A grain-like cerium oxide nanostructure: synthesis and uric acid sensing application
Rafiq
Ahmad†
 *a,
Sakeena
Masrat†
*a,
Sakeena
Masrat†
 b,
Md. Tabish
Rehman
b,
Md. Tabish
Rehman
 c,
Mohamed F.
AlAjmi
c,
Shamshad
Alam
d,
Prabhash
Mishra
c,
Mohamed F.
AlAjmi
c,
Shamshad
Alam
d,
Prabhash
Mishra
 e and
Byeong-Il
Lee
*fgh
e and
Byeong-Il
Lee
*fgh
a‘New-Senior’ Oriented Smart Health Care Education Center, Pukyong National University, Busan 48513, Republic of Korea. E-mail: ahmadrafiq38@gmail.com
bCentre for Nanoscience and Nanotechnology, Jamia Millia Islamia, New Delhi 110025, India. E-mail: rafia.masrat@gmail.com
cDepartment of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia. E-mail: malajmii@ksu.edu.sa; mrehman@ksu.edu.sa
dDepartment of Pharmacology & Therapeutics, Roswell Park Cancer Institute, Buffalo, New York 14263, USA. E-mail: shamshad.alam@roswellpark.org
eQuantum Materials and Devices Laboratory, Faculty of Engineering and Technology, Jamia Millia Islamia (Central University), New Delhi 110025, India. E-mail: pmishra@jmi.ac.in
fIndustry 4.0 Convergence Bionics Engineering, Pukyong National University, Busan 48513, Republic of Korea. E-mail: bilee@pknu.ac.kr
gDigital Healthcare Research Center, Institute of Information Technology and Convergence, Pukyong National University, Busan 48513, Republic of Korea
hDivision of Smart Healthcare, College of Information Technology and Convergence, Pukyong National University, Busan 48513, Republic of Korea
First published on 6th September 2024
Abstract
Utilizing nanomaterials on the working electrode of sensors enables the fabrication of highly sensitive devices for the detection of various analytes. Herein, a facile synthesis method is used to formulate a grain-like cerium oxide (CeO2) nanostructure. The structural features and surface properties of the synthesized CeO2 nanostructure were studied, which showed that the CeO2 nanostructure exhibited grain-like morphology, good crystalline structure, and excellent vibrational properties. To evaluate the sensing properties of grain-like CeO2 nanostructure, nanomaterial slurry was prepared in butyldiglycol acetate binder. Then, the nanomaterial slurry was drop-casted onto the working electrode of the screen-printed carbon electrode (SPCE) to fabricate the CeO2-modified SPCE sensor. The sensor's electrochemical properties were analysed, which showed excellent charge-transfer behavior compared to the bare SPCE. CV-based electrochemical sensing of uric acid (UA) on a CeO2-modified SPCE sensor exhibited excellent linear performance up to 1070 μM UA. Moreover, the sensor offers good sensitivity, low detection limit, reproducibility, selectivity, and long-term stability. The CeO2-modified SPCE sensor demonstrated a promising application for UA detection in real samples, addressing the need for timely UA concentration monitoring.
1. Introduction
In recent years, electrochemical investigations of uric acid (UA) have garnered significant attention as an important field of study.1 UA is humans’ final metabolite product of purine nucleotide breakdown.2 Notably, at normal pH, UA primarily exists as urate in its ionized form.2 The typical concentration of UA varies across different biological fluids. For instance, it ranges from 100 to 250 μM in saliva, 300 to 500 μM in plasma, and 20 to 25 μM in sweat for a healthy individual.3 It's important to note that several disorders, including gout, hypouricemia, hyperuricemia, UA stone formation, tumor lysis syndrome, diabetes, and Lesch-Nyhan syndrome are associated with deviations from the normal concentration of UA.4 Given the implications of elevated UA levels in various health conditions, routine monitoring of UA levels in saliva, serum, or sweat is crucial for disease prevention, diagnosis, and treatment. Therefore, there is a pressing need to develop precise and accurate methods for quantifying UA concentration in biological systems with high sensitivity and rapid detection capabilities.Currently, research communities employ various conventional methods to determine the concentration of UA. These methods include the enzyme electrode method, capillary electrophoresis, fluorescence spectroscopy, liquid chromatography, and flow injection analysis method.5–10 However, several of these techniques possess significant drawbacks. These shortcomings include laborious sample preparation, protracted and time-consuming procedures, high operational precision requirements, sluggish testing speed, the need for specialized staff, and limited applicability beyond traditional laboratory settings. On the other hand, electrochemical sensing is a preferred choice due to its wide range of applications. Electrochemical sensing offers advantages such as low cost, ease of use, simplicity in concept, rapid analysis, and high sensitivity.11–19 These attributes make electrochemical sensing particularly attractive for UA detection in various settings.
There are two types of electrochemical sensing assays for UA detection: non-enzymatic11–17 and enzymatic.5,6,18,19 In comparison to non-enzymatic sensors, the latter requires the laborious use of enzymes, such as enzyme immobilization, which often results in less repeatable results and a smaller range of detection limits. Additionally, enzymatic sensors tend to be more expensive and require specific environmental conditions (i.e., pH and temperature). Conversely, the increasing popularity of non-enzymatic sensors stems from their simplicity, as they only require the oxidation of UA on an appropriate electrode for detection purposes.20,21 Various electrochemical detection techniques, such as square wave voltammetry (SWV),22,23 amperometric analysis,24,25 differential pulse voltammetry (DPV),26 and cyclic voltammetry (CV),27–32 are employed for non-enzymatic sensors. Among these techniques, CV stands out for its convenience and straightforward operation. Therefore, CV is chosen to assess the sensing performance due to its sensitivity, selectivity, and immunity to interference. Since the components of the electrode primarily influence the electrochemical response, the research community has shown interest in exploring new materials to enhance their sensing capabilities.33
Non-enzymatic sensors based on nanostructured materials offer several advantages over enzyme-based sensors. They are highly stable, reproducible, inexpensive, and unaffected by pH and other environmental factors. Consequently, these nanostructured non-enzymatic sensors have garnered significant scientific interest for various analyte detection.34–39 A wide range of nanomaterials, including manganese oxide (MnO2), copper oxide (CuO), MXene, iron(III) oxide (Fe2O3), carbon black, nickel oxide (NiO), tungsten disulfide (WS2), cobalt oxide (Co3O4), cerium oxide (CeO2), tin(IV) oxide (SnO2), molybdenum disulfide (MoS2), etc., and their nanocomposites (e.g., ZnO-CuO, ZnO-polyaniline, ZnO-Au, ZnO-Fe2O3, ZnO-MWCNTs, etc.), have been employed for the development of electrochemical sensors fabrication.40–50 Among these nanomaterials, CeO2 nanostructures are considered excellent materials for sensing applications due to several inherent properties that enhance their performance.51–59 Firstly, CeO2 has a high surface area-to-volume ratio, which increases the active sites available for chemical reactions, thereby improving sensitivity and detection limits. The unique redox properties of CeO2, characterized by the facile transition between Ce3+ and Ce4+ oxidation states, enable efficient electron transfer, which is crucial for electrochemical sensing. Additionally, CeO2 exhibits excellent chemical stability and resistance to harsh environmental conditions, ensuring long-term durability and consistent performance of the sensor. The oxygen vacancies present in CeO2 nanostructures also play a significant role in enhancing their catalytic activity, making them highly effective for detecting various analytes. These vacancies facilitate the adsorption and desorption of target molecules, further improving the sensor's responsiveness and selectivity. Moreover, the ease of synthesis and the ability to tailor the morphology of CeO2 nanostructures through various fabrication methods allow for the optimization of their electrochemical properties for specific sensing applications. Furthermore, the utilization of screen-printed electrodes (SPEs) further enhances the affordability and reliability of these sensors. The past few years have witnessed a surge in the use of SPEs, which is anticipated to expedite the development of SPE-based electrochemical sensors across various industries, including health monitoring.60
In this study, grain-like CeO2 nanostructure was synthesized using a low-temperature solution route followed by characterization. To fabricate the sensor, the working electrode surface of the screen-printed carbon electrode (SPCE) was modified with grain-like CeO2 nanostructure to detect UA. The interface properties of the bare and grain-like CeO2 nanostructure-modified SPCE were studied by CV and EIS analysis in the redox probe solution. The electrocatalytic activity of grain-like CeO2 nanostructure-based sensor was thoroughly examined with increasing concentrations of UA. Our results demonstrated that grain-like CeO2 nanostructure-modified sensors exhibit excellent electrochemical performance (i.e., good sensitivity and linear range), making them well-suited for detecting UA even in the presence of other interferents. Moreover, fabrication reproducibility, stability, and real sample analysis were examined, highlighting the sensor's excellent stability and practical application in real samples.
2. Experimental section
2.1. Materials
Cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O; extra pure), urea (ACS reagent, 99.0–100.5%), ethanol, potassium hexacyanoferrate(II) trihydrate (K4[Fe(CN)6]·3H2O; 98.5–102.0%), dopamine (DA), potassium hexacyanoferrate(III) (K3Fe(CN)6; ≥99.0%), potassium chloride (KCl; 99.0–100.5%), phosphate-buffered saline (PBS; tablet), butyldiglycol acetate (≥99.2%), UA (≥99%, crystalline), sodium chloride (NaCl; ACS reagent, ≥99.0%), lactic acid (LA; ≥98%), L-Cystine (L-Cyst; ≥98%, crystalline), glucose (≥99%), and fructose (≥99%) were obtained from Sigma-Aldrich (St Louis, MO, USA).2.2. Grain-like CeO2 nanostructure synthesis
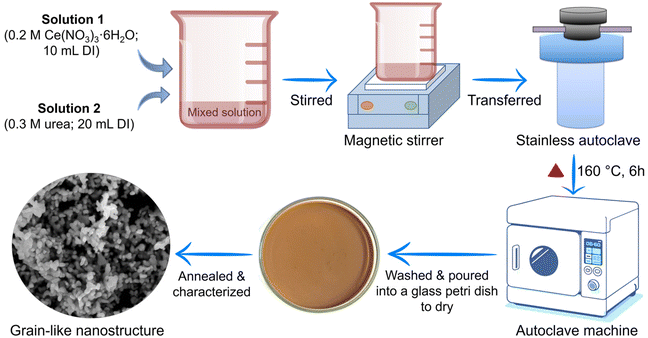
To synthesize the grain-like CeO2 nanostructure, first, 0.2 M Ce(NO3)3·6H2O was dissolved in 10 mL deionized water (DIW) and stirred for 30 minutes. In another beaker, 0.3 M urea was added in 20 mL DIW and stirred for 30 minutes. The prepared urea solution was slowly added to the Ce(NO3)3·6H2O solution to increase the solubility of ceria. The obtained homogeneous solution of Ce(NO3)3·6H2O and urea was poured into an autoclave and heated at 160 °C for 6 hours. A schematic of the synthesis is shown in Fig. 1. After the reaction process, the ivory-colored solution was transferred to centrifuge tubes for centrifugation. The solution was centrifuged with DIW and ethanol to remove impurities. The final precipitate was dried at 60 °C overnight. The resulting fine powder was vacuum annealed at 450 °C for 2 hours and then characterized.2.3. Fabrication of grain-like CeO2 nanostructure-based sensor
The grain-like CeO2 nanostructure suspension was prepared in the 50 μL butyldiglycol acetate binder by mixing 0.01 g of the synthesized material. Grain-like CeO2 nanostructure-modified SPC electrodes were fabricated by drop-casting the different amounts of prepared nanomaterial suspension. The drop-casted CeO2/SPCE sensors were kept to dry at 40 °C overnight. The 5 μL amount of nanomaterial-based sensor resulted in an optimum sensor response, which was used for further analysis.2.4. Characterizations
The structural and morphological analysis of as-synthesized grain-like CeO2 nanostructure was characterized using FESEM (Hitachi S-4700, Hitachi, Tokyo, Japan), TEM (120 kV; Hitachi/HT7800), and XRD (Rigaku, The Woodlands, TX, USA) equipped with 1.54178 Å wavelength Cu-Kα radiation. The XRD scan range was in the range of 10°–90° diffraction angle. The vibrational properties of the grain-like CeO2 nanostructure were analysed using Raman spectroscopy (JASCO/NRS-5100). The Raman analysis was done using an excitation source of 532 nm and an Ar+ laser.The Palmsens4 potentiostat/impedance analyzer (PalmSens, Houten, the Netherlands) was used for electrochemical measurements of the fabricated sensor. The bare and modified SPCE were connected to the instrument using an SPE connector. The CV and EIS were conducted in 5 mM K4Fe(CN)6 and 5 mM K3Fe(CN)6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) having 0.1 M KCl. The EIS measurement was performed at 0.1 V applied potential in a fixed frequency range (0.1 Hz–100 kHz). The electrochemical analysis was performed in PBS solution (pH 7.4).
1 ratio) having 0.1 M KCl. The EIS measurement was performed at 0.1 V applied potential in a fixed frequency range (0.1 Hz–100 kHz). The electrochemical analysis was performed in PBS solution (pH 7.4).
3. Results and discussion
3.1. Characterization of grain-like CeO2 nanostructure
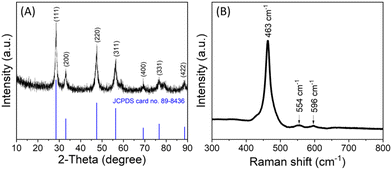
The morphology of the as-prepared CeO2 nanostructures was studied using FESEM. Fig. 2A–D shows the surface morphology of the synthesized CeO2 nanostructures at both low and high magnifications. The results indicate that the synthesized CeO2 nanostructures are small, grain-like in shape, and appear to be agglomerated. This agglomeration may be attributed to the high temperature and duration of the reaction process. TEM images at low and high magnification show the well-designed grain-like shape and confirm the crystalline nature of the synthesized CeO2 nanostructure (Fig. 2E and F). XRD analysis verified the phase purity, crystalline nature, and atomic structure of the CeO2 nanostructures, as shown in Fig. 3A. The XRD spectra reveal that the nanostructures are crystallized in the cubic fluorite phase with Miller indices (111), (200), (220), (311), (400), and (422), consistent with JCPDS card no. 89-8436.51 The sharp and strong peaks observed indicate that the CeO2 diffraction powders have a high crystalline nature. A characteristic high-intensity diffraction peak was observed at 2θ = 28.5°, corresponding to the Miller index plane (111). No other impurity peaks were detected up to 90 degrees, confirming the high phase purity of the synthesized nanomaterial. The XRD pattern confirms that the CeO2 nanostructures were obtained in a single-phase structure.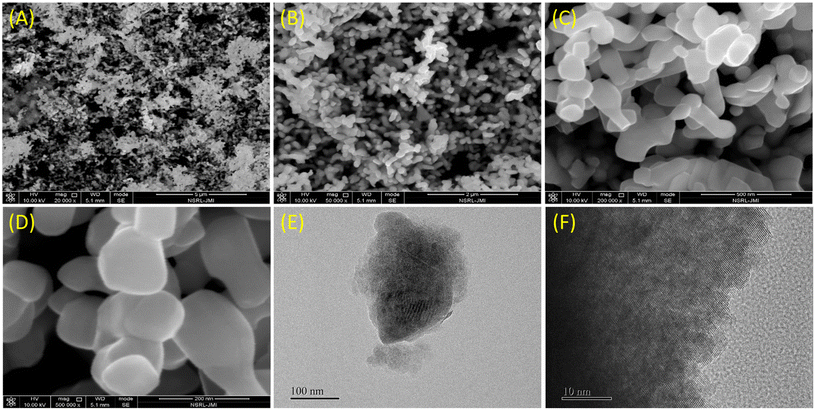 | ||
| Fig. 2 FESEM (A–D) and TEM images (E and F) of grain-like CeO2 nanostructure synthesised using the hydrothermal method at different magnifications. | ||
Furthermore, the Raman scattering analysis of the synthesized grain-like CeO2 nanostructure was characterized, substantiating the CeO2 nanostructure's crystallinity and vibrational properties by displaying the peaks for fluorite crystal structure (Fig. 3B). The Raman spectra exhibit characteristic Raman active modes that are indicative of its crystalline phase and the presence of oxygen vacancies. The most prominent feature in the Raman spectrum of CeO2 is the F2g symmetric stretching mode, which typically appears around 463 cm−1. This mode is associated with the symmetric breathing of the oxygen atoms around the cerium ions in the fluorite structure, reflecting the crystallinity of the material.61 Additionally, the Raman spectrum shows peaks at 554 cm−1 and 596 cm−1 wavenumbers, which are attributed to the presence of oxygen vacancies. These peaks provide information about the defect structure and the level of non-stoichiometry in the CeO2 nanostructures.61,62 The position and intensity of these peaks can vary based on the synthesis method, nanoparticle size, and surface morphology of the nanostructures.
3.2. Optimization of grain-like CeO2-modified/SPCE sensor
Ensuring the appropriate amount of nanostructure material on the working area of the sensor electrode is crucial for optimal performance. To evaluate, we investigated the effect of loading grain-like CeO2 nanostructures onto the working area of the sensor. Various amounts of nanomaterial slurry (2, 5, and 10 μL), prepared in a conductive binder, were applied to the SPCE surface using the drop-casting method. The CV responses of these electrodes were then tested in a probe solution containing 5 mM Fe(CN)3−/4− and 0.1 M KCl at a sweep rate of 50 mV s−1. As shown in Fig. 4A, the sensor fabricated with a 5 μL nanomaterial suspension exhibited the most favorable current response, establishing this quantity as the optimal amount for sensor fabrication and subsequent analysis of its sensing properties. Furthermore, the buffer pH was carefully optimized by analyzing the CV response of the CeO2 nanostructure (optimized 5 μL)-modified SPCE sensor, in the presence of 100 μM UA across a range of PBS buffer pH levels (Fig. 4B). The sensor showed an optimum current response between 7–8 pH. Hence, the physiological pH (7.4) was opted for the CeO2-modified/SPCE sensor analysis.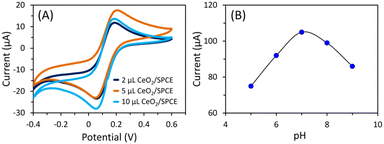 | ||
| Fig. 4 Optimization of the grain-like CeO2 loading amount (A) and pH (B) of the grain-like CeO2-modified/SPCE sensor. | ||
3.3. Electrochemical analysis of grain-like CeO2-modified/SPCE sensor
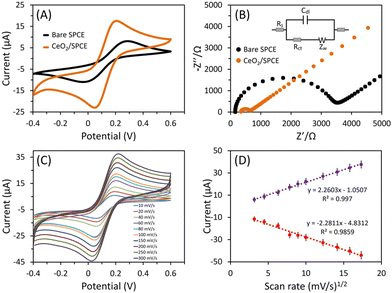
Fig. 5A shows the CV curves of the bare and CeO2-modified SPCE sensor measured in a probe solution of 5 mM Fe(CN)63−/4− and 0.1 M KCl at a sweep rate of 50 mV s−1. From the CV curves, the redox peak potential differences (ΔEp) for bare and CeO2-modified SPCE were 0.30 and 0.15 V, respectively. The anodic peak current (Ipa) of the CeO2-modified SPCE sensor was ∼2.1 times higher compared to bare SPCE. The changes in the ΔEp and Ipa are attributed to the SPCE surface modification with grain-like CeO2 nanostructure. Further, the EIS was used to confirm the CV results. The EIS is useful in characterizing the electron transfer rate over the electrode surface and shows variations in impedance at the electrode interface. Fig. 5B presents the Nyquist plots of bare SPCE and CeO2-modified SPCE. The plots show the distinct semicircular curve observed in the high-frequency region corresponds to the electron transfer process, while the linear portion in the low-frequency region indicates the diffusion-limited process of the electrochemical reaction.63 The Randle circuit (shown in the inset of Fig. 5B) model was used for impedance data fitting. From Nyquist plots, the estimated charge transfer resistance (Rct) value of CeO2-modified SPCE (∼3474 Ω) was lower than the bare SPCE (∼646 Ω). The CV and EIS analysis indicate that the CeO2-modified SPCE have better conductivity than bare SPCE, which is attributed to the conductive and electrocatalytic properties of CeO2.Additionally, the effect of increasing the sweep rates (10–300 mV s−1) for CeO2-modified SPCE on the electrocatalytic efficacy was examined using CV in the probe solution [5 mM Fe(CN)63−/4− and 0.1 M KCl]. Fig. 5C shows the CV response of the CeO2-modified SPCE sensor at different (10–300 mV s−1) sweep rates. With increasing sweep rates, the peak current increases and establishes the linear association with the square root of the sweeping rate (Fig. 5D). The plots of the oxidation and reduction peak current versus the square root of the sweeping rate were linear with excellent correlation coefficient values. This confirms the diffusion-controlled nature of the CeO2-modified SPCE sensors’ electrochemical reaction in the probe solution.64 To illustrate that the SPCE surface modification with CeO2 nanostructure improves the conductivity and response in probe solution, the calibrated linear fitting of current intensity versus square root of sweep rates shown in Fig. 5D was used to calculate the electrochemically active surface area (EASA) with the Randles–Sevcik equation.65 The effective EASA of the fabricated CeO2/SPCE sensor was calculated to be 0.062 cm2, which represented a slight decrease compared to the geometrical area of the bare electrode. However, the CV response represents an improved current response of the CeO2/SPCE sensor compared to the bare SPCE (Fig. 5A).
3.4. Electrochemical sensing of UA
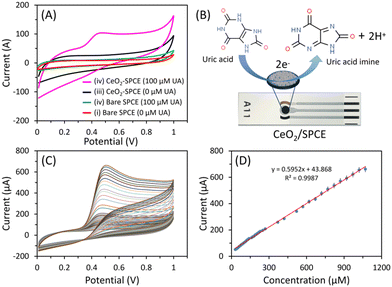
To investigate the electrochemical properties of the bare SPCE and CeO2-modified SPCE sensor, the CV response of the sensors was measured in the absence (curve (i) and (iii)) and presence (100 μM; curve (ii) and (iv)) of UA in a PBS solution (pH 7.4). During bare SPCE analysis in the presence of 0 and 100 μM UA, the CV response showed no response peak (Fig. 6A). However, the CeO2-modified SPCE sensor displayed a prominent oxidation peak at 0.41 V in UA presence, corresponding to UA oxidation. Additionally, irreversible oxidation was observed with a single peak in the CV curve, indicating rapid electron transfer between the SPCE and the grain-like CeO2 nanostructure during the UA detection. The most widely accepted detection mechanism for UA oxidation involves a two-electron/two-proton transfer process (Fig. 6B).53–59Fig. 6C shows the CV responses with increasing UA concentrations in PBS solution (pH 7.4; sweep rate = 50 mV s−1). The peak current increased with excellent linearity over a wide UA concentration range from 30 μM to 1070 μM. Fig. 6D shows the fitted calibration curve of current intensity and UA concentration, which exhibit a linear regression equation (y = 0.5952x + 43.868) with a correlation coefficient (R2) of 0.9987. The sensitivity of the CeO2-modified SPCE sensor was calculated to be 8.38 μAμM−1cm−2 using the slope of the calibration curve 0.5952 μA μM−1 and geometrical area (0.071 cm2) of the working electrode. The LOD was 0.7 μM, based on the signal and noise ratio (S/N) of 3. The sensing properties of the CeO2-modified SPCE sensor are compared with the other works in Table 1. Compared to previous reports, this sensor showed good sensitivity in a wide-linear range.
| Working electrode | Detection method | Sensitivity (μAμM−1 cm−2) | Linear range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|---|
| CeO2 nanoparticles/glassy carbon electrode (GCE) | CV | — | 0.2–500 | 0.10 | 53 |
| CeO2 hollow spheres/GCE | Amperometric | 0.11614 | 1–950 | 0.30 | 54 |
| ZnO-CeO2 hollow nanospheres/GCE | DPV | 0.90853 | 10–1000 | 0.49 | 55 |
| Porous CeO2/CPE | DPV | — | 0.25–10, 10–300 | 0.06 | 56 |
| CeO2 nanocubes/GCE | DPV | — | 10–700 | 4.3 | 57 |
| CeO2 NPs@sulfur-doped scaly carbon/GCE | DPV | — | 0.005–175 | 0.0031 | 59 |
| Co-CeO2 nanoparticles/GCE | DPV | — | 1–2200 | 0.12 | 59 |
| Grain-like CeO2/SPCE | CV | 8.38 | 30–1070 | 0.70 | This work |
3.5. Selectivity, reproducibility, and stability analysis
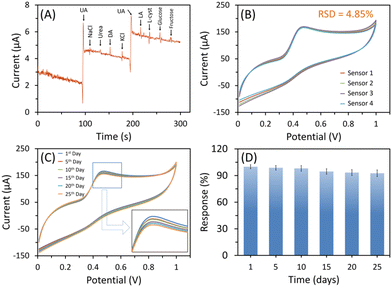
The selectivity of a sensor determines its ability to provide accurate, reliable, and specific measurements in various applications, thereby enhancing its effectiveness and utility. Initially, selectivity was investigated in the presence of ascorbic acid (AA), which often coexists with UA using the CV method. The CV response was measured in the presence of 200 μM UA and then in the presence of 200 μM UA along with 100 μM AA (Fig. 7). With the introduction of AA, there is a negligible increase in the current response. However, recognizing the need for a more precise quantification of the effect of these interfering species, we subsequently employed amperometric studies. The amperometric measurement was performed at the applied potential of 0.41 V with stepwise addition of 25 μM UA and 25 μM of each interferent, as shown in Fig. 8A. The CeO2-modified SPCE sensor responded to UA addition, however, the amperometric response remained unchanged when potential interferents were spiked. This indicates that the CeO2-modified SPCE sensor is selective for UA determination in the presence of potential interferents. Both, CV and amperometric methods, demonstrated outstanding selectivity in the detection of UA.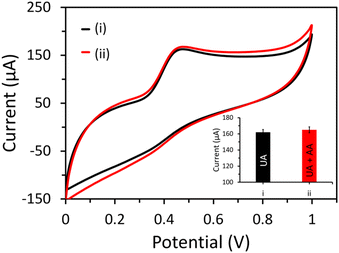 | ||
| Fig. 7 CV response of the CeO2-modified SPCE sensor in the presence of (i) 200 μM UA and (ii) 200 μM UA and 100 μM AA (sweep rate, 50 mV s−1). | ||
The reproducibility of the CeO2-modified SPCE sensor was assessed with four identical fabricated sensors through CV response measurements in 0.1 M PBS having 200 μM UA. Fig. 8B shows an almost similar response with a low relative standard deviation (RSD) of 4.85%, which confirms excellent fabrication reproducibility. Additionally, the stability of the CeO2-modified SPCE sensor was examined for 25 days (Fig. 8C). The sensors were rinsed in DIW, dried, and stored in ambient conditions after every use. The CeO2-modified SPCE sensor retained a 92.6% response after 25 days of storage compared to its initial current response (Fig. 8D). This indicates fabricated sensor is suitable for long-term storage with excellent stability.
3.6. Serum sample analysis
The CeO2-modified SPCE sensor was used to examine the UA in serum samples with spiked known UA concentrations using CV. The commercial serum sample (H4522) was obtained from Sigma-Aldrich. Variations in the UA concentrations were done using the standard addition-based method, where fixed UA concentrations (i.e., 100, 200, 400, and 600 μM) were added to the serum sample. The CeO2-modified SPCE sensor was used to estimate the total UA concentration. The recovery percentage and RSD percentage values for the CeO2-modified SPCE sensor are provided in Table 2. The recovery and RSD rates fell within the 97.8–102 and 2.62–5.34% ranges, respectively. This consistency highlights the successful application of the CeO2-modified SPCE sensor in serum samples.| Sample | UA (μM) added | Estimated (μM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Serum sample | 0 | 280 | — | — |
| 100 | 377.8 | 97.8 | 2.62 | |
| 200 | 478.6 | 99.3 | 3.46 | |
| 400 | 685.6 | 101.4 | 4.28 | |
| 600 | 892 | 102 | 5.34 |
4. Conclusions
In this study, a straightforward synthesis process for grain-like CeO2 nanostructure is used at low temperatures followed by characterization with standard techniques like FESEM, TEM, XRD, and Raman spectroscopy. The FESEM and TEM images confirmed the grain-like morphology of the synthesized CeO2 nanostructure. The XRD and Raman analysis revealed the crystallinity, purity, and vibrational properties of the CeO2 nanostructure. To analyse the non-enzymatic sensing property of the synthesized grain-like CeO2 nanostructure toward UA, the working electrode surface of the SPCE was modified with grain-like CeO2 nanostructure after mixing with conductive binder. The electrochemical investigations of the CeO2-modified SPCE sensor were estimated by employing CV and EIS techniques. These analyses of CeO2-modified SPCE sensors demonstrated excellent charge-transfer behavior compared to bare SPCE. CV measurements at different scan rates confirmed the diffusion-controlled process at the surface of the CeO2-modified SPCE sensor. The fabricated nonenzymatic electrochemical CeO2-modified SPCE sensor was directly employed for UA detection at various concentrations. The excellent UA oxidation and favorable electrical conductivity on the CeO2-modified SPCE sensor resulted in linear performance enhancement up to 1070 μM UA concentration. Also, the sensor displayed good sensitivity and low LOD. Moreover, the excellent reproducibility, selectivity, stability, and real sample application of the CeO2-modified SPCE sensor addresses its suitability and holds promise for future applications. Therefore, grain-like CeO2 nanostructure-based sensor development could be coupled to enzyme functionalization for sensing various analytes.Data availability
All data generated or analysed during this study are included in this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SM would like to thank the Ministry of Education, Government of India, for the Prime Minister's Research Fellows (PMRF) award. M. T. R. and M. F. A. acknowledge the generous support from the Researchers Supporting project number (RSP 2024R122), King Saud University, Riyadh, Saudi Arabia.References
- S. Aafria, P. Kumari, S. Sharma, S. Yadav, B. Batra, J. S. Rana and M. Sharma, Microchem. J., 2022, 182, 107945 CrossRef CAS.
- F. Cortese, P. Giordano, P. Scicchitano, M. F. Faienza, G. D. Pergola, G. Calculli, G. Meliota and M. M. Ciccone, Vasc. Pharmacol., 2019, 120, 106565 CrossRef CAS PubMed.
- K. Shibasaki, M. Kimura, R. Ikarashi, A. Yamaguchi and T. Watanabe, Metabolomics, 2012, 8, 484–491 CrossRef CAS.
- J. Maiuolo, F. Oppedisano, S. Gratteri, C. Muscoli and V. Mollace, Int. J. Cardiol., 2016, 213, 8–14 CrossRef PubMed.
- R. Ahmad, N. Tripathy, M.-S. Ahn and Y.-B. Hahn, Sci. Rep., 2017, 7, 46475 CrossRef CAS PubMed.
- S. H. Han, Y.-J. Ha, E. H. Kang, K. Shin, Y. J. Lee and G.-J. Lee, Sci. Rep., 2022, 12, 12033 CrossRef CAS PubMed.
- O. Moravčík, M. Dvořák and P. Kubáň, Anal. Chim. Acta, 2023, 1267, 341390 CrossRef PubMed.
- F. Li, J. Chen, J. Wen, Y. Peng, X. Tang and P. Qiu, Sens. Actuators, B, 2022, 369, 132381 CrossRef CAS.
- Q. Li, Y. Qiu, W. Han, Y. Zheng, X. Wang, D. Xiao, M. Mao and Q. Li, RSC Adv., 2018, 8, 25808–25814 RSC.
- A. Wong, A. M. Santos, M. H. A. Feitosa, O. Fatibello-Filho, F. C. Moraes and M. D. P. T. Sotomayor, Biosensors, 2023, 13, 690 CrossRef CAS PubMed.
- S. Masrat, V. Nagal, M. Khan, A. Ahmad, M. B. Alshammari, S. Alam, U. T. Nakate, B. Lee, P. Mishra, K. S. Bhat and R. Ahmad, ACS Appl. Nano Mater., 2023, 6, 16615–16624 CrossRef CAS.
- S. Rajendrachari, H. Arslanoglu, A. Yaras and S. M. Golabhanvi, ACS Omega, 2023, 8, 46946–46954 CrossRef CAS PubMed.
- V. Nagal, M. Khan, S. Masrat, S. Alam, A. Ahmad, M. B. Alshammari, K. S. Bhat and R. Ahmad, New J. Chem., 2023, 47, 4206–4212 RSC.
- M. Sun, C. Cui, H. Chen, D. Wang, W. Zhang and W. Guo, ChemPlusChem, 2023, 88, e202300262 CrossRef CAS PubMed.
- Y. Chen, G. Li, W. Mu, X. Wan, D. Lu, J. Gao and D. Wen, Anal. Chem., 2023, 95, 3864–3872 CrossRef CAS PubMed.
- Y. Xia, G. Li, Y. Zhu, Q. He and C. Hu, Microchem. J., 2023, 190, 108726 CrossRef CAS.
- Q. Li, Y. Xia, X. Wan, S. Yang, Z. Cai, Y. Ye and G. Li, Mater. Sci. Eng., 2020, 109, 110615 CrossRef CAS PubMed.
- V. Nagal, V. Kumar, M. Khan, S. Alomar, N. Tripathy, K. Singh, A. Khosla, N. Ahmad, A. K. Hafiz and R. Ahmad, New J. Chem., 2021, 45, 18863–18870 RSC.
- R. Ahmad, N. Tripathy, N. K. Jang, G. Khang and Y.-B. Hahn, Sens. Actuators, B, 2015, 206, 146–151 CrossRef CAS.
- F. Mazzara, B. Patella, G. Aiello, A. O'Riordan, C. Torino, A. Vilasi and R. Inguanta, Electrochim. Acta, 2021, 388, 138652 CrossRef CAS.
- B. Wu, L. Xiao, M. Zhang, C. Yang, Q. Li, G. Li, Q. He and J. Liu, J. Solid State Chem., 2021, 296, 122023 CrossRef CAS.
- M. Arvand, A. Pourhabib and M. Giahi, J. Pharm. Anal., 2017, 7, 110–117 CrossRef PubMed.
- P. Kokoskarova, L. Stojanov, K. Najkov, N. Ristovska, T. Ruskovska, S. Skrzypek and V. Mirceski, Sci. Rep., 2023, 13, 8485 CrossRef CAS PubMed.
- S. Tvorynska, J. Barek and B. Josypčuk, Sens. Actuators, B, 2021, 344, 130252 CrossRef CAS.
- P. E. Erden and E. Kılıç, Talanta, 2013, 107, 312–323 CrossRef CAS PubMed.
- S. P. Selvam, M. Hansa and K. Yun, Sens. Actuators, B, 2020, 307, 127683 CrossRef.
- M. Motshakeri, A. R. J. Phillips and P. A. Kilmartin, Food Chem., 2019, 293, 23–31 CrossRef CAS PubMed.
- D. Dhinasekaran, J. F. John and S. Subashchandran, J. Electrochem. Soc., 2024, 171, 057505 CrossRef.
- S. Zhang, P. Ling, Y. Chen, J. Liu and C. Yang, Diamond Relat. Mater., 2023, 135, 109811 CrossRef CAS.
- T. Wang, Y. Xia, X. Wan, Y. Zhang, N. Chen, Y. Jin and G. Li, Microchem. J., 2024, 201, 110673 CrossRef CAS.
- G. Li, X. Wan, Q. Zheng, M. Yang, Y. Xia, X. Qi, T. Wang and Z. Wu, Colloids Surf., A, 2024, 699, 134713 CrossRef CAS.
- X. Wan, H. Du, D. Tuo, X. Qi, T. Wang, J. Wu and G. Li, ACS Appl. Nano Mater., 2023, 6, 19403–19413 CrossRef CAS.
- L. Chelmea, M. Badea, I. Scarneciu, M. A. Moga, L. Dima, P. Restani, C. Murdaca, D. Ciurescu and L. E. Gaman, Chemosensors, 2023, 11, 341 CrossRef CAS.
- G. Martínez-Saucedo, M. Ugalde-Reygadas, J. J. Alcántar Peña, G. Lastra-Medina, J. Márquez-Marín, G. Torres-Delgado, R. Castanedo-Pérez and I. R. Chávez-Urbiola, Surf. Interfaces, 2023, 37, 102702 CrossRef.
- D. Jiang, T. Liu, Z. Chu and Y. Wang, Anal. Methods, 2023, 15, 6344–6361 RSC.
- S. Masrat, V. Nagal, M. Khan, I. Moid, S. Alam, K. S. Bhat, A. Khosla and R. Ahmad, Biosensors, 2022, 12, 1140 CrossRef CAS PubMed.
- F. Franceschini and I. Taurino, Phys. Med., 2022, 14, 100054 CrossRef.
- X. Niu, M. Lan, H. Zhao and C. Chen, Anal. Chem., 2013, 85, 3561–3569 CrossRef CAS PubMed.
- G. A. Naikoo, H. Salim, I. U. Hassan, T. Awan, F. Arshad, M. Z. Pedram, W. Ahmed and A. Qurashi, Front. Chem., 2021, 22, 748957 CrossRef PubMed.
- D. Thatikayala, D. Ponnamma, K. K. Sadasivuni, J.-J. Cabibihan, A. K. Al-Ali, R. A. Malik and B. Min, Biosensors, 2020, 10, 151 CrossRef CAS PubMed.
- F. Bakhshandeh, H. Zheng, N. G. Barra, S. Sadeghzadeh, I. Ausri, P. Sen, F. Keyvani, F. Rahman, J. Quadrilatero, J. Liu, J. D. Schertzer, L. Soleymani and M. Poudineh, Adv. Mater., 2024, 2313743 CrossRef CAS PubMed.
- M. M. Foroughi, S. Jahani and S. Rashidi, Microchem. J., 2024, 198, 110156 CrossRef.
- V. Nagal, S. Masrat, M. Khan, S. Alam, A. Ahmad, M. B. Alshammari, K. S. Bhat, S. M. Novikov, P. Mishra, A. Khosla and R. Ahmad, Biosensors, 2023, 13, 375 CrossRef CAS PubMed.
- P. Kanagavalli and S. Eissa, Biosens. Bioelectron., 2024, 259, 116388 CrossRef CAS PubMed.
- A. Raucci, M. Metitiero, C. Cuzzi, P. M. Kalligosfyri, M. Messina, M. Spinelli, A. Amoresano, S. L. Woo, I. Cacciotti and S. Cinti, Analyst, 2024, 149, 3302–3308 RSC.
- V. Nagal, T. Tuba, V. Kumar, S. Alam, A. Ahmad, M. B. Alshammari, A. K. Hafiz and R. Ahmad, New J. Chem., 2022, 46, 12333–12341 RSC.
- R. Baretta, A. Raucci, S. Cinti and M. Frasconi, Sens. Actuators, B, 2023, 376, 132985 CrossRef CAS.
- Z. Shi, P. Deng, L. Zhou, M. Jin, F. Fang, T. Chen, G. Liu, H. Wen, Z. An, H. Liang, Y. Lu, J. Liu and Q. Liu, Biosens. Bioelectron., 2024, 251, 116136 CrossRef CAS PubMed.
- A. C. Anithaa, N. Lavanya, K. Asokan and C. Sekar, Electrochim. Acta, 2015, 167, 294–302 CrossRef CAS.
- X. Zhao, P. Pachfule and A. Thomas, Chem. Soc. Rev., 2021, 50, 6871–6913 RSC.
- S. N. Matussin, F. Khan, P. Chandika, M. H. Harunsani, N. Ahmad, Y.-M. Kim, W.-K. Jung and M. M. Khan, ACS Omega, 2024, 9, 157–165 CrossRef CAS PubMed.
- A. A. Atran, F. A. Ibrahim and M. S. Hamdy, Inorg. Chem. Commun., 2024, 163, 112359 CrossRef CAS.
- Y. Wei, M.-G. Li and B. Fang, Chin. J. Chem., 2007, 25, 1622–1626 CrossRef CAS.
- L. Han, R. Liu, C. Li, H. Li, C. Li, G. Zhang and J. Yao, J. Mater. Chem., 2012, 22, 17079–17085 RSC.
- Y. Zhang, X. Yan, Y. Chen, D. Deng, H. He, Y. Lei and L. Luo, Molecules, 2024, 29, 1786 CrossRef CAS PubMed.
- Z. Hashemzaei, H. Saravani, M. Sharifitabar and M. Shahbakhsh, J. Part. Sci. Technol., 2021, 7, 73–82 CAS.
- D. S. Tharani and R. Sivasubramanian, J. Chem. Sci., 2023, 135, 93 CrossRef CAS.
- D. Zhu, X. Li, L. Fu, T. Bai, Q. Wang, C. Ma, X. Sun, L. Lin and X. Li, New J. Chem., 2023, 47, 18712–18720 RSC.
- N. Lavanya, C. Sekar, R. Murugan and G. Ravi, Mater. Sci. Eng., C, 2016, 65, 278–286 CrossRef CAS PubMed.
- A. M. Musa, J. Kiely, R. Luxton and K. C. Honeychurch, TrAC, Trends Anal. Chem., 2021, 139, 116254 CrossRef CAS.
- N. Kainbayev, M. Sriubas, D. Virbukas, Z. Rutkuniene, K. Bockute, S. Bolegenova and G. Laukaitis, Coatings, 2020, 10, 432 CrossRef CAS.
- M. Scavini, F. Bertolotti, J. Mlloja, F. Umbri, A. Bosc, S. Cappelli, S. Checchia, C. Oliva, P. Fumagalli, D. Ceresoli, M. Longhi, A. Guagliardi and M. Coduri, Nanomaterials, 2022, 12, 3385 CrossRef CAS PubMed.
- N. Hallemans, D. Howey, A. Battistel, N. F. Saniee, F. Scarpioni, B. Wouters, F. L. Mantia, A. Hubin, W. D. Widanage and J. Lataire, Electrochim. Acta, 2023, 466, 142939 CrossRef CAS.
- L. Zhang, M. Yin, J. Qiu, T. Qiu, Y. Chen, S. Qi, X. Wei, X. Tian and D. Xu, Biosens. Bioelectron.: X, 2022, 10, 100102 CAS.
- R. Ahmad and B. I. Lee, Chem. Eng. J., 2024, 492, 152432 CrossRef CAS.
Footnote |
| † These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2024 |