Nitrogen-doped carbon dots: a novel biosensing platform for selective norfloxacin detection and bioimaging†
S.
Sivaselvam
,
R. S.
Anjana
,
N. S.
Dhujana
,
Marina
Victor
and
Ramapurath S.
Jayasree
 *
*
Division of Biophotonics and Imaging, Department of Biomaterial Sciences and Technology, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), Trivandrum 695012, India. E-mail: jayasree@sctimst.ac.in
First published on 1st July 2024
Abstract
Incomplete metabolism and non-biodegradable nature of norfloxacin (NORx) lead to its persistent residues in the environment and food, potentially fostering the emergence of antibiotic resistance and posing a significant threat to public health. Hence, we developed a norfloxacin sensor employing hydrothermally synthesized N-doped carbon dots (N-Ch-CQDs) from chitosan and PEI demonstrated high sensitivity and specificity towards the antibiotic detection. The quantum yield of excitation-dependent emission of N-Ch-CQDs was effectively tuned from 4.6 to 21.5% by varying the concentration of PEI (5–15%). With the enhanced fluorescence in the presence of norfloxacin, N-Ch-CQDs exhibited a linear detection range of 20–1400 nM with a limit of detection (LoD) of 9.3 nM. The high biocompatibility of N-Ch-CQDs was confirmed in the in vitro and in vivo model and showed the environment-friendly nature of the sensor. Detailed study elucidated the formation of strong hydrogen bonds between N-Ch-CQDs and NORx, leading to fluorescence enhancement. The developed sensor's capability to detect NORx was evaluated in water and milk samples. The recovery rate ranged from 98.5% to 103.5%, demonstrating the sensor's practical applicability. Further, the bioimaging potential of N-Ch-CQDs was demonstrated in both the in vitro (L929 cells) and in vivo model (C. elegans). The synergistic influence of the defecation pattern and functioning of intestinal barrier mitigates the translocation of N-Ch-CQDs into the reproductive organ of nematodes. This study revealed the bioimaging and fluorescent sensing ability of N-Ch-CQDs, which holds significant promise for extensive application in the biomedical field.
1. Introduction
The discovery of antibiotics remodeled the healthcare system and has been safeguarding human and animal health for many decades. The increase in the use and subsequent presence of the same in the environment and water bodies is a growing global concern. Most of the antibiotics are non-biodegradable, and their presence even at very low concentration (ng L−1) could contribute to antimicrobial resistance, which is a serious environmental concern.1 Norfloxacin (NORx), a potent third-generation fluoroquinolone, is a broad-spectrum antibiotic commonly employed in human and veterinary care to combat various diseases.2 However, its persistence in animal meat and milk due to incomplete metabolism has raised concern. The prolonged consumption of milk with NORx residues can lead to adverse side effects in humans, including gastrointestinal discomfort, dizziness and nausea.3,4 Moreover, it may lead to the emergence of drug-resistant bacteria, posing a significant threat to human and animal well-being. To ensure the safer consumption, monitoring NORx concentration in water and milk is mandated. To date, many analytical techniques such as electrochemical, HPLC (high performance liquid chromatography), immunoassay, capillary electrophoresis, immune chromatographic assay, fluorescent spectroscopy and colorimetric methods have been used for the detection of NORx in food samples such as milk and meat and water.5–8 However, time consumption, complex sample preparation, requirement of costly equipment and poor reproducibility in real samples hinder their practical applications. Hence, it is essential to develop a simple, cost-effective, sensitive and rapid method to detect NORx in environmental and food samples.This background encouraged us to design a nanosensor with suitable material, which itself will not pose environmental threat. In the last decade, fluorescence-based materials have garnered significant interest in sensing metal ions, antibiotics and toxic compounds due to their high sensitivity, ease of operation, and prompt response time. Semiconductor-based quantum dots possess potential application in fluorescence sensing because of their high quantum yield (QY) and electrical conductivity. However, its toxicity to environmental organisms and high photo-bleaching hampers its practical application.9–11 Recently, carbon quantum dots (CQDs) have garnered tremendous attention because of their notable properties like high biocompatibility, tunable size with strong luminescent property and high photostability. Due to the tunable fluorescent and electrical properties of quantum dots, they are used in photocatalysis, biosensing, CO2 reduction, energy conversion, drug delivery and as a fluorescent probe in bioimaging applications.12–15 However, enhancing the low quantum yield of CQDs is imperative for their effective utilization in applications such as bioimaging. Bioimaging application enables the comprehensive monitoring of nanomaterial uptake and biodistribution in the targeted site or organs. The fluorescence of CQDs is influenced by surface charge, functional group and size, and they can be specifically tailored for specific biological applications. The doping of foreign atom into CQDs structure alters its electronic property, thereby enhancing its QY. Previous reports showed that doping CQDs with elements like S, Cu, Zn, B and N and functionalizing its surface alter the electronic band gap between the energy levels and enhance the QY.16–18 Among all the dopants, nitrogen doping is the most intensively used method that enhances fluorescence emission by facilitating the upward transfer of the conduction band electron and influences the Fermi level.19
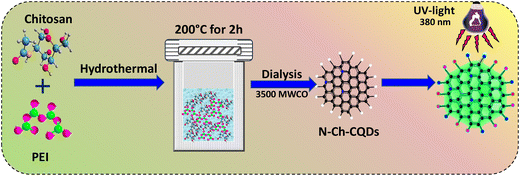
In this work, nitrogen-doped CQDs (N-Ch-CQDs) were prepared as the sensor for NORx via the hydrothermal method employing chitosan and PEI as the precursors. Further, the impact of varying PEI concentrations on the optical property of N-Ch-CQDs was investigated. The biological response of N-Ch-CQDs was validated via the in vitro model using L929 cells, and the prolonged in vivo effect was evaluated using C. elegans. The inherent fluorescence of N-Ch-CQDs was utilized to develop a rapid, selective, and highly sensitive fluorescence sensor for NORx and its mechanism was elucidated. The fabricated sensor was successfully employed to quantify NORx in water and milk samples. The proposed N-Ch-CQDs with high fluorescence and their potential application in the bioimaging and sensing of NORx paved a new research platform with future perspective in biomedical applications.
2. Experimental section
2.1. Chemicals
Chitosan, acetic acid, polyethyleneimine (PEI), sodium acetate, magnesium sulphate, ethidium bromide (EtBr), sodium hydroxide, agarose, sodium chloride, 2′,7′-dichlorofluorescein diacetate (H2 DCF-DA), and norfloxacin (NORx) were procured from Sigma Aldrich. Dimethylsulfoxide (DMSO), acetonitrile (ACN), dimethylformamide (DMF) and other chemicals employed in this study were of analytical grade and purchased from Hi-Media. Millipore water (18 MΩ cm−1) was used throughout the work.2.2. Characterization
The size and morphology of N-Ch-CQDs was unveiled by high-resolution transmission electron microscopy (JEOL JEM, 2100) at 200 kV operating voltage. The photoluminescence (PL) lifetime of the Ch-QDs was measured using an IBH time-correlated single-photon counting system (TCSPC). A Fourier transform infrared spectrometer (Bruker Tensor 27) was used to study the functional groups present in N-Ch-QDs. The PL excitation and emission of Ch-QDs were recorded using a Fluoromax-4 spectrometer (Horiba). The elemental composition and oxidation state were revealed by X-ray photoelectron spectroscopy (K-Alpha Surface Analysis, Thermo Scientific). The crystallographic structure of N-Ch-CQDs was elucidated by X-ray diffraction (XRD) analysis (Rigaku, Japan).2.3. Synthesis of chitosan quantum dots (N-Ch-QDs)
A single step, facile hydrothermal method was used to synthesize chitosan quantum dots (Ch-QDs). Briefly, 50 mL of acetic acid solution (4%) containing chitosan (1 mg mL−1) was mixed with 10 mL of PEI (5%, 10% and 15%) and stirred under N2 atmosphere for 20 min. Subsequently, the resultant mixture was shifted to a Teflon-lined autoclave and subjected to heating at 200 °C for 2 h. After cooling, the reaction mixture was centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min. The supernatant was filtered (0.22 μm) and subsequently dialyzed using a 3.5 kDa membrane. Finally, the quantum yield (Φ) was calculated, and the detailed procedure for quantum yield calculation is given in the ESI† (SI.1).
000 rpm for 20 min. The supernatant was filtered (0.22 μm) and subsequently dialyzed using a 3.5 kDa membrane. Finally, the quantum yield (Φ) was calculated, and the detailed procedure for quantum yield calculation is given in the ESI† (SI.1).
2.4. Cytocompatibility of Ch-QDs
Mouse fibroblast L929 cells were grown in DMEM supplement containing 10% FBS. The cytocompatibility of N-Ch-CQDs was evaluated via the MTT assay.20 Briefly, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well were seeded in a 96-well plate. After 24 h, the old media was removed and each well was supplemented with fresh DMEM containing various concentrations of N-Ch-CQDs (0–500 μg mL−1). After the co-incubation of N-Ch-CQDs with cells for 24 h, the viability was quantified using MTT dye (0.5 mg mL−1) and the absorbance was recorded at 570 nm.
000 cells per well were seeded in a 96-well plate. After 24 h, the old media was removed and each well was supplemented with fresh DMEM containing various concentrations of N-Ch-CQDs (0–500 μg mL−1). After the co-incubation of N-Ch-CQDs with cells for 24 h, the viability was quantified using MTT dye (0.5 mg mL−1) and the absorbance was recorded at 570 nm.
2.5. Maintenance of C. elegans
Prolonged assay systems of wild-type N2 worms were used for the in vivo evaluation of N-Ch-CQDs. Prolonged assay system involved exposing C. elegans to N-Ch-CQDs from L1 to L4 larval stages. According to the standard procedure, the worms were age synchronized before each experiment.21 Detailed protocols for lifespan evaluation, primary and secondary target organ analyses are given in the ESI† (SI.2–SI.4).2.6. Bioimaging, chemotaxis and food sensing behavior in C. elegans
Age-synchronized wild-type (N2) worms were grown in nematode growth medium (NGM) following established protocols.22 To assess the interaction with N-Ch-CQDs, worms at the L4 larval stage were gently washed and transferred to 1 mL of M9 buffer containing 100 μL of N-Ch-CQDs suspension (200 μg mL−1 final concentration), and E. coli OP50 was added as the food source. After 20 min, the worms were gently rinsed thrice using M9 buffer. Subsequently, individual worms were mounted on 1.5% agar padded glass slides for laser scanning confocal microscopy (LSCM) analysis. For food sensing and chemotaxis, the treated worms were analyzed according to the method described earlier.2.7. Fluorescence sensing of norfloxacin
For fluorescence sensing, NORx solution (50 μL) with varying concentration (0–1.6 mM) was mixed with 2 mL of PBS solution containing 200 μL of N-Ch-CQDs (0.25 mg mL−1). The solution was gently mixed at RT for 60 s and fluorescence measurement was recorded at 460 nm excitation. For selectivity analysis, different metal and antibiotic stock solutions (10 mM) were prepared. Then, 1 mM of different analyte solutions was added to N-Ch-CQDs and the fluorescence was quantified.2.8. Determination of NORx concentration in real water and milk sample
The application of this fluorescence sensor was assessed by determining the concentration of NORx in real water sample. For determining the concentration of NORx in real water, predetermined concentrations of NORx were added to the real water samples. The solution was mixed with N-Ch-CQDs and the fluorescence was recorded and quantified. For determining the concentration of NORx in the milk sample (collected from supermarket), it was pretreated as described earlier.23 Briefly, the milk was heated at 80 °C for 10 min and subsequently added with 500 μL of acetonitrile under vigorous stirring. The milk tends to curdle and the supernatant was collected, pH was adjusted to neutral and centrifuged for 5 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The supernatant was again filtered using a 0.22 μm filter. The final solution was spiked with NORx and their concentration was quantified as described above.
000 rpm. The supernatant was again filtered using a 0.22 μm filter. The final solution was spiked with NORx and their concentration was quantified as described above.
3. Results and discussion
Nitrogen-doped chitosan carbon quantum dots (N-Ch-CQDs) were prepared using a facile hydrothermal method. At 200 °C, the chitosan precursor undergoes fragmentation through the cleavage of its glycosidic bonds (C–O–C), resulting in the formation of smaller chitosan oligomers and monosaccharides. The inherent hydronium ions from water dissociation acts as the catalyst for the fragmentation of chitosan. Moreover, the addition of polyethyleneimine (PEI) led to its decomposition, releasing amine-containing monomers. These monomers then reacted with chitosan oligomers through dehydration and condensation, leading to the formation of aromatic clusters via aldol condensation. Upon reaching a critical point, the aromatic clusters undergo burst nucleation, resulting in the formation of nitrogen-doped Ch-CQDs. The schematic representation of N-Ch-CQDs by the hydrothermal method is given in Scheme 1.3.1. Optical and functional group characterization
The UV-Visible spectra of all the N-Ch-CQDs reveal two main absorbance peaks in the ultraviolet region (Fig. SI, ESI†). The dominant peak observed at about 224 nm is assigned to the π–π* transition of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C framework within the sp2 hybridized carbon core. The other weak absorbance peak at about 274–279 nm involves the n to π* transition of the C
C framework within the sp2 hybridized carbon core. The other weak absorbance peak at about 274–279 nm involves the n to π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–O group. Interestingly, this peak exhibits a progressive red-shift with increasing N doping concentration in the N-Ch-CQDs. This shift can be attributed to the presence of unshared electron pairs associated with N doping on the CQD surface. These electron pairs act as auxochromes, influencing the electronic transitions and leading to the observed shift in the absorbance. Our results agree with a previous report.1
N/C–O group. Interestingly, this peak exhibits a progressive red-shift with increasing N doping concentration in the N-Ch-CQDs. This shift can be attributed to the presence of unshared electron pairs associated with N doping on the CQD surface. These electron pairs act as auxochromes, influencing the electronic transitions and leading to the observed shift in the absorbance. Our results agree with a previous report.1
Three distinct N-Ch-CQDs were prepared by the hydrothermal method by varying the PEI concentration (5, 10 and 15%). FTIR analysis was harnessed to identify the functional groups present in N-Ch-CQDs. The FTIR spectra exhibited similar peak positions with varying peak intensity (Fig. 1a). The broad absorption peak at about 3333 and 2931 cm−1 corresponds to the N–H and C–H groups, respectively. Oxygen-containing functional groups are inferred from the peaks observed at 1155 and 1030 cm−1, representing the stretching vibration of the C–O and C–O–C groups, respectively. The intense absorbance at 1420 and 1640 cm−1 is attributed to the bending vibrations of C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and this indicates the functionalization of amino groups in the Ch-CQDs structure. Further, the intensity of the amino group peaks progressively increased with increasing PEI concentration (5% to 15%), suggesting the successful incorporation of nitrogen-containing amino groups onto the carbon framework of the N-Ch-CQDs (Fig. 1b–d). Notably, all N-Ch-CQDs prepared with varying PEI concentrations (5%, 10%, and 15%) demonstrated excitation-dependent emission behavior. The excitation and emission maxima showed progressive bathochromic shift from 400/495 nm, 420/520 nm and 460/535 nm for N-Ch-CQDs with 5, 10 and 15% of PEI, respectively. The observed red shift could be due to the incorporation of the N-containing group on the CQDs surface that influence their electronic transition responsible for fluorescence. Further, the quantum yield (QY) of N-Ch-CQDs was found to be 4.5, 9.6 and 21.5% for 5, 10 and 15% of PEI, respectively. The N-Ch-CQD with the highest quantum yield (15% PEI) was further characterized and used for bioimaging and sensing applications.
C, and this indicates the functionalization of amino groups in the Ch-CQDs structure. Further, the intensity of the amino group peaks progressively increased with increasing PEI concentration (5% to 15%), suggesting the successful incorporation of nitrogen-containing amino groups onto the carbon framework of the N-Ch-CQDs (Fig. 1b–d). Notably, all N-Ch-CQDs prepared with varying PEI concentrations (5%, 10%, and 15%) demonstrated excitation-dependent emission behavior. The excitation and emission maxima showed progressive bathochromic shift from 400/495 nm, 420/520 nm and 460/535 nm for N-Ch-CQDs with 5, 10 and 15% of PEI, respectively. The observed red shift could be due to the incorporation of the N-containing group on the CQDs surface that influence their electronic transition responsible for fluorescence. Further, the quantum yield (QY) of N-Ch-CQDs was found to be 4.5, 9.6 and 21.5% for 5, 10 and 15% of PEI, respectively. The N-Ch-CQD with the highest quantum yield (15% PEI) was further characterized and used for bioimaging and sensing applications.
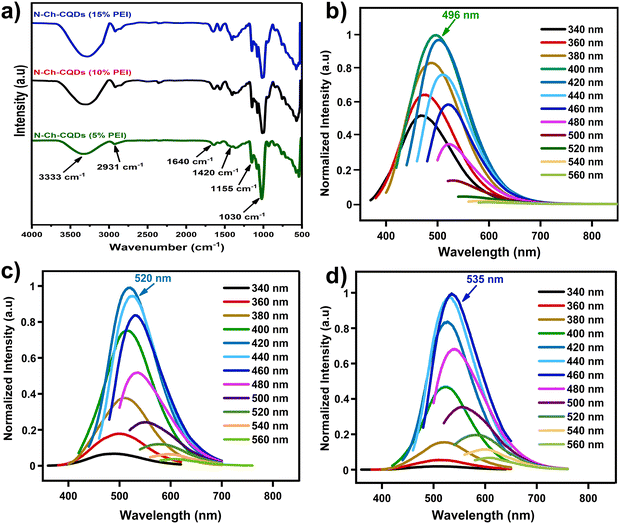 | ||
| Fig. 1 (a) FTIR spectra of N-Ch-CQDs and photoluminescent spectra of N-Ch-CQDs with (b) 5%, (c) 10% and (d) 15% of PEI. | ||
3.2. Structural, morphological and physiochemical characterization
HR-TEM analysis revealed uniformly-sized, quasi-spherical shaped N-Ch-CQDs, exhibiting an average diameter of 3 nm (Fig. 2a, b and Fig. S2, ESI†). The SAED pattern exhibited a diffused ring pattern, indicating the amorphous nature of N-Ch-CQDs (Fig. 2c). A full scan elemental analysis of N-Ch-CQDs was obtained by XPS spectroscopy. The XPS survey spectrum of N-Ch-CQDs confirmed the elemental composition with the peaks attributed to C 1s (284.5 eV), N 1s (401.2 eV) and O 1s (532.8 eV) (Fig. 2d). The deconvoluted C 1s spectra identified three peaks attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.7 eV), C–N/C–O (285.9 eV) and C
C (284.7 eV), C–N/C–O (285.9 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.07 eV) (Fig. 2e). The deconvoluted O 1s spectra displayed peaks corresponding to C
O (288.07 eV) (Fig. 2e). The deconvoluted O 1s spectra displayed peaks corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (531 eV) and C–OH (532.7 eV) (Fig. S3, ESI†). The deconvoluted N 1s spectra displayed peaks attributed to pyridinic N (399.9 eV), pyrrolic N (401.2 eV), and graphitic N (402.5 eV) (Fig. 2f). The N 1s deconvoluted spectra displayed peaks at 399.9 eV, 401.2 eV, and 402.5 eV, attributed to pyridinic N, pyrrolic N, and graphitic N, respectively (Fig. 2f). The presence of N peaks in the XPS corroborate with our FTIR data. The notable pyridinic N and pyrrolic N peaks suggest the incorporation of N at the edges and within the carbon structure, respectively. The percentage of N gradually increases from 3.64% to 8.13% and finally to 11.18% for the 5, 10 and 15% PEI of N-Ch-CQDs (Fig. S4 and Table S1, ESI†). This trend aligns with our FTIR spectrum, where the amine peak intensity also increases with a higher PEI content. Such a monotonous increase in the N content alters its electronic transition by inducing the defect in the graphitic structure and increases the fluorescence.
O (531 eV) and C–OH (532.7 eV) (Fig. S3, ESI†). The deconvoluted N 1s spectra displayed peaks attributed to pyridinic N (399.9 eV), pyrrolic N (401.2 eV), and graphitic N (402.5 eV) (Fig. 2f). The N 1s deconvoluted spectra displayed peaks at 399.9 eV, 401.2 eV, and 402.5 eV, attributed to pyridinic N, pyrrolic N, and graphitic N, respectively (Fig. 2f). The presence of N peaks in the XPS corroborate with our FTIR data. The notable pyridinic N and pyrrolic N peaks suggest the incorporation of N at the edges and within the carbon structure, respectively. The percentage of N gradually increases from 3.64% to 8.13% and finally to 11.18% for the 5, 10 and 15% PEI of N-Ch-CQDs (Fig. S4 and Table S1, ESI†). This trend aligns with our FTIR spectrum, where the amine peak intensity also increases with a higher PEI content. Such a monotonous increase in the N content alters its electronic transition by inducing the defect in the graphitic structure and increases the fluorescence.
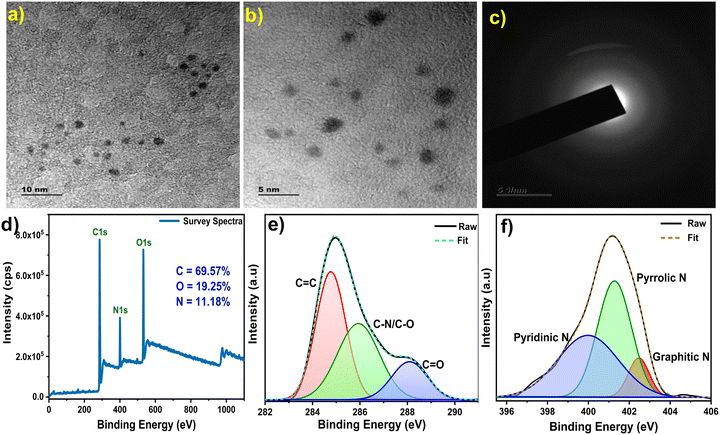 | ||
| Fig. 2 HR-TEM and XPS analysis of N-Ch-CQDs. (a) and (b) High-resolution TEM images, (c) SAED pattern, (d) full survey spectrum, deconvoluted (e) C 1s and (f) N 1s spectra. | ||
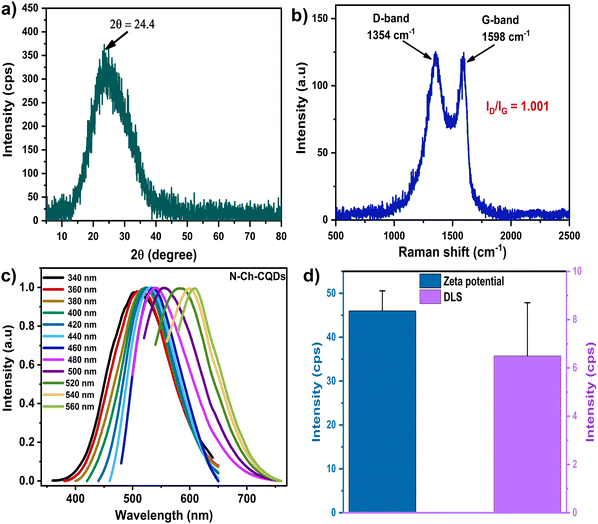
The XRD pattern of N-Ch-CQDs exhibited a broad diffraction peak centered at 2θ = 23.5 corresponding to the (002) plane, which indicates the graphitic nature (Fig. 3a). The broad peak also revealed the amorphous nature, which is consistent with our SAED pattern. Microstructure analysis by Raman spectroscopy revealed two notable peaks centered at 1354 and 1598 cm−1 (Fig. 3b). The former peak at 1354 cm−1 signifies the defect in the carbon structure, and the peak at 1598 cm−1 signifies the in-plane vibration of sp2 hybridized carbon. The calculated ID/IG value of 1.01 reflects a high degree of defect, which is potentially attributed to nitrogen incorporation into the Ch-CQDs framework, as corroborated by FTIR and XPS analyses. The normalized spectra in Fig. 3c clearly depicts the excitation-dependent emission phenomenon of N-Ch-CQDs. The observed excitation-dependent emission nature of N-Ch-CQDs may be attributed to the incorporation of nitrogen-containing groups in their structure, which could introduce structural defects, thereby influencing their electronic transitions responsible for fluorescence. This unique property offers significant advantages in bioimaging applications by enhancing the signal-to-noise ratio through selective excitation, enabling improved visualization.
Moreover, N-Ch-CQDs displayed good photostability with no significant change in the PL intensity even after 60 min irradiation of 365 nm light (Fig. S5a, ESI†). The fluorescence decay curve of N-Ch-CQDs revealed the average lifetime of 1.06 ns (Fig. S5b, ESI†). A positive charge zeta potential of 46 mV indicates the higher colloidal stability of N-Ch-CQDs (Fig. 3d). The positive charge could arise from the incorporation of amino group from PEI, as observed in the FTIR and XPS results. The DLS analysis revealed the average hydrodynamic size of 6.4 nm (Fig. 3d).
3.3. Cytocompatibility of N-Ch-CQDs
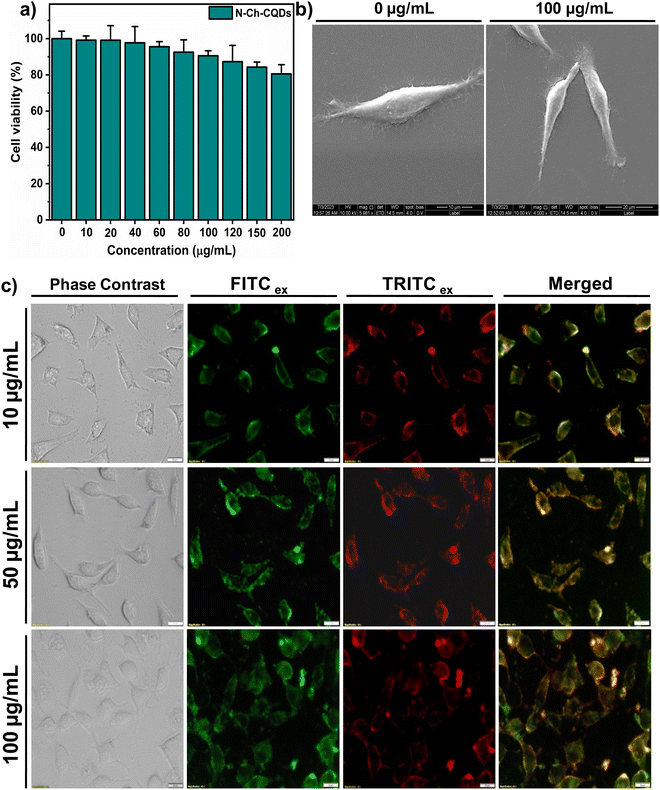
N-doped carbon quantum dots (N-Ch-CQDs) possess a unique combination of optical properties, including high photostability with ultrasmall size, making them highly versatile nanomaterials for biomedical applications. Most semiconductor-based quantum dots like CdSe, CdTe, InP, PbS, and PbSe exhibit high fluorescence and quantum yield. However, their inherent bio-incompatibility hinders their biological application. Hence, the cytocompatibility of N-Ch-CQDs was evaluated using the MTT assay on mouse fibroblast L929 cells. Following 24 h of co-incubation with N-Ch-CQDs, only negligible cytotoxicity was observed at a concentration of 200 μg mL−1 (Fig. 4a). FE-SEM analysis revealed the intact and smooth cellular morphology of the cells treated with 100 μg mL−1 of N-Ch-CQDs, suggesting no observable damage to the cell integrity (Fig. 4b). This result corroborates with the MTT analysis and highlights their potential and safe use in biological applications. Further, the bioimaging potential of N-Ch-CQDs was evaluated. After 3 h of co-incubation with L929 cells, the N-Ch-CQDs was readily observed in the cytoplasm via a fluorescent microscope due to the inherent photoluminescent property of the material (Fig. 4c). Fluorescent microscopy analysis revealed that N-Ch-CQDs are primarily localized in the cell membrane at a lower concentration (10 μg mL−1), which suggests the possible interaction of positively charged N-Ch-CQDs and the cell membrane components. However, at higher concentrations of 50 and 100 μg mL−1, N-Ch-CQDs exhibit internalization into the cytoplasm of the cells, indicating a potential concentration-dependent uptake mechanism. Previous studies showed that the positively charged CQDs exhibit enhanced cell interaction and internalization than negatively charged CQDs, potentially due to the initial electrostatic interaction, followed by endocytosis.24 Moreover, the ultrasmall size and good colloidal stability of N-Ch-CQDs also contribute to their higher cellular uptake. The in vitro analysis in L929 cells revealed the higher cytocompatibility and bioimaging potential of N-Ch-CQDs.3.4. Biocompatibility and bioimaging analysis of N-Ch-CQDs in wild-type nematodes
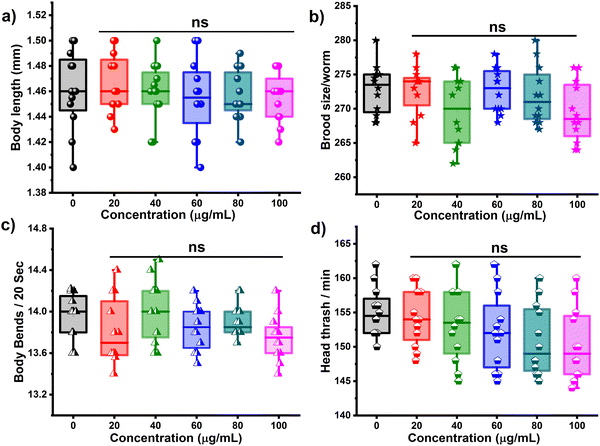
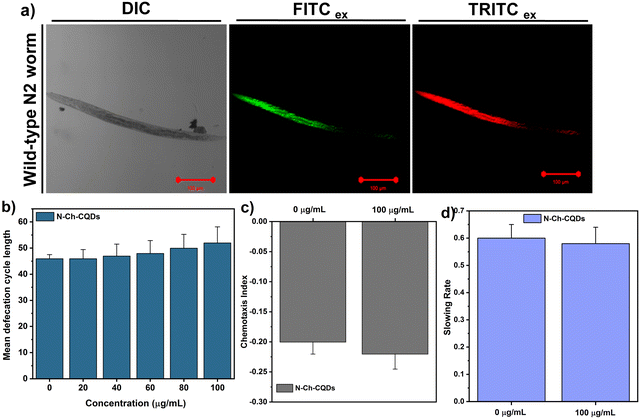
C. elagans, a simple transparent worm with well-defined cell lineage and high progeny rate, has emerged as a powerful in vivo model organism in biological research due to its genetic similarities with humans (60–80%). The transparent anatomy of C. elegans facilitates live imaging and the easy maintenance in the lab makes it a cost-effective option for diverse applications. Apart from its contribution in neurobiology, developmental biology and aging research, at present, C. elegans is used in assessing environmental pollutants. Previous investigation with nanoparticles like graphene, graphene quantum dots, carbon nanotubes, silica and iron oxide nanoparticles in C. elegans has offered a better understanding and valuable insight into the biocompatibility and toxicity mechanism.22,25–27 Herein, the in vivo safety of N-Ch-CQDs was assessed in wild-type N2 nematodes via the survival graph, initially. The survival of worms was monitored at varying concentrations of N-Ch-CQDs (0–1000 μg mL−1) and no obvious change was observed in the survival of worms treated with 0–200 μg mL−1 (Fig. S6, ESI†), whereas treatment with 0.5 and 1 mg mL−1 of N-Ch-CQDs displayed a significant drop in the survival percentage and was found to be toxic at higher concentration. The survival curve further corroborated these findings. Treatment with 25 and 50 μg mL−1 showed no significant reduction in the mean lifespan (p = 0.7257 and p = 0.7075, respectively) and even displayed a slight increase in the mean lifespan with 1.02 and 0.93%, respectively (Fig. S7 and Table S2, ESI†). Likewise, concentration in the range of 75–200 μg mL−1 showed no obvious decrease in the lifespan. However, consistent with the survival analysis, concentration of 500 (p = 0.0001) and 1000 μg mL−1 (p = 0.0001) significantly reduced the lifespan by −19.5 and 21.0%, respectively. These results suggest that N-Ch-CQDs (0–200 μg mL−1) are relatively safe to nematodes at lower concentrations.Further, the effect of N-Ch-CQDs on the essential physiological functions of nematodes was investigated using the prolonged exposure system (L1–L4 larvae). The result showed no significant change in the body length and egg laying capacity of worms treated with N-Ch-CQDs (0–100 μg mL−1) (Fig. 5a and b). The normal brooding behavior of nematodes depicts the proper functioning of secondary-targeted organs even at 100 μg mL−1 of N-Ch-CQDs. Further, the locomotion behavior of nematodes was assessed using head thrash and body bends of the nematodes. Similarly, the result showed no significant changes in the locomotion behavior of nematodes (Fig. 5c and d). In addition, the pharyngeal pumping behavior of 100 μg mL−1-treated nematodes also showed no obvious change compared to the control (Fig. S8, ESI†). The proper pharyngeal pumping rate of nematodes depicts the motor behavior, which indicated the normal functioning of the nervous system. All these results collectively suggest that N-Ch-CQDs do not induce any significant change in the primary and secondary organs of nematodes. The inherent fluorescence property of N-Ch-CQDs was utilized to observe its translocation pattern within the nematode body. The translocation of nanomaterials into different organs can significantly impact their toxicity.28 Following co-incubation with 100 μg mL−1 of N-Ch-CQDs for 6 h, confocal microscopy analysis revealed prominent localization within the gut and intestine of worms (Fig. 6). Very low fluorescence signal was observed in the vulva region, which depicts the effective functioning of the intestinal barrier. Additionally, the proper functioning of defecation behavior in nematodes treated with N-Ch-CQDs also contributes to their mitigation and preventing their accumulation in the reproductive organs. The chemotaxis and food searching behavior analysis showed no obvious change in the nematodes exposed to N-Ch-CQDs (Fig. 6c and d). All these results indicate the biocompatible nature of N-Ch-CQDs in the in vivo model C. elegans. Moreover, the inherent fluorescent property and good photostability of N-Ch-CQDs indicates its potential application as a bioimaging agent.
3.5. Fluorescence sensing of norfloxacin (NORx) using N-Ch-CQDs
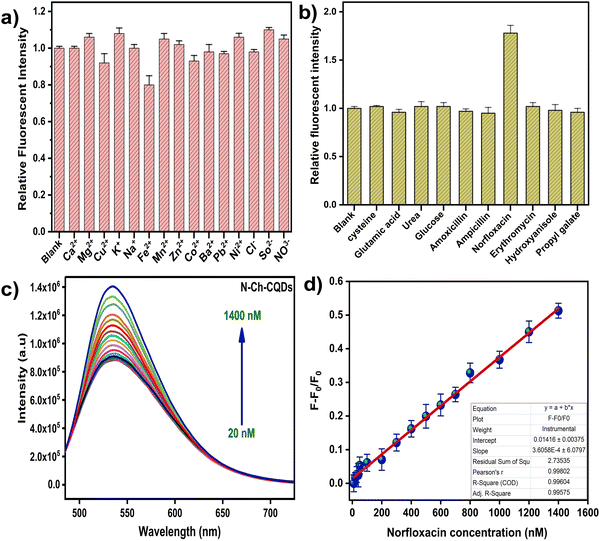
The selectivity of N-Ch-CQDs for NORx sensing was initially evaluated in the presence of various metals and antibiotics (Ca2+, Mg2+, Cu2+, K+, Na+, Fe2+, Mn2+, cysteine, glutamic acid, urea, glucose, tryptophan, histidine, amoxicillin, ampicillin, norfloxacin, erythromycin, methionine, ciprofloxacin, 3,4-hydroxyanisole and propyl gallate) at a concentration of 1 mM (Fig. 7a and b). The presence of norfloxacin led to a notable increase in the fluorescence intensity of N-Ch-CQDs, whereas the addition of other interfering agents did not elicit a significant change in the fluorescence intensity. This observation highlights the high selectivity and sensitivity of N-Ch-CQDs towards norfloxacin. The selectivity of norfloxacin (NORx) is due to the establishment of hydrogen bonding between the carboxyl and amino groups of N-Ch-CQDs and the piperazine ring of NORx, respectively.29 By adding different concentrations of NORx to N-Ch-CQDs at the optimized condition, the linear response range and the limit of detection (LoD) was measured. As the concentration of NORx increases, a gradual rise in the fluorescent intensity of N-Ch-CQDs was observed, which reached a saturation point (Fig. 7c). With increasing concentration of NORx, the fluorescence intensity of N-Ch-CQDs gradually increased and reached a saturation point. Good linear relationship exists between the NORx concentration and fluorescence intensity of N-Ch-CQDs in the linear range of 20–1400 nM (R2 = 0.9963) with an LoD of 9.3 nM and a strong indication of the potential of N-Ch-CQDs to detect NORx with high sensitivity (Fig. 7d).3.6. Plausible norfloxacin sensing mechanism by N-Ch-CQDs
The observed high selectivity of NORx by N-Ch-CQDs may possibly be attributed to its unique structure. The NORx contain fluorinated 4-quinolone ring with carboxyl group, F atom and piperazine ring at the position of 3, 7 and 8, respectively. The presence of carboxyl and hydroxyl groups in N-Ch-CQDs preferentially forms a strong hydrogen bond with the fluorine/amine groups of the piperazine ring in NORx and leads to fluorescence enhancement.30 Moreover, good electrostatic attraction between positively charged N-Ch-CQDs with negatively charged NORx could potentially vary the surface defect of N-Ch-CQDs and result in fluorescence enhancement. The FTIR results further validated the formation of hydrogen bonding in fluorescence enhancement. After the addition of NORx, the C-O and C–O–C groups in N-Ch-CQDs at 1155 and 1030 cm−1 was red shifted to 1174 and 1052 cm−1, respectively (Fig. S9, ESI†). In order to confirm that the fluorescent enhancement is due to hydrogen bonding formation, the fluorescence intensity of N-Ch-CQDs with NORx was studied in 3 different aprotic solvents (DMF, DMSO and ACN). As the concentration of aprotic solvent increased, the hydrogen bonding ability with N-Ch-CQDs and NORx decreased gradually (Fig. S10a–c, ESI†). These results confirm the role of hydrogen bonding in the fluorescence enhancement of N-Ch-CQDs/NORx. When compared with the existing report, the N-Ch-CQDs showed higher sensitivity with low LoD (Table 1).| S. no. | Sensing system | Linear range (μg mL−1) | LoD (nM) | Ref. |
|---|---|---|---|---|
| NAC-Cu@AuNCs: Au-doped copper nanocluster, SQDs: sulphur quantum dots, Y3+@CdTe QDs: Yttrium-doped cadmium telluride quantum dots. | ||||
| 1 | Carbon dots/metal–organic framework | 1–8 | 82 | 31 |
| 2 | NAC-Cu@AuNCs | 0.02–16 | 38.3 | 32 |
| 3 | Carbon dot | 0.038–100 | 38 | 33 |
| 4 | SQDs | 0–90 | 20 | 34 |
| 5 | Y3+@CdTe QDs | 1–150 | 31.8 | 35 |
| 6 | TGA-coated CdTe QDs | 0.1–100 | 31 | 36 |
| 7 | N-Ch-CQDs | 0.02–1.4 | 9.3 | This work |
3.7. Analytical validation of N-Ch-CQDs for NORx sensing
The practical feasibility of N-Ch-CQDs for detecting NORx was examined by performing the analytical tests in real water (tap water from SCTIMST) and milk sample (purchased from supermarket) by the standard addition method. The concentration of NORx in real water and milk samples was spiked between 0.25 and 1 μM and mixed with N-Ch-CQDs, the resulting fluorescence intensity was recorded and quantified (Table 2). As a result, the recovery rate of NORx in real water and milk samples was in the range of 98.51–103.58%. These results suggest that the N-Ch-CQDs have practical capability for NORx determination in real samples.| Sample | Spiked (nM) | Found (nM) | Recovery (%) (mean ± RSD) (n = 3) |
|---|---|---|---|
| Tap water | 250 | 255.35 | 102.1 ± 0.015 |
| Tap water | 500 | 506.86 | 101.37 ± 0.017 |
| Tap water | 750 | 745.3 | 99.37 ± 0.011 |
| Tap water | 1000 | 1015.89 | 101.58 ± 0.008 |
| Milk sample | 250 | 258.75 | 103.58 ± 0.052 |
| Milk sample | 500 | 492.54 | 98.51 ± 0.895 |
| Milk sample | 750 | 744.63 | 99.284 ± 0.527 |
| Milk sample | 1000 | 987.04 | 98.704 ± 0.284 |
4. Conclusion
In summary, employing chitosan and PEI as starting materials, excitation-dependent N-Ch-CQDs were prepared through a simple hydrothermal method. Increasing the PEI concentration (5 to 15%) enhanced the QY (4.5 to 21.5%) and exhibited a shift in the excitation and emission maximum of the N-Ch-CQDs towards higher wavelength. The average diameter of N-Ch-CQDs (15%) was about 3 nm. MTT and FESEM analysis confirmed the excellent cytocompatibility of N-Ch-CQDs in L929 cells. The in vivo assessment of N-Ch-CQDs with the prolonged assay system of C. elegans revealed good biocompatibility with no significant effect to the primary and secondary targeted organs. The alleviated signal of N-Ch-CQDs to the reproductive organ of N2-worms is attributed to the intact functioning of intestinal barriers and the defecation behavior. N-Ch-CQDs as a fluorescent probe displayed remarkable sensing ability towards NORx with a wide linearity in the range of 20–1400 nM. It also displayed high sensitivity and selectivity towards NORx with an LoD of 9.3 nm. Moreover, N-Ch-CQDs displayed superior capability for detecting NORx in real samples like water and milk. These results displayed the bioimaging and fluorescence sensing ability of N-Ch-CQDs, which holds significant promise for extensive application in the biomedical field.Author contributions
SS: experiments, analysis of data and writing the original draft. RSA, MV and DNS: review & editing the original draft. RSJ: conceptualization, funding acquisition, resources, review & editing, supervision.Data availability
The raw data of the manuscript entitled “Nitrogen-Doped Carbon Dots: A Novel Biosensing Platform for Selective Norfloxacin Detection and Bioimaging” authored by S. Sivaselvam, R. S. Anjana, Dhujana N. S., Marina Victor and R. S. Jayasree are available on request from the authors.Conflicts of interest
The authors declare no conflicting interests.Acknowledgements
RSJ acknowledges the financial support received from Life Science Research Board, Defence Research and Development Organisation, Government of India, under the grant no. LSRB/01/15001/LSRB-412/SH&DDI2023. The author S. S. acknowledges the Department of Science and Technology (DST), Science and Engineering Research Board (SERB) and the Government of India for the financial support of the N-PDF fellowship (PDF/2022/002036). R. S. A. acknowledges Department of Science and Technology, Government of India, for the INSPIRE fellowship (DST/INSPIRE/03/2019/003028). The author DNS acknowledges Department of Biotechnology, Government of India, for the DBT-JRF fellowship (DBT/2022-23/SCTMIST/2102).References
- S. Sivaselvam, C. Viswanathan and N. Ponpandian, Biomater. Adv., 2022, 135, 212731 CrossRef CAS.
- M. Aboubakr, M. Elbadawy, A. Soliman and M. El-Hewaity, Adv. Pharmacol. Sci., 2014, 1, 924706 Search PubMed.
- A. Rusu, A. C. Munteanu, E. M. Arbănaşi and V. Uivarosi, Pharmaceutics, 2023, 15, 804 CrossRef CAS.
- R. Moreau, L. Elkrief, C. Bureau, J. M. Perarnau, T. Thévenot, F. Saliba, A. Louvet, P. Nahon, A. Lannes, R. Anty, S. Hillaire, B. Pasquet, V. Ozenne, M. Rudler, I. Ollivier-Hourmand, M. A. Robic, L. d’Alteroche, V. Di Martino, M. P. Ripault, A. Pauwels, J. D. Grangé, N. Carbonell, J. P. Bronowicki, A. Payancé, P. E. Rautou, D. Valla, N. Gault and D. Lebrec, Gastroenterology, 2018, 155, 1816–1827 CrossRef CAS.
- L. Chierentin and H. R. N. Salgado, Crit. Rev. Anal. Chem., 2016, 46, 22–39 CrossRef CAS.
- Z. Han, C. Xia, B. Ning, Z. Xu, X. Liu, H. Zuo, L. Cai, T. Sun and Y. Liu, Microchem. J., 2022, 179, 107660 CrossRef CAS.
- N. Yang, Q. L. Wen, Y. B. Fu, L. F. Long, Y. J. Liao, S. B. Hou, P. Qian, P. Liu, J. Ling and Q. Cao, Spectrochim. Acta, Part A, 2022, 281, 121568 CrossRef CAS PubMed.
- S. Hu, J. Liu, G. Zhang, Z. Wang, X. Xiao, D. Li, J. Peng and W. Lai, Talanta, 2020, 219, 121245 CrossRef CAS PubMed.
- S. Filali, F. Pirot and P. Miossec, Trends Biotechnol., 2020, 38, 163–177 CrossRef CAS PubMed.
- N. J. Orfield, S. Majumder, J. R. McBride, F. Yik-Ching Koh, A. Singh, S. J. Bouquin, J. L. Casson, A. D. Johnson, L. Sun, X. Li, C. K. Shih, S. J. Rosenthal, J. A. Hollingsworth and H. Htoon, ACS Nano, 2018, 12, 4206–4217 CrossRef CAS.
- L. V. Nair, R. V. Nair and R. S. Jayasree, J. Polym. Sci. Eng., 2024, 6, 3208 CrossRef.
- R. V. Nair, P. Radhakrishna Pillai Suma and R. S. Jayasree, Mater. Sci. Eng. C, 2020, 109, 110569 CrossRef CAS PubMed.
- N. Nimi, A. Saraswathy, S. S. Nazeer, N. Francis, S. J. Shenoy and R. S. Jayasree, Biomaterials, 2018, 171, 46–56 CrossRef CAS PubMed.
- L. V. Nair, D. S. Philips, R. S. Jayasree and A. Ajayaghosh, Small, 2013, 9, 2673–2677 CrossRef CAS PubMed.
- H. Santhakumar, R. V. Nair, D. S. Philips, S. J. Shenoy, A. Thekkuveettil, A. Ajayaghosh and R. S. Jayasree, Sci. Rep., 2018, 8(1), 9069 CrossRef.
- H. He, Y. Yang, J. Li, X. Lai, X. Chen, L. Wang, W. Zhang, Y. Huang and P. Zhang, Mater. Sci. Eng. B, 2021, 264, 114955 CrossRef CAS.
- S. Liao, X. Zhao, F. Zhu, M. Chen, Z. Wu, X. Song, H. Yang and X. Chen, Talanta, 2018, 180, 300–308 CrossRef CAS.
- C. Ya Wang, S. Shang, X. Zheng, P. Lei, J. Han, L. Yuan, Z. Li, R. Wang, W. Gong, J. Tang and Y. Yang, J. Braz. Chem. Soc., 2019, 30, 988–996 Search PubMed.
- Y. Sun, M. Zhang, B. Bhandari and C. Yang, Food Rev. Int., 2022, 38(7), 1513–1532 CrossRef CAS.
- A. J. Anjusha, S. Thirunavukkarasu, A. N. Resmi, R. S. Jayasree, S. Dhanapandian and N. Krishnakumar, Inorg. Chem. Commun., 2023, 149, 110428 CrossRef CAS.
- S. Sivaselvam, A. Mohankumar, R. Narmadha, R. Selvakumar, P. Sundararaj, C. Viswanathan and N. Ponpandian, Environ. Pollut., 2023, 318, 120933 CrossRef CAS PubMed.
- S. Sivaselvam, A. Mohankumar, G. Thiruppathi, P. Sundararaj, C. Viswanathan and N. Ponpandian, Nanoscale Adv., 2020, 2, 5219–5230 RSC.
- Q. Pu, L. Zhang, N. Ye and Y. Xiang, Dyes Pigm., 2024, 222, 111815 CrossRef CAS.
- L. N. Ramana, L. N. M. Dinh and V. Agarwal, Nanoscale Adv., 2021, 3, 3513–3521 RSC.
- C. Acosta, J. M. Barat, R. Martínez-Máñez, F. Sancenón, S. Llopis, N. González, S. Genovés, D. Ramón and P. Martorell, Environ. Res., 2018, 166, 61–70 CrossRef CAS PubMed.
- L. Gonzalez-Moragas, S. M. Yu, E. Carenza, A. Laromaine and A. Roig, ACS Biomater. Sci. Eng., 2015, 1, 1129–1138 CrossRef CAS.
- A. Nouara, Q. Wu, Y. Li, M. Tang, H. Wang, Y. Zhao and D. Wang, Nanoscale, 2013, 5, 6088–6096 RSC.
- L. Gonzalez-Moragas, A. Roig and A. Laromaine, Adv. Colloid Interface Sci., 2015, 219, 10–26 CrossRef CAS PubMed.
- A. Kundu, B. Maity and S. Basu, ACS Biomater. Sci. Eng., 2022, 8, 4764–4776 CrossRef CAS.
- W. Lu, Y. Jiao, Y. Gao, J. Qiao, M. Mozneb, S. Shuang, C. Dong and C. Z. Li, ACS Appl. Mater. Interfaces, 2018, 10, 42915–42924 CrossRef CAS.
- S. Wu, C. Chen, J. Chen, W. Li, M. Sun, J. Zhuang, J. Lin, Y. Liu, H. Xu, M. Zheng, X. Zhang, B. Lei and H. Zhang, J. Mater. Chem. C, 2022, 10, 15508–15515 RSC.
- J. Shen, X. Wen and Z. Fan, Sens Actuators, B, 2023, 381, 133436 CrossRef CAS.
- M. Yang, H. Li, J. Liu, W. Kong, S. Zhao, C. Li, H. Huang, Y. Liu and Z. Kang, J. Mater. Chem. B, 2014, 2, 7964–7970 RSC.
- S. Wang, X. Bao, B. Gao and M. Li, Dalton Trans., 2019, 48, 8288–8296 RSC.
- X. Chen, Y. Jiang, Y. Liu and C. Yao, Analyst, 2023, 148, 3776–3784 RSC.
- X. Wei, Z. Zhou, T. Hao, H. Li, J. Dai, L. Gao, X. Zheng, J. Wang and Y. Yan, J. Lumin., 2015, 161, 47–53 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01006j |
| This journal is © The Royal Society of Chemistry 2024 |