Open Access Article
Open Access ArticleDecoding eumelanin's spin label signature: a comprehensive EPR analysis
João V.
Paulin
 *a,
Carlos F. O.
Graeff
*a,
Carlos F. O.
Graeff
 *a and
A. Bernardus
Mostert
*a and
A. Bernardus
Mostert
 *b
*b
aSão Paulo State University (UNESP), School of Sciences, Department of Physics and Meteorology, Bauru/SP, Brazil. E-mail: jv.paulin@unesp.br; carlos.graeff@unesp.br
bDepartment of Physics and Centre for Integrative Semiconductor Materials, Swansea University Bay Campus, Fabian Way, Swansea, SA1 8EN, UK. E-mail: a.b.mostert@swansea.ac.uk
First published on 9th January 2024
Abstract
Eumelanin is a black-brown natural pigment found throughout nature. As a material class, it possesses unique physical and chemical properties that entice much attention from chemists, physicists, and material scientists due to its potential use in medical and sustainable organic (bio)electronics settings. To harness its potential, accurate spectroscopical characterization is a primary requirement for the efficient engineering of eumelanin-based electronic devices. A key spectroscopic technique is electron paramagnetic resonance (EPR) since the persistent paramagnetism of eumelanin is a spin-label signature of its molecular state. This review provides a comprehensive discussion on the origin of eumelanin paramagnetism through a multifrequency EPR approach and highlights the current multi-species point-of-view.
1. Introduction
Nature has employed organic matter as the base to develop complex systems capable of performing sensing, energy storage, light-harvesting, photoconversion, and free-radical scavenging. Inspired by these functional materials and their properties, humankind has been able to build all kinds of (bio)devices ranging from sensors, solar cells, ion-to-electron transducers, memory, and energy storage.1–7Among a wide variety of candidates, melanins are one of the most thought-provoking bio-sourced materials in nature. It is a class of biological pigments of diverse chemical structures originating in animals from the oxidation and polymerization of tyrosine or in lower organisms from phenolic compounds.5,8–10 In humans, the melanin class is split into black-brown eumelanin, reddish-yellow pheomelanin, and neuromelanin (spherical aggregates consisting of eumelanin encasing a central core of pheomelanin). These subclasses are variously responsible for antioxidant and anti-inflammatory functions as well as protection from harmful UV-light.8,9,11 In addition, they are also of high medical relevance due to the potential relation to diseased states, such as melanoma (a type of skin cancer formed in the epidermal melanocytes),12–14 Parksinson's disease15–19 and Alzheimer's disease.19,20
Melanins, especially the main subclass eumelanin, are also receiving attention in chemistry, physics, and materials science due to potential technological applications in biomedicine, organic electronics, and bioelectronics.5,6,9 Indeed, several devices’ platforms, such as electrochemical transistors,21–23 energy storage,24–27 memory,28 optoelectronic skins,29 phototransistors,30 and sensors,31–35 can be found in the literature. The variety of applications is attributable to the ability to form smooth and homogenous thin films35–37 and to many relevant physicochemical properties, including UV-Vis broadband optical absorption, photoluminescence quantum efficiency to almost null, metal-ion chelation, radical scavenging, redox activity, paramagnetism, and hydration-dependent charge-transport.5,6,8,9,11,38–40
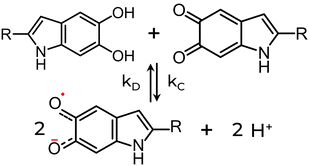
The physicochemical functionalities result from the highly heterogeneous nature of eumelanin's molecular and supramolecular structural features at the nano- and microscale. At the molecular level, the eumelanin class is assembled by chemically heterogeneous oligomers made up of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA).5,6,9,10 These monomer building blocks can undergo two processes of one-electron one-proton removal to be reversibly oxidized into their ortho-quinone forms and tautomers, Fig. 1a.10,41,42 Synthetic intermediate residues such as uncyclized and charged structures (like the protonated nitrogen,5,43Fig. 1b) can also be found in eumelanin structure. These structures bind together, forming graphene-like layers of up to tens of units (Fig. 1c, Levels 1 & 2). Such layers are then self-assembled into nanoaggregates with different extensions via apparent π–π stacking and H-bonding in a combination of planar and twisted segments (Fig. 1c, Level 3).38 The characteristic stacking distances of ∼3.2–4.0 Å depend on hydration level and type of eumelanin.44–48 Finally, the small aggregates agglomerate via edge-to-edge stacking (Fig. 1c, Level 4).
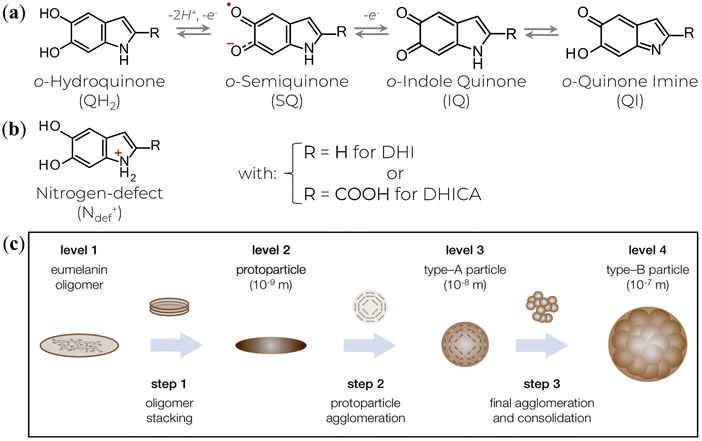 | ||
| Fig. 1 (a) The various redox states and tautomers of eumelanin monomer building blocks. (b) A charged structure formed during the synthesis and aggregation steps. (c) The current three-step version for eumelanin particle formation. (c) is a reprint from A. Büngeler et al.49 under Creative Common CC BY license. | ||
The high structural disorder and the low solubility hindered the design and engineering of eumelanin-based materials in earlier days due to challenges in precise characterization with traditional molecular chemical and physical methodologies. However, modern approaches indicate that UV-Vis, X-ray photoelectron spectroscopy (XPS), and electron paramagnetic resonance (EPR) techniques are the best primary tools for eumelanin's core characterization.6
Of particular interest to the present work, the EPR spectroscopic approach relies on eumelanin's strong and persistent electronic paramagnetic signal to obtain evidence of its structural and dynamic processes. Indeed, since the first report of eumelanin’ EPR signal in 1954,50 numerous papers have covered a wide range of paramagnetic responses to physical parameter changes. However, the precise nature of the paramagnetic signal in eumelanin still needs to be clarified. This review aims to provide a comprehensive understanding of the eumelanin paramagnetic system using multifrequency EPR in both solution and solid-state. The in-depth analysis presented herein will illuminate the current state-of-the-art knowledge of eumelanin's paramagnetic signal, including its origins and potential future research directions.
2. Overview of EPR spectroscopy
EPR is a spectroscopy technique used to probe systems with paramagnetic centers (i.e., unpaired spin states) such as free-radicals, ionic radicals, and molecules in triplet states. It can explore the chemical structure of these species, their interactions with the chemical environment, and the dynamics of processes in which they may be involved.51–55This technique is based on the absorption of electromagnetic radiation, usually in the microwave frequency region, by a paramagnetic sample in the presence of an external magnetic field. The presence of the external magnetic field is commonly a necessary condition for the occurrence of the Zeeman effect, which is the splitting of paramagnetic levels for the different spin states of a sample; in other words, the magnetic field breaks the degeneracy of the spin states. Nonetheless, zero-field EPR signals are observed in specific cases.56
As a quantum mechanical construction, EPR spectroscopy uses the spin Hamiltonian to describe all the magnetic interactions of the spin with its surrounding environment. For a paramagnetic species at the ground energetic state with an electron spin S and n nuclei of spin I, the static spin Hamiltonian (![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) 0) will be a contribution of the electronic spin with the external magnetic fields and internal magnetic moments57 given by,
0) will be a contribution of the electronic spin with the external magnetic fields and internal magnetic moments57 given by,
![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) 0 = 0 = ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) EZ + EZ + ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) HF + HF + ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) NZ NZ | (1) |
The different terms in eqn (1) indicate the electron Zeeman interaction (![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) EZ), electronic and nuclear spins hyperfine couplings (
EZ), electronic and nuclear spins hyperfine couplings (![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) HF), nuclear Zeeman interactions (
HF), nuclear Zeeman interactions (![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) NZ). For the following term descriptions, we will show conventional forms utilizing the Cartesian spin vector operator ST = (Sx, Sy, Sz)T, with T indicating the matrix transpose.
NZ). For the following term descriptions, we will show conventional forms utilizing the Cartesian spin vector operator ST = (Sx, Sy, Sz)T, with T indicating the matrix transpose.
The electron Zeeman term (eqn (2)), which represents the interaction between the electron spin and the external magnetic field B,52–55,58 is the dominant term of ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) 0.
0.
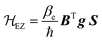 | (2) |
In eqn (2), 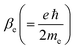 is an atomic unit of the magnetic moment called the Bohr magneton. The parameters e, ℏ and me represent the values of the electron fundamental charge, the reduced Planck's constant (i.e.,
is an atomic unit of the magnetic moment called the Bohr magneton. The parameters e, ℏ and me represent the values of the electron fundamental charge, the reduced Planck's constant (i.e., 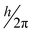 ) and the electron mass respectively. S represents the spin angular momentum. Since S and B are orientation-dependent, the magnetic dipole moment g (denoted by g-factor or g-value) will assume the general form of a tensor that in the radical molecular coordinate system results in eqn (3) or (4) if the orientation-dependence is averaged by fast molecular motion (i.e., isotropic g-value).53,55
) and the electron mass respectively. S represents the spin angular momentum. Since S and B are orientation-dependent, the magnetic dipole moment g (denoted by g-factor or g-value) will assume the general form of a tensor that in the radical molecular coordinate system results in eqn (3) or (4) if the orientation-dependence is averaged by fast molecular motion (i.e., isotropic g-value).53,55
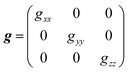 | (3) |
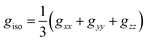 | (4) |
The interaction between the magnetic dipole of unpaired electrons and the nuclear spin in its vicinity is characterized by the hyperfine interaction ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) HF (eqn (5)).52–55,59 This interaction brings direct information about the magnetic environment of the spin.
HF (eqn (5)).52–55,59 This interaction brings direct information about the magnetic environment of the spin.
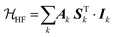 | (5) |
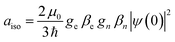 | (6) |
Like the electron Zeeman splitting, the nuclear Zeeman term (eqn (7)) describes the coupling of the nucleus spin with the external magnetic field.53,55,59 However, it can be a few orders of magnitude lower than the ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) EZ due to the smaller gyromagnetic ratio.59
EZ due to the smaller gyromagnetic ratio.59
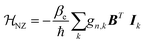 | (7) |
Experimentally, a static magnetic field is varied across the sample to align the electronic spin in a specific direction, while a perpendicular microwave frequency is kept fixed. Hence, the microwave's absorption will only occur when the energy hν of the radiation coincides with the energy difference (ΔE) between the spin states,51–53 causing the spin to flip in the opposite direction of the polarizing field. The resonance condition (eqn (8)) determines the energy needed for this spin flip.
| hυ = ΔE = Eα − Eγ = geβeB0 | (8) |
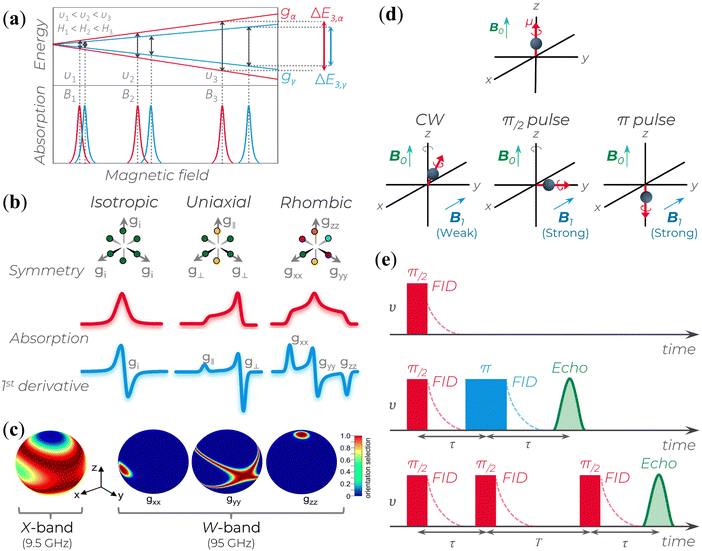 | ||
| Fig. 2 (a) Splitting of the energy levels of a two-spin system with isotropic lineshapes and similar g-values. At υi, the microwave frequency (or the corresponding Bi magnetic field), the two EPR lines almost completely overlap; as υi (or Bi) increases, the two lines get more separated. gα and gγ represents the g-values of two different spins. The splitting behavior represents the enhanced spectral resolution of high-frequency/high-field EPR (HFEPR). (b) Schematic relation of a powder paramagnetic system based on different symmetry condition and their respective absorption and first derivative EPR spectra for S = ½. (c) Enhanced orientational selectivity of disordered materials by HFEPR. It is possible to determine the orientations of the dominant interaction tensor in spin systems that are randomly oriented and have a small g-anisotropy as long as the anisotropy of the primary interaction in the spin Hamiltonian exceeds the inhomogeneous linewidth. Reprinted with permission from K. Möbius et al. (2013).58 Copyright© 2013, Elsevier. (d) B0 indicates the direction of the polarizing field along which a magnetic dipole moment becomes aligned. The B1 field induces magnetic interactions. B1 applies a torque to the electron's magnetic dipole, resulting in a deviation from the B0 axis. This deviation can be small (in CW experiments) or large (in pulse experiments). If the magnetic moment is aligned with or opposite to the B0 field (as in π pulse case), there is no gyroscopic precession around the z-axis due to the electron spin. (e) Three common signal types generated in pulse EPR experiments: (top) free-induction decay (FID), (middle) echoes generated using a two-pulse sequence, and (bottom) echoes generated using a three-pulse sequence. | ||
As mentioned above, due to the spin–orbit coupling of the electron residing on a molecule, its g-value will deviate from the ge and can display an anisotropic character; that is, the system will undergo different interactions of the magnetic field depending on the molecule's orientation axis. As a result, different g-values for each molecular axis will be obtained, i.e., gxx≠ gyy≠ gzz (rhombic symmetry). However, in many organic materials, paramagnetic systems have little spin–orbit interaction, and the value of the effective g-value is independent of the magnetic field direction. When the effective g-value is identical across all sample axes, gxx= gyy = gzz = gi, it is said to have isotropic symmetry.51–53,55 An intermediary condition is also possible. In this case, the symmetry is uniaxial, with gxx = gyy ≠ gzz or gxx≠ gyy= gzz. Here, the unique g-value is ascribed as “g‖” (g parallel), whereas the other two are “g⊥” (g perpendicular). The overall absorption shape will be altered depending on the symmetrical orientations (Fig. 2b).
The microwaves’ absorption is obtained by varying the magnetic field during data collection. Empirically, this absorption results in a Gaussian-like line shape shown in Fig. 2b. However, since the measurement is carried out with a modulated oscillating magnetic field and lock-in detection, the signal obtained corresponds to the first derivative of the absorption (Fig. 2b).51–53 Based on this representation, the main experimental parameters are the signal lineshape, the number of peaks, the resonant magnetic field B0 position, the linewidth, and the peak-to-peak signal amplitude.
At low microwave power (P), the EPR signal intensity (Y) can increase proportionally to 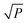 due to the absence of perturbation on the equilibrium of the spin state population. However, as P continues to increase, the signal eventually reaches a saturation point, after which it may either continue to increase or start to decrease.51,52,61–63 This behavior is described by eqn (9).
due to the absence of perturbation on the equilibrium of the spin state population. However, as P continues to increase, the signal eventually reaches a saturation point, after which it may either continue to increase or start to decrease.51,52,61–63 This behavior is described by eqn (9).
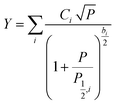 | (9) |
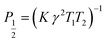 | (10) |
In addition to the experimental parameters and spectral behavior, another helpful parameter is the spin concentration in a sample. For the spin concentration determination, the signal from the studied sample is compared to a standard model containing a known spin number to minimize errors.64 In this way, the spin concentration (ρN) can be estimated using eqn (11), where it considers the area ARC under the resonance curve, a constant CE specific to each spectrometer, the temperature T, the modulation amplitude AM, and the applied microwave power P.52,64
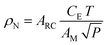 | (11) |
As eqn (11) is based on the double integral of the EPR spectra first-derivative, it is expected to obtain good baselines on both sides of the spectrum (i.e., low and high-field sides). Indeed, a constant baseline is required or, at least, a baseline correction after each integration.52,64 Additionally, extended measurements far from the signal center should be used to minimize potential error resulting from finite truncation, especially for the extensive wings of Lorentzian-shaped lines.52
The standard X-band EPR spectrum has limited resolution for disordered materials, and the broad lines may hide information on molecular orientation and magnetic parameters.58 Hence, higher microwave frequencies and higher magnetic fields (above W-band) are often employed, Table 1.
| Microwave band | υ (GHz) | B 0 (mT) gCCR = 2.0032 | B 0 (mT) gSFR = 2.0045 |
|---|---|---|---|
| L | 1 | 35.670 | 35.644 |
| S | 4 | 142.667 | 142.575 |
| X | 9 | 321.001 | 320.793 |
| K | 24 | 856.003 | 855.448 |
| Q | 35 | 1248.338 | 1247.528 |
| U | 50 | 1783.340 | 1782.183 |
| V | 65 | 2318.342 | 2316.838 |
| E | 75 | 2675.010 | 2673.275 |
| W | 95 | 3388.346 | 3386.148 |
| F | 111 | 3959.015 | 3956.447 |
| D | 140 | 4993.352 | 4990.113 |
| — | 190 | 6776.692 | 6772.297 |
| — | 265 | 9451.702 | 9445.572 |
| J | 285 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165.038 165.038 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158.445 158.445 |
| — | 360 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840.048 840.048 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831.720 831.720 |
High-frequency/high-field EPR (HFEPR) has two important characteristics:
(i) Enhanced spectral (or g-value) resolution: g-values are directly proportional to the EPR spectrometer microwave frequency. Hence, a higher external Zeeman field will be able to increase the resolution of the g-values in relation to the hyperfine coupling (Fig. 2a and Table 1) since it can separate the field-dependent spin interactions from the field-independent ones.
(ii) Enhanced orientational selectivity in disordered samples: materials with different spin–orbit coupling can yield different responses with respect to the Zeeman-field. Hence, as the external magnetic field is increased, a specific orientation relative to the high field is obtained, which enables differentiation of the g-tensor anisotropy (Fig. 2c), i.e., the orientation of their main axes within the g-matrix system.
Additional advantages of HFEPR over standard EPR measurements are the enhancement in detection sensitivity for restricted-volume materials, enhancement for probing fast motion dynamics, and enhancement of low-temperature electron-spin polarization. For a detailed explanation of these particularities, the authors suggest to the readers the references.55,58,59,65
One efficient manner to obtain an EPR signal is through continuous wave (CW) methods, in which microwave or radio frequency (rf) fields are continuously applied. However, a drawback to this approach is that the resulting spectra have complete ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) 0 information and often manifest overlapping contributions that are not easily resolved into individual components.66,67 Therefore, pulse EPR is a powerful tool to precisely manipulate the spin system by applying microwave pulses of a specific frequency to induce transitions that contain only the desired
0 information and often manifest overlapping contributions that are not easily resolved into individual components.66,67 Therefore, pulse EPR is a powerful tool to precisely manipulate the spin system by applying microwave pulses of a specific frequency to induce transitions that contain only the desired ![[script letter H]](https://www.rsc.org/images/entities/i_char_e142.gif) 0 contribution of one particular spin system.66,67
0 contribution of one particular spin system.66,67
During pulse EPR experiments, the spin evolution of the electron is recorded after applying a strong B1 field for brief durations, typically ranging from tens to hundreds of nanoseconds, while maintaining a constant external magnetic field B0. Compared to CW EPR, the B1 pulses used are considerably more intense, allowing for a more pronounced tilting of the electron's magnetic moment into the xy plane in a short period of time (Fig. 2d). This tilting enables the precession and dephasing of electron spins around the z-axis within the same plane. To obtain the description of the spin evolution under the influence of applied pulses using quantum mechanics, visit the fundamental works.51,66,67 Differences in the structural features of molecules that contain the unpaired spin or variations in the spin states of nearby magnetic nuclei can perturbate the precession frequency of each spin, leading to changes in the g and hyperfine coupling values.
In pulse EPR, there are several variations to generate and detect signals. The signal can be detected when the spin magnetic moments precess in the xy plane and add up to a total non-zero magnetic moment. A free induction decay (FID) signal can be observed after a π/2 pulse is applied along the x-axis. In this situation, the magnetic moment will gradually diminish in the y direction due to the different rates of the electron spin precession. This decrease is the FID (Fig. 2e, top). However, as a single microwave pulse is often insufficient to excite the broad spectra of a short-lived FID, a second pulse followed by a time τ twice as long as the first (Fig. 2e, middle) is applied to rephase the spins. This results in an echo signal that can be detected and analyzed. A stimulated echo can also be obtained using a third pulse at any time T after the second one (Fig. 2e, bottom). In a typical pulse EPR, the integrated FID or integrated echo intensity is collected as a function of another experimental variable (usually the magnetic field B0).
The relaxation behavior (T1 and T2) are fundamental limitations in pulse EPR experiments, as T1 dictates the speed at which an experiment can be repeated, and T2 affects the persistence of the spin-echo, restricting the resolution of frequencies and the distances that the technique can measure.
For a detailed interpretation of the EPR signals, simulations can be used to accurately determine critical spectral features such as the g and hyperfine values. Among the various computational tools available, EasySpin is commonly used and is available as free software.68,69 This computational package has several high-level functions that separate the resonance lines (g-value, linewidth, and intensity) of the paramagnetic species present in the studied systems and thus identify their spectroscopic characteristics. Not only that, but it is also possible to simulate EPR spectra with isotropic and/or anisotropic g-values, different line forms and linewidths, hyperfine interactions, and an arbitrary number of electronic and nuclear spins of samples in several physical states (powders, crystals or liquids) and experimental conditions (continuous or pulsed wave, low or high temperature).68 WinSim,70 XSophe,71 and SpinDynamica72 are other available options. In-house written simulation programs are also a possibility.
3. X-band EPR signal overview of eumelanin
The X-band EPR signal of eumelanin is usually reported by a slight asymmetric spectrum with a width of about 4–6 G, g-value ranging from 2.003–2.006, and no observable hyperfine coupling (Fig. 3a). Additionally, such spectra features are intriguing by being unusually broad for an organic radical and by being remarkably similar in different experimental conditions (hydrated suspensions or solid-state) and sample source (natural or synthetic). This behavior made the EPR technique an efficient tool for identifying different melanins (eu- and pheomelanin) in pigmented systems (e.g., eye, hair, and skin).73,74 The difference that allows such differentiation arises from the 1,4-benzothiazine subunits in pheomelanin, which provides a distinct hyperfine coupling from a partial localization of the unpaired electron on the nitrogen atom of the o-semiquinonimine (Fig. 3b and c).75 The featureless EPR signal of eumelanin is the result of inhomogeneous broadening of the lineshape that hinders the amount of information one can get, especially the hyperfine interactions from immobilized randomly oriented o-semiquinone radicals76 and the various hyperfine couplings of the protons in the eumelanin subunits.77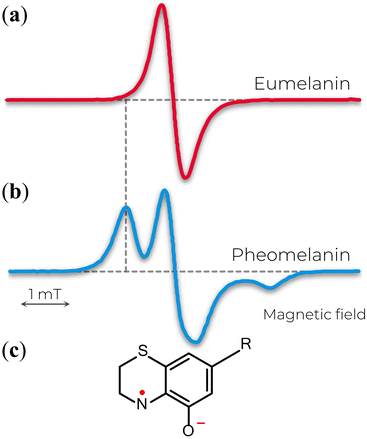 | ||
| Fig. 3 X-band EPR spectra of (a) eumelanin and (b) pheomelanin. The extra feature in b is attributed to the nitrogen hyperfine coupling of (c) o-semiquinonimine. (a) Adapted with permission from J.V. Paulin et al. (2019).78 Copyright© 2019, American Chemical Society. (b) Adapted with permission from A. Zadlo et al. (2018).79 Copyright© 2018, John Wiley & Sons. | ||
The nature of the EPR signal of eumelanin has been speculated in the seminal paper of M.S. Blois and co-workers back in 1964.80 They proposed three possibilities: transition element ions, semiconductor-like behavior, and triplet-singlet band overlap. The first case was quickly ruled out by the similarities between natural and ultra-pure synthetic eumelanin (Fig. 4, as examples),80–82 which indicates that the unpaired electrons should arise from a common origin. The semiconductor model was rejected as the free-radical concentration in the dry state does not follow an exponential function with temperature, implying that the thermally excited electrons cannot be the origin of the eumelanin resonance.80 In the same line, the observed g-values suggest that the unpaired electron would be restricted to one or two monomeric units.83 In the case of the triplet–singlet band model, an extensive conjugation length would be a necessary condition; however, this is not the case. The EPR signal did not suffer any alteration when ascorbic acid was used to reduce the conjugation, and the spin–lattice relaxation values were large, which would be counter-intuitive if dealing with conjugation. Also, a higher concentration of cupric ions can quench the eumelanin's signal. Such a behavior would be an odd feature if they were just splitting the overlapping bands.80 Later studies on temperature-dependent EPR measurements on eumelanin hydrated suspension agree that the spectroscopy features (g-value) do not fit with the triplet idea.84 Instead, it suggests that the unpaired electron should be restricted to a few monomeric units in radical or biradical states.80,84 The idea of the eumelanin's paramagnetism being part of monomeric units remains strong to the current date, and several models were built on it.
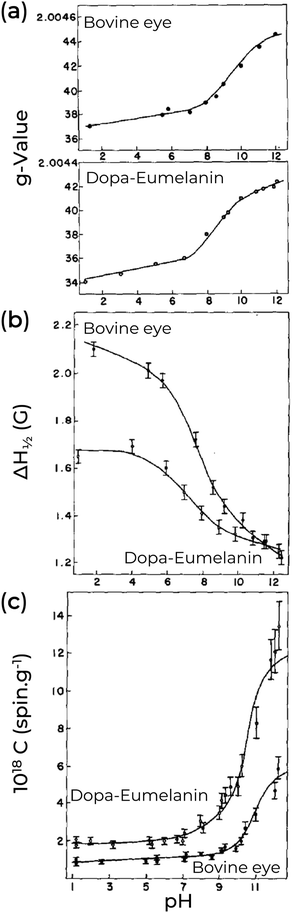 | ||
| Fig. 4 Effect of pH on (a) g-value, (b) linewidth, and (c) spin concentration of natural Bovine eye pigment (majoritarian eumelanin) and synthetic Dopa-Eumelanin. (a)–(c) Adapted with permission from S-S Chio et al. (1982).82 Copyright© 1982, Elsevier Inc. | ||
A key point of eumelanin's EPR signal is that it is extremely persistent; however, the free-radical concentration is not. Indeed, only the degradation of the eumelanin backbone structure was able to quench its EPR signal irreversibly.80 As an example of changes in the EPR signal, we turn our attention to the pH effect of Dopa-Eumelanin shown in Fig. 4. As the pH increases from low (pH 1) to high (pH 12), the g-values range from 2.0034 to 2.0042, and the EPR signal becomes narrower and more asymmetric.82 Additionally, it is possible to observe variation in the spin concentration (2.0 × 1018 at pH 2 to 1.2 × 1019 at pH 12).82 Similar changes can be seen in Cu2+-eumelanin complexes where the free radical's g-value and intensity show similar pH dependent behavior as for the uncomplexed material.85 However, a key difference is that the line shape broadens due to the presence of the Cu2+.85 This allows investigation of how the radical centers bind to metal cores (see below). Other external agents can also change the eumelanin EPR signal, as presented in Table 2. In Table 3, we provide a concise overview of the prevailing EPR signal behavior corresponding to the commonly utilized external agents.
| Eumelanin | Experimental condition | Physicochemical agent | Spectral alteration | Ref. |
|---|---|---|---|---|
FM: Field modulation; ΔYPP: peak-to-peak height; MA: Modulation amplitude; MWP: microwave power; DMPO: 5,5-dimethyl-l-pyrroline-1-oxide; ΔH½: first-derivative peak’ half-width at half-height of the positive-signal component; P½: microwave power at which the saturation effect reduces the signal intensity by half; PBS: Phosphate-buffered saline; DMPO: 5,5’-dimethylpyrroline-1-oxide; EDFS: Echo detected field sweep; PFSR: Picket fence saturation recovery; CCR: carbon-centered radical; SFR: semiquinone free-radical; 4-MeO-PhN2BF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-methoxybenzenediazonium tetrafluoroborate; RH: relative humidity.a Presumably, but not inferred in the paper. 4-methoxybenzenediazonium tetrafluoroborate; RH: relative humidity.a Presumably, but not inferred in the paper. |
||||
| Bovine eye Eumelanin | - CWa | Visible light (not specified) | - Reversible photo induced radical observed. | 86 |
| - 9.5 GHz | ||||
| - Photo induced radical stabilized in alkaline solution. | ||||
| - FM: 100 kHz | ||||
| - 296 K | ||||
| - Suspension | ||||
| Bovine-eye (Primarily eumelanin) | - CWa | Visible light (not specified) | - Reversible ΔYPP increase with light. | 87 |
| - 9.5 GHz | - Increase in signal after a plateau in pH curve. | |||
| - FM: 100 kHz | pH (pH 1 to pH 10.5, both light and dark) | - Additional increase in signal with light. | ||
| - Dark/light | - Reversible increase in signal with increasing O2. | |||
| Dopa-Eumelanin | - Aqueous suspensions | Dissolved aqueous O2 (0–100 %) | - No change in signal for dried bovine-eye sample. | |
| - ∼ pH 7 | ||||
| Dopa-Eumelanin | - CWa | Light (Dopa: Visible Light at 200 W cm−2; Human skin pigment: 600–700 nm at 200 W cm−2; Black hair: Visible Light at 300 W cm−2) | MWP data shows an increase, saturation and then a decrease in signal intensity. | 88 |
| Human skin pigment | - 9.3 GHz | |||
| - FM: 100 kHz | - Power saturation curves are offset by a constant (on a log scale) to a higher signal intensity after irradiation of all samples. | |||
| - Black human hair eumelanin | - Suspensions (Dopa) | |||
| - Solid State (Skin and Hair) | - Wet hair showed a bigger signal increase after irradiation vis-à-vis dry sample. | |||
| - 100 K (Dopa) | - Microwave Power | - Wet sample had lower signal intensity than dry sample before irradiation. | ||
| - 77 K (Skin and Hair) | - Hydration (Black human hair: Dry and wet) | |||
| - Aqueous suspensions | Dissolved aqueous O2 (0–100 %) | - No change in signal for dried bovine-eye sample. | ||
| - ∼ pH 7 | ||||
| Squid-ink (Logigo opalescens) in Calcium-Magnesium salt form (“A-melanin”) | - CWa | - Metal ion (Cu2+) | - Generally, auto-oxidized and enzyme-oxidized materials spectra taken at 295 K fell within a giso of ∼2.0036–2.0040. | 80 |
| - 9–10 GHz | - Generally, eumelanins from natural sources have a broader range of giso values of 2.0030–2.0048. | |||
| Squid-ink (Logigo opalescens) Calcium & Magnesium removed (“B-melanin”) | - FM: 100 kHz | - Temperature (4.2 K, 77 K, 295 K, 450 K, 500 K) | - A-melanin lineshape in-between Gaussian and Lorentzian shapes, with a slight asymmetry. | |
| - MWP: 4–100 mW (most experiments) | - A-melanin giso is pretty insensitive to temperature. Line width increases with lowering temperature. | |||
| - MWP: 10–1000 μW (B-melanin, at 77 & 295 K) | - Hydration (Dry & Wet [0–30% by weight]) | - A-melanin giso slight increase with Cu addition, with a significant increase in line width. | ||
| Harding-Passey mouse melanome | - Powders | - Ink from Logigo opalescens has a lower spin concentration than Sepia officinalis. | ||
| UV irradiated (55 min, unfiltered) | - B-melanin and Cu-doped A-melanin exhibit Curie–Weiss law temperature dependence. | |||
| Human hair-eumelanin (alkaline extraction) | - B-melanin line width broadens with decreasing temperature. | |||
| - T2 relaxation times are similar between all samples and MWP insensitive (CW-EPR inferred). | ||||
| Human hair-eumelanin (intact hair) | - T1 relaxation times for A & B melanins varied depending on MWP. | |||
| - T1 relaxation times for A-melanin decreased with increasing copper content. | ||||
| Potato-eumelanin | - T1 relaxation times for B-melanin increased with decreasing temperature. | |||
| - There is an effect on T1 relaxation times due to hydration. | ||||
| o-hydroquinone (catechol)-Eumelanin (auto-oxidation and enzyme oxidized) | - Decrease in signal amplitude with increased Cu2+ concentration for A-melanin and hydroquinone. Most detail on A-melanin. | |||
| - Decrease in signal for Cu-doped A-melanin when hydrated. | ||||
| p-hydroquinone-eumelanin (auto-oxidation and enzyme oxidized) | ||||
| m-hydroquinone (resorcinol)-eumelanin (auto-oxidation and enzyme oxidized) | ||||
| L-Dopa-Eumelanin (auto-oxidation and enzyme oxidized) | ||||
| D-Dopa-Eumelanin (auto-oxidation and enzyme oxidized) | ||||
| L-adrenalin-eumelanin (auto-oxidation and enzyme oxidized) | ||||
| Bovine-eye (With protein (A-type) and without protein (B-type)) | - CWa | - Metal ions (Mn2+, Cu2+, Ni2+, Co2+, Gd3+, Ho3+, Tm3+, Dy3+, Er3+, Nd3+, Sm3+, Pr3+) | - Decrease in signal amplitude with increasing concentration. | 89 |
| - 9–10 GHz | - Increase in P½ with increasing concentration. | |||
| - Aqueous suspensions | - Effects are more muted in B-type vs. A-type. | |||
| - 295 K | - Binding of Gd3+ preferential to eumelanin vs. ethylenediaminetetraacetic acid. | |||
| Bovine-eye | - CWa | - Metal ions (Mg2+, Ca2+, Sr2+, Zn2+, Cd2+, Al3+, Sc3+, La3+, In3+) | - Increase in signal amplitude. | 90 |
| - 9.5 GHz | - Higher g-value for Bovine vs. dopa samples. | |||
| - FM: 100 kHz | - Reversible complexation of metal ions with the eumelanin. | |||
| - MWP: 20 μW to 200 mW | - Generally, no to small lower changes in g-values relative to Bovine melanin upon complexation | |||
| Dopa-Eumelanin | - Aqueous suspensions | - Generally higher g-values for synthetic samples upon complexation. | ||
| - pH 5.5 | - Predominantly an increase in linewidth upon complexation. | |||
| - 77 K and room temperature | - Generally, a decrease in P½ for bovine eumelanin upon chelation. | |||
| - Similar P½ values were observed for Dopa-Eumelanin upon chelation. | ||||
| - Smallest increases in signal are for alkaline earth metal ions. | ||||
| Dopa-Eumelanin | - CWa | Microwave Power | - MWP saturation point with associated high power signal attenuation increases with oxygen content in solution. | 91 |
| - 9–10 GHza | - O2 (Oxygenated, aerated & deoxygenated) | - Line shape independent of oxygen content. | ||
| - MWP: Power saturation experiment | - UV-Visible Light (Filtered light of 320-600 nm) | - With irradiation, oxygenated samples’ power saturation point decreases. | ||
| - 298 K | - Spin Traps (Superoxide dismutase, catalase, DMPO) | |||
| - Suspensions | ||||
| Bovine eye eumelanin (with protein) | - CWa | - pH (acidic (0.5 M in HCl) and basic (0.5 M in KOH)) | - ΔH½ and spin lattice relaxation time increase with decreasing temperature. | 92 |
| - 9–10 GHza | - P½ decreases with decreases with temperature. | |||
| - FM: 100 kHz | - Metal ion content (0.5 M Zn2+) | - Free radical concentration did not change with temperature. | ||
| Bovine eye eumelanin (reduced protein content) | - Solid state (powder) | - ΔH½ and P½ decrease going from solid to suspension (any suspension). | ||
| - Suspensions | - Temperature (123 K, 193 K, 303 K) | - pH had greater effect on ΔH½ than Zn2+. | ||
| - Power saturation recovery experiments | - Spin concentration: alkaline > solid > Zn2+ > pH 6 > acidic. | |||
| - ΔH½ increase going from material with protein to without protein. | ||||
| - Protein free had higher spin concentration. | ||||
| Bovine-eye | - CWa | UV-vis light (320-600 nm) | - Increase in g-values and lineshape. | 93 |
| Dopa-Eumelanin | - Time-resolved EPR | - Significant enhancement on microwave saturation characteristics. | ||
| - Dark/Light | ||||
| - Deoxygenated aqueous suspension | ||||
| - pH 7 | ||||
| - 77 K and 353 K | ||||
| Bovine-eye | - CWa | - Temperature (77–353 K) | - Spin concentration is temperature-dependent, with a decrease with increasing temperature. Arrhenius behavior in liquid solution. There is no change when the solution is frozen. | 84 |
| - 9–10 GHz | - ΔH½ is temperature-independent for frozen suspensions. | |||
| - FM: 100 kHz | - ΔH½ decrease for T > 273 K. | |||
| Dopa-Eumelanin | - Aqueous suspensions | |||
| - pH 7 | ||||
| Catechol-Eumelanin | - CWa | - pH (pH 2 to pH 10.7) | - g-value increases from pH 2.0 to pH of 10.7. | 85 |
| - 9–10 GHza | - ΔYPP has slow decrease with pH and then under alkaline conditions (> pH 8) rapidly increases up to 5 times to initial acidic signal. | |||
| - FM: 100 kHz | - ΔH½ increases with pH under concentrated acidic conditions and then levels off. | |||
| - MA 5 G | - Dopa-Eumelanin has an overall broader signal compared to Catechol-Eumelanin. | |||
| - MWP: 5 mW | - P½ decreases with increasing pH, with a similar qualitative trend in size as ΔH½. | |||
| Dopa-Eumelanin | - 77 K | |||
| - Complexed to Cu2+ | ||||
| - Suspensions | ||||
| Dopa-Eumelanin (neat, oxidized, reduced and methylated) | - CWa | - pH (pH 5.5 to pH 12) | - Eumelanin consumes oxygen, especially SFR. | 94 |
| - 9–10 GHza | - O2 (Native consumption monitored) | |||
| Bovine eye eumelanin (with protein and reduced protein content) | - FM: 100 kHz | - Spin Traps (3-carbamoyl-2,2,5,5-tetramethyl-3- pyrroline-1-yloxy) | ||
| - MA: 1.6 G | - Visible light (power 360 W m−2) | |||
| - MWP: 1 mW | ||||
| - 296 K | ||||
| - Suspensions | ||||
| Bovine-eye | - CWa | - pH (pH 1 to pH 12) | - Increase in free-radical concentration after a plateau. | 82 |
| - Temperature (278–353 K) | ||||
| - 9.3 GHz | - g-value and concentration increase with pH. | |||
| Dopa-Eumelanin | - FM: 100 kHz | - ΔH½ decreases with pH. | ||
| - MWP: 50 μW | - Radical concentration higher in Dopa-Eumelanin vs. Bovine-eye Eumelanin. | |||
| - Aqueous suspensions | - Apparent Arrhenius behavior. | |||
| - 296 K (pH isotherm results) | - Increasing Arrhenius constant with increasing pH. | |||
| - 278–353 K | - Increasing enthalpy with pH up to neutral, then there is a slight decrease. | |||
| - pH 1 to 12 | ||||
| Human hair-Eumelanin | - CWa | - UV-vis light (280–560 nm) | - Increase in the signal intensity. | 95 |
| - 9.52 GHz | - At low temperatures, the signal decreases with increasing temperature (Curie–Weiss law). | |||
| - FM: 100 kHz | - Temperature (∼120–500 K) | - Signal increases at higher temperatures. | ||
| - MA: 0.5–2 G | - Low pH, decrease in signal intensity, line width remains same vis-à-vis neutral. | |||
| - MWP: 7.6 mW | - pH (Low, neutral and high) | - High pH, increase in signal intensity, line width increases. | ||
| - KBr pellets | - In an aqueous solution, the signal increases, and there is no change in line width. | |||
| - Initial 296 K | pH (pH 0 to pH 13) | |||
| - Poly or monochromatic | ||||
| - Aqueous solution | ||||
| Bovine-eye | - CWa | UV-vis light (230-600 nm) | - Increase in signal intensity. | 96 |
| - 9.5 GHz | - Increasing O2 content does not lead to an increase in radical production. | |||
| - FM: 100 kHz | - Radical formation is higher for natural eumelanin | |||
| - MA: 8 G | - Radical production is more effective at shorter wavelengths. | |||
| - MWP: 1 mW | - Proteins in natural eumelanin had no effect in generating light-induced radicals. | |||
| Dopa-Eumelanin | - 296 K | |||
| - Dark/Light | ||||
| - Aqueous suspension | ||||
| - pH 7.6 | ||||
| Bovine-eye | - CWa | - UV-vis light (250 and 544 nm) | - Increase in free-radical concentration. | 97 |
| Dopa-Eumelanin | - Aqueous suspension | |||
| - pH 7.6 | ||||
| Dopa-Eumelanin (auto-oxidation and enzyme oxidation) | - CWa | - UV-Visible Light (230-580 nm) | - Rate of reaction increases with pH. | 98 |
| Bovine-eye Eumelanin | - 9–10 GHza | - Spin Traps (Nitroxides) | - Oxidation and reduction of the eumelanins under light conditions depending on wavelength and spin trap compound. | |
| - FM: 100 kHz | - pH (pH 5 to pH 10) | |||
| - 298 K | ||||
| - Initial suspensions in PBS (pH 7.6) | ||||
| Dopa-Eumelanin | - CWa | - pH (pH 3 to pH 12) | - Increase in linewidth and g-values. | 99 |
| - 35 GHz | - Low anisotropy in acid and neutral pH. | |||
| - Aqueous suspensions | - Moderate anisotropy at higher pH. | |||
| - 233 K | - Increase in total spin concentration with pH. | |||
| Dopa-Eumelanin (auto-oxidation and enzyme oxidation) | - CWa | - Superoxide anion (Potassium superoxide in dimethyl sulfoxide) | - Higher spin concentration for Dopa-Eumelanin vs. Bovine-eye eumelanin. | 100 |
| - 9.5 GHz | - pH (pH 7.5 to pH 9.7) | - Increase in linewidth and g-values. | ||
| Bovine-eye eumelanin | - FM: 100 kHz | - Increase in EPR signal amplitude. | ||
| - MWP: 1 mW (eumelanin radicals), and 20 mW (Phosphate buffer solution; 0.1 M, pH 8.0). | - Superoxide-induced eumelanin radical formation rate following: auto-oxidized eumelanin > enzyme-oxidized eumelanin > bovine eumelanin. | |||
| - Rate of formation is linearly dependent on eumelanin and superoxide concentrations. | ||||
| - Rate of formation increased with pH. | ||||
| - Increase in radical concentration as a function of Mg2+ concentration. | ||||
| - The rate of induced radicals reduces in the presence of Mg2+. | ||||
| - Similar effects seen in Ca2+ and Zn2+ as for Mg2+. | - 296 K | |||
| Human red hair | - CWa | - Relative Humidity (Dry, 32.3%, 58% and 79.8%) | - Radical concentration decreases with increasing humidity. | 101 |
| - 9.75 GHz | - Visible light (UV and infrared filtered, 50 mW.cm−2) | - Light induced radical concentration increases with increasing humidity. | ||
| -298 Ka | - Decay of light induced radicals increases with increasing humidity. | |||
| - Solid state | ||||
| Dopa-Eumelanin | - CWa | - 4-MeO-PhN2BF4 | - Increase in relative (i.e., vs. 4-MeO-PhN2+) signal amplitude with increasing concentration for [4-MeO-PhN2+] < 2 mM. | 102 |
| - 9 GHz | - [4-MeO-PhN2+] > 2 mM, relative EPR signal amplitude reaches a plateau. | |||
| - FM: 100 kHz | ||||
| - MA: 3.3 G | ||||
| - MWP: 1 mW | ||||
| - Time constant: 0.25 s | ||||
| - Scan rate: 4 min | ||||
| - Phosphate buffer solution (50 mM, pH 7.0) | ||||
| - 296 K | ||||
| Dopa-Eumelanin | - CWa | - UV light (366 nm) | - Benzophenone absence: reversible increase of eumelanin free-radical. | 103 |
| - X-Band | - Benzophenone presence: unstable but persistent formation of photoinduced radicals even after the light is turned off; increase in signal intensity. | |||
| - FM: 100 kHz | - Radicals can be destroyed with continuous illumination. | |||
| - MA: 0.5–3 G | ||||
| - MWP: 1 or 10 mW | ||||
| - Dark/Light | ||||
- DMPO and benzophenone in water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), deaerated 1), deaerated |
||||
| Dopa-Eumelanin | - CWa | Visible Light (> 300 nm) | - Increase in signal intensity upon illumination. | 104 |
| - 9.4 GHz | ||||
| - FM: 100 kHz | ||||
| - Suspension at pH 4.6 | ||||
| - 296 Ka | ||||
| Dopa-Eumelanin (Centrifuged from pH 7.6) | - CWa | - Microwave Power (Attenuation 20 to 0.5 dB) | - Slight decrease in g-value, ΔH½, radical concentration and spin lattice relaxation from neutral sample to acid treated sample. | 105 |
| Dopa-Eumelanin (Acid treated and washed) | - 9.3 GHz | - Slight increase in spin–spin relaxation time. | ||
| - FM: 100 kHz | - No clear discernable difference in power saturation. | |||
| -MWP: Power saturation experiment | ||||
| - 298 K | ||||
| - Powder samples | ||||
| Dopa-Eumelanin | - CWa | Temperature (333–373 K) | - Increase in g-values and linewidth. | 106 |
| - 9.1 GHz | - Increase in spin-concentration and tend towards saturation with time. | |||
| - Powder | - Decrease of bounded water mass. | |||
| DHI-Eumelanin | - CWa | pH (5.59–11.02) | - ΔYPP increases with pH. | 107 |
| - 9–10 GHza | - g-value shifts to higher values with increasing pH. | |||
| - Suspension | - Line asymmetry become more pronounced with increasing pH. | |||
| - 10 K | ||||
| Dopa-Eumelanin | - CWa | - Light with time (390 nm–520 nm, 0.4 and 0.24 W cm−2) | - Signal intensity decreased with time, i.e., photo bleaching was achieved over 200 h. | 108 |
| - Human RPE Eumelanin | - 9–10 GHza | |||
| - FM: 100 kHz | ||||
| - MA: 2.0 G | ||||
| - MWP: 20 μW | ||||
| - 283 K | ||||
| - Suspension | ||||
| Dopa-Eumelanin | - CWa | - Metal ion (Cu2+ and Zn2+ content varied) | - a systematic higher g-value when Cu2+ present, but no trend in concentration. | 109 |
| - 9.3 GHz | - g-value does not significantly change with Zn2 addition. | |||
| - FM: 100 kHz | - Increased ΔH½ with Cu2+ and Zn2+ addition | |||
| - MWP: 0.7 mW and power saturation experiment | - Systematic decrease in spin concentration with increasing Cu2+. | |||
| - Solid-State | - Systematic increase in spin concentration with increasing Zn2+. | |||
| - 298 K | - Power saturation observed for neat material, and then a decrease of signal intensity with power. | |||
| - Line width of neat material increased linearly with MWP½. | ||||
| - Intensity increased linearly with MWP½ for Cu2+ containing material. | ||||
| Human-eye (eumelanin and low concentration of pheomelanin) | - CWa | - UV light (355 nm) | - Increase in signal intensity. | 110 |
| - Time-resolved EPR | - Slight increase in linewidth. | |||
| - 9–10 GHz | - Negative baseline after light illumination observed, which recovers to the original baseline with time. | |||
| - Dark/Light | ||||
| - Oxygenated & deoxygenated aqueous solution. | ||||
| Dopa-Eumelanin | - CWa | - Temperature (180–470 K) | - Sample was tested as prepared, heat treated to 373 K, cooled, and then tested. | 111 |
| - 9.5 GHz | - Heat Treatment/Annealing (373 K) | - Increase in g-values and linewidth after treatment. | ||
| - Powder | - Decrease in EPR signal intensity for T < 350 K. | |||
| - Wet and dried | - Increase in EPR signal intensity for T > 350 K. | |||
| - For wet sample at low temperatures, temperature dependence obeys Curie–Weiss law, i.e., signal decrease with increasing temperature (180–350 K) | ||||
| - Signal increases at higher temperatures (350-470 K), linear in T−1. | ||||
| - The dry sample has a relatively higher signal than the wet; it obeys the Curie–Weiss law (470–240 K). | ||||
| Dopa-Eumelanin | - CWa | - No specific variable | - g-value = 2.004. | 112 |
| - 9.5 GHz | - ΔH½ = 0.45 mT. | |||
| - FM: 100 kHz | ||||
| - MA: 0.1 mT | ||||
| - MWP: 5 mW | ||||
| - 298 K | ||||
| - Solid-State | ||||
| DHI-Eumelanin | - CWa | - DHI vs. DHICA starting material. | - g-values remained the same for poly-DHI and poly-DHICA. | 38 |
| DHICA-Eumelanin | - 9 GHz | - Desiccated vs. Lyophilized | - ΔH½ not affected by preparation state, but it is broader for poly-DHI compared to poly-DHICA. | |
| - FM: 100 kHz | - Preparation state did not show discernable trend in spin concentration changes. | |||
| - MA: 2.0 G | - Systematic decrease in spin concentration from poly-DHI to poly-DHICA. | |||
| - MWP: 0.6 mW and power saturated experiment | - Poly-DHICA power saturates under lower microwave power and then decreases quickly. In contrast poly-DHI power saturates at high microwave power and then decreases slowly. | |||
| - 298 K | ||||
| - Solid-State | ||||
| Dopa-Eumelanin (acidic, neutral and alkaline) | - CW | - pH (Acidic, neutral and alkaline)Hydration (0 to 80% RH) | - Acid and neutral signal inferred to be dominated by CCR. | 113 |
| - 9–10 GHz | - Alkaline signal has an additional feature, inferred as an SFR. | |||
| - FM: 100 kHz | - Spin concentration is modulated by pH. | |||
| - MA: 0.036 mT | - Decrease in signal intensity with hydration. | |||
| - MWP: 0.2 μW to 2 mW | - ΔYPP decreases with the increase in hydration for acidic and neutral samples. | |||
| - Solid-state | - ΔYPP is independent of hydration for alkaline samples. | |||
| - Vacuum | - Spin concentration is modulated by hydration. | |||
| - H2O vapor pressure | ||||
| Dopa-Eumelanin (acidic, neutral and alkaline) | - CW | - Hydration (0 to 80% RH) | - Decrease in EPR signal intensity with hydration. | 114 |
| - 9–10 GHz | - pH (Acidic, Neutral & Alkaline) | - ΔYPP decreases with the increase of hydration for acidic and neutral samples. | ||
| - FM: 100 kHz | - ΔYPP is independent of hydration for alkaline samples. | |||
| - MA: 0.036 mT | - Spin concentration is modulated by hydration. | |||
| - MWP: 2 μW to 2 mW | - Line width narrows with hydration for high pH sample. Probable reduction in hyperfine interaction with deuterium addition. | |||
| - Solid-state | ||||
| - D2O vapor pressure | ||||
| - 296 K | ||||
| Dopa-Eumelanin | - CW | - pH (Neutral and alkaline) | - Neutral EPR signal dominated by CCR (lower g-value inferred). | 115 |
| - 9–10 GHz | - Hydration (0 to 80% RH) | - Alkaline EPR signal has an additional feature at a high g-value, inferred as SFR. | ||
| - FM: 100 kHz | - Optical Light (White LED spectrum) | - Ammonia treatment and MWP increase the relative strength of SFR. | ||
| - MA: 0.25, 0.36 or 9.8 G | - Sample probed at low MWP gave a negative photo response when dry. The photo signal becomes systematically positive with increasing hydration. It is inferred as CCR behavior. | |||
| - MWP: 0.063, 2, 199.2 or 585 mW | - Sample probed at high MWP gave positive photo response in dry and wet conditions. Inferred as SFR behavior. | |||
| - Solid-state | ||||
| - Vacuum | ||||
| - 296 K | ||||
| Tyrosinase Natural Eumelanin | - CWa | - Multifrequency experiment | - At 3.9 GHz: Natural eumelanin has a higher g-value vs. synthetic material. | 116 |
| Tyrosinase Synthesized Dopa-Eumelanin | - 3.9 GHz | - At 9.8 GHz: ΔH½ is larger for the natural eumelanin vs. synthetic material. | ||
| -Tv laccase Synthesized Dopa-Eumelanin | - FM: 100 kHz | |||
| - MA: 0.2 mT | ||||
| - MWP: 1.90 mW | ||||
| - 296 K | ||||
| - Solid state (powder) | ||||
| Tyrosinase Natural Eumelanin | - CWa | - Multifrequency experiment | - Complex spectra vs. S and X band data. Consistent with multiple species but not sufficient to resolve the species spectra. | 116 |
| Tyrosinase Synthesized Dopa Eumelanin | - 33.9 GHz | |||
| Tv laccase Synthesized Dopa Eumelanin | - FM: 50 kHz | |||
| - MA: 0.2 mT | ||||
| - MWP: 0.06 mW | ||||
| - 296 K | ||||
| - Solid state (powder) | ||||
| Dopa-Eumelanin | - CW | - Hydration (0 and 80% RH) | - Decrease in EPR signal intensity with hydration. | 117 |
| Oxidized Dopa-Eumelanin | - 9.3 GHz | - g-value statistically independent on hydration. | ||
| - FM: 100 kHz | - ΔYPP decreases with hydration at low MWP. | |||
| - MA: 0.025 mT | - ΔYPP increases with hydration at high MWP. | |||
| - MWP: 0.03 and 10 mW | - Linewidth decreases with hydration. | |||
| - Solid-state (Pellets) | ||||
| Sulphonated-Eumelanin | - CW | - Hydration (0 and 80% RH) | - EPR signal intensity is independent of hydration. | 117 |
| Oxidized sulphonated-Eumelanin | - 9.3 GHz | - g-value independent on hydration. | ||
| - FM: 100 kHz | - ΔYPP decreases with hydration at low MWP. | |||
| - MA: 0.025 mT | - ΔYPP increases with hydration at high MWP. | |||
| - MWP: 0.03 and 10 mW | - Linewidth is independent of hydration. | |||
| - Solid-state (Pellets) | ||||
| Dopa-Eumelanin | - CW | - Microwave Power (max. 144.5 mW) | - Signal increase with microwave power until saturation and then decreases. | 118 |
| - 9.871 GHz | ||||
| - MWP: Power saturation experiment (max. 144.5 mW) | ||||
| - 296 K | ||||
| - Solid-state (powders) | ||||
| Dopa-Eumelanin | - CW | - Microwave Power (max. 6.3 mW) | - Signal increase with microwave power until saturation and then decreases. | 118 |
| - 33.843 GHz | ||||
| - MWP: Power saturation experiment (max. 6.3 mW) | ||||
| - 296 K | ||||
| - Solid-state (powders) | ||||
| Dopa-Eumelanin | - Pulsed | - Temperature (20–110 K) | - T1 relaxation time decreased with increasing temperature. | 118 |
| - 34 GHz | ||||
| - EDFS: π/2 – τ – π sequence (π/2 = 42 ns, π = 84 ns) | ||||
| - PFSR | ||||
| - Initial 296 K | ||||
| - Solid-state (powders) | ||||
| Black Soldier Fly (Hermetia illucens) Eumelanin | - CWa | - No specific variable | - Slight asymmetric signal. | 119 |
| - 9 GHz | ||||
| - Solid-State and PBS Solution | ||||
| Physicochemical agent | Spectral alteration |
|---|---|
| — | - Voight-like line shape with slight asymmetry |
| - Homogeneous power saturation | |
| - Broader range of g-values for natural sourced eumelanin compared to synthetic derivative | |
| - g-values remained the same for poly-DHI and poly-DHICA. | |
| - Broader ΔH½ and higher spin concentration for poly-DHI compared to poly-DHICA. | |
| Hydration | - Hydration-dependent spin concentration/signal intensity |
| - g-value statistically independent on hydration. | |
| - ΔYPP increases with hydration at high MWP | |
| - ΔYPP decreases with hydration at low MWP (& acidic and neutral materials) | |
| - ΔYPP is independent of hydration for alkaline samples | |
| - Linewidth decreases with hydration | |
| Light irradiation | - Reversible increase signal amplitude |
| - g-values increases | |
| - Lineshape increases | |
| - Signal amplitude increases | |
| - Enhancement of saturation characteristics | |
| - Radicals can be destroyed with continuous illumination | |
| - More effective at shorter wavelengths | |
| Metal ions | - Type of ions influences signal amplitude and P½ |
| - Increase in linewidth | |
| - Reversible behavior | |
| pH | - increasing pH: g-value increases, concentration increases, ΔH½ decreases, and line asymmetry gets more pronounced |
| Temperature | - Arrhenius behavior in solution |
| - Curie–Weiss law behavior in solid-state and frozen solutions | |
In light of the monomer-based perspective, the changes in the EPR signal can be attributed to a comproportionation equilibrium reaction, as depicted in Scheme 1. This reaction describes interactions between fully reduced and fully oxidized subunits forming an intermediate semi-reduced/semi-oxidized state.82,90 External agents, some of which are listed in Table 2, can influence or alter this equilibrium by changing the forward (comproportionation) and reverse (disproportionation) reaction rate constants (kC & kD, respectively). In the case of eumelanin, the radical is the intermediate oxidative product. One way more radicals can be generated, or the reaction rates modified, is by increasing the pH as deprotonation shifts the equilibrium towards the product, or SQ radicals.42,120
Diamagnetic metal ions such as zinc (Zn2+) and cadmium (Cd2+) can also generate additional radicals. They can form chelate complexes with the radicals, essentially removing them from the product “ledger” of the comproportionation reaction, leading to further SQ formation. In contrast, paramagnetic metal ions, such as copper (Cu2+), iron (Fe3+), or manganese (Mn2+), can dramatically reduce eumelanin's radical EPR signal intensity.80 The origin of the quench was not ascribed to a chemical reaction with eumelanin radicals but rather to the magnetic nature of the metal ions.89 Indeed, it was observed consistent changes in amplitude (with no apparent differences in the linewidth) and microwave power saturation with the type and concentration of lanthanide ions with similar chemical properties but different magnetic properties, like Gadolinium (Gd3+; paramagnetic) and Lanthanum (La3+; diamagnetic). For instance, the addition of Gd3+ almost totally reduced the free-radical signal amplitude, whereas no significant signal alteration was obtained with La3+.
Sarna et al. showed experimentally that Leigh's theory could be employed to explain the effect of transition metal ions on the radical signal mentioned above.89 Leigh's approach considers magnetic dipoles fixed in space to a system composed of a metal ion in the vicinity of a radical. The interaction between the metal ion and the radical will induce a dipolar broadening of the narrow radical EPR signal, and the magnitude will depend on the ion's concentration. Additionally, the spin–lattice relaxation of the metal ion will modulate the interaction of the magnetic dipole by efficiently decreasing the dipolar broadening of the radical EPR signal.89,120,121 Consequently, the higher the spin–lattice relaxation rate of the metal ion, the weaker the dipolar broadening; therefore, the smaller the decrease in the radical EPR signal intensity. In eumelanin's case, the Leigh-type effect decreases for metal ions with a short T1, followed by an increase in the free-radical P½. Except for Gd3+, the lanthanide metals analyzed (and especially Dysprosium Dy3+ and Thulium Tm3+) increased P½ due to their high magnetic moments and 2πυT1 ≈ 1. Nonetheless, it should be noted that a recent study on Cu-doped eumelanin shown that a chemical reaction with eumelanin radicals (eqn (12)) can also lead to a loss of signal.22
| Cu2+-complex + Anionic SQ ⇄ Cu1+-complex + IQ | (12) |
Eumelanin EPR spectra analysis was also used to determine the molecular nature of the binding sites on natural and synthetic models using 63Cu2+ as a molecular probe. Depending on the system's pH, the several functional groups in eumelanin lead to different copper complexes.85,120,122 At pH < 7, the binding of cupric ions in eumelanin is predominantly to monodentate carboxyl complexes and bidentate nitrogen-carboxyl groups in the synthetic models only (Fig. 5a and b). The corresponding EPR spectral parameters for the ions were g‖ = 2.26–2.34, A‖ = 460–560 MHz and g⊥ = 2.066–2.076. At 7 ≤ pH ≤ 11, the preferential binding site was to bidentate hydroxyl, but tri- or even tetradentate oxygen and nitrogen complexes can also happen (Fig. 5c and d). At these mild alkaline conditions, g‖ = 2.24–2.26, A‖ = 560–579 MHz, and g⊥ = 2.054–2.064. Above pH 11, Cu2+-eumelanin complexes with multiple monomeric units could be obtained. The spectroscopic parameter would be g‖ = 2.18, A‖ = 610–620 MHz, and g⊥ = 2.050.
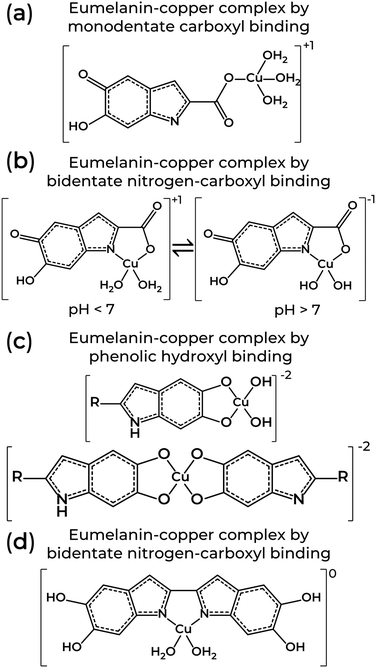 | ||
| Fig. 5 Structures of eumelanin-copper coordination involving one or two monomeric units from eumelanin through (a) monodentate carboxyl, (b) bidentate nitrogen-carboxyl, (c) phenolic hydroxyl, and (d) bidentate nitrogen-carboxyl binding. Structures adapted from T. Sarna et al. (1980)122 and W. Froncisz et al. (1980).85 | ||
When the eumelanin's molecular structure remains intact, the EPR signal does not quench entirely.83,84,120,123 In fact, as seen in Fig. 4c, the radical concentration of natural and synthetic eumelanin reaches a plateau below a certain pH level. This feature has led to the idea that SQ may not be the only free-radical in eumelanin but rather two independent free-radical types: intrinsic and extrinsic paramagnetic centers. The extrinsic center would be an inducible eumelanin free-radical capable of reporting the redox state of its functional groups and the molecular nature of the monomer units on the macrostructure outer surface.120 On the other hand, the intrinsic center would be related to the eumelanin core, representing its molecular state and integrity.108,120 It has been speculated that the intrinsic centers would be trapped entities within the growing oligomers and aggregation of its particles (steps 1 & 2, Fig. 1c), where they would have low chemical reactivity due to the unapproachability of any reactive extraneous agents.80,120 This has support from kinetic EPR studies that found an enhancement of redox activity at high pH levels vs. at lower pH.98 The intrinsic and extrinsic paramagnetic centers were years later named carbon-centered radicals (CCR) and semiquinone-free radicals (SFR), respectively.113 We should reinforce here that, although SFR center was defined as extrinsic in the past, it does not mean that SFR does not belong to eumelanin but rather just its position on the structure surface. Indeed, the formation of superoxide and hydrogen peroxide on the eumelanin surface being attributed to the intrinsic eumelanin radical,91 the extrinsic terminology is strengthened as this reduction activity should exclusively take place at the eumelanin particle's surface. Recent infrared spectroscopy analysis supports this idea.124 Note that some authors prefer the use of intrinsic and extrinsic for radicals that are present in the dark and those that are photo-generated.110 Given that the SFR can be photo-generated,115 we believe our use of these terms is more consistent.
Motivated by this context, systematic spectral simulation was carried out on different pH-dependent EPR signals.78 The computational analysis in Fig. 6 indicates that more than one paramagnetic center should be present in the eumelanin system, with one having a strong pH dependence. The study showed that Lines 1 & 2 dominate the EPR signal at acidic and neutral states, whereas alkaline media is dominated by Line 3. As low pH is the experimental condition to probe the intrinsic free-radicals, such a behavior is compatible with the presence of the CCR and SFR centers mentioned above. This idea of multiple radicals is consistent with older observations of multiple radical reactive centers under photo-irradiated (pH 7.6) conditions, though no commentary was given as to these redox centers’ g-values.98
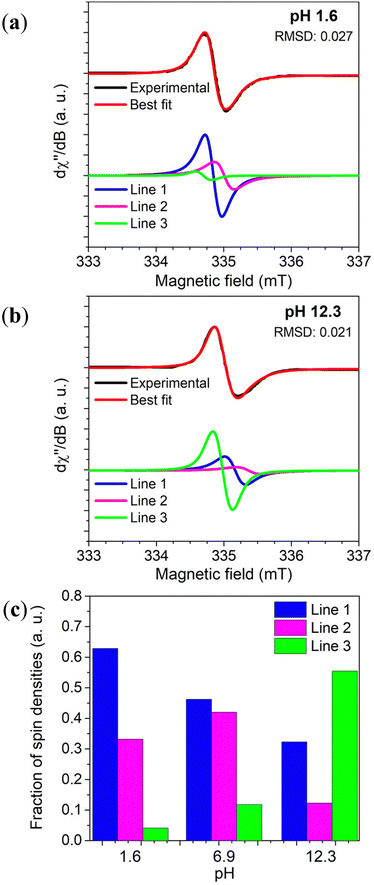 | ||
| Fig. 6 Variation of Dopa-Eumelanin spin populations with pH obtained from X-band EPR spectrum simulation considering three different Voight lines. In (a) pH 1.6, and (b) pH 12.3. (c) The spin concentration estimation of the three-component fitting. Reproduced with permission from J.V. Paulin et al. (2019).78 Copyright© 2019, American Chemical Society. | ||
The solid-state investigations revealed analogous characteristics to those observed in solution.78,113–115 The prevailing presence of CCR radicals is evident at low microwave power, as illustrated in Fig. 7a (black line). However, an increase in microwave power not only diminishes the signal intensity but also introduces a distinctive feature at high g-values (Fig. 7a, gold line).115 This feature is an indicative of radicals with close g-values but differing spin–lattice relaxation times.51,52,78,113–115,117 The additional feature is further enhanced by exposing eumelanin to ammonia vapor,115 suggesting an induced pH-dependent free-radical concentration. Such a behavior implies that CCR alone cannot be responsible for the solid-state EPR signal. To add on, owing to a consistent absence of water content variation, the temperature dependence of solid-state eumelanin's EPR signal follows a typical Curie–Weiss behavior (signal intensity ∝ T−1),80,111 as opposed to the Arrhenius dependence observed for semiquinone in solution.84
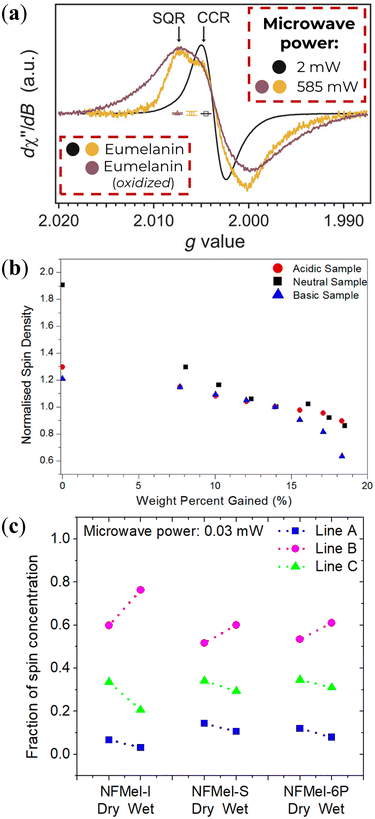 | ||
| Fig. 7 (a) Solid-State CW-EPR spectra of eumelanin under vacuum. Adapted from A.B. Mostert et al. (2018)115 under Creative Commons Attribution-NonCommercial License 4.0 (CC BY-NC). (b) Normalized spin-density variation as a function of water percent adsorbed of eumelanin. Reprinted with permission from A.B. Mostert et al. (2013).113 Copyright© 2013, American Chemical Society. (c) Estimation of each radical species concentration of eumelanin (NFMel-I) and eumelanin-inspired materials (NFMel-S and NFMel-6P) at dry (vacuum) and wet (80% RH) conditions. Reprinted with permission from J.V. Paulin et al. (2020).117 Copyright© 2020, American Chemical Society. | ||
Similarly to pH, hydration can also affect the EPR signal of solid-state eumelanin materials. Hydration is known to decrease linewidth and signal intensity, as shown in Fig. 7b and inferred from Fig. 7c. Assuming the three radical species model of Fig. 5, it is possible to infer what component does as pH changes (Fig. 7c). These changes could be associated with the radicals’ interaction with the number of water molecules in their close surrounding.117
Although the molecular nature of SFR is most likely related to anionic SQ species, the CCR, on the other hand, is not that well understood as to its molecular origin. To bring light to this situation, modern density functional theory (DFT) calculations were performed to model distinct monomers and dimers of potential eumelanin moieties in their cationic, anionic, and radical structures.77 In this study, two distinct groups were observed, one with a g value around 2.0030 and the other with g ranging between 2.0045 and 2.0050 (Fig. 8a). Moreover, SQ species, usually associated with SFR centers, were not the only structure with high g-values. IQ-anion and QI-anion species also showed g-values close to 2.0050. In contrast, CCR centers are associated with Ndef+, QH2-anion, and QH2-cation. Due to the broader linewidth of the EPR signal at low pH, the low hyperfine coupling of QH2-cation in relation to SQR, was initially considered an improbable origin. Based on the alignment of the energy levels around the frontier orbitals of the eumelanin monomeric structures, the high LUMO (lowest unoccupied molecular orbital) levels imply that QH2 cannot act as electron traps.78 Hence, the formation of QH2-anion would be hindered. Instead, it was proposed that QH2 species would donate electrons to Ndef+ originating the paramagnetic species of the CCR center: Ndef-neutral and QH2-cation (QH20 + Ndef+ → QH2+ + Ndef0).78 Note that the stabilization of free radicals through electron trapping at deep defect states has also been proposed using the Hückel theory for band structures.125 The co-existence of these two systems would give origin to the CCR's broader spectral line features. Coincidentally, the formation of Ndef+ during synthesis126,127 at the inner part of eumelanin's macro-structure would make this CCR system protect from reactive exogenous entities capable of affecting the kC/kD constant and, as a consequence, exhibit low reactivity.
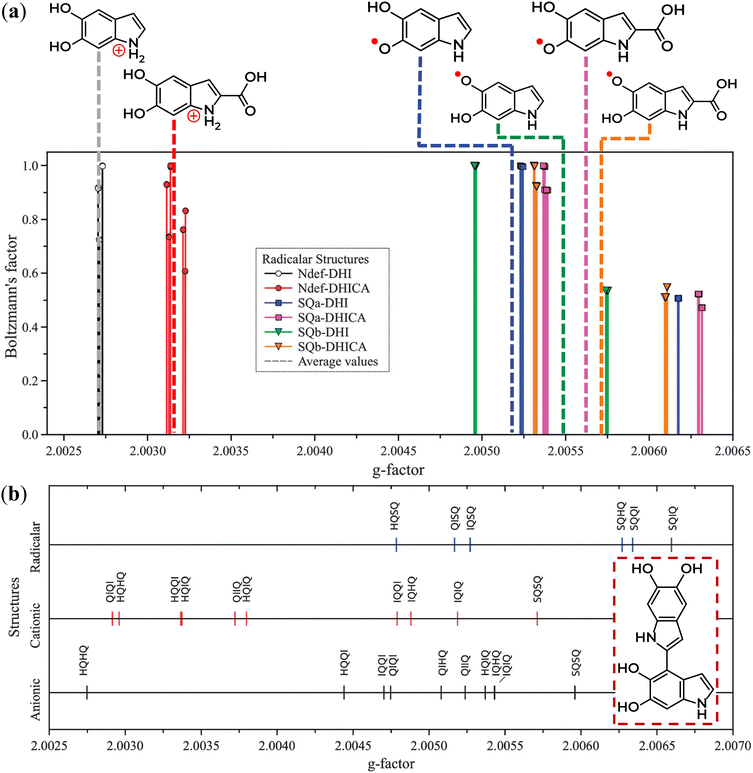 | ||
| Fig. 8 Distribution of the theoretical g-values from eumelanin moieties that are: (a) radical monomers and (b) homo- and hetero-dimers. Compatible behavior was obtained for anionic and cationic monomers. The inset in (b) represents an example of a homo-dimer. Reproduced from A. Batagin-Neto et al. (2015)77 with permission from the PCCP Owner Societies. | ||
There is a monomer entity that has not been thoroughly considered as a potential contribution to the CCR signal, which may have a connection to the SFR: a protonated semiquinone. The SFR, as depicted above, is almost always considered in an anionic form and not in its protonated form. Even though there is currently no computational EPR work produced on this monomer, considering that the experimental pKa of a protonated DHI-semiquinone has been established to be 6.8,128 one should anticipate that changes in the pH may lead to changes in the EPR signal around this value. This is consistent with the observations of Chio et al. (Fig. 4).82 If careful attention is given to the literature, it is clear that a few authors have considered the protonated moiety as a viable contributor to the EPR signal behavior.82,84,99,129,130 In essence, if the semiquinone is protonated, the electron density's symmetry for the anionic form would be broken, which likely may lead to a concentration of the electron density on a carbon system, yielding a signal similar to a carbon-centered radical. If this is the case, the signal line width changes with pH can be neatly explained. However, what prevents us from ascribing CCR to a protonated semiquinone is the sheer persistence of the CCR. As established previously, the EPR signal in eumelanin is quite robust to several extreme conditions, such as high temperature and oxygenation, which does not comport with the easy manipulation of the semiquinone by “softer” variables such as hydration and moderate pH changes. As such, if the protonated semiquinone is present and is aligned with the CCR g-values, it may be that this signal adds to an additional internal or intrinsic radical, e.g., those discussed previously as CCR candidates.
As eumelanin has an oligomeric macro-structure, a natural question would be whether one can use the spectral parameters of the monomeric units to evaluate the EPR spectrum of larger structures. Hence, DFT was applied to commonly reported dimers of two equal units (homo-structured dimers) or two different units (hetero-structured dimers). The data is depicted in Fig. 8b.77 The g-values for homo-dimers remained consistent, with no discernible difference. In the hetero-dimers' case, the unprotected lateral oxygen units influence the spectroscopic features, following the IQ > QI > QH2 order. This trend held true for most cases, except for the elevated g-values in SQ dimers in both scenarios. These results suggest that dimer or closely situated SQ species may not be entirely related to the SFR part of the EPR signal, which diverges from the prevailing consensus within the literature. This specific conclusion is consistent with what is known since the concentration of the induced radicals centers is relatively low, around one radical per 1000 monomer moieties,85 making the probability of two SQ units in close neighboring also low. These computational studies may also form the future basis for understanding the differences between eumelanins that are DHI-rich vs. DHICA-rich, where their respective EPR spectra is modified, most likely due to different stacking morphologies.38
4. Multifrequency EPR and relaxation dynamics of eumelanin
High-frequency EPR spectroscopy has also been applied to eumelanin due to the higher spectral resolution avialable, which enables a better understanding of eumelanin's free-radical origin and molecular nature.99,116,118,129,131 One of the first reports on Q-band EPR of a series of eumelanin and eumelanin-inspired materials was obtained by F.J. Grady and D.C. Borg back in 1968.129 They showed that these materials had a broader asymmetrical spectrum in the Q-band with strong pH dependencies. These authors interpreted the asymmetry based on the presence of protonated radicals at lower pH and, at high pH, the formation of anionic radicals with high g-value. The most important result from their work lies in the Q-band EPR spectrum of a simple 5-hydroxyindole-eumelanin (5-OH indole). The 5-OH indole EPR signal has a single broad line, which did not change with pH, that aligns with the low g-value components of the other natural and synthetic eumelanin (see Fig. 9a). Given that the formation of semiquinone would be difficult in such a system, the observed EPR signal makes plausible the presence of a paramagnetic species (potentially indole-like) protected from environmental variations, i.e., the CCR or component thereof.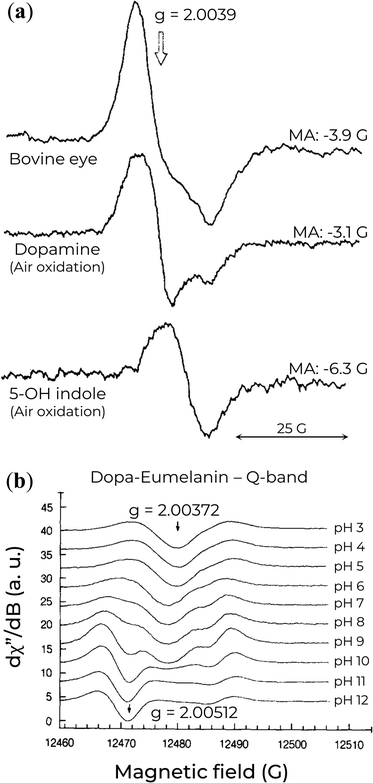 | ||
| Fig. 9 (a) Q-band EPR spectra of eumelanin and eumelanin-inspired materials. MA indicates the modulation amplitude. Reprinted with permission from F.J. Grady and D.C. Borg (1968).129 Copyright© 1968, American Chemical Society. (b) Variation of Q-band EPR signal of Dopa-Eumelanin as a function of pH. Reprinted with permission from M. Pasenkiewicz-Gierula and R.C. Sealy (1986).99 Copyright© 1986, Elsevier B.V. | ||
A few years later, a detailed computer simulations analysis on the EPR spectra recorded at Q-band (35 GHz) of frozen solutions of eumelanin at a wide range of pH was performed, Fig. 9b.99 A combination of anionic o-SQ and cationic radicals was necessary for their analysis to simulate the EPR spectra adequately. At low pH, the unpaired electron of the protonated eumelanin would extend over several monomeric units. Nonetheless, with the increase of the pH, the eumelanin starts to deprotonate, converting the delocalized radical into the relatively localized SQ-anion. The authors also propose that a second ionization would originate another localized anion at an extremely high pH. This species would be divided into two distinct radical groups. One of them would be pH-dependent and like anionic o-SQ (g‖ = 2.0023 and g⊥ = 2.0055), whereas the other would be associated with backbone defects and be pH-independent (g‖ = 2.0023 and g⊥ = 2.0038).132
The results obtained by Q-band EPR analysis are particularly gripping as they align with the current understanding of both CCR and SFR species, which have been extensively investigated through spectral analysis and DFT calculations, as discussed in Section 3.
With the advances in EPR spectroscopy instrumentation, the examination and analysis of eumelanin materials have been measured at a very high frequency (263 GHz)131 in the solid-state. The eumelanin high-resolution EPR spectra, shown in Fig. 10a, revealed two overlapping signals with relatively different g-anisotropies. One radical species is characterized by gx ≈ 2.0042, gy ≈ 2.0032, and gz ≈ 2.0022, while the other is characterized by gx ≈ 2.0037, gy ≈ 2.0032, and gz ≈ 2.0022.
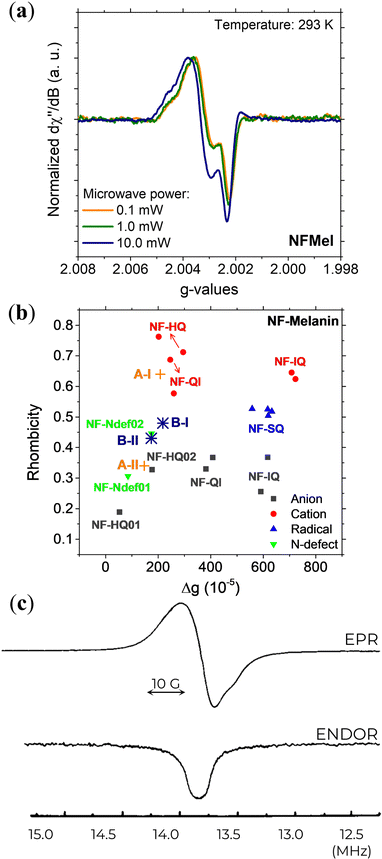 | ||
| Fig. 10 (a) 263 GHz EPR signal at different microwave powers and (b) Rhombicity as a function of g-value anisotropy of Dopa-Eumelanin. (a) and (b) Reprint from J.V. Paulin et al. (2021)131 under Creative Commons Attribution-NonCommercial License 3.0 (CC BY-NC). (c) X-band EPR (top) and ENDOR (bottom) signal of natural eumelanin at 4.2 K with 3 mW of microwave power, 1.25 G of modulation amplitude, and −3 dB of RF power. Reprint with permission from T. Sarna et al. (1976).76 Copyright© 1976, Elsevier Inc. | ||
DFT evaluation on the dependence of the g-tensor evaluated by the rhombicity 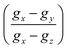 as a function of g-anisotropy (Δg = gx − gz) of the different monomeric units in anionic, cationic, and neutral forms are summarized in Fig. 10b (colored squares, circles, and triangles).131 Based on this plot, the two mentioned radicals can be ascribed to the previous species predicted (Ndef and QH2) by X-band EPR spectral analysis for CCR. The absence of SFR was explained by potential signal saturation or the neutral and solid-state nature of the samples, the two conditions known to increase the concentration of CCR.
as a function of g-anisotropy (Δg = gx − gz) of the different monomeric units in anionic, cationic, and neutral forms are summarized in Fig. 10b (colored squares, circles, and triangles).131 Based on this plot, the two mentioned radicals can be ascribed to the previous species predicted (Ndef and QH2) by X-band EPR spectral analysis for CCR. The absence of SFR was explained by potential signal saturation or the neutral and solid-state nature of the samples, the two conditions known to increase the concentration of CCR.
To probe the intricate relaxation dynamics and electron-nuclear interactions in eumelanin (and eumelanin-inspired materials), advanced EPR techniques like electron-nuclear double resonance (ENDOR) have also been employed in a limited number of studies.76,92,118,133,134 The implementation of this method has the potential to significantly contribute to enhancing the understanding of the molecular structure and dynamics of eumelanin radicals, as well as their spatial distribution and surrounding environment.55
The first result from ENDOR is the lack of a resolved structure or shoulders in the spectral wings (Fig. 10c), which indicates that eumelanin has a heterogeneity of trapping sites on its macrostructure, which is consistent with the widely accepted disorder model of eumelanin structure.5,10
The second result from the ENDOR technique shows that the relaxation time T1 of eumelanin is greatly influenced by the radical and the local environment in which it exists.76,92 Also, the degree of spatial uniformity of the radical distribution plays a significant role in determining T2.133 Notably, T1 is consistent across different sources of eumelanin, and T2 exhibits striking variation among them.76,92,133
In the solid state, the EPR signal of synthetic eumelanin quickly becomes saturated at low microwave powers and is demonstrated by very long relaxation time, T1 = 4.3 ms and T2 = 101 μs,133 suggesting that the spins are decoupled from one another. These findings highlight the intricate interplay between radical type, local environment, and spatial uniformity in influencing the relaxation times of eumelanin, which could be further evidence of different spin populations found across various regions of the eumelanin's macrostructure. Indeed, when eumelanin was incubated in D2O, there was observed minimal effect on its relaxation properties.76 This behavior indicates that eumelanin has a free-radical (or at least one) well-protected or buried, compatible with CCR species.
6. Future directions
The understanding of the eumelanin free-radical species is commonly investigated by X-band CW EPR. However, this methodology can exhibit certain limitations in providing comprehensive information on the electronic structure of these species. This limitation is being overcome using pH-dependent studies in multifrequency CW or pulsed EPR spectroscopy. Additionally, with the advance in magnetic resonance of the last decades, many advanced EPR techniques not yet explored in the eumelanin field could also be extremely important to understand the nature of each paramagnetic species and their dynamics. Therefore, future research could focus on implementing the proposed strategies outlined below to address these gaps.(i) EPR imaging:135–138 this technique exposes a sample placed in a magnetic field to electromagnetic radiation, allowing the paramagnetic species to emit radiation detected by a receiver coil. A two- or three-dimensional image of the sample will be constructed by obtaining the emitted radiation from various points. Although this method may not be useful to understanding the nature of the paramagnetic species of melanin and their dynamics, it could assist in investigating their distribution and concentration.
As we mentioned before, pulsed EPR can provide detailed information on the electronic and nuclear structure of paramagnetic species. It relies on the alignment of the electron spin magnetization in the presence of a constant field. This approach encompasses several techniques, each with unique characteristics,55,67,139–142 that will be elaborated in the subsequent subsections.
(ii) Electron-nuclear double resonance (ENDOR) and electron spin echo envelope modulation (ESEEM): these techniques can probe the transitions involving nuclear spin flips driven by radiofrequency radiation and provide information on the electronic and nuclear spin interactions and the local structure of paramagnetic species. ENDOR often provides information on strongly coupled nuclei, whereas ESEEM detects more weakly coupled nuclei.
(iii) Hyperfine-sublevel-correlation spectroscopy (HYSCORE): it analyzes the hyperfine interactions between an unpaired electron and its neighboring nuclear spins. Besides determining the strength of the hyperfine interactions, obtaining information on the number, type, and position of the coupled nuclei is possible.
(iv) Double electron–electron resonance (DEER): this is a two-frequency technique capable of estimating the distance between two weakly-coupled electron spins based on the magnetic dipole–dipole. It elucidates distances, orientation, and conformational flexibility of specific structural features on the nanometer scale.
It is speculated that the paramagnetic species of eumelanin is a key feature that determines some of its physicochemical properties, for instance, charge transport,40,42,114,143,144 photoconductivity,145 and photodynamics.115,146 Hence, other techniques that could be interesting to investigate are:
(v) Optically Detected Magnetic Resonance (ODMR):147–151 it is a powerful spectroscopic technique capable of detecting the electron spin resonance transitions of paramagnetic species using optical techniques. The method involves optically exciting the sample and then monitoring the fluorescence, phosphorescence, or absorption from the excited state as a function of an applied magnetic field over a wide range of temperature, pH, and excitation wavelength parameters. This methodology is commonly used for higher quantum efficiency materials, which is not the case for eumelanin.152,153 However, spin-locking, pulse sequence, and cryogenic temperatures can be applied to improve the signal-to-noise ratio and obtain a stronger ODMR signal.
(vi) Electrically detected magnetic resonance (EDMR):154–157 this magnetic resonance spectroscopy can provide information on free radicals' electronic and magnetic properties by measuring the spin-dependent transport of charge carriers under the influence of a magnetic field. Compared to conventional EPR, EDMR is extremely sensitive, down to 100 spins (at liquid helium temperatures).158 The study of EDMR on eumelanin can be of high complexity due to the presence of multiple paramagnetic species and their environmental-dependent stability.
An additional avenue for future research may leverage recent advancements in engineering eumelanin's chemical structure. For example, the manipulation of the number of carboxylate (DHICA) and non-carboxylate (DHI) units, as well as the availability of different polymerization positions, packing arrangements, and macrostructures, along with covalent-bounded protection of co-existing redox-states5,38,159 may facilitate the determination of the type and environment of the radicals. Accordingly, incorporating the methods mentioned in this review with controlled parameters of pH and temperature, along with a variety of eumelanin-related materials, can significantly enhance the comprehension of eumelanin's paramagnetic system.
To bring matters to a close, it's worth noting that some of the studies referenced here are rather dated. Performing a pH-dependent study, similar to the one conducted by Chio et al.82 (Fig. 4), on solutions using a modern spectrometer with additional advanced modeling would be immensely useful, especially when coupled with power saturation and pulsed EPR techniques. Additionally, the choice of solvent in these experiments might play a crucial role in influencing the paramagnetic properties of eumelanin and should be carefully considered in future investigations. These cutting-edge approaches and considerations will undoubtedly contribute to a deeper understanding of the paramagnetic nature of eumelanin and its potential implications.
7. Conclusion
The central subclass of the melanin family of natural pigments known as eumelanin has unique physical and chemical properties. The strong and persistent paramagnetic signal is one of them, and it is not fully comprehended yet. This review summarizes the rich literature on eumelanin's multifrequency and time-dependent EPR characterization. Solution and solid-state results are showcased to discuss the potential origin of the paramagnetic signal. The in-depth analysis indicates a complex paramagnetic system for eumelanin. Indeed, the superposition of at least two different paramagnetic signals is expected to compose eumelanin's EPR spectrum.Data availability statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.Author contributions
J.V. Paulin: conceptualization, validation, investigation, writing – original draft, visualization. C.F.O. Graeff: conceptualization, validation, investigation, writing – review & editing. A.B. Mostert: conceptualization, validation, investigation, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. V. P. thanks the support of the São Paulo Research Foundation (FAPESP, grants: 2014/25979-2, 2020/15869-6). C. F. O. G. thank the support of FAPESP under the Research, Innovation and Dissemination Center (CEPID) no 2013/07296-2 and Brazilian National Council for Scientific and Technological Development (CNPq) under the Instituto Nacional de Ciência e Tecnologia de Nanomateriais para a Vida (NanoVIDA) n° 406079/2022-6. A. B. M. contribution was under the Sêr Cymru II fellowship and the results incorporated in this work had received funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 663830. For A. B. M. this work was also supported by the UKRI Research Partnerships Investment Fund through the Centre for Integrative Semiconductor Materials.References
- P. Meredith, C. J. Bettinger, M. Irimia-Vladu, A. B. Mostert and P. E. Schwenn, Rep. Prog. Phys., 2013, 76, 034501 CrossRef CAS PubMed.
- M. Irimia-Vladu, Chem. Soc. Rev., 2014, 43, 588–610 RSC.
- M. Irimia-Vladu, Y. Kanbur, F. Camaioni, M. E. Coppola, C. Yumusak, C. V. Irimia, A. Vlad, A. Operamolla, G. M. Farinola, G. P. Suranna, N. González-Benitez, M. C. Molina, L. F. Bautista, H. Langhals, B. Stadlober, E. D. Głowacki and N. S. Sariciftci, Chem. Mater., 2019, 31, 6315–6346 CrossRef CAS PubMed.
- N. Amdursky, E. D. Głowacki and P. Meredith, Adv. Mater., 2019, 31, 1802221 CrossRef PubMed.
- J. V. Paulin and C. F. O. Graeff, J. Mater. Chem. C, 2021, 9, 14514–14531 RSC.
- A. B. Mostert, Polymers, 2021, 13, 1670 CrossRef CAS PubMed.
- J. V. Paulin and C. C. B. Bufon, Submitted Search PubMed.
- M. D’Ischia, K. Wakamatsu, A. Napolitano, S. Briganti, J.-C. Garcia-Borron, D. Kovacs, P. Meredith, A. Pezzella, M. Picardo, T. Sarna, J. D. Simon and S. Ito, Pigm. Cell Melanoma Res., 2013, 26, 616–633 CrossRef PubMed.
- M. D’Ischia, K. Wakamatsu, F. Cicoira, E. Di Mauro, J. C. Garcia-Borron, S. Commo, I. Galvàn, G. Ghanem, K. Koike, P. Meredith, A. Pezzella, C. Santato, T. Sarna, J. D. Simon, L. Zecca, F. A. Zucca, A. Napolitano and S. Ito, Pigm. Cell Melanoma Res., 2015, 28, 520–544 CrossRef PubMed.
- M. d’Ischia, A. Napolitano, A. Pezzella, P. Meredith and M. J. Buehler, Angew. Chem., Int. Ed., 2020, 59, 11196–11205 CrossRef PubMed.
- P. Meredith and T. Sarna, Pigm. Cell Res., 2006, 19, 572–594 CrossRef CAS PubMed.
- W. H. Koch and M. R. Chedekel, Photochem. Photobiol., 1986, 44, 703–710 CrossRef CAS PubMed.
- B. A. Gilchrest, M. S. Eller, A. C. Geller and M. Yaar, N. Engl. J. Med., 1999, 340, 1341–1348 CrossRef CAS PubMed.
- F. P. Noonan, M. R. Zaidi, A. Wolnicka-Glubisz, M. R. Anver, J. Bahn, A. Wielgus, J. Cadet, T. Douki, S. Mouret, M. A. Tucker, A. Popratiloff, G. Merlino and E. C. De Fabo, Nat. Commun., 2012, 3, 884 CrossRef PubMed.
- D. M. A. Mann and P. O. Yates, Mech. Ageing Dev., 1983, 21, 193–203 CrossRef CAS PubMed.
- R. J. D’Amato, Z. P. Lipman and S. H. Snyder, Science, 1986, 231, 987–989 CrossRef PubMed.
- F. A. Zucca, G. Giaveri, M. Gallorini, A. Albertini, M. Toscani, G. Pezzoli, R. Lucius, H. Wilms, D. Sulzer, S. Ito, K. Wakamatsu and L. Zecca, Pigm. Cell Res., 2004, 17, 610–617 CrossRef CAS PubMed.
- H. Fedorow, F. Tribl, G. Halliday, M. Gerlach, P. Riederer and K. L. Double, Prog. Neurobiol., 2005, 75, 109–124 CrossRef CAS PubMed.
- S. Z. Berg and J. Berg, Front. Immunol., 2023, 14, 1–17 Search PubMed.
- M. G. Reyes, F. Faraldi, R. Rydman and C. C. Wang, Neurol. Res., 2003, 25, 179–182 CrossRef PubMed.
- M. Sheliakina, A. B. Mostert and P. Meredith, Mater. Horiz., 2018, 5, 256–263 RSC.
- A. B. Mostert, S. Rienecker, M. Sheliakina, P. Zierep, G. R. Hanson, J. R. Harmer, G. Schenk and P. Meredith, J. Mater. Chem. B, 2020, 8, 8050–8060 RSC.
- N. L. Nozella, J. V. M. Lima, R. F. de Oliveira and C. F. D. O. Graeff, Mater. Adv., 2023, 4, 4732–4743 RSC.
- Y. J. Kim, W. Wu, S. Chun, J. F. Whitacre and C. J. Bettinger, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 20912–20917 CrossRef CAS PubMed.
- P. Kumar, E. Di Mauro, S. Zhang, A. Pezzella, F. Soavi, C. Santato and F. Cicoira, J. Mater. Chem. C, 2016, 4, 9516–9525 RSC.
- R. Xu, A. Gouda, M. F. Caso, F. Soavi and C. Santato, ACS Omega, 2019, 4, 12244–12251 CrossRef CAS PubMed.
- J. V. Paulin, S. L. Fernandes and C. F. O. Graeff, Electrochem, 2021, 2, 264–273 CrossRef CAS.
- M. Ambrico, P. F. Ambrico, T. Ligonzo, A. Cardone, S. R. Cicco, A. Lavizzera, V. Augelli and G. M. Farinola, Appl. Phys. Lett., 2012, 100, 253702 CrossRef.
- A. Wahab, N. Gogurla, J. Y. Park and S. Kim, Adv. Mater. Technol., 2022, 7, 2101271 CrossRef CAS.
- H. J. Nam, J. Cha, S. H. Lee, W. J. Yoo and D. Y. Jung, Chem. Commun., 2014, 50, 1458–1461 RSC.
- M. Piacenti-Silva, J. C. Fernandes, N. B. de Figueiredo, M. Congiu, M. Mulato and C. F. D. O. Graeff, AIP Adv., 2014, 4, 037120 CrossRef.
- T. F. Wu, B. H. Wee and J. D. Hong, Adv. Mater. Interfaces, 2015, 2, 1500203 CrossRef.
- Z. Tehrani, S. P. Whelan, B. Mostert, J. V. Paulin, M. M. Ali, E. D. Ahmadi, C. F. O. Graeff, O. J. Guy and D. T. Gethin, 2D Mater., 2020, 7, 024008 CrossRef CAS.
- S. P. Whelan, Z. Tehrani, M. Peacock, J. V. Paulin, O. Guy and D. Gethin, J. Electroanal. Chem., 2022, 904, 115868 CrossRef CAS.
- J. V. Paulin, L. G. S. Albano, D. H. S. Camargo, M. P. Pereira, B. A. Bregadiolli, C. F. O. Graeff and C. C. B. Bufon, Appl. Mater. Today, 2022, 28, 101525 CrossRef.
- S. N. Dezidério, C. A. Brunello, M. I. N. da Silva, M. A. Cotta and C. F. O. Graeff, J. Non Cryst. Solids, 2004, 338–340, 634–638 CrossRef.
- J. P. Bothma, J. de Boor, U. Divakar, P. E. Schwenn and P. Meredith, Adv. Mater., 2008, 20, 3539–3542 CrossRef CAS.
- L. Panzella, G. Gentile, G. D’Errico, N. F. Della Vecchia, M. E. Errico, A. Napolitano, C. Carfagna and M. D’Ischia, Angew. Chem., 2013, 52, 12684–12687 CrossRef CAS PubMed.
- M. Reali, P. Saini and C. Santato, Mater. Adv., 2021, 2, 15–31 RSC.
- J. V. Paulin, M. P. Pereira, B. A. Bregadiolli, J. P. Cachaneski-Lopes, C. F. O. Graeff and C. C. B. Bufon, J. Mater. Chem. C, 2023, 11, 6107–6118 RSC.
- E. V. Gan, H. F. Haberman and I. A. Menon, Arch. Biochem. Biophys., 1976, 173, 666–672 CrossRef CAS PubMed.
- A. B. Mostert, Chem. Phys., 2021, 546, 111158 CrossRef CAS.
- F. G. Cánovas, F. García-Carmona, J. V. Sánchez, J. L. I. Pastor and J. A. L. Teruel, J. Biol. Chem., 1982, 257, 8738–8744 CrossRef.
- J. Cheng, S. C. Moss and M. Eisner, Pigm. Cell Res., 1994, 7, 263–273 CrossRef CAS PubMed.
- K. B. Stark, J. M. Gallas, G. W. Zajac, J. T. Golab, S. Gidanian, T. McIntire and P. J. Farmer, J. Phys. Chem. B, 2005, 109, 1970–1977 CrossRef CAS PubMed.
- A. A. R. Watt, J. P. Bothma and P. Meredith, Soft Matter, 2009, 5, 3754–3760 RSC.
- C.-T. Chen, V. Ball, J. J. A. Gracio, M. K. Singh, V. Toniazzo, D. Ruch and M. J. Buehler, ACS Nano, 2013, 7, 1524–1532 CrossRef CAS PubMed.
- P. A. Abramov, O. I. Ivankov, A. B. Mostert and K. A. Motovilo, Phys. Chem. Chem. Phys., 2023, 25, 16212–16216 RSC.
- A. Büngeler, B. Hämisch and O. I. Strube, Int. J. Mol. Sci., 2017, 18, 1901 CrossRef PubMed.
- B. Commoner, J. Townsend and G. E. Pake, Nature, 1954, 174, 689–691 CrossRef CAS PubMed.
- C. P. Poole, Electron Spin Resonance: A Comprehensive Treatise on Experimental Techniques, John Wiley & Sons, New York, 2nd edn, 1983 Search PubMed.
- J. A. Weil and J. R. Bolton, Electron Paramagnetic Resonance: Elementary Theory and Practical Applications, Wiley, New York, 2nd edn, 2007 Search PubMed.
- M. Brustolon, Electron Paramagnetic Resonance: A Practitioner's Toolkit, Wiley, 1st edn, 2009 Search PubMed.
- K. U. R. Naveed, L. Wang, H. Yu, R. S. Ullah, M. Haroon, S. Fahad, J. Li, T. Elshaarani, R. U. Khan and A. Nazir, Polym. Chem., 2018, 9, 3306–3335 RSC.
- M. M. Roessler and E. Salvadori, Chem. Soc. Rev., 2018, 47, 2534–2553 RSC.
- R. Bramley and S. J. Strach, Chem. Rev., 1983, 83, 49–82 CrossRef CAS.
- A. Abragam and M. H. L. Pryce, Proc. R. Soc. A, 1951, 205, 135–153 CAS.
- K. Möbius, W. Lubitz and A. Savitsky, Prog. Nucl. Magn. Reson. Spectrosc., 2013, 75, 1–49 CrossRef PubMed.
- M. Bennati and T. F. Prisner, Rep. Prog. Phys., 2005, 68, 411–448 CrossRef CAS.
- B. Odom, D. Hanneke, B. D’urso and G. Gabrielse, Phys. Rev. Lett., 2006, 97, 030801 CrossRef CAS PubMed.
- C. Hägerhäll, S. Magnitsky, V. D. Sled, I. Schröder, R. P. Gunsalus, G. Cecchini and T. Ohnishi, J. Biol. Chem., 1999, 274, 26157–26164 CrossRef PubMed.
- D. J. Hirsh and G. W. Brudvig, Nat. Protoc., 2007, 2, 1770–1781 CrossRef CAS PubMed.
- M. Brustolon, Electron paramagnetic spectroscopy a practitioner's toolkit, Wiley-VCH, Hoboken, NJ., 2009 Search PubMed.
- G. R. Eaton, S. S. Eaton, D. P. Barr and R. T. Weber, Quantitative EPR, Springer, Vienna, 1st edn, 2010, pp. 107–113 Search PubMed.
- K. Möbius and A. Savitsky, High-Field EPR Spectroscopy on Proteins and their Model Systems, Royal Society of Chemistry, 2008 Search PubMed.
- A. Schweiger and G. Jeschke, Principles of Pulse Electron Paramagnetic Resonance, Oxford University Press, 1st edn, 2001 Search PubMed.
- EPR Spectroscopy: Fundamentals and Methods, ed. D. Goldfarb and S. Stoll, John Wiley & Sons Ltd, 2018 Search PubMed.
- S. Stoll and A. Schweiger, J. Magn. Reson., 2006, 178, 42–55 CrossRef CAS PubMed.
- S. Stoll, EasySpin - EPR spectrum simulation, https://www.easyspin.org/.
- D. R. Duling, J. Magn. Reson B, 1994, 104, 105–110 CrossRef CAS PubMed.
- G. R. Hanson, K. E. Gates, C. J. Noble, M. Griffin, A. Mitchell and S. Benson, J. Inorg. Biochem., 2004, 98, 903–916 CrossRef CAS PubMed.
- C. Bengs and M. H. Levitt, Magn. Reson. Chem., 2018, 56, 374–414 CrossRef CAS PubMed.
- R. Sealy, J. Hyde, C. Felix, I. Menon and G. Prota, Science, 1982, 217, 545–547 CrossRef CAS PubMed.
- P. M. Plonka, Exp. Dermatol., 2009, 18, 472–484 CrossRef CAS PubMed.
- R. Sealy, J. Hyde, C. Felix, I. Menon, G. Prota, H. Swartz, S. Persad and H. Haberman, Proc. Natl. Acad. Sci. U. S. A., 1982, 79, 2885–2889 CrossRef CAS PubMed.
- T. Sarna, C. Mailer, J. S. Hyde, H. M. Swartz and B. M. Hoffman, Biophys J., 1976, 16, 1165–1170 CrossRef CAS PubMed.
- A. Batagin-Neto, E. S. Bronze-Uhle and C. F. O. Graeff, Phys. Chem. Chem. Phys., 2015, 17, 7264–7274 RSC.
- J. V. Paulin, A. Batagin-Neto and C. F. O. Graeff, J. Phys. Chem. B, 2019, 123, 1248–1255 CrossRef CAS PubMed.
- A. Zadlo, G. Szewczyk, M. Sarna, T. G. Camenisch, J. W. Sidabras, S. Ito, K. Wakamatsu, F. Sagan, M. Mitoraj and T. Sarna, Pigm. Cell Melanoma Res., 2019, 32, 359–372 CrossRef CAS PubMed.
- M. S. Blois, A. B. Zahlan and J. E. Maling, Biophys J., 1964, 4, 471–490 CrossRef CAS PubMed.
- H. S. Mason, D. J. E. Ingram and B. Allen, Arch. Biochem. Biophys., 1960, 86, 225–230 CrossRef CAS PubMed.
- S. Chio, J. S. Hyde and R. C. Sealy, Arch. Biochem. Biophys., 1982, 215, 100–106 CrossRef CAS PubMed.
- R. C. Sealy, C. C. Felix, J. S. Hyde and H. M. Swartz, in Free Radicals in Biology, ed. W. A. Pryor, Academic Press, New York, 1980, pp. 209–259 Search PubMed.
- S. Chio, J. S. Hyde and R. C. Sealy, Arch. Biochem. Biophys., 1980, 199, 133–139 CrossRef CAS PubMed.
- W. Froncisz, T. Sarna and J. S. Hyde, Arch. Biochem. Biophys., 1980, 202, 289–303 CrossRef CAS PubMed.
- R. J. Sever, F. W. Cope and B. D. Polis, Science, 1962, 137, 128–129 CrossRef CAS PubMed.
- F. W. Cope, R. J. Sever and B. D. Polis, Arch. Biochem. Biophys., 1963, 100, 171–177 CrossRef CAS.
- K. Stratton and M. A. Pathak, Arch. Biochem. Biophys., 1968, 123, 477–483 CrossRef CAS PubMed.
- T. Sarna, J. S. Hyde and H. M. Swartz, Science, 1976, 192, 1132–1134 CrossRef CAS PubMed.
- C. C. Felix, J. S. Hyde, T. Sarna and R. C. Sealy, J. Am. Chem. Soc., 1978, 100, 3922–3926 CrossRef CAS.
- C. C. Felix, J. S. Hyde, T. Sarna and R. C. Sealy, Biochem. Biophys. Res. Commun., 1978, 84, 335–341 CrossRef CAS PubMed.
- T. Sarna and J. S. Hyde, J. Chem. Phys., 1978, 69, 1945–1948 CrossRef CAS.
- C. C. Felix, J. S. Hyde and R. C. Sealy, Biochem. Biophys. Res. Commun., 1979, 88, 456–461 CrossRef CAS PubMed.
- T. Sarna, A. Duleba, W. Korytowski and H. Swartz, Arch. Biochem. Biophys., 1980, 200, 140–148 CrossRef CAS PubMed.
- R. Arnaud, G. Perbet, A. Deflandre and G. Lang, Photochem. Photobiol., 1983, 38, 161–168 CrossRef CAS PubMed.
- T. Sarna and R. C. Sealy, Arch. Biochem. Biophys., 1984, 232, 574–578 CrossRef CAS PubMed.
- T. Sarna, I. A. Menon and R. C. Sealy, Photochem. Photobiol., 1985, 42, 529–532 CrossRef CAS PubMed.
- T. Sarna, W. Korytowski and R. C. Sealy, Arch. Biochem. Biophys., 1985, 239, 226–233 CrossRef CAS PubMed.
- M. Pasenkiewicz-Gierula and R. C. Sealy, Biochim. Biophys. Acta, 1986, 884, 510–516 CrossRef CAS PubMed.
- W. Korytowski, B. Kalyanaraman, I. A. Menon, T. Sarna and R. C. Sealy, BBA, Biochim. Biophys. Acta, Gen. Subj., 1986, 882, 145–153 CrossRef CAS PubMed.
- M. S. Jahan, T. R. Drouin and R. M. Sayre, Photochem. Photobiol., 1987, 45, 543–546 CrossRef CAS PubMed.
- K. J. Reszka and C. F. Chignell, J. Am. Chem. Soc., 1993, 115, 7752–7760 CrossRef CAS.
- R. Dunford, E. J. Land, M. Rozanowska, T. Sarna and T. G. Truscott, Free Radic. Biol. Med., 1995, 19, 735–740 CrossRef CAS PubMed.
- K. Jimbow, K. Reszka, S. Schmitz, T. Salopek and P. Thomas, Distribution of eu- and pheomelanins in human skin and melanocytic tumors, and their photoprotective vs. phototoxic properties, in Melanin: Its role in human photoprotection, ed. L. Zeise, M. R. ChedekelandT. B. Fitzpatrick, Valdenmar Publishing Company, Overland Park, KS, 1995, pp. 155–175 Search PubMed.
- M. M. Jastrzebska, H. Isotalo, J. Paloheimo, H. Stubb and B. Pilawa, J. Biomater. Sci. Polym., 1996, 7, 781–793 Search PubMed.
- P. Goncalves, C. A. Brunello and C. F. O. Graeff, Mol. Cryst. Liq. Cryst., 2002, 374, 39–44 CrossRef CAS.
- B. Szpoganicz, S. Gidanian, P. Kong and P. Farmer, J. Inorg. Biochem., 2002, 89, 45–53 CrossRef CAS PubMed.
- T. Sarna, J. M. Burke, W. Korytowski, M. Rozanowska, C. M. B. Skumatz, A. Zareba and M. Zareba, Exp. Eye Res., 2003, 76, 89–98 CrossRef CAS PubMed.
- E. Chodurek, B. Pilawa, A. Dzierzega-Lecznar, S. Kurkiewicz, L. Swiatkowska and T. Wilczok, J. Anal. Appl. Pyrolysis, 2003, 70, 43–54 CrossRef CAS.
- B.-L. Seagle, K. A. Rezai, E. M. Gasyna, Y. Kobori, K. A. Rezaei and J. R. Norris, J. Am. Chem. Soc., 2005, 127, 11220–11221 CrossRef CAS PubMed.
- P. J. Gonçalves, O. B. Filho and C. F. O. Graeff, J. Appl. Phys., 2006, 99, 104701 CrossRef.
- M. E. Cano, R. Castañeda-Priego, A. Gil-Villegas, M. A. Sosa, P. Schio, A. J. A. De Oliveira, F. Chen, O. Baffa and C. F. O. Graeff, Photochem. Photobiol., 2008, 84, 627–631 CrossRef CAS PubMed.
- A. B. Mostert, G. R. Hanson, T. Sarna, I. R. Gentle, B. J. Powell and P. Meredith, J. Phys. Chem. B, 2013, 117, 4965–4972 CrossRef CAS PubMed.
- S. B. Rienecker, A. B. Mostert, G. Schenk, G. R. Hanson and P. Meredith, J. Phys. Chem. B, 2015, 119, 14994–15000 CrossRef CAS PubMed.
- A. B. Mostert, S. B. Rienecker, C. Noble, G. R. Hanson and P. Meredith, Sci. Adv., 2018, 4, eaaq1293 CrossRef PubMed.
- M. Al Khatib, M. Harir, J. Costa, M. C. Baratto, I. Schiavo, L. Trabalzini, S. Pollini, G. M. Rossolini, R. Basosi and R. Pogni, Molecules, 2018, 23, 1916 CrossRef PubMed.
- J. V. Paulin, A. Batagin-Neto, P. Meredith, C. F. O. Graeff and A. B. Mostert, J. Phys. Chem. B, 2020, 124, 10365–10373 CrossRef CAS PubMed.
- M. Al Khatib, J. Costa, M. C. Baratto, R. Basosi and R. Pogni, J. Phys. Chem. B, 2020, 124, 2110–2115 CrossRef CAS PubMed.
- U. D’amora, A. Soriente, A. Ronca, S. Scialla, M. Perrella, P. Manini, J. W. Phua, C. Ottenheim, R. Di Girolamo, A. Pezzella, M. G. Raucci and L. Ambrosio, Biomedicines, 2022, 10, 2945 CrossRef PubMed.
- T. Sarna and H. A. Swartz, in The Pigmentary System: Physiology and Pathophysiology, ed. J. J. Nordlund, R. E. Boissy, V. J. Hearing, R. A. King, W. S. Oetting and J. Ortonne, Blackwell Publishing, 2nd edn, 2006, pp. 191–212 Search PubMed.
- J. S. Leigh, J. Chem. Phys., 1970, 52, 2594–2608 CrossRef.
- T. Sarna, W. Froncisz and J. S. Hyde, Arch. Biochem. Biophys., 1980, 202, 304–313 CrossRef CAS PubMed.
- T. Sarna, J. Photochem. Photobiol., B, 1992, 12, 215–258 CrossRef CAS PubMed.
- Z. V. Bedran, S. S. Zhukov, P. A. Abramov, I. O. Tyurenkov, B. P. Gorshunov, A. B. Mostert and K. A. Motovilov, Polymers, 2021, 13, 4403 CrossRef CAS PubMed.
- D. S. Galvão and M. J. Caldas, J. Chem. Phys., 1988, 88, 4088–4091 CrossRef.
- H. S. Raper, Physiol Rev., 1928, 8, 245–282 CrossRef CAS.
- F. G. Cánovas, F. García-Carmona, J. V. Sánchez, J. L. I. Pastor and J. A. L. Teruel, J. Biol. Chem., 1982, 257, 8738–8744 CrossRef.
- A. T. Al-Kazwini, P. O’Neill, G. E. Adams, R. B. Cundall, B. Jacquet, G. Lang and A. Junino, J. Phys. Chem., 1990, 94, 6666–6670 CrossRef CAS.
- F. J. Grady and D. C. Borg, J. Am. Chem. Soc., 1968, 90, 2949–2952 CrossRef CAS PubMed.
- T. Sarna and H. M. Swartz, in Atmospheric Oxidation and Antioxidants, ed. G. Scott, Elsevier, Amsterdam, 1993, pp. 129–169 Search PubMed.
- J. V. Paulin, A. Batagin-Neto, B. Naydenov, K. Lips and C. F. O. Graeff, Mater. Adv., 2021, 2, 6297–6305 RSC.
- T. Sarna and P. M. Plonka, in Biomedical EPR, Part A: Free Radicals, Metals, Medicine, and Physiology, ed. S. S. Eaton, G. R. Eaton and L. J. Berliner, Springer, US, 1st edn, 2005, pp. 125–146 Search PubMed.
- M. Okazaki, K. Kuwata, Y. Miki, S. Shiga and T. Shiga, Arch. Biochem. Biophys., 1985, 242, 197–205 CrossRef CAS PubMed.
- K. Tadyszak, R. Mrówczyński and R. Carmieli, J. Phys. Chem. B, 2021, 125, 841–849 CrossRef CAS PubMed.
- G. R. Eaton, S. S. Eaton and M. M. Maltempo, Int. J. Radiat. Appl. Instrum., Part A, 1989, 40, 1227–1231 CrossRef CAS.
- H. Jang, S. Subramanian, N. Devasahayam, K. Saito, S. Matsumoto, M. C. Krishna and A. B. McMillan, Magn. Reson. Med., 2013, 70, 1173–1181 CrossRef PubMed.
- S. Steinert, F. Ziem, L. T. Hall, A. Zappe, M. Schweikert, N. Götz, A. Aird, G. Balasubramanian, L. Hollenberg and J. Wrachtrup, Nat. Commun., 2013, 4, 1607 CrossRef CAS PubMed.
- R. Mrówczyński, L. E. Coy, B. Scheibe, T. Czechowski, M. Augustyniak-Jabłokow, S. Jurga and K. Tadyszak, J. Phys. Chem. B, 2015, 119, 10341–10347 CrossRef PubMed.
- D. M. Murphy and R. D. Farley, Chem. Soc. Rev., 2006, 35, 249–268 RSC.
- J. Harmer, G. Mitrikas and A. Schweiger, in High Resolution EPR: Applications to Metalloenzymes and Metals in Medicine, ed. L. Berliner and G. Hanson, Springer, New York, NY, 2009, pp. 13–61 Search PubMed.
- M. J. N. Junk, Assessing the Functional Structure of Molecular Transporters by EPR Spectroscopy, Springer, Berlin, Heidelberg, 2012, pp. 7–52 Search PubMed.
- R. A. Stein, A. H. Beth and E. J. Hustedt, Methods Enzymol., 2015, 563, 531–567 CAS.
- A. B. Mostert, B. J. Powell, I. R. Gentle and P. Meredith, Appl. Phys. Lett., 2012, 100, 093701 CrossRef.
- J. Wünsche, Y. Deng, P. Kumar, E. Di Mauro, E. Josberger, J. Sayago, A. Pezzella, F. Soavi, F. Cicoira, M. Rolandi and C. Santato, Chem. Mater., 2015, 27, 436–442 CrossRef.
- A. B. Mostert, B. J. Powell, F. L. Pratt, G. R. Hanson, T. Sarna, I. R. Gentle and P. Meredith, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 8943–8947 CrossRef CAS PubMed.
- A. Wang, A. R. Marino, E. M. Gasyna, T. Sarna and J. R. Norris, J. Phys. Chem. B, 2009, 113, 10480–10482 CrossRef CAS PubMed.
- E. T. Harrigan and N. Hirota, J. Am. Chem. Soc., 1975, 97, 6647–6652 CrossRef CAS.
- J. J. Davies, Contemp. Phys., 1976, 17, 275–294 CrossRef CAS.
- A. H. Maki, in Biological Magnetic Resonance, ed. L. J. Berliner and J. Reuben, Springer, Boston, MA, 1984, pp. 187–294 Search PubMed.
- D. Carbonera, Photosynth. Res., 2009, 102, 403–414 CrossRef CAS PubMed.
- E. Goovaerts, eMagRes, 2017, 6, 343–358 CAS.
- P. Meredith and J. Riesz, Photochem. Photobiol., 2004, 79, 211–216 CrossRef CAS PubMed.
- J. V. Paulin, A. G. Veiga, Y. Garcia-Basabe, M. L. M. Rocco and C. F. Graeff, Polym. Int., 2018, 67, 550–556 CrossRef CAS.
- C. F. O. Graeff, C. A. Brunello and R. M. Faria, Synth. Met., 1999, 101, 805–806 CrossRef CAS.
- C. Boehme and K. Lips, Phys. Rev. B. Condens. Matter. Mater. Phys., 2003, 68, 245105 CrossRef.
- K. Kato and Y. Teki, Phys. Chem. Chem. Phys., 2021, 23, 6361–6369 RSC.
- K. J. Myers, P. M. Lenahan, J. P. Ashton and J. T. Ryan, J. Appl. Phys., 2022, 132, 115301 CrossRef CAS.
- D. R. McCamey, H. Huebl, M. S. Brandt, W. D. Hutchison, J. C. McCallum, R. G. Clark and A. R. Hamilton, Appl. Phys. Lett., 2006, 89, 182115 CrossRef.
- X. Wang, L. Kinziabulatova, M. Bortoli, A. Manickoth, M. A. Barilla, H. Huang, L. Blancafort, B. Kohler and J. P. Lumb, Nat. Chem., 2023, 15, 787–793 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |