One-dimensional hybrid copper halides with high-efficiency photoluminescence as scintillator†
Zhongliang
Gong‡
a,
Jie
Zhang‡
ab,
Ying-Yue
Liu
a,
Lu-Xin
Zhang
a,
Qing
Zhang
a,
Lingyun
Xiao
a,
Bingqiang
Cao
*b,
Bing
Hu
 *c and
Xiao-Wu
Lei
*c and
Xiao-Wu
Lei
 *a
*a
aResearch Institute of Optoelectronic Functional Materials, School of Chemistry, Chemical Engineering and Materials, Jining University, Qufu, Shandong 273155, P. R. China. E-mail: xwlei_jnu@163.com
bSchool of Material Science and Engineering, University of Jinan, Jinan, Shandong 250022, P. R. China. E-mail: mse_caobq@ujn.edu.cn
cState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: hubing@fjirsm.ac.cn
First published on 22nd August 2024
Abstract
A new one-dimensional hybrid [APCHA]Cu2I4 was designed and applied as an X-ray scintillator. It exhibits broad-band green emission with a high PLQY of 74.80% and excellent stability. It demonstrates radioluminescence property with a light yield of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336 photons MeV−1, detection limit of 41 nGyair s−1, and high spatial limit of 13.95 lp mm−1 in X-ray imaging.
336 photons MeV−1, detection limit of 41 nGyair s−1, and high spatial limit of 13.95 lp mm−1 in X-ray imaging.
Semiconducting metal halides have sparked significant research interest due to their excellent optical properties and promising practical applications in photovoltaics, light-emitting diodes, photoelectric detectors, and scintillators.1–5 Especially, scintillators can convert high-energy radiation into visible or near-infrared light, broadly used in medical diagnostics, security detection, and space exploration.6–10 Three-dimensional (3D) all-inorganic perovskite nanocrystals of CsPbX3 (X = Cl, Br, I) showed unique advantages as scintillators owing to their simple synthesis method, tunable photoluminescence (PL) wavelength, and negligible afterglow.11–14 However, the inherent toxicity of Pb2+ and the instability of perovskite nanocrystals hinder their application in more sophisticated and extensive optical devices. Therefore, it is urgent to explore lead-free, low-cost, and diverse luminous metal halides at the molecular level.
Recently, a series of lead-free low-dimensional metal halides (LDMHs) with good PL performance have emerged. Owing to their various organic cations and abundant metal centers (In3+, Sb3+, Cd2+, Cu+, Zn2+), LDMHs offered more varied spatial arrangement structures and tunable PL properties, making them promising candidates in LEDs, displays, and X-ray detectors.15–19 Furthermore, the inherent quantum confinement endows LDMHs with highly localized excitons and high luminescent efficiency. For example, featuring ns2 electron configuration, the Sb3+-based organic–inorganic hybrid of C50H44P2SbCl5 crystal demonstrated strong yellow light emission with a near-unity PL quantum yield (PLQY) of 98.42% when excited by 370-nm light. This material also showed great environmental and irradiation stability, with a good linear response to X-ray dose rates and a light yield of 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 photons MeV−1.20 Sun et al. developed a [DBA]4Cu4I4 wafer with a high PLQY of 94.9%, which demonstrated a light yield higher than 1 × 104 photons MeV−1 and a high spatial resolution of 5.0 lp mm−1, showing potential in practical X-ray imaging applications.21 Mohammed et al. reported a 0D Cu4I4L4 crystal with a broad emission band and a high PLQY above 85%, exhibiting a high light yield of 30
460 photons MeV−1.20 Sun et al. developed a [DBA]4Cu4I4 wafer with a high PLQY of 94.9%, which demonstrated a light yield higher than 1 × 104 photons MeV−1 and a high spatial resolution of 5.0 lp mm−1, showing potential in practical X-ray imaging applications.21 Mohammed et al. reported a 0D Cu4I4L4 crystal with a broad emission band and a high PLQY above 85%, exhibiting a high light yield of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons MeV−1 and a low detection limit of 150 nGy s−1.22 A manganese-based hybrid (C38H34P2)MnBr4 exhibits narrow green light emission with a high light yield of 80
000 photons MeV−1 and a low detection limit of 150 nGy s−1.22 A manganese-based hybrid (C38H34P2)MnBr4 exhibits narrow green light emission with a high light yield of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photons MeV−1, a low detection limit of 72.8 nGy s−1 and a strong afterglow of 10 ms.23 Nevertheless, although organic–inorganic metal halides are very promising luminescent materials, the low light yields of Sb3+-based hybrids and the long afterglow of Mn2+-based hybrids limited their applications in scintillation materials field. Recently, low-dimensional cuprous halides have demonstrated unique superiorities as scintillators due to their nanosecond afterglow, shedding light on the way forward for the research of metal halide scintillators.24 Short afterglow, high light yields, and various spatial arrangement structures offer copper-based hybrids with advantages as scintillators.
000 photons MeV−1, a low detection limit of 72.8 nGy s−1 and a strong afterglow of 10 ms.23 Nevertheless, although organic–inorganic metal halides are very promising luminescent materials, the low light yields of Sb3+-based hybrids and the long afterglow of Mn2+-based hybrids limited their applications in scintillation materials field. Recently, low-dimensional cuprous halides have demonstrated unique superiorities as scintillators due to their nanosecond afterglow, shedding light on the way forward for the research of metal halide scintillators.24 Short afterglow, high light yields, and various spatial arrangement structures offer copper-based hybrids with advantages as scintillators.
Inspired by the previous study, we decided to focus on X-ray scintillation materials and strive to explore Cu-based halides in this field. In this work, 1D copper halide of [APCHA]Cu2I4 (APCHA = N-(3-aminopropyl)cyclohexylamine) was assembled using a simple solvent method. Under the excitation of ultraviolet light, [APCHA]Cu2I4 displays strong green light emission with a high PLQY of 74.80%. Furthermore, as a scintillation material, [APCHA]Cu2I4 revealed radioluminescence with a light yield of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336 photons MeV−1 and a detection limit of 41 nGyair s−1. In addition, the halide exhibits good optical stability when exposed to air and X-ray doses. This work paves the way for the designing of copper-based metal halides in X-ray imaging and detectors.
336 photons MeV−1 and a detection limit of 41 nGyair s−1. In addition, the halide exhibits good optical stability when exposed to air and X-ray doses. This work paves the way for the designing of copper-based metal halides in X-ray imaging and detectors.
A high-quality [APCHA]Cu2I4 crystal was synthesized at room temperature using a saturated crystallization method. The single crystal was identified as belonging to the P21 space group within a monoclinic system, composed of [APCHA]2+ organic cations and [Cu2I4]2− chains (Fig. 1a). The configuration of the distorted [Cu2I4]2− chain is formed by two approximately mirror-symmetric tetrahedra with Cu–I bond lengths in a normal range of 0.259–0.281 Å.25 The 1D [Cu2I4]2− chains are separated and surrounded by organic cations, forming a hybrid structure with abundant hydrogen bonds between them. The PXRD pattern of [APCHA]Cu2I4 crystal matches well with the simulated result from single-crystal data, confirming high purity without impurity (Fig. 1b). Thermogravimetric analysis (TGA) shown in Fig. 1c reveals a sharp weight loss when the temperature was above 280 °C, suggesting good thermostability of [APCHA]Cu2I4. When the temperature reached 420 °C, the crystal melted and decomposed with the organic species evaporating completely, leaving the final substance of cuprous iodide (Fig. S1, ESI†).
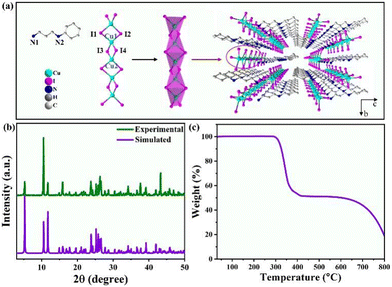 | ||
| Fig. 1 (a) The detailed and arrangement structure of [APCHA]Cu2I4. (b) The simulated and experimental PXRD patterns of [APCHA]Cu2I4. (c) TGA curve of [APCHA]Cu2I4. | ||
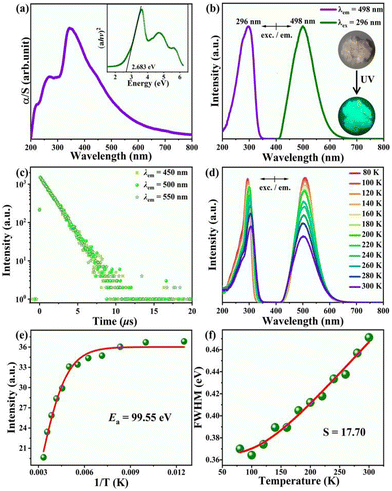
UV-vis absorption spectrum shown in Fig. 2a features a broad band with several sub-bands in the range 200–750 nm and a sharp slope, corresponding to a bandgap of 2.68 eV from the Tauc's Plot. Owing to the partial absorption in the visible region, this crystal appears light pink under daylight. It exhibits green light emission under excitation of 254 nm UV light (Fig. 2b). To understand the luminescence mechanism, we recorded the PL excitation (PLE) and PL spectra of the [APCHA]Cu2I4 crystal (Fig. S2 and S3, ESI†). Monitoring the wavelength of the maximum emission peak at 498 nm, the excitation spectrum consisted of an intense band with a maximum peak of 296 nm and a shoulder peak around 270 nm. Upon UV excitation at 296 nm, the halide exhibited a single broad emission band with a full width at half maximum (FWHM) of ≈492.8 meV. The Commission Internationale del'Eclairage (CIE) chromaticity coordinates were (0.18, 0.36) and the absolute PLQY of 74.80% (Fig. S4 and S5, ESI†). Furthermore, by monitoring different emission wavelengths, the time-resolved PL decay curves showed an identical long-lived monoexponential decay lifetime of 1.332 μs. This indicates that there is only one radiative carrier recombination pathway in this compound (Fig. 2c).
To gain deep insights into the excited-stated dynamics of [APCHA]Cu2I4 crystal, we measured the temperature-dependent PL spectra in the range of 80–300 K. As shown in Fig. 2d, both the excitation and emission spectra reveal a gradual decrease in intensity and a broadening of the bandwidth with increasing temperature. Moreover, the PL lifetimes decrease from 2.124 to 1.431 μs, due to enhanced electron–phonon coupling accelerating nonradiative relaxation (Fig. S6 and Table S4, ESI†). Temperature-dependent PL intensity and FWHM provide information about the compound's activation energy (Ea) and electron–phonon coupling strength. A higher Ea value of 99.55 meV is obtained by fitting the integrated PL intensity as a function of inverse temperature based on the Arrhenius function.This value is higher than the room temperature thermal energy (∼25 meV), indicating excellent stability of the excitons (Fig. 2e). The Huang–Rhys factor (S), representing electron–phonon coupling parameter, is fitted by the equation with the FWHM as a function of temperature. Compared to the inorganic halides Cs3Cu2I5 (40.33)26 and CsCu2I3 (38.4),27 [APCA]Cu2I4 has a smaller S value of 17.70, suggesting weaker electron–phonon coupling, which is consistent with the compact tetrahedral coordination (Fig. 2f).
To gain deeper insight into the photophysical properties of [APCHA]Cu2I4, we performed density functional theory (DFT) calculations on the band structure and density of states (DOS). The results reveal that [APCHA]Cu2I4 has a direct bandgap of 2.696 eV with both valence band maximum (VBM) and conduction band minimum (CBM) being at G point, which is in good agreement with the experimental results from the absorption spectrum (Fig. 3a). The VBM is mostly contributed by Cu-3d state while the CBM mainly comprises Cu-4s and I-5s states (Fig. 3b). Therefore, the excited electron is generally located on the [Cu2I4]2− chain. Based on the above analysis, the photophysical process of [APCHA]Cu2I4 is depicted in the diagram as shown in Fig. 3c. Upon excitation with UV light, the electrons transition from the ground state to an excited state. The exciton–phonon interaction then leads to the formation of STEs from free carriers, followed by radiative decay from STEs as broad-band emission. In addition, [APCHA]Cu2I4 exhibits superior optical stability, as evidenced by an unchanged XRD pattern and sufficient PL emission intensity after being stored in humid air for 2 months (Fig. 3d and Fig. S7, ESI†). The sample of [APCHA]Cu2I4 also exhibits structural and spectral stability toward various organic solutions, such as acetone, acetonitrile, and dichloromethane, for one day. (Fig. S8 and S9, ESI†).
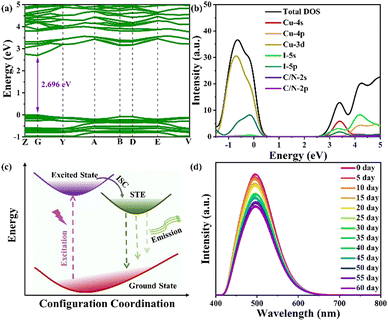 | ||
| Fig. 3 Electronic band structures (a) and densities of state (b) for the [APCHA]Cu2I4 computed by DFT. (c) Schematic PL mechanism of [APCHA]Cu2I4. (d) Time-dependent PL spectra of [APCHA]Cu2I4. | ||
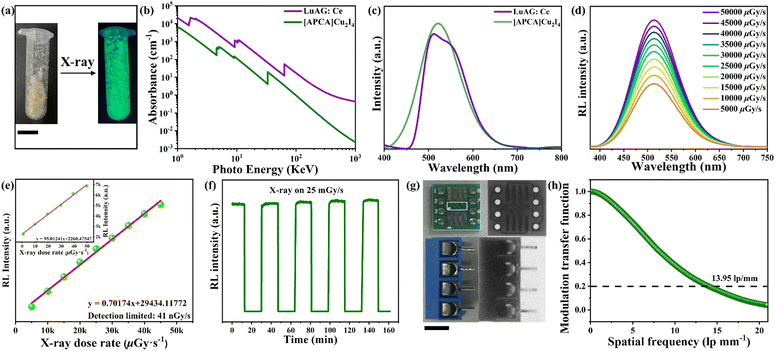
The [APCHA]Cu2I4 crystal also exhibits bright green light under X-ray irradiation, which benefits from high PLQY and large Stokes shift (Fig. 4a). The similar radioluminescence (RL) and PL emission bands indicate the same recombination pathway under both X-ray and UV light. The absorption coefficient of [APCHA]Cu2I4, one of the critical performance indicators, as a function of X-ray photon energy is smaller than that of LuAG: Ce, due to its lower-atomic-number elements (Fig. 4b). The scintillation light yield of [APHCA]Cu2I4 is calculated to be 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336 photons MeV−1 by using a commercially available LuAG: Ce as standard reference (Fig. 4c). The detection limit is a key parameter for evaluating the performance of X-ray scintillators. When irradiated with an X-ray dose rate in the range of 5000–50
336 photons MeV−1 by using a commercially available LuAG: Ce as standard reference (Fig. 4c). The detection limit is a key parameter for evaluating the performance of X-ray scintillators. When irradiated with an X-ray dose rate in the range of 5000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μGyair s−1, the RL emission spectra revealed that [APCHA]Cu2I4 shows a linear response to the X-ray dose rate with a low detection limit of 41 nGyair s−1 (Fig. 4d and e). Fig. 4f shows the temporal response of the [APCHA]Cu2I4 under a square-wave modulated X-ray dose of 25 mGyair s−1, indicating excellent repeatability and stability under X-ray irradiation. In addition, we prepared a film by mixing microscale [APCHA]Cu2I4 with polydimethylsiloxane (PDMS) to verify its X-ray imaging performance (Fig. S8, ESI†). The metal wire embedded in the integrated circuit can be clearly distinguished in the scintillator screen under X-ray irradiation owing to the difference in the absorption intensity of different materials (Fig. 4g). To further evaluate the image quality, the modulation transfer function (MTF) is calculated based on the slanted-edge method, which gives a spatial resolution of 13.95 lp mm−1 at an MTF value of 0.2 (Fig. 4h).
000 μGyair s−1, the RL emission spectra revealed that [APCHA]Cu2I4 shows a linear response to the X-ray dose rate with a low detection limit of 41 nGyair s−1 (Fig. 4d and e). Fig. 4f shows the temporal response of the [APCHA]Cu2I4 under a square-wave modulated X-ray dose of 25 mGyair s−1, indicating excellent repeatability and stability under X-ray irradiation. In addition, we prepared a film by mixing microscale [APCHA]Cu2I4 with polydimethylsiloxane (PDMS) to verify its X-ray imaging performance (Fig. S8, ESI†). The metal wire embedded in the integrated circuit can be clearly distinguished in the scintillator screen under X-ray irradiation owing to the difference in the absorption intensity of different materials (Fig. 4g). To further evaluate the image quality, the modulation transfer function (MTF) is calculated based on the slanted-edge method, which gives a spatial resolution of 13.95 lp mm−1 at an MTF value of 0.2 (Fig. 4h).
In summary, we successfully synthesized a new LDMH of [APCHA]Cu2I4 using a room-temperature saturated crystallization method. Excited by UV light at 300 nm, this crystal displays bright green light with a high PLQY of 74.80% originating from the STE recombination process. Furthermore, this compound exhibits intensive scintillation performance with a light yield of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 336 photons MeV−1 and a detection limit of 41 nGyair s−1 under X-ray irradiation. This work thus provides a new structural platform for the application in optoelectronic devices and promotes the potential application as scintillation materials. We believe this work will encourage the development of molecular-level optoelectronic devices by demonstrating the great application potential of low-dimensional hybrid Cu-based halides.
336 photons MeV−1 and a detection limit of 41 nGyair s−1 under X-ray irradiation. This work thus provides a new structural platform for the application in optoelectronic devices and promotes the potential application as scintillation materials. We believe this work will encourage the development of molecular-level optoelectronic devices by demonstrating the great application potential of low-dimensional hybrid Cu-based halides.
We express thanks for financial support from the National Nature Science Foundation of China (22171105), Shandong Provincial Natural Science Foundation (ZR2022YQ14 and ZR2021MB001), and Special Foundation of Taishan Scholar Project.
Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
There are no conflicts to declare.Notes and references
- X. C. Wang, T. X. Bai, J. L. Sun, J. Y. Liu, Y. Su and J. S. Chen, Chem. Eng. J., 2024, 486, 150257 CrossRef CAS
.
- D. H. Liang, H. B. Xiao, W. S. Cai, S. R. Lu, S. Y. Zhao, Z. G. Zang and L. Xie, Adv. Opt. Mater., 2023, 11, 2202997 CrossRef CAS
.
- S. S. Li, P. F. Cheng, H. X. Liu, J. T. Li, S. J. Wang, C. L. Xiao, J. Y. Liu, J. S. Chen and K. F. Wu, Angew. Chem., Int. Ed., 2024, 63, e202319969 CrossRef CAS
.
- D. H. Chang, Y. P. Chen, L. R. Wang, J. X. Wang, Y. J. Feng, Y. F. Yuan, H. Gao, M. Wu, R. J. Fu, G. Yang, K. Wang and H. Z. Guo, Adv. Opt. Mater., 2024, 12, 2302829 CrossRef CAS
.
- J. Zhang, X. Wang, W. Q. Wang, X. Y. Deng, C. Y. Yue, X. W. Lei and Z. L. Gong, Inorg. Chem., 2024, 63, 2647–2654 CrossRef CAS
.
- L. Zi, J. Song, N. Wang, T. Y. Wang, W. Li, H. C. Zhu, W. Xu and H. W. Song, Laser Photonics Rev., 2023, 17, 2200852 CrossRef CAS
.
- H. M. Chen, M. Lin, C. B. Zhao, D. W. Zhang, Y. Zhang, F. H. Chen, Y. Chen, X. Fang, Q. Liao, H. Meng and M. J. Lin, Adv. Opt. Mater., 2023, 11, 2300365 CrossRef CAS
.
- H. P. Xu, W. Q. Liang, Z. Z. Zhang, C. Cao, W. S. Yang, H. M. Zeng, Z. E. Lin, D. W. Zhao and G. H. Zou, Adv. Mater., 2023, 35, 2300136 CrossRef CAS
.
- Z. Z. Zhang, Z. L. He, J. B. Luo, J. H. Wei, X. X. Guo, J. H. Chen and D. B. Kuang, Adv. Opt. Mater., 2024, 12, 2302434 CrossRef CAS
.
- D. Y. Li, Y. B. Shang, Q. Liu, H. W. Zhang, X. Y. Zhang, C. Y. Yue and X. W. Lei, Mater. Horiz., 2023, 10, 5004–5015 RSC
.
- S. H. Shi, H. H. Yao, D. X. Chen, Z. Z. Li, Z. Xu and Q. Wang, Adv. Opt. Mater., 2023, 11, 2300795 CrossRef CAS
.
- X. C. Wu, Z. Guo, S. Zhu, B. B. Zhang, S. M. Guo, X. H. Dong, L. Q. Mei, R. X. Liu, C. J. Su and Z. J. Gu, Adv. Sci., 2022, 9, 2200831 CrossRef CAS
.
- W. B. Ma, T. M. Jiang, Z. Yang, H. Zhang, Y. R. Su, Z. Chen, X. Y. Chen, Y. G. Ma, W. J. Zhu, X. Yu, H. M. Zhu, J. B. Qiu, X. Liu, X. H. Xu and Y. Yang, Adv. Sci., 2021, 8, 2003728 CrossRef CAS
.
- H. Y. Chen, Q. Wang, G. Q. Peng, S. Wang, Y. T. Lei, H. X. Wang, Z. Yang, J. Sun, N. Li, L. Zhao, W. Lan and Z. W. Jin, Adv. Opt. Mater., 2022, 10, 2102790 CrossRef CAS
.
- M. S. Molokeev, B. B. Su, A. S. Aleksandrovsky, N. N. Golovnev, M. E. Plyaskin and Z. G. Xia, Chem. Mater., 2022, 34, 537–546 CrossRef CAS
.
- Z. Tang, X. Meng, H. Y. Zhao, S. J. Ji, Q. J. Wang, T. X. Bai, R. L. Zhang, J. K. Jiang, C. Katan, J. Even and F. Liu, Adv. Opt. Mater., 2024, 12, 2301282 CrossRef CAS
.
- X. Meng, S. J. Ji, Q. J. Wang, X. C. Wang, T. X. Bai, R. L. Zhang, B. Yang, Y. M. Li, Z. P. Shao, J. K. Jiang, K. L. Han and F. Liu, Adv. Sci., 2022, 9, 2203596 CrossRef CAS
.
- G. D. Zhang, P. P. Dang, H. Xiao, H. Z. Lian, S. Liang, L. Yang, Z. Y. Cheng, G. G. Li and J. Lin, Adv. Opt. Mater., 2021, 9, 2101637 CrossRef CAS
.
- S. H. He, S. Q. Hao, L. B. Fan, K. J. Liu, C. X. Cai, C. Wolverton, J. Zhao and Q. L. Liu, Adv. Opt. Mater., 2023, 11, 2300218 CrossRef CAS
.
- H. X. Meng, B. Chen, W. J. Zhu, Z. J. Zhou, T. X. Jiang, X. W. Xu, S. J. Liu and Q. Zhao, Laser Photonics Rev., 2023, 17, 2201007 CrossRef CAS
.
- Q. S. Hu, C. K. Zhang, X. Wu, G. J. Liang, L. Wang, X. W. Niu, Z. Wang, W. D. Si, Y. B. Han, R. Q. Huang, J. W. Xiao and D. Sun, Angew. Chem. Int. Ed., 2023, 135, e202217784 CrossRef
.
- Y. Zhou, T. Y. He, P. Yuan, J. Yin, S. L. Chen, L. Gutiérrez-Arzaluz, L. J. Wang, O. M. Bakr and O. F. Mohammed, ACS Mater. Lett., 2023, 5, 2002–2008 CrossRef CAS
.
- L. J. Xu, X. S. Lin, Q. Q. He, M. Worku and B. W. Ma, Nat. Commun., 2020, 11, 4329 CrossRef CAS
.
- T. T. Xu, Y. Y. Li, M. Nikl, R. Kucerkova, Z. Y. Zhou, J. Chen, Y. Y. Sun, G. D. Niu, J. Tang, Q. Wang, G. H. Ren and Y. T. Wu, ACS Appl. Mater. Interfaces, 2022, 14, 14157–14164 CrossRef CAS
.
- H. Z. Chen, D. L. Wang, R. X. Hou, D. F. Sun, L. Q. Meng, K. Wu, J. Y. Wang and C. Y. Shen, ACS Appl. Mater. Interfaces, 2024, 16, 10325–10334 CrossRef CAS PubMed
.
- L. Y. Lian, M. Y. Zheng, W. Z. Zhang, L. X. Yin, X. Y. Du, P. Zhang, X. W. Zhang, J. B. Gao, D. L. Zhang, L. Gao, G. D. Niu, H. S. Song, R. Chen, X. Z. Lan, J. Tang and J. B. Zhang, Adv. Sci., 2020, 7, 2000195 CrossRef CAS
.
- Z. Z. Ma, Z. F. Shi, C. C. Qin, M. H. Cui, D. W. Yang, X. J. Wang, L. T. Wang, X. Z. Ji, X. Chen, J. L. Sun, D. Wu, Y. Zhang, X. J. Li, L. J. Zhang and C. X. Shan, ACS Nano, 2020, 14, 4475–4486 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, theoretical, analytical and crystallographic details. CCDC 2361461. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03296a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |