Open Access Article
Open Access ArticleTailored MXenes and graphene as efficient telemedicine platforms for personalized health wellness
Kamil Reza
Khondakar
 *a,
Divya
Tripathi
b,
Hirak
Mazumdar
*c,
Kirti
Ahuja
b and
Ajeet
Kaushik
*a,
Divya
Tripathi
b,
Hirak
Mazumdar
*c,
Kirti
Ahuja
b and
Ajeet
Kaushik
 d
d
aSchool of Technology, Woxsen University, Telangana 502345, India. E-mail: kamilreza@gmail.com; kamil.reza@woxsen.edu.in
bSchool of Health Sciences and Technology, University of Petroleum and Energy Studies (UPES), Dehradun, India
cDepartment of Computer Science and Engineering, Graphic Era Deemed to be University, Dehradun, Uttarakhand 248002, India
dNano Biotech Laboratory, Department of Environmental Engineering, Florida Polytechnic University, Lakeland, FL 33805-8531, USA
First published on 16th April 2024
Abstract
This comprehensive review paper provides an insightful exploration of the burgeoning field of 2D nanostructures and their development as telemedicine platforms for futuristic smart healthcare systems. A remote health monitoring device is known as telemedicine for continuous surveillance of body parameters. 2D materials, such as graphene and MXenes with their unique physical, chemical, and electrical properties, have recently garnered significant attention in this field. Graphene, composed of a single layer of carbon atoms in a hexagonal lattice, boasts a high surface-to-volume ratio, exceptional electrical and thermal conductivity, and remarkable mechanical strength. In the realm of telemedicine, graphene-based nanostructures have emerged as versatile tools for biosensing, targeted drug delivery, and bioimaging. The exceptional attributes of graphene facilitate efficient drug loading and controlled release. Additionally, its outstanding electrical conductivity supports the development of biosensors with heightened sensitivity and selectivity. On the other hand, MXenes, a family of 2D transition metal carbides and nitrides, offer distinct surface chemistry and excellent biocompatibility. These materials exhibit promise in various biological applications, including biosensing and drug delivery. Their substantial surface area and diverse surface characteristics enhance drug-loading capabilities and cellular interactions. Furthermore, the integration of hybrid nanocomposites, combining graphene and MXenes, presents an opportunity to synergistically enhance the functionality and performance of telemedicine platforms. Moreover, the integration of 2D nanomaterials with artificial intelligence (AI) amplifies the potential of telemedicine. AI-powered systems optimize the release of therapeutic agents in drug delivery systems and improve the precision of disease detection through data analysis and modeling. Real-time data analysis allows for personalized healthcare, enabling immediate adjustments and tailored interventions. This convergence of 2D nanomaterials and AI fosters dynamic synergy, offering the prospect of elevated patient outcomes, reduced side effects, and the expedited development of cutting-edge healthcare solutions. The future of telemedicine is undeniably promising with these advancements.
1. Introduction
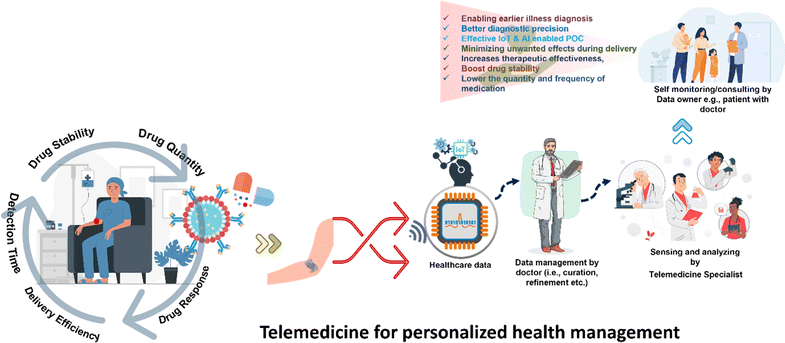
Telemedicine has emerged as the next-generation diagnostic tool and has the potential to revolutionize the practice of medicine and healthcare.1–4 It introduces innovative approaches for disease prevention, drug delivery, and treatment by modifying and designing materials at the nanoscale level. Telemedicine-based technology makes it possible to create extremely sensitive and focused diagnostic instruments for enhancing better diagnostic capabilities.5,6 Early biomarker detection is made possible by nanoscale sensors and imaging tools, enabling earlier illness diagnosis and better diagnostic precision. Nanostructures are useful in imaging procedures like MRI, CT scans, and molecular imaging due to their capacity to be tailored for precise targeting of particular cells or areas. Targeted drug delivery has been the most explored area in the telemedicine field. Drugs can be packaged, and transported to the target site and can be released directly to target cells or tissues, overcoming obstacles, and minimizing unwanted effects. Targeted drug delivery methods increase therapeutic effectiveness, boost drug stability, and lower the quantity and frequency of medication needed. Telemedicine also provides controlled release systems that can deliver medications or therapeutic chemicals over a prolonged period or in response to triggers. With this strategy, therapeutic levels are sustained, fluctuations are reduced, and patient compliance is improved. Controlled release systems are especially useful for long-term medication therapy or chronic illnesses. Also, improved treatment efficacy can be achieved by combining many medications for therapeutic and diagnostic purposes, and more efficiently crossing biological barriers. This adaptability increases therapeutic efficacy and lowers the risk of developing medication resistance. Therefore, personalized medicine by allowing for the customization of treatments to meet the unique demands of each patient can be addressed. Fig. 1 depicts the importance of telemedicine for personalized disease management.Advancements in science and nanotechnology are driving continuous evolution in nanomaterials and their composites. These materials, with their size and composition-dependent properties, play a pivotal role in solving various scientific challenges in healthcare because of their versatility.7 Nanomaterials can be categorized according to their size, agglomeration state, morphology, dimensions, and composition, and depending on each characteristic, they can be used for a wide range of healthcare applications.8,9 Two-dimensional (2D) nanomaterials are ideal for a variety of applications, most notably in energy storage, electronics, sensors, catalysis, and biomedical applications because they have an atomic thickness, large active surface sites, a large surface area to volume ratio, and excellent mechanical properties.10–20 Size, shape, surface chemistry, and other features of 2D nanostructures can all be customized. Furthermore, the introduction of nanostructures like graphene and MXenes as 2D materials has revolutionized biomedical technology providing precision telemedicine platforms for disease management.21 They can operate as scaffolds for sensing materials, bioimaging, pathogen killers, wound healers, and tissue regeneration.22 Also, these 2D layer structures can deliver drugs to the desired locations, aiding in tissue regeneration, and repair.
Graphene is an allotrope of carbon possessing ultrathin thickness, a planar structure with a large active surface area.23 Graphene also exhibits excellent biocompatibility and can directly disperse in aqueous solutions without modification, and ease of functionalization making it very suitable for biosensing applications.24,25 Graphene is successfully used in the fabrication of optical devices, and biosensors due to its high electron mobility and atomic sheet structure. Graphene-based nanocarriers provide notable benefits for medication delivery. Therapeutic compounds may be encapsulated by them, which prevents their deterioration and permits controlled release at certain disease areas.26 The great drug-loading capacity provided by graphene's huge surface area improves the effectiveness of therapies. Additionally, by using electro-responsive devices, graphene's special electrical properties enable tailored drug delivery. Graphene-based biosensors have shown outstanding sensitivity and selectivity for detecting biomarkers in addition to medication delivery.26,27 Graphene scaffolds can support tissues mechanically, encourage cell adhesion, and accelerate tissue growth for tissue engineering applications in healthcare. Additionally, the optical qualities of graphene have made it easier to use it in imaging processes, enabling high-resolution imaging of cells and tissues. Overall, due to its adaptability and distinctive qualities, graphene is a promising material for developing telemedicine and opening the door for novel treatments, diagnostics, and tissue engineering techniques.
Recently, a class of novel materials known as MXenes have had a significant impact on creative ideas in various scientific disciplines.28 With their intricate atomic configuration and numerous adjustable properties within their multilayered structures, MXenes demonstrate enhanced multifunctional behaviours. Their multilayered structure, which has a complex atomic arrangement and a lot of tunable properties, enhances their multifunctional behaviors. MXenes are a family of 2D materials (transition metal carbides/nitrides) that have shown significant applications in medical diagnostics because of their unique physicochemical properties, ultrathin lamellar structure, and high specific surface area.28 These new remarkable nanostructures have been utilized as therapeutics for anticancer treatment, and in photothermal therapy as drug delivery agents.29 MXenes can be precisely designed and synthesized in the pristine structure for a variety of applications including biosensing, precision medicine, bioimaging, antimicrobial activity, etc.30 The high specific surface area, metallic conductivity, and hydrophilic nature of MXene nanosheets make them potential drug or protein carriers with abundant anchoring sites and reservoirs for precision nanomedicine. Furthermore, MXenes have been explored as a contrast agent for computer tomography (CT) scans and magnetic resonance imaging (MRI). MXenes thus enable new applications and modify/improve the functionality of existing ones by tailoring the functional groups on the surface.
The emergence of these 2D smart nanomaterials in developing sophisticated tools for telemedicine applications and integration of AI and ML for data analysis have improved the innovations and breakthroughs in medical technology in elevating the quality of healthcare. AI in telemedicine is transforming diagnostics and treatment by leveraging data analysis for precise disease detection and drug delivery optimization at the nanoscale. It accelerates the development of innovative telemedicine solutions through simulations and modeling. In clinical practice, AI enables real-time patient monitoring and personalized interventions. The synergy of AI and telemedicine holds tremendous potential for more effective and tailored healthcare solutions. This review will include discussions on current applications of 2D nanostructures (graphene, MXenes, and their composites) in the field of telemedicine with a focus on AI-based systems for future applications.
2. Graphene and MXenes, emerging 2D-materials for biomedical applications
We briefly introduce the basics of MXenes and graphene. MXenes are considered as 2D material where M represents an early transition metal, X depicts carbon or nitrogen, and T represents terminal groups with the basic formula of Mn+1XnTx, (where n = 1, 2, 3, and x represents the number of terminal groups).31 This unique combination of elemental arrangements and structures of MXenes has opened numerous possibilities to synthesize a variety of 2D materials. MXenes display a variety of electronic applications due to their excellent electrical conductivity and unique physicochemical properties.32 Because of their metallic conductivity, which is a result of transition metals being present in their structure, MXenes are effective conductors in electrical devices. Furthermore, MXenes have great mechanical strength and flexibility, which makes them suitable for application in composites and innovative materials. They show high surface reactivity of their large surface area and abundance of functional groups, such as hydroxyl or oxygen groups. These terminations allow for improved interactions with ions and molecules. Due to their customizable surface chemistry and catalytic activity, MXenes have also demonstrated potential in catalysis. MXenes have a distinctive combination of layered structures, excellent electrical conductivity, mechanical strength, and versatile surface chemistry for biomedical applications.33They are being explored in various industries including energy storage and conversion, the environment and catalysis, separation membranes, medicine, optics, and electronics, etc. According to Gogotsi et al., MXenes have a unique combination of properties, including the high electrical conductivity and mechanical properties of transition metal carbides/nitrides.34 They have functionalized surfaces that make MXenes hydrophilic and can be bonded to various species; high negative zeta-potential, enabling stable colloidal solutions in water; and efficient absorption of electromagnetic waves, which have led to many applications. MXenes, possessing numerous appealing physicochemical characteristics, such as a large specific surface area, significant electrical conductivity, magnetism, low toxicity, luminescence, and high biocompatibility, have emerged as a promising contender for cancer therapy and theragnostics. These two-dimensional (2D) nanostructures, which exhibit photothermal, chemotherapeutic synergistic, and photodynamic effects, hold great potential for effective and non-invasive anticancer treatments. They have been extensively investigated for applications in photothermal/chemo-photothermal therapy (PTT) and targeted delivery of anticancer drugs. Notably, MXenes’ unique optical properties have facilitated their use in bioimaging and biosensing, and their exceptional ability to convert light to heat makes them highly suitable as biocompatible and efficient nano-scaled agents for PTT applications. Nevertheless, there remain significant challenges that need to be addressed, such as ensuring their stability in physiological environments, achieving sustained and controlled drug release, and enhancing biodegradability.35
Earlier, graphene was considered one of the most important materials of the century, having numerous applications in areas including electronics, medicine, agriculture, structural engineering, and many others that are under investigation.36,37 Graphene is a single layer of carbon atoms arranged in a hexagonal lattice that exhibits extraordinary properties.13 It is a perfect material for electronics applications because of its incredibly fast electrons resulting in high electrical conductivity. The planar shape is facilitated by the pi-bonds that form between carbon atoms, which create a continuous network that facilitates faster electron movement. In terms of physical characteristics, graphene is almost transparent and has exceptional mechanical strength. It is one of the strongest materials known, despite only being one atom in thickness. Graphene's distinct blend of mechanical and electrical characteristics has sparked interest in applications such as composite materials and flexible electronics. It may also be used in thermal devices for photothermal therapy management due to its high thermal conductivity. Graphene's chemical reactivity is largely dependent on its high surface area and two-dimensional structure. The graphene surface can be functionalized to add functional groups to the surfaces or edges of the sheets for customized chemical interactions. Graphene's strong carbon–carbon bonds prevent it from degrading chemically, which makes it a durable material in a variety of settings. Because of these characteristics, graphene is a used as biocompatible material and for sophisticated coatings. These properties are essential to fabricating sensors for various healthcare applications. In conclusion, graphene's unique physicochemical properties, including exceptional electrical conductivity, mechanical strength, transparency, and chemical reactivity, make it a material with vast potential for revolutionizing numerous industries, from electronics to materials science and beyond.27,38,39 Recently, graphene has been explored as a potential material for biomedical applications in areas such as biosensing, cancer therapies, drug delivery systems (DDSs), and tissue engineering, allowing the development of new research fields associated with the interactions of biomolecular systems and graphene.24,26 The surface of the two-dimensional structure, enriched with oxygen and sp2 domains, facilitates interaction and anchoring with a diverse array of biomolecules, including DNA, peptides, proteins (such as enzymes), viruses, and more. These interactions can occur through both covalent and noncovalent bonds. The functionalization of graphene with these biomolecules holds great promise for applications in materials science, bioengineering, nanotechnology, and nanomedicine.40
Furthermore, among the transition-metal dichalcogenides (TMDs), molybdenum disulfide (MoS2) has been explored in the nanotechnology industry due to its high mobility of charge carriers, excellent optical absorption properties, and high thermal and chemical stability. Due to these properties, MoS2 has found applications in different industries such as optical and electronic materials.
Similarly, boron nitride (BN) is one of the emerging insulating 2D layered materials that has shown high mechanical strength, chemical inertness, exceptional thermal stability, and superior ion conductivity. Scientists are exploring BN in the fields of optoelectronics, quantum optics, electronics, and sensing as the next-generation smart material.
In conclusion, each material has its advantages and disadvantages. Graphene has remarkable mechanical strength, thermal stability, and electrical conductivity with applications in energy storage, electronics, and sensor industries. However, its applicability in several semiconductor applications is limited due to its absence of an intrinsic bandgap. However, MXenes overcome some of graphene's drawbacks with their metallic conductivity and adjustable qualities, making them a promising material for electronic devices and energy storage. TMDs with a natural bandgap, such as MoS2, are good candidates for transistor applications. It can be difficult to synthesize them and integrate them into electronics, though. Despite being electrically insulating, boron nitride is a great substrate and insulator for electronic devices. Depending on the particular needs of the application, one can choose between MXenes, graphene, and TMDs while taking conductivity, bandgap, and simplicity of use into consideration. A comparison table has been included to summarize the various properties and characteristics of the emerging 2D materials (Table 1).
| 2D materials | Properties | Types of layer | Synthesis | Applications | Ref. |
|---|---|---|---|---|---|
| Graphene | High surface area, high electrical conductivity, biocompatible | Single layer, few layers | Chemical exfoliation, epitaxial growth (MBE), mechanical exfoliation, arc discharge, chemical vapor deposition (CVD) | Sensors energy electronics | 13 |
| MoS2 (molybdenum disulfide) | Excellent electrochemical and luminescence properties, superconducting properties. | Monolayer, bilayer, hexalayer | Atomic layer deposition (ALD), pulsed laser deposition (PLD), chemical vapor deposition (CVD) | Sensors LED fabrication, solar cell | 10 |
| MXenes | Large specific surface area, high electrical conductivity, high mechanical strength, tunable band gap | Single layer, few layers | Molten salt etching, hydrofluoric acid (HF) etching, electrochemical, hydrothermal | Energy storage, sensors nanomedicine | 11 |
| BN (boron nitride) | High thermal conductivity, low thermal expansion, high chemical resistance | Monolayer, bilayer, few layers | Chemical vapor deposition (CVD), hydrothermal, laser-ablative aqueous synthesis | Detecting corrosive chemicals, high-temperature monitoring, harsh industrial environments | 12 |
| Tungsten disulfide (WS2) | Excellent optical properties, large surface area, high mechanical strength, valley polarization | Monolayer, bilayer, multilayer | Chemical vapor deposition (CVD) microwave-assisted synthesis | Light emitters, photodetectors/sensors, valleytronics, and flexible nanoelectronics | 14 |
| Niobium disulfide (NbS2) | Electronic properties polymorphism and polytypism, superconducting properties | Monolayer, few layers | Chemical vapor deposition (CVD) | Superconductor | 15 |
| Tungsten diselenide (WSe2) | Large surface area, high thermal conductivity, good photostability | Single layer, few layers | Chemical vapor deposition (CVD) and flux zone growth (flux) | Sensors, photocatalysis and solar energy quantum computing | 16 |
| Tungsten telluride (WTe2) | Excellent thermoelectric properties, Superconducting properties, magnetoresistance | Single or few layers | Chemical vapor deposition (CVD), molecular beam epitaxy (MBE), and mechanical exfoliation | Energy harvesting and waste heat recovery, energy storage and conversion, quantum computing | 17 |
| Carbon nitride (C3N4) | Large surface area, high mechanical strength, high conductivity, biocompatible | Monolayer, few layers | Hydrothermal, co-precipitation, acid etching, etc. | Nanomedicine, water filtration, energy storage | 18 |
| Molybdenum diselenide (MoSe2) | Large surface area, electroactive material, electrocatalytic properties | Single layer, few layers | Solvo-thermal chemical root method, chemical vapor deposition (CVD) | Lubricants and energy storage devices | 19 |
| Molybdenum ditelluride (MoTe2) | High surface-to-volume ratio, superconducting properties | Single layer, few layers | Flux method, liquid exfoliation, thermal treatment powder mixture | Sensors, light emitting diodes, solar cells, ultra-fast photodetectors and single-photon emitters | 20 |
3. Telemedicine as a smart analytical approach for health wellness
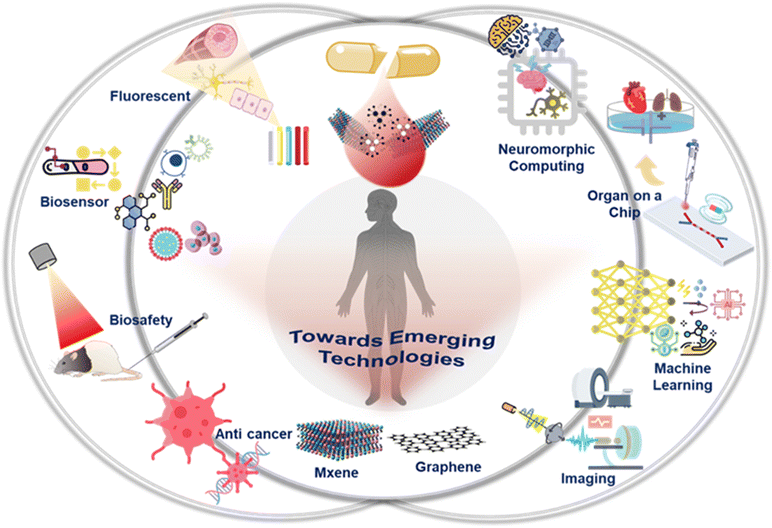
Telemedicine as a new frontier in healthcare promises ground-breaking improvements in disease detection, therapy, and prevention. The field of telemedicine in healthcare has the potential to completely alter how we identify, treat, and comprehend diseases. Telemedicine provides precise drug targeting, early disease detection, and customized treatments by utilizing the unique qualities of nanoscale materials, eventually resulting in more efficient and personalized healthcare. It addresses issues like poor drug solubility, minimizes side effects, and improves imaging methods used in medicine. Nanomedicine also facilitates tissue regeneration, overcomes biological obstacles, and provides drug delivery. It provides precision targeting at the cellular and molecular levels by utilizing nanoscale materials and technology, minimizing adverse effects, and increasing therapeutic efficacy.The integration of intelligent nanomaterials into biological systems has sparked numerous groundbreaking applications in precision telemedicine. These applications encompass innovative therapeutics, novel drug delivery systems, the creation of rapid and highly sensitive diagnostic tools, and many more. Among the next-generation smart materials, MXenes and graphene, are poised to revolutionize healthcare across multiple categories.41 These nanoparticles’ excellent electrical conductivity and surface area make them perfect for creating extremely sensitive biosensors in diagnostics. MXene-based biosensors can accurately diagnose early disease by detecting biomarkers at exceedingly low concentrations.42 Graphene nanoparticles can act as adaptable drug carriers in cancer therapy, effectively delivering therapeutic compounds to tumor areas while causing the least amount of harm to healthy tissues. Additionally, MXenes are an excellent candidate for tissue engineering applications due to their distinct electrical and mechanical characteristics, which will help with the regeneration of injured tissues in regenerative medicine. The antibacterial properties of graphene oxide can be used to provide novel approaches for managing infectious diseases and enhancing wound healing. The combination of MXenes and graphene nanoparticles holds enormous potential to advance telemedicine as it develops. These two smart materials (MXenes and graphene) have been extensively explored for innovative applications in the telemedicine field for developing smart healthcare systems. In Fig. 2, we described some of the emerging applications of MXene and graphene-based systems with AI.
4. MXenes and graphene for advanced biosensing applications
The incorporation of cutting-edge two-dimensional (2D) functional materials like graphene, borophene, and MXenes has ushered in the era of next-generation biosensing devices, characterized by enhanced spatiotemporal attributes.43 There are several reports of graphene-based sensors for biomedical applications.44 Single-atom thickness, biocompatibility, and large active surface area of graphene act as a better sensing platform for a variety of molecules. Ease of functionalization, wide potential window as well as high electron transfer rate, etc. make graphene an excellent sensing material. However, the application of graphene sensors in clinical settings has faced challenges such as reproducibility, high manufacturing yields, Debye screening, and nonspecific absorption on electrodes. Yin et al. recently developed an on-chip-based POCT (point-of-care testing) graphene field effect transistor (GFET) sensor where they addressed those above-mentioned problems of graphene-based sensors and successfully detected pancreatic cancerous exosomes.45 The chip consists of GFET sensor arrays and a portable read-in/out electronic system was built to measure the real-time electrical response from patient samples in less than 1 hour. A schematic of the GEFT sensor along with its operation as a portable sensor for exosome detection is shown in Fig. 3.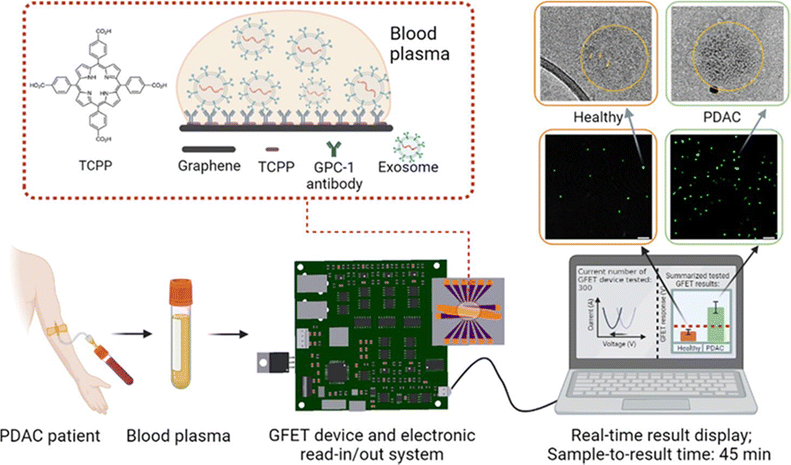 | ||
| Fig. 3 Schematics of detection of PDAC exosomes using graphene-based sensors (GFETs with portable electronics and real-time detection results). The total detection time from applying blood plasma on the GFETs to results is less than 45 min. Reproduced with permission.45 Copyright 2023 American Chemical Society. | ||
In the field of telemedicine, wound healing is one of the sectors gaining the attention of material scientists toward improving the condition of patients. Graphene has gained significant attention in the field of wound healing due to its versatile applications. It can be used in wound dressings, where its vast surface area acts as a scaffold for cell growth, and its flexibility allows for easy application to various wound types. Graphene's inherent antibacterial properties also aid in preventing infections, a crucial factor for accelerating the wound healing process. Moreover, functionalized graphene can serve as a vehicle for drug delivery, enabling the targeted administration of healing agents to wounds, thereby promoting tissue regeneration. Graphene's surface characteristics enhance cell adhesion, proliferation, and migration, crucial processes for wound healing. It has also shown promise in promoting angiogenesis, ensuring adequate blood supply to healing tissues, and supporting cell development and tissue regeneration. Additionally, graphene's superior thermal conductivity makes it suitable for thermal therapy applications like photothermal therapy (PTT), which can expedite tissue repair, reduce pain, and facilitate wound cleansing. Zhu et al. utilized these properties of graphene and designed a unique material – ciprofloxacin (CF)-encapsulated graphene–silk fibroin macromolecular hydrogel dressings for the treatment of burn wound injuries. This advanced blend improved the graphene's superior thermal conductivity to enhance the effectiveness of thermal therapy and provided an excellent dressing material for the treatment of burn wound injuries. This hydrogel composite was effective for wound healing with enhanced antibacterial activity for preventing bacterial infection.46
MXenes have emerged as a prominent interfacing material within the domains of portable healthcare and environmental sensing, showcasing exceptional sensitivity and selectivity. Ongoing research into the use of MXenes in wound healing has revealed promising applications. MXene-based wound dressings, characterized by their high surface area, offer flexible and protective barriers for wounds. These dressings have the potential to not only safeguard wounds but also promote tissue regeneration, potentially possessing antibacterial properties.36,47 Moreover, MXenes can be tailored for drug delivery purposes, encapsulating medications or growth factors that enhance tissue regeneration and wound healing. This controlled drug release from MXene carriers can optimize the healing process and improve wound healing outcomes. Additionally, MXene materials have demonstrated the ability to enhance cell adhesion, proliferation, and migration, essential elements of wound healing, thereby fostering favorable conditions for cell growth at wound sites.48 Furthermore, MXene-based materials show promise in their antimicrobial properties, which can aid in infection prevention when integrated into wound dressings or coatings.49 Notably, MXenes may also play a role in modulating inflammation, a critical factor in wound healing, by regulating excessive inflammation at the wound site and potentially expediting the healing process.
Similarly, MXenes have excellent sensing properties. Among the emerging applications of MXenes, application in biosensors has gained a lot of attention due to their flexible structure for ease of functionalization, high electron mobility, good stability, and high active surface area. Recently, MXene QDs have demonstrated high photo-thermal stability, hydrophilicity, and multimodal sensing/imaging capacities for cellular imaging. Based on the above properties of MXenes, Xue et al. successfully designed and developed photoluminescent Ti3C2 MXene QDs for multicolor cellular imaging using a simple hydrothermal technique. They demonstrated sensing properties with quantum efficiency and were deployed as zinc ion sensors.50 Wang et al. developed an MXene-integrated microneedle to slowly release asiaticoside (AS) to accelerate chronic wound healing.51 Their work showed that MXenes as a drug delivery platform not only enhance the mechanical durability of microneedles, enabling them to penetrate the skin's barrier for subcutaneous drug administration, but also prolong the release duration of the active substance (AS) (a typical microneedle patch for diabetic foot ulcer treatment shown in Fig. 4). An enzymatic electrochemical biosensor was fabricated using 2D niobium carbide MXene for pesticide phosmet detection. This new biosensor possessed low cytotoxicity, good chemical stability, high porosity, excellent conductivity, and enhanced electrochemical activity towards phosmet detection. The fabricated sensor had a limit of detection (LOD) of 0.046 ng mL−1 towards the phosmet analyte showing promising results as a high-quality detection tool.52 In the context of glucose monitoring, the intrinsic heterogeneous electron transfer (HET) characteristics and electrical properties of MXenes have been explored to propel the development of second-generation glucose-sensing devices. Swift and direct measurement of blood glucose levels hold pivotal significance in personalized diabetes mellitus management. Novel strategies involving hybrid nanocomposite materials, amalgamating 2D MXenes with 1D nanostructures, have been pursued to amplify adhesion stability on transducer surfaces, ultimately leading to extended-term monitoring capabilities.53 Leveraging the physicochemical properties with superior mechanical strength as well as HET characteristics of MXenes have been explored by Chia et al. to fabricate multilayered MXene titanium carbide-based hydrogen biosensors for glucose quantification.54 This hydrogel-based nanocomposite sensor demonstrated excellent chronoamperometric results to quantify glucose concentration as they showed excellent electrocatalytic activity toward glucose analytes. The biosensing platform showed high selectivity with very long linear ranges of (50–27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 μM) and a low limit of detection of 23.0 μM. In the age of smart technology, scientists are exploring multifunctional, highly stable, electronic materials for healthcare applications taking inspiration from the environment. Scientists have developed a bean pod-inspired self-healable pressure sensor for human arterial pulse monitoring and gait detection using graphene and polystyrene beads as a wearable (Fig. 5).55
750 μM) and a low limit of detection of 23.0 μM. In the age of smart technology, scientists are exploring multifunctional, highly stable, electronic materials for healthcare applications taking inspiration from the environment. Scientists have developed a bean pod-inspired self-healable pressure sensor for human arterial pulse monitoring and gait detection using graphene and polystyrene beads as a wearable (Fig. 5).55
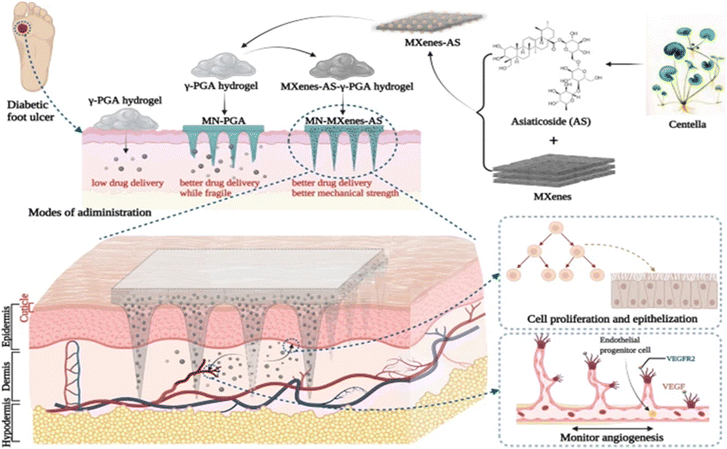 | ||
| Fig. 4 A diagram depicting the design of a microneedle patch using MXenes (referred to as MN-MOF-GO-Ag) aimed at expediting the healing process of diabetic wounds. Reproduced with permission.51 Copyright 2022 Springer Nature. | ||
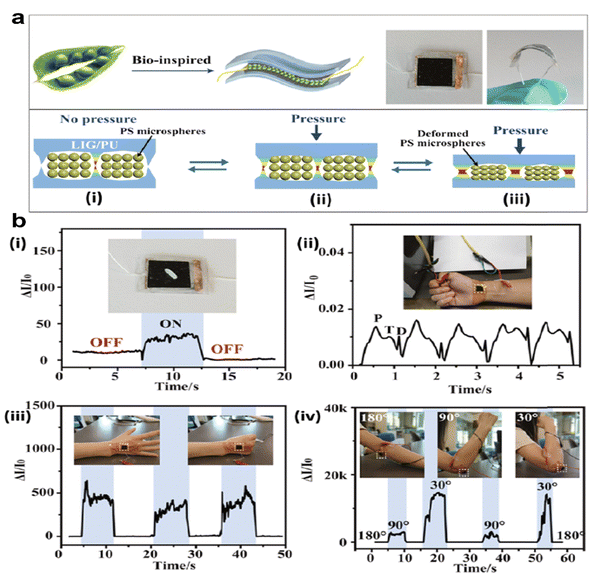 | ||
| Fig. 5 (a) Schematic illustration of the bean pod inspired healable pressure sensor and its mechanism. (b) Wearable sensing applications of the pressure sensor in the detection of (i) rice as a light object, (ii) blood pulse on the wrist, (iii) hand clenching, and (iv) elbow bending. Reproduced with permission.55 Copyright 2020 American Chemical Society. | ||
Furthermore, MXene–graphene integrated electronic skin (e-skin) sensors designed to detect human movements via pressure transduction principles have been recently reported. Remarkably breathable and biodegradable, the MXene pressure sensor is amenable to interfacing with wireless smart sensing platforms, opening avenues for practical applications encompassing human locomotion monitoring, biodegradable implanted devices, intelligent electronic skins, and therapeutic oversight. In this direction, a self-healable high-performance composite has been designed with the help of MXene and graphene nanomaterials.56 Currently, self-healing graphene and MXene composites are being explored in soft robotics, electronic skin technology, and wearable sensors for a variety of reasons. These new composites exhibited excellent arbitrary shape adaptability, suitable adhesiveness, ideal durability, high stretchability, immediate self-healing responsibility, and outstanding electromagnetic features.57 However, the Integration of multifunctional sensors into a common substrate for simultaneous detection of human body parameters is quite challenging. Recently, Zhang et al. fabricated a multifunctional sensor for strain, temperature, and electrocardiogram (ECG) monitoring using MXene-Ti3C2Tx and 3,4-ethylene dioxythiophene (EDOT) deposited on laser-induced graphene (LIG) as a wearable sensor (Fig. 6). They also demonstrated on-site detection of human body-induced deformations and physiological health indicators on the human body for wearables.58 This work looks very promising as future wearables for smart skin and healthcare applications.
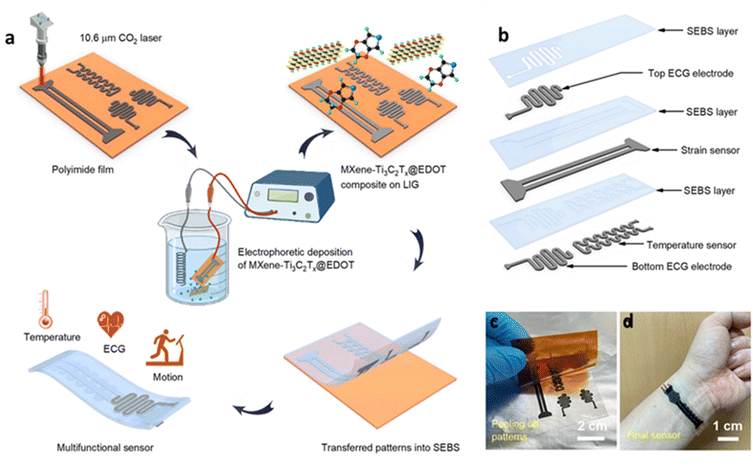 | ||
| Fig. 6 A wearable multifunctional sensor has been developed with an MXene and graphene composite for health monitoring. (a) Fabrication of the sensor. (b) Various parts of the sensor, (c) & (d) The fabricated sensor on the hand. Reproduced with permission.58 Copyright 2022 Springer Nature. | ||
Currently, lab-on-chip platforms are being explored for developing personalized therapy-based treatment.59–61 In particular, lab-on-chip-based medical devices are being investigated for organoid application using MXenes and graphene as the next-generation smart materials. These new age smart nanomaterials have been integrated into lab-on-a-chip platforms with optical detection systems providing myriad possibilities for the study of personalized telemedicine. These smart materials have exhibited outstanding prospects for boosting the functioning of lab-on-a-chip devices because of their high electrical conductivity, exceptional surface area, and biocompatibility. In integration with LOC, MXenes and graphene can be utilized for precise and quick cellular analysis with focused medication delivery, and diagnostics due to their low cytotoxicity, high porosity, and excellent conductivity. Such applications have a significant deal of potential to revolutionize customized medicine and disease management. For example, new generation smart composites are being investigated for designing innovative tissue engineering materials for organoid chip applications. Wychowaniec designed a porous hydrogel made of two-dimensional flakes from graphene oxide and MXenes (Ti3C2Tx). They fabricated a three-dimensional porous hydrogel with a unique porous architecture of well-suited chemical surfaces to carry out three different types of cultured cells on these scaffolds with extended three-dimensional networks for organoid applications.62 A completely revolutionary work has been reported by the team of Prof. Wei Gao from Caltech, USA where they developed a wireless electrochemical biosensor for the automatic, non-invasive, and wireless monitoring of inflammation in the human body. In this work, they did not require any time-consuming steps like incubation and washing steps for the quantification of protein biomarkers in the blood. They fabricated a skin-interfaced graphene array-based microfluidic chip that capitalizes on sweat flow to achieve fully automated protein and detector antibody (dAb) capturing, subsequent washing and picomolar-level electrochemical detection on the skin.63Fig. 7 depicts the circulating CRP mechanism in the human body (a), the scheme of the fabricated sensor (b), the image of the sensor (c) and (d), and the detection mechanism of the biosensor from the sweat sample (e). This prototype wearable biosensing technology can be utilized for real-time monitoring of sweat for multiple body parameter analysis at home.
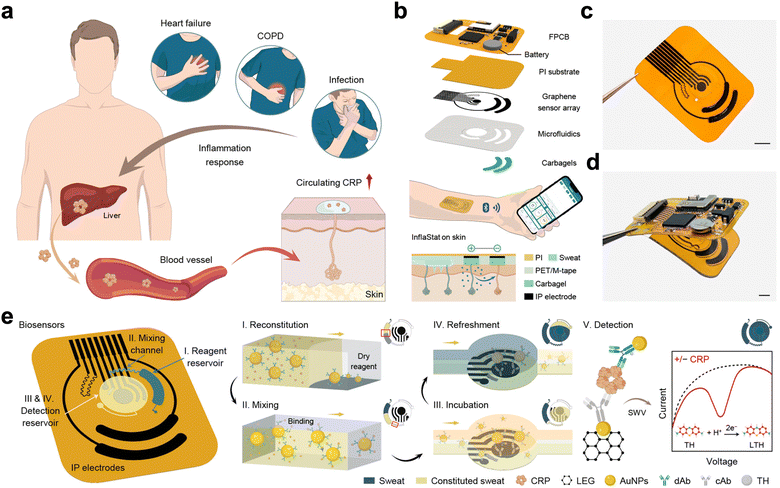 | ||
| Fig. 7 Graphene-based microfluidic chip for detection of inflammatory biomarkers from sweat. The schematic illustrations of the sensor along with its fabrication steps for biomarker detection have been described from A. Reproduced with permission.63 Copyright 2023 Springer Nature. | ||
5. Graphene and MXenes: advancing bioimaging for precision medicine
Cancer stands as one of the foremost contributors to global mortality.64,65 Thus, the imperative to devise novel, sophisticated, and precise approaches within the realm of cancer research has grown significantly.65–67 These approaches target early detection and treatment, aiming to enhance diagnostic outcomes while minimizing the adverse effects of therapies. Graphene and more recently MXenes, both belonging to the family of two-dimensional (2D) materials, have emerged as pivotal contenders in this field. They are extensively investigated as versatile nanoplatforms, particularly for the diagnosis and treatment of cancer, harnessing their potential as agents for photodynamic therapy. Their exceptional physicochemical attributes render them valuable assets in the realm of photodynamic therapy (PDT), effectively synergizing with bioimaging, photothermal therapy, and drug and gene delivery. Diverse nanoplatforms with varying compositions and nanostructures have been created as nano-agents for photothermal conversion, aiming to amplify the therapeutic impact of photothermal therapy (PTT). These platforms encompass well-established entities like gold nanoparticles, graphene, and its derived forms, black phosphorus, transition-metal dichalcogenides, and certain organic nano-systems. Moreover, the precise determination of tumor localization holds significant importance in refining therapeutic precision and minimizing the harm caused to neighboring healthy cells and tissues through PTT. This objective can be realized through imaging guidance and continuous monitoring before and during PTT hyperthermia.68 The distinctive advantages of each diagnostic imaging modality have been clearly illustrated. For instance, computed tomography stands out as a potent tool in medical diagnosis due to its exceptional spatial resolution and valuable tomographic insights into anatomical structures.69 Additionally, MRI offers enhanced information on soft tissue anatomical structures through a non-invasive and nonionizing approach, setting it apart from other imaging methods.70 As a burgeoning diagnostic imaging technique, photoacoustic (PA) imaging has the potential to surpass the penetration limitations of optical imaging.71,72 It achieves this by capturing pressure waves induced in laser-irradiated tissues, making use of its low tissue-attenuation coefficient. This capability enables real-time visualization of biological structures and functional details. The integration of various imaging modalities holds significant promise in comprehensively and synergistically collecting information from living subjects to enhance diagnostic accuracy. MXenes, renowned for their exceptional photothermal attributes, have also been explored in the context of antimicrobial therapy.73 In comparison to alternative photothermal agents, MXenes exhibit the subsequent advantages: (1) they demonstrate biocompatibility both in vitro and in vivo; (2) their unique two-dimensional (2D) structural makeup facilitates more effective interaction with bacteria, thereby enhancing their antibacterial efficiency; (3). the distinct edges of MXene nanosheets possess the ability to disrupt bacterial membranes, showcasing innate antibacterial properties. These distinct anti-bacterial properties of MXenes have been explored by scientists. One of the interesting pieces of work was carried out by Yang et al. They reported a novel clinical implant into cells, utilizing 2D niobium carbide (Nb2C) MXene on titanium, which offers advanced antimicrobial and antibiofilm functions (Fig. 8).74 It combats infections by disrupting biofilms, sensitizing bacteria through photothermal therapy, and reducing inflammation for improved tissue healing. This innovation addresses critical challenges in antibiotic resistance and biofilm-related infections.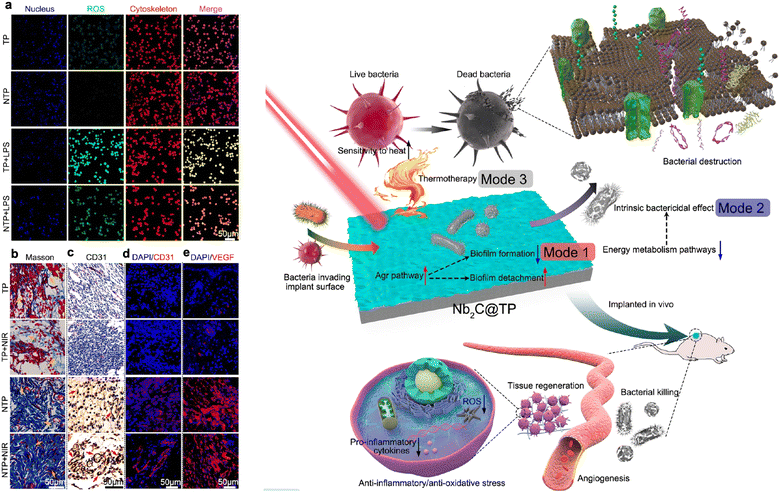 | ||
| Fig. 8 (a) Fluorescent images of reactive oxygen species. (b) Masson and immunohistochemical staining for CD31 cells. (c) Immunohistochemical staining was performed for CD31, with red arrows indicating CD31-positive cells. (d) and (e) Immunofluorescence staining of cells. (f) Schematic description of trimodal bacterial killing by an MXene composite and its application in tissue regeneration. Reproduced with permission.74 Copyright 2020 American Chemical Society. | ||
However, the conventional employment of PTT for tackling bacterial infections often necessitates elevated localized temperatures, which can inadvertently induce nonselective thermal effects, leading to inflammation and harm to nearby healthy tissues. Therefore, more comprehensive research is required before clinical application.
6. Revolutionizing theragnostics using graphene and MXenes at the forefront
In the realm of theragnostic applications utilizing 2D materials, the traditional concept of theragnostic agents demands the integration of both therapeutic and diagnostic components within a single formulation.75 However, an evolving perspective expands the definition of theragnostics to incorporate tools where imaging serves to guide therapy rather than solely for diagnosis. Theragnostic nanomedicines, utilizing 2D materials, open opportunities for integrating multiple imaging techniques and therapeutic functions. This includes passive and active targeting for various disease treatments, effectively serving as theragnostics for drug delivery, as well as enabling stimuli-responsive drug release, such as temperature- and pH-dependent therapy. Among the 2D materials, graphene offers exceptional attributes including remarkable lightweight and flexibility, a high surface-to-volume ratio, near-infrared (NIR) light absorption, and distinctive Raman spectra, making it highly appealing for disease therapy applications.76Additionally, graphene oxide (GO) stands out as a promising photoacoustic (PA) imaging agent due to its intrinsic high photothermal conversion efficiency. Graphene and its derivatives have found widespread application in diverse therapeutic approaches, including photothermal therapy, photodynamic therapy, and multimodal therapy, offering promising strategies for treating various diseases, notably cancer.77 Their versatility in these treatments showcases the significant potential of graphene-based materials in advancing medical interventions and improving patient outcomes.77 Kumawat et al. synthesized and applied a fluorescent graphene-based nanohybrid, termed “GO-PEI-GQDs,” as a promising nano-theragnostic system for cancer treatment.78 This hybrid capitalized on the excellent intrinsic properties of GQDs and GO, demonstrating remarkable biocompatibility and proficient bioimaging in both L929 and MDA-MB-231 cells. Importantly, this nanohybrid proved highly effective even at very low concentrations (50 μg mL−1) and under low-power laser density (0.5 W cm−2) using an 808 nm laser. The combined photothermal and photodynamic attributes of the nanohybrid resulted in a synergistic treatment approach for combating cancer cells.
Recently, significant attention has been directed toward MXenes and their composite materials within the domain of cancer nano-theranostics. Lu et al. demonstrated the application of MXene nanosheets as an effective photothermal therapy agent (Fig. 9).79 This method can effectively kill cancer cells with synergistic photothermal/chemotherapy and provides remarkable photoacoustic imaging with high photothermal conversion efficiency in near-infrared regions. This attraction is owed to their captivating mechanical, optical, electronic, and thermal attributes. Their hydrophilicity and expansive surface area, amenable to functionalization and modification, position them as promising contenders for targeted cancer monotherapy as well as precise imaging and diagnosis of cancer cells and tumor sites.28,80,81 Innovative MXene-based systems, encompassing enhanced solubility, heightened targeting/selectivity, multifunctionality, biocompatibility, and minimal toxicity, have emerged as viable options for targeted delivery of anticancer drugs, and for employing photothermal, photodynamic, and chemo-dynamic therapies, in conjunction with magnetic resonance and computed tomography imaging.82,83 Zhu et al. demonstrated that the combination of MXene (particularly Ti3C2) nanosheets, exhibiting remarkable near-infrared (NIR) responsiveness, with gold nanorods resulted in nanohybrids with exceptional photothermal conversion efficiency.84 This synergy between the gold nanorods and an MXene showed great promise for effective cancer therapy. Furthermore, MXenes have been utilized to load anticancer drugs, like doxorubicin, and have shown distinct pH/NIR responsive drug release behaviors when subjected to NIR irradiation, attributed to strong π–π stacking interactions between MXene-based composites and doxorubicin.85
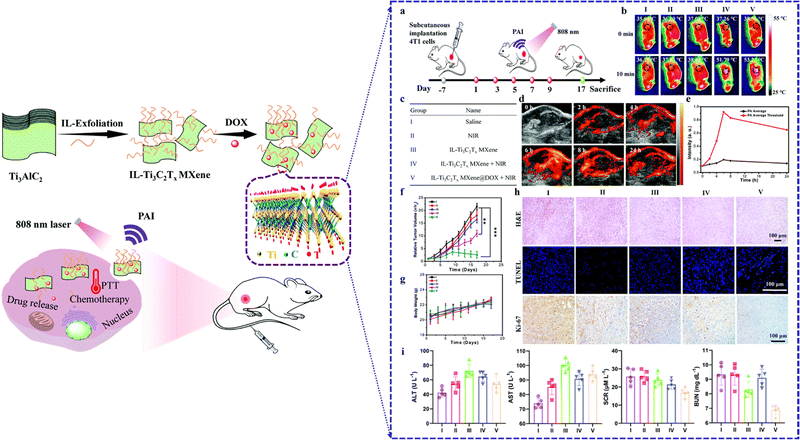 | ||
| Fig. 9 Schematic illustration of MXene nanosheets investigated for photothermal/chemo-photothermal therapy (PTT), and targeted anticancer drug delivery treatment. Various in vivo treatment and imaging of MXene nanosheets (a)–(i). Reproduced with permission.79 Copyright 2022 Royal Society of Chemistry. | ||
Moreover, MXenes possessing high drug-loading capacities and efficient photothermal conversion capabilities has exhibited pH-responsive and NIR laser-triggered on-demand drug-release behaviors. This breakthrough paves the way for synergistic photothermal tumor ablation and chemotherapy both in vitro and in vivo.85 Furthermore, these materials hold potential as contrast agents for photoacoustic imaging, presenting a promising avenue for diagnostic imaging guidance and real-time monitoring throughout therapeutic interventions. This discussion thus delves into the latest advancements in the realm of cancer nanotheranostic applications involving MXenes and their composite counterparts. Numerous investigations have concentrated on the formulation of MXenes and their composites with potential for both diagnostics and therapeutics.29,86
Nonetheless, in comparison to other scrutinized 2D structures such as graphene and its derivatives, the field has witnessed fewer endeavors in exploring the concurrent therapeutic and diagnostic utility of these materials. MXenes, characterized by their distinct architectures and surface chemistry conducive to the in situ growth of superparamagnetic Fe3O4 nanocrystals, have been harnessed in constructing superparamagnetic 2D MXene (Ti3C2)-based structures tailored for precise cancer theragnostic applications.87 These biocompatible composite entities have demonstrated impressive features including a considerable photothermal conversion efficiency (∼48.6%) suitable for the photothermal eradication of cancer cells and the efficient ablation of tumor tissues both in vitro and in vivo. Additionally, they exhibit excellent T2 relaxivity (∼394.2 mM−1 s−1) and effectively enhance contrast in tumor imaging through MRI. This advancement charts a novel course for the realm of cancer theragnostics.87
Similarly, ultrathin Ta4C3 MXene nanosheets were synthesized and then leveraged for the on-surface in situ growth of superparamagnetic iron oxide nanomaterials.88 The inherent merits of hydrophilicity and low cytotoxicity position MXene-based structures as promising candidates for cancer diagnosis and therapy, carrying the potential for biosafety and eventual clinical translation.89 Furthermore, the notable capacity of MXenes to absorb a wide range of near-infrared (NIR) light and their significant efficacy in converting light to heat encourage further exploration in domains such as photoacoustic imaging and photothermal therapy.90 It is worth noting that MXenes exhibit substantial surface engineering capabilities due to the abundance of oxygen-containing groups on their surfaces, leading to enhanced colloidal stability and prolonged blood circulation upon in vivo administration.81 Despite these advantageous traits, numerous MXene variations remain largely unexplored in the context of their biomedical viability.
The prospect of designing novel MXene-based structures featuring multifaceted theragnostic potential, strong biocompatibility, and rapid biodegradability holds the promise of unlocking their versatile biomedical application and potential clinical translation. Additionally, it is worthwhile to consider hyperthermia-amplified nano-zyme catalytic therapy using MXenes as an alternative approach for treating cancers. Furthermore, the introduction of biocompatible MXene (Ti3C2)-based composites (MnOx/Ti3C2) has ushered in an era of cancer theragnostics, offering effective platforms for photothermal cancer/tumor monotherapy. These platforms have demonstrated remarkable effects in tumor ablation and suppression of tumor growth, guided by both MR and photoacoustic imaging.91
The distinctive chemical, physical, and biological attributes of both graphene and MXenes have demonstrated their potential as formidable tools in the domain of cancer theragnostics, specifically in PDT.92 These two-dimensional nano-systems offer the unique advantage of enabling concurrent utilization of non-invasive bioimaging alongside therapeutic techniques that can be seamlessly integrated with PDT. These techniques encompass photothermal therapy, magnetic therapy, and remotely controlled drug and gene delivery for chemotherapy.
Of note, while MXenes have been harnessed for photothermal cancer monotherapy, there is room for improvement in terms of enhancing their cellular internalization, potentially achieved through the application of ligands with high specificity toward cancer cells to coat their surfaces. Moreover, innovative design strategies should be employed to craft MXene-based structures responsive to biological cues such as pH, temperature, and enzymes, thereby paving the way for heightened therapeutic outcomes.
The efficacy of these promising materials as agents for photothermal therapy and imaging in tumor treatment stems from their ability to absorb light within the near-infrared (NIR) spectrum.93 Additionally, their inherent capacity for facile functionalization, facilitated by their substantial surface-to-volume ratio, permits the incorporation of photosensitizer agents onto these nanoplatforms. This augmentation enhances targeting precision and efficacy, resulting in a more localized therapeutic impact that is marked by diminished side effects and heightened therapeutic effectiveness.
7. Graphene and MXenes: enabling the next frontier of AI-driven telemedicine
The application of nanotechnology-enabled wearable continuous-monitoring devices is popular in the healthcare sector.94 Advanced wearable devices are embedded nano-enabled sensors, enabling the device to monitor physiological parameters continuously.95 However, comprehending the role of nanotechnology requires a deeper understanding, and leveraging AI for enhanced sensitivity and miniaturization is imperative. Extensive clinical datasets necessitate AI-driven analysis and training to achieve precise diagnosis and prognosis.96 Various ML techniques have recently been integrated with graphene-based sensors for data analysis, classification, and diagnosis. The integration of a sensor with a machine learning approach facilitates quicker and more comprehensive identification and tracking of both chemical substances and movements.97 Recently, the application of graphene in AI has aroused considerable attention.Graphene sensors are combined with ML techniques to implement task recognition, where graphene sensors detect the external environments and generate output signals. In contrast, ML techniques employ the information to establish the relationship between the known environments (e.g., liquid, gas, motion, voice, and image) and output signals (usually electrical signals). In addition, ML methods are suitable for property predictions, structure recognition, and inverse design of graphene.98 Gas sensing is essential for many applications, such as environmental monitoring, drug screening, medical diagnosis, food storage, and alcohol testing. The electronic properties of graphene enable the construction of electronic nose (e-nose) sensors for developing gas sensors that mimic the human olfactory system. Based on this, Hayaska et al. developed an artificial olfactory system (e-nose) for detecting volatile organic gases using graphene-based field-effect transistors (GFETs).99 ML methods have been used in the atomic-level analysis of graphene and GO, such as defect detection of graphene, bandgap regulation of doped graphene, structural analysis of GO, and the exploration of fractured C–C bonds in GO.100 Graphene strain sensors coupled with the ML system represent a great artificial tactile system to identify material species and micro-sculpture patterns, showing a complete superiority to human fingers.101 The graphene-based sensors combined with ML systems have been widely used as wearable human–machine interface (HMI) systems for applications in motion recognition. The high-aspect ratio graphene offers gel-free, high-fidelity recording of muscle activities. W. H. Yeo et al. used biocompatible solderable graphene to fabricate an all-printed wearable and wireless device that incorporates ML algorithms to implement multi-class and versatile HMI scenarios.102 Similarly, Ravenscroft et al. developed an approach to classify recorded resistance signals into predicted words. A graphene strain gauge sensor was first fabricated and worn on the throat to detect signals of small muscle movements and vocal vibrations as the training dataset of the ML.103 These ML-assisted graphene sensors have achieved ultra sensitivity through iterative analysis of data-driven sensing outcomes for developing intelligent smart sensor systems.
Incorporation of two-dimensional (2D) nanomaterials (NMs) with IoTs/5G/AI/ML technologies has transformed a wide range of sensor applications in healthcare and wearable electronics for the safety, environment, military, space, and agriculture sectors.104 Owing to their unique physicochemical characteristics and surface functionalities, borophene and MXenes have emerged as advanced 2D materials (A2M) to architect future-generation sensors. ML-based theoretical modeling has guided the research and development of A2M sensors economically by reducing cost, human resources, and contamination. A2M sensors are flexible, wearable, intelligent, biocompatible, portable, energy-efficient, self-sustained, point-of-care, and economical, and can drastically transform conventional sensing strategies.105,106 This throws light on various advancements in the artificial synapse (AS) devices by using 2D materials like MXenes with the features of AI and ML.107 These materials hold great promise for applications in AI and neural computing, due to their swift switching speed, high storage density, low energy consumption, exceptional data processing capabilities, and potential for biological-scale simulation. Moreover, efforts have been made to develop hybrid nanocomposite materials by combining 2D MXenes with 1D nanostructures, aimed at improving the adhesion stability on the transducer surface for prolonged monitoring.108 The interfacial integration of a 2D MXene/1D graphene nanoribbon has been investigated for developing the desired pressure sensor with an improved life cycle. ML approaches were utilized for training the sensors for detecting various sitting postures with >95% accuracy. Flexible, breathable, and degradable pressure sensors with excellent sensing performance are drawing tremendous attention for various practical applications in wearable artificial skins, healthcare monitoring, and artificial intelligence due to their flexibility, breathability, lightweight, decreased electronic rubbish, and environmentally friendly impact.109 Recently, on-site detection has been clubbed with solution-on-chip MXenes by interfacing biosensors with modern-age technologies, including 5G communication, internet-of-medical-things (IoMT), artificial intelligence (AI), and data clouding to progress toward hospital-on-chip (HOC) modules.43 A new bifunctional intelligent nano-sensing platform based on graphene-like titanium carbide MXene (Ti2C MXene)/Au–Ag nano-shuttles (NSs) for both electrochemical and surface-enhanced Raman scattering (SERS) intelligent analysis of ultra-trace carbendazim (CBZ) residues in tea and rice coupled with machine learning (ML) was successfully designed by Zhu et al.110 Ti2C MXene was synthesized by selectively etching Al layers of Ti2AlC with hydrofluoric acid and high-temperature calcination. Ti2C MXene/Au–Ag NSs prepared by the ultrasonic dispersion of graphene-like Ti2C MXene into Au–Ag NS solution under dark conditions displayed a large and rough surface, enhanced conductivity, excellent electrochemical response, prominent Raman enhancement, and high stability. The ML via different algorithms such as artificial neural network, support vector machine, and relevance vector machine (RVM) was contrasted for the intelligent analysis of CBZ. Ge et al. have developed a fast portable intelligent method for electrochemical detection of MPA in silage with a variable-pH microenvironment using Zn–Co MOF/Ti3C2 MXene/Fe3O4–MGO coupling with machine learning (ML).111 Integration of wearables with AI & ML has been investigated by Song et al. to fabricate a 3d printed electronic skin (e3 skin) for health monitoring.112 They fabricated epifluidic elastic electronic skin-based wearables using MXene ink with multiple electrochemical sweat biosensors (e.g., glucose, alcohol, and pH sensors) and biophysical sensors (e.g., temperature and pulse sensors) depicted in Fig. 10. Furthermore, through the integration of e3 skin data with machine learning, they successfully forecasted an individual's level of impairment in behaviors, such as reaction time and inhibitory control, following alcohol consumption. The e3-skin sets the stage for the autonomous production of personalized wearable systems, facilitating broad use in routine health monitoring and clinical contexts. Furthermore, the assembly of 2D materials for real-world diagnostics needs material selection, stability, and connectivity for advanced clinical trial and error. Prior research interest in graphene and MXenes, towards telemedicine applications exhibits tremendous attention for their utmost flexibility and conductivity, enabling the development of wearable health monitoring devices that continuously track vital signs and transmit data wirelessly to healthcare providers for remote assessment. Also, another research study illustrated in Fig. 11 [left side (a–g)] developed a highly durable and adaptable pressure sensor utilizing a composite film of MXene and polydopamine (PDA) for future clinical contexts.113 The sensor boasts a distinctive structure integrating spherical PDA molecules, leading to exceptional sensing capabilities. With a sensitivity of 138.8 kPa within the pressure range of 0.18–6.20 kPa with rapid response and recovery times (t1 < 100 ms; t2 < 50 ms), it proves ideal for portable and wearable applications. Its versatility extends to real-time monitoring of various health-related signals such as wrist pulse, finger motions, vocalization, and facial expressions, exhibiting both high sensitivity and accuracy that meets potential clinical healthcare requirements.
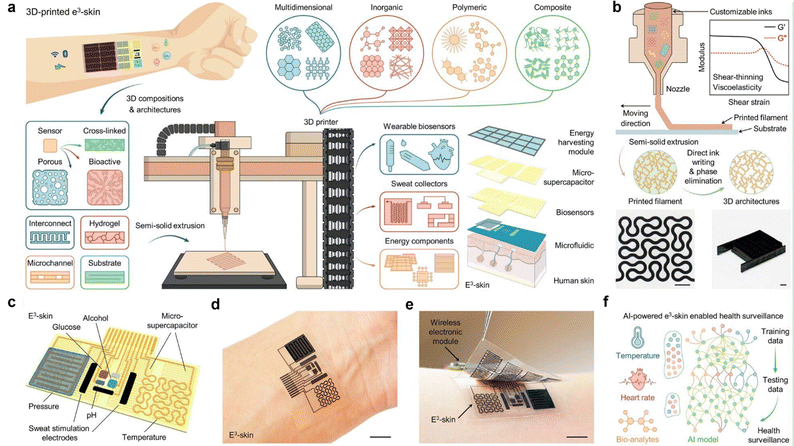 | ||
| Fig. 10 (a) Schematic depiction of SSE-based 3D printing, enabling customizable inks for wearable e3-skin with diverse sensing and power management capabilities. (b) Fabrication procedure. (c)–(e) 3D-printed e3-skin integrates biophysical and biochemical sensors, microfluidic sweat sampling, and energy storage, as shown in optical images of a human subject. (f) Machine learning-enhanced e3-skin offers personalized health monitoring, combining AI with multisensory data from the skin. Reproduced with permission.112 Copyright 2023 American Association for the Advancement of Science. | ||
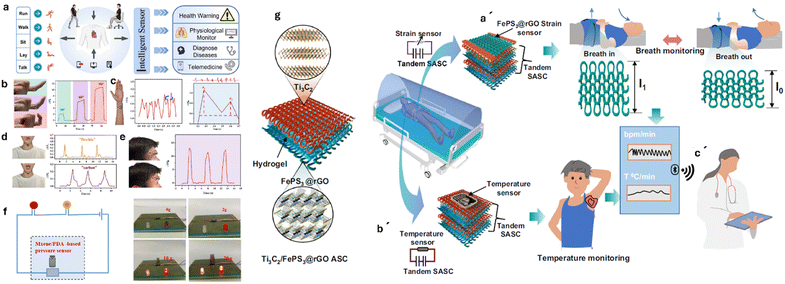 | ||
| Fig. 11 (a) Schematic of health monitoring applications of an MXene/PDA film based flexible sensor. (b)–(f) Demonstration of the sensing performance and various characterizations of the pressure sensor on different parts of the body and (g) fabrication of the sensor. (a′)–(c′) A real time application of the strain sensor in a hospital setting for continues health monitoring of a patient depicting the telemedicine approach. Reproduced with permission.113 Copyright 2023, Wiley. Reproduced with permission.3 Copyright 2022 Springer Nature. | ||
There are certain challenges to the practical implementation of these devices. Integration into existing diagnostic systems requires careful consideration of scalability, cost-effectiveness, and regulatory issues for various critical diseases like Alzheimer, epilepsy, cancer, etc.39,114,115 Concerns related to large-scale production, ensuring reproducibility and public trial must be addressed. Additionally, compatibility with current diagnostic systems is critical for seamless integration in healthcare. Collaborative efforts between researchers, industry stakeholders, and regulatory bodies are crucial for navigating these challenges and realizing the transformative impact of advanced materials in practical diagnostic applications. Most importantly, human trials are needed which address the clinical promise of these sensors for future healthcare applications. Amidst the rise of infectious diseases, remote surveillance of infected individuals is crucial, particularly for hospital settings aiming to prevent pathogen spread.116,117 Hence, a remote health monitoring system was reported by merging a stretchable asymmetric supercapacitor (SASC) as a portable power source with sensors capable of real-time monitoring of human health indicators.3 An abnormal body temperature or breathing rate can signal illness or infection. The system combines a FePS3@graphene-based strain sensor and SASC into a textile system, wrapped around the abdomen to continuously monitor breathing cycles shown in Fig. 11 [right side: (a′–c′)]. Real-time body temperature is recorded by integrating a temperature sensor with the SASC. This setup enables remote monitoring and serves as a screening tool for infection status. The system, affixed directly to the body, allows accurate monitoring of breathing rate and body temperature without direct contact with healthcare personnel. Data from monitoring are wirelessly transmitted to hospital cloud systems for clinical assessment. This wearable health monitoring system integrates sensors with SASC for real-time physiological monitoring, aiding in the early identification of infections or emergencies. The assembly method involves spray-coating deposition for Ti3C2 and FePS3@rGO-based stretchable electrodes, assembled with a polymer gel electrolyte. The resulting SASC device exhibits high electrochemical performance. Additionally, FePS3@rGO-based energy storage and strain sensors are fabricated for flexible and stretchable power sources, successfully monitoring live breathing patterns. Integration with a temperature sensor enables real-time body temperature monitoring, wirelessly relaying data to smartphones. These wireless devices can be vital in emergency rooms for monitoring various infectious diseases and ensuring the safety of healthcare personnel. We have summarized a comparative table of various sensors based on graphene, MXenes, and their composites for telemedicine applications (Table 2).
| Material | Type of sensing | Application | Analyte | Remarks | Ref. |
|---|---|---|---|---|---|
| Graphene | Electrochemical | Wearable sensor for inflammation monitoring | Sweat | Detection of C-reactive protein (CRP), a key indicator of inflammation | 63 |
| Graphene field effect transistor (GFET) | Electrical | Point of care cancer detection | Exosome as cancer biomarker | Detection of (GPC-1 expression) | 45 |
| MXene hydrogel | Hydrogel as drug loading system | Microneedle patch for wound healing | Asiaticoside as therapeutic agent | Diabetic foot ulcer treatment | 51 |
| MXene composite | Electrochemical | Glucose monitoring | Glucose detection using enzyme glucose oxidase | Probable applications of pristine MXenes toward the field of biosensors | 54 |
| Niobium carbide (Nb2C) MXene titanium plate nanosheets | Thermotherapy and nanomedicine | Bacterial infection elimination and tissue regeneration | MXene composite showing anti-bacterial activity | Thermotherapy not only killed bacteria in vivo but also promoted angiogenesis | 74 |
| MXene and graphene composite | Electromechanical | Wearables as a multifunctional sensor for strain, temperature, and electrocardiogram (ECG) monitoring | Body deformations including biopotential | High strain sensitivity (2075; >22% strain) a good TCR (0.52% K−1) and low skin-electrode impedance (51.08 kΩ at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hz) Hz) |
58 |
| MXene nanosheets | Electrochemical chip | 3D-printed microfluidic wearable sensor | Sweat, body temperature, heart rate | AI driven chip for health monitoring | 113 |
8. Challenges
The evolution of telemedicine has unlocked novel avenues for healthcare improvements. A fundamental necessity is exploring how nanomaterials engage with diverse surfaces, compartments, organelles, living cells, tissues, and organisms within the human body. Despite the promising potential of nanotechnology, there remain numerous unresolved inquiries and safety issues that require careful consideration.There are some key limitations in these next-generation 2D materials for developing wearable devices as they directly interact with human skin, tissue, and vital organs. Concerns over long-term impacts on human health are raised by the high surface area and propensity for oxidative stress, especially in wearable sensor applications. Therefore, to mitigate the potential adverse effects on the human body we must bring forth changes for smooth integration of these advanced materials into wearable technologies. In this paragraph, we will discuss the major challenges associated with MXenes and graphene in developing devices and possible solutions to reduce the effect.
The complex synthesis process of MXenes and graphene may introduce residual chemicals, raising concerns about skin irritation and potential long-term effects.118 Employing rigorous purification methods and incorporating protective coatings can enhance stability and reduce the risk of adverse reactions.119–121 The synthesis and application of MXene and graphene-based materials require sustainable techniques due to potential environmental implications associated with their manufacture and disposal. Tailoring the surface properties of MXenes and graphene through functionalization during synthesis with bio-friendly molecules can reduce the chemical residue, making them suitable for prolonged skin contact in healthcare applications.
There are concerns about MXenes and graphene's biocompatibility—a critical factor for wearable sensor applications—brought up by their interactions with biological systems. According to studies, some graphene compounds, like graphene oxide, can cause inflammation and cytotoxicity in biological tissues.122 Wearable sensor design must carefully examine MXenes’ biocompatibility, which differs depending on parameters including surface functionalization and synthesis techniques.123,124 Applying biocompatible coatings on MXene and graphene surfaces can serve as a protective barrier, preventing direct contact with the skin and reducing the risk of adverse reactions. MXenes and graphene along with their derivatives exhibit excellent electrical conductivity; therefore, considerations must be made to prevent excessive exposure to the human body. This could lead to skin soreness or thermal discomfort for wearables.125 Designing wearable devices with flexible substrates ensures mechanical compatibility, allowing the device to conform to body contours without causing discomfort or potential injury. Integrating within insulating layers or adopting lower conductivity variants may provide safer options.126
While in vivo studies of nanomedicine show promising outcomes, most of them are still limited to laboratory research, with only a few being applied in clinical settings. The toxicity, side effects, efficacy, and internal metabolic conversion of nanoparticles remain areas where our understanding is limited. Due to their smaller size, nanomaterials require different assessment methods compared to conventional substances, and new routes of exposure have emerged, allowing them to interact with cellular portals differently. As this technology is still in its early stages, new biological interactions and undiscovered toxicities may arise.
One significant concern is nanotoxicology, as there is no internationally accepted standard protocol for toxicity testing of nanomaterials.127 Different researchers use their protocols, making it challenging to establish consistent nanotoxicity results. Furthermore, certain issues such as the local supply chain of nanotechnology-based products and the potential for local invention or fabrication have not received sufficient attention. In addition to the numerous benefits, some limitations hinder commercial use, such as mono-dispersity, unregulated size, and cumbersome production procedures of nanomaterials. These factors need to be addressed to unlock the full potential of telemedicine for practical applications.
Conquering these hurdles is imperative, given that the future of telemedicine in the field of medicine holds significant promise. With its remarkable advantages, telemedicine has the potential to revolutionize the healthcare industry. However, ensuring safety and addressing any uncertainties will be key to fully harnessing its potential to improve health outcomes. While the advent of MXenes has significantly broadened the realm of two-dimensional materials and their diverse applications, the strategic design of MXenes and their composites for cancer nano-theragnostics encompassing photothermal/photodynamic therapy, radiotherapy, catalytic therapy, and imaging attributes remains an enduring challenge in the field of biomedicine. Enhancing the mechanical, electronic, thermal, and optical properties of these materials through hybridization and surface functionalization/modification holds potential for their effective utilization in cancer diagnosis and therapy. Given that clinical trials involving MXenes in nanomedicine are still in their nascent stages, further investigations should prioritize crucial aspects such as optimization conditions, facile and environmentally benign synthesis methodologies, clinical translational assessments, potential long-term toxicity/biosafety concerns, pharmacokinetics, targeting capabilities, and their responsiveness to stimuli. To comprehensively address critical concerns in terms of biosafety, various avenues of inquiry are necessary, encompassing biocompatibility, biodegradability (including degradation rate and extent), blood circulation, and excretion behaviors. The extended presence of nanomaterials raises concerns about potential inflammation, oxidative harm, and fibrosis. Conversely, a specific subset of MXenes, predominantly Ti3C2, has recently captured significant attention for its exceptional chemical and physical attributes in biomedical contexts. Therefore, forthcoming explorations in cancer theranostics should expand their scope to encompass other MXene types beyond Ti3C2, all while meticulously considering optimal synthesis conditions and functionalization techniques.
9. Conclusions and viewpoint
Telemedicine plays a pivotal role in shaping the trajectory of medical research and patient care, particularly in an era where personalized medicine and innovative therapies take precedence in healthcare. In summary, this review underscores significant advancements in utilizing graphene-based materials and MXenes for telemedicine applications. We have focused on cancer to demonstrate the importance of graphene-based materials and MXenes for telemedicine applications. However, this can be extended to other diseases as well. Nonetheless, three primary factors warrant increased attention: (i) the possible non-targeted toxicity, (ii) the careful selection of physical–chemical attributes of materials before their assessment in cancer therapy thorough characterization (iii), and (iv) the evaluation of their potential biodegradability. Despite the existing gaps in our understanding of this field, more than 30 analyzed studies have revealed promising prospects for GBMs and MXenes. These materials have emerged as the most auspicious candidates among 2D nanomaterials, holding the potential to reshape the landscape of conventional cancer theragnostics and ushering in novel protocols for personalized therapeutic approaches.In summary, the synergy between AI, ML, and telemedicine is reshaping the healthcare landscape. This alliance enables the realization of personalized medicine by harnessing the analytical power of AI and ML to decode vast datasets, detect subtle patterns, and predict patient-specific needs. With AI and ML at the forefront, healthcare is on the cusp of a revolution where treatment plans are tailored to the genetic and health profiles of each patient. Complementing this revolution, telemedicine provides the tools at the nanoscale, allowing for precise drug delivery, real-time health monitoring, and unprecedented insights into cellular and molecular processes. By manipulating materials at this tiny scale, telemedicine opens the doors to diagnostics and treatments that were once deemed science fiction. Collectively, this integration promises to make healthcare genuinely patient-centric. It enhances our understanding of diseases at a molecular level and unlocks the potential to develop innovative therapies and diagnostics that were previously unimaginable. The possibilities are limitless, and the future of healthcare is both thrilling and auspicious. In conclusion, the interplay of AI, ML, and telemedicine is not only defining a new era in healthcare but also pushing the boundaries of medical research and patient care. As we continue to explore these emerging frontiers, it is evident that AI, ML, and telemedicine will continue to be fundamental forces shaping the healthcare landscape for years to come. In Fig. 12, we depicted the futuristic applications of graphene and MXene in some of the emerging areas, including implantable sensors, artificial organs, advanced sensors, etc. in conjunction with AI for smart healthcare systems.
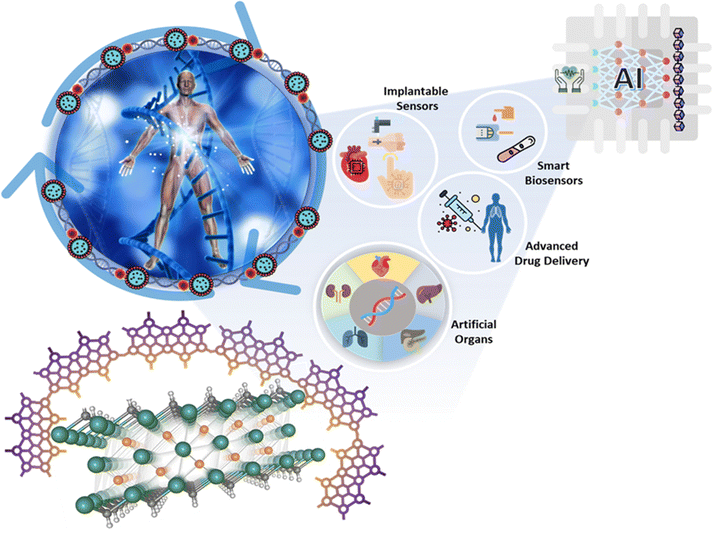 | ||
| Fig. 12 Graphene and MXenes exhibit promising futuristic applications within the field of telemedicine. | ||
Nevertheless, a more comprehensive grasp of the interplay between nanotechnology and its synergy with AI is imperative to achieve the desired levels of sensitivity and miniaturization. Establishing a connection between nanotechnology and AI-driven systems that interface with IoMT technology is of paramount importance in forging innovative healthcare solutions, including the realms of telemedicine and nanorobotics. The healthcare domain is witnessing a surge in the popularity of nanotechnology-enabled wearable continuous-monitoring devices. These advanced wearable devices are embedded with nano-enabled sensors, enabling uninterrupted monitoring of physiological parameters. For more precise diagnosis and prognosis, the abundant clinical dataset necessitates AI-driven analysis and training.
Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript. K. R. K. – visualized, conceptualizing, and writing. D. T. writing and editing. H. M. – writing, editing, conceptualizing. K. A. – writing and editing. A. K. – editing and mentoring.Conflicts of interest
There are no conflicts of interest among the authors.Acknowledgements
The authors want to acknowledge their respective institutions for their support.References
- K. R. Khondakar, S. Dey, A. Wuethrich, A. A. I. Sina and M. Trau, Acc. Chem. Res., 2019, 52, 2113–2123 CrossRef CAS PubMed.
- R. M. Torrente-Rodríguez, H. Lukas, J. Tu, J. Min, Y. Yang, C. Xu, H. B. Rossiter and W. Gao, Matter, 2020, 3, 1981–1998 CrossRef PubMed.
- J. V. Vaghasiya, C. C. Mayorga-Martinez and M. Pumera, npj Flexible Electron., 2022, 6, 73 CrossRef CAS PubMed.
- R. van der Meel, E. Sulheim, Y. Shi, F. Kiessling, W. J. Mulder and T. Lammers, Nat. Nanotechnol., 2019, 14, 1007–1017 CrossRef CAS PubMed.
- J. Bedolla and J. M. Pines, Evidence-Based Emergency Care: Diagnostic Testing and Clinical Decision Rules, 2023, pp. 699–722 Search PubMed.
- Z. Su, C. Li, H. Fu, L. Wang, M. Wu and X. Feng, Intell. Med., 2024, 4, 1–9 CrossRef.
- K. K. Reza, S. Srivastava, S. K. Yadav and A. Biradar, Mater. Lett., 2014, 126, 126–130 CrossRef.
- N. Singh, K. K. Reza, M. A. Ali, V. V. Agrawal and A. Biradar, Biosens. Bioelectron., 2015, 68, 633–641 CrossRef CAS PubMed.
- K. K. Reza, M. K. Singh, S. K. Yadav, J. Singh, V. V. Agrawal and B. Malhotra, Sens. Actuators, B, 2013, 177, 627–633 CrossRef.
- O. Samy, S. Zeng, M. D. Birowosuto and A. El Moutaouakil, Crystals, 2021, 11, 355 CrossRef CAS.
- Y. Li, S. Huang, S. Peng, H. Jia, J. Pang, B. Ibarlucea, C. Hou, Y. Cao, W. Zhou and H. Liu, Small, 2023, 19, 2206126 CrossRef CAS PubMed.
- J. Rawat, D. Sajwan, S. V. Garimella, H. Sharma and C. Dwivedi, Nano Trends, 2023, 100008 CrossRef.
- W. Yu, L. Sisi, Y. Haiyan and L. Jie, RSC Adv., 2020, 10, 15328–15345 RSC.
- C. Cong, J. Shang, Y. Wang and T. Yu, Adv. Opt. Mater., 2018, 6, 1700767 CrossRef.
- C. Witteveen, K. Górnicka, J. Chang, M. Månsson, T. Klimczuk and F. O. von Rohr, Dalton Trans., 2021, 50, 3216–3223 RSC.
- A. Eftekhari, J. Mater. Chem. A, 2017, 5, 18299–18325 RSC.
- Y. Sun, K. Fujisawa, M. Terrones and R. E. Schaak, J. Mater. Chem. C, 2017, 5, 11317–11323 RSC.
- N. Talreja, D. Chuahan and M. Ashfaq, Mater. Adv., 2024, 5, 1454–1461 RSC.
- N. Lundt, A. Maryński, E. Cherotchenko, A. Pant, X. Fan, S. Tongay, G. Sęk, A. V. Kavokin, S. Höfling and C. Schneider, 2D Mater., 2016, 4, 015006 CrossRef.
- R. Zazpe, H. Sopha, J. Charvot, R. Krumpolec, J. Rodriguez-Pereira, J. Michalička, J. Mistrík, D. Bača, M. Motola and F. Bureš, Appl. Mater. Today, 2021, 23, 101017 CrossRef.
- A. Jayakumar, S. Mathew, S. Radoor, J. T. Kim, J.-W. Rhim and S. Siengchin, Mater. Today Chem., 2023, 30, 101492 CrossRef CAS.
- Y. Wang, X. Xu, X. Chen and J. Li, Adv. Mater., 2022, 34, 2107406 CrossRef CAS PubMed.
- F. R. Fan, R. Wang, H. Zhang and W. Wu, Chem. Soc. Rev., 2021, 50, 10983–11031 RSC.
- M. A. Ali, K. Kamil Reza, S. Srivastava, V. V. Agrawal, R. John and B. D. Malhotra, Langmuir, 2014, 30, 4192–4201 CrossRef CAS PubMed.
- K. K. Reza, M. A. Ali, S. Srivastava, V. V. Agrawal and A. Biradar, Biosens. Bioelectron., 2015, 74, 644–651 CrossRef CAS PubMed.
- K. K. Reza, S. Dey, A. Wuethrich, A. A. I. Sina, D. Korbie, Y. Wang and M. Trau, Nanoscale, 2018, 10, 18482–18491 RSC.
- A. Gosai, K. R. Khondakar, X. Ma and M. A. Ali, Biosensors, 2021, 11, 384 CrossRef CAS PubMed.
- L. Chen, X. Dai, W. Feng and Y. Chen, Acc. Mater. Res., 2022, 3, 785–798 CrossRef CAS.
- A. Szuplewska, D. Kulpińska, A. Dybko, M. Chudy, A. M. Jastrzębska, A. Olszyna and Z. Brzózka, Trends Biotechnol., 2020, 38, 264–279 CrossRef CAS PubMed.
- H. Lin, Y. Chen and J. Shi, Adv. Sci., 2018, 5, 1800518 CrossRef PubMed.
- T. Habib, X. Zhao, S. A. Shah, Y. Chen, W. Sun, H. An, J. L. Lutkenhaus, M. Radovic and M. J. Green, npj 2D Mater. Appl., 2019, 3, 8 CrossRef.
- X. Li, Z. Huang, C. E. Shuck, G. Liang, Y. Gogotsi and C. Zhi, Nat. Rev. Chem., 2022, 6, 389–404 CrossRef PubMed.
- K. Huang, Z. Li, J. Lin, G. Han and P. Huang, Chem. Soc. Rev., 2018, 47, 5109–5124 RSC.
- Y. Gogotsi and B. Anasori, ACS Nano, 2019, 13, 8491–8494 CrossRef CAS PubMed.
- S. Iravani and R. S. Varma, ACS Biomater. Sci. Eng., 2021, 7, 1900–1913 CrossRef CAS PubMed.
- M. Machado, A. M. Oliveira, G. A. Silva, D. B. Bitoque, J. Tavares Ferreira, L. A. Pinto and Q. Ferreira, Nanomaterials, 2022, 12, 1624 CrossRef CAS PubMed.
- B. Fang, D. Chang, Z. Xu and C. Gao, Adv. Mater., 2020, 32, 1902664 CrossRef CAS PubMed.
- M. Christian, R. Mazzaro and V. Morandi, Adv. Funct. Mater., 2020, 30, 2007458 CrossRef CAS.
- K. R. Khondakar, M. S. Anwar, H. Mazumdar and A. Kaushik, Mater. Adv., 2023, 4, 4991–5002 RSC.
- J. A. Carrasco, P. Congost-Escoin, M. Assebban and G. Abellán, Chem. Soc. Rev., 2022, 36, e00180 Search PubMed.
- A. Aziz, M. Asif, G. Ashraf, T. Iftikhar, W. Hussain and S. Wang, Trends Environ. Anal. Chem., 2022, e00180 CrossRef CAS.
- V. Chaudhary, R. Chowdhury, P. Thukral, D. Pathania, S. Saklani, S. Rustagi, A. Gautam, Y. K. Mishra, P. Singh and A. Kaushik, Environ. Res., 2023, 115933 CrossRef CAS PubMed.
- V. Chaudhary, V. Khanna, H. T. A. Awan, K. Singh, M. Khalid, Y. K. Mishra, S. Bhansali, C.-Z. Li and A. Kaushik, Biosens. Bioelectron., 2023, 220, 114847 CrossRef CAS PubMed.
- G. Li, Z. Liu, W. Gao and B. Tang, Coord. Chem. Rev., 2023, 478, 214966 CrossRef CAS.
- T. Yin, L. Xu, B. Gil, N. Merali, M. S. Sokolikova, D. C. Gaboriau, D. S. Liu, A. N. Muhammad Mustafa, S. Alodan and M. Chen, ACS Nano, 2023, 17, 14619–14631 CrossRef CAS PubMed.
- L. Zhu and L. Chen, Polym. Bull., 2022, 1–16 Search PubMed.
- A. R. Monteiro, M. G. P. Neves and T. Trindade, ChemPlusChem, 2020, 85, 1857–1880 CrossRef CAS PubMed.
- S. Ushiba, T. Ono, Y. Kanai, K. Inoue, M. Kimura and K. Matsumoto, ACS Omega, 2018, 3, 3137–3142 CrossRef CAS PubMed.
- N. Dwivedi, C. Dhand, P. Kumar and A. Srivastava, Mater. Adv., 2021, 2, 2892–2905 RSC.
- Q. Xue, H. Zhang, M. Zhu, Z. Pei, H. Li, Z. Wang, Y. Huang, Y. Huang, Q. Deng and J. Zhou, Adv. Mater., 2017, 29, 1604847 CrossRef PubMed.
- P. Wang, Y. Wang, Y. Yi, Y. Gong, H. Ji, Y. Gan, F. Xie, J. Fan and X. Wang, J. Nanobiotechnol., 2022, 20, 259 CrossRef CAS PubMed.
- M. Song, S. Y. Pang, F. Guo, M. C. Wong and J. Hao, Adv. Sci., 2020, 7, 2001546 CrossRef CAS PubMed.
- M. R. Farani, B. N. Khiarak, R. Tao, Z. Wang, S. Ahmadi, M. Hassanpour, M. Rabiee, M. R. Saeb, E. C. Lima and N. Rabiee, Environ. Sci.: Nano, 2022, 9, 4038–4068 RSC.
- H. L. Chia, C. C. Mayorga-Martinez, N. Antonatos, Z. K. Sofer, J. J. Gonzalez-Julian, R. D. Webster and M. Pumera, Anal. Chem., 2020, 92, 2452–2459 CrossRef CAS PubMed.
- Q. Tian, W. Yan, Y. Li and D. Ho, ACS Appl. Mater. Interfaces, 2020, 12, 9710–9717 CrossRef CAS PubMed.
- Y. Yue, N. Liu, Y. Ma, S. Wang, W. Liu, C. Luo, H. Zhang, F. Cheng, J. Rao and X. Hu, ACS Nano, 2018, 12, 4224–4232 CrossRef CAS PubMed.
- A. Zarepour, S. Ahmadi, N. Rabiee, A. Zarrabi and S. Iravani, Nano-Micro Lett., 2023, 15, 100 CrossRef CAS PubMed.
- S. Zhang, A. Chhetry, M. A. Zahed, S. Sharma, C. Park, S. Yoon and J. Y. Park, npj Flexible Electron., 2022, 6, 11 CrossRef CAS.
- K. K. Reza, S. Dey, A. Wuethrich, J. Wang, A. Behren, F. Antaw, Y. Wang, A. A. I. Sina and M. Trau, ACS Nano, 2021, 15, 11231–11243 CrossRef CAS PubMed.
- K. K. Reza, A. A. I. Sina, A. Wuethrich, Y. S. Grewal, C. B. Howard, D. Korbie and M. Trau, Biosens. Bioelectron., 2019, 126, 178–186 CrossRef CAS PubMed.
- K. Kamil Reza, J. Wang, R. Vaidyanathan, S. Dey, Y. Wang and M. Trau, Small, 2017, 13, 1602902 CrossRef PubMed.
- J. K. Wychowaniec, J. Litowczenko, K. Tadyszak, V. Natu, C. Aparicio, B. Peplińska, M. W. Barsoum, M. Otyepka and B. Scheibe, Acta Biomater., 2020, 115, 104–115 CrossRef CAS PubMed.
- J. Tu, J. Min, Y. Song, C. Xu, J. Li, J. Moore, J. Hanson, E. Hu, T. Parimon, T.-Y. Wang, E. Davoodi, T.-F. Chou, P. Chen, J. J. Hsu, H. B. Rossiter and W. Gao, Nat. Biomed. Eng., 2023, 7, 1293–1306 CrossRef CAS PubMed.
- K. R. Khondakar, M. Ataei Kachouei, F. E. E. Erukainure and M. A. Ali, ECS Sens. Plus, 2023, 2, 043403 CrossRef CAS.
- K. R. Khondakar and A. K. Kaushik, Nanotechnology in Cancer Management: Precise Diagnostics Toward Personalized Health Care, Elsevier, 2021 Search PubMed.
- S. Dey, K. K. Reza, A. Wuethrich, D. Korbie, A. A. I. Sina and M. Trau, Biotechnol. Adv., 2019, 37, 145–153 CrossRef CAS PubMed.
- S. Dey, R. Vaidyanathan, K. K. Reza, J. Wang, Y. Wang, H. J. Nel, S.-C. Law, J. Tyler, J. Rossjohn and H. H. Reid, Sens. Actuators, B, 2019, 284, 281–288 CrossRef CAS.
- C. Dai, Y. Chen, X. Jing, L. Xiang, D. Yang, H. Lin, Z. Liu, X. Han and R. Wu, ACS Nano, 2017, 11, 12696–12712 CrossRef CAS PubMed.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu and H. Xing, Adv. Mater., 2014, 26, 1886–1893 CrossRef CAS PubMed.
- P. Mi, D. Kokuryo, H. Cabral, H. Wu, Y. Terada, T. Saga, I. Aoki, N. Nishiyama and K. Kataoka, Nat. Nanotechnol., 2016, 11, 724–730 CrossRef CAS PubMed.
- I. Hasan, S. Roy, B. Guo, S. Du, W. Tao and C. Chang, Biomater. Sci., 2023, 11, 1270–1310 RSC.
- K. Zhang, Y. Sun, S. Wu, M. Zhou, X. Zhang, R. Zhou, T. Zhang, Y. Gao, T. Chen and Y. Chen, Eur. J. Nucl. Med. Mol. Imaging, 2021, 48, 1736–1758 CrossRef PubMed.
- S. Hao, H. Han, Z. Yang, M. Chen, Y. Jiang, G. Lu, L. Dong, H. Wen, H. Li and J. Liu, Nano-Micro Lett., 2022, 14, 178 CrossRef CAS PubMed.
- C. Yang, Y. Luo, H. Lin, M. Ge, J. Shi and X. Zhang, ACS Nano, 2020, 15, 1086–1099 CrossRef PubMed.
- L. Fusco, A. Gazzi, G. Peng, Y. Shin, S. Vranic, D. Bedognetti, F. Vitale, A. Yilmazer, X. Feng and B. Fadeel, Theranostics, 2020, 10, 5435 CrossRef CAS PubMed.
- C.-H. Wu, H. J. H. Ma, P. Baessler, R. K. Balanay and T. R. Ray, Sci. Adv., 2023, 9, eadg4272 CrossRef CAS PubMed.
- S. Song, H. Shen, Y. Wang, X. Chu, J. Xie, N. Zhou and J. Shen, Colloids Surf., B, 2020, 185, 110596 CrossRef CAS PubMed.
- M. K. Kumawat, M. Thakur, R. Bahadur, T. Kaku, R. Prabhuraj, A. Suchitta and R. Srivastava, Mater. Sci. Eng., C, 2019, 103, 109774 CrossRef CAS PubMed.
- B. Lu, S. Hu, D. Wu, C. Wu, Z. Zhu, L. Hu and J. Zhang, J. Mater. Chem. B, 2022, 10, 1226–1235 RSC.
- L. M. Dong, C. Ye, L. L. Zheng, Z. F. Gao and F. Xia, Nanophotonics, 2020, 9, 2125–2145 CrossRef CAS.
- A. Sundaram, J. S. Ponraj, C. Wang, W. K. Peng, R. K. Manavalan, S. C. Dhanabalan, H. Zhang and J. Gaspar, J. Mater. Chem. B, 2020, 8, 4990–5013 RSC.
- H. Huang, C. Dong, W. Feng, Y. Wang, B. Huang and Y. Chen, Adv. Drug Delivery Rev., 2022, 184, 114178 CrossRef CAS PubMed.
- S. Nikazar, Z. Mofidi and M. Mortazavi, Age of MXenes, Applications in Diagnostics, Therapeutics, and Environmental Remediation, ACS Publications, 2023, vol. 2, pp. 19–46 Search PubMed.
- X. Zhu, Y. Zhang, M. Liu and Y. Liu, Biosens. Bioelectron., 2021, 171, 112730 CrossRef CAS PubMed.
- X. Han, J. Huang, H. Lin, Z. Wang, P. Li and Y. Chen, Adv. Healthcare Mater., 2018, 7, 1701394 CrossRef PubMed.
- M. Soleymaniha, M. A. Shahbazi, A. R. Rafieerad, A. Maleki and A. Amiri, Adv. Healthcare Mater., 2019, 8, 1801137 CrossRef PubMed.
- Z. Liu, M. Zhao, H. Lin, C. Dai, C. Ren, S. Zhang, W. Peng and Y. Chen, J. Mater. Chem. B, 2018, 6, 3541–3548 RSC.
- Z. Liu, H. Lin, M. Zhao, C. Dai, S. Zhang, W. Peng and Y. Chen, Theranostics, 2018, 8, 1648 CrossRef CAS PubMed.
- G. Jamalipour Soufi, P. Iravani, A. Hekmatnia, E. Mostafavi, M. Khatami and S. Iravani, Comments Inorg. Chem., 2022, 42, 174–207 CrossRef CAS.
- B. Zhou, H. Yin, C. Dong, L. Sun, W. Feng, Y. Pu, X. Han, X. Li, D. Du and H. Xu, Adv. Sci., 2021, 8, 2101043 CrossRef CAS.
- C. Dai, H. Lin, G. Xu, Z. Liu, R. Wu and Y. Chen, Chem. Mater., 2017, 29, 8637–8652 CrossRef CAS.
- A. Gazzi, L. Fusco, A. Khan, D. Bedognetti, B. Zavan, F. Vitale, A. Yilmazer and L. G. Delogu, Front. Bioeng. Biotechnol., 2019, 7, 295 CrossRef PubMed.
- C. Xu and K. Pu, Chem. Soc. Rev., 2021, 50, 1111–1137 RSC.
- K. R. Khondakar and A. Kaushik, Biosensors, 2023, 13, 62 CrossRef PubMed.
- P. Manickam, S. A. Mariappan, S. M. Murugesan, S. Hansda, A. Kaushik, R. Shinde and S. Thipperudraswamy, Biosensors, 2022, 12, 562 CrossRef CAS PubMed.
- M. A. Mujawar, H. Gohel, S. K. Bhardwaj, S. Srinivasan, N. Hickman and A. Kaushik, Mater. Today Chem., 2020, 17, 100306 CrossRef CAS PubMed.
- M. Huang, Z. Li and H. Zhu, Adv. Intell. Syst., 2022, 4, 2200077 CrossRef.
- S. Mudhulu, M. Channegowda, S. Balaji, A. Khosla and P. Sekhar, IEEE Sens. J., 2023, 23, 18963–18976 CAS.
- T. Hayasaka, A. Lin, V. C. Copa, L. P. Lopez, R. A. Loberternos, L. I. M. Ballesteros, Y. Kubota, Y. Liu, A. A. Salvador and L. Lin, Microsyst. Nanoeng., 2020, 6, 50 CrossRef CAS PubMed.
- B. Motevalli, A. J. Parker, B. Sun and A. S. Barnard, Nano Futures, 2019, 3, 045001 CrossRef CAS.
- K. Pang, X. Song, Z. Xu, X. Liu, Y. Liu, L. Zhong, Y. Peng, J. Wang, J. Zhou, F. Meng, J. Wang and C. Gao, Sci. Adv., 2020, 6, eabd4045 CrossRef CAS PubMed.
- Y.-T. Kwon, Y.-S. Kim, S. Kwon, M. Mahmood, H.-R. Lim, S.-W. Park, S.-O. Kang, J. J. Choi, R. Herbert, Y. C. Jang, Y.-H. Choa and W.-H. Yeo, Nat. Commun., 2020, 11, 3450 CrossRef CAS PubMed.
- D. Ravenscroft, I. Prattis, T. Kandukuri, Y. A. Samad, G. Mallia and L. G. Occhipinti, Sensors, 2022, 22, 299 CrossRef PubMed.
- H. Yang, J. Li, X. Xiao, J. Wang, Y. Li, K. Li, Z. Li, H. Yang, Q. Wang and J. Yang, Nat. Commun., 2022, 13, 5311 CrossRef CAS PubMed.
- V. Chaudhary, V. Khanna, H. T. A. Awan, K. Singh, M. Khalid, Y. Mishra, S. Bhansali, C.-Z. Li and A. Kaushik, Biosens. Bioelectron., 2022, 114847 Search PubMed.
- C. Li, A. K. Tareen, K. Khan, J. Long, I. Hussain, M. F. Khan, M. Iqbal, Z. Xie, Y. Zhang and A. Mahmood, Prog. Solid State Chem., 2023, 100392 CrossRef CAS.
- J. Swapnalin, T. Ghosh, B. Koneru and P. Banerjee, Age of MXenes, Applications in Diagnostics, Therapeutics, and Environmental Remediation, ACS Publications, 2023, vol. 2, pp. 85–106 Search PubMed.
- H. J. Lee, J. C. Yang, J. Choi, J. Kim, G. S. Lee, S. P. Sasikala, G.-H. Lee, S.-H. K. Park, H. M. Lee and J. Y. Sim, ACS Nano, 2021, 15, 10347–10356 CrossRef CAS PubMed.
- M. Chao, L. He, M. Gong, N. Li, X. Li, L. Peng, F. Shi, L. Zhang and P. Wan, ACS Nano, 2021, 15, 9746–9758 CrossRef CAS PubMed.
- X. Zhu, P. Liu, T. Xue, Y. Ge, S. Ai, Y. Sheng, R. Wu, L. Xu, K. Tang and Y. Wen, Ceram. Int., 2021, 47, 173–184 CrossRef CAS.
- Y. Ge, M. B. Camarada, P. Liu, M. Qu, Y. Wen, L. Xu, H. Liang, E. Liu, X. Zhang and W. Hao, Sens. Actuators, B, 2022, 372, 132627 CrossRef CAS.
- Y. Song, R. Y. Tay, J. Li, C. Xu, J. Min, E. Shirzaei Sani, G. Kim, W. Heng, I. Kim and W. Gao, Sci. Adv., 2023, 9, eadi6492 CrossRef CAS PubMed.
- S. Han, M. Zou, X. Pu, Y. Lu, Y. Tian, H. Li, Y. Liu, F. Wu, N. Huang and M. Shen, View, 2023, 20230005 CrossRef CAS.
- A. Kaushik, Neural Regener. Res., 2024, 19, 1185–1186 CrossRef PubMed.
- K. R. Khondakar and A. K. Kaushik, in Nanotechnology in Cancer Management, ed. K. R. Khondakar and A. K. Kaushik, Elsevier, 2021, pp. 229–233 DOI:10.1016/B978-0-12-818154-6.00001-9.
- I. A. Yasmin and K. R. Khondakar, Next-Generation Smart Biosensing, Elsevier, 2024, pp. 261–286 Search PubMed.
- I. Farid and K. R. Khondakar, Next-Generation Smart Biosensing, Elsevier, 2024, pp. 149–187 Search PubMed.
- C. E. Shuck, K. Ventura-Martinez, A. Goad, S. Uzun, M. Shekhirev and Y. Gogotsi, ACS Chem. Health Saf., 2021, 28, 326–338 CrossRef CAS.
- G. Murali, J. K. Reddy Modigunta, Y. H. Park, J.-H. Lee, J. Rawal, S.-Y. Lee, I. In and S.-J. Park, ACS Nano, 2022, 16, 13370–13429 CrossRef CAS PubMed.
- P. Lakhe, E. M. Prehn, T. Habib, J. L. Lutkenhaus, M. Radovic, M. S. Mannan and M. J. Green, Ind. Eng. Chem. Res., 2019, 58, 1570–1579 CrossRef CAS.
- J. Li, H. Zeng, Z. Zeng, Y. Zeng and T. Xie, ACS Biomater. Sci. Eng., 2021, 7, 5363–5396 CrossRef CAS PubMed.
- S. Yadav, A. P. Singh Raman, H. Meena, A. G. Goswami, Bhawna, V. Kumar, P. Jain, G. Kumar, M. Sagar and D. K. Rana, ACS Omega, 2022, 7, 35387–35445 CrossRef CAS PubMed.
- P. Cravedi, S. Farouk, A. Angeletti, L. Edgar, R. Tamburrini, J. Duisit, L. Perin and G. Orlando, Transplant Int., 2017, 30, 1199–1208 CrossRef PubMed.
- V. Kedambaimoole, K. Harsh, K. Rajanna, P. Sen, M. Nayak and S. Kumar, Mater. Adv., 2022, 3, 3784–3808 RSC.
- M. O. Faruk, A. Ahmed, M. A. Jalil, M. T. Islam, B. Adak, M. M. Hossain and S. Mukhopadhyay, Appl. Mater. Today, 2021, 23, 101025 CrossRef.
- J. V. Vaghasiya, C. C. Mayorga-Martinez and M. Pumera, npj Flexible Electron., 2023, 7, 26 CrossRef PubMed.
- S. I. Gomes, J. J. Scott-Fordsmand and M. J. Amorim, Nano Today, 2021, 40, 101242 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |