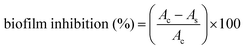Surface modification of medical grade biomaterials by using a low-temperature-processed dual functional Ag–TiO2 coating for preventing biofilm formation†
Lipi
Pradhan
a,
Sobhan
Hazra
 b,
Satya Veer
Singh
b,
Satya Veer
Singh
 b,
Bajrang
a,
Anjali
Upadhyay
a,
Bhola Nath
Pal
b,
Bajrang
a,
Anjali
Upadhyay
a,
Bhola Nath
Pal
 *b and
Sudip
Mukherjee
*b and
Sudip
Mukherjee
 *a
*a
aSchool of Biomedical Engineering, Indian Institute of Technology (BHU), Varanasi, 221005, UP, India. E-mail: sudip.bme@iitbhu.ac.in
bSchool of Materials Science and Technology, Indian Institute of Technology (BHU), Varanasi, UP 221005, India. E-mail: bnpal.mst@iitbhu.ac.in
First published on 2nd September 2024
Abstract
Biofilm development in medical devices is considered the major virulence component that leads to increased mortality and morbidity among patients. Removing a biofilm once formed is challenging and frequently results in persistent infections. Many current antibiofilm coating strategies involve harsh conditions causing damage to the surface of the medical devices. To address the issue of bacterial attachment in medical devices, we propose a novel antibacterial surface modification approach. In this paper, we developed a novel low-temperature based solution-processed approach to deposit silver nanoparticles (Ag NPs) inside a titanium oxide (TiO2) matrix to obtain a Ag–TiO2 nanoparticle coating. The low temperature (120 °C)-based UV annealed drop cast method is novel and ensures no surface damage to the medical devices. Various medical-grade biomaterials were then coated using Ag–TiO2 to modify the surface of the materials. Several studies were performed to observe the antibacterial and antibiofilm properties of Ag–TiO2-coated medical devices and biomaterials. Moreover, the Ag–TiO2 NPs did not show any skin irritation in rats and showed biocompatibility in the chicken egg model. This study indicates that Ag–TiO2 coating has promising potential for healthcare applications to combat microbial infection and biofilm formation.
Introduction
Medical science and technology advancements have led to the progression of medical devices and artificial organs.1 Many healthcare centers heavily depend on modern medical technologies for diagnosis and treatment. These include vascular prostheses, contact lenses, breast implants, intravenous and urinary catheters, joint prostheses, dental implants, orthopedic devices, and many more.2,3 These devices are manufactured using a wide range of materials such as silicone, ceramic, glass, polymers, metals, composites, etc., often providing an ideal environment for the growth and development of biofilms.4 A biofilm can be defined as a group of microbial cells attached and enclosed within a self-secreted extracellular polymeric matrix (EPM).5,6 It has been estimated that EMP contributes to 90% of the dry mass of the biofilm. Biofilm formation is regarded as a survival strategy of microbes that helps them to adapt to the challenging environment.7 EPM plays a crucial role in the adhesion and aggregation of tiny microbial cells to the substrate.8 Moreover, they provide enhanced resistance from host immune cells and various antibiotics compared to planktonic cells by generating a hydrated barrier between the internal and external environment. Biofilm development is considered the major virulence component that leads to increased mortality and morbidity among the patients receiving medical treatments.9,10 Studies reveal that a significant proportion of microbial infections observed in humans are associated with biofilms. It is estimated that approximately 26% of hospital-acquired infections in the United States are the result of biofilm deposition over medical devices.11 Furthermore, around 1.7 million people experience severe health issues, resulting in more than 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fatalities attributed to hospital-acquired infections.12 According to a survey conducted by the CDC (Centre for Disease Control and Prevention), an overall annual expense of $0.67–$2.68 billion is solely caused by infections in central venous catheters.13 Once the medical devices get infected by a biofilm, it is tough to eradicate them using traditional methods, necessitating prolonged antibacterial treatments.14
000 fatalities attributed to hospital-acquired infections.12 According to a survey conducted by the CDC (Centre for Disease Control and Prevention), an overall annual expense of $0.67–$2.68 billion is solely caused by infections in central venous catheters.13 Once the medical devices get infected by a biofilm, it is tough to eradicate them using traditional methods, necessitating prolonged antibacterial treatments.14
In response to these challenges, numerous strategies are employed to inhibit the growth and development of biofilms.15 Various chemical, physical, light-based, and nanotechnology-mediated therapies prevent biofilm formation.7,14,16,17 Conventional antibiotic therapies, commonly employed to treat infections, typically fail to work against biofilms due to their difficulty penetrating the EPM.18 Antibiotics can also cause side effects, including cytotoxicity, immunogenicity, antibiotic resistance, etc.19–21 There has been rising interest in the creation and investigation of novel antimicrobial agents due to the high expense and increased drug resistance of microbes to existing antimicrobial therapy, as current antimicrobial treatment is costly and is expected to boost the drug resistance of pathogens.22 In recent years, Ag NP coating-based technology has gained significant progress in developing an antibacterial coating for medical devices, which is cost-effective compared to conventional strategies.23,24 However, a significant issue arises as Ag undergoes an oxidation process when it comes in contact with air, compromising the inherent properties of Ag and affecting the long-term coating stability. Additionally, the growth process of Ag NPs can be expensive in some cases, which are the main drawbacks, potentially limiting the widespread use of this technology in the medical sector.
To overcome these challenges, our study focuses on developing a Ag–TiO2 based coating using a combination of silver (Ag) and titanium dioxide (TiO2) nanoparticles. We have developed an innovative coating method that works at a relatively low temperature of 120 °C combined with UV light exposure, in contrast to traditional coating methods that require higher temperatures, which could potentially harm sensitive medical devices or cause degradation of materials. This low-temperature procedure is intended to shield medical equipment from degradation and loss of functionality. From surgical tools to implantable devices, various medical devices utilized in clinical settings can benefit from this novel coating process.
A low-temperature and solution process coating method of Ag–TiO2 has been employed for various substrates commonly found in medical devices, including silicone, latex, glass, metal, ceramic, and PTFE. The in situ grown TiO2 embedded Ag NP processing temperature is ∼120 °C, which allows us to coat this material on a wide range of surfaces, including flexible plastic, ceramic, metals, and alloys. In this material combination, TiO2 is chosen for its high chemical stability and photocatalytic activity in the presence of light, resulting in the formation of reactive oxygen species (ROS) and contributing to antimicrobial activity.25,26 Furthermore, its chemical stability ensures a prolonged lifespan of the Ag NPs present in the antibiofilm coating. The coated materials are then thoroughly characterized, employing various techniques to confirm the presence of Ag and Ti coating over medical-grade biomaterials. Several in vitro and in vivo studies were conducted to observe the antibacterial and antibiofilm properties of Ag–TiO2 coated medical grade biomaterials. This study demonstrates an exciting method to create low-temperature, low-cost, long-term stable, and environment-friendly antimicrobial coatings to prevent bacterial biofilms on medical devices.
Results
Characterization studies confirmed the formation of Ag–TiO2
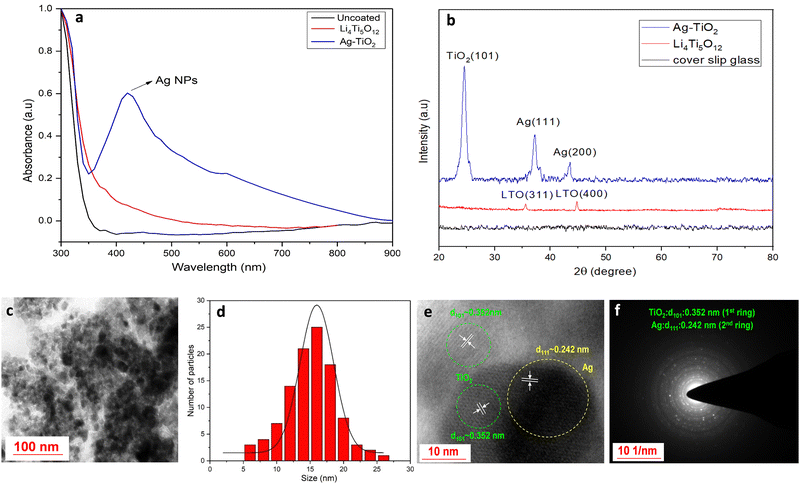
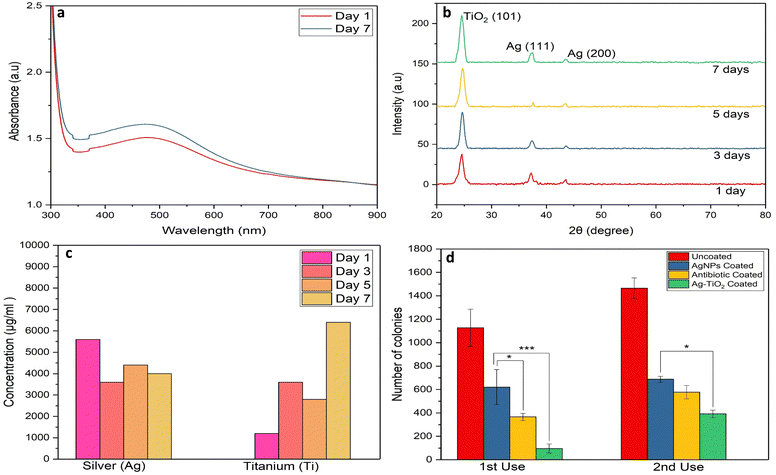
The visible absorption spectrum of the active antimicrobial Ag–TiO2 thin film shows strong plasmonic absorption of Ag NPs with a plasmonic peak at 420 nm (Fig. 1a). Initial ion-conducting Li4Ti5O12 (LTO) is a wide band-gap dielectric (energy band gap typically around ∼3.8 eV), and its UV absorption mainly appears in the 300–380 nm wavelength region. Fig. 1b shows the XRD data of a thin films of LTO, Ag–TiO2 coated, and uncoated cover slip to compare the presence of Ag NPs inside the TiO2 matrix for coated materials. The uncoated cover slip glass was kept as a reference sample, showing no significant absorption in that range (Fig. 1b). The XRD peak of the LTO thin film demonstrated a strong peak position at 2θ ∼35.54° and 44.79°, associated with the reflection planes of (311) and (400), respectively (JCPDS 490207).The XRD pattern of the Ag–TiO2 thin film exhibits peaks at 2θ of 24.81°, 37.64° and 43.58°, respectively (JCPDS 897372), which corresponds to TiO2 (101), Ag (111), and Ag (200) planes, respectively. The XRD peak located at 2θ of 24.81° confirms the formation of the anatase phase of TiO2. Additional XRD peaks, which are ordinated at 2θ of 37.64° and 43.58°, are due to the formation of Ag NPs within the TiO2 matrix (Fig. 1b). Detailed microstructural studies of the Ag–TiO2 film have been investigated through a TEM analysis (Fig. 1c–f). The figures clearly show that Ag NPs have grown inside the TiO2 matrix (Fig. 1c). The size of the Ag NPs is within the diameter of 5–25 nm with an average particle size of 15.4 nm (Fig. 1d). The high-resolution image of Ag–TiO2 (Fig. 1e) showed independent lattice fringe development of Ag NPs and TiO2, suggesting their coexistence (Fig. 1e). The Ag (111) and anatase TiO2 (101) planes are represented by the average d spacings of 0.241 and 0.352 nm, respectively, which is also supported by the XRD study (Fig. 1b). Besides, the selected area electron diffraction (SAED) pattern also defines all these spacings (Fig. 1f), supporting the co-existence of TiO2 (101) and Ag (111) phases.
AFM and SEM images revealed the surface modification of the Ag–TiO2 coated substrate
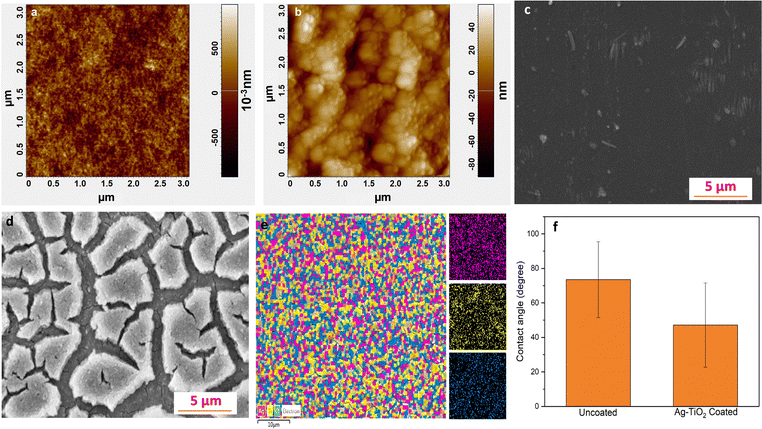
An AFM was used to analyze the surface morphology and roughness (Rr.ms) of the Ag–TiO2 coating. The uncoated (Fig. 2a) glass substrate showed r.m.s roughness of 0.22 nm while the surface roughness of the Ag–TiO2 coated glass (Fig. 2b) was 24.176 nm. In these figures, small bright spots indicate Ag NPs, while slightly more prominent and darker colored spots are TiO2. Fig. 2c and d represent the SEM images of uncoated and coated glass substrates. The coated glass substrate exhibits a unique and clear pattern.The observation of the distinctive pattern of Ag–TiO2 (Fig. 2d) serves as confirmation that the coating has been effectively applied to the glass substrate. Furthermore, we calculated the thickness of the Ag–TiO2 film. The average thickness obtained through the cross-sectional FE-SEM image of the Ag–TiO2 film was found to be near ∼50 nm. A cross-sectional FE-SEM image of the Ag–TiO2 film has been provided in the ESI† (Fig. S1). EDX color mapping was performed to ensure the uniformity of the Ag–TiO2 nanoparticle coating over the coverslip substrates (Fig. 2e). We also performed EDAX color mapping for various substrates, including silicon, PTFE and latex to ensure the uniformity of the Ag–TiO2 coating (Fig. S2a–c, ESI†). The color mapping for all substrates revealed an even distribution of Ag NPs on the TiO2 matrix. Pink, yellow, and blue colored dots represent silver, titanium, and oxygen, respectively. The contact angle was analyzed to investigate the wetting ability of water on both Ag–TiO2 and the uncoated coverslip. The results demonstrated the contact angle of 47.14 and 73.51 degrees for Ag–TiO2 coated and uncoated glass, respectively (Fig. 2f). Comparatively, a lower contact angle suggests the high wettability of the liquid over an Ag–TiO2 coated surface. This hydrophilic nature of Ag–TiO2 enhances biocompatibility by mimicking the natural environment of biological fluids, which are predominantly water-based. Additionally, the hydrophilic surface repels protein adsorptions, preventing biofilm attachment and suggesting the potential application of Ag–TiO2 as an antibiofilm coating over medical devices.27
In vivo biocompatibility study revealed that Ag–TiO2 is non-toxic and promotes the development of blood vessels in a chick embryo model
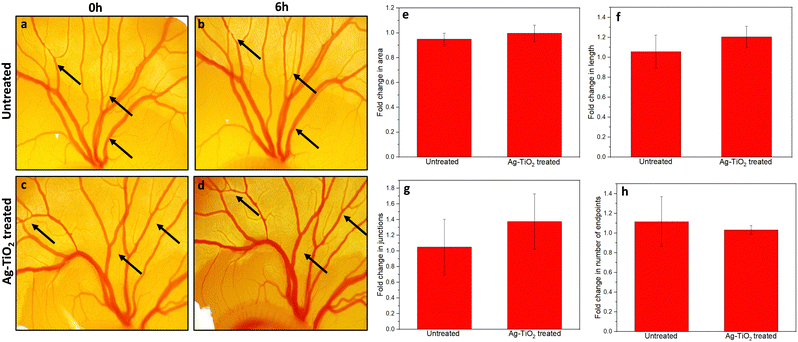
A material intended for use in a medical setting should be biocompatible to avoid any side effects as it comes in direct contact with the human body. The biocompatibility of the Ag–TiO2 coated material was analyzed using a Chick CAM (chorioallantoic membrane) assay.28 The microscopic images taken at various time points indicated that the chicken embryo exposed to Ag–TiO2 substrate (Fig. S3f–j, ESI†) showed similar growth of blood vessels as the untreated group (Fig. S3a–e, ESI†). Fold changes in various parameters of blood vessels in the untreated (Fig. S3a and b, ESI†) and Ag–TiO2 treated groups (Fig. S3c and d, ESI†) at 0 h and 6 h were evaluated using Angiotool software. In the chicken eggs implanted with AgTiO2 coated PTFE disc, the fold change in area and number of endpoints remained nearly constant when compared to the untreated group (Fig. 3e–h). This indicates that Ag–TiO2 did not cause any significant change in the overall size and branching pattern of the blood vessels in the CAM model, indicating biocompatibility. Moreover, a slight increase in fold change in length and junctions compared to the control was observed, suggesting AgTiO2 to be non-toxic and it might have some angiogenic properties. Detailed studies are needed to confirm the angiogenic potential of these materials that are out of scope for this study.Moreover, to evaluate the biocompatibility of the Ag–TiO2 coating for a longer duration, we incubated the developing embryo with Ag–TiO2 coated PTFE disc for 2 days and compared its growth to the control (untreated) chicken embryo. We observed that the chicken embryo exposed to Ag–TiO2 coating showed similar growth as the untreated control group, indicating long-term biocompatibility.
A skin irritation test demonstrated Ag–TiO2 as non-irritant and immunologically inert
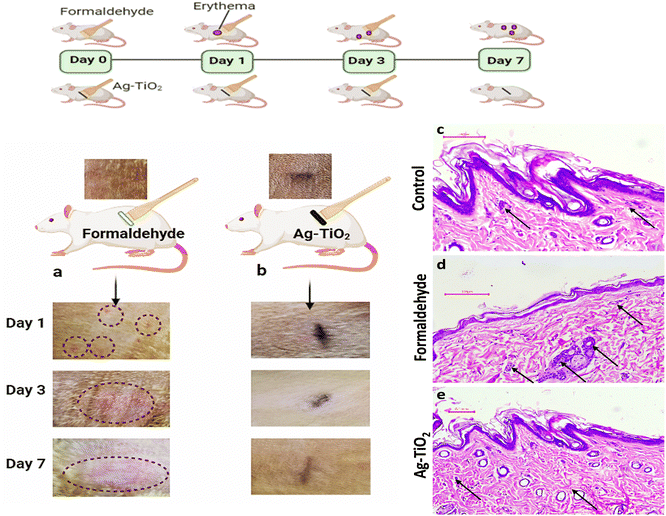
An in vivo dermal irritation study was conducted to visualize the effects of Ag–TiO2 on rat skin and compare the observed effects with standard skin irritant (0.8% formaldehyde). Even after prolonged 7-day exposure to Ag–TiO2, no immune reactions, redness, or irritation were observed over the skin (Fig. 4b).In contrast, the skin region exposed to formaldehyde showed irritation, redness, and inflammation within 24 hours of application, which increased as the days passed (Fig. 4a).
Histological analysis of the rat skin treated with 0.8% formaldehyde (a common skin irritant) demonstrated a decline in epidermal cells (Fig. 4d) when compared to normal skin (Fig. 4c), suggesting loss of cells present in the outermost layer of the skin. Furthermore, a notable inflammatory response in the skin exposed to formaldehyde was observed by significant neutrophil infiltration into the dermis and subcutaneous muscles. This inflammatory response suggests an immunological response to the irritant and tissue damage.29 In contrast, despite being exposed to Ag–TiO2 for seven days, the skin showed no signs of degenerative changes in the epidermal cells. Significantly few neutrophils were observed in the tissues, confirming minimum inflammatory response in the Ag–TiO2 treated skin (Fig. 4e). The results suggest that Ag–TiO2 is non-irritant and is safe for antibiofilm applications.
Ag–TiO2 showed enhanced antibacterial efficacy, outperforming conventional antibiotics
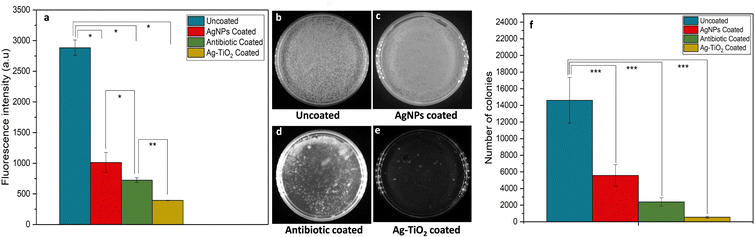
To check the efficacy of Ag–TiO2 in combating bacterial growth, several in vitro studies were conducted using a Ag–TiO2 coated coverslip. The studies aimed to evaluate the effectiveness of Ag–TiO2 with traditional antibiotics (ciprofloxacin) and only Ag NPs. We incubated bacterial (GFP-E. coli, OD nearly 0.6) samples separately with Ag–TiO2, antibiotic, and Ag NPs coated coverslip and kept the uncoated coverslip as a control. The fluorescence intensity following 24 hours of incubation was measured and found to be minimal in the Ag–TiO2 coated group compared to the others, suggesting it to be more effective than antibiotics and Ag NPs (Fig. 5a).Moreover, few colonies were observed when bacterial suspensions collected from differently treated samples were plated (Fig. 5b–f). The results of these two experiments highlight the excellent antibacterial properties of Ag–TiO2, indicating its ability to mitigate bacterial growth compared to currently used commercial treatments.
Ag–TiO2 facilitates normal growth of an E. coli infected chick embryo model by suppressing bacterial growth
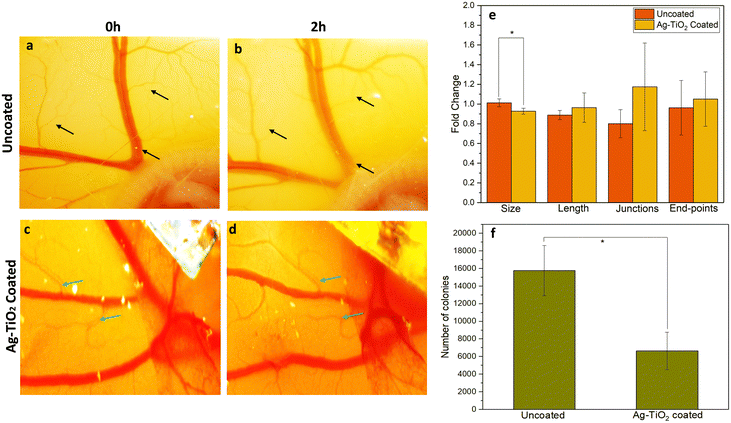
To evaluate the antibacterial role of Ag–TiO2 coating in an in vivo setting, we employed an infected four-day old chick embryo model and implanted Ag–TiO2 coated coverslips, including uncoated coverslips as a control.Following the implantation, the growth of the blood vessels was monitored for 2 hours. Loss of some of the blood vessels was observed (marked by black arrows) in the uncoated coverslip group (Fig. 6a and b), whereas a marked increase and stable growth of blood vessels were observed (marked by green arrow) in the Ag–TiO2 coated group (Fig. 6c and d). In the Ag–TiO2 treated group, there was an increase in the length, junctions, and endpoints of blood vessels, suggesting normal development of blood vessels (Fig. 6e). On the other hand, uncoated groups showed a reduction in these parameters, reflecting damage and loss of blood vessel growth (Fig. 6e). To further analyze the impact of Ag–TiO2, the allantoic fluid was collected from both groups of embryos after 4 hours and plated for 24 hours, following which, the colonies were counted using ImageJ software. The results revealed that the uncoated group had significantly higher colonies, about three times more than those formed in the Ag–TiO2 treated sample (Fig. 6f). These findings support that the Ag–TiO2 film helped combat the invaded bacteria to maintain the natural growth and development of the chicken embryos.
Ag–TiO2 coating disrupts the bacterial structural framework, causing mortality
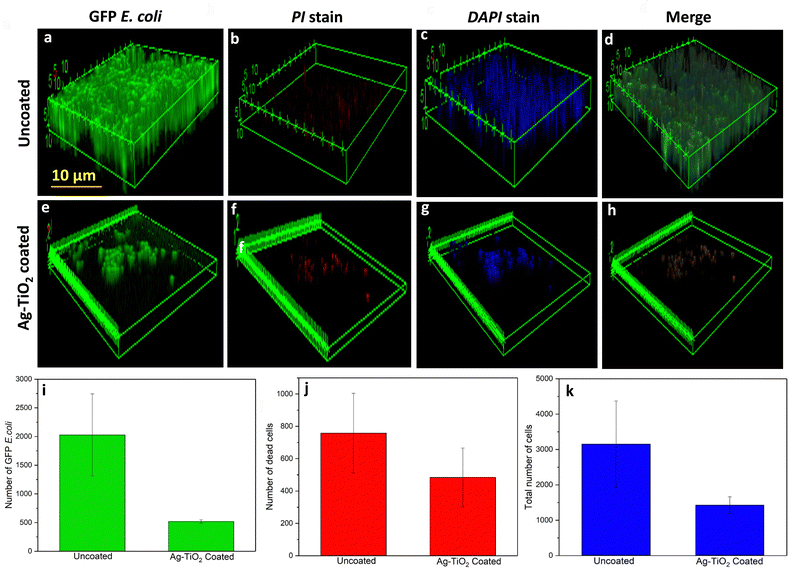
Confocal microscopy was performed to visualize the bacterial attachment over the uncoated (Fig. 7a–d) and Ag–TiO2 coated coverslip (Fig. 7e–h). The confocal images demonstrated higher attachment of GFP E. coli in the uncoated coverslip (Fig. 7a) than to the Ag–TiO2 coated coverslip (Fig. 7e). Moreover, the bacterium maintains its structural integrity over the uncoated sample, which is compromised in the Ag–TiO2 coated sample, causing bacterial death and destruction of the biofilm. DAPI and PI staining were further utilized to quantify the bacteria.30 These results demonstrated higher attachment of bacteria in the uncoated coverslip compared to the Ag–TiO2 coated coverslip (Fig. 7i–k). DAPI staining further corroborated the results, confirming minimum biofilm attachment in the Ag–TiO2 coated coverslip group (Fig. 7g and k). The PI staining of the Ag–TiO2 coated coverslip demonstrates significant dead bacterial cell attachment, supporting the loss of structural integrity of the biofilm on the Ag–TiO2 coated coverslip (Fig. 7f and j). The confocal microscopy images also showed that very few bacteria were attached to the Ag–TiO2 coated catheter (Fig. S4c and d, ESI†) when compared to the uncoated catheter (Fig. S4a and b, ESI†). Furthermore, the majority of the bacteria had lost their structural integrity. Additionally, some of the cells were deformed and fragmented. The number of cells deposited over the uncoated catheter was nearly 4 times more than that of the Ag–TiO2 coated catheter (Fig. S4e, ESI†).Ag–TiO2 coating exhibited versatile antibiofilm activity on diverse medically used substrates
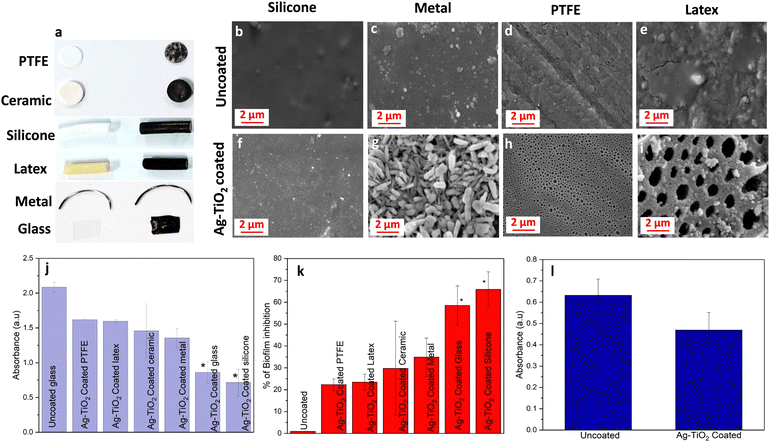
To examine the antibiofilm property of Ag–TiO2 across diverse medical-grade biomaterials, we coated various types of substrates (PTFE, ceramic, silicone, latex, metal, and glass) using Ag–TiO2 by a similar fabrication strategy (Fig. 8a). Fig. 8b–e shows the SEM images of uncoated silicone, metal, PTFE, and Latex. The SEM pictures in Fig. 8f–i demonstrated that the Ag–TiO2 coating had been successfully applied over various substrates. The images exhibit discrete and unique coating patterns, highlighting the flexibility and efficiency of the coating method for different substrates. A difference in the appearance of the SEM images of the Ag–TiO2 coating was observed on different substrates. The surface roughness of a substrate plays a crucial role in the process of coating, affecting nucleation and growth.31–33 Surface roughness, wetting properties, hydrophobicity, and porosity of a substrate highly influence the pattern of coating.34–36 As various substrates, including PTFE, glass, silicone, latex, and metal, have differences in their surface topography, a difference in coating pattern was noted.A CV staining assay was performed to evaluate biofilm attachment on these substrates coated with Ag–TiO2 quantitatively. Ag–TiO2-coated silicone showed excellent antibiofilm activity and inhibited around 65% of biofilm growth (Fig. 8j–k).
The glass coated with Ag–TiO2 could impede 60% of the bacterial growth. The Ag–TiO2 coated PTFE, latex, ceramic, and metal exhibited more than 20% biofilm inhibition in contrast to the uncoated coverslip, which can further be improved by coating optimization and increasing the concentration of Ag–TiO2. These results support the universal use of Ag–TiO2 coating as an antibiofilm coating across various medical-grade biomaterials currently used to develop different medical devices. Additionally, the results of the CV staining assay utilizing Bacillus subtilis revealed the efficient role of Ag–TiO2 in killing Gram-positive bacteria (Fig. 8l).
UV-visible spectroscopy and XRD analysis revealed remarkable optical and structural integrity of the Ag–TiO2 coated sample
The stability of a coating on the biomaterials plays a crucial role in ensuring biocompatibility, effectiveness, and long-term success of the therapeutic outcome. Various characterization techniques, such as UV visible spectroscopy, XRD, and ICP-MS, were employed to study the stability of the Ag–TiO2 coating. The UV spectra obtained after day one and day seven did not show any significant difference (Fig. 9a), indicating that the optical properties of the coating remained the same over this period, suggesting good stability. Besides, the XRD analysis at different time points up to 7 days revealed similar crystallographic patterns (Fig. 9b), indicating the high strength of the coating. We also evaluated the stability of Ag–TiO2-coated silicone and latex for time-dependent XRD analysis (Fig. S5a and b, ESI†). The results demonstrated the presence of similar peaks of Ag and TiO2 even after 7 days, indicating the stability of the Ag–TiO2 coating over various substrates over time.Lastly, to test the long-term stability and durability of the coating, we compared UV-visible absorption spectra and XRD of the Ag–TiO2 coated glass slide after 1 day and 3 months. UV visible spectra (Fig. S6a, ESI†) and XRD analysis (Fig. S6b, ESI†) of a 3-month-old Ag–TiO2 coated glass slide showed a nearly similar peak and intensity when compared with a 1-day-old Ag–TiO2 coated slide, indicating the long-term stability and durability of the Ag–TiO2 coating.
Continuous release of Ag and Ti leads to prolonged antibacterial and antibiofilm properties of Ag–TiO2 coated medical devices
Furthermore, the Ag+ and Ti4+ release kinetics were studied using ICP-MS. ICP-MS analysis of FBS incubated with Ag–TiO2 coated coverslips at different intervals shows continuous release of Ag+ and Ti4+ from the Ag–TiO2 coated material into the surrounding environment (Fig. 9c). The concentration of Ti4+ released on days 1, 3, 5, and 7 days is 1200, 3600, 2800, and 6400 μg mL−1, which shows a gradual increase over time. On the other hand, the concentration of Ag+ released on days 1, 3, 5, and 7 was found to be 5600, 3600, 4400, and 4000 μg mL−1, respectively, indicating a sustained steady release kinetics. Continuous and sustained release of Ag and Ti can protect medical devices from bacterial attachment and inhibit biofilm generation for an extended period.Reusability study demonstrated exceptionally strong antibacterial efficacy of Ag–TiO2 in subsequent use
We designed a reusability study to evaluate if the efficacy of Ag–TiO2 remains the same in the second use. The results depicted that even in the second use, the Ag–TiO2 managed to maintain its excellent antibacterial properties compared to antibiotic and only AgNP coated groups (Fig. 9d). This is useful for enhancing the reusability of the medical equipment and materials coated with Ag–TiO2, preventing long-term bacterial infections. An Ag–TiO2 coated glass substrate, coated 3 months before, was incubated with E. coli for 24 hours to evaluate the long-term antibacterial effects of Ag–TiO2. E. coli exposed to uncoated glass served as a control. After the incubation period, the absorbance of the bacterial suspension treated with both coated and uncoated glass was measured using a multiple reader. The lower absorbance of bacteria treated with Ag–TiO2 coated glass revealed that it could inhibit bacterial attachment over the substrate even after 3 months of coating, suggesting the stability and durable antibacterial effects of coating over a longer period of time (Fig. S6c, ESI†).Ag–TiO2 coating exhibited ROS-mediated inhibition of both Gram-positive as well as Gram-negative bacteria
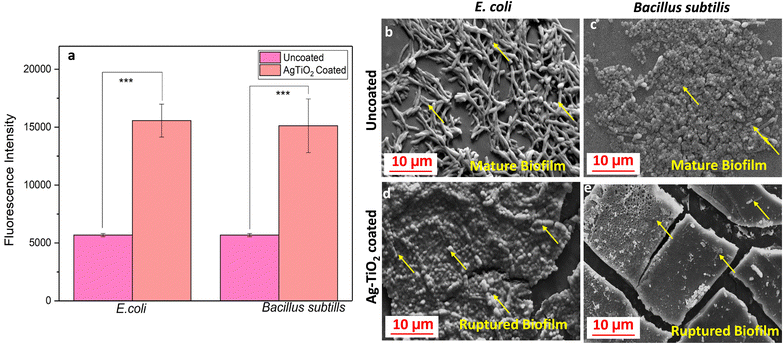
A reactive oxygen species (ROS) generation assay was performed to quantify the amount of ROS generated by bacterial cells when they encounter Ag–TiO2 using DCFDA staining. We allowed both E. coli and Bacillus subtilis to grow in the presence of Ag–TiO2 coated and uncoated medical catheters. The fluorescence intensity of the bacterial suspension was higher in the Ag–TiO2 treated suspension than the uncoated catheters, indicating a higher amount of ROS production in the Ag–TiO2 coated group in both E. coli and Bacillus subtilis (Fig. 10a). ROS generates oxidative stress inside the bacterial cell, hindering the basic physiological activity of the bacterium, leading to nucleotide damage, disruption of vital proteins and enzymes, cell death, and destruction of the biofilm.37,38SEM analysis revealed that Ag–TiO2 coating acts as a barrier against bacterial adherence on medical catheters
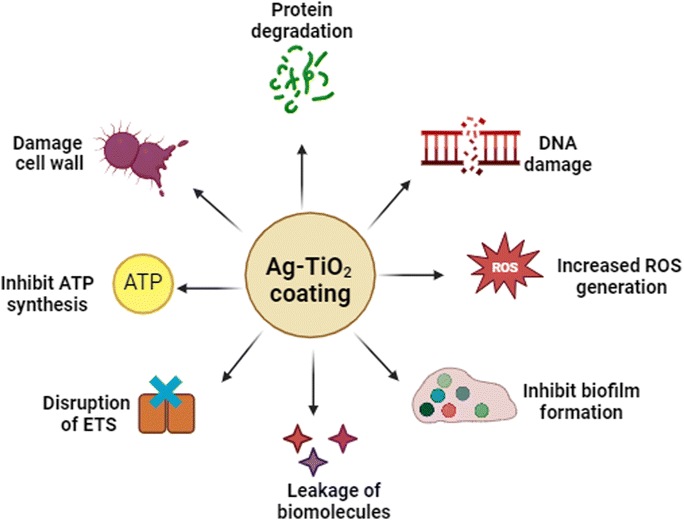
Biofilm was allowed to grow on Ag–TiO2 coated and uncoated catheters to assess the impact of coating on bacterial survival. Following 48 hours of incubation, the biofilm was visualized using SEM. The SEM analysis highlighted the presence of several layers of bacterial attachment over the uncoated medical catheter (Fig. 10b and c). Moreover, the elongated bacterial cells suggest that the structure of bacteria is well maintained and might have been replicated to increase its population, facilitating strong biofilm formation. In comparison, few bacterial cells were attached to the Ag–TiO2 coated catheter (Fig. 10d and e). Furthermore, the bacterial cells on the surface of the Ag–TiO2 coated catheter showed compromised cell walls, causing leakage of essential proteins and organelles and significant damage to the overall morphology (Fig. 10d and e). Confocal microscopy of the catheter similarly confirmed that little attachment of bacteria to the Ag–TiO2 coated catheter was observed compared to the uncoated catheter, supporting the antibiofilm property (Fig. S4, ESI†). The findings from the above studies strongly suggest that Ag–TiO2 directly interacts with bacteria and inhibits their growth and development. Ag–TiO2 facilitates the destruction of bacterial cells, causing leakage of vital proteins and organelles. Moreover, Ag–TiO2 coating triggers the generation of ROS, causing further damage and eventual bacterial cell death (Scheme 1).Discussion
There has been a significant increase in medical-acquired infections caused by biofilm formation over medical devices. Biofilms act as a major virulence factor, hindering the effectiveness of antibiotics and the host immune system. To address this issue of bacterial infections, novel approaches in antimicrobial treatment have been developed, including antibiotic combinations, innovative surface coatings, and the application of antimicrobial nanoparticle-based formulations.39 Notably, recent reports have highlighted the antimicrobial activity of bare silver nanoparticles (Ag NPs) and only titanium oxide nanoparticles (TiO2 NPs) for topical administration against hospital-acquired infections.25,40–42 The combination of AgNPs within a TiO2 matrix significantly improves the antimicrobial and antibiofilm properties of the combined entity over the individual materials.We developed an innovative low-temperature (120 °C) annealed antimicrobial dip coating technique under UV light treatment to coat various medical devices and reduce the risks of hospital-acquired infections. The combination of low-temperature and UV exposure is a unique approach that differentiates our coating method from the other existing methods. Dip coating at low temperatures will minimize the risk of damage to sensitive medical devices, ensuring their continued functionality. This technique can coat various substrates, including latex, glass, silicone, metal, ceramic, and PTFE, expanding its potential application for coating multiple medical devices. Additionally, the combination of silver and titanium oxide makes the coating exhibit an excellent antimicrobial performance, as both silver and titanium are well known for their antimicrobial applications.
In this research, we modified the surface of various medical grade biomaterials by applying a Ag–TiO2 thin film using a novel solution-based dip/spin coating approach followed by low-temperature annealing (120 °C) under UV-illumination, which is compatible with a wide range of substrates, including flexible plastic, ceramic, glass, etc. Chemical ion exchange allows for the selective exchange of ions between two materials. In this case, lithium ions from lithium titanate (Li4Ti5O12) are exchanged with silver ions, resulting in the formation of Ag–TiO2. This selective exchange mechanism enables precise control over the composition and distribution of silver within the TiO2 matrix, which can lead to unique properties not achievable through other synthesis methods. By adjusting parameters such as reaction time, temperature, and precursor concentrations, one can tailor the composition, structure, and morphology of the Ag–TiO2 material. Detailed functional studies demonstrated the utility of Ag–TiO2 surface coating to mitigate biofilm formation and exhibit strong antibacterial properties. Various characterization studies confirmed the durability of the coating along with the slow and sustained release of Ag+ and Ti4+ ions, suggesting the stability and long-term efficacy of the Ag–TiO2 coating. The TiO2 matrix improves the homogeneity and durability of the coating and allows slow and sustained release of Ag+, facilitating long-term antibiofilm protection. Since Ag NPs and TiO2 are well known for their antimicrobial efficacy, combining these two enhances antimicrobial properties, providing a synergistic effect. While Ag NPs have demonstrated more efficient inhibitory activity against Gram-negative bacteria than Gram-positive bacteria,43 the combined Ag–TiO2 can inhibit both Gram-positive and Gram-negative bacteria. As per the published literature, Ag NPs get aggregated over time and cause adverse effects on the living system.44 Furthermore, TiO2-NPs exhibited toxicity in various tissues, compromising their biocompatibility when tested in fish.45 As per our findings, combined Ag–TiO2 did not exhibit any toxic effects in the development of blood vessels in the chick embryo model and did not cause any inflammatory response when applied over rat skin. Additionally, it reduced the infection more efficiently than the embryo treated with an uncoated sample. Comparably, better antibacterial and antibiofilm efficacy was exhibited by Ag–TiO2 in contrast to bare Ag NPs and existing antibiotics. The Ag–TiO2 coated sample showed durable effectiveness even after repeated use, outperforming both Ag NPs and antibiotics in current use. Lastly, this Ag–TiO2 coating prevented bacterial adhesion over medical urinary catheter surfaces and altered the shape of the attached bacteria. This morphological alteration and inability of biofilm formation makes the bacteria more susceptible to antimicrobial coating, leading to bacterial death. This ultimately enhances the effectiveness of AgTiO2 over similar coating materials.
Unlike earlier approaches, this Ag–TiO2 thin film has not developed by mixing Ag NPs and TiO2 NPs, but instead grown through a chemically synthesized in situ growth technique inside an oxide film that gives a stable coating. Most importantly, unlike other published reports, a facile and rapid low-temperature solution-processed dip/spin coating method was developed under UV treatment.46 This coating method is low-cost, and it facilitates a smooth surface with homogeneous coating across a diverse range of biomaterials. Our work shows potential antibacterial and antibiofilm efficacy by generating reactive oxygen species and destroying the bacterial cell walls that can inhibit both Gram-positive and Gram-negative bacteria. Moreover, it is biocompatible, which is necessary for biomedical applications. We further tested the efficacy of Ag–TiO2 in combating infection in an in vivo model, providing a more realistic representation of the potential applications of the coated material. Various articles based on Ag–TiO2 failed to show their biological compatibility and effectiveness in in vivo models. Coating compatibility with a diverse range of medical grade biomaterials (latex, silicone, ceramic, PTFE, glass, and metal) was tested, confirming the medical utility across a range of substrates, unlike other published reports. The size of the nanoparticles and surface roughness were controlled and smoother in our fabrication process compared to some of the reported work (Table 1).47–49
| S. No. | Material | Coating substrate | Process of coating | Characterization | Properties | Bacterial inhibition | In vivo animal model | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. | Ag–TiO2 | Latex silicone ceramic PTFE glass metal | Solution process Low temperature (120 °C) UV annealed drop cast method | Average particle between 5 to 25 nm, roughness of 24.18 nm | Antibacterial, antibiofilm, biocompatibility and ROS generation | E. coli, Bacillus subtilis | 4 days old chick embryo and Rat model | This work |
| 2. | Ag NPs-TiO2 nanotubes | — | Anodic oxidation of polished titanium foil and fabrication of AgNPs | Roughness measured by AFM was approximately 24.34 nm | Antibacterial properties | S. aureus, E. coli, and P. aeruginosa | — | 50 |
| 3. | Epoxy/Ag–TiO2 Polymer Nanocomposite | Glass | Solution process high temperature (500 °C) annealed spin coat | Nanocrystals of 18 nm size | Antibiofilm | S. aureus, E. coli, | — | 46 |
| 4. | Bio-synthesized Ag–TiO2 Nanocomposites | — | Green synthesis using plant flower (A. haussknechtii) | The average size of nanocomposites is 36.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.03 12.03 |
Anti planktonic, antibiofilm, anti-swarming motility, and antiquorum sensing activities | S. aureus, E. coli, and P. aeruginosa | — | 47 |
| 5. | Ag–TiO2/polymer nanohybrid films | Glass | Degussa P25 TiO2 reduced with Ag ions under irradiation with UV light and spray-coated with polyacrylate. | — | Reactive oxygen species (ROS) generation, Antibacterial | S. aureus, E. coli, and P. aeruginosa | — | 51 |
| 6. | Corn starch (CS) film/Satureja khuzestanica essential oil (SEO)/Ag–TiO2 nanocomposites | — | Green synthesized using S. khuzestanica plant | 30–60 nm | Antimicrobial | E. coli, S. typhimurium S. aureus | — | 48 |
| 7. | G-PDA-Ag/TiO2 | Glass | Solution process chemical synthesized method under high annealing temperature (400 °C) | 150–200 nm. | Antibacterial | E. coli and B. subtilis | — | 49 |
| 8. | Ag–TiO2 catalyst | — | Synthesized by a Eucalyptus globulus L. extract as a reductive agent through sol–gel and microwave-assisted sol–gel processes | 11–14 nm | Antimicrobial properties | E. coli and S. aureus | — | 52 |
| 9. | Ag@TiO2-PPDO (poly(p-dioxanone)) | PLGA/PLCA electrospinning membranes | Chemically induced Ag@TiO2-PPDO coated onto PLGA membrane via electro-spraying | — | Antibacterial | S. aureus | Rats | 52 |
While Ag–TiO2 coating has outstanding antimicrobial properties, it is important to acknowledge certain limitations associated with its coating. The effectiveness of Ag–TiO2 coating appears to vary depending on the substrate, as evidenced by excellent results on catheters but less satisfactory outcomes on Ag–TiO2-coated polytetrafluoroethylene (PTFE). One potential explanation for this difference could be a lower deposition of the coating material on the PTFE substrate. Additional studies are required to reduce the uneven distribution of AgTiO2 on the substrate. Addressing these challenges requires thorough investigations to realize the full potential of Ag–TiO2 for effective medical coating. Future studies focus on refining the coating process and optimizing substrate compatibility and concentration to enhance the overall efficacy and versatility of Ag–TiO2 in antibiofilm coating applications for medical materials and devices.
Conclusion
In our current work, we coated different medical grade substrates widely used to prepare medical devices and diagnostic tools using Ag–TiO2 thin film using a low-temperature coating method. The coating process employed was simple, economically cheap, and did not cause any damage to various biomaterial surfaces. Physiochemical characterization showed excellent stability of the coating material. In vivo, CAM assay and skin irritation tests in the rat model further supported the biocompatible nature of Ag–TiO2. Additionally, Ag–TiO2 demonstrated outstanding antibacterial and antibiofilm properties opposing the growth of Gram-negative (E. coli) and Gram-positive (B. subtilis) bacteria. The findings altogether show the potential of this typical in situ grown low-temperature processed Ag–TiO2 surface coating over medical devices to combat bacterial infections.Experimental section
Synthesis of Ag–TiO2 film
As mentioned earlier, Ag–TiO2 film has been deposited in a low-temperature solution process technique where Ag NPs are grown into a TiO2 (titanium oxide) matrix via an in situ growth technique. In the beginning, a sol–gel method was utilized to deposit an ion-conducting Li4Ti5O12 (lithium titanium oxide)53 ceramic thin film on various substrates. For this synthesis, titanium(IV) butoxide [Ti(OC4H9)4] (>97% pure, obtained from Sigma-Aldrich) and lithium acetate (99% extra pure, purchased from Alfa-aesar) were used as precursor reagents.54 Two different precursor solutions of titanium(IV) butoxide and lithium acetate of concentration 300 mM are prepared separately using 2-methoxy ethanol as the solvent through continuous stirring for 30 minutes. The resulting solutions are added in a volume ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 followed by rapid stirring for two hours to prepare the precursor solution of Li4Ti5O12 (LTO). This precursor solution is then coated on various substrates, including catheters and coverslip glass, through a dip or spin coating method. Before coating, all the substrates are cleaned ultrasonically with DI water, acetone, and isopropanol, respectively. At the end of this wet cleaning, substrates are dried with an air-blower and placed inside an oxygen plasma chamber for plasma cleaning (power 20 W, for 6 minutes), which makes the substrates hydrophilic. Afterward, those clean plastic substrates are spin-coated at 3000 r.p.m for 30 seconds with a precursor solution of Li4Ti5O12 and then placed upon a calibrated hot plate at 120 °C and UV light is exposure for one hour to form the polycrystalline Li4Ti5O12 film. Subsequently, these polycrystalline thin films are immersed in a 100 mM AgNO3 solution for an hour for the anion-exchange process,55 which chemically exchanges silver ions (Ag+) of the solution with the loosely bound lithium ions (Li+) of the Li4Ti5O12 film to obtain Ag4Ti5O12 thin film, changing the substrate color from white to yellowish.56 Following this process, the samples were washed utilizing distilled water to eliminate excess AgNO3 from the film's surface. After that, those substrates are placed in a NaBH4 solution57 for one hour, where the Ag+ ion is reduced to Ag0 to form Ag NPs inside the TiO2 matrix. In that process, the substrate color changes from yellow to blackish, confirming that the reduction is completed. This chemical reaction associated with these ion exchange and reduction processes is illustrated in eqn (1) and (2), respectively. Besides, Ag–TiO2 thin film synthesis steps are schematically presented in Scheme 2.
5 followed by rapid stirring for two hours to prepare the precursor solution of Li4Ti5O12 (LTO). This precursor solution is then coated on various substrates, including catheters and coverslip glass, through a dip or spin coating method. Before coating, all the substrates are cleaned ultrasonically with DI water, acetone, and isopropanol, respectively. At the end of this wet cleaning, substrates are dried with an air-blower and placed inside an oxygen plasma chamber for plasma cleaning (power 20 W, for 6 minutes), which makes the substrates hydrophilic. Afterward, those clean plastic substrates are spin-coated at 3000 r.p.m for 30 seconds with a precursor solution of Li4Ti5O12 and then placed upon a calibrated hot plate at 120 °C and UV light is exposure for one hour to form the polycrystalline Li4Ti5O12 film. Subsequently, these polycrystalline thin films are immersed in a 100 mM AgNO3 solution for an hour for the anion-exchange process,55 which chemically exchanges silver ions (Ag+) of the solution with the loosely bound lithium ions (Li+) of the Li4Ti5O12 film to obtain Ag4Ti5O12 thin film, changing the substrate color from white to yellowish.56 Following this process, the samples were washed utilizing distilled water to eliminate excess AgNO3 from the film's surface. After that, those substrates are placed in a NaBH4 solution57 for one hour, where the Ag+ ion is reduced to Ag0 to form Ag NPs inside the TiO2 matrix. In that process, the substrate color changes from yellow to blackish, confirming that the reduction is completed. This chemical reaction associated with these ion exchange and reduction processes is illustrated in eqn (1) and (2), respectively. Besides, Ag–TiO2 thin film synthesis steps are schematically presented in Scheme 2.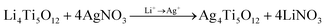 | (1) |
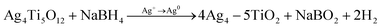 | (2) |
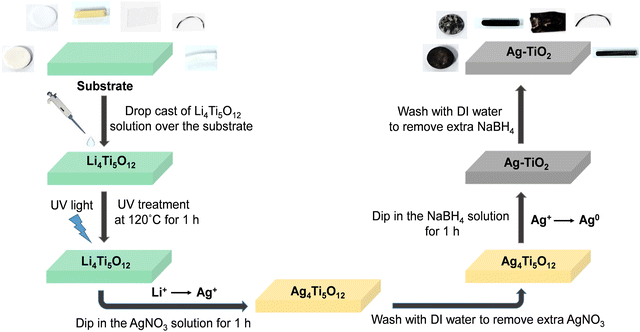 | ||
| Scheme 2 Schematic presentation of the step-by-step synthesis process of low-temperature annealed Ag4Ti5O12 anti-microbial thin-film under UV light treatment. | ||
Material characterization
For the UV-Vis absorption, thin film samples are made on a quartz surface to ensure optimal absorption by the sample that have been investigated by using an integrated spectrometer (Enlitech QE-R EQE measurement unit), in the range of 300–900 nm. A high resolution (HR) scanning electron microscope (SEM) study has been performed by using a NOVA NANOSEM 40 FEITM to check the surface morphology of the Ag–TiO2 coated substrate. The metallic elemental composition was determined by an EDX (energy-dispersive X-ray spectrometer) coupled to the HR-SEM. The roughness and surface morphology of the prepared samples were examined employing atomic force microscopy (NTEGRA Prima from NT-MDT Service & Logistics Ltd) applying a cantilever tip of thickness (h), width (w) and length (l), as 1.84 μm, 35 μm and 123 μm, respectively. The contact angle of the uncoated and Ag–TiO2 coated samples was investigated using a KRUSS Tensiometer K100.Biocompatibility study using chicken egg model
A Chick CAM (chorioallantoic membrane) assay study has been performed to investigate the toxic effects of Ag–TiO2 coated samples.58 The detailed procedure is provided in the ESI† file.59In vivo skin irritation test
A dermal skin irritation study was performed after IAEC approval (IIT(BHU)/IAEC/2023/068; Approval Date: February 9, 2023). Healthy Wistar rats (6–8 weeks old; 150–175 g) were obtained from CDRI-Lucknow and employed for in vivo skin irritation experiments. A skin irritation assay was performed to study the effects of Ag–TiO2 coating when exposed to rat skin. Initially, the dorsal region of the skin was shaved and classified into three sections. Ag–TiO2 powder and formaldehyde, a common irritant, were applied to the respective areas. Images were taken after 0, 1, 3, and 7 days to visualize any immediate or delayed immune reactions such as swelling, redness, etc.60 After seven days, the rats were sacrificed, and the skin tissue sections were retrieved and fixed in paraformaldehyde, followed by histological analysis.Antibacterial studies
Several in-vitro and in-vivo experiments were performed to evaluate the antibacterial effects of Ag–TiO2 coated substrates, including colony counting methods, LB broth study, an in vivo infected CAM model study, etc. Detailed experimental details are provided in the ESI† file.CV staining assay
A CV (crystal violet) staining assay was used to assess biofilm attachment.61–63 The detailed experimental protocol is provided in the ESI† file. Further analysis involves the calculation of percentage biofilm inhibition in AgTiO2 coated medical devices with respect to the uncoated control by applying the given formula:where Ac denotes the absorbance of the control, and As denotes the absorbance of the sample at 595 nm.
Confocal microscopy
We employed confocal microscopy (Zeiss 510 Meta confocal microscopy system) to check the attachment of bacteria over the Ag–TiO2 coated and uncoated sample.64,65 The detailed process is mentioned in the ESI.†ROS generation assay
2,7-Dichlorofluorescein diacetate (DCFDA) is a frequently employed reagent to examine the presence of ROS (reactive oxygen species) inside cells. It can easily cross the plasma membrane because of its non-polar nature. Cellular esterase hydrolyzes DCFDA, cleaving its acetate groups and yielding a non-fluorescent molecule named 2,7-dichlorofluorescein (DCFH). DCFH, in contact with ROS, gets oxidized, resulting in 2,7-dichlorofluorescein (DCF), which shows green fluorescence.66 There is a direct correlation between the quantity of ROS in the cells and a rise in fluorescence intensity. Ag–TiO2 coated and uncoated samples were incubated with Gram-positive (Bacillus) and Gram-negative (E. coli) bacteria for a full day after being adjusted to OD600 around 0.5. The next day, 50 μL of 10 mM DCFH-DA (procured from Sisco Research Laboratories Pvt. Ltd) was mixed with 150 μL of bacterial solution, which was then kept for 5 min in black 96-well plates at room temperature. The fluorescence intensity was then assessed employing a microplate reader (emission; 525 nm and excitation; 485).67SEM of catheters
SEM analysis was carried out to determine the effects of Ag–TiO2 on biofilm development. Initially, Ag–TiO2 coated and uncoated medical catheters (Codman HOLTER Atrial Distal catheter, 821670) were incubated with E. coli (Escherichia coli) and Bacillus subtilis to allow the development of a biofilm. After the incubation duration of 2 days, the catheters were removed from the bacterial suspension and washed twice utilizing PBS. The biofilm developed over the catheter was fixed using 200 μL of 2.5% glutaraldehyde followed by overnight incubation.68 Next, the catheters were rinsed twice with PBS. Following this, the catheters underwent dehydration by sequential changes in ethanol concentration, starting with 30%, followed by increments to 50%, 70%, 80%, 90%, 95%, and ultimately reaching 100%. The samples were then submitted for SEM analysis.68Data availability
Data for this article, including raw data are available at Science data bank at https://www.scidb.cn/en/anonymous/eTZyTUJm.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
SM acknowledges research fund support from ICMR, New Delhi (No. 33/01/2023/RD/BMS) and DBT, India (BT/PR49530/MED/32/839/2023) for providing funding support for this work. BNP thanks SERB, India (CRG/2019/001826) and DST, India (DST/INT/SWD/VR/P-12/2019) for financial support. The authors are grateful to the Central Instrument Facility Centre, IIT (BHU), for providing the FE-SEM, HR-TEM, and XRD measurement facility. Mr Sobhan Hazra thanks IIT(BHU) for providing a PhD fellowship. We also acknowledge the director of the Indian Institute of Technology (BHU), Varanasi, India, for continuous support. LP and AU are grateful to MHRD and UGC for their fellowship support.References
- P. S. Stewart and T. Bjarnsholt, Risk factors for chronic biofilm-related infection associated with implanted medical devices, Clin. Microbiol. Infect., 2020, 26(8), 1034–1038 CrossRef CAS PubMed
.
- M. K. Chug and E. J. Brisbois, Recent Developments in Multifunctional Antimicrobial Surfaces and Applications toward Advanced Nitric Oxide-Based Biomaterials, ACS Mater. Au, 2022, 2(5), 525–551 CrossRef CAS PubMed
.
- S. Amin Yavari, S. M. Castenmiller, J. A. van Strijp and M. Croes, Combating implant infections: shifting focus from bacteria to host, Adv. Mater., 2020, 32(43), 2002962 CrossRef CAS PubMed
.
- L. Yao, K. Fu and G. Liu, Solvent-Directed Hierarchical Self-Assembly of Tetraphenylpyrazine-Cholesterol with Amplified Circularly Polarized Luminescence, ACS Appl. Mater. Interfaces, 2023, 15(34), 40817–40827 CrossRef CAS PubMed
.
- R. Roy, M. Tiwari, G. Donelli and V. Tiwari, Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action, Virulence, 2018, 9(1), 522–554 CrossRef CAS PubMed
.
- F. Li, Q. Pan, Y. Ling, J. Guo, Y. Huo, C. Xu, M. Xiong, M. Yuan, Z. Cheng, M. Liu and J. Lin, Gold − Titanium dioxide heterojunction for enhanced sonodynamic mediated biofilm eradication and peri-implant infection treatment, Chem. Eng. J., 2023, 460, 141791 CrossRef CAS
.
- F. J. Geissel, V. Platania, A. Gogos, I. K. Herrmann, G. N. Belibasakis, M. Chatzinikolaidou and G. A. Sotiriou, Antibiofilm activity of nanosilver coatings against Staphylococcus aureus, J. Colloid Interface Sci., 2022, 608, 3141–3150 CrossRef CAS PubMed
.
- J. Mei, D. Xu, L. Wang, L. Kong, Q. Liu, Q. Li, X. Zhang, Z. Su, X. Hu and W. Zhu, Biofilm Microenvironment-Responsive Self-Assembly Nanoreactors for All-Stage Biofilm Associated Infection through Bacterial Cuproptosis-like Death and Macrophage Re-Rousing, Adv. Mater., 2023, 35(36), 2303432 CrossRef CAS PubMed
.
- M. Tumbarello, B. Posteraro, E. M. Trecarichi, B. Fiori, M. Rossi, R. Porta, K. de Gaetano Donati, M. La Sorda, T. Spanu, G. Fadda, R. Cauda and M. Sanguinetti, Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia, J. Clin. Microbiol., 2007, 45(6), 1843 CrossRef CAS PubMed
.
- S. Mohanty, T. Bharadwaj, D. Verma and S. Paul, Development of Ag doped ZnO nanostructure and tranexamic acid infused chitosan-guargum film: A multifunctional antimicrobial dressing with haemostatic and wound closure potential, Chem. Eng. J., 2023, 472, 144976 CrossRef CAS
.
- H. Wu, C. Moser, H. Z. Wang, N. Høiby and Z. J. Song, Strategies for combating bacterial biofilm infections, Int. J. Oral Sci., 2015, 7(1), 1–7 CrossRef CAS PubMed
.
- S. A. Khan and A. Shakoor, Recent Strategies and Future Recommendations for the Fabrication of Antimicrobial, Antibiofilm, and Antibiofouling Biomaterials, Int. J. Nanomed., 2023, 3377–3405 CrossRef CAS PubMed
.
- M. Assefa and A. Amare, Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review, Infect. Drug Resist., 2022, 15, 5061–5068 CrossRef CAS PubMed
.
- Y. Luo, Q. Yang, D. Zhang and W. Yan, Mechanisms and Control Strategies of Antibiotic Resistance in Pathological Biofilms, J. Microbiol. Biotechnol., 2021, 31(1), 1–7 CrossRef CAS PubMed
.
- C. Sahli, S. E. Moya, J. S. Lomas, C. Gravier-Pelletier, R. Briandet and M. Hémadi, Recent advances in nanotechnology for eradicating bacterial biofilm, Theranostics, 2022, 12(5), 2383–2405 CrossRef CAS PubMed
.
- H. Torkzadeh, K. R. Zodrow, W. C. Bridges and E. L. Cates, Quantification and modeling of the response of surface biofilm growth to continuous low intensity UVC irradiation, Water Res., 2021, 193, 116895 CrossRef CAS PubMed
.
- A. Mukherjee, S. Bose, A. Shaoo and S. K. Das, Nanotechnology based therapeutic approaches: an advanced strategy to target the biofilm of ESKAPE pathogens, Mater. Adv., 2023, 4, 2544–2572 RSC
.
- J. S. Fernandes, P. Gentile, R. A. Pires, R. L. Reis and P. V. Hatton, Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue, Acta Biomater., 2017, 59, 2–11 CrossRef CAS PubMed
.
- M. Vallet-Regí, D. Lozano, B. González and I. Izquierdo-Barba, Biomaterials against bone infection, Adv. Healthcare Mater., 2020, 9(13), 2000310 CrossRef PubMed
.
- C. Zhao, W. Liu, M. Zhu, C. Wu and Y. Zhu, Bioceramic-based scaffolds with antibacterial function for bone tissue engineering: A review, Bioactive Mater., 2022, 18, 383–398 CrossRef CAS PubMed
.
- L. Burroughs, W. Ashraf, S. Singh, L. Martinez-Pomares, R. Bayston and A. L. Hook, Development of dual anti-biofilm and anti-bacterial medical devices, Biomater. Sci., 2020, 8(14), 3926–3934 RSC
.
- S. Mishra, A. Gupta, V. Upadhye, S. C. Singh, R. P. Sinha and D.-P. Häder, Therapeutic strategies against biofilm infections, Life, 2023, 13(1), 172 CrossRef CAS PubMed
.
- H. Yazdani-Ahmadabadi, D. F. Felix, K. Yu, H. H. Yeh, H. D. Luo, S. Khoddami, L. E. Takeuchi, A. Alzahrani, S. Abbina, Y. Mei, L. Fazli, D. Grecov, D. Lange and J. N. Kizhakkedathu, Durable Surfaces from Film-Forming Silver Assemblies for Long-Term Zero Bacterial Adhesion without Toxicity, ACS Cent. Sci., 2022, 8(5), 546–561 CrossRef CAS PubMed
.
- S. A. Ahmad, S. S. Das, A. Khatoon, M. T. Ansari, M. Afzal, M. S. Hasnain and A. K. Nayak, Bactericidal activity of silver nanoparticles: A mechanistic review, Mater. Sci. Energy Technol., 2020, 3, 756–769 Search PubMed
.
- V. Kumaravel, K. M. Nair, S. Mathew, J. Bartlett, J. E. Kennedy, H. G. Manning, B. J. Whelan, N. S. Leyland and S. C. Pillai, Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants, Chem. Eng. J., 2021, 416, 129071 CrossRef CAS PubMed
.
- E. Bletsa, P. Merkl, T. Thersleff, S. Normark, B. Henriques-Normark and G. A. Sotiriou, Highly durable photocatalytic titanium suboxide–polymer nanocomposite films with visible light-triggered antibiofilm activity, Chem. Eng. J., 2023, 454, 139971 CrossRef CAS
.
- E. J. Falde, S. T. Yohe, Y. L. Colson and M. W. Grinstaff, Superhydrophobic materials for biomedical applications, Biomaterials, 2016, 104, 87–103 CrossRef CAS PubMed
.
- L. Pradhan, P. Sah, M. Nayak, A. Upadhyay, P. Pragya, S. Tripathi, G. Singh, B. Mounika, P. Paik and S. Mukherjee, Biosynthesized silver nanoparticles prevent bacterial infection in chicken egg model and mitigate biofilm formation on medical catheters, JBIC, J. Biol. Inorg. Chem., 2024, 29(3), 353–373 CrossRef CAS PubMed
.
- H. M. Anter, H. I. I. Abu, W. Awadin and M. M. Meshali, Novel anti-inflammatory film as a delivery system for the external medication with bioactive phytochemical Apocynin, Drug Des., Dev. Ther., 2018, 12, 2981–3001 CrossRef CAS PubMed
.
- M. Nayak, L. Sonowal, L. Pradhan, A. Upadhyay, P. Kamath and S. Mukherjee, Multifunctional (4-in-1) Therapeutic Applications of Nickel Thiocyanate Nanoparticles Impregnated Cotton Gauze as Antibacterial, Antibiofilm, Antioxidant and Wound Healing Agent, Chem. - Asian J., 2024, e202400187 CrossRef PubMed
.
- F. Xi, Y. Huang, Y. Zhao, Y. Liu, W. Dai and Y. Tian, Effects of Substrate Roughness on Microstructure and Fatigue Behavior of Plasma Electrolytic Oxidation-Coated Ti-6Al-4V Alloy, Materials, 2022, 15(12), 4256 CrossRef CAS PubMed
.
- Q. Li, L. Liang, J. Tan and G. Xie, The influence mechanism of micron surface roughness on slime coating and bubble attachment on coal surface, Miner. Eng., 2022, 189, 107895 CrossRef CAS
.
- Z. Qi, Z. Wu, D. Zhang, B. Wei, J. Wang and Z. Wang, Effect of sputtering power on the chemical composition, microstructure and mechanical properties of CrNx hard coatings deposited by reactive magnetron sputtering, Vacuum, 2017, 145, 136–143 CrossRef CAS
.
- J. Ruhkopf, S. Sawallich, M. Nagel, M. Otto, U. Plachetka, T. Kremers, U. Schnakenberg, S. Kataria and M. C. Lemme, Role of Substrate Surface Morphology on the Performance of Graphene Inks for Flexible Electronics, ACS Appl. Electron. Mater., 2019, 1(9), 1909–1916 CrossRef CAS PubMed
.
- V. A. Tarbokov; M. Slobodyan; S. Pavlov; E. Smolyanskiy; V. Uglov and G. E. Remnev, Changes in adhesion of crn coatings on zr-1% nb alloy substrates preliminary irradiated with high-intense pulsed ion beams, High Temperature Material Processes: An International Quarterly of High-Technology Plasma Processes, 2022, 26, (3) Search PubMed.
- S. Mondal, Impact of the process conditions on polymer pattern morphology during spin coating over topological surfaces, Soft Matter, 2021, 17(5), 1346–1358 RSC
.
- A. Krisko and M. Radman, Protein damage and death by radiation in Escherichia coli and Deinococcus radiodurans, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(32), 14373–14377 CrossRef CAS PubMed
.
- I. Matic, The major contribution of the DNA damage-triggered reactive oxygen species production to cell death: implications for antimicrobial and cancer therapy, Curr. Genet., 2018, 64(3), 567–569 CrossRef CAS PubMed
.
- N. Hassan, U. Farooq, A. K. Das, K. Sharma, M. A. Mirza, S. Fatima, O. Singh, M. J. Ansari, A. Ali and Z. Iqbal, In Silico Guided Nanoformulation Strategy for Circumvention of Candida albicans Biofilm for Effective Therapy of Candidal Vulvovaginitis, ACS Omega, 2023, 8(7), 6918–6930 CrossRef CAS PubMed
.
- S. Jacob Inbaneson, S. Ravikumar and N. Manikandan, Antibacterial potential of silver nanoparticles against isolated urinary tract infectious bacterial pathogens, Appl. Nanosci., 2011, 1, 231–236 CrossRef CAS
.
- J. M. Khaled, N. S. Alharbi, M. Z. Siddiqi, A. S. Alobaidi, K. Nauman, S. Alahmedi, A. O. Almazyed, M. A. Almosallam and A. N. Al Jurayyan, A synergic action of colistin, imipenem, and silver nanoparticles against pandrug-resistant Acinetobacter baumannii isolated from patients, J. Infect. Public Health, 2021, 14(11), 1679–1685 CrossRef PubMed
.
- X. Ge, C. Ren, Y. Ding, G. Chen, X. Lu, K. Wang, F. Ren, M. Yang, Z. Wang and J. Li, Micro/nano-structured TiO2 surface with dual-functional antibacterial effects for biomedical applications, Bioactive Mater., 2019, 4, 346–357 CrossRef PubMed
.
- H. F. Hetta, I. M. S. Al-Kadmy, S. S. Khazaal, S. Abbas, A. Suhail, M. A. El-Mokhtar, N. H. A. Ellah, E. A. Ahmed, R. B. Abd-ellatief, E. A. El-Masry, G. E.-S. Batiha, A. A. Elkady, N. A. Mohamed and A. M. Algammal, Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii, Sci. Rep., 2021, 11(1), 10751 CrossRef CAS PubMed
.
- P. Bélteky, A. Rónavári, N. Igaz, B. Szerencsés, I. Y. Tóth, I. Pfeiffer, M. Kiricsi and Z. Kónya, Silver nanoparticles: Aggregation behavior in biorelevant conditions and its impact on biological activity, Int. J. Nanomed., 2019, 667–687 CrossRef PubMed
.
- T. L. Carmo, P. R. Siqueira, V. C. Azevedo, D. Tavares, E. C. Pesenti, M. M. Cestari, C. B. Martinez and M. N. Fernandes, Overview of the toxic effects of titanium dioxide nanoparticles in blood, liver, muscles, and brain of a Neotropical detritivorous fish, Environ. Toxicol., 2019, 34(4), 457–468 CrossRef CAS PubMed
.
- K. Natarajan, Antibiofilm activity of epoxy/Ag-TiO2 polymer nanocomposite coatings against Staphylococcus aureus and Escherichia coli, Coatings, 2015, 5(2), 95–114 CrossRef
.
- M. Alavi and N. Karimi, Antiplanktonic, antibiofilm, antiswarming motility and antiquorum sensing activities of green synthesized Ag–TiO2, TiO2–Ag, Ag–Cu and Cu–Ag nanocomposites against multi-drug-resistant bacteria, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 399–413 CrossRef PubMed
.
- N. Sallak, A. M. Moghanjoughi, M. Ataee, A. Anvar and L. Golestan, Antimicrobial biodegradable film based on corn starch/Satureja khuzestanica essential oil/Ag–TiO2 nanocomposites, Nanotechnology, 2021, 32(40), 405703 CrossRef CAS PubMed
.
- Z. Lu, H. F. Zhou, J. J. Liao, Y. Y. Yang, K. Wang, L. M. Che, N. He, X. D. Chen, R. Song and W. F. Cai, A facile dopamine-assisted method for the preparation of antibacterial surfaces based on Ag/TiO2 nanoparticles, Appl. Surf. Sci., 2019, 481, 1270–1276 CrossRef CAS
.
- O. Bilek, T. Fialova, A. Otahal, V. Adam, K. Smerkova and Z. Fohlerova, Antibacterial activity of AgNPs–TiO 2 nanotubes: influence of different nanoparticle stabilizers, RSC Adv., 2020, 10(72), 44601–44610 RSC
.
- S. P. Tallósy, L. Janovák, E. Nagy, Á. Deák, Á. Juhász, E. Csapó, N. Buzás and I. Dékány, Adhesion and inactivation of Gram-negative and Gram-positive bacteria on photoreactive TiO2/polymer and Ag–TiO2/polymer nanohybrid films, Appl. Surf. Sci., 2016, 371, 139–150 CrossRef
.
- J. Torres-Limiñana, A. A. Feregrino-Pérez, M. Vega-González, L. Escobar-Alarcón, J. A. Cervantes-Chávez and K. Esquivel, Green synthesis via eucalyptus globulus l. extract of Ag-TiO2 catalyst: Antimicrobial activity evaluation toward water disinfection process, Nanomaterials, 2022, 12(11), 1944 CrossRef PubMed
.
- S. Hazra, S. V. Singh, S. Dahiya, P. K. Aich and B. N. Pal, Solution-Processed Ag-TiO2 Nanostructure-Based Schottky Junction Thin Films for Narrowband Hot-Electron Photodetectors, ACS Appl. Nano Mater., 2023, 6(16), 15119–15127 CrossRef CAS
.
- S. V. Singh, M. P. Kumar, S. Anantharaj, B. Mukherjee, S. Kundu and B. N. Pal, Direct evidence of an efficient plasmon-induced hot-electron transfer at an in situ grown Ag/TiO2 interface for highly enhanced solar H2 generation, ACS Appl. Energy Mater., 2020, 3(2), 1821–1830 CrossRef CAS
.
- U. Qamar, S. Hazra, C. Kant, U. U. Ghosh, B. N. Pal and S. Das, Two-dimensional silver nanonetwork on Ag4Ti5O12 film as highly efficient SERS substrate, Microchem. J., 2023, 109686 Search PubMed
.
- S. V. Singh, U. Gupta, S. Biring, B. Mukherjee and B. N. Pal,
In situ grown nanoscale pn heterojuction of Cu2S-TiO2 thin film for efficient photoelectrocatalytic H2 evolution, Surf. Interfaces, 2022, 28, 101660 CrossRef CAS
.
- S. V. Singh, A. Sharma, S. Biring and B. N. Pal, Solution processed Cu2S/TiO2 heterojunction for visible-near infrared photodetector, Thin Solid Films, 2020, 710, 138275 CrossRef CAS
.
- I. Moreno-Jiménez, J. M. Kanczler, G. Hulsart-Billstrom, S. Inglis and R. O. C. Oreffo, The Chorioallantoic Membrane Assay for Biomaterial Testing in Tissue Engineering: A Short-Term In Vivo Preclinical Model, Tissue Eng., Part C, 2017, 23(12), 938–952 CrossRef PubMed
.
- S. Mukherjee, S. Sau, D. Madhuri, V. S. Bollu, K. Madhusudana, B. Sreedhar, R. Banerjee and C. R. Patra, Green Synthesis and Characterization of Monodispersed Gold Nanoparticles: Toxicity Study, Delivery of Doxorubicin and Its Bio-Distribution in Mouse Model, J. Biomed. Nanotechnol., 2016, 12(1), 165 CrossRef CAS PubMed
.
- L. Rahman, Y. Sarwar, S. Khaliq, Inayatullah, W. Abbas, A. Mobeen, A. Ullah, S. Z. Hussain, W. S. Khan, M.-E. Kyriazi, I. Hussain, A. G. Kanaras and A. Rehman, Surfactin-Conjugated Silver Nanoparticles as an Antibacterial and Antibiofilm Agent against Pseudomonas aeruginosa, ACS Appl. Mater. Interfaces, 2023, 15(37), 43321–43331 CrossRef CAS PubMed
.
- E. F. Haney, M. J. Trimble, J. T. Cheng, Q. Vallé and R. E. W. Hancock, Critical Assessment of Methods to Quantify Biofilm Growth and Evaluate Antibiofilm Activity of Host Defence Peptides, Biomolecules, 2018, 8, 2 CrossRef PubMed
.
- K. N. Kragh, M. Alhede, L. Kvich and T. Bjarnsholt, Into the well-A close look at the complex structures of a microtiter biofilm and the crystal violet assay, Biofilm, 2019, 1, 100006 CrossRef PubMed
.
- A. Das, M. Das, P. Sandhu, N. Das, P. Tribedi, U. De, Y. Akhter and S. Bhattacharjee, Antibiofilm activity of: Parkia javanica against Pseudomonas aeruginosa: A study with fruit extract, RSC Adv., 2017, 7 Search PubMed
.
- K. Murata, Applicability of LIVE/DEAD BacLight Stain with Glutaraldehyde Fixation for the Measurement of Bacterial Cell Concentration and Viability in the Air, Aerosol Air Qual. Res., 2013, 13 Search PubMed
.
- P. N. Tawakoli, A. Al-Ahmad, W. Hoth-Hannig, M. Hannig and C. Hannig, Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm, Clin Oral Investig, 2013, 17(3), 841 CrossRef CAS PubMed
.
- S. Ghosh, G. Amariei, M. E. G. Mosquera and R. Rosal, Polymeric ruthenium precursor as a photoactivated antimicrobial agent, J. Hazard. Mater., 2021, 402, 123788 CrossRef CAS PubMed
.
- E. Piktel, L. Suprewicz, J. Depciuch, S. Chmielewska, K. Skłodowski, T. Daniluk, G. Król, P. Kołat-Brodecka, P. Bijak, A. Pajor-Świerzy, K. Fiedoruk, M. Parlinska-Wojtan and R. Bucki, Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices, Sci. Rep., 2021, 11(1), 12546 CrossRef CAS PubMed
.
- W. Huang, J. Q. Wang, H. Y. Song, Q. Zhang and G. F. Liu, Chemical analysis and in vitro antimicrobial effects and mechanism of action of Trachyspermum copticum essential oil against Escherichia coli, Asian Pac. J. Trop. Med., 2017, 10(7), 663–669 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb00701h |
| This journal is © The Royal Society of Chemistry 2024 |