nano-Micro Lett., 2018, 10, 75 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, A. Vasileff and S. Z. Qiao, Angew. Chem., Int. Ed., 2018, 57, 7568–7579 CrossRef CAS PubMed.
- H. Fan, H. Yu, Y. Zhang, Y. Zheng, Y. Luo, Z. Dai, B. Li, Y. Zong and Q. Yan, Angew. Chem., Int. Ed., 2017, 56, 12566–12570 CrossRef CAS PubMed.
- H. Cheng, Y.-Z. Su, P.-Y. Kuang, G.-F. Chen and Z.-Q. Liu, J. Mater. Chem. A, 2015, 3, 19314–19321 RSC.
- L. Jin, H. Zhao, Z. M. Wang and F. Rosei, Adv. Energy Mater., 2021, 11, 2003233 CrossRef CAS.
- D. Kandi, S. Martha and K. M. Parida, Int. J. Hydrogen Energy, 2017, 42, 9467–9481 CrossRef CAS.
- X. B. Li, C. H. Tung and L. Z. Wu, Angew. Chem., 2019, 131, 10918–10925 CrossRef.
- M. Pohanka, Mini Rev. Med. Chem., 2017, 17, 650–656 CrossRef CAS PubMed.
- Y. Zhang, Y. Liu, C. Li, X. Chen and Q. Wang, J. Phys. Chem. C, 2014, 118, 4918–4923 CrossRef CAS.
- W. C. Chan and S. Nie, Science, 1998, 281, 2016–2018 CrossRef CAS PubMed.
- M. Bruchez Jr, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013–2016 CrossRef CAS PubMed.
- A. S. Rasal, S. Yadav, A. Yadav, A. A. Kashale, S. T. Manjunatha, A. Altaee and J.-Y. Chang, ACS Appl. Nano Mater., 2021, 4, 6515–6541 CrossRef CAS.
- R. J. Warburton, Contemp. Phys., 2002, 43, 351–364 CrossRef CAS.
- H.-L. Wu, X.-B. Li, C.-H. Tung and L.-Z. Wu, Adv. Mater., 2019, 31, 1900709 CrossRef PubMed.
- Q. Wu, X. Gong, D. Zhao, Y. B. Zhao, F. Cao, H. Wang, S. Wang, J. Zhang, R. Quintero-Bermudez and E. H. Sargent, Adv. Mater., 2022, 34, 2108150 CrossRef CAS PubMed.
- S. Anantharaj, M. O. Valappil, K. Karthick, V. K. Pillai, S. Alwarappan and S. Kundu, Catal. Sci. Technol., 2019, 9, 223–231 RSC.
- R. Han, Q. Zhao, A. Hazarika, J. Li, H. Cai, J. Ni and J. Zhang, ACS Appl. Mater. Interfaces, 2022, 14(3), 4061–4070 CrossRef CAS PubMed.
- V. Balakumar, M. Ramalingam, K. Sekar, C. Chuaicham and K. Sasaki, Chem. Eng. J., 2021, 426, 131739 CrossRef CAS.
- W. Wang and S. A. Asher, J. Am. Chem. Soc., 2001, 123, 12528–12535 CrossRef CAS PubMed.
- C. W. Wang, C. Orrison and D. H. Son, Bull. Korean Chem. Soc., 2022, 43, 492 CrossRef CAS.
- S. E. Shirsath, X. Liu, M. Assadi, A. Younis, Y. Yasukawa, S. K. Karan, J. Zhang, J. Kim, D. Wang and A. Morisako, Nanoscale Horiz., 2019, 4, 434–444 RSC.
- X. Kou, S. Jiang, S.-J. Park and L.-Y. Meng, Dalton Trans., 2020, 49, 6915–6938 RSC.
- Y. Lee, H. Lee, P. B. Messersmith and T. G. Park, Macromol. Rapid Commun., 2010, 31, 2109–2114 CrossRef CAS PubMed.
- C. Rocha, A. Latgé and L. Chico, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 085419 CrossRef.
- M. Kim, B.-H. Kwon, C. W. Joo, M. S. Cho, H. Jang, H. Cho, D. Y. Jeon, E. N. Cho and Y. S. Jung, Nat. Commun., 2022, 13, 1–9 Search PubMed.
- N. Amin and A. J. Peter, Phys. B, 2022, 413693 CrossRef CAS.
- Z. Li, C. Zhao, Q. Fu, J. Ye, L. Su, X. Ge, L. Chen, J. Song and H. Yang, Small, 2022, 18, 2105160 CrossRef CAS PubMed.
- M. Li, J. Sun, G. Chen, S. Yao and B. Cong, Appl. Catal., B, 2022, 301, 120792 CrossRef CAS.
- A. Saraiva, W. H. Lim, C. H. Yang, C. C. Escott, A. Laucht and A. S. Dzurak, Adv. Funct. Mater., 2022, 32, 2105488 CrossRef CAS.
- A. Rastelli, F. Ding, J. D. Plumhof, S. Kumar, R. Trotta, C. Deneke, A. Malachias, P. Atkinson, E. Zallo and T. Zander, Phys. Status Solidi B, 2012, 249, 687–696 CrossRef CAS.
- A. Mittelstädt, A. Schliwa and P. Klenovský, Light: Sci. Appl., 2022, 11, 1–14 CrossRef PubMed.
- X. Zhang, H. Xie, Z. Liu, C. Tan, Z. Luo, H. Li, J. Lin, L. Sun, W. Chen, Z. Xu, L. Xie, W. Huang and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 3653–3657 CrossRef CAS PubMed.
- Z. Sun, H. Xie, S. Tang, X. F. Yu, Z. Guo, J. Shao, H. Zhang, H. Huang, H. Wang and P. K. Chu, Angew. Chem., Int. Ed., 2015, 54, 11526–11530 CrossRef CAS PubMed.
- H. Gerischer, Electrochim. Acta, 1990, 35, 1677–1699 CrossRef CAS.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC.
- H. Mattoussi, G. Palui and H. B. Na, Adv. Drug Delivery Rev., 2012, 64, 138–166 CrossRef CAS PubMed.
- C. Burda, X. Chen, R. Narayanan and M. A. El-Sayed, Chem. Rev., 2005, 105, 1025–1102 CrossRef CAS PubMed.
- A. Valizadeh, H. Mikaeili, M. Samiei, S. M. Farkhani, N. Zarghami, M. Kouhi, A. Akbarzadeh and S. Davaran, Nanoscale Res. Lett., 2012, 7, 480 CrossRef PubMed.
- D. Bera, L. Qian, T.-K. Tseng and P. H. Holloway, Materials, 2010, 3, 2260–2345 CrossRef CAS.
- K. Lasek, P. M. Coelho, K. Zberecki, Y. Xin, S. K. Kolekar, J. Li and M. Batzill, ACS Nano, 2020, 14, 8473–8484 CrossRef CAS PubMed.
- A. Bouravleuv, V. Sapega, V. Nevedomskii, A. Khrebtov, Y. Samsonenko, G. Cirlin and V. Strocov, J. Cryst. Grow., 2017, 468, 680–682 CrossRef CAS.
- S. Thevuthasan, V. Shutthanandan, C. M. Wang, W. J. Weber, W. Jiang, A. Cavanagh, J. Lian and L. M. Wang, J. Mater. Res., 2011, 19, 1311–1314 CrossRef.
- A. Ueda, D. O. Henderson, R. Mu, Y. S. Tung, C. Hall, J. G. Zhu, C. W. White and R. A. Zuhr, MRS Proc., 2011, 396, 441 CrossRef.
- W. Chen, T. Shi, J. Du, Z. Zang, Z. Yao, M. Li, K. Sun, W. Hu, Y. Leng and X. Tang, ACS Appl. Mater. Interfaces, 2018, 10, 43978–43986 CrossRef CAS PubMed.
- F. Palazon, Q. A. Akkerman, M. Prato and L. Manna, ACS Nano, 2016, 10, 1224–1230 CrossRef CAS PubMed.
- S. Xin, P. Wang, A. Yin, C. Kim, M. Dobrowolska, J. Merz and J. Furdyna, Appl. Phys. Lett., 1996, 69, 3884–3886 CrossRef CAS.
- E. Kurtz, J. Shen, M. Schmidt, M. Grün, S. Hong, D. Litvinov, D. Gerthsen, T. Oka, T. Yao and C. Klingshirn, Thin Solid Films, 2000, 367, 68–74 CrossRef CAS.
- K. Leonardi, H. Selke, H. Heinke, K. Ohkawa, D. Hommel, F. Gindele and U. Woggon, J. Cryst. Grow., 1998, 184, 259–263 CrossRef.
- M. T. Swihart, Curr. Opin. Colloid Interface Sci., 2003, 8, 127–133 CrossRef CAS.
- A. Forleo, L. Francioso, S. Capone, P. Siciliano, P. Lommens and Z. Hens, Sens. Actuators, B, 2010, 146, 111–115 CrossRef CAS.
- H. Zhang, J. Yu, C. Sun, W. Xu, J. Chen, H. Sun, C. Zong, Z. Liu, Y. Tang and D. Zhao, J. Am. Chem. Soc., 2020, 142, 16177–16181 CrossRef CAS PubMed.
- L. Hu, H. Zhong and Z. He, Colloids Surf., B, 2021, 200, 111609 CrossRef CAS PubMed.
- Y. Liu, W. Zhang and W. Zheng, Nano-Micro Lett., 2022, 14, 158 CrossRef CAS PubMed.
- S. Mei, X. Wei, D. Yang, D. Su, W. Yang, G. Zhang, Z. Hu, B. Yang, H. Dai, F. Xie, W. Zhang and R. Guo, J. Lumin., 2019, 212, 264–270 CrossRef CAS.
- B. Mohanty, L. Giri and B. K. Jena, Energy Fuels, 2021, 35, 14304–14324 CrossRef CAS.
- Y. Xu, L. Yan, X. Li and H. Xu, Sci. Rep., 2019, 9, 2931 CrossRef PubMed.
- B. Shao, Z. Liu, G. Zeng, H. Wang, Q. Liang, Q. He, M. Cheng, C. Zhou, L. Jiang and B. Song, J. Mater. Chem. A, 2020, 8, 7508–7535 RSC.
- J. Zhang, C. Ling, W. Zang, X. Li, S. Huang, X. L. Li, D. Yan, Z. Kou, L. Liu and J. Wang, Nanoscale, 2020, 12, 10964–10971 RSC.
- H. Huang, Y. Xue, Y. Xie, Y. Yang, L. Yang, H. He, Q. Jiang and G. Ying, Inorg. Chem. Front., 2022, 9, 1171–1178 RSC.
- Y. Liu, X. Li, Q. Zhang, W. Li, Y. Xie, H. Liu, L. Shang, Z. Liu, Z. Chen and L. Gu, Angew. Chem., Int. Ed., 2020, 59, 1718–1726 CrossRef CAS PubMed.
- G. Ou, P. Fan, X. Ke, Y. Xu, K. Huang, H. Wei, W. Yu, H. Zhang, M. Zhong and H. Wu, Nano Res., 2018, 11, 751–761 CrossRef CAS.
- X. Ren, L. Pang, Y. Zhang, X. Ren, H. Fan and S. F. Liu, J. Mater. Chem. A, 2015, 3, 10693–10697 RSC.
- L. Zhang, J. Xiao, H. Wang and M. Shao, ACS Catal., 2017, 7, 7855–7865 CrossRef CAS.
- F. Li, J. Li, Z. Cao, X. Lin, X. Li, Y. Fang, X. An, Y. Fu, J. Jin and R. Li, J. Mater. Chem. A, 2015, 3, 21772–21778 RSC.
- C. Zhang, Z. Pu, I. S. Amiinu, Y. Zhao, J. Zhu, Y. Tang and S. Mu, Nanoscale, 2018, 10, 2902–2907 RSC.
- Z. Pu, I. S. Amiinu, X. Liu, M. Wang and S. Mu, Nanoscale, 2016, 8, 17256–17261 RSC.
- S. Xu, D. Li and P. Wu, Adv. Funct. Mater., 2015, 25, 1127–1136 CrossRef CAS.
- X.-P. Yu, C. Yang, P. Song and J. Peng, Tungsten, 2020, 2, 194–202 CrossRef.
- W. Li, Y. Liu, M. Wu, X. Feng, S. A. Redfern, Y. Shang, X. Yong, T. Feng, K. Wu and Z. Liu, Adv. Mater., 2018, 30, 1800676 CrossRef PubMed.
- Q. Dang, F. Liao, Y. Sun, S. Zhang, H. Huang, W. Shen, Z. Kang, Y. Shi and M. Shao, Electrochim. Acta, 2019, 299, 828–834 CrossRef CAS.
- H. Cheng, M. L. Li, C. Y. Su, N. Li and Z. Q. Liu, Adv. Funct. Mater., 2017, 27, 1701833 CrossRef.
- L. B. Huang, L. Zhao, Y. Zhang, Y. Y. Chen, Q. H. Zhang, H. Luo, X. Zhang, T. Tang, L. Gu and J. S. Hu, Adv. Energy Mater., 2018, 8, 1800734 CrossRef.
- Y. Gong, L. Wang, H. Xiong, M. Shao, L. Xu, A. Xie, S. Zhuang, Y. Tang, X. Yang and Y. Chen, J. Mater. Chem. A, 2019, 7, 13671–13678 RSC.
- W. Zhang, X. Zhao, Y. Zhao, J. Zhang, X. Li, L. Fang and L. Li, ACS Appl. Mater. Interfaces, 2020, 12, 10280–10290 CrossRef CAS PubMed.
- L. Huo, B. Liu, Z. Gao and J. Zhang, J. Mater. Chem. A, 2017, 5, 18494–18501 RSC.
- B. Ye, L. Huang, Y. Hou, R. Jiang, L. Sun, Z. Yu, B. Zhang, Y. Huang and Y. Zhang, J. Mater. Chem. A, 2019, 7, 11379–11386 RSC.
- S. Das, R. Ghosh, P. Routh, A. Shit, S. Mondal, A. Panja and A. K. Nandi, ACS Appl. Nano Mater., 2018, 1, 2306–2316 CrossRef CAS.
- Y. Liu, X. Zhang, W. Zhang, X. Ge, Y. Wang, X. Zou, X. Zhou and W. Zheng, Energy Environ. Mater., 2022 Search PubMed.
- D. Dinda, M. E. Ahmed, S. Mandal, B. Mondal and S. K. Saha, J. Mater. Chem. A, 2016, 4, 15486–15493 RSC.
- F. Li, J. Li, Z. Cao, X. Lin, X. Li, Y. Fang, X. An, Y. Fu, J. Jin and R. Li, J. Mater. Chem. A, 2015, 3, 21772–21778 RSC.
- X. Ren, L. Pang, Y. Zhang, X. Ren, H. Fan and S. Liu, J. Mater. Chem. A, 2015, 3, 10693–10697 RSC.
- L. Najafi, S. Bellani, B. Martín-García, R. Oropesa-Nuñez, A. E. Del Rio Castillo, M. Prato, I. Moreels and F. Bonaccorso, Chem. Mater., 2017, 29, 5782–5786 CrossRef CAS.
- Z. Chen, H. Xu, Y. Ha, X. Li, M. Liu and R. Wu, Appl. Catal., B, 2019, 250, 213–223 CrossRef CAS.
- T. Ali, X. Wang, K. Tang, Q. Li, S. Sajjad, S. Khan, S. A. Farooqi and C. Yan, Electrochim. Acta, 2019, 300, 45–52 CrossRef CAS.
- Q. Zhou, X. Luo, Y. Li, Y. Nan, H. Deng, E. Ou and W. Xu, Int. J. Hydrogen Energy, 2020, 45, 433–442 CrossRef CAS.
- C. Wang, M. Jin, D. Liu, F. Liang, C. Luo, P. Li, C. Cai, H. Bi, X. Wu and Z. Di, J. Phys. D: Appl. Phys., 2021, 54, 214006 CrossRef CAS.
- S.-Y. Pang, W.-F. Io and J. Hao, Adv. Sci., 2021, 8, 2102207 CrossRef CAS PubMed.
- L. Chen, J. Liang, Q. Zhang, X. Hu, W. Peng, Y. Li, F. Zhang and X. Fan, Int. J. Hydrogen Energy, 2022, 47, 10583–10593 CrossRef CAS.
- S. Jeong, H. D. Mai, T. K. Nguyen, J.-S. Youn, K.-H. Nam, C.-M. Park and K.-J. Jeon, Appl. Catal., B, 2021, 293, 120227 CrossRef CAS.
- S.-Y. Lu, S. Li, M. Jin, J. Gao and Y. Zhang, Appl. Catal., B, 2020, 267, 118675 CrossRef CAS.
- Q. Shi and H. Liu, Int. J. Hydrogen Energy, 2021, 46, 36763–36770 CrossRef CAS.
- J. Dong, X. Zhang, J. Huang, S. Gao, J. Mao, J. Cai, Z. Chen, S. Sathasivam, C. J. Carmalt and Y. Lai, Electrochem. Commun., 2018, 93, 152–157 CrossRef CAS.
- G. Zhao, P. Li, K. Rui, Y. Chen, S. X. Dou and W. Sun, Chem. – Eur. J., 2018, 24, 11158–11165 CrossRef CAS PubMed.
- S. Gao, B. Wang, X. Liu, Z. Guo, Z. Liu and Y. Wang, Nanoscale, 2018, 10, 10288–10295 RSC.
- Z. Pu, M. Wang, Z. Kou, I. S. Amiinu and S. Mu, Chem. Commun., 2016, 52, 12753–12756 RSC.
- L. Lin, Z. Sun, M. Yuan, H. Yang, H. Li, C. Nan, H. Jiang, S. Ge and G. Sun, ACS Sustainable Chem. Eng., 2019, 7, 9637–9645 CrossRef CAS.
- W. Li, F. Li, X. Wang, Y. Tang, Y. Yang, W. Gao and R. Li, Appl. Surf. Sci., 2017, 401, 190–197 CrossRef CAS.
- I. W. P. Chen, C.-H. Hsiao, J.-Y. Huang, Y.-H. Peng and C.-Y. Chang, ACS Appl. Mater. Interfaces, 2019, 11, 14159–14165 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |