Circularly polarized luminescence and nonlinear optical harmonic generation based on chiral zinc halides†
Xiao
Han
a,
Puxin
Cheng
a,
Wenqing
Han
a,
Rongchao
Shi
a,
Junjie
Guan
a,
Geng
Li
b and
Jialiang
Xu
 *a
*a
aSchool of Materials Science and Engineering, Smart Sensing Interdisciplinary Science Center, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tongyan Road 38, Tianjin 300350, P. R. China. E-mail: jialiang.xu@nankai.edu.cn
bNational Supercomputer Center in Tianjin, Tianjin 300457, China
First published on 23rd May 2023
Abstract
Chiral hybrid metal halides have grabbed extensive attention in linear and nonlinear chiroptics. Herein, 0D chiral zinc halides, (R-/S-2-MP)ZnCl4, have been fabricated, which demonstrate high efficiency second-order nonlinear optical responses. Incorporating Sb3+ into the chiral zinc halide matrix triggers the circularly polarized luminescence effectively with a balance between quantum yield and luminescence dissymmetry factor.
Chiral materials possess a plethora of distinctive properties, stemming from their inherent asymmetry, particularly their capability to rotate the polarization of light.1 Consequently, chiral materials have flourished in various research fields such as catalysis,2 molecular recognition,3 optics,4,5 and magnetics,6etc.7 Hybrid organic-inorganic metal halides (HOMHs) have been regarded as ideal candidates for the next-generation semiconductor materials in virtue of their facile solution-process syntheses, tuneable bandgaps, large exciton binding energies, and high optical-absorption coefficient.8 Importantly, when compared to all-inorganic metal halides, the organic cations at the A site offer a greater abundance of diversity and substitutability. This has led to a significant expansion in the architecture of HOMHs, including the formation of low-dimensional polynuclear metal clusters, which in turn further broadens their potential applications in optoelectronics.9
Chiral metal halides combine the merits of chiral materials and HOMHs.10 It has been also certificated that the chirality of chiral organic ligands can be conveyed to the inorganic scaffolds in metal halides.11 Since Billing et al. reported the chiral lead-based halides, i.e., (S-β-phenethylammonium)PbBr3,12 various constructions among chiral metal halides, including 2D, quasi 2D, 1D, and 0D, have sprung up in the past decade. Chiral metal halides currently represent a crucial division of hybrid organic-inorganic materials.13,14 On the one hand, the transfer of chirality from organic components eliminates inversion symmetry, which is a prerequisite for second-order nonlinear optical (NLO) effects15 including second harmonic generation (SHG).16–18 On the other hand, enantiomeric metal halides exhibit fascinating chiroptical activity19,20 and chiral-induced spin selectivity (CISS).21,22 Here, we reported the chiral zinc-based halides via introducing the organic ligands (R)-(−)/(S)-(+)-2-methylpiperazine (abbreviated as R-/S-MP). The NLO properties of the chiral zinc halides have been explored, whose SHG response is 19.6 times that of Z-cut quartz at 480 nm. Moreover, the successful alloying of Sb3+ into chiral zinc halide hosts results in the generation of circularly polarized luminescence (CPL) from self-trapped excitons (STEs). Accordingly, a quantum yield of 34.5% and luminescence dissymmetry factor (glum) of 1.81 × 10−3 can be achieved for the 10% Sb feeding.
A pair of chiral zinc halides are prepared via slow evaporation from their corresponding hydrochloric acid solutions. Single-crystal X-ray diffraction (SCXRD) analyses confirm that R-/S-2-MP-ZnCl crystalizes in the monoclinic P21 space group. Within their inorganic structural units, a tetrahedron is constructed by four Cl− anions coordinated with a Zn2+ cation. The incorporation of a chiral amine isolates each tetrahedron, forming the typical zero-dimensional (0D) Zn-based halide.23 Simultaneously, the chiral ligands also transfer the original chirality to the inorganic block buildings, successfully constructing the hybrid zinc halide enantiomer (Fig. 1a). As depicted in Fig. 1b and c, the as-synthesized chiral zinc halides display a colourless bar-like shape. The optical microscope pictures of the obtained samples indicate the orderly growth of the crystals. Powder XRD patterns of the two chiral Zn-based halides all present sharp peaks, and are in good agreement with the simulated results, implying high crystallinity and phase purity.24
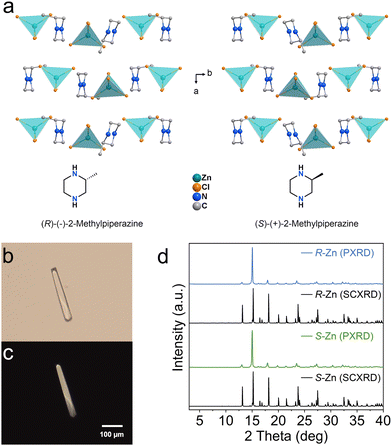 | ||
| Fig. 1 (a) Crystal structures of R- and S-Zn. (b) Optical microscope image and (c) the corresponding crossing polarized microscope image of an R-Zn crystal. (d) Powder XRD patterns of R- and S-Zn. | ||
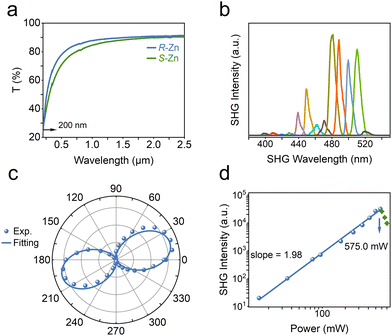
Chiral spacer cations, (R-/S-2-MP)2+, have been successfully incorporated, forming an enantiomeric pair of chiral zinc halides with acentric architectures. Induced by N–H⋯Cl–Zn hydrogen bonds, inorganic (ZnCl4)2− tetrahedra engender the corresponding asymmetric tilts or distortions (Fig. S1, ESI†).25,26 The chirality-induced asymmetric materials are generally employed to study second-order NLO features such as SHG.27–30 As indicated in Fig. 2a, the (R-/S-2-MP)ZnCl4 (abbreviated as R-/S-Zn) can both showcase a wide transparency window, retaining 70% optical transparency spanning from 0.5 to 2.5 μm.31 The NLO signals of chiral zinc halides have been collected in reflection geometry via a home-built microscope equipped with a femtosecond pulsed laser (100 fs, 80 MHz). The wavelength-dependent SHG characterizations have been conducted at the same incident power by switching the pump wavelengths from 800 to 1040 nm at 20 nm intervals. Chiral zinc halide R-Zn manifests clear SHG responses in a wide wavelength range, especially at the excitation of 960 nm. Polarization dependent measurements of the NLO response of R-Zn at 480 nm have been carried out. Fig. 4c illustrates that the polarization plots agree well with the cos4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ function32 with the maximum SHG intensity at polarized angles of 20 and 200 deg. The corresponding polarization ratio, ρ = (Imax − Imin)/(Imax + Imin), is derived to be 97.7% for the p-analyzer, indicating the high anisotropy of the NLO responses. Analysis of the power-dependent SHG spectra (Fig. 2d) pumped at 960 nm unveils a quadratic dependence for the power-SHG intensity function, confirming the two-photon nature of the second-order NLO effect. Furthermore, the quadratic dependence on laser power can be retained until the incident power exceeds 575.0 mW. Thus, the laser damage threshold (LDT) of R-Zn is estimated to be ∼2.29 mJ cm−2 in pump fluence, given the laser spot of ∼20 μm in diameter (100 fs, 80 MHz). In addition, the SHG efficiency of R-Zn has been evaluated via utilizing the Z-cut quartz as the benchmark. The obtained SHG response of R-Zn is 19.6 times that of Z-cut quartz at the optimized pump wavelength (Fig. S3, ESI†).
θ function32 with the maximum SHG intensity at polarized angles of 20 and 200 deg. The corresponding polarization ratio, ρ = (Imax − Imin)/(Imax + Imin), is derived to be 97.7% for the p-analyzer, indicating the high anisotropy of the NLO responses. Analysis of the power-dependent SHG spectra (Fig. 2d) pumped at 960 nm unveils a quadratic dependence for the power-SHG intensity function, confirming the two-photon nature of the second-order NLO effect. Furthermore, the quadratic dependence on laser power can be retained until the incident power exceeds 575.0 mW. Thus, the laser damage threshold (LDT) of R-Zn is estimated to be ∼2.29 mJ cm−2 in pump fluence, given the laser spot of ∼20 μm in diameter (100 fs, 80 MHz). In addition, the SHG efficiency of R-Zn has been evaluated via utilizing the Z-cut quartz as the benchmark. The obtained SHG response of R-Zn is 19.6 times that of Z-cut quartz at the optimized pump wavelength (Fig. S3, ESI†).
Numerous chiral metal halides have been utilized for NLOs and the detection of circularly polarized emission. However, many of these compounds demonstrate negligible photoluminescence (PL) at room temperature.33 For the majority of zinc halides, luminescence is scarcely observed owing to its completely filled d10 electron configuration, which makes it difficult for Zn2+ ions to participate in luminescence processes.34 The R-Zn and S-Zn are indeed non-luminescent at ambient temperature, as shown in Fig. S11 (ESI†). To improve this, doping strategies based on metal ions have been explored, particularly with 5s2 metal ions (Sb3+, Sn2+, Bi3+, etc.).35,36 In this study, various amounts of Sb3+ have been then introduced into the chiral zinc chloride host. The powder XRD patterns (Fig. S4, ESI†) demonstrate that the R-Zn:x%Sb preserve the original undoped phase if the Sb3+ feeding concentration is less than 30%; otherwise, the (R-2-MP)SbCl5 (abbreviated as R-Sb) phase replaces R-Zn as the dominant phase. For the structure of R-Sb (CCDC 80302637), a tetrahedron (SbCl4)− with a free Cl− constitutes each 0D inorganic unit. Moreover, when the Sb feeding level is less than 30%, as the feeding level increases, the diffraction peak shifts slightly to lower angles, indicating that the doping of Sb3+ ions (r = 0.74 Å, CN = 4) with a larger atomic radius than that of Zn2+ (r = 0.60 Å, CN = 4) ions causes lattice expansion.38
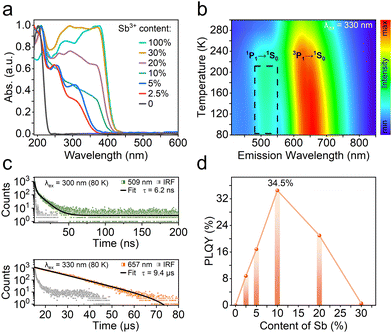
UV-vis diffuse reflection absorption spectra have been conducted to investigate the photophysical features of R-Zn:x%Sb. With increasing the Sb3+ feeding level, the absorption intensity between 230–350 nm rises up significantly along with a bathochromic-shift of the absorption edge. The X-ray photoelectron spectra (XPS) testify the successful incorporation of Sb3+ into the chiral zinc halide host (Fig. S5, ESI†), as evidenced by the peaks at 539.8 and 530.4 eV, attributed to the Sb(III) 3d3/2 and 3d5/2 orbitals.39 Thermogravimetric analysis (TGA) curves indicate that R-Zn:x%Sb are thermally stable up to 240 °C (Fig. S6, ESI†). After alloying Sb3+ ions, the PL spectra unveil broadband emission at around 616 nm with large Stokes shift for R-Zn:x%Sb (Fig. S7a, ESI†). However, no emission can be observed for R-Sb at room temperature, excluding the effect of impurities on the PL process. Time-resolved photoluminescence spectra indicate that R-Zn:10%Sb features a lifetime of 7.5 μs with a single exponential decay at the excitation of 350 nm (Fig. S7b, ESI†). This is consistent with the characteristics of self-trapped exciton (STE) emission.39–41
To validate the nature of the STE emission, varied-temperature PL measurements of R-Zn:10%Sb have been carried out (Fig. 3b). The emission band at 509 nm gradually emerges when the ambient temperature drops below 240 K. Meanwhile, as presented in Fig. S8 (ESI†), the emission intensity at 616 nm rises up while its full width at half-maximum (FWHM) narrows as the temperature declines, implying the enhanced radiative recombination from less phonon-exciton coupling.42,43 The lifetimes (Fig. 3c) of R-Zn:10%Sb at 509 and 657 nm are confirmed to be 6.2 ns and 9.4 μs, conforming to the features of singlet (1P1) and triplet (3P1) STEs.44 The excitation-emission mapping pattern of R-Zn:10%Sb at 80 K (Fig. S9, ESI†) shows that the peak at 509 nm is only observed at the short excitation wavelengths (<340 nm), revealing that the dual-emission stems from the various excited states. Modulating the doping ratio of Sb3+ in the zinc halide host offers a facile pathway to optimize their emission efficiency. In this study, a maximum photoluminescence quantum yield (PLQY) of 34.5% could be obtained for R-Zn:10%Sb by alternating the dopant ratio. However, excessive Sb content has been found to quench the original luminescence (Fig. 3d).
This chiral framework not only eliminates the spatial symmetry in the metal halide, but also facilitates chiral optical activity. The chiroptical performance of R-/S-Zn:10%Sb has been examined by circular dichroism (CD). The two chiral enantiomeric zinc halides present nearly reverse CD signals (Fig. 4a) at the same location between 300 and 600 nm on account of the Cotton effect, suggesting the chirality transfer from chiral organic ligands to achiral inorganic skeletons.45,46 Moreover, the R-/S-Zn:10%Sb reveal clear mirror-image outlines for the CPL spectra (Fig. 4b), where the derived luminescent asymmetry factor (glum) values have been determined to be 1.81 × 10−3 for R-Zn:10%Sb and −1.43 × 10−3 for S-Zn:10%Sb at 616 nm, respectively.
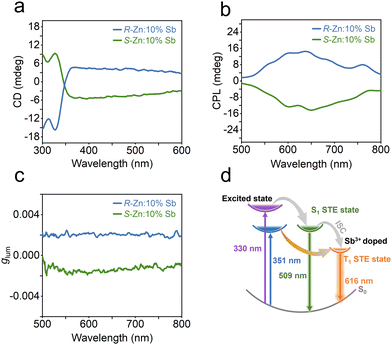 | ||
| Fig. 4 (a) CD spectra, (b) CPL spectra and (c) corresponding glum of R-/S-Zn:10%Sb. (d) Schematic illustration of the proposed PL process in R-Zn:x%Sb. | ||
A possible photoexcited emission process in R-Zn:10%Sb has been illustrated in Fig. 4d. Upon excitation by high-energy photons (330 nm), electrons will enter the singlet STE state. Then, partial electrons transfer to the triplet STE state via intersystem crossing (ISC).44,47 Thus, both the 509 and 616 nm bands can be observed. However, the band at 509 nm only appears at low temperatures. Excitation by low-energy photons (351 nm) directly shifts the electrons to the triplet STE state and back to the ground state, resulting in the broadband emission solely at 616 nm.48
In summary, a pair of chiral zinc chlorides have been fabricated via introducing chiral organic cations into the system. Grounded on a noncentrosymmetric framework, the R-Zn crystals exhibit clear NLO responses, with an SHG intensity 19.6 times that of Z-cut quartz. Furthermore, melding trivalent antimony (Sb3+) ions with 5s2 lone pair electrons into the R-Zn host can activate CPL, with a PLQY of 34.5% and a glum of 1.81 × 10−3. Relevant PL measurements argue that the broadband emission originates from the STEs. This work offers a novel channel to design and manufacture second-order NLO materials and CPL material emitters based on low-dimensional chiral metal halides.
This work was supported by the National Natural Science Foundation of China (Grant No. 52172045) and the Fundamental Research Funds for the Central Universities. Dr A. Liu and Dr B.-H. Li from the Beijing Academy of Quantum Information Sciences are acknowledged for technical assistance and fruitful discussions. We appreciate Prof. Wei Li for the assistance with DFT calculations.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. P. Rodrigues, S. Lan, L. Kang, Y. Cui, P. W. Panuski, S. Wang, A. M. Urbas and W. Cai, Nat. Commun., 2017, 8, 14602 CrossRef PubMed.
- S. Mondal, F. Dumur, D. Gigmes, M. P. Sibi, M. P. Bertrand and M. Nechab, Chem. Rev., 2022, 122, 5842–5976 CrossRef CAS PubMed.
- S. Tashiro and M. Shionoya, Acc. Chem. Res., 2020, 53, 632–643 CrossRef CAS PubMed.
- C. Zhang, S. Li, X.-Y. Dong and S.-Q. Zang, Aggregate, 2021, 2, e48 CrossRef CAS.
- H. Hübener, U. De Giovannini, C. Schäfer, J. Andberger, M. Ruggenthaler, J. Faist and A. Rubio, Nat. Mater., 2021, 20, 438–442 CrossRef PubMed.
- D. E. Kharzeev and J. Liao, Nat. Rev. Phys., 2021, 3, 55–63 CrossRef CAS.
- Y. Wang, J. Xu, Y. Wang and H. Chen, Chem. Soc. Rev., 2013, 42, 2930–2962 RSC.
- A. Younis, C.-H. Lin, X. Guan, S. Shahrokhi, C.-Y. Huang, Y. Wang, T. He, S. Singh, L. Hu, J. R. D. Retamal, J.-H. He and T. Wu, Adv. Mater., 2021, 33, 2005000 CrossRef CAS PubMed.
- C. Zhou, H. Lin, Q. He, L. Xu, M. Worku, M. Chaaban, S. Lee, X. Shi, M.-H. Du and B. Ma, Mater. Sci. Eng., R, 2019, 137, 38–65 CrossRef.
- H. Duim and M. A. Loi, Matter, 2021, 4, 3835–3851 CrossRef CAS.
- Z. Guo, J. Li, R. Chen and T. He, Prog. Quantum Electron., 2022, 82, 100375 CrossRef.
- D. G. Billing and A. Lemmerer, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2003, 59, m381–m383 CrossRef CAS.
- J. Ma, H. Wang and D. Li, Adv. Mater., 2021, 33, 2008785 CrossRef CAS PubMed.
- J. Crassous, M. J. Fuchter, D. E. Freedman, N. A. Kotov, J. Moon, M. C. Beard and S. Feldmann, Nat. Rev. Mater., 2023 DOI:10.1038/s41578-023-00543-3.
- X. Han, F. Ge, J. Xu and X.-H. Bu, Aggregate, 2021, 2, e28 CrossRef CAS.
- C. Yuan, X. Li, S. Semin, Y. Feng, T. Rasing and J. Xu, Nano Lett., 2018, 18, 5411–5417 CrossRef CAS PubMed.
- X. Fu, Z. Zeng, S. Jiao, X. Wang, J. Wang, Y. Jiang, W. Zheng, D. Zhang, Z. Tian, Q. Li and A. Pan, Nano Lett., 2023, 23, 606–613 CrossRef CAS PubMed.
- Y. Peng, X. Liu, L. Li, Y. Yao, H. Ye, X. Shang, X. Chen and J. Luo, J. Am. Chem. Soc., 2021, 143, 14077–14082 CrossRef CAS PubMed.
- X. Li, F. Wu, Y. Yao, W. Wu, C. Ji, L. Li, Z. Sun, J. Luo and X. Liu, J. Am. Chem. Soc., 2022, 144, 14031–14036 CrossRef CAS PubMed.
- X. Ji, S. Geng, S. Zhang, Y. Gong, X. Zhang, R. Li, Y. Liu, J. Chen, R. Chen, Z. Xiao and L. Mao, Chem. Mater., 2022, 34, 8262–8270 CrossRef CAS.
- H. Lu, Z. V. Vardeny and M. C. Beard, Nat. Rev. Chem., 2022, 6, 470–485 CrossRef PubMed.
- R. Naaman, Y. Paltiel and D. H. Waldeck, Acc. Chem. Res., 2020, 53, 2659–2667 CrossRef CAS PubMed.
- X. Liu, C. Peng, L. Zhang, D. Guo and Y. Pan, J. Mater. Chem. C, 2022, 10, 204–209 RSC.
- L. Wang, Y. Xue, M. Cui, Y. Huang, H. Xu, C. Qin, J. Yang, H. Dai and M. Yuan, Angew. Chem., Int. Ed., 2020, 59, 6442–6450 CrossRef CAS PubMed.
- L. Yao, Z. Zeng, C. Cai, P. Xu, H. Gu, L. Gao, J. Han, X. Zhang, X. Wang, X. Wang, A. Pan, J. Wang, W. Liang, S. Liu, C. Chen and J. Tang, J. Am. Chem. Soc., 2021, 143, 16095–16104 CrossRef CAS PubMed.
- J.-H. Lee, N. C. Bristowe, J. H. Lee, S.-H. Lee, P. D. Bristowe, A. K. Cheetham and H. M. Jang, Chem. Mater., 2016, 28, 4259–4266 CrossRef CAS.
- Q. Wen, S. Tenenholtz, L. J. W. Shimon, O. Bar-Elli, L. M. Beck, L. Houben, S. R. Cohen, Y. Feldman, D. Oron, M. Lahav and M. E. van der Boom, J. Am. Chem. Soc., 2020, 142, 14210–14221 CrossRef CAS PubMed.
- Q. Qian, R. Zu, Q. Ji, G. S. Jung, K. Zhang, Y. Zhang, M. J. Buehler, J. Kong, V. Gopalan and S. Huang, ACS Nano, 2020, 14, 13333–13342 CrossRef CAS PubMed.
- Z. Guo, J. Li, J. Liang, C. Wang, X. Zhu and T. He, Nano Lett., 2022, 22, 846–852 CrossRef CAS PubMed.
- Z. Yu, S. Cao, Y. Zhao, Y. Guo, M. Dong, Y. Fu, J. Zhao, J. Yang, L. Jiang and Y. Wu, ACS Appl. Mater. Interfaces, 2022, 14, 39451–39458 CrossRef CAS PubMed.
- J. Chen, H. Chen, F. Xu, L. Cao, X. Jiang, S. Yang, Y. Sun, X. Zhao, C. Lin and N. Ye, J. Am. Chem. Soc., 2021, 143, 10309–10316 CrossRef CAS PubMed.
- Y. Zheng, X. Han, P. Cheng, X. Jia, J. Xu and X.-H. Bu, J. Am. Chem. Soc., 2022, 144, 16471–16479 CrossRef CAS PubMed.
- Z. Wang, X. Wang, Z. Chen, Y. Liu, H. Xie, J. Xue, L. Mao, Y. Yan and H. Lu, Angew. Chem., Int. Ed., 2022, 62, e202215206 CrossRef PubMed.
- E. Merzlyakova, S. Wolf, S. Lebedkin, L. Bayarjargal, B. L. Neumeier, D. Bartenbach, C. Holzer, W. Klopper, B. Winkler, M. Kappes and C. Feldmann, J. Am. Chem. Soc., 2021, 143, 798–804 CrossRef CAS PubMed.
- Y. Wei, C. Li, Y. Li, Z. Luo, X. Wu, Y. Liu, L. Zhang, X. He, W. Wang and Z. Quan, Angew. Chem., Int. Ed., 2022, 61, e202212685 CAS.
- W. Zhang, W. Zheng, L. Li, P. Huang, Z. Gong, Z. Zhou, J. Sun, Y. Yu and X. Chen, Angew. Chem., Int. Ed., 2022, 61, e202116085 CAS.
- L. Li and G.-X. Wang, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, m1629 CrossRef CAS PubMed.
- Y. Dai, Q. Wei, T. Chang, J. Zhao, S. Cao, B. Zou and R. Zeng, J. Phys. Chem. C, 2022, 126, 11238–11245 CrossRef CAS.
- B. Su, M. Li, E. Song and Z. Xia, Adv. Funct. Mater., 2021, 31, 2105316 CrossRef CAS.
- Q. Guo, X. Zhao, B. Song, J. Luo and J. Tang, Adv. Mater., 2022, 34, 2201008 CrossRef CAS PubMed.
- V. Pinchetti, F. Moro, B. Zhang, M. Fanciulli, L. De Trizio, F. Meinardi, L. Manna and S. Brovelli, ACS Energy Lett., 2022, 7, 1566–1573 CrossRef CAS.
- M. Li and Z. Xia, Chem. Soc. Rev., 2021, 50, 2626–2662 RSC.
- L. Zhou, L. Zhang, H. Li, W. Shen, M. Li and R. He, Adv. Funct. Mater., 2021, 31, 2108561 CrossRef CAS.
- J.-H. Wei, J.-F. Liao, L. Zhou, J.-B. Luo, X.-D. Wang and D.-B. Kuang, Sci. Adv., 2021, 7, eabg3989 CrossRef CAS PubMed.
- S. Ma, J. Ahn and J. Moon, Adv. Mater., 2021, 33, 2005760 CrossRef CAS PubMed.
- S. Ma, Y.-K. Jung, J. Ahn, J. Kyhm, J. Tan, H. Lee, G. Jang, C. U. Lee, A. Walsh and J. Moon, Nat. Commun., 2022, 13, 3259 CrossRef CAS PubMed.
- C. Sun, Z. Deng, Z. Li, Z. Chen, X. Zhang, J. Chen, H. Lu, P. Canepa, R. Chen and L. Mao, Angew. Chem., Int. Ed., 2023, 62, e202216720 CAS.
- J. Wu, X. Li, X. Lian, B. Su, J. Pang, M.-D. Li, Z. Xia, J. Z. Zhang, B. Luo and X.-C. Huang, ACS Nano, 2021, 15, 15354–15361 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, material characterization, theoretical calculations and crystallographic details. CCDC 2241401 and 2241402. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc01461d |
| This journal is © The Royal Society of Chemistry 2023 |