Recent advances in 3D bioprinting of polysaccharide-based bioinks for fabrication of bioengineered tissues
Kasula
Nagaraja†
a,
Pratik
Dhokare†
a,
Amitava
Bhattacharyya
 abc and
Insup
Noh
abc and
Insup
Noh
 *ab
*ab
aDepartment of Chemical and Biomolecular Engineering, Seoul National University of Science and Technology, Seoul 01811, Republic of Korea. E-mail: insup@seoultech.ac.kr
bConvergence Institute of Biomedical Engineering and Biomaterials, Seoul National University of Science and Technology, Seoul 01811, Republic of Korea
cMedical Electronics Research Center, Seoul National University of Science and Technology, Seoul 01811, Republic of Korea
First published on 16th May 2024
Abstract
Bioink in three-dimensional (3D) bioprinting of biomimetic tissue scaffolds has emerged as a key factor for the success of tissue engineering and regenerative medicine. The bioinks used for extrusion 3D bioprinting have hydrogel matrices with different kinds of polymeric biomaterials such as proteins, peptides, polysaccharides, hydrophilic synthetic polymers, and others. Natural polysaccharides such as alginate, chitosan, and hyaluronic acid have garnered significant attention as bioink materials due to their excellent biocompatibility, extracellular matrix mimetic properties, biodegradability, injectability, bioprintablilty and structural versatility among their many advantages, even though many research groups focus on the study of protein-based bioinks to utilize their high potential of cell adhesiveness. This review encompasses recent advancements of polysaccharide-based hydrogels and bioinks for bioengineered tissue regeneration and reconstruction, especially by focusing on fabrication of multilayered complex structures for biomimetic tissue engineering applications.
Design, System, ApplicationA bioprinting technology is a layer-by-layer 3D deposition of cell-laden hydrogel, i.e. a bioink, by extrusion-based printing technology to fabricate 3D constructs for its applications to regeneration of diseased tissues such as cartilage, bones, skin, skeletal muscle, and neural tissues. Even though this technology has attracted much attention in tissue engineering and regenerative medicine, the design and fabrication of tissue engineering scaffolds have yet to be solved by overcoming the difficulties of multilayered 3D scaffold construction in stable 3D structures. We here report the design of polysaccharide-based hydrogels, bioinks and 3D bioprinting scaffolds, targeting tissue engineering applications. For the 3D bioprinting and regeneration of diverse damaged tissues, the issues such as cell sources, biocompatibility, bioink and bioprinting properties, polymeric biomaterial selection, bioprint-ability performance, scaffold fabrication, and their applications to tissue regeneration have been described. Furthermore, the benefits, difficulties, and future directions of 3D bioprinting of polysaccharide-based bioinks for multilayered tissue engineering are discussed. |
1. Introduction
Complex and multilayered tissues represent a fascinating frontier in the fields of biomaterials, 3D bioprinting, regenerative medicine, and biomedical engineering.1–4 These tissues are composed of various cell types and extracellular matrices (ECM) intricately arranged in three-dimensional molecular structures, mimicking the natural complexity of human tissues and organs. 3D bioprinting allows the molecular, structural systems to be designed as a tailored scaffold construction of multilayered tissues, with the potential to revolutionize regenerative medicine. This cutting-edge technique leverages the precision of 3D bioprinting to create intricate, multilayered constructs that closely mimic the native architecture of human tissues and organs.5–7 By combining various natural biomaterials, including polysaccharides, with living cells and bioactive factors, 3D bioprinting allows the bioengineering of multilayered, functional and biocompatible tissue scaffolds. Within native tissues, diverse cell types like endothelial, epithelial, muscular, and adipose cells exist or co-exist in an intricate ECM in different tissues, intricately woven with interconnected networks.8,9 The goal of tissue engineering is to emulate this natural milieu, thereby fostering the creation and revitalization of fully functional tissues and organs through designed biodegradable scaffolds. Recent technological advancements like bioprinting and advanced manufacturing have illuminated a promising trajectory for tissue engineering and reproductive medicine, profoundly shaping their future.10,11Polysaccharides serve a variety of purposes, such as structural properties, tissue regeneration,12 antibacterial,13 antiviral,14 antitumor,15,16 and immune regulatory properties in tissue engineering.17,18 Hyaluronic acid and chitosan, derived from human connective tissue and arthropod exoskeletons respectively, along with alginic acid from brown algae, are key polysaccharides used in tissue engineering. These materials are processed into hydrogels and bioinks using various synthesis technologies. Hydrogels, known for their ability to retain significant amounts of water in a 3D polymeric network, differ from bioinks, which incorporate living cells within their polymer structure. Additionally, these materials can be enhanced with bioactive molecules like growth factors, nanoparticles, and drugs to tailor their properties for specific applications.19 The bioink is employed as a biomaterial for the fabrication of tissue engineering scaffolds. Thus, hydrogels are composed of highly hydrated 3D environments that resemble the ECM of tissues. Furthermore, because of their special architecture, which is porous and flexible, cells can migrate and communicate with each other, as well as receive nutrients, oxygen, and other water-soluble substances in a specific tissue.19 Different techniques can be used to design a hydrogel, based on the intended attributes and purpose in tissue engineering. Physically cross-linked hydrogels are created through molecular interactions.20 The interactions can be influenced by changing the surrounding environment or applying mechanical forces. They are reversible and regenerated (associations break and continuously reform).21 For instance, ionic interactions with calcium ions (Ca2+) can cross-link alginate to create a hydrophilic 3D network that resembles an egg-box model structure.22 Polymer chains joined by covalent bonds make up chemically cross-linked hydrogels.23 There are two general methods for preparing these hydrogels, the first involves using a cross-linking agent to polymerize a hydrophilic monomer; the second method involves directly cross-linking water-soluble polymers.24 Due to the strong covalent connections that form between polymeric chains, chemical crosslinking is typically more persistent than physical cross linking and has stronger mechanical qualities, making it appropriate for long-term use.21 Chemical hydrogels are formed by reacting polysaccharide functional groups (–COOH, –OH, –NH2) with cross-linkers and other chemicals, ensuring that these groups network to create the gel. This process enhances the hydrogel's physical properties like viscosity, elasticity, and stability by forming a durable 3D structure with improved mechanical qualities.25 These crosslinking induces formation of a hydrogel and makes layer-by-layer additive manufacturing of tissue engineering scaffolds by 3D bioprinting technology.
Proteins and their domain, i.e. peptides and oligopeptides, are essential for constructing hydrogel/bioink scaffolds in tissue engineering, thanks to their biocompatibility and ability to foster cell adhesion and then tissue regeneration. Proteins like collagen, fibronectin, gelatin, fibrin, and silk are crucial for their roles in enhancing cell interactions and scaffold functionality in tissue engineering.26 Collagen, which is an abundant component in the ECM, supports cell adhesion and tissue regeneration, while its denatured form, gelatin, and its modified form (GelMA) have been employed as typical bioink hydrogels and allow for precise scaffold structures through photo cross-linking while maintaining biodegradability.26 Fibrin is valued for its natural ability to form scaffolds that promote cell growth, especially in wound healing,27 while silk fibroin's strength makes it suitable for structural repairs in bone and ligament contexts.28 Incorporating peptides such as RGD, YIGSR, and IVAK have been employed as alternative biomaterials for proteins to prevent the issue of complex handling in synthesis by higher molecular weight. Peptides are the specific interaction domains of cell-adhesive proteins.26 Even though this strategic application underscores their pivotal role in developing advanced, functional scaffolds for tissue engineering, they have limitations in their bioink applications, such as difficulty in gel synthesis compared to polysaccharide polymers.
Polysaccharides, with their diverse biophysical and chemical properties, are crucial in tissue engineering and 3D bioprinting. Classified as anionic, cationic, or neutral based on their natural charges, these polysaccharides such as alginate (ALG), chitosan, HA, gelatin (GEG), pectin, guar gum, and chondroitin sulfate are transformed into hydrogels through chemical and physical modifications. They are essential in various tissues within animal bodies, performing key biological, physiological, and structural functions influenced by their chemical composition, molecular weight, shape, and hydrophilicity.29,30 For example, sulfated marine polysaccharides like fucoidan and chondroitin sulfate have anti-inflammatory and antiviral properties, potentially blocking viruses from attaching to host cells. Chitosan, deacetylated from chitin, shows antibacterial effects by interfering with anions on bacterial cell walls, which can disrupt vital processes and kill the bacteria. Meanwhile, neutral dextran, due to its water solubility, resists protein adsorption.31,32
The development of 3D bioprinting has been emphasized as a particular solution for creating unique 3D matrices (fabrication) similar to native tissues and organs, the regulated deposition of different biomaterials in layer-by-layer forms, and the shortcomings of the traditional approach. It is undoubtedly a groundbreaking method with several uses in tissue engineering, regenerative medicine, food industry, drug delivery and other bioengineering fields.33,34 3D bioprinting is a biotechnology that uses biomacromolecules and biomedical engineering to create 3D structures that regulate cell functions, tissue regeneration and disease treatment. It has applications in drug screening models, medical engineering, dentistry, surgery, and human-created structures like meniscus, vascular junctions, bone, tumor models, nerve and skin tissues. By harnessing the power of 3D bioprinting, researchers and medical professionals can advance healthcare and improve patient outcomes, making it a promising technology with significant implications in medical and biological fields.35 However, there are no established standards or criteria for bioprinting or bioinks, which makes them challenging for researchers to determine which type of bioprinting or bioink they require for given tissue engineering applications.
Even while there are now a lot more commercially available bioprinting systems and bioinks than ever before, certain research organizations will probably still need to use unique setups and custom formulations of bioinks for specific tissues with different shapes and compositions.36 Extrusion-based 3D bioprinting is one of the most frequently employed 3D bioprinting methods in tissue engineering applications, which deposits layers of tissue-specific cell-filled bioink on top of one another, indicating the importance of each layer of the bioprinted scaffolds. Natural polysaccharides have consistently attracted considerable attention in tissue engineering and extrusion-based bioprinting,37,38 and their hydrogels have gained broader applications in tissue engineering in recent decades. Notably, polysaccharides have diverse applications, for example, regeneration of encompassing bone and cartilage, muscle and cardiac tissue, neural tissue, skin, and other tissues. However, for the fabrication of large, multilayered complex structures through the techniques of polysaccharide-based hydrogel printing, the self-standing gel strength and post-printing shape fidelity are the issues of major concerns for the researchers working with such biomaterials in tissue engineering.39,40
This review discusses the key properties of polysaccharides, their application in extrusion-based bioprinting technologies, and their role in tissue engineering. It delves into recent developments in polysaccharide-based hydrogels for creating multilayered scaffolds suitable for complex tissue structures. The review underscores the adaptability of these natural polymers in developing 3D bioprintable hydrogels and outlines their advantages and challenges in bioprinting. It also emphasizes the importance of bioink composition for effective 3D bioprinting and highlights extrusion-based bioprinting as a favorable method due to its versatility and reduced cell damage.
2. Characteristics of bioinks
Bioinks come in two categories: those that utilize scaffolds and those that do not. Scaffolds are fibrous or 3D structural biomaterials that permit not only transformation into tissues, but also biological liquids and gases to pass through. This is accomplished by making it simpler for cells to interact, deposit ECM and maintain viability with no or mild toxicity and inflammation.41 The bioink should be printable and capable of maintaining cells without any cell damage, and also preserve the physio-mechanical and biological characteristics of the targeted tissue after bioprinting. The bioinks can be altered depending on the printing systems used to print on the target tissue for all the qualities to be achieved. Combining two or more nano-biomaterials is frequently necessary to improve the mechanical and biological properties, particularly for extrusion-based bioprinting.42 Multi-biomaterial bioinks are superior in bioprinting to those formed from a single biomaterial. Most single polymer-based bioinks lack the bioprintability, outstanding mechanical qualities, and functionality required to create tissues that mirror biological ones. The selection of polysaccharides is more constrained in 3D bioprinting because cells and post-printing fidelity are involved. It is important to consider mechanical qualities, biocompatibility, porosity, pH neutrality, hydrophilicity, and biodegradability while currently selecting the biomaterials for bioink.43,44 The majority of bioprinting techniques use additive manufacturing concepts by depositing tissue-specific cells and biomaterials collectively known as “bioinks” spatially and temporally in a regulated and sequential manner. Even though numerous bioinks have been created, no single bioprinter/bioink combination has proven effective in all tissue engineering applications to date, which is why the sector is still quite experimental. The following are some of the important characteristics that a bioink must have.2.1 Cell source
When living cells are incorporated into a hydrogel, it becomes a measurable bioink. Since a necessary, key element of bioink is cells, the cell types and sources should be considered important factors. Since the chosen cell must continue to operate, reproduce successfully, imitate its physiological state, and regenerate the target tissues throughout the bioproduction process,45 the right cell type, source, and density should be chosen carefully to create bioink and tissue engineering scaffolds. One of the most fundamental prerequisites for bioink's compatibility is that it must be robust and secure to support the loaded cells' survival and differentiation.462.2 Hydrogel and its synthesis
A hydrogel, a 3D network of polymer, transforms into a bioink by adding cells into a hydrogel in 3D bioprinting and tissue engineering. It is synthesized by using diverse polymers such as HA, chitosan, gelatin, and chondroitin sulfate through the methods of chemical or physical crosslinking. The gelation process with cell incorporation should be easy to be handled, sterilizable and nontoxic because it can affect not only the cell viability but also the bioprinting processing and post-printing fidelity of the scaffolds.47 Depending on how the composition and properties of the hydrogel and bioink are prepared, various alternatives are available for the gelation procedure such as photo-crosslinking, ionic, hydrophobic, thermal, enzymatic, and stereo-complexing methods.2.3 Biocompatibility
For the development of tissue engineering constructs by 3D bioprinting, cells in bioink should maintain their capacity to differentiate and proliferate in vitro without any damage. Biocompatibility and biodegradation were the primary concerns for the hydrogels and 3D printing.48 Cutting edge researchers have expanded the concept of biocompatibility from the need for minimal cytotoxic effects in cell and biomaterial levels to a favorable biological relationship with the host, especially in tissue engineering and regenerative medicine area. Because they can simulate the natural cellular environment, the bioinks from natural biomaterials offer a nontoxic biomanufacturing approach, but they have to overcome the issues of poor mechanical qualities and immune response issues.492.4 Bioprintability
Bioprintability refers to a biomaterial's ability to be printed in a controlled manner and shapes within a certain time frame. The idea behind 3D bioprinting is the layer-by-layer, controlled production of three-dimensional living objects using computer-aided design.50 Several techniques, such as extrusion printing,51 inkjet printing,52 laser-assisted printing,53 and stereolithography,54 are used to bioprint cell-laden biomaterials, or bioinks. At present, 3D bioprinting technology has garnered significant interest and is being extensively researched for a variety of biomedical uses.55 Rheology is essential to the extrusion-based 3D printing of hydrogels because it influences the behavior of the material during the printing process through its flow and deformation characteristics. Viscosity, shear thinning behavior, and viscoelasticity are examples of rheological qualities that are important in influencing the extrudability, shape retention, and general quality of printed structures.56 Organ and tissue bioprinting relies on bioprinting of a gel with cells; however, the demanding bioprinting environment limits the bioink material options. Since the bioink's nature can substantially impact the bioprintability, the scaffold properties such as size accuracy and structural construction are all important considerations in 3D bioprinting and tissue engineering. Bioprinting technology varies based on its purposes; for example, micro-extrusion bioprinting requires adequate rheological properties and shear-thinning behaviors of bioinks, and preserves the preliminary shape fidelity of the post-bioprinting scaffolds.572.5 Mechanical properties
Bioprinted hydrogels should have convincing mechanical qualities to withstand diverse biopressures throughout the bioprinting process and to sustain the scaffold structure after bioprinting for successful practical use as well as differentiation during its biodegradation into specific tissue regeneration. At the same time, the bioink should guarantee the scaffold's mechanical qualities that can adapt to the target tissue's native environment. Due to their low mechanical properties, natural polysaccharide hydrogels do not provide good sacrificial nourishment during bioprinting and implant in the patient's body. Hence, synthetic polymers or nano-biomaterials are often used for higher mechanical qualities, keeping the bioink structurally and mechanically stable like genuine tissue, especially under mechanical stress like cartilage, cardiovascular tissues or bone.583. Natural polysaccharides in bioinks
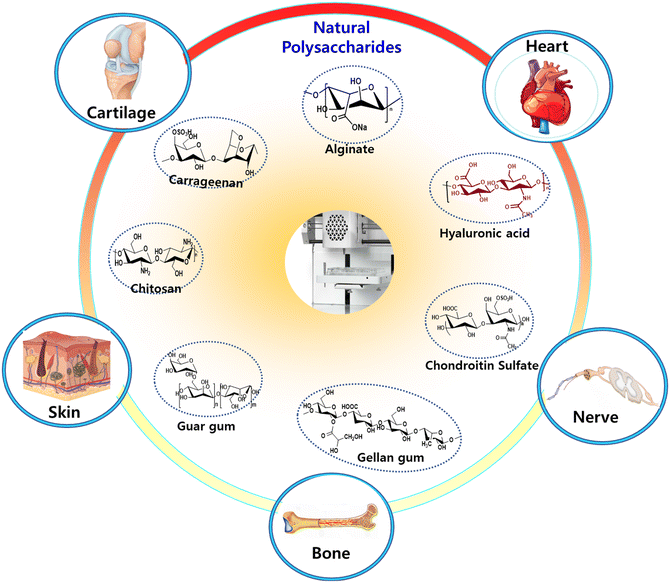
Polysaccharides and their derivatives are becoming more and more popular among the variety of biopolymers that can create hydrogels for 3D bioprinting applications, especially for the engineering and development of bioink formulations. Their primary characteristics—which include their vast diversity of chemical structures, ease of derivatization and functionalization, suitable mechanical and rheological qualities, and inherent biocompatibility and biodegradability—are what make them so appealing.59 When it comes to bioprinting of the intricate and multilayered tissues, natural polysaccharides are essential building elements. Natural sources of polysaccharides as depicted in (Fig. 1) have the extraordinary capacity to produce hydrogels, which offer an environment that is favorable for cell encapsulation and growth as well as tissue regeneration. These polysaccharides function as the structural backbone of bioinks, enabling precise multilayer deposition during bioprinting procedures.29 This integration gives the printed structures both mechanical stability and tissue engineering potential, by simulating the intricacy of original tissues in physical and biological aspects, presenting a possible path toward the creation of complex and useful biological constructs. The appropriate utilization of natural polysaccharides in bioprinting has enormous promise to transform regenerative medicine and advance the creation of realistic, multilayered tissues as researchers explore the frontier of tissue engineering.603.1 Alginate or sodium salt of alginic acid (ALG)
One of the most common products made from brown algae is an alginate, ALG, with anionic, edible and hydrophilic properties. Due to its extremely low cost and biocompatibility, ALG is widely employed in biomedical applications.61 While ALG's low bioactivity also prevents the growth and proliferation of encapsulated cells, its stability is obtained by oxidation processing.62,63 Ca2+ and other divalent and trivalent cations are commonly used in the ionic gelation process to create ALG hydrogels in a stable state.64 The drug release rate and the stability of the gels are modulated by control of the increase in ionic cross-linking.65 ALG hydrogels' gel formation, mucoadhesive ability, biodegradability and nontoxicity, among other qualities, have encouraged the use of these biomaterials in multilayer 3D bioprinting, tissue engineering and drug delivery applications including wound healing, bioinks for 3D printing, in vitro models, or the delivery of anticancer medications.66In order to create hydrogels with potential uses in various multilayer 3D bioprinting and tissue engineering techniques, ALG has also been mixed with other polymers, in order to create skin tissue analogues using extrusion bioprinting; Somasekharan et al. reported a bioink based on alginate mixed with gelatin and diethylaminoethyl cellulose (DCEL). Because the gelatin backbone naturally contains RGD peptide sequences, the inclusion of gelatin boosted cell adherence, while the fibrous character of DCEL gave matrix stability and improved mechanical qualities. When compared to skin tissue, the bioprinted scaffolds had appropriate mechanical properties, such as elasticity, with an elongation of break of 91.7 ± 9.36% and a Young's modulus of 125 ± 22 kPa. For 21 days, the co-culturing of fibroblasts and keratinocytes was seen, along with the progression of cell differentiation within the scaffolds. Histological examination at this stage revealed the development of comparable dermal and epidermal components.67
Dogan et al. went one step further in a recent study, creating bioprinted scaffolds using an alginate–collagen I bioink loaded with mesodermal progenitor cells (hiMPCs) derived from human-induced pluripotent stem cells that were cultured with the addition of vascular endothelial growth factor (VEGF) to induce blood vessel formation. Following a 21 day incubation period, the bioprinted scaffolds showed signs of both small and big vessel creation. To evaluate their functionalities, the scaffolds were subsequently inserted into a chicken embryo's chorioallantoic membrane (CAM). The CAM model's appropriate blood perfusion was made possible by the printed vessels.68
3.2 Chitosan
Chitosan is a linear polysaccharide, made up of randomly distributed-(1 → 4)-linked D-glucosamine (deacetylated unit molecules from chitin).69 Chitosan's motivating properties are its low renewability, lack of toxicity, lower cost, biocompatibility, bio-adhesiveness, anti-bacterial activity, and biodegradability. Chitosan degrades more slowly than animal-derived polymers like collagen and fibrin70,71 and has several functional groups, including amine, amino, and hydroxyl groups, which can interact with both cationic and anionic molecules. This is particularly significant in the case of proteins. With the addition of various groups along the polymer chain,72 the functionalization of the polysaccharide was possible, opening up a variety of applications for it, including hydrogel, bioink, 3D bioprinting, tissue engineering73 and drug administration.74Chitosan hydrogels have been used for diverse purposes such as the repair of bone and cartilage, and other tissues. As examples, polyelectrolyte hydrogels can be produced in a simple way by combining chitosan with polysaccharides or proteins with opposed charges.75 In recent years, further applications for chitosan hydrogels have included heart regeneration and wound healing.76,77 Tonda-Turo et al. created a bioink based on chitosan that combines photocrosslinking and thermally induced gelation in a dual crosslinking mechanism. To do this, β-glycerol phosphate salt and chitosan–methacrylate were combined to provide thermosensitive behavior, having an elastic modulus of about 6 kPa. After 48 hours, cell proliferation was observed from the extrusion bioprinting of these hydrogels loaded with NIH 3T3 cells, resulting in 3D constructs with good cell dispersion even after 24 hours.78 The simultaneous inclusion of additional ingredients or nanostructures in the bioinks is another popular tactic used to boost the effectiveness of chitosan-based bioinks. For example, the development of a bioink based on chitosan and poly(gamma-glutamic acid) γPGA is described in the work of Pisani et al. The amine groups in chitosan and the carboxylic acid groups in γPGA interacted ionically to produce the hydrogel. Human dermal fibroblasts were utilized by the authors to 3D bioprint grid-shaped constructs using this hydrogel. Cell viabilities of about 70% were seen 24 hours after the bioprinting procedure and maintained for 14 days in culture.79
3.3 Hyaluronic acid (HA)
HA is available in all joints, neural, connective, and epithelial tissues in animals. The extracellular membrane of mammalian tissue (muscle), present in some organs including the central nervous system, contains HA with a repeating unit of N-acetyl-D-glucosamine and D-glucuronic acid in its chemical structure.80 The capacity of HA to maintain moisture levels makes it a perfect substance for promoting wound healing. Depending its molecular weight and water solubility, HA has diverse properties of bioprinting, mechanical strength and stability.81,82By adding gelling agents, or chemical modifications, HA hydrogels can be created.83 Combining HA with gelling agents, such as poloxamer,84 ALG,2,85 collagen,86 methylcellulose (MC),87 or gelatin,6 is one of the frequently used techniques for producing physically cross-linked HA hydrogels. Cross-linking substances like divinyl sulfone or glutaraldehyde can be added to HA polymers to create crosslinked HA hydrogels.88 However, the usage of cross-linking agents may be constrained since they might be hazardous or produce toxic byproducts to tissue engineering applications. For the creation of HA hydrogels, more biocompatible chemical modification techniques including enzymatic cross-linking, click chemistry, or physical crosslinking have been investigated89 such as oligonucleotides. For these new options to work, HA must be functionalized with additional chemical groups, which can be added to the carboxylate, hydroxyl, or amide groups.90
Methacrylate groups can be chemically crosslinked with HA to generate HA hydrogels, a method that is frequently employed with various biopolymers. These platforms have been used to release PLs and GF. Further, even MSCs have been cultivated in these matrices to stimulate their differentiation for uses in periodontal regeneration,91 cartilage regeneration,92 bone regeneration, or skin regeneration.93 A HA-based bioink made of HA, hydroxyethyl acrylate, and gelatin-methacryloyl was reported by Noh et al. They demonstrated that the HA-based bioink did not adversely affect the viability of embedded bone cells when used to manufacture multilayered construction. The hydrogel consisting of three components was biocompatible, and the gel printing procedure was good.81
3.4 Cellulose
Due to its rigid structure, cellulose, one of the most prevalent polymers in plant cell walls, contributes to its construction. It consists of a repeating unit of (1 → 4) connected-D-glucopyranosyl. As a renewable polysaccharide, cellulose offers intriguing properties due to its adaptability and sustainability.94,95 Natural cellulose possesses specific chain length, crystallinity, and intra-intermolecular hydrogen bonding, among other physicochemical properties. The great thickness of free OH groups in cellulose allows for hydrogen bonding, but they also contribute to the insoluble nature of cellulose.96,97Given that cellulose is not soluble in water, it makes sense to alter its structure to develop soluble derivatives with a high affinity for water, which can then be used to make hydrogels. Methylcellulose (MC), one of them, has been used in the creation of several hydrogels for their multilayered bioprinting.98 For instance, Boonlai et al. combined CNCs, methylcellulose, and κ-carrageenan, and then used ionic crosslinking (with aqueous KCl) to make an appropriate hydrogel for 3D extrusion-based bioprinting of live constructions for applications such as bone tissue creation and regeneration. Better shear-thinning behavior resulted from adding 2 and 4 wt% of CNCs to the hydrogels because nanocellulose enhanced the bioink's rheological characteristics. Furthermore, while increasing the CNC content from 2 to 4 wt%, the compressive stress at 30% strain increased in the CNC-reinforced hydrogels from 20.03 ± 0.02 to 23.28 ± 0.01 kPa. The vitality of L929-loaded printed constructs was greater than 90% five days after bioprinting, demonstrating that the bioprinting procedure and the prepared biomaterials did not damage these cells.99
3.5 Dextran
Dextran polymer is a naturally occurring, non-toxic, hydrophilic homopolysaccharide containing alpha-(1 → 6)-linked-D-glucopyranose. Dextran comprises solely hydroxyl(–OH) groups, which do not contribute to cellular adhesion,100,101 but its hydroxyl groups are simple to functionalize, making hydrogel preparation possible. DEX hydrogels can be created using physical or chemical cross-linking, including photo-crosslinking,102 Michael addition,103 Schiff-base reaction,104 and enzymatic cross-linking. Physically cross-linked hydrogels typically have lower mechanical strengths than chemically cross-linked ones, but because they may be made at softer circumstances, there is less risk of harm to the included biomacromolecules.Actually, utilizing a promising core/shell extrusion-based 3D bioprinting technology, only one study to date has described the bioprinting of a vascularized construct for wound care employing oxidized dextran.69,105 This was accomplished by using a hydrogel consisting of periodate-oxidized dextran and peptide-functionalized succinylated chitosan (C) as the shell and GelMA as the core. In this experiment, two cell types were used: BMSC in the shell and HUVECs in the core. With HUVEC-specific markers visible 21 days after bioprinting, the 3D bioprinted peptide-CD/GelMA constructs offered a suitable milieu for cell growth and development, indicating the presence of endothelial cells within the tube-like structures. Although this work is the only one on the usage of dextran-based bioinks to date, it provides a way to employ this polysaccharide to create vascularized structures, which addresses one of the primary obstacles in 3D bioprinting live tissues.106
3.6 Gellan gum
An anionic polysaccharide with a linear chemical structure is GEG. As a result of the fermentation process, it is secreted by the bacteria P. elodea. L-rhamnose, two D-glucose subunit chains, and repeated chain units of D-glucuronic acid are all present in gellan gum. It has effective mechanical and gelling capabilities and exhibits cytocompatibility, biocompatibility, and biodegradation.107,108 Gellan gum is used in tissue engineering to bio print skeletal cartilage, tissue, and structures that resemble the human brain.109 In a different direction, gellan gum and pure starch were mixed to create 3D-printed scaffolds with varied printing gaps for Schwann cell seeding. The printed constructs were shown to be stable, to have sufficient swelling ratios, and not to be harmful to the L929 fibroblast cell line. Because these methods work well for 3D bioprinting, the usage of gellan gum is expected to be increased in the formulation of cell-laden bioinks in the future.1103.7 Agarose
A repeating chain like arrangement of D-galactose and 3,6-anhydro-L-galactose units makes up the agarose polymer. Along with agaropectin, agarose is a naturally occurring linear polysaccharide composed of D-galactose and 3,6-anhydro-L-galactopyranose units.111Because of its thermo-responsive reversible gelling, agarose polysaccharide, generated from red seaweed, is frequently used in the tissue engineering sector.112,113 Instead of using bioink components, alternative uses for agarose hydrogels in bioprinting procedures have also been investigated.114 Agarose hydrogels are easily molded and used to cast sub-millimetric geometries at low concentrations (less than 1% (w/v)).114 Utilizing this characteristic, Aydin et al. created a bioink using alginate and agarose that was created using a microwave-assisted technique. The agarose acted as a self-eroding sacrificial component to cast tubular structures within the alginate that was packed with cells, creating living printed constructs with a vascularized network. In particular, this sacrificial bioink made it possible to bioprint a 2 cm tubular structure in 2 minutes, preserving its shape and enabling very high cell survival (up to 95%) in a 3 day culture of MSCs generated from human adipose tissue (AT MSCs).115
3.8 Konjac gum
A water-soluble hydrophilic polymer derived from konjac tubers is called konjac glucomannan (KJG). China, Japan, and Southeast Asia have traditionally used KJG as a food source and traditional medicine. The α-(1 → 4)-pyranoside bond between D-glucose and D-mannose polymerizes the main structural chain of KJG, with a decreased number of acetyl groups at C-6 locations in the side chain unit.116 KJG is a desirable biopolymer because of its notable properties, which include biodegradability, biocompatibility, the ability to form films, and the capacity to gel.117 Alves et al. presented a KJG–xanthan gum hydrogel for wound dressing application. The dressing was then assessed by physicochemical and biological experiments.116 The use of a KJG hydrogel in the treatment of acute wounds was investigated by Yang et al. after its synthesis by adjusting the KJG concentration during the preparation process. The hydrogel was demonstrated to have antibacterial activity, biocompatibility, and water-holding capacity.1183.9 Guar gum(GG)
GG is a plant-based polysaccharide derived from Cyamopsis tetragonolobus and has a high molecular weight. It is made up of alpha (α)-(1 → 6), connected galactose side chain units and beta β-1 → 4-D-mannan linear chains.108 An affordable hydrophilic polyhydroxy polysaccharide is guar gum. Due to its strong biocompatibility, biodegradability, and outstanding rheological qualities, it is frequently utilized in multilayered and complex tissue engineering application.119,120 Indurkar et al. presented a novel, cost-effective bioink made of gelatin and varying guar gum concentrations. This novel biopolymer combination was created to provide bloom strengths, G′ and G′′, that range widely, and then tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∂ was computed using G′ and G′′, and then the bloom test was conducted. Following the study of filament creation, these physical parameters were associated with the printability of the bioink. The guar gum–gelatin bioinks with bloom strength between 480 and 750 and tan
∂ was computed using G′ and G′′, and then the bloom test was conducted. Following the study of filament creation, these physical parameters were associated with the printability of the bioink. The guar gum–gelatin bioinks with bloom strength between 480 and 750 and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∂ between 0.15 and 0.2 were reported to be printable because they could form filaments during extrusion.120
∂ between 0.15 and 0.2 were reported to be printable because they could form filaments during extrusion.120
3.10 Xanthan gum (XTG)
XTG is an anionic polysaccharide generated from Xanthomonas bacteria that produces a viscous solution (high molecular weight) and has a low shearing rate. The side chains of XTG are composed of a trisaccharide made up of D-mannose beta (β)-(1 → 4), D-glucuronic acid beta(β)-(1 → 2), and D-mannose, which are linked to substitute residues of glucose groups by alpha(α)-(1 → 3)-linkage in the backbone. XTG is a branched polysaccharide.121 The remarkable qualities of XTG, which include exceptional physicochemical properties, stability over a wide pH range and temperature range, high viscosity at low concentration, high degree of pseudoplastic behavior, biodegradability, and non-toxicity, are suitable for multilayered and complex tissue engineering application.122Since xanthan gum has outstanding rheological properties, it is often used to enhance the mechanical and rheological properties of bioinks.123,124 To be more precise, Lim et al. used xanthan gum's ability to thin shear and mixed it with CMC as well as with alginate, GelMA, and hMSCs to create a suitable bioink for extrusion-based bioprinting.125
4. Polysaccharide-based hydrogels in complex/multilayered tissue engineering applications
Polysaccharide-based hydrogels are increasingly used in tissue engineering for regenerating various tissue types, including soft, hard, connective, and functional tissues. These hydrogels are formulated into bioinks that encapsulate cells and bioactive molecules, offering properties such as biocompatibility, appropriate rheology, and elasticity, crucial for bioprinting of complex or multilayered scaffolds like those needed for cartilage, bone, skin and nerves. However, single-polymer hydrogels often fall short of meeting the complex demands of multilayered tissues. To address this, combinations of multiple bioactive polymers, nanobiomaterials, and crosslinking techniques are used to enhance the mechanical, biological, and post-printing properties of the constructs, ensuring effective regeneration of multilayered complex tissues. Further control of the interactions between layers is a crucial requirement to achieve multi-layer post-printing stability and integration.Table 1 summarizes polysaccharide-based hydrogels used for 3D bioprinting of multilayered complex structures. To successfully regenerate specific functional tissues, cellular level bioengineering is essential during and after 3D bioprinting. The details of each tissue reconstruction using polysaccharide-based bioinks are discussed in the subsections below.
| S. No | Polysaccharide | Preparation of bioink | Cell type | TE application | Features/limitations | Ref. |
|---|---|---|---|---|---|---|
| 1 | HAMA-CSMA-gel | UV-crosslink | SMSCs | Cartilage | 1. Easy extrusion and stable 15-layer scaffolds w/ fidelity | 126 |
| 2. Significant elastic and compressive strengths; robust after cross-linking | ||||||
| 3. In vitro proliferation and differentiation of SMSCs | ||||||
| 4. In vivo cartilage regeneration and functional recovery | ||||||
| 2 | HA–ALG | Ionic Ca+2 | ADSCs | Cartilage | 1. Double cross-linking w/ biotinylated HA and streptavidin for printability and structural integrity | 127 |
| 2. Strengthened w/ Ca2+ for mechanical stability & durability | ||||||
| 3. High precision in printing both square & round shape | ||||||
| 4. Chondrogenic differentiation for cartilage tissue engineering | ||||||
| 3 | GEG/lignin | Magnesium (Mg+2) | hMSCs | Cartilage | 1. Elation properties & lignin's shear-thinning behavior | 128 |
| 2. High stability mimicking the cartilage environment | ||||||
| 3. Chondrogenic differentiation | ||||||
| 4 | GEG/gel | Ionic Ca+2 | MSCs | Cartilage | 1. Rheological properties, shear recovery, and shape fidelity | 129 |
| 2. The 30-layer scaffold w/ mechanical strength | ||||||
| 3. Initial cell viability in scaffolds is over 80% | ||||||
| 5 | ALG/gel | Ionic Ca+2 | MSCs | Bone | 1. Stiffness controlled by alginate concentrations | 130 |
| 2. Matrix mineralization and cellular organization | ||||||
| 3. Osteogenic development effectively at high cell densities | ||||||
| 4. Softer scaffolds excel in matrix deposition and cellular activity | ||||||
| 6 | GEG | UV-crosslink | MC3T3-E1 | Bone | 1. Good shear-thinning properties | 131 |
| 2. UV cross-linking post-printing significantly w/ mechanical stability | ||||||
| 3. High shape accuracy and structural integrity in complex structures | ||||||
| 7 | ALG | UV-crosslink | HASCs | Bone | 1. Osteogenic potential of bioink by bone-derived ECM | 132 |
| 2. Modified dECM enhances the mechanical properties of the bioink | ||||||
| 3. Printing of complex, cell-laden structures w/ precise multi-layered, micro-sized struts | ||||||
| 4. Osteogenic development w/ high in situ cell viability (>90%) | ||||||
| 8 | GEG MA/GelMA | Ionic Ca+2 and UV-crosslink | BMSCs | Bone | 1. Structural stability of scaffolds w/ double cross-linking | 133 |
| 2. Post-printing shape integrity | ||||||
| 3. Diverse printing across a broad temperature range | ||||||
| 4. Angiogenic and osteogenic properties | ||||||
| 9 | XTG/ALG | Ionic Ca+2 (electrostatics interaction) | CCD-986 | Skin | 1. Viscoelastic properties, printability & structural stability w/ catechol-functionalization | 134 |
| 2. Compressive and tensile strengths of the gel | ||||||
| 3. Catechol functionalization w/ the biocompatibility | ||||||
| 10 | ALG/gelatin | Ionic Ca+2 | PBMCs | Skin | 1. Gel stability and offers strong antibacterial properties w/ gallium | 135 |
| 2. Bioink's reproducibility and scalability | ||||||
| 3. High-resolution & free printing of refined structures | ||||||
| 11 | Pectin methacrylate | Ionic Ca+2 | Human neonatal dermal fibroblast | Skin | 1. Tunable mechanical properties w/ UV cross-linking & ionic gelation | 136 |
| 2. Sear-thinning behavior in extrusion printing | ||||||
| 3. Mechanical strength for dermal tissue support | ||||||
| 12 | ALG/DCEl/gel | Ionic Ca+2 | hFBs | Skin | 1. Enhanced mechanical integrity and stacking | 67 |
| 2. Printing various shapes durable for manual handling | ||||||
| 3. Supports growth and function of skin cells | ||||||
| 13 | ALG/gel | Ionic Ca+2 | Schwann cells | Nerve | 1. Post-printing stability | 137 |
| 2. High viability rate of 91.87% in the scaffold | ||||||
| 3. Enhanced release of neurotrophic factors (NGF, BDNF, GDNF etc.) | ||||||
| 4. Positive immunofluorescence after 4 weeks | ||||||
| 14 | HA acid/ALG | Ionic Ca+2 | Schwann cells | Nerve | 1. Multilayer scaffolds w/ varied geometries | 138 |
| 2. Balance stabilization speed & flowability for effective bioprinting | ||||||
| 3. High Schwann cell viability and functional activity | ||||||
| 15 | ALG/BNC/GelMA | Ionic Ca+2 | Schwann cells | Nerve | 1. BNC addition improves mechanical & rheological properties | 139 |
| 2. Pos-printing shape fidelity & line thickness | ||||||
| 3. Oriented growth and high viability of Schwann cells | ||||||
| 16 | HA acid/ALG/gel | Ionic Ca+2 | Schwann cells | Nerve | 1. Curable via iron(III) chelation or photocuring, adjusting mechanical properties & degradation rates | 140 |
| 2. Intricate tissue architecture printing w/ post-printing stability | ||||||
| 3. High cell viability in Schwann cells, neuronal cells, and glioma cell-laden constructs | ||||||
| 17 | HA/gelatin | Genipin | MC3T3 | 1. Gelatin concentration affects hydrogel's viscosity and shear-thinning, improving extrusion | 6 | |
| 2. Printed complex, multi-layer (50 and 100 layers) structures | ||||||
| 3. Robust cell growth & high cell viability | ||||||
| 18 | ALG/HA | Ionic Ca+2 | MC3T3 | 1. Good printability for complex structures w/ stable viscosity | 2 | |
| 2. 100 layers printing w/ post-printing integrity under stress by Ca2+ | ||||||
| 3. Cell survival and controlled release of medicinal substances |
4.1 Cartilage
Collagen fibers, matrix, and chondrocytes make up cartilage tissue.141 Cartilage is devoid of lymphatic and blood vessels. From the perichondrium's blood arteries, nutrients enter the intercellular matrix and feed the osteocytes.142 This histological trait significantly restricts the injured cartilage's ability to regenerate itself. Thus, physicians and researchers have been baffled by the damage healing challenge.143 Combining cartilage scaffolds with 3D bioprinting technology offers a potent strategy to address and revitalize cartilage imperfections. These scaffolds are central in fostering essential cellular processes such as adhesion, differentiation, proliferation, and migration of the cells in the 3D bioprinted scaffolds. Moreover, they actively promote the regeneration of new tissue after implantation.144,1453D printing of bone and cartilage has utilized ALG, a biocompatible and organic polymer, for bioink synthesis. Although progress has been made in 3D bioprinting of ALG for orthopedic applications, issues such as poor bioink component distributions and mechanical properties, lack of long-term stability, the absence of functional moieties for cell adhesion and proliferation, etc. remained as problems to be solved.146 Hence, it is mixed with other proteins, polypeptides, and bioactive molecules to strengthen its structure and improve its biological activities. The uniform biomixing of multiple components present in todays advanced bioink is crucial for the controlled and homogeneous tissue regeneration. In a recent study, Bhattacharyya et al. demonstrated a screw extruder head with an ionic gel (ALG), alpha-tricalcium phosphate (α-TCP) micro/nanoparticles, and osteoblast cells. 10 layers were 3D bioprinted using the bioink samples with a screw extruder which showed better bioprintability, with better mechanical and biological properties than the conventional mixing ones. The live cell distribution in the printed constructs was significantly superior to conventional mixing even with continuous feeding and extrusion-based bioink printing. The extrusion head ensured the control of uniform micro/nano-materials and cell distribution throughout the directly mixed printable bioink with minimal cell damage as shown in Fig. 2(a and b).147
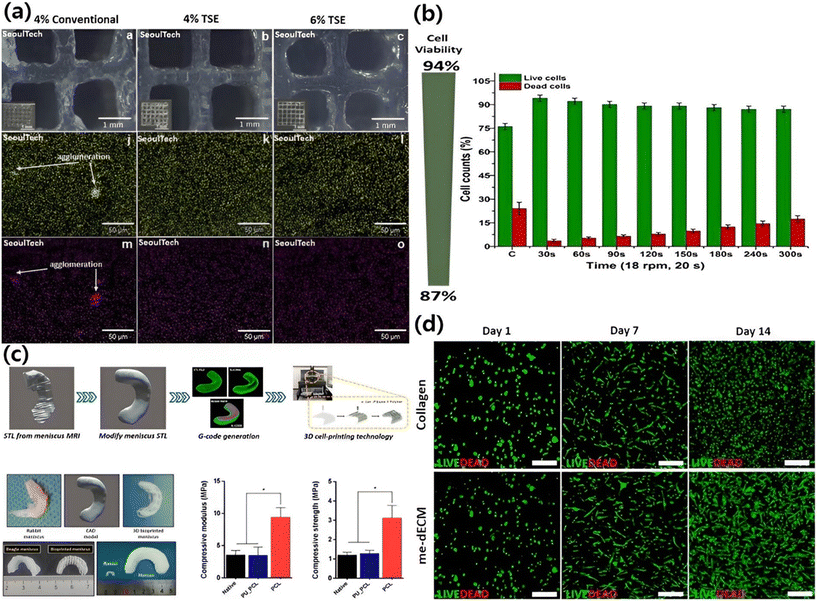 | ||
| Fig. 2 (a) 3D bioprinted (top view) micro/nanocomposite ALG gels showing improved dispersion of α-TCP particles in the bioprinting pen process; (b) cell viability of ALG gels (permission from Elsevier147); (c) bioink from the meniscus was used to 3D print living, functional meniscus grafts; (d) live/dead staining (permission from Elsevier148). | ||
Govindharaj et al. developed a bioink hydrogel combining dECM with ALG and Phallusia nigra supports. When 3D bioprinted using extrusion with human mesenchymal stem cells (hMSCs), the hydrogel exhibited high cell viability, substantially increasing hMSC proliferation and promoting chondrogenesis for cartilage repair applications.149 Chae et al. developed a 3D meniscus graft using mesenchymal stem cells and ECM. The implants showed good biocompatibility and strong mechanical properties in a rabbit meniscectomy model. They also stimulated the development of neo-fibro cartilage in vivo due to a meniscus-specific environment. This approach, combining cells, biomaterials, and bioactive proteins, holds promise for promoting regenerative healing of complex cartilage tissues (as depicted in Fig. 2(c) and (d)).148 Sadeghianmaryan et al. enhanced 3D bioprinting using a chitosan-based bioink with optimized crosslinking methods. The biocompatibility of the chitosan-printed bioink was validated through ATDC5 cell testing. This bioink holds promise for applications in cartilage regeneration and joint repair due to the articular cartilage's role as a shock absorber and lubricant in the joints.150
To increase the strength of the chitosan hydrogel, He et al. first treated the chitosan with calcium and ethylene diamine tetraacetic acid (EDTA), and then 3D printed a scaffold. The bioink has been altered to exhibit enhanced mechanical characteristics and to encourage the growth of chondrocytes and the expression of genes related to cartilage.151 One derivative of chitosan with outstanding injectability and biocompatibility is hydroxybutyl-chitosan (HBC). Liu et al. synthesized the HBC–nanofiber (NF) hydrogel by combining HBC hydrogel with poly(lactic acid-co-glycolic acid) copolymer nanofiber. The use of nanofibers considerably improved the HBC–NF hydrogel's capacity to encourage cartilage development. The HBC–NF hydrogel was then injected into a 3D printed PCL skeleton that had internal microchannels to produce a mechanical concentration scaffold resembling a real cartilage.152 Antich et al. printed 3D scaffolds for articular cartilage by combining HA and alginate. The novel biological ink that encourages the regeneration of articular cartilage and has appropriate mechanical qualities is made of HA and alginate.153 Angelis et al. printed 3D CH/HAa scaffolds using chitosan (CH) and hydroxyapatite (HAa) as raw materials. During a protracted culturing period, the printed scaffold's adequate porosity offers ideal conditions for chondrocyte colonization and synthesis. A unique idea for articular cartilage tissue engineering is provided by the scaffold cultured in platelet lysate (PL), which can better retain the phenotype of chondrocytes and enhance gene expression of the most relevant ECM components, utilizing the fact that HAa encourages cartilage regeneration.154
Olate-Moya et al. mixed alginate, gelatin, and chondroitin sulfate (CS). To enhance the shape fidelity and resolution of the 3D printing scaffold, graphene oxide was further added to the composite hydrogel as a nanofiller material. A 30 × 30 × 1 mm3 (length × width × height) scaffold was designed. An interior pattern resembling a mesh was established, with a thread spacing of 1.5 mm. Human adipose tissue mesenchymal stem cells were used to demonstrate that the novel nanocomposite hydrogel had a higher rate of cell proliferation than pure sodium alginate. Additionally, the scaffold made with the innovative hydrogel nanocomposite shows superior cartilage induction.155
4.2 Bone
Bone is a sophisticated composition comprising hierarchical tissue, mineralized collagen fibers, and a network of blood vessels. Remarkably, bones can autonomously mend minor cracks and fractures due to their strong regenerative capacity. However, when a bone injury exceeds the size of two centimeters, its natural healing mechanisms become insufficient. Restoring both form and function necessitates tailored grafts with tissue regeneration capacity. Conventional hydrogels lack precise control for internal structure and growth factor distribution.Innovative 3D bioprinting methods have been proposed to get over these constraints.156,157 In a recent advancement, Curti et al. created a scaffold-based 3D bioprint bioink from fish gelatin(FG), cuttlebone (CB), and ALG. It was accomplished by bonding the bioink after it had been produced. Conical nozzles with an inner diameter of 0.25 mm (G25) were employed; eight layers with a deposition direction ranging from 0 to 90°, and a layer thickness equal to 75% of the nozzle diameter. The composite FG–CB–ALG bio-ink hydrogel encapsulated to pre-osteoclast cells MC3T3-E1 and most bone ECMs were replicated using marine materials. The in vitro biocompatibility assay revealed that the composites could support MC3T3-E1 murine pre-osteoblast growth and proliferation and that the hydrogel may boost the bone regeneration potential of the 3D bioprint scaffold.158 Seunghyun et al. introduced PDANPs for their notable affinity to bind with calcium ions, ALG, and temporally oxidized cellulose nanofibrils, all vital components within the bioink designed for bone tissue construction. Once integrated into each bioink, osteoblasts were extruded using 3D printing technology, and hydrogels were printed as a grid structure of 6 layers, followed by a curing period lasting up to 7 days. Notably, most cells in each sample remained viable. The printed cells' viability rate (% of living cells) surpassed 80% for all three models on both days 1 and 7.159
For the objective of bone tissue engineering, a unique MeGC-based cell-laden 3D bioink was created. For creating biocompatible and structurally stable cell structures, the bioink solutions were tuned. MeGC bioink was created by photocuring the precursor solution for 70 seconds. The produced five-layer 3D bioprinting constructions showed increased cell proliferation, distinct bone differentiation, and good shape fidelity.160
Wu et al. introduced a dual-network hydrogel approach, by combining the shear-thinning and recovery properties of GEG with the rapid photo crosslinking capabilities of poly(ethylene glycol)-diacrylate (PEGDA). The bioprinted multilayered scaffold provided an open and interconnected network (Fig. 3(a to l)), facilitating efficient oxygen and nutrient exchange. Within these hydrogels, several human-scale structures were printed with good stability and fidelity. Furthermore, researchers incorporated MC3T3-E1 cells and murine BMSCs. Throughout the 21 day cell culture, both cell types demonstrated both stability and viability, with cell survival exceeding 87%.131 The deferoxamine-loaded GelMA/GGMA-based hybrid bio-ink was improved by Zhihui et al. using ionic and light crossing techniques to create a 3D-printed scaffold of 60 layers as shown in (Fig. 3(m)–(o)). The hydrogels made of GGMA/GelMA had the best printing and tensile qualities. The regulated release of pharmaceuticals from GGMA/GelMA hydrogels could be used to simulate in vivo bone reserve, HUVEC tube improvement, and cell migration. A rat cranial defect model study demonstrated that angiogenesis and bone repair might occur in vivo by activating the hypoxia-inducible factor 1 (one)-alpha signaling pathway. To repair the bond defect, hybrid hydrogel 3D bioprinting can assist with osteogenesis and angiogenesis.133
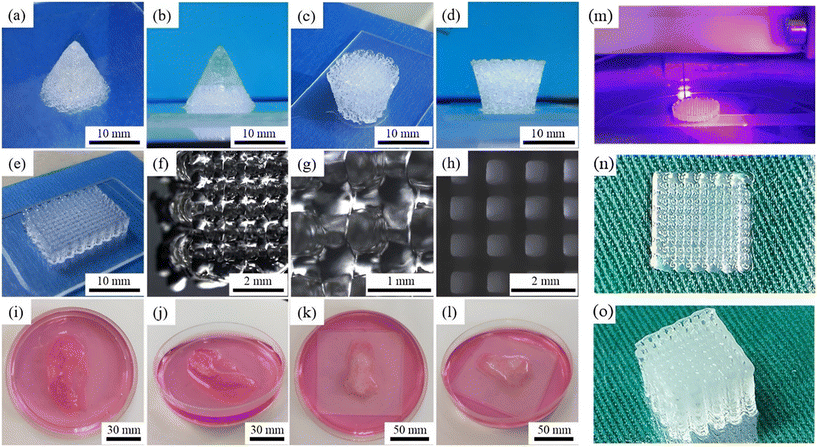 | ||
| Fig. 3 Images of several 3D printed scaffolds by Ink G1.5P10. Aerial view (a) and left view (b) images of a sharp cone structure. Aerial view (c) and left view (d) images of a reverse square prism structure. (e) The aerial view image of a cuboid structure. Its partial enlarged images obtained by a stereoscope shown as (f)–(h) (after being treated with liquid nitrogen). Top view (i) and aerial view (j) images of a 3D printed gel human ear. Top view (k) and aerial view (l) images of a 3D printed gel human nose. (permission from Elsevier).131 (m)–(o) Printing process of the scaffold, top view and side view of the scaffold with 60 layers (permission from Elsevier133). | ||
4.3 Skin
The body's largest organ, the skin, serves as a protective barrier against the external environment. Bioprinted skin must possess key attributes to facilitate effective nutrient/waste exchange and promote robust skin regeneration. These include biocompatibility, mechanical properties aligned with natural skin tissue, and a meticulously interconnected network of pores.161,162 Lately, there has been a growing trend in developing 3D multicellular HA-based constructs.6 In addition to HA, researchers have explored various other biocompatible materials like collagen, fibrin, decellularized dermis and epidermis, ALG,147 chitosan,7,147 gelatin, and cellulose,95 aiming to facilitate skin wound healing. Liu et al. introduced an innovative approach by combining ALG/gelatin to produce a bioink with enhanced printability of multilayered scaffold structures. Through an in-depth rheological analysis, they identified the optimal blend comprising 2% ALG and 15% gelatin. Introducing 2% CaCl2 triggered a remarkable ionic gelation process driven by the ALG component, which substantially stabilizes the fabricated structures. This hybrid bioink encapsulated hAECs and was employed for bioprinting a skin-like, bi-layered membrane construct. The outcomes of the in vitro study demonstrated a notable enhancement in the epithelial cell phenotype of the hAECs.163 In a study by Zhao et al., the approach involved the incorporation of poly(p-phenylene vinyl acetate) (PPV) and HA into ALG/gelatin ink blends, resulting in skin patches imbued with distinctive biological properties. The ALG/HA/Gel-A5G81/PPV skin patches proved instrumental in effectively healing the wound and fostering the development of new skin tissue. Importantly, these patches exhibited in vivo biodegradability, signifying their potential as a promising avenue for advanced wound healing approaches.164 Due to the uniform distribution of chitosan and kaolinite nanoclay utilizing the screw-based pen-type extruder, Bhattacharyya et al. in this study demonstrated the structural stability of the multicomponent ALG-based hydrogel. Extrudable multilayered big and sophisticated tissue engineering constructs can be created using this multiple crosslinking method of the self-standing hydrogel with good post-printing shape fidelity. For large scale 3D bioprinting, the “Biowork™” pen with a roller head attached to a 3D bioprinter is utilized. Bioinks made from osteoblast and fibroblast cells in varying concentrations of kaolin-dispersed alginate/chitosan hydrogels were used, and various multilayered 3D bioprinted constructs were demonstrated as given in (Fig. 4(a)–(h)). Additionally, it increases the cellular activity of two distinct cell types (osteoblast and fibroblast), which are targeted towards bone and skin tissue engineering.7 The double crosslinked hydrogel with HA as its foundation showed excellent swelling characteristics, biocompatibility, and extremely controlled breakdown. To treat diabetic foot ulcers, this material is being evaluated as a novel gel wound dressing. Zhou et al. printed functional live skin using a newly developed biomimetic GelMA/HA-NB/LAP hydrogel using digital light processing based 3D printing technology (Fig. 4 (i), (i.1)–(i.3) and (j)).165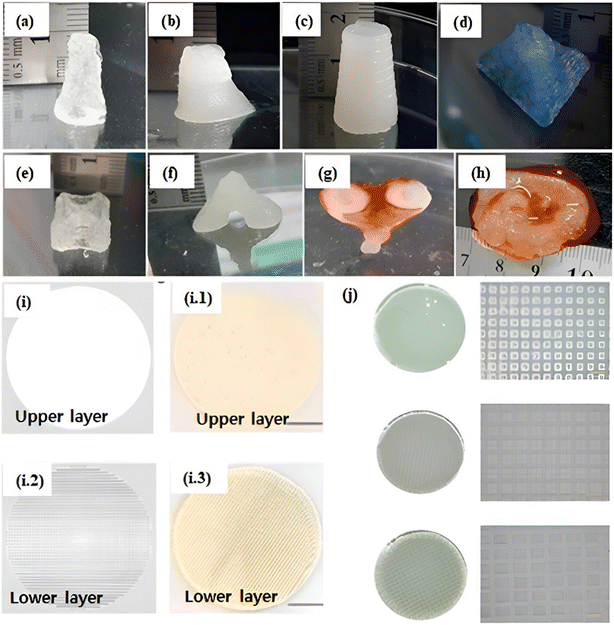 | ||
| Fig. 4 (a)–(c) 3D printed hollow cylinder with varying nanocomposite concentration, (d) pyramid, (e) star, (f) top view of the bifurcated tube, (g) bottom view of the bifurcated tube, (h) and human ear (permission from Elsevier7). (i) & (i.1) The upper dense layer is dense mimicking the epidermis, (i.2) and (i.3) the lower porous layer resembling the dermis. (j) The printed products by DLP-based 3D printing showed complex structures of CAD images of 3D bioprinted products.165 | ||
4.4 Nerve
The nervous system is pivotal in transmitting impulses across different body regions, thereby governing their coordinated movement. In addressing central and peripheral nerve injuries, the restoration of nerve function through regeneration is of paramount importance. However, the intricacy lies in the fact that nerve regeneration is particularly challenging for the central nervous system compared to the peripheral nervous system, as the former exhibits limited inherent self-repair capabilities.166 Efforts directed at brain tissue regeneration through bioprinting entail the creation of 3D models that encompass elevated cell densities and a diverse array of cell types. This orchestrated complexity serves as a vital substrate for mimicking the intricate architecture of brain tissue. Moreover, the success of these models hinges upon the precise administration of external cues, which govern cell behavior and sustain the intricate web of cellular interactions.167 By harnessing bioprinting, a dynamic platform is established for fostering the renewal and preservation of the nervous system, addressing the challenges posed by nerve injuries and contributing to the advancement of regenerative therapies.168 Peripheral nerve damage presents a clinically intricate and challenging scenario, often leading to compromised sensory and motor capabilities due to inherent regenerative potential limitations. Despite various efforts, their efficacy is frequently confined. In the pursuit of addressing peripheral nerve regeneration, nerve-guided catheters have emerged as captivating alternatives. Among the therapeutic options available, autologous nerve transplantation, while popular, is burdened with shortcomings such as donor scarcity and potential neurological issues in the donor area.169,170 Presently, the predominant choice for malleable nerve cells in neural tissue engineering revolves around Schwann cells. These versatile cells possess the capacity to express an array of nerve growth factors (NGF), encompassing NGF, BDNF, GDNF, and PDGF, thereby instigating the formation of nerve synapses. Particularly adept at promoting the regeneration of the peripheral nervous system, Schwann cells find extensive application. Consequently, integrating ALG-based hydrogels housing Schwann cells can effectively facilitate the expression of multiple nerve growth factors, contributing to a conducive environment for nerve development.137 As an illustration, the hydrogel framework, crafted from a blend of low-viscosity ALG, HA, and fibrinogen, notably fosters heightened activity and proliferation of Schwann cells, accompanied by the expression of essential biological proteins. Moreover, this scaffold exerts a regulatory influence on the maturation of the dorsal root ganglion, concurrently promoting the alignment of Schwann cells.138 The study explored the printability aspects of ALG, GelMA and a hybrid bioink composed of bacterial nano-cellulose (BNC), accompanied by an in-depth analysis of the resulting material's physicochemical attributes reported by Wu et al. Subsequently, RSC96 cells were integrated into the bioink and used for bioprinting. The incorporation of ALG and BNC in the composition reveals that post photo-crosslinking and chemical crosslinking, the BNC and GelMA composite exhibited the most delicate lines compared to other groups. This suggests that the addition of BNCs enables the preservation of the scaffold's original shape, highlighting its potential to maintain inherent structural integrity as shown in (Fig. 5a). Furthermore, the biocompatibility of the engineered construct was confirmed through experiments involving unclothed mice. To facilitate nerve regeneration, the combination of BNC with ALG/GelMA was employed in the hydrogel construct. Notably, in vivo investigations revealed compelling outcomes, wherein directed growth, proliferation, and gene expression of rat Schwann cells were promoted as shown in (Fig. 5b and c).139 Haring et al. employed a combination of natural and synthetic polymers to successfully bioprint 3D micro-physiological brain tissue, facilitating research on disease models and regenerative potential. The bioprinting process involved using a specially formulated bioink of HA, ALG, and gelatin, which could be crosslinked using CaCl2. This investigation was conducted in comparison to the stiffness of the original brain tissue. Intriguingly, it was discovered that the hydrogel attained a stiffness level comparable to that of native human tissue at higher concentrations of gelatin.140 Similarly, Khatun et al. demonstrated genipin crosslinked with a gelatin–HA bioresponsive hydrogel for high shape fidelity and resolution. A variety of forms, including hollow tubes and star shapes that were each made up of about 50 layers and could reach a height of 1 cm (Fig. 5d and e), were successfully printed. These intricate printed forms have a porous surface morphology that was visible after lyophilization. The gelatin–HA gel-based 3D printed structures, in particular the 1.5 cm tall pyramid-shaped structure (Fig. 5f) with about 100 layers, demonstrated exceptional stability and shape integrity. An intriguing finding was that the printed structures stuck to the substrates very well without the need for further adhesive, indicating how highly sticky the hydrogel is to the surface.6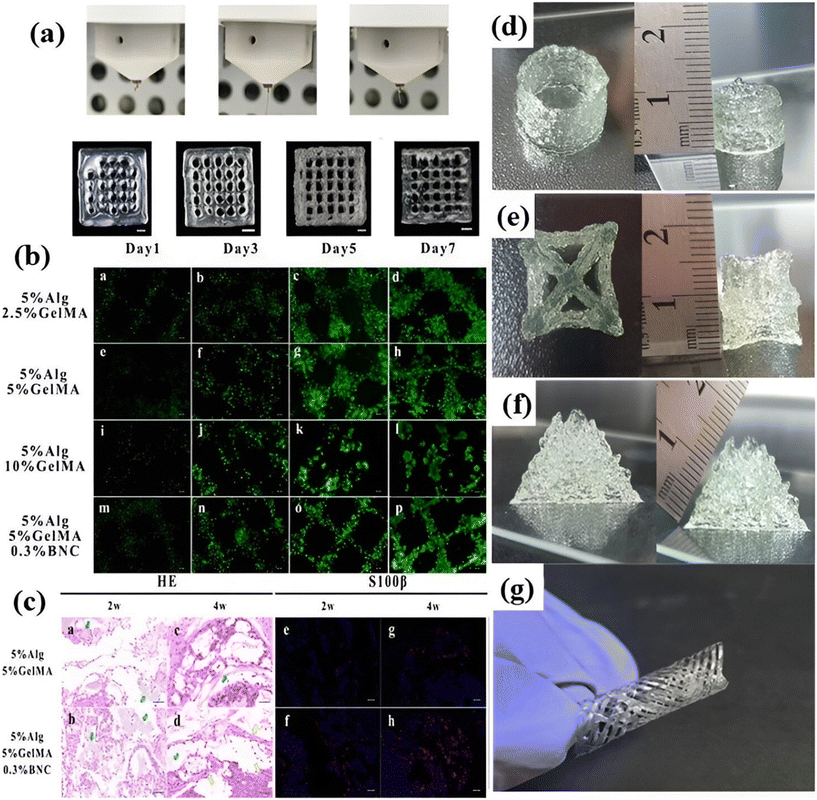 | ||
| Fig. 5 Application of nerve tissue regeneration: (a) different concentrations of ALG BNC, and GelMA, excessive fluidity of extruded liquid, extruded lines suitable for printing. (b) and (c) Cell viability staining is performed on the hydrogel (permission Elsevier).139 3D bioprinted structures: (d) hollow tube structure, (e) star shape, (f) pyramid shape & (g) four-axis printed tubular shape with a bio responsive gelatin–hyaluronic acid hydrogel (permission MDPI6). | ||
4.5 Muscle
Muscle tissue provides for around 45% of total body weight and accounts for a considerable percentage of the body's composition, with a significant portion interwoven with bones. Peripheral nerves intricately innervate muscles, particularly those within motor organs. In nerve injury, particularly to peripheral nerves, the innervated muscles undergo a process of atrophy, leading to a decline in size and function.171 Hence, the restoration of muscle tissue involves a combination of strategies encompassing nerve and vascular regeneration techniques. Currently, the principal avenues for promoting muscle regeneration involve administering growth factors and cell transplantation. Notably, ALG is a widely favored biomaterial for 3D printing muscle constructs, particularly in ALG-based bioink with cells.172 Lee et al. demonstrated a bioink composed of collagen, GelMA, and dECM, restoring muscle tissue using 3D bioprinting with a novel photo-crosslinking technique. This crosslinking method was used to create multilayered 3D cell-laden structures, mechanically stable and biologically safe, cell-laden struts. When administered to a volumetric muscle defect injury in mice, the bioprinted hASC-containing constructs showed noticeably improved muscle regeneration over those made with a traditional bioprinting method.173 The C2C12 myogenic cells, a widely used cell type in tissue engineering for muscle regeneration, were studied in those 3 commercial biomaterials. The results showed robust cell proliferation and successful differentiation across all 3 muscular scaffolds. The cells also demonstrated significant myotubular organization along a linear structure and the ability to coalesce and fuse, generating structurally aligned multinucleated myotubes.1744.6 Other tissues
Tuning the polysaccharide-based hydrogels through a simple modification or crosslinking method enabled researchers to use them in tissue engineering and bioprinting tissues such as retina, liver, heart, etc.175 The kidney's substantial complexity and intricate structure pose a formidable challenge for complete bioprinting in a single endeavor. The formidable nature of this task arises from both the intricacy of the organ's design and its considerable size. The most practical approach involves employing 3D bioprinting to create microscopic components, often called “mini tissue building blocks.” Subsequently, these intricate building blocks can be assembled to construct larger, functional kidney structures.175 To circumvent the intricacies of organ size and cellular diversity, the utilization of organoids presents a viable avenue for bioprinting endeavors. However, achieving functional viability for the kidney necessitates addressing critical concerns related to the organ's ductal system and vasculature, which present their own complex challenges.176 Cardiovascular disease (CD) is the foremost global cause of mortality, accounting for 31% of all deaths. This prevalent health issue primarily arises from plaque accumulation, a condition known as atherosclerosis, which constricts arteries and other vital blood vessels. This broader category of cardiovascular ailments encompasses conditions like myocardial infarction, congestive heart failure, stroke, and valvular heart disease.177 A composite bioink (ALG/GelMA) is intricately deposited using the coaxial extrusion bioprinting technology, encasing endothelial cells, and creating micro fibrous scaffolds with intricate micro-channels. This novel approach significantly promotes the process of scaffold reendothelialization, encouraging the growth and proliferation of HUVECs. After this, the deendothelialized scaffold serves as a foundation for introducing cardiomyocytes, culminating in developing a deendothelialized myocardial scaffold. This scaffold configuration closely mimics the structural attributes of native myocardial tissue. Remarkably, the scaffold orchestrates synchronized and spontaneous contractions and maintains a consistent rhythm, yielding an impressive heartbeat rate ranging from 55 to 75 beats per minute.178 LA et al. created axially symmetric aortic valve scaffolds with a 12 mm internal diameter using PEGDA and ALG, similar to double nozzle extrusion printing. The hybrid scaffolds had an elastic modulus of 5.3–74.6 kPa and a nearly 100% survival rate for cultured PAVIC over 21 days.179 The bioink derived from ALG can be used to create 3D scaffolds that enhance the mechanical and biological properties of the hydrogels. These scaffolds, when adapted to the liver's honeycomb architecture, can significantly improve hepatocyte proliferation, replication, and overall liver function preservation.180 Rania et al. used a core–shell bioprinting technique to enhance the complexity of hepatocytes' microenvironment. They used distinct core–shell structures housing different cell types and incorporated Matrigel into ALG/MA to mimic the honeycomb-like arrangement in liver tissue. This led to significant increase in hepatocyte activity and proliferation within the scaffold. The introduction of plasma or fibrin into the core of ALG/MA facilitated the organization of fibroblasts into a network, creating a more intricate and realistic microenvironment.181 A 3D retina-equivalent construct was 3D printed using GM and triethylamine to methacrylate–HA at different degrees. Cell-binding motifs were added to HA–GM polymers, followed by photoirradiation using a LAP photo initiator. The hydrogels were further enhanced with RGD to improve adhesion properties. The construct, with a base layer of 125 μm and a top layer of 250 μm, showed over 70% vitality and matured into retinal photoreceptors, ganglion cells, and bipolar cells within 14 days, resembling real retina layers.182,1835. Future directions
3D bioprinting has made significant progress, but the challenge lies in creating tissue engineering structures with desired bioink properties. Natural polysaccharides continue to be the preferred option for creating bioinks for 3D bioprinting tissue engineering. Because of their greater bioactivity, lower immune response and biocompatibility, polysaccharides are frequently preferred over proteins and synthetic polymers like PCL, PEG, and Pluronic. These naturally occurring biomaterials are excellent at simulating the ECM and offer vital signals that support cell adhesion, proliferation, differentiation, and tissue regeneration, all of which are necessary for not only tissue regeneration but also proper integration with host tissues and operation of bioengineered tissues. Although they have limitations in terms of processing and cell interactions in 3D bioprinting tissue engineering, a number of interesting methods and chemical alterations have already shown how to overcome these restrictions. 3D bioprinting has made substantial progress using polysaccharide-based bioinks due to their biocompatibility and effective mimicry of the ECM in different tissues. These hydrogels can work as a major bioink component for engineering complex, multilayered structures and are favored for their non-toxicity, natural degradability with minimal side effects, and diverse crosslinking properties. Current research is enhancing these bioinks' properties with modifications of hydrogels to their chemical and physical structures similar to ECM's proteoglycans including cell-adhesive oligopeptides, aiming to closely replicate native tissue environments and improve the functionality of the engineered tissues. The field is moving towards integrating bioactive molecules such as growth factors and nanoparticles, as well as incorporating real-time monitoring technologies to refine the bioprinting process. This integration is expected to drive significant advances in personalized regenerative medicine, leading to more effective tissue and organ replacements. Especially in complex tissue regeneration, fabrication of multilayered tissues such as skin, blood vessel and osteocartilage emerges as an essential tool. Furthermore, development of bioinks and hydrogels is critical in tissue engineering and regenerative medicine with post-printing fidelity, i.e. higher interaction between the layers of the tissue engineering constructs. The convergence of these technologies holds promise for accelerating the development of personalized, functional tissue replacements, ushering in a new era of 3D bioprinting of multilayered complex tissue scaffolds, regenerative medicine and tissue engineering.6. Abbreviations
| DECL | Diethylaminoethyl cellulose |
| ALG | Alginate (sodium salt) |
| CHN | Chitosan |
| HA | Hyaluronic acid |
| CEL | Cellulose |
| DEX | Dextran |
| ECM | Extracellular matrix |
| GEG | Gellan gum |
| AGR | Agarose |
| KJG | Konjac gum |
| GG | Guar gum |
| XTG | Xanthan gum |
| CS | Chondroitin sulfate |
| PRP | Platelet-rich plasma |
| BMSCs | Bone marrow mesenchymal stromal cells |
| dECM | Decellularized extracellular matrix |
| MFC | Meniscal fibrocartilage cells |
| TGF | Transforming growth factor beta-1 |
| MSCs | Mesenchymal stem cells |
| MECM | Meniscal extracellular matrix |
| SMSc | Synovial mesenchymal stem cells |
| hMSCs | Human mesenchymal stem cells |
| ATDC5 | Mouse teratocarcinoma cell |
| MC3T3-E1 | Clonal murine cell line of immature osteoblasts |
| MA | Methacrylate |
| HASCs | Human adipose-derived stem cells |
| BMP-2 | Bone morphogenetic protein-2 |
| GGMA | Gellan gum methacrylate |
| HUVEC | Human umbilical vein endothelial cells |
| hAECs | Human amniotic epithelial cells |
| NGF | Nerve growth factor |
| BDNF | Brain-derived neurotrophic factor |
| GDNF | Glial cell-derived neurotrophic factor |
| PDGF | Platelet-derived growth factor |
| BNC | Bacterial nanocellulose |
| RSc | Rat Schwann Cell 96 |
| CaCl2 | Calcium chloride |
| CD | Cardiovascular disease |
| PAVIC | Porcine aortic valve interstitial cells |
| BTE | Bone tissue engineering |
| CCD-986 | Human skin fibroblast cells |
| PBMCs | Human peripheral blood mononuclear cells |
| hFBs | Human fibroblasts |
| MeGC | Methacrylated glycol chitosan |
| FG | Fish gelatin |
| CB | Cuttle bone |
| CMP | Cuttlefish melanin nanoparticles |
| PLs | Platelet lysates |
| GFs | Growth factor |
| CNC | Carboxylated-cellulose nanocrystal |
| PDANP | Polydopamine nanoparticles |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by Seoul National University of Science and Technology.References
- D. Lee, J.-Y. Park, Y.-W. Seo, X. Jin, J. Hong and A. Bhattacharyya, Photo-crosslinked gelatin methacryloyl strengthened with nanoparticles for regeneration of rabbit calvarial defects, J. Periodontal Implant Sci., 2023, 53, 321–335 CrossRef CAS PubMed.
- G. Janarthanan, J. H. Kim, I. Kim, C. Lee, E.-J. Chung and I. Noh, Manufacturing of self-standing multi-layered 3D-bioprinted alginate-hyaluronate constructs by controlling the cross-linking mechanisms for tissue engineering applications, Biofabrication, 2022, 14(3), 035013 CrossRef CAS PubMed.
- A. Bhattacharyya, V. K. Priya, J.-h. Kim, M. R. Khatun, R. Nagarajan and I. Noh, Nanodiamond enhanced mechanical and biological properties of extrudable gelatin hydrogel cross-linked with tannic acid and ferrous sulphate, Biomater. Res., 2022, 26(1), 37 CrossRef CAS PubMed.
- A. Bhattacharyya, M. R. Khatun, S. Narmatha, R. Nagarajan and I. Noh, Modulation of 3D Bioprintability in Polysaccharide Bioink by Bioglass Nanoparticles and Multiple Metal Ions for Tissue Engineering, Tissue Eng. Regener. Med., 2023, 1–15 Search PubMed.
- H. N. Tran, I. G. Kim, J. H. Kim, A. Bhattacharyya, E. J. Chung and I. Noh, Incorporation of Cell-Adhesive Proteins in 3D-Printed Lipoic Acid-Maleic Acid-Poly(Propylene Glycol)-Based Tough Gel Ink for Cell-Supportive Microenvironment, Macromol. Biosci., 2023, 23(11), 2300316 CrossRef CAS.
- M. R. Khatun, A. Bhattacharyya, M. Gunbayar, M. Jung and I. Noh, Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting, Gels, 2023, 9, 601 CrossRef CAS PubMed.
- A. Bhattacharyya, H.-w. Ham, J. Sonh, M. Gunbayar, R. Jeffy and R. Nagarajan, et al., 3D bioprinting of complex tissue scaffolds with in situ homogeneously mixed alginate-chitosan-kaolin bioink using advanced portable biopen, Carbohydr. Polym., 2023, 121046 CrossRef CAS PubMed.
- M. P. Sekar, H. Budharaju, A. Zennifer, S. Sethuraman, N. Vermeulen, D. Sundaramurthi and D. M. Kalaskar, Current standards and ethical landscape of engineered tissues—3D bioprinting perspective, J. Tissue Eng., 2021, 12, 20417314211027677 Search PubMed.
- H. Budharaju, A. Subramanian and S. Sethuraman, Recent advancements in cardiovascular bioprinting and bioprinted cardiac constructs, Biomater. Sci., 2021, 9(6), 1974–1994 RSC.
- P. M. Sivakumar, A. A. Yetisgin, S. B. Sahin, E. Demir and S. Cetinel, Bone tissue engineering: Anionic polysaccharides as promising scaffolds, Carbohydr. Polym., 2022, 283, 119142 CrossRef PubMed.
- A. Zaszczyńska, M. Moczulska-Heljak, A. Gradys and P. Sajkiewicz, Advances in 3D printing for tissue engineering, Materials, 2021, 14(12), 3149 CrossRef.
- P. Wu, X. Xi, R.Li and G. Sun, Engineering polysaccharides for tissue repair and regeneration, Macromol. Biosci., 2021, 21(9), 2100141 CrossRef CAS.
- Y. Zhou, X. Chen, T. Chen and X. Chen, A review of the antibacterial activity and mechanisms of plant polysaccharides, Trends Food Sci. Technol., 2022, 123, 264–280 CrossRef CAS.
- H. Claus-Desbonnet, E. Nikly, V. Nalbantova, D. Karcheva-Bahchevanska, S. Ivanova and G. Pierre, et al., Polysaccharides and their derivatives as potential antiviral molecules, Viruses, 2022, 14(2), 426 CrossRef CAS PubMed.
- R. Guo, M. Chen, Y. Ding, P. Yang, M. Wang and H. Zhang, et al., Polysaccharides as potential anti-tumor biomacromolecules—a review, Front. Nutr., 2022, 9, 838179 CrossRef PubMed.
- H. Jin, M. Li, F. Tian, F. Yu and W. Zhao, An overview of antitumour activity of polysaccharides, Molecules, 2022, 27(22), 8083 CrossRef CAS PubMed.
- E. J. Murphy, G. W. Fehrenbach, I. Z. Abidin, C. Buckley, T. Montgomery and R. Pogue, et al., Polysaccharides—Naturally Occurring Immune Modulators, Polymer, 2023, 15(10), 2373 CAS.
- Y. Hu, Y. He, Z. Niu, T. Shen, J. Zhang and X. Wang, et al., A review of the immunomodulatory activities of polysaccharides isolated from Panax species, J. Ginseng Res., 2022, 46(1), 23–32 CrossRef PubMed.
- M. Mahinroosta, Z. J. Farsangi, A. Allahverdi and Z. Shakoori, Hydrogels as intelligent materials: A brief review of synthesis, properties and applications, Mater. Today Chem., 2018, 8, 42–55 CrossRef CAS.
- J. N. Israelachvili, Intermolecular and surface forces, Academic press, 2011 Search PubMed.
- Y. Liu, J. Wang, H. Chen and D. Cheng, Environmentally friendly hydrogel: A review of classification, preparation and application in agriculture, Sci. Total Environ., 2022, 846, 157303 CrossRef CAS PubMed.
- D. Kühbeck, J. Mayr, M. Häring, M. Hofmann, F. Quignard and D. D. Díaz, Evaluation of the nitroaldol reaction in the presence of metal ion-crosslinked alginates, New J. Chem., 2015, 39(3), 2306–2315 RSC.
- M. F. Akhtar, M. Hanif and N. M. Ranjha, Methods of synthesis of hydrogels… A review, Saudi Pharm. J., 2016, 24(5), 554–559 CrossRef PubMed.
- E. Caló and V. V. Khutoryanskiy, Biomedical applications of hydrogels: A review of patents and commercial products, Eur. Polym. J., 2015, 65, 252–267 CrossRef.
- N. Reddy, R. Reddy and Q. Jiang, Crosslinking biopolymers for biomedical applications, Trends Biotechnol., 2015, 33(6), 362–369 CrossRef CAS PubMed.
- A. Sheikhi, J. de Rutte, R. Haghniaz, O. Akouissi, A. Sohrabi, D. Di Carlo and A. Khademhosseini, Microfluidic-enabled bottom-up hydrogels from annealable naturally-derived protein microbeads, Biomaterials, 2019, 192, 560–568 CrossRef CAS PubMed.
- T. A. Ahmed, E. V. Dare and M. Hincke, Fibrin: a versatile scaffold for tissue engineering applications, Tissue Eng., Part B, 2008, 14(2), 199–215 CrossRef CAS PubMed.
- M. A. de Moraes, E. Paternotte, D. Mantovani and M. M. Beppu, Mechanical and biological performances of new scaffolds made of collagen hydrogels and fibroin microfibers for vascular tissue engineering, Macromol. Biosci., 2012, 12(9), 1253–1264 CrossRef PubMed.
- A. Gandini, T. M. Lacerda, A. J. Carvalho and E. Trovatti, Progress of polymers from renewable resources: furans, vegetable oils, and polysaccharides, Chem. Rev., 2016, 116(3), 1637–1669 CrossRef CAS PubMed.
- D. Impellizzeri, R. Siracusa, M. Cordaro, A. F. Peritore, E. Gugliandolo and R. D'amico, et al., Protective effect of a new hyaluronic acid-carnosine conjugate on the modulation of the inflammatory response in mice subjected to collagen-induced arthritis, Biomed. Pharmacother., 2020, 125, 110023 CrossRef CAS PubMed.
- H. Q. Song, Y. Fan, Y. Hu, G. Cheng and F. J. Xu, Polysaccharide–peptide conjugates: a versatile material platform for biomedical applications, Adv. Funct. Mater., 2021, 31(6), 2005978 CrossRef CAS.
- P. Sahariah and M. Másson, Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship, Biomacromolecules, 2017, 18(11), 3846–3868 CrossRef CAS PubMed.
- L. Lin, Y. Fang, Y. Liao, G. Chen, C. Gao and P. Zhu, 3D printing and digital processing techniques in dentistry: a review of literature, Adv. Eng. Mater., 2019, 21(6), 1801013 CrossRef.
- L. Pugliese, S. Marconi, E. Negrello, V. Mauri, A. Peri and V. Gallo, et al., The clinical use of 3D printing in surgery, Updates Surg., 2018, 70, 381–388 CrossRef PubMed.
- S. Song, X. Liu, J. Huang and Z. Zhang, Neural stem cell-laden 3D bioprinting of polyphenol-doped electroconductive hydrogel scaffolds for enhanced neuronal differentiation, Biomater. Adv., 2022, 133, 112639 CrossRef CAS PubMed.
- S. Freeman, S. Calabro, R. Williams, S. Jin and K. Ye, Bioink formulation and machine learning-empowered bioprinting optimization, Front. bioeng. biotechnol., 2022, 10, 913579 CrossRef PubMed.
- A. I. Cernencu and M. Ioniţă, The current state of the art in gellan-based printing inks in tissue engineering, Carbohydr. Polym., 2023, 120676 CrossRef CAS PubMed.
- M. Jin, J. Shi, W. Zhu, H. Yao and D.-A. Wang, Polysaccharide-based biomaterials in tissue engineering: a review, Tissue Eng., Part B, 2021, 27(6), 604–626 CrossRef CAS PubMed.
- M. S. Algahtani, A. A. Mohammed and J. Ahmad, Extrusion-based 3D printing for pharmaceuticals: contemporary research and applications, Curr. Pharm. Des., 2018, 24(42), 4991–5008 CrossRef CAS PubMed.
- J. K. Placone and A. J. Engler, Recent advances in extrusion-based 3D printing for biomedical applications, Adv. Healthcare Mater., 2018, 7(8), 1701161 CrossRef PubMed.
- X. Cui, J. Li, Y. Hartanto, M. Durham, J. Tang and H. Zhang, et al., Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks, Adv. Healthcare Mater., 2020, 9(15), 1901648 CrossRef CAS PubMed.
- N. Khoshnood and A. Zamanian, A comprehensive review on scaffold-free bioinks for bioprinting, Bioprinting, 2020, 19, e00088 CrossRef.
- N. Ashammakhi, S. Ahadian, C. Xu, H. Montazerian, H. Ko and R. Nasiri, et al., Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs, Mater. Today Bio, 2019, 1, 100008 CrossRef CAS PubMed.
- A. Dhawan, P. M. Kennedy, E. B. Rizk and I. T. Ozbolat, Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery, J. Am. Acad. Orthop. Surg., 2019, 27(5), e215–e226 CrossRef PubMed.
- G. Gao, H. Kim, B. S. Kim, J. S. Kong, J. Y. Lee and B. W. Park, et al., Tissue-engineering of vascular grafts containing endothelium and smooth-muscle using triple-coaxial cell printing, Appl. Phys. Rev., 2019, 6, 041402 Search PubMed.
- R. El Khoury, N. Nagiah, J. A. Mudloff, V. Thakur, M. Chattopadhyay and B. Joddar, 3D bioprinted spheroidal droplets for engineering the heterocellular coupling between cardiomyocytes and cardiac fibroblasts, Cyborg Bionic Syst., 2021, 2021, 9864212 Search PubMed.
- M. Kesti, M. Müller, J. Becher, M. Schnabelrauch, M. D'Este, D. Eglin and M. Zenobi-Wong, A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation, Acta Biomater., 2015, 11, 162–172 CrossRef CAS PubMed.
- C. B. Rodell, J. E. Mealy and J. A. Burdick, Supramolecular guest–host interactions for the preparation of biomedical materials, Bioconjugate Chem., 2015, 26(12), 2279–2289 CrossRef CAS PubMed.
- R. Khoeini, H. Nosrati, A. Akbarzadeh, A. Eftekhari, T. Kavetskyy and R. Khalilov, et al., Natural and synthetic bioinks for 3D bioprinting, Adv. NanoBiomed Res., 2021, 1(8), 2000097 CrossRef CAS.
- M. Burke, B. M. Carter and A. W. Perriman, Bioprinting: uncovering the utility layer-by-layer, J. 3D Print. Med., 2017, 1(3), 165–179 CrossRef CAS.
- D. X. Chen and D. X. Chen, Extrusion bioprinting of scaffolds, Springer, 2019 Search PubMed.
- P. Kumar, S. Ebbens and X. Zhao, Inkjet printing of mammalian cells–Theory and applications, Bioprinting, 2021, 23, e00157 CrossRef.
- A. Zennifer, A. Subramanian and S. Sethuraman, Design considerations of bioinks for laser bioprinting technique towards tissue regenerative applications, Bioprinting, 2022, 27, e00205 CrossRef.
- A. Zennifer, S. Manivannan, S. Sethuraman, S. G. Kumbar and D. Sundaramurthi, 3D bioprinting and photocrosslinking: emerging strategies & future perspectives, Biomater. Adv., 2022, 134, 112576 CrossRef CAS PubMed.
- I. T. Ozbolat, W. Peng and V. Ozbolat, Application areas of 3D bioprinting, Drug Discovery Today, 2016, 21(8), 1257–1271 CrossRef CAS PubMed.
- H. Herrada-Manchón, M. A. Fernández and E. Aguilar, Essential guide to hydrogel rheology in extrusion 3D printing: how to measure it and why it matters?, Gels, 2023, 9(7), 517 CrossRef PubMed.
- D. M. Kirchmajer and I. R. Gorkin, An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing, J. Mater. Chem. B, 2015, 3(20), 4105–4117 RSC.
- L. Han, K. Liu, M. Wang, K. Wang, L. Fang and H. Chen, et al., Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance, Adv. Funct. Mater., 2018, 28(3), 1704195 CrossRef.
- D. Diekjürgen and D. W. Grainger, Polysaccharide matrices used in 3D in vitro cell culture systems, Biomaterials, 2017, 141, 96–115 CrossRef PubMed.
- M. R. Khatun, A. Bhattacharyya, S. Taheri, H. Hw, H. Kim, S. H. Chang and I. Noh, High Molecular Weight Fucoidan Loading Into and Release from Hyaluronate-Based Prefabricated Hydrogel and its Nanogel Particles Controlled by Variable Pitch and Differential Extensional Shear Technology of Advanced Twin Screw-Based System, Adv. Mater. Technol., 2023, 8(5), 2201478 CrossRef CAS.
- C. Benwood, J. Chrenek, R. L. Kirsch, N. Z. Masri, H. Richards, K. Teetzen and S. M. Willerth, Natural biomaterials and their use as bioinks for printing tissues, Bioengineering, 2021, 8(2), 27 CrossRef CAS PubMed.
- Q. Zhou, H. Kang, M. Bielec, X. Wu, Q. Cheng, W. Wei and H. Dai, Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing, Carbohydr. Polym., 2018, 197, 292–304 CrossRef CAS PubMed.
- S. Khalil and W. Sun, Bioprinting endothelial cells with alginate for 3D tissue constructs, J. Biomech. Eng., 2009, 131(11), 111002 CrossRef PubMed.
- G. Janarthanan, S. Lee and I. Noh, 3D printing of bioinspired alginate-albumin based instant gel ink with electroconductivity and its expansion to direct four-axis printing of hollow porous tubular constructs without supporting materials, Adv. Funct. Mater., 2021, 31(45), 2104441 CrossRef CAS.
- I. Braccini and S. Pérez, Molecular basis of Ca2+−induced gelation in alginates and pectins: the egg-box model revisited, Biomacromolecules, 2001, 2(4), 1089–1096 CrossRef CAS PubMed.
- K. Y. Lee and S. H. Yuk, Polymeric protein delivery systems, Prog. Polym. Sci., 2007, 32(7), 669–697 CrossRef CAS.
- L. T. Somasekharan, R. Raju, S. Kumar, R. Geevarghese, R. P. Nair, N. Kasoju and A. Bhatt, Biofabrication of skin tissue constructs using alginate, gelatin and diethylaminoethyl cellulose bioink, Int. J. Biol. Macromol., 2021, 189, 398–409 CrossRef CAS PubMed.
- L. Dogan, R. Scheuring, N. Wagner, Y. Ueda, S. Schmidt and P. Wörsdörfer, et al., Human iPSC-derived mesodermal progenitor cells preserve their vasculogenesis potential after extrusion and form hierarchically organized blood vessels, Biofabrication, 2021, 13(4), 045028 CrossRef CAS PubMed.
- M. C. Teixeira, N. S. Lameirinhas, J. P. Carvalho, A. J. Silvestre, C. Vilela and C. S. Freire, A guide to polysaccharide-based hydrogel bioinks for 3D bioprinting applications, Int. J. Mol. Sci., 2022, 23(12), 6564 CrossRef CAS PubMed.
- M. Rajabi, M. McConnell, J. Cabral and M. A. Ali, Chitosan hydrogels in 3D printing for biomedical applications, Carbohydr. Polym., 2021, 260, 117768 CrossRef CAS PubMed.
- Q. Wu, D. Therriault and M.-C. Heuzey, Processing and properties of chitosan inks for 3D printing of hydrogel microstructures, ACS Biomater. Sci. Eng., 2018, 4(7), 2643–2652 CrossRef CAS PubMed.
- N. M. Alves and J. F. Mano, Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications, Int. J. Biol. Macromol., 2008, 43(5), 401–414 CrossRef CAS PubMed.
- M. Sahranavard, A. Zamanian, F. Ghorbani and M. H. Shahrezaee, A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering, Bioprinting, 2020, 17, e00063 CrossRef.
- S. Peers, A. Montembault and C. Ladavière, Chitosan hydrogels for sustained drug delivery, J. Controlled Release, 2020, 326, 150–163 CrossRef CAS PubMed.
- M. B. Oliveira, H. X. Bastos and J. F. Mano, Sequentially moldable and bondable four-dimensional hydrogels compatible with cell encapsulation, Biomacromolecules, 2018, 19(7), 2742–2749 CrossRef CAS PubMed.
- Y.-H. Lee, Y.-L. Hong and T.-L. Wu, Novel silver and nanoparticle-encapsulated growth factor co-loaded chitosan composite hydrogel with sustained antimicrobility and promoted biological properties for diabetic wound healing, Mater. Sci. Eng., C, 2021, 118, 111385 CrossRef CAS PubMed.
- T. Lim, Q. Tang, Z. Zhu, X. Wei and C. Zhang, Sustained release of human platelet lysate growth factors by thermosensitive hydroxybutyl chitosan hydrogel promotes skin wound healing in rats, J. Biomed. Mater. Res., Part A, 2020, 108(10), 2111–2122 CrossRef CAS PubMed.
- C. Tonda-Turo, I. Carmagnola, A. Chiappone, Z. Feng, G. Ciardelli, M. Hakkarainen and M. Sangermano, Photocurable chitosan as bioink for cellularized therapies towards personalized scaffold architecture, Bioprinting, 2020, 18, e00082 CrossRef.
- S. Pisani, R. Dorati, F. Scocozza, C. Mariotti, E. Chiesa and G. Bruni, et al., Preliminary investigation on a new natural based poly(gamma-glutamic acid)/Chitosan bioink, J. Biomed. Mater. Res., Part B, 2020, 108(7), 2718–2732 CrossRef CAS PubMed.
- J. A. Burdick, C. Chung, X. Jia, M. A. Randolph and R. Langer, Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks, Biomacromolecules, 2005, 6(1), 386–391 CrossRef CAS PubMed.
- I. Noh, N. Kim, H. N. Tran, J. Lee and C. Lee, 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering, Biomater. Res., 2019, 23(1), 1–9 CrossRef PubMed.
- J. K. Carrow, P. Kerativitayanan, M. K. Jaiswal, G. Lokhande and A. K. Gaharwar, Polymers for bioprinting Essentials of 3D biofabrication and translation, Elsevier, 2015, pp. 229–248 Search PubMed.
- S. Trombino, C. Servidio, F. Curcio and R. Cassano, Strategies for hyaluronic acid-based hydrogel design in drug delivery, Pharmaceutics, 2019, 11(8), 407 CrossRef CAS PubMed.
- H.-Y. Hsieh, W.-Y. Lin, A. L. Lee, Y.-C. Li, Y.-J. Chen, K.-C. Chen and T.-H. Young, Hyaluronic Acid on the Urokinase Sustained Release with a Hydrogel System Composed of Poloxamer 407, bioRxiv, 2020, preprint, DOI:10.1371/journal.pone.0227784.
- S. Ansari, I. M. Diniz, C. Chen, T. Aghaloo, B. M. Wu, S. Shi and A. Moshaverinia, Alginate/hyaluronic acid hydrogel delivery system characteristics regulate the differentiation of periodontal ligament stem cells toward chondrogenic lineage, J. Mater. Sci.: Mater. Med., 2017, 28, 1–12 CrossRef CAS PubMed.
- R. Tsaryk, A. Gloria, T. Russo, L. Anspach, R. De Santis and S. Ghanaati, et al. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration, Acta Biomater., 2015, 20, 10–21 CrossRef CAS PubMed.
- Z. He, H. Zang, L. Zhu, K. Huang, T. Yi, S. Zhang and S. Cheng, An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury, Int. J. Nanomed., 2019, 721–732 CrossRef PubMed.
- L. Li, G. Jiang, W. Yu, D. Liu, H. Chen and Y. Liu, et al., A composite hydrogel system containing glucose-responsive nanocarriers for oral delivery of insulin, Mater. Sci. Eng., C, 2016, 69, 37–45 CrossRef CAS PubMed.
- N. K. Agrawal, P. Allen, Y. H. Song, R. A. Wachs, Y. Du, A. D. Ellington and C. E. Schmidt, Oligonucleotide-functionalized hydrogels for sustained release of small molecule(aptamer) therapeutics, Acta Biomater., 2020, 102, 315–325 CrossRef CAS PubMed.
- G. Janarthanan, H. S. Shin, I.-G. Kim, P. Ji, E.-J. Chung, C. Lee and I. Noh, Self-crosslinking hyaluronic acid–carboxymethylcellulose hydrogel enhances multilayered 3D-printed construct shape integrity and mechanical stability for soft tissue engineering, Biofabrication, 2020, 12(4), 045026 CrossRef CAS PubMed.
- P. S. Babo, R. L. Pires, L. Santos, A. Franco, F. Rodrigues and I. Leonor, et al., Platelet lysate-loaded photocrosslinkable hyaluronic acid hydrogels for periodontal endogenous regenerative technology, ACS Biomater. Sci. Eng., 2017, 3(7), 1359–1369 CrossRef CAS PubMed.
- Y. Deng, A. X. Sun, K. J. Overholt, Z. Y. Gary, M. R. Fritch and P. G. Alexander, et al., Enhancing chondrogenesis and mechanical strength retention in physiologically relevant hydrogels with incorporation of hyaluronic acid and direct loading of TGF-β, Acta Biomater., 2019, 83, 167–176 CrossRef CAS PubMed.
- S. Thönes, S. Rother, T. Wippold, J. Blaszkiewicz, K. Balamurugan and S. Moeller, et al., Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor, Acta Biomater., 2019, 86, 135–147 CrossRef PubMed.
- H. Mao, L. Yang, H. Zhu, L. Wu, P. Ji, J. Yang and Z. Gu, Recent advances and challenges in materials for 3D bioprinting, Prog. Nat. Sci.: Mater. Int., 2020, 30(5), 618–634 CrossRef CAS.
- M. M. Pillai, H. N. Tran, G. Sathishkumar, K. Manimekalai, J. Yoon and D. Lim, et al., Symbiotic culture of nanocellulose pellicle: A potential matrix for 3D bioprinting, Mater. Sci. Eng., C, 2021, 119, 111552 CrossRef CAS PubMed.
- J. Y. Shin, Y. H. Yeo, J. E. Jeong, S. A. Park and W. H. Park, Dual-crosslinked methylcellulose hydrogels for 3D bioprinting applications, Carbohydr. Polym., 2020, 238, 116192 CrossRef CAS PubMed.
- N. Reddy and Y. Yang, Citric acid cross-linking of starch films, Food Chem., 2010, 118(3), 702–711 CrossRef CAS.
- M. I. Rial-Hermida, A. Rey-Rico, B. Blanco-Fernandez, N. Carballo-Pedrares, E. M. Byrne and J. F. Mano, Recent progress on polysaccharide-based hydrogels for controlled delivery of therapeutic biomolecules, ACS Biomater. Sci. Eng., 2021, 7(9), 4102–4127 CrossRef CAS PubMed.
- W. Boonlai, V. Tantishaiyakul and N. Hirun, Characterization of κ-carrageenan/methylcellulose/cellulose nanocrystal hydrogels for 3D bioprinting, Polym. Int., 2022, 71(2), 181–191 CrossRef CAS.
- S. G. Lévesque, R. M. Lim and M. S. Shoichet, Macroporous interconnected dextran scaffolds of controlled porosity for tissue-engineering applications, Biomaterials, 2005, 26(35), 7436–7446 CrossRef PubMed.
- L. Pescosolido, W. Schuurman, J. Malda, P. Matricardi, F. Alhaique and T. Coviello, et al., Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting, Biomacromolecules, 2011, 12(5), 1831–1838 CrossRef CAS PubMed.
- A. Brunsen, U. Ritz, A. Mateescu, I. Hoefer, P. Frank and B. Menges, et al., Photocrosslinkable dextran hydrogel films as substrates for osteoblast and endothelial cell growth, J. Mater. Chem., 2012, 22(37), 19590–19604 RSC.
- G. Peng, J. Wang, F. Yang, S. Zhang, J. Hou and W. Xing, et al., In situ formation of biodegradable dextran-based hydrogel via Michael addition, J. Appl. Polym. Sci., 2013, 127(1), 577–584 CrossRef CAS.
- Z. Wei and S. Gerecht, A self-healing hydrogel as an injectable instructive carrier for cellular morphogenesis, Biomaterials, 2018, 185, 86–96 CrossRef CAS PubMed.
- P. R. Turner, E. Murray, C. J. McAdam, M. A. McConnell and J. D. Cabral, Peptide chitosan/dextran core/shell vascularized 3D constructs for wound healing, ACS Appl. Mater. Interfaces, 2020, 12(29), 32328–32339 CrossRef CAS PubMed.
- P. Datta, B. Ayan and I. T. Ozbolat, Bioprinting for vascular and vascularized tissue biofabrication, Acta Biomater., 2017, 51, 1–20 CrossRef CAS PubMed.
- R. Levato, J. Visser, J. A. Planell, E. Engel, J. Malda and M. A. Mateos-Timoneda, Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers, Biofabrication, 2014, 6(3), 035020 CrossRef CAS PubMed.
- R. Mohammadinejad, A. Kumar, M. Ranjbar-Mohammadi, M. Ashrafizadeh, S. S. Han, G. Khang and Z. Roveimiab, Recent advances in natural gum-based biomaterials for tissue engineering and regenerative medicine: A review, Polymer, 2020, 12(1), 176 CAS.
- Y. Niu, T. Yang, R. Ke and C. Wang, Preparation and characterization of pH-responsive sodium alginate/humic acid/konjac hydrogel for L-ascorbic acid controlled release, Mater. Express, 2019, 9(6), 563–569 CrossRef CAS.
- L. Zhang, T. Zheng, L. Wu, Q. Han, S. Chen and Y. Kong, et al., Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth, Nanotechnol. Rev., 2021, 10(1), 50–61 CrossRef CAS.
- P. Zarrintaj, S. Manouchehri, Z. Ahmadi, M. R. Saeb, A. M. Urbanska, D. L. Kaplan and M. Mozafari, Agarose-based biomaterials for tissue engineering, Carbohydr. Polym., 2018, 187, 66–84 CrossRef CAS PubMed.
- L. E. Bertassoni, J. C. Cardoso, V. Manoharan, A. L. Cristino, N. S. Bhise and W. A. Araujo, et al., Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels, Biofabrication, 2014, 6(2), 024105 CrossRef CAS PubMed.
- S. H. Moon, H. N. Choi and Y. J. Yang, Natural/synthetic polymer materials for bioink development, Biotechnol. Bioprocess Eng., 2022, 27(4), 482–493 CrossRef CAS.
- B. Pokusaev, A. Vyazmin, N. Zakharov, S. Karlov, D. Nekrasov, V. Reznik and D. Khramtsov, Thermokinetics and rheology of agarose gel applied to bioprinting technology, Therm. Sci., 2020, 24(1 Part A), 347–353 CrossRef.
- L. Aydin, S. Kucuk and H. Kenar, A universal self-eroding sacrificial bioink that enables bioprinting at room temperature, Polym. Adv. Technol., 2020, 31(7), 1634–1647 CrossRef CAS.
- A. Alves, S. P. Miguel, A. R. Araujo, M. J. de Jesús Valle, A. Sánchez Navarro and I. J. Correia, et al. Xanthan gum–Konjac glucomannan blend hydrogel for wound healing, Polymer, 2020, 12(1), 99 CAS.
- D. Yang, Y. Yuan, L. Wang, X. Wang, R. Mu and J. Pang, et al., A review on konjac glucomannan gels: Microstructure and application, Int. J. Mol. Sci., 2017, 18(11), 2250 CrossRef PubMed.
- L. Yang, Q. Zhao, Z. Guo, Y. Liu, W. Gao, Y. Pu and B. He, Konjac glucomannan hydrogel dressing and its combination with Chinese medicine for the wound treatment, New J. Chem., 2022, 46(48), 23077–23087 RSC.
- X. Pan, Q. Wang, D. Ning, L. Dai, K. Liu and Y. Ni, et al., Ultraflexible self-healing guar gum-glycerol hydrogel with injectable, antifreeze, and strain-sensitive properties, ACS Biomater. Sci. Eng., 2018, 4(9), 3397–3404 CrossRef CAS PubMed.
- A. Indurkar, P. Bangde, M. Gore, P. Reddy, R. Jain and P. Dandekar, Optimization of guar gum-gelatin bioink for 3D printing of mammalian cells, Bioprinting, 2020, 20, e00101 CrossRef.
- E. Bakirci, B. Toprakhisar, M. C. Zeybek, G. Ince and B. Koç, Cell sheet based bioink for 3D bioprinting applications, Biofabrication, 2017, 9(2), 024105 CrossRef CAS PubMed.
- H. Baniasadi, E. Kimiaei, R. T. Polez, R. Ajdary, O. J. Rojas, M. Österberg and J. Seppälä, High-resolution 3D printing of xanthan gum/nanocellulose bio-inks, Int. J. Biol. Macromol., 2022, 209, 2020–2031 CrossRef CAS PubMed.
- B. Piola, M. Sabbatini, S. Gino, M. Invernizzi and F. Renò, 3D bioprinting of gelatin–xanthan gum composite hydrogels for growth of human skin cells, Int. J. Mol. Sci., 2022, 23(1), 539 CrossRef CAS PubMed.
- S. Muthusamy, S. Kannan, M. Lee, V. Sanjairaj, W. F. Lu and J. Y. Fuh, et al., 3D bioprinting and microscale organization of vascularized tissue constructs using collagen-based bioink, Biotechnol. Bioeng., 2021, 118(8), 3150–3163 CrossRef CAS PubMed.
- W. Lim, S. Y. Shin, J. M. Cha and H. Bae, Optimization of polysaccharide hydrocolloid for the development of bioink with high printability/biocompatibility for Coextrusion 3D bioprinting, Polymer, 2021, 13(11), 1773 CAS.
- S. Sang, X. Mao, Y. Cao, Z. Liu, Z. Shen and M. Li, et al., 3D Bioprinting using synovium-derived MSC-laden photo-cross-linked ECM bioink for cartilage regeneration, ACS Appl. Mater. Interfaces, 2023, 15(7), 8895–8913 CrossRef CAS PubMed.
- S. Nedunchezian, P. Banerjee, C.-Y. Lee, S.-S. Lee, C.-W. Lin and C.-W. Wu, et al., Generating adipose stem cell-laden hyaluronic acid-based scaffolds using 3D bioprinting via the double crosslinked strategy for chondrogenesis, Mater. Sci. Eng., C, 2021, 124, 112072 CrossRef CAS PubMed.
- M. A. Bonifacio, S. Cometa, A. Cochis, A. Scalzone, P. Gentile and A. C. Scalia, et al., A bioprintable gellan gum/lignin hydrogel: a smart and sustainable route for cartilage regeneration, Int. J. Biol. Macromol., 2022, 216, 336–346 CrossRef CAS PubMed.
- A. R. Akkineni, B. S. Elci, A. Lode and M. Gelinsky, Addition of high acyl gellan gum to low acyl gellan gum enables the blends 3d bioprintable, Gels, 2022, 8(4), 199 CrossRef CAS PubMed.
- J. Zhang, E. Wehrle, P. Adamek, G. R. Paul, X.-H. Qin, M. Rubert and R. Müller, Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering, Acta Biomater., 2020, 114, 307–322 CrossRef CAS PubMed.
- D. Wu, Y. Yu, J. Tan, L. Huang, B. Luo, L. Lu and C. Zhou, 3D bioprinting of gellan gum and poly(ethylene glycol) diacrylate based hydrogels to produce human-scale constructs with high-fidelity, Mater. Des., 2018, 160, 486–495 CrossRef CAS.
- J. Lee, J. Hong, W. Kim and G. H. Kim, Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering, Carbohydr. Polym., 2020, 250, 116914 CrossRef CAS PubMed.
- Z. Li, S. Li, J. Yang, Y. Ha, Q. Zhang, X. Zhou and C. He, 3D bioprinted gelatin/gellan gum-based scaffold with double-crosslinking network for vascularized bone regeneration, Carbohydr. Polym., 2022, 290, 119469 CrossRef CAS PubMed.
- M. Puertas-Bartolomé, M. K. Włodarczyk-Biegun, A. Del Campo, B. Vázquez-Lasa and J. San Román, Development of bioactive catechol functionalized nanoparticles applicable for 3D bioprinting, Mater. Sci. Eng., C, 2021, 131, 112515 CrossRef PubMed.
- H. Rastin, M. Ramezanpour, K. Hassan, A. Mazinani, T. T. Tung, S. Vreugde and D. Losic, 3D bioprinting of a cell-laden antibacterial polysaccharide hydrogel composite, Carbohydr. Polym., 2021, 264, 117989 CrossRef CAS PubMed.
- R. F. Pereira, A. Sousa, C. C. Barrias, P. J. Bártolo and P. L. Granja, A single-component hydrogel bioink for bioprinting of bioengineered 3D constructs for dermal tissue engineering, Mater. Horiz., 2018, 5(6), 1100–1111 RSC.
- Z. Wu, Q. Li, S. Xie, X. Shan and Z. Cai, In vitro and in vivo biocompatibility evaluation of a 3D bioprinted gelatin-sodium alginate/rat Schwann-cell scaffold, Mater. Sci. Eng., C, 2020, 109, 110530 CrossRef CAS PubMed.
- L. Ning, H. Sun, T. Lelong, R. Guilloteau, N. Zhu, D. J. Schreyer and X. Chen, 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications, Biofabrication, 2018, 10(3), 035014 CrossRef PubMed.
- Z. Wu, S. Xie, Y. Kang, X. Shan, Q. Li and Z. Cai, Biocompatibility evaluation of a 3D-bioprinted alginate-GelMA-bacteria nanocellulose(BNC) scaffold laden with oriented-growth RSC96 cells, Mater. Sci. Eng., C, 2021, 129, 112393 CrossRef CAS PubMed.
- A. P. Haring, E. G. Thompson, Y. Tong, S. Laheri, E. Cesewski, H. Sontheimer and B. N. Johnson, Process-and bio-inspired hydrogels for 3D bioprinting of soft free-standing neural and glial tissues, Biofabrication, 2019, 11(2), 025009 CrossRef CAS PubMed.
- N. Li, R. Guo and Z. J. Zhang, Bioink formulations for bone tissue regeneration, Front. bioeng. biotechnol., 2021, 9, 630488 CrossRef PubMed.
- S. Taheri, H. S. Ghazali, Z. S. Ghazali, A. Bhattacharyya and I. Noh, Progress in biomechanical stimuli on the cell-encapsulated hydrogels for cartilage tissue regeneration, Biomater. Res., 2023, 27(1), 1–17 CrossRef PubMed.
- C. Qi, J. Liu, Y. Jin, L. Xu, G. Wang, Z. Wang and L. Wang, Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage, Biomaterials, 2018, 163, 89–104 CrossRef CAS PubMed.
- N. Liu, X. Zhang, Q. Guo, T. Wu and Y. Wang, 3D bioprinted scaffolds for tissue repair and regeneration, Front. Mater., 2022, 9, 925321 CrossRef.
- M. Li, D. Sun, J. Zhang, Y. Wang, Q. Wei and Y. Wang, Application and development of 3D bioprinting in cartilage tissue engineering, Biomater. Sci., 2022, 10(19), 5430–5458 RSC.
- S. Murab, A. Gupta, M. K. Włodarczyk-Biegun, A. Kumar, P. van Rijn and P. Whitlock, et al. Alginate based hydrogel inks for 3D bioprinting of engineered orthopedic tissues, Carbohydr. Polym., 2022, 119964 CrossRef CAS PubMed.
- A. Bhattacharyya, G. Janarthanan, H. N. Tran, H. J. Ham, J. Yoon and I. Noh, Bioink homogeneity control during 3D bioprinting of multicomponent micro/nanocomposite hydrogel for even tissue regeneration using novel twin screw extrusion system, Chem. Eng. J., 2021, 415, 128971 CrossRef CAS.
- S. Chae, S.-S. Lee, Y.-J. Choi, G. Gao, J. H. Wang and D.-W. Cho, 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink, Biomaterials, 2021, 267, 120466 CrossRef CAS PubMed.
- M. Govindharaj, N. Al Hashimi, S. S. Soman, S. Kanwar and S. Vijayavenkataraman, 3D Bioprinting of human Mesenchymal Stem Cells in a novel tunic decellularized ECM bioink for Cartilage Tissue Engineering, Materialia, 2022, 23, 101457 CrossRef CAS.
- A. Sadeghianmaryan, S. Naghieh, H. A. Sardroud, Z. Yazdanpanah, Y. A. Soltani, J. Sernaglia and X. Chen, Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering, Int. J. Biol. Macromol., 2020, 164, 3179–3192 CrossRef CAS PubMed.
- Y. He, S. Derakhshanfar, W. Zhong, B. Li, F. Lu, M. Xing and X. Li, Characterization and application of carboxymethyl chitosan-based bioink in cartilage tissue engineering, J. Nanomater., 2020, 2020, 1–11 Search PubMed.
- X. Liu, S. Song, J. Huang, H. Fu, X. Ning, Y. He and Z. Zhang, HBC-nanofiber hydrogel scaffolds with 3D printed internal microchannels for enhanced cartilage differentiation, J. Mater. Chem. B, 2020, 8(28), 6115–6127 RSC.
- C. Antich, J. de Vicente, G. Jiménez, C. Chocarro, E. Carrillo and E. Montañez, et al. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs, Acta Biomater., 2020, 106, 114–123 CrossRef CAS PubMed.
- E. De Angelis, R. Saleri, P. Martelli, L. Elviri, A. Bianchera and C. Bergonzi, et al. Cultured horse articular chondrocytes in 3d-printed chitosan scaffold with hyaluronic acid and platelet lysate, Front. Vet. Sci., 2021, 8, 671776 CrossRef PubMed.
- F. Olate-Moya, L. Arens, M. Wilhelm, M. A. Mateos-Timoneda, E. Engel and H. Palza, Chondroinductive alginate-based hydrogels having graphene oxide for 3D printed scaffold fabrication, ACS Appl. Mater. Interfaces, 2020, 12(4), 4343–4357 CrossRef CAS PubMed.
- K. Elkhoury, M. Morsink, L. Sanchez-Gonzalez, C. Kahn, A. Tamayol and E. Arab-Tehrany, Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications, Bioact. Mater., 2021, 6(11), 3904–3923 CAS.
- M. Ojansivu, A. Rashad, A. Ahlinder, J. Massera, A. Mishra and K. Syverud, et al., Wood-based nanocellulose and bioactive glass modified gelatin–alginate bioinks for 3D bioprinting of bone cells, Biofabrication, 2019, 11(3), 035010 CrossRef CAS PubMed.
- F. Curti, A. Serafim, E. Olaret, S. Dinescu, I. Samoila and B. S. Vasile, et al., Development of Biocomposite Alginate-Cuttlebone-Gelatin 3D Printing Inks Designed for Scaffolds with Bone Regeneration Potential, Mar. Drugs, 2022, 20(11), 670 CrossRef CAS PubMed.
- S. Im, G. Choe, J. M. Seok, S. J. Yeo, J. H. Lee and W. D. Kim, et al., An osteogenic bioink composed of alginate, cellulose nanofibrils, and polydopamine nanoparticles for 3D bioprinting and bone tissue engineering, Int. J. Biol. Macromol., 2022, 205, 520–529 CrossRef CAS PubMed.
- H. K. Chang, D. H. Yang, M. Y. Ha, H. J. Kim, C. H. Kim and S. H. Kim, et al., 3D printing of cell-laden visible light curable glycol chitosan bioink for bone tissue engineering, Carbohydr. Polym., 2022, 287, 119328 CrossRef CAS PubMed.
- C. Gao, C. Lu, Z. Jian, T. Zhang, Z. Chen and Q. Zhu, et al., 3D bioprinting for fabricating artificial skin tissue, Colloids Surf., B, 2021, 208, 112041 CrossRef CAS PubMed.
- R. Augustine, Skin bioprinting: a novel approach for creating artificial skin from synthetic and natural building blocks, Prog. Biomater., 2018, 7(2), 77–92 CrossRef CAS PubMed.
- P. Liu, H. Shen, Y. Zhi, J. Si, J. Shi, L. Guo and S. G. Shen, 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels, Colloids Surf., B, 2019, 181, 1026–1034 CrossRef CAS PubMed.
- H. Zhao, J. Xu, H. Yuan, E. Zhang, N. Dai and Z. Gao, et al., 3D printing of artificial skin patches with bioactive and optically active polymer materials for anti-infection and augmenting wound repair, Mater. Horiz., 2022, 9(1), 342–349 RSC.
- F. Zhou, Y. Hong, R. Liang, X. Zhang, Y. Liao and D. Jiang, et al., Rapid printing of bio-inspired 3D tissue constructs for skin regeneration, Biomaterials, 2020, 258, 120287 CrossRef CAS PubMed.
- R. Poongodi, Y.-L. Chen, T.-H. Yang, Y.-H. Huang, K. D. Yang, H.-C. Lin and J.-K. Cheng, Bio-scaffolds as cell or exosome carriers for nerve injury repair, Int. J. Mol. Sci., 2021, 22(24), 13347 CrossRef CAS PubMed.
- P. Zhuang, A. X. Sun, J. An, C. K. Chua and S. Y. Chew, 3D neural tissue models: From spheroids to bioprinting, Biomaterials, 2018, 154, 113–133 CrossRef CAS PubMed.
- M. Habibizadeh, S. Nadri, A. Fattahi, K. Rostamizadeh, P. Mohammadi and S. Andalib, et al., Surface modification of neurotrophin-3 loaded PCL/chitosan nanofiber/net by alginate hydrogel microlayer for enhanced biocompatibility in neural tissue engineering, J. Biomed. Mater. Res., Part A, 2021, 109(11), 2237–2254 CrossRef CAS PubMed.
- G. Zhou, Y. Chen, F. Dai and X. Yu, Chitosan-based nerve guidance conduit with microchannels and nanofibers promotes schwann cells migration and neurite growth, Colloids Surf., B, 2023, 221, 112929 CrossRef CAS PubMed.
- S. Houshyar, A. Bhattacharyya and R. Shanks, Peripheral nerve conduit: materials and structures, ACS Chem. Neurosci., 2019, 10(8), 3349–3365 CrossRef CAS PubMed.
- Q. Lian, L. Zhou, X. Li, W. Mao and D. Li, Perfusive and osmotic capabilities of 3D printed hollow tube for fabricating large-scaled muscle scaffold, Rapid Prototyp. J., 2020, 26(1), 1–10 CrossRef.
- S. Ostrovidov, S. Salehi, M. Costantini, K. Suthiwanich, M. Ebrahimi and R. B. Sadeghian, et al., 3D bioprinting in skeletal muscle tissue engineering, Small, 2019, 15(24), 1805530 CrossRef PubMed.
- J. Lee, H. Lee, E.-J. Jin, D. Ryu and G. H. Kim, 3D bioprinting using a new photo-crosslinking method for muscle tissue restoration, npj Regener. Med., 2023, 8(1), 18 CrossRef CAS PubMed.
- F. L. Ronzoni, F. Aliberti, F. Scocozza, L. Benedetti, F. Auricchio and M. Sampaolesi, et al., Myoblast 3D bioprinting to burst in vitro skeletal muscle differentiation, J. Tissue Eng. Regener. Med., 2022, 16(5), 484–495 CrossRef CAS PubMed.
- A. Bhattacharyya, G. Janarthanan and I. Noh, Nano-biomaterials for designing functional bioinks towards complex tissue and organ regeneration in 3D bioprinting, Addit. Manuf., 2021, 37, 101639 CAS.
- H.-W. Kang, S. J. Lee, I. K. Ko, C. Kengla, J. J. Yoo and A. Atala, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nat. Biotechnol., 2016, 34(3), 312–319 CrossRef CAS PubMed.
- G. Cattelan, A. Guerrero Gerbolés, R. Foresti, P. P. Pramstaller, A. Rossini, M. Miragoli and M. C. Caffarra, Alginate formulations: current developments in the race for hydrogel-based cardiac regeneration, Front. bioeng. biotechnol., 2020, 8, 414 CrossRef PubMed.
- Y. S. Zhang, A. Arneri, S. Bersini, S.-R. Shin, K. Zhu and Z. Goli-Malekabadi, et al., Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip, Biomaterials, 2016, 110, 45–59 CrossRef CAS PubMed.
- L. Hockaday, K. Kang, N. Colangelo, P. Cheung, B. Duan and E. Malone, et al., Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds, Biofabrication, 2012, 4(3), 035005 CrossRef CAS PubMed.
- Y. Wu, A. Wenger, H. Golzar and X. Tang, 3D bioprinting of bicellular liver lobule-mimetic structures via microextrusion of cellulose nanocrystal-incorporated shear-thinning bioink, Sci. Rep., 2020, 10(1), 20648 CrossRef CAS PubMed.
- R. Taymour, D. Kilian, T. Ahlfeld, M. Gelinsky and A. Lode, 3D bioprinting of hepatocytes: Core–shell structured co-cultures with fibroblasts for enhanced functionality, Sci. Rep., 2021, 11(1), 5130 CrossRef CAS PubMed.
- P. Wang, X. Li, W. Zhu, Z. Zhong, A. Moran and W. Wang, et al., 3D bioprinting of hydrogels for retina cell culturing, Bioprinting, 2018, 12, e00029 CrossRef PubMed.
- M. P. Sekar, S. Suresh, A. Zennifer, S. Sethuraman and D. Sundaramurthi, Hyaluronic Acid as Bioink and Hydrogel Scaffolds for Tissue Engineering Applications, ACS Biomater. Sci. Eng., 2023, 9(6), 3134–3159 CrossRef CAS PubMed.
Footnote |
| † Kasula Nagaraja and Pratik Dhokare are co-first authors with equal contribution and importance. |
| This journal is © The Royal Society of Chemistry 2024 |