Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
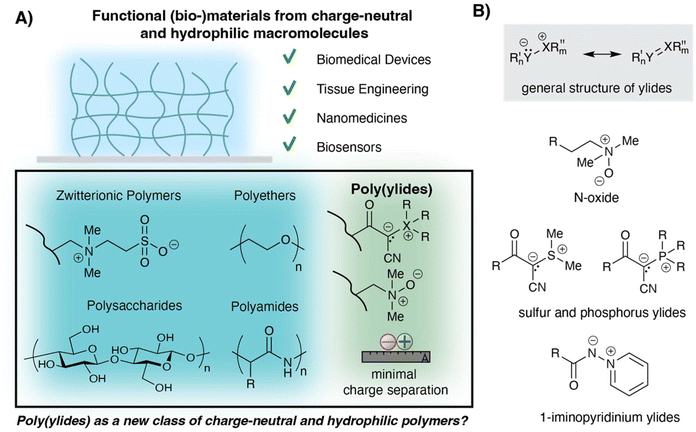
The case for poly(ylides) as a class of charge-neutral, hydrophilic polymers with applications in biomaterials science
Kevin Neumann
Institute for Molecules and Materials, Radboud University, The Netherlands. E-mail: kevin.neumann@ru.nl
First published on 10th September 2024
Abstract
Many applications of biomaterials require hydrophilic polymers as building blocks, including hydrogels and nanomedicinal devices. Besides enabling sufficient swelling properties in aqueous environments, hydrophilic polymers provide hydration layers, which are considered a major requirement when designing non-fouling surfaces and materials. For the last few decades, polyethylene glycol has been seen as the gold standard for such applications. However, reports on its stability and immunogenicity have urged chemists to identify alternatives with comparable or superior properties. In addition to biopolymers, zwitterionic polymers have gained increasing attention by effectively offering an overall charge-neutral scaffold capable of forming strong hydration layers. Driven by an enhanced understanding of the structure–property relationship of zwitterionic materials, poly(ylides) have emerged as a new class of hydrophilic and charge-neutral polymers. By having the negative charge adjacent to the positive charge, ylides offer not only a minimal dipole moment but also maintain their overall charge-neutral nature. Despite some early reports on their synthesis during the 1980s, polymeric ylides were largely overlooked as a class of polymers, and their utility as unique hydrophilic building blocks for the design of biomaterials and nanomedicinal tools remained elusive. In recent years, several groups have reported N-oxide and carbon-centered ylide-based polymers as highly effective building blocks for the design of antifouling materials and nanomedicines. Here, by reviewing recent progress and understanding of structure–property relationships, arguments are provided explaining why polymeric ylides should be classified as a standalone class of hydrophilic polymers. Consequently, the author concludes that the term ‘poly(ylide)’ or ‘polymeric ylides’ should be routinely used to adequately describe this emerging class of polymers.
1. Introduction
Hydrophilic polymers play a pivotal role not only in designing new biomaterials but increasingly also in the field of drug delivery, for example, in the design of nanocarriers and protein-polymer conjugates, and may be derived from synthetic or natural origin.1 While hydrophilic polymers from natural resources typically display high biocompatibility, synthetic variants often display higher stability and can be synthetically tailored. In particular polyethylene glycol (PEG) is considered as a key component for the majority of biomedical materials and nanomedicinal systems because of its ability to reduce the undesired process of biofouling (Fig. 1).2–4 Mechanistically, the adhesion of biomolecules such as proteins to surfaces is enabled by electrostatic and hydrophobic interactions. PEGylated surfaces are overall charge-neutral and prevent such adhesion by forming a so-called hydration layer. While PEG is still considered as the gold standard for biomedical applications, recent concerns related to immunogenic responses, stability, and chemical inertness have urged scientists to investigate alternative materials with comparable or superior properties.5,6 Ultimately, this transformative and multidisciplinary challenge has resulted in an increasing interest in zwitterionic polymers. Zwitterionic polymers contain both positive and negative charges in stoichiometric amounts and therefore remain overall charge neutral.7 Because of their strongly hydrophilic nature and overall charge-neutral state, zwitterionic polymers are attractive building blocks for a variety of medical applications including as antifouling materials, components for drug delivery, and self-healing materials.8,9 Unlike PEG-derived materials, which form hydrogen bonds with water, zwitterionic materials hold water more effectively via ionic solvation.10,11 The strong electrostatic interactions also result in a so-called anti-polyelectrolyte effect, which describes the phenomenon of zwitterionic materials becoming more soluble in the presence of higher salt concentrations as a result of dissociation of intermolecular chain interactions.12,13 So far, polymeric zwitterions are classified into two categories, namely polyampholytes and polybetaines, describing scaffolds with charges on different repeating units and on the same repeating unit, respectively.14,15 Since precise balance of negative and positive charges within a single polymer chain is synthetically unfeasible, polyampholytes are also referred to as pseudozwitterionic polymer. In contrast, polybetaines provide a high level of control over their molecular structure with an exact balance between positive and negative charge, making these polymers subject to extensive research. Polybetaines can further be classified by the nature of their ionic groups.16 For example, polybetaines with carboxylates are referred to as poly(carboxybetaines), and those with sulfonates as poly(sulfobetaines). Additional anions that are often employed for the design of polybetaines include phosphates and sulfates. Typically, when not otherwise specified, betaines bear a positively charged ammonium residue. Further examples of positively charged groups include pyridinium and phosphonium residues. Synthetically, zwitterionic polymers are often accessed via post-polymerization modifications, either because the corresponding zwitterionic residues are not compatible with common polymerization techniques such as RAFT or ring-opening polymerization or because of solubility issues encountered during copolymerisation with more hydrophobic monomers.17 From a chemistry perspective, zwitterionic polymers offer great flexibility in terms of their structures, thereby differing strongly from other synthetic polymer scaffolds such as polyethylene glycol. The vast chemical landscape offered by such zwitterionic polymers has certainly contributed to their popularity in academic research.Besides finding application in drug delivery, polybetaines are frequently encountered as antifouling coatings on the surfaces of medical devices.18 Their ability to form strong hydration layers alongside with their overall charge-neutral nature has made them a building block of choice when designing next-generation biomaterials. Naturally, with increasing use, the demand for understanding structure–property relationships has evolved. The large structural diversity displays both, a strength and challenge with even small structural modifications significantly impacting the overall performance of zwitterionic polymers. Here, the extensive work of A. Rosenhahn, A. Laschewsky and coworkers must be highlighted which in more than one way lay the foundation for our modern understanding of zwitterionic materials and their interplay with the biological environment. Systematically, these two groups have screened various structures and revealed that, in addition to the nature of the ionic residues and the type of backbone, the distance separating the two charges from the polymer backbone, as well as the linker between the two charges, play pivotal roles.19–21
Inspired by the increasing understanding of structure–property relationships, we and others begun to investigate the use of polymeric ylides as hydrophilic building blocks. In stark contrast to betaines, ylides display a positive charge directly adjacent to a negative charge.22 According to IUPACs Gold book of nomenclature, ylides may be classified as zwitterionic species by carrying opposite formal electrical charges; yet, several chemists restrict the definition of zwitterions to compounds with the charges on non-adjacent elements. The purpose of this article is not to clarify this definition and prove one definition right or wrong. Instead, with this work, the author suggests establishing the term ‘polymeric ylide’ or ‘poly(ylide)’ and to consider these polymers as an independent group with distinct properties from polybetaines and polyampholytes.
While ylides were already reported in the early 1900s, they were only established as powerful reagents in organic chemistry in 1953 by Wittig.23 Since then, several different structures of ylides with varying reactivities have been reported.24 Consequently, the chemical landscape of ylides is now vast and generally describes structures with an anionic residue (Y−) directly covalently linked to a heteroatom (X+), often phosphorus, nitrogen, or sulfur, displaying a formal positive charge. Historically, the negative charge was displayed on a carbon atom (Y = C) but has since been extended to other atoms as well (e.g., Y = C, O, N). Thus, they are 1,2-dipolar species and can be described as RInX+–Y−RIIm. Structural analysis and computational studies focusing on phosphorus and sulfur ylides suggest that the general stability of ylides arises from negative hyperconjugation, involving the transfer of electron density from a filled orbital belonging to the (carb)anion to an antibonding orbital of the adjacent heteroatom-containing residue.25,26 Further stability of ylides depends strongly on the stabilization of the two charges, for example by aryl residues (pyridinium vs. ammonium ylides) or by electron-withdrawing groups in case for the negative charge, such as nitriles and carbonyls. While many reactions with unstabilized ylides exist, nowadays most processes employ stabilized versions, allowing ylides to be used routinely in organic chemistry laboratories, sometimes even as bench-stable reagents. Most commonly, ylides are accessible by deprotonation of the corresponding salt, for example, tetrasubstituted phosphonium, trisubstituted sulfonium, or monosubstituted N-pyridinium species.
Somewhat surprising, poly(ylides) remain a so far largely overlooked class of charge neutral polymers, despite early reports in the 80s by H. Ringsdorf of 1-iminopyridinium ylides and their use as photoresponsive residues, which was demonstrated on monolayers and liposomes using 1-iminopyridinium ylide displaying lipids.27 The unique photochemistry of such ylides was extended to polymeric systems by H. Ringsdorf and later by the group of P. Théato.28,29
It was only in 2019 when S. Jiang and coworkers first reported the use of N-oxides as antifouling surface coatings.30 Since then, polymeric N-oxides have found numerous applications in drug delivery, and naturally, new forms of poly(ylides) have been reported, significantly enhancing their chemical space. Much to our surprise, the term poly(ylide) is still not used to describe and define this specific type of polymer. Here, the recent emergence of polymeric ylides and their utility as unique building blocks for biomaterials is reviewed, and arguments are provided as to why polymeric ylides should be categorized as a distinct class of hydrophilic polymers, which also offer plentiful and distinct alternatives to polybetaines. This article does not serve as a comprehensive review but instead provides reflections on recent understanding, ultimately helping to navigate and define a dynamic field of macromolecular chemistry with significant implications for biomaterials science.
2. N-Oxides: first reported class of polymeric ylides for the use in (bio)materials science
2.1 N-Oxides as antifouling coatings
In 2019, Jiang and co-workers reported poly(trimethylamine-N-oxide) (PTMAO) as a new type of superhydrophilic polymer, taking inspiration from trimethylamine N-oxide (TMAO), a naturally occurring zwitterionic osmolyte (Fig. 2).30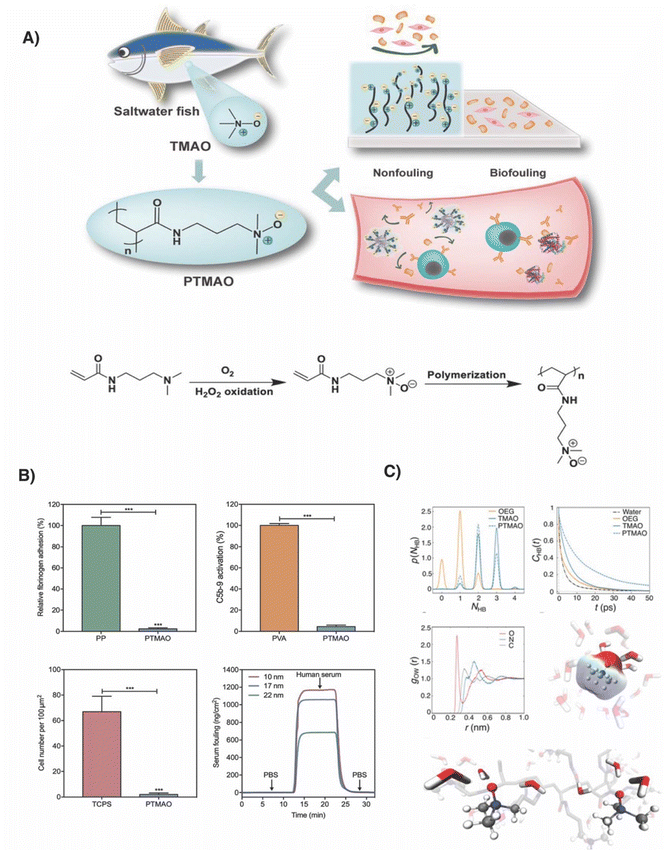 | ||
| Fig. 2 (A) Concept of using N-oxides as ultralow-fouling materials and synthetic route towards polymeric N-oxide. (B) Low-fouling determination by fibrinogen adhesion to PP and PTMAO hydrogel surfaces as determined by ELISA; C5b-9 activation by PVA and PTMAO hydrogels; Adhered NIH 3T3 cells to polystyrene and PTMAO hydrogel surfaces, after 3 days and SPR sensorgram of PTMAO films with varying thicknesses in blood serum. (C) Computational simulations with probability distributions of hydrogen bonds; autocorrelation function; RDF of water oxygen with respect to oxygen, nitrogen and carbon of TMAO; image of TMAO in aqueous environment; snapshot of PTMAO in aqueous solution with interactions being highlighted. Adapated from ref. 30, reprinted with permission from AAAS, copyright (2019). | ||
Structurally, N-oxides differ significantly from betaines by having the positive charge directly displayed next to the negative charge. While in respect to other chemical bonds, N-oxides display a relatively high dipole moment (4.0 to 5.0 D), in comparison to betaine residues this dipole moment may be considered as relatively low.31 PTMAO was readily accessible by oxidizing N-[3-(dimethylamino)propyl]acrylamide with hydrogen peroxide in the presence of pentetic acid. Eventually, the monomers were polymerized by free radical polymerization in the presence of a cross-linker. To demonstrate and confirm the highly hydrophilic character of polymeric N-oxide-derived materials, hydrogels were prepared by photo-crosslinking TMAO in the presence of N,N′-methylenebis(acrylamide) as a crosslinker. The resulting hydrogels displayed ultralow fouling properties with fibrinogen adhesion being reduced by nearly 98% in comparison to polypropylene disks. Fibrinogen is a clinically relevant blood-plasma protein with high hydrophobicity, which readily adsorbs on surfaces; thus, a strong decrease in adhesion provides the first indication of reduced binding of the protein to the surface, likely caused by a strong hydration layer. The authors additionally prepared polymer-coated gold surfaces by SI-ATRP, which enabled the quantification of fouling when exposed to undiluted blood serum. With no significant binding of biomolecules being detected when flowing blood serum over the coated surfaces, first observations that N-oxide coated surfaces present ultralow fouling properties were confirmed. Notably, these assays were conducted under extremely challenging conditions using undiluted blood serum. The experimental data suggested a strong hydration layer caused by the superhydrophilic TMAO residues. To obtain molecular insights, the authors simulated the interaction of TMAO with water (Fig. 2C). These simulations suggest that the oxygen of TMAO accepting either two or three hydrogen bonds with approximately equal probability. For comparison, the oxygen of PEG forms only one hydrogen bond with water.
Salt responsiveness in form of decreased hydration layer displays a major challenge for most zwitterionic coatings. This phenomenon stems from binding of cations to betaine residues, therefore directly affecting the hydration layer. For many potential applications, it is highly desirable to work under high salt concentration, for example when applied for ship coatings. In a joint effort, the groups of Tao Wei, Shaoyi Jiang and Zhan Chen investigated theoretically and experimentally the impact of high salt concentrations on N-oxide bearing coatings and probed further the underlying mechanism responsible for the strong non-fouling properties of N-oxides derived materials.32 With the help of surface-sensitive sum frequency generation (SGF) vibrational spectroscopy, the groups quantified the hydration layer formed by PTMAO coating. Analysis of SGF spectra revealed strongly hydrogen-bonded water molecules on PTMAO-coated surfaces. Surprisingly, the observed hydration layer is resistant to hydration loss induced by salt, which is opposed to many betaine derived coatings. Notably, besides high concentration of NaCl and MgCl2, the authors applied also seawater to study the effect of salts on the hydration layer. The team around Z. Chen attributed this effect to the short distance between oxygen anion and ammonium, which prevents binding to cations via repulsion with the positively charged nitrogen. This observation indicates that ylides do not only offer superhydrophilic building blocks, but remarkably also offer properties distinct from those of betaines, for example resistance to salt. The work by Z. Chen et al. strongly suggests that this characteristic behaviour originates from the adjacent charges, a structural element that exclusively is provided by ylide residues, thus raising the question whether polymeric ylides should be treated as a standalone group of hydrophilic polymers. The initial work by the Jiang group on N-oxide bearing polymers as superhydrophilic building blocks has inspired others to explore similar scaffolds for the design of advanced materials displaying strong hydration layers.
Maison and co-workers hypothesized that N-oxide displaying surfaces should not only prevent adhesion of blood plasma proteins but should be also effective against adhesion of microorganisms.33 For this purpose, PE surfaces were grafted with a variety of tertiary amine containing building blocks including styrene, acrylamide and acrylate derived monomers (Fig. 3). The zwitterionic surface was obtained upon oxidation with hydrogen peroxide and confirmed by 3D mass spectrometric and scanning probe microscopy analysis. The surface roughness Ra and the brush layer thickness were determined with 1 to 2.5 μm and 50 to 100 nm, respectively.
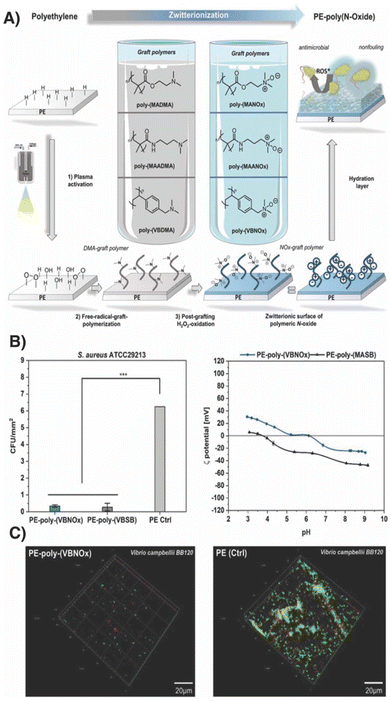 | ||
| Fig. 3 (A) PE surfaces were decorated with N-oxides via surface grafting using plasma activation and free radical polymerization, with subsequent oxidation of tertiary amines to N-oxides using H2O2. (B) Left: assessment of N-oxide fouling surfaces with bacterial adhesion assay with S. aureus (strain ATCC29213) after 24 h. Right: zeta potential of PE-poly-(VBNOx) and PE-poly-(MASB) between pH 3 and 9. (C) Confocal microscopy of PE (right) PE-poly-(VBNOx) (left) after 24 h incubation with V. campbellii (strain BB120) in seawater. Adapted from ref. 33, with permission of wiley, copyright (2023). | ||
The coated surfaces displayed low water contact angles, once more confirming the highly hydrophilic nature of N-oxides. Interestingly, styrene-derived poly(VBNOx) displayed the lowest water contact angle (θ = 10°) compared to methacrylate- and acrylamide-derived polymers with θ = 20° and 29°, respectively. The team around Maison emphasized that surfaces containing N-oxides displayed nearly neutral zeta potentials at a physiologically relevant pH range between 5 and 7. This ylide-specific property sets N-oxides apart from other polymeric zwitterions, such as sulfobetaines, which typically have negative zeta potentials even at acidic pH values. When exposed to Gram-positive S. aureus and Gram-negative V. campbellii - an important pathogen for clinically relevant infections and a potential pathogen encountered in coastal water, respectively - N-oxide-coated surfaces displayed low-fouling properties towards bacteria with similar effectiveness to conventionally used sulfobetaines. Strikingly, the team around Maison observed a significant antimicrobial effect in the growth controls, suggesting a bactericidal effect of the N-oxide grafted materials. Interestingly, this phenomenon was only observed for grafted N-oxides and could not be confirmed for monomeric N-oxides. In fact, the minimal inhibitory concentrations of the corresponding monomer were determined to be >1024 μg mL−1 for both S. aureus and E. coli. To this end, the authors propose ROS formation as one reason for the observed toxicity caused by the reductive transformation of poly-N-oxides when exposed to microorganisms.
Synthetically, all so far discussed work has been grafted from surfaces using tertiary amine bearing monomers, either via free radical polymerisation or copper catalysed ATRP. Amines were either oxidized prior or after polymerisation upon treatment with hydrogen peroxide. While generally this method guarantees high surface coverage, precise polymer characterisation remains a large challenge. Chia-Chih Chang and coworkers reported an alternative approach, namely synthesis of N-oxide bearing poly(methacrylates) using RAFT polymerisation with incorporation of a benzophenone containing monomer.34 The obtained polymers were photo-crosslinked with obtained film thickness between 27 and 32 nm. Besides confirming low binding of E. coli., the authors showed that N-oxides displaying surfaces prevented also the adhesion of blood cells.
Whether for antimicrobial applications or as stealth coating against adhesion of blood plasma proteins, N-oxides decorated surfaces offer an attractive, alternative approach compared to existing hydrophilic polymers including polybetaines and PEG. The work of Maison indicates that the antimicrobial effect of N-oxides – at least to a certain degree – relies on the release of reactive oxygen species, thus originating from a chemical mechanism. Yet, the ability to form strong hydration shells with water and subsequently provide superhydrophilic materials alongside their salt resistant nature, sets N-oxides apart from other zwitterionic and hydrophilic polymers in general.
What remained elusive was the impact of the short linker separating the positive and negative charges on possible interactions with biofoulers. Recent work by T. Wei and coworkers provided valuable insights by revealing that, besides a strong hydration shell, the overall neutral charge and reduced dipole moment of N-oxides play an essential role by preventing dipole–dipole interactions and decreasing their attractive electrostatic interactions with biofoulers.35
2.2 N-Oxide based materials beyond coatings
Besides being employed as components for antifouling coatings, N-oxide-displaying polymers have found numerous applications as hydrophilic building blocks for the design of advanced materials, including drug delivery carriers and membrane technologies.A research team led by Y. Shen reported on polyzwitterionic micelles fabricated from poly(tertiary amine-oxide)-block-poly(ε-caprolactone) (PTAO-PCL), with each block displaying an equal molecular weight of around 5 kDa.36 Upon self-assembly, the authors obtained micellar structures with average diameters of 29 and 25 nm and zeta potentials of −2.08 and −3.46 mV for OPDMA-PCL and OPDEA-PCL, respectively. The ability of the poly(ylide) corona to prevent protein adsorption provided good blood circulation times, with values similar to those obtained for PEG-derived micelles. Interestingly, PTAO-PCL micelles exhibited significantly enhanced DOX accumulation in tumors (up to 2.7-fold). The authors attributed this observation to tumor tissue uptake via transcytosis-mediated extravasation. This finding was further confirmed by determining the diffusion into tumor tissues. While PTAO-PCL micelles diffused up to 100 μm away from vessels, PEG-PCL did not diffuse further than 40 μm. These beneficial attributes were confirmed by in vivo antitumor studies. For this purpose, DOX-loaded PTAO-PCL micelles were compared to PEG-PCL micelles when administered to an MCF-7/ADR orthotopic tumor mice model. In contrast to the DOX-loaded PEG-PCL micelles, PTAO-PCL micelles suppressed tumor growth.
The use of N-oxide-containing materials extends to applications in the field of analytical chemistry. Y. Yu and co-workers designed an anti-nonspecific adsorption immunomagnetic platform for capturing and quantifying heterogeneous circulating tumor cells with high efficiency.37 This was achieved by coating polymeric N-oxides on magnetic Fe3O4@SiO2 beads, which were further modified with aptamers for selective cell binding. After in vitro cell binding validation, the authors exposed the platform to blood samples, which pose significant challenges due to non-specific adhesion. The beads not only allowed for the detection of patient-derived SW480 cells in blood samples but also enabled the quantification of small amounts of SW480 cells added to healthy blood donor samples. Next, the authors determined changes in the heterogeneity of circulating tumor cells before and after surgery in patients by distinguishing different phenotypes based on the epithelial–mesenchymal transition, specifically epithelial, mesenchymal, and mixed (epithelial, mesenchymal) circulating tumor cells. It was found that only the proportion of mesenchymal circulating tumor cells changed across all stages of cancer patients, while the number of epithelial circulating tumor cells was significantly decreased only in early colorectal cancer patients (stages I and II).
Beyond applications in biomedical science, N-oxide-derived materials are also used in membrane technologies. Similar to previous examples, the ability of N-oxide-containing materials to prevent non-specific protein adhesion, combined with their hydrophilicity, makes them attractive building blocks for membranes. The team led by Y. Wang developed nanoporous membranes from poly(2-dimethylaminoethyl methacrylate)-block-polystyrene via selective swelling-induced pore generation and subsequent oxidation with hydrogen peroxide.38 While SEM suggested pore sizes of approximately 25 nm, BSA as a sample protein was still rejected, indicating that the N-oxides on the interface collapse in the absence of water, such as during SEM measurement. The obtained membranes displayed flux recovery of up to 97% during BSA exposure and fouling. This study demonstrates that N-oxides hold great potential not only in biomedical fields but also in advancing membrane technologies.
3. Stabilized carbon-ylides offer a large chemical space for the design of hydrophilic polymers
The impact of the short linker length that separates charges in N-oxides on the ability to form strong hydration layers has been highlighted by several research groups. Although N-oxides are not the prototypical form of ylides, their use as antifouling coatings has revitalized the field of poly(ylides), which was last actively explored by H. Ringsdorf and P. Theato.28,29 Surprisingly, the term poly(ylide) has been rarely used in recent literature, possibly due to the lack of reported properties characteristic to poly(ylides). In recent years, various forms of polymeric N-oxides have been utilized as building blocks for functional materials. Simultaneously, a second type of poly(ylide) has emerged, namely stabilized sulfonium and phosphorus ylides, where the negative charge is partially localized on a carbon atom.Inspired by reports from T. Wei and S. Jiang on the importance of the linker lengths between the positive and negative charges, our group sought to investigate stabilized ylides with the negative charge placed on a carbon as building blocks for hydrophilic polymers. We were intrigued by the large – yet unexplored – chemical space offered by these carbon-ylides, which stands in contrast to N-oxides. Ylides that display a negative charge on the carbon are often associated with high reactivity and reactive intermediates in organic chemistry, such as during the Wittig reaction, which we also believe is responsible for their absence in polymer chemistry and biomaterial science. Despite their reputation as reactive building blocks, several forms of carbon-ylide exist that display sufficient stability and chemical inertness. Here, the extensive work of H. Wasserman, carried out in the 1980s and 1990s, must be mentioned, as it discloses several forms of highly stabilized phosphorus ylides.39,40 In the context of polymer science, the work of H. Ringsdorf and P. Theato, who used 1-iminopyridinium ylides as photoresponsive residues, serves as one of the very few examples in which ylides are employed to design functional materials.28,29 Their use for the design of biomaterials, however, remained so far elusive.
In 2022, our group reported poly(sulfur ylides) composed of stabilized sulfonium ylides as a synthetically accessible form of poly(ylides) with remarkable stability.41 The synthesis of the corresponding monomers was readily achieved starting from a carboxylic acid, and subsequent polymerization was performed using RAFT polymerization. Despite a polystyrene backbone, these polymers showed excellent solubility in protic organic solvents as well as in mixtures of aqueous and organic solvents. Water contact angles confirmed the hydrophilic character of poly(sulfur ylide) when immobilized on surfaces. Inspired by the impact of the spacer lengths separating the positive and negative charges on the ability to prevent fouling of surfaces, we investigated whether poly(ylides) could serve as a new chemical tool for the design of antifouling materials.42 Indeed, when incubating poly(sulfur ylide)-coated surfaces with fibrinogen, we observed decreased adhesion with similar levels of biomolecules attached compared to polyethylene glycol-coated surfaces, confirming a somewhat hydrophilic character (Fig. 4). When incubating these surfaces with Gram-negative bacteria, we found that: (i) biomass accumulated by adhered bacteria was reduced for both polyethylene glycol and poly(sulfur ylide)-coated surfaces, and (ii) the viability of those bacteria that overcame the initial physical barrier in the form of the hydration layer differed significantly. While bacterial cells displayed high viability when attached to polyethylene glycol surfaces, cells experienced a hostile environment when attached to poly(sulfur ylide)-coated surfaces. By seeding NIH/3T3 cells on ylide-coated glass slides, we assessed the compatibility of poly(sulfur ylides) with fibroblasts. In contrast to cells seeded on PEG-coated surfaces, fibroblast cells not only grew in high density but also exhibited elongated morphologies on ylide-coated surfaces, indicating that mammalian and human cells are not affected by poly(sulfur ylides), while these materials display toxicity towards bacterial cells. It is noteworthy that the bactericidal properties of poly(sulfur ylides) were more pronounced on surfaces but could only be observed to a lower degree when using poly(sulfur ylides) in solution. When conducting surface energy analysis, an enhanced Lewis-base component was observed, while the obtained value for surface energy indicated an only moderately hydrated surface likely related to the hydrophobic styrene backbone.
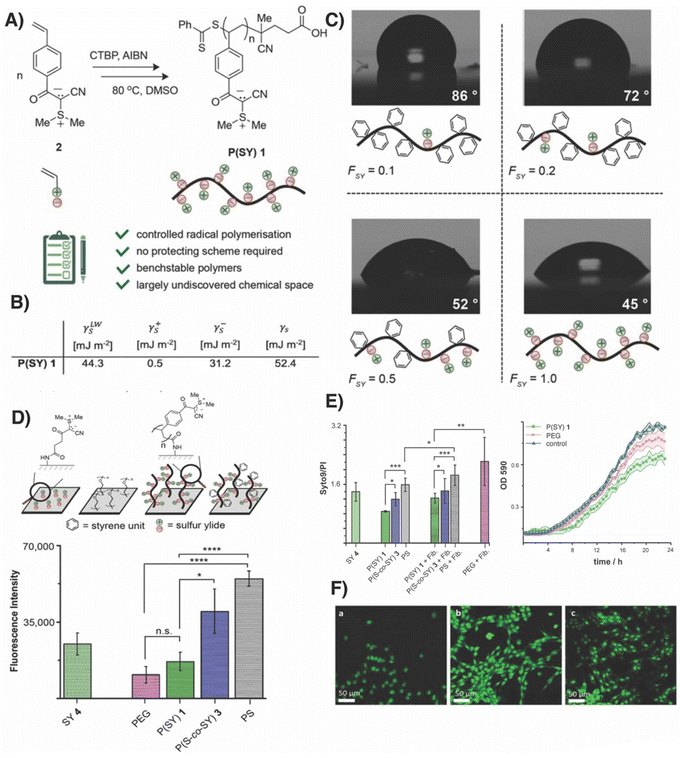 | ||
| Fig. 4 (A) Polymerisation of sulfur ylide homopolymer P(SY) 1. (B) Surface energy analysis revealed that surfaces coated with P(SY) 1 displayed a relatively high Lewis base component. (C) Water contact angles are reduced with increasing fractions of sulfur ylides residues in copolymers. (D) Surfaces were coated by covalently immobilising sulfur ylide polymers and small molecules alongside PEG as a reference. PS = polystyrene. Fluorescence intensity corelates to adhesion of fibrinogen. (E) Live/dead staining of bacteria adhered to surfaces and bacterial growth upon incubation with polymers (0.1 mg mL−1). (F) NIH/3T3 cells seeded on surfaces coated with (a) PEG, (b) P(SY) 1, (c) collagen. Adapted from ref. 42, with permission of wily, copyright (2023). | ||
The discovery that sulfur ylides serve as building blocks for bactericidal materials while maintaining high biocompatibility with mammalian cells opens up numerous new directions for the design of biomaterials. Yet, at this point, it remained unclear to what extent these findings apply to the entire field of stabilized carbon-ylides or if they are restricted specifically to sulfur ylides. For this reason, our group accessed poly(phosphorous ylides) using a styrenic backbone.43 In contrast to sulfur ylides, phosphorous ylides can be derived from the corresponding trialkyl or triaryl phosphines, hence offering a significantly larger chemical space. In particular, trimethyl-derived poly(phosphorous ylides) displayed similar properties to sulfur ylides. Simulations confirmed a dipole moment similar to that of N-oxides, a key requirement for preventing interactions with biomolecules alongside an overall charge-neutral nature. Importantly, poly(phosphorus ylides) displayed a comparable trend to poly(sulfur ylides) when incubated with Gram-negative bacteria, namely a reduction in biomass and an hostile environment for bacterial cells. These findings provide further indication that ylides offer distinct properties and represent a powerful addition to the toolbox of hydrophilic building blocks.
4. Conclusion
Future applications of next-generation functional materials increasingly demand multi-functional building blocks and an enhanced understanding of structure–property relationships. In particular the design of future biomaterials requires hydrophilic building blocks as a key component. Zwitterionic polymers, particularly polybetaines, have emerged as the go-to building block for the fabrication of non-fouling and overall charge-neutral materials and surfaces. Recent studies on structure–property relationships have revealed that one key aspect in the choice of betaine residues is a short linker length separating the positive and negative charges, thereby reducing dipole–dipole interactions with biomolecules.Based on this improved understanding, several groups have re-evaluated the use of polymeric ylides, a so far largely overlooked class of hydrophilic polymers. Besides N-oxides, ylides with the negative charge localized on a carbon atom have been reported for the design of antifouling materials. With the increasing use of these building blocks for the fabrication of new biomaterials, the author suggests that poly(ylides) may be classified as an independent class of charge-neutral and hydrophilic polymers. By displaying a minimal linker length between the two charges, ylides are distinct from betaines. Importantly, recent reports by T. Wei and coworkers suggest that the distance between the charges not only impacts the dipole moment but also reduces salt-responsive behaviour. While resonance stabilization makes it arguable whether all ylides necessarily can be classified as zwitterionic structures, poly(ylides) certainly open new avenues as hydrophilic building blocks for the design of functional (bio)materials.
Currently, research is primarily focused on elucidating the properties of these materials, such as their low-salt responsiveness, and understanding the origins of these behaviors. In this context, various research groups are modifying existing structures to better correlate structure with function. For example, the two alkyl residues of an N-oxide functionality can be easily altered, which affects the hydrophobic/hydrophilic balance, also known as amphiphilic balance.44,45 These structural modifications have shown a significant impact on the performance of antifouling agents. The author anticipates that future research will provide deeper insights into the structure–property relationship. Another area of focus is the development of new poly(ylide) residues, an approach that combines organic, polymer, and materials science. For instance, one might consider substituting sulfur and phosphorus with other elements that exhibit similar chemistry, such as arsenic. The choice of heteroatom and their covalently linked surrounding are not trivial and T. Wei has shown that in case of ammonium moieties (as part of the N-oxides or betaines), the net charge varies significantly and depends on neighbouring alkyl residues.35 Consequently, the localisation of the charge depends not only on the nature of the heteroatom but also on the surrounding environment, something that still needs to be considered when comparing different substituents in the case of polymeric N-oxides or C-ylides. However, the author sees a more pressing need in advancing this field, namely, to further define this class of polymers, enabling more precise comparisons and correlations of structure–property relationships. While the examples presented here can be clearly defined as ylide structures, other examples with less clear definitions are existing. For example, the groups around K. Whittaker and T. P. Davis reported sulfoxide-containing polymers that not only serve as hydrophilic building blocks but also as antifouling coatings.46,47 Although sulfoxides are commonly represented by an SO double bond, theoretical studies support a charge-separated representation, which helps to explain their observed properties.26 The author hopes that this review will help clarify and guide current and future research in this area.
In summary, in the future, the author foresees an increased use of poly(ylides) when designing new functional materials including stimuli-responsive nanomedicinal tools.48–50 On the one hand, this is due to the largely unexplored chemical space, providing plenty of possibilities to further chemically fine-tune ylide scaffolds. In addition, currently used zwitterionic polybetaines have yet to be routinely translated into commercial applications, and poly(ylides) offer a promising alternative scaffold with distinct properties, – including a certain inertness to salt concentrations – while remaining overall charge-neutral. As with all new materials, further applications will provide additional insights into structure–property relationships and ultimately determine the utility of poly(ylides) as a key building block for the design of functional (bio)materials.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author acknowledges funding from Radboud University (starting grant). The author thanks Prof. Daniela Wilson for fruitful and insightful discussions.References
- N. Bayliss and B. V. K. J. Schmidt, Prog. Polym. Sci., 2023, 147, 101753 CrossRef.
- Z. Wang, Q. Ye, S. Yu and B. Akhavan, Adv. Healthc. Mater., 2023, 12, 2300105 CrossRef PubMed.
- L. Shi, J. Zhang, M. Zhao, S. Tang, X. Cheng, W. Zhang, W. Li, X. Liu, H. Peng and Q. Wang, Nanoscale, 2021, 13, 10748–10764 RSC.
- Y. J. Eng, T. M. Nguyen, H. K. Luo and J. M. W. Chan, Nanoscale, 2023, 15, 15472–15512 RSC.
- Q. Yang and S. K. Lai, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2015, 7, 655–677 CAS.
- B. Li, Z. Yuan, H. C. Hung, J. Ma, P. Jain, C. Tsao, J. Xie, P. Zhang, X. Lin, K. Wu and S. Jiang, Angew. Chem., Int. Ed., 2018, 57, 13873–13876 CrossRef CAS PubMed.
- A. Laschewsky, Polymers, 2014, 6, 1544–1601 CrossRef.
- Q. Li, C. Wen, J. Yang, X. Zhou, Y. Zhu, J. Zheng, G. Cheng, J. Bai, T. Xu, J. Ji, S. Jiang, L. Zhang and P. Zhang, Chem. Rev., 2022, 122, 17073–17154 CrossRef CAS PubMed.
- M. Harijan and M. Singh, J. Mol. Recognit., 2022, 35(1), e2944 CrossRef CAS PubMed.
- Q. Shao, S. Jiang, Q. Shao and S. Jiang, Adv. Mater., 2015, 27, 15–26 CrossRef CAS PubMed.
- A. J. Keefe and S. Jiang, Nat. Chem., 2011, 4, 59–63 CrossRef PubMed.
- O. Azzaroni, A. A. Brown and W. T. S. Huck, Angew. Chem., Int. Ed., 2006, 45, 1770–1774 CrossRef CAS PubMed.
- S. Aleid, M. Wu, R. Li, W. Wang, C. Zhang, L. Zhang and P. Wang, ACS Mater. Lett., 2022, 4(3), 511–520 CrossRef CAS.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177–4189 CrossRef CAS PubMed.
- L. D. Blackman, P. A. Gunatillake, P. Cass and K. E. S. Locock, Chem. Soc. Rev., 2019, 48, 757–770 RSC.
- E. Schönemann, A. Laschewsky, E. Wischerhoff, J. Koc and A. Rosenhahn, Polymers, 2019, 11, 1014 CrossRef PubMed.
- P. A. Woodfield, Y. Zhu, Y. Pei and P. J. Roth, Macromolecules, 2014, 47, 750–762 CrossRef CAS.
- J. B. Schlenoff, Langmuir, 2014, 30, 9625–9636 CrossRef PubMed.
- J. F. Karthäuser, R. Kopecz, E. Schönemann, A. M. Guajardo, A. Laschewsky and A. Rosenhahn, Adv. Mater. Interfaces, 2024, 11, 2300873 CrossRef.
- V. Hildebrand, A. Laschewsky and E. Wischerhoff, Polym. Chem., 2016, 7, 731–740 RSC.
- J. F. Karthäuser, J. Koc, E. Schönemann, R. Wanka, N. Aldred, A. S. Clare, A. Rosenhahn and A. Laschewsky, Adv. Mater. Interfaces, 2022, 9, 2200677 CrossRef.
- M. Kumar, I. Sadaf, J. Pamidighantam, Anamika and S. Kumar, J. Heterocycl. Chem., 2024, 61, 29–70 CrossRef.
- G. Wittig and G. Geissler, Justus Liebigs Ann. Chem., 1953, 580, 44–57 CrossRef CAS.
- A. H. Li, L. X. Dai and V. K. Aggarwal, Chem. Rev., 1997, 97, 2341–2372 CrossRef CAS PubMed.
- D. G. Gilheany, Chem. Rev., 1994, 94, 1339–1374 CrossRef CAS PubMed.
- J. M. Standard, B. A. Copack, T. K. Johnson, D. E. Przybyla, S. R. Graham and R. J. Steidl, J. Phys. Chem. A, 2008, 112, 336–341 CrossRef CAS PubMed.
- M. Haubs and H. Ringsdorf, Angew. Chem., Int. Ed. Engl., 1985, 24, 882–883 CrossRef.
- L. D. Taylor, H. S. Kolesinski, B. Edwards, M. Haubs and H. Ringsdorf, J. Polym. Sci., Part C: Polym. Lett., 1988, 26, 177–180 CrossRef CAS.
- D. Klinger, K. Nilles and P. Theato, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 832–844 CrossRef.
- B. Li, P. Jain, J. Ma, J. K. Smith, Z. Yuan, H. C. Hung, Y. He, X. Lin, K. Wu, J. Pfaendtner and S. Jiang, Sci. Adv., 2019, 5, 9562–9576 CrossRef.
- M. Kobus, T. Friedrich, E. Zorn, N. Burmeister and W. Maison, J. Med. Chem., 2024, 67, 5168–5184 CrossRef PubMed.
- H. Huang, C. Zhang, R. Crisci, T. Lu, H. C. Hung, M. S. J. Sajib, P. Sarker, J. Ma, T. Wei, S. Jiang and Z. Chen, J. Am. Chem. Soc., 2021, 143, 16786–16795 CrossRef PubMed.
- N. Burmeister, E. Zorn, A. Farooq, L. Preuss, C. Vollstedt, T. Friedrich, T. Mantel, N. Scharnagl, M. Rohnke, M. Ernst, S. G. Wicha, W. R. Streit and W. Maison, Adv. Mater. Interfaces, 2023, 10, 2300505 CrossRef.
- V.-S. Luc, C.-C. Lin, S.-Y. Wang, H.-P. Lin, B.-R. Li, Y.-N. Chou and C.-C. Chang, Biomacromolecules, 2023, 24, 5467–5477 CrossRef CAS.
- P. Sarker, T. Lu, D. Liu, G. Wu, H. Chen, M. S. J. Sajib, S. Jiang, Z. Chen and T. Wei, Chem. Sci., 2023, 14, 7500–7511 RSC.
- J. Xiang, Y. Shen, Y. Zhang, X. Liu, Q. Zhou, Z. Zhou, J. Tang, S. Shao and Y. Shen, Adv. Sci., 2022, 9, 2200173 CrossRef CAS PubMed.
- C. Li, R. Li, X. Wu, Y. Zuo, G. Xiong, M. Huang, Y. Sun, R. Liao, Y. Xiao, L. Hu, C. Gao and Y. Yu, Anal. Chem., 2022, 94, 15240–15249 CrossRef CAS PubMed.
- C. Zhang, J. Zhou, X. Ye, Z. Li and Y. Wang, Macromolecules, 2021, 54, 4236–4245 CrossRef CAS.
- H. H. Wasserman and G. H. Kuo, Tetrahedron, 1992, 48, 7071–7082 CrossRef CAS.
- H. H. Wasserman and W. B. Ho, J. Org. Chem., 1994, 59, 4364–4366 CrossRef CAS.
- G. Poulladofonou and K. Neumann, Polym. Chem., 2022, 13, 4416–4420 RSC.
- B. B. Berking, G. Poulladofonou, D. Karagrigoriou, D. A. Wilson and K. Neumann, Angew. Chem., Int. Ed., 2023, 62, e202308971 CrossRef CAS PubMed.
- D. Karagrigoriou, B. B. Berking, Q. Wang, D. M. Sánchez-Cerrillo, D. R. Galimberti, D. A. Wilson and K. Neumann, ACS Macro Lett., 2023, 12, 1608–1613 CrossRef CAS PubMed.
- E. F. Palermo, K. Lienkamp, E. R. Gillies and P. J. Ragogna, Angew. Chem., Int. Ed., 2019, 58, 3690–3693 CrossRef CAS PubMed.
- V. Sambhy, B. R. Peterson, A. Sen, V. Sambhy, B. R. Peterson and A. Sen, Angew. Chem., Int. Ed., 2008, 47, 1250–1254 CrossRef CAS PubMed.
- R. Qiao, C. Fu, Y. Li, X. Qi, D. Ni, A. Nandakumar, G. Siddiqui, H. Wang, Z. Zhang, T. Wu, J. Zhong, S. Y. Tang, S. Pan, C. Zhang, M. R. Whittaker, J. W. Engle, D. J. Creek, F. Caruso, P. C. Ke, W. Cai, A. K. Whittaker and T. P. Davis, Adv. Sci., 2020, 7, 2000406 CrossRef PubMed.
- X. Xu, X. Huang, Y. Chang, Y. Yu, J. Zhao, N. Isahak, J. Teng, R. Qiao, H. Peng, C. X. Zhao, T. P. Davis, C. Fu and A. K. Whittaker, Biomacromolecules, 2021, 22, 330–339 CrossRef PubMed.
- C. H. Kim, E. S. Lee, H. Ko, S. Son, S. H. Kim, C. H. Lee, H. K. Na, J. M. Shin and J. H. Park, Chem. Mater., 2024, 36, 1088–1112 CrossRef.
- P. Rana, C. Singh, A. Kaushik, S. Saleem and A. Kumar, J. Mater. Chem. B, 2024, 12, 382–412 RSC.
- R. C. P. A. Remmers and K. Neumann, Biomater. Sci., 2023, 11, 1607–1624 RSC.
| This journal is © The Royal Society of Chemistry 2024 |