Fine tuning the steric hindrance of the Eu(II) tris(pyrazolyl)borate complex for a blue organic light-emitting diode†
Wenchao
Yan‡
a,
Yuqin
Li‡
bc,
Peihao
Huo‡
a,
Ruoyao
Guo
a,
Gang
Yu
 a,
Zifeng
Zhao
a,
Zifeng
Zhao
 a,
Kezhi
Wang
a,
Kezhi
Wang
 b,
Zuqiang
Bian
b,
Zuqiang
Bian
 a and
Zhiwei
Liu
a and
Zhiwei
Liu
 *a
*a
aBeijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: zwliu@pku.edu.cn
bBeijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, Beijing Normal University, Beijing 100875, China
cSchool of Chemical Engineering and Biotechnology, Xingtai University, Xingtai 054001, China
First published on 5th June 2024
Abstract
Eu(II) complexes with characteristic d–f transitions demonstrate their potential applications in organic light-emitting diodes (OLEDs) due to their advantages including short excited-state lifetimes, 100% theoretical exciton utilization efficiency and tunable emission colors. Thermally stable and efficient Eu(II) complexes are necessary for blue OLEDs, which are still the bottleneck as compared to red and green OLEDs. A blue-emitting Eu(II) complex can be obtained by appropriate ligand design because the 5d energy levels are sensitive to the external environments. Herein, a series of Eu(II) complexes based on tris(pyrazolyl)borate ligands were designed and synthesized. By carefully adjusting the steric hindrance, a thermally stable sky-blue emission Eu(II) complex bis[hydrotris(3-tert-butylpyrazolyl)borate]europium(II) (Eu–tBu) was obtained by employing the bulky tert-butyl group. The sky-blue OLED based on Eu–tBu was fabricated, showing a maximum external quantum efficiency of 15.7%, a maximum luminance of 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 cd m−2, and Commission Internationale del Eclairage coordinates of (0.13, 0.27) at 1000 cd m−2.
240 cd m−2, and Commission Internationale del Eclairage coordinates of (0.13, 0.27) at 1000 cd m−2.
1. Introduction
Organic light-emitting diodes (OLEDs) have developed rapidly over the past few years. A series of materials, such as fluorescence,1 phosphorescence,2 thermally activated delayed fluorescence3 and organic radical4,5 materials, have been investigated as emitters to construct OLEDs with high efficiency. Nevertheless, the blue OLEDs are still the bottleneck as compared to red and green OLEDs.6,7 As a new type of emitter in OLEDs, d–f transition complexes, such as Ce(III)8,9 and Eu(II)10,11 complexes, have demonstrated advantages including short excited-state lifetimes, 100% theoretical exciton utilization efficiency and tunable emission colors. Compared with noble metal phosphorescence materials, Ce(III) and Eu(II) complexes exhibit nanosecond excited-state lifetimes, which is beneficial to OLEDs. Meanwhile, the cost of Ce(III) and Eu(II) complexes may be lower than that of noble metal complexes because of the richer abundance of Ce and Eu on Earth.8 Moreover, the Eu(II) complex is usually characterized by a single emission peak, which can realize high chromatic purity. A variety of thermally stable Ce(III) complexes have been reported to fabricate vacuum thermal deposited blue OLEDs with high efficiency,8,9,12 and a maximum external quantum efficiency (EQE) of 20.8% was achieved in sky-blue OLED based on the Ce(III) complex.9 However, the OLEDs based on Eu(II) complexes are mainly orange11,13,14 and green emitting.10 Blue-emitting Eu(II) complexes were obtained by using crown ethers,15 cryptands,16–18 tris(2-aminoethyl) amine and triethanolamine19 ligands. However, these Eu(II) complexes are mainly ionic compounds. The poor thermal stability of these Eu(II) complexes restricts their application in vacuum thermal deposited OLEDs. The best blue OLED based on the Eu(II) complex was fabricated by spin-coating, showing a maximum EQE of 9.2% and a maximum luminance of 1780 cd m−2,19 which is inferior to vacuum thermal deposited OLEDs. Therefore, thermally stable blue emission Eu(II) complexes are necessary to construct d–f transition blue OLEDs.Tris(pyrazolyl)borate ligands were widely used to coordinate with lanthanide ions,20–24 and thermally stable neutral Eu(II) complexes can be obtained by coordinating with two tris(pyrazolyl)borate ligands. Nevertheless, the emission colors of Eu(II) complexes based on tris(pyrazolyl)borate ligands are mainly located in the region of red to green.11,13,25–27 In our previous work,11 the orange-emitting Eu(II) complex Eu(TpCH3)2 showed promising performance in OLEDs with a maximum EQE of 6.5% and a maximum luminance of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 cd m−2. The introduction of the trifluoromethyl group results in a weaker ligand field, and therefore, Eu(TpCF3)2 exhibits a strong hypsochromic shift with a maximum emission wavelength (λm) of 480 nm in a solid; however the OLED using Eu(TpCF3)2 as an emitter exhibits unsatisfactory performance with a λm of 510 nm and a maximum EQE of 0.75%.
620 cd m−2. The introduction of the trifluoromethyl group results in a weaker ligand field, and therefore, Eu(TpCF3)2 exhibits a strong hypsochromic shift with a maximum emission wavelength (λm) of 480 nm in a solid; however the OLED using Eu(TpCF3)2 as an emitter exhibits unsatisfactory performance with a λm of 510 nm and a maximum EQE of 0.75%.
To construct a blue emission Eu(II) complex, a weak ligand field is necessary to obtain a weak splitting of 5d levels, since the energy of 4f levels is hardly influenced by the ligands; therefore the energy of 5d–4f transition increases in a weak ligand field, and the emission band blue shifts. The ligand field generally relates to the average coordination bond length and coordination number. The larger steric hindrance usually corresponds to a longer coordination bond length and lower coordination number, and finally results in a weaker ligand field. However, the large steric hindrance may also lead to a weak coordination between the ligand and central metal ion, resulting in poor thermal stability. Hence it is necessary to fine tune the steric hindrance of the tris(pyrazolyl)borate ligand to simultaneously achieve blue emission and good thermal stability. Herein, a series of Eu(II) complexes based on tris(pyrazolyl)borate ligands were designed and synthesized. With the increase of steric hindrance, the corresponding Eu(II) complexes exhibit tunable emission colors changing from orange-red to sky-blue. A thermally stable sky-blue emission Eu(II) complex bis[hydrotris(3-tert-butylpyrazolyl)borate]europium(II) (Eu–tBu) was obtained by employing the bulky tert-butyl group. The sky-blue OLED device using Eu–tBu as an emitter was fabricated, showing a maximum external quantum efficiency of 15.7%, a maximum luminance of 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 cd m−2, and Commission Internationale de l’Eclairage coordinates of (0.13, 0.27) at 1000 cd m−2.
240 cd m−2, and Commission Internationale de l’Eclairage coordinates of (0.13, 0.27) at 1000 cd m−2.
2. Results and discussion
2.1. Synthesis and structures
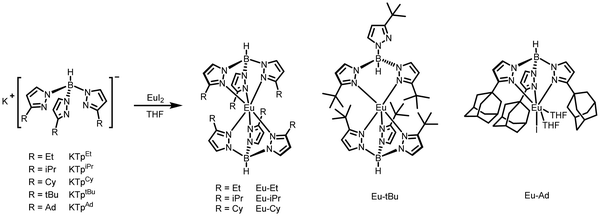
The different substituent groups, ethyl (Et), isopropyl (iPr), cyclohexyl (Cy), tert-butyl (tBu) and adamantyl (Ad), were introduced into the 3-position of pyrazolyl to systematically regulate the steric hindrance of tris(pyrazolyl)borate ligands and further adjust the coordination environments of Eu2+ ions. All the potassium salts of ligands, potassium hydrotris(3-ethylpyrazolyl)borate (KTpEt), potassium hydrotris(3-isopropylpyrazolyl)borate (KTpiPr), potassium hydrotris(3-cyclohexylpyrazolyl)borate (KTpCy), potassium hydrotris(3-tert-butylpyrazolyl)borate (KTptBu) and potassium hydrotris(3-adamantylpyrazolyl)borate (KTpAd) were synthesized by melting pyrazole with KBH4, and were further purified by sublimation or recrystallization as shown in the ESI.† The Eu(II) complexes, Eu(TpEt)2 (Eu–Et), Eu(TpiPr)2 (Eu–iPr), Eu(TpCy)2 (Eu–Cy), Eu(TptBu)2 (Eu–tBu) and Eu(TpAd)I(THF)2 (THF = tetrahydrofuran, Eu–Ad), were synthesized by mixing EuI2 and corresponding ligands in THF in a glovebox (Scheme 1), and the purified products were identified by elemental analysis. When the substituent groups are Et, iPr, Cy and tBu, two tris(pyrazolyl)borate ligands coordinate with central Eu2+ ion simultaneously. While the larger Ad group results in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 coordination pattern between Eu2+ and TpAd ligand, one I− ion and two THF molecules coordinate with Eu2+ as speculated by elemental analysis.
1 coordination pattern between Eu2+ and TpAd ligand, one I− ion and two THF molecules coordinate with Eu2+ as speculated by elemental analysis.
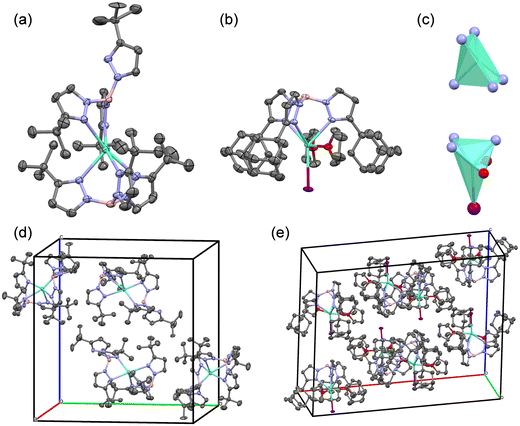
The single crystal X-ray diffraction was performed to investigate the coordinate geometry of these Eu(II) complexes. The crystals of Eu–tBu and Eu–Ad were obtained by slow evaporation of n-hexane/dichloromethane solution and n-hexane/THF solution, respectively. The crystal structures of Eu–tBu and Eu–Ad are shown in Fig. 1, and the relevant data are shown in Tables S1 and S2 (ESI†). Eu–tBu crystallizes in the space group P21/c and one unit contains four Eu–tBu molecules. In the crystal structure of Eu–tBu, two TptBu ligands coordinate with the central Eu2+ ion simultaneously. However, the coordination number of central Eu2+ ion is five as a quadrangular pyramid geometry, and one of the TptBu ligands is bidentate due to the increased steric hindrance. Eu–Ad crystallizes in the space group I2/a and one unit contains eight Eu–Ad molecules. The central Eu2+ ion is coordinated by one bulky TpAd ligand, one I− ion, and two THF molecules as a distorted octahedron geometry. The crystal of Eu–Et was also obtained by evaporation of its n-hexane/dichloromethane solution; however no exact data of Eu–Et were acquired due to the existence of twin crystals. Only a coordination model of Eu–Et was obtained and is shown in Fig. S1 (ESI†). The central Eu2+ ion is coordinated by six nitrogens of TpEt as an anti-triangular prism geometry, which is similar to that of Eu(TpCH3)2.11 We did not obtain the crystals of Eu–iPr and Eu–Cy because the introduction of flexible alkyl groups leads to the difficulty in crystallization. The increased steric hindrance of tris(pyrazolyl)borate ligands leads to the coordination number of central Eu2+ changing from six to five, and finally results in only one tris(pyrazolyl)borate ligand coordinating with Eu2+.
2.2. Photophysical properties
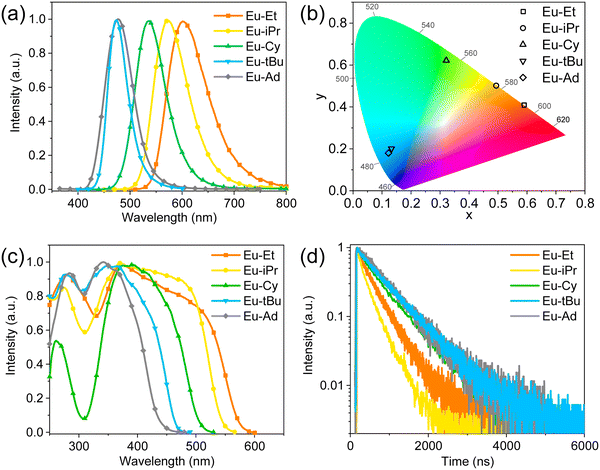
The emission spectra of these Eu(II) complexes in solid powder were recorded and are shown in Fig. 2a. The increased steric hindrance of tris(pyrazolyl)borate ligands results in the strong hypsochromic shift. The λm of Eu–Et, Eu–iPr, Eu–Cy, Eu–tBu and Eu–Ad in solid powder are 601, 572, 537, 474 and 476 nm, respectively, corresponding to emission colors change from orange-red to sky-blue (Fig. 2b). These broad emission bands are attributed to the d–f transitions of Eu2+ ions. The full widths at half maximums (FWHMs) for Eu–Et, Eu–iPr, Eu–Cy and Eu–tBu are 89, 77, 68 and 45 nm, respectively (Table 1). The gradual decrease of FWHMs is attributed to the increase of rigidity caused by the increased steric hindrance while Eu–Ad exhibits a slight broad FWHM of 62 nm because the coordination of THF molecules reduces the rigidity around Eu2+. The excitation spectra of these Eu(II) complexes are broad and featureless as shown in Fig. 2c, and two excitation bands can be observed in their excitation spectra. The emission of these Eu(II) complexes originates from the d–f transition between the lowest 5d energy level and the ground 4f energy level. Therefore, these Eu(II) complexes exhibit almost same emission spectra under different excitation wavelengths (Fig. S2, ESI†). The excited-state lifetimes (τ) for these Eu(II) complexes in solid powder were found to be hundreds of nanoseconds (245–691 ns, Fig. 2d, and Fig. S3, ESI†), which are similar to other Eu(II) complexes.11,26 The PLQYs of Eu–Et, Eu–iPr, Eu–Cy, Eu–tBu and Eu–Ad in solid powder were measured as 32%, 17%, 83%, 95% and 98%, respectively (Table S1, ESI†). The long excited-state lifetimes and high PLQY values were obtained in Eu–Cy, Eu–tBu and Eu–Ad, due to the large steric hindrance reducing the vibration quenching of the excited state.| Complex | Solid | Solution | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ m (nm) | FWHM (nm) | τ (ns) | PLQY (%) | CIE | λ m (nm) | FWHM (nm) | τ (ns) | PLQY (%) | CIE | |
| a The excitation wavelength is 400 nm during the PLQY measurements. b The excitation wavelength is 360 nm during the PLQY measurements. | ||||||||||
| Eu–Eta | 601 | 89 | 413 | 32 | (0.59, 0.41) | 590 | 88 | 56 | 5 | (0.55, 0.45) |
| Eu–iPra | 572 | 77 | 245 | 17 | (0.49, 0.50) | 561 | 76 | 11, 285 | 10 | (0.44, 0.55) |
| Eu–Cya | 537 | 68 | 619 | 83 | (0.32, 0.62) | 538 | 70 | 101 | 2 | (0.34, 0.62) |
| Eu–tBub | 474 | 45 | 691 | 95 | (0.13, 0.20) | 479 | 48 | 724 | 89 | (0.12, 0.20) |
| Eu–Adb | 476 | 62 | 648 | 98 | (0.12, 0.18) | 443, 473 | 80 | 512 | 58 | (0.14, 0.13) |
The photophysical properties of these Eu(II) complexes in dichloromethane solutions (10−3 M) were also studied. The UV-visible spectra show that Eu–Et, Eu–iPr and Eu–Cy have similar high-energy absorption below 250 nm and low-energy absorption peaks at 399, 390 and 374 nm, respectively (Fig. S4, ESI†). The similar absorption features of the three Eu(II) complexes indicate the similar coordination patterns of central Eu2+ ions. Eu–tBu exhibits a low-energy absorption peak at 343 nm and an additional band at 265 nm, while Eu–Ad shows three low-energy absorption peaks at 337, 293 and 259 nm. These absorption bands are attributed to the 4f–5d transitions of Eu2+ ions. The broad emission bands were also observed in the solutions of Eu–Et, Eu–iPr, Eu–Cy and Eu–tBu with λm at 590, 561, 538 and 479 nm, respectively (Fig. S5, ESI†). However, Eu–Ad reveals two emission peaks at 443 and 473 nm, and we attributed this phenomenon to the dissociation of THF molecules in its solution. These Eu(II) complexes exhibit broad excitation bands (Fig. S6, ESI†) in solutions similarly to their solid powder. The PLQYs of Eu–Et, Eu–iPr, Eu–Cy and Eu–Ad in solutions show a dramatic decrease compared with their solids due to the quenching of solvent and ligand vibrations (Table 1), and Eu–tBu exhibits the highest PLQY of 89% in solution due to its more rigid and robust coordination. The excited-state lifetimes of Eu–Et, Eu–Cy and Eu–Ad in solutions also exhibit a decrease compared with their solids, and a double-exponential lifetime (11 and![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 ns) was observed in the solution of Eu–iPr (Fig. S7, ESI†). We temporarily attributed the double-exponential lifetime of Eu–iPr to the partial degeneration of Eu–iPr in solution.
285 ns) was observed in the solution of Eu–iPr (Fig. S7, ESI†). We temporarily attributed the double-exponential lifetime of Eu–iPr to the partial degeneration of Eu–iPr in solution.
2.3. Electroluminescence performance
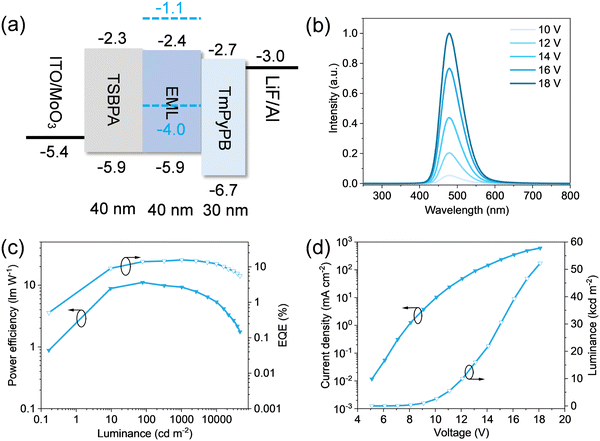
Two sky-blue emitting Eu(II) complexes Eu–tBu and Eu–Ad were obtained by adjusting the steric hindrance of tris(pyrazolyl)borate ligands. Considering there are two coordinated THF molecules in Eu–Ad, which are not suitable for OLED fabrication, Eu–tBu was chosen to further study its electroluminescence performance. Prior to this, the thermal and electrochemical properties of Eu–tBu were studied by thermogravimetric analysis (Fig. S8, ESI†) and cyclic voltammetry (Fig. S9, ESI†). Eu–tBu exhibits a decomposition temperature (Tg, corresponding to 5% weight loss) around 242 °C, which is higher than its sublimation temperature (180 °C). Therefore, Eu–tBu can be vacuum-deposited to fabricate OLEDs. The highest occupied molecular orbital (HOMO) of Eu–tBu was estimated from its oxidation peak as −4.0 eV, the optical band gap was determined from its absorption edge as 2.9 eV, and the lowest unoccupied molecular orbital (LUMO) of Eu–tBu was calculated to be −1.1 eV. N,N-Dicarbazolyl-3,5-benzene (mCP) with a high triplet state energy was chosen as the host material, and the PLQY of its doped film was measured to be 96%. The corresponding device structure was designed as ITO/MoO3 (2 nm)/4,4′-(diphenylsilanediyl)bis(N,N-diphenylaniline) (TSBPA, 40 nm)/mCP:Eu–tBu (12 wt%, 40 nm)/1,3,5-tri(m-pyrid-3-yl-phenyl)benzene (TmPyPB, 30 nm)/LiF/Al (Fig. 3a). The device exhibits a sky-blue emission with a λm of 478 nm, and no emission of mCP was observed even under high voltage (Fig. 3b). This device gives pretty good performance with a turn-on voltage of 5.7 V, a maximum EQE of 15.7%, a maximum luminance of 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 cd m−2, and CIE coordinates of (0.13, 0.27) at 1000 cd m−2 (Fig. 3c and d). The EQE are retained to be 13.4% and 12.1% even at a high luminance of 5000 and 10
240 cd m−2, and CIE coordinates of (0.13, 0.27) at 1000 cd m−2 (Fig. 3c and d). The EQE are retained to be 13.4% and 12.1% even at a high luminance of 5000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, respectively, demonstrating low efficiency roll-off.
000 cd m−2, respectively, demonstrating low efficiency roll-off.
Compared with the state-of-the-art blue OLEDs,28–37 there are some deficiencies in the OLED based on Eu–tBu. Firstly, the exciton utilization efficiency (EUE) of this OLED is calculated as 82% by the equation of EQE = ηe−h × ηPL × ηEUE × ηout, considering a hole–electron recombination efficiency (ηe−h) around 100%, a photoluminescence quantum yield (ηPL) of 96% and a light out-coupling efficiency (ηout) of around 20%. The EUE is higher than the OLEDs based on fluorescence materials but does not attain the 100% theoretical EUE. The further device optimizations, such as attempting more function materials and designing more delicate device structures, are needed to improve the EQE. Secondly, the FWHM of its electroluminescence spectra is 64 nm, and the broader emission band than these observed in solid powder and solution (45 and 48 nm, respectively) hinders the chromatic purity. Narrowband blue emissions from Eu(II) are necessary to improve the color purity of blue OLEDs, which may be achieved by increasing both the rigidity of Eu(II) complexes and the doped emission layers. The rigid coordination environment around Eu2+ can restrain the spectra broadening caused by ligand vibrations. Except for the above deficiencies, the low efficiency roll-off and high luminance were achieved in this OLED benefiting from the short excited-state lifetime of the d–f transition Eu(II) complex.
3. Conclusions
In summary, a series of Eu(II) complexes based on tris(pyrazolyl)borate ligands were designed and synthesized. By adjusting the steric hindrance of the 3-position of pyrazolyl, these Eu(II) complexes exhibit tunable emission colors with maximum emission wavelengths in the range of 601–474 nm. A thermally stable sky-blue emission Eu(II) complex Eu–tBu was obtained by employing the bulky tert-butyl group. The OLED using Eu–tBu as an emitter shows sky-blue emission with a maximum EQE of 15.7% and a maximum luminance of 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 cd m−2. This work demonstrates a strategy to construct blue emission and thermally stable Eu(II) complexes, and sheds light on the design and fabrication of efficient blue OLEDs by vacuum thermal deposition of a Eu(II) complex as an emitter.
240 cd m−2. This work demonstrates a strategy to construct blue emission and thermally stable Eu(II) complexes, and sheds light on the design and fabrication of efficient blue OLEDs by vacuum thermal deposition of a Eu(II) complex as an emitter.
Author contributions
W. Y. and Y. L. synthesized and characterized the complexes. P. H. fabricated and measured the OLEDs. R. G. measured the PLQYs. G. Y., Z. Z. and K. W. helped synthesize the ligands. W. Y. wrote the draft. Z. B. and Z. L. supervised the project. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts of declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071003, 92156016, 21621061, and 62104013), the National Key R&D Program of China (2021YFB3501800), and the Beijing Natural Science Foundation (2202015).References
- C. W. Tang and S. A. VanSlyke, Organic electroluminescent diodes, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Highly efficient phosphorescent emission from organic electroluminescent devices, Nature, 1998, 395, 151–154 CrossRef CAS.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- Q. Peng, A. Obolda, M. Zhang and F. Li, Organic light-emitting diodes using a neutral π radical as emitter: The emission from a doublet, Angew. Chem., Int. Ed., 2015, 54, 7091–7095 CrossRef CAS PubMed.
- X. Ai, E. W. Evans, S. Dong, A. J. Gillett, H. Guo, Y. Chen, T. J. H. Hele, R. H. Friend and F. Li, Efficient radical-based light-emitting diodes with doublet emission, Nature, 2018, 563, 536–540 CrossRef CAS PubMed.
- C.-Y. Chan, M. Tanaka, Y.-T. Lee, Y.-W. Wong, H. Nakanotani, T. Hatakeyama and C. Adachi, Stable pure-blue hyperfluorescence organic light-emitting diodes with high-efficiency and narrow emission, Nat. Photon., 2021, 15, 203–207 CrossRef CAS.
- A. Monkman, Why do we still need a stable long lifetime deep blue OLED emitter, ACS Appl. Mater. Interfaces, 2021, 14, 20463–20467 CrossRef PubMed.
- L. Wang, Z. Zhao, G. Zhan, H. Fang, H. Yang, T. Huang, Y. Zhang, N. Jiang, L. Duan, Z. Liu, Z. Bian, Z. Lu and C. Huang, Deep-blue organic light-emitting diodes based on a doublet d–f transition cerium(III) complex with 100% exciton utilization efficiency, Light: Sci. Appl., 2020, 9, 157–165 CrossRef PubMed.
- Z. Zhao, L. Wang, G. Zhan, Z. Liu, Z. Bian and C. Huang, Efficient rare earth cerium(III) complex with nanosecond d–f emission for blue organic light-emitting diodes, Natl. Sci. Rev., 2021, 8, nwaa193 CrossRef CAS PubMed.
- J. Li, L. Wang, Z. Zhao, B. Sun, G. Zhan, H. Liu, Z. Bian and Z. Liu, Highly efficient and air-stable Eu(II)-containing azacryptates ready for organic light-emitting diodes, Nat. Commun., 2020, 11, 5218–5225 CrossRef CAS PubMed.
- G. Zhan, L. Wang, Z. Zhao, P. Fang, Z. Bian and Z. Liu, Highly efficient and air-stable lanthanide Eu(II) complex: New emitter in organic light emitting diodes, Angew. Chem., Int. Ed., 2020, 59, 19011–19015 CrossRef CAS PubMed.
- P. Fang, L. Wang, G. Zhan, W. Yan, P. Huo, A. Ying, Y. Zhang, Z. Zhao, G. Yu, Y. Huang, S. Gong, L. Duan, Z. Liu, Z. Bian and C. Huang, Lanthanide cerium(III) tris(pyrazolyl)borate complexes: Efficient blue emitters for doublet organic light-emitting diodes, ACS Appl. Mater. Interfaces, 2021, 13, 45686–45695 CrossRef CAS PubMed.
- C. P. Shipley, S. Capecchi, O. V. Salata, M. Etchells, P. J. Dobson and V. Christou, Orange electroluminescence from a divalent europium complex, Adv. Mater., 1999, 11, 533–536 CrossRef CAS.
- H. Qi, P. Huo, B. Han, J. Zheng, L. Wang, W. Yan, R. Guo, T. Li, K. Yu, Z. Liu and Z. Bian, High-performance circularly polarized electroluminescence from d–f transition europium(II) complexes, Cell Rep. Phys. Sci., 2022, 3, 101107 CrossRef CAS.
- J. Jiang, N. Higashiyama, K. Machida and G. Adachi, The luminescent properties of divalent europium complexes of crown ethers and cryptands, Coord. Chem. Rev., 1998, 170, 1–29 CrossRef CAS.
- T. C. Jenks, M. D. Bailey, B. A. Corbin, A. N. W. Kuda-Wedagedara, P. D. Martin, H. B. Schlegel, F. A. Rabuffetti and M. J. Allen, Photophysical characterization of a highly luminescent divalent-europium-containing azacryptate, Chem. Commun., 2018, 54, 4545–4548 RSC.
- C. U. Lenora, F. Carniato, Y. Shen, Z. Latif, E. M. Haacke, P. D. Martin, M. Botta and M. J. Allen, Structural features of europium(II)-containing cryptates that influence relaxivity, Chem. Eur. J., 2017, 23, 15404–15414 CrossRef CAS PubMed.
- W. Yan, T. Li, Z. Cai, H. Qi, R. Guo, P. Huo, Z. Liu and Z. Bian, Systematic tuning of the emission colors and redox potential of Eu(II)-containing cryptates by changing the N/O ratio of cryptands, Inorg. Chem. Front., 2022, 9, 4794–4800 RSC.
- A. Wu, P. Huo, G. Yu, R. Guo, Z. Zhao, W. Yan, L. Wang, Z. Bian and Z. Liu, Europium(II) complexes with substituted tris(2-aminoethyl)amine/triethanolamine ligand and their application in blue spin-coated organic light-emitting diodes, Adv. Opt. Mater., 2022, 10, 2200952 CrossRef CAS.
- S. Y. Liu, G. H. Maunder, A. Sella, M. Stevenson and D. A. Tocher, Synthesis and molecular structures of hydrotris(dimethylpyrazolyl)borate complexes of the lanthanides, Inorg. Chem., 1996, 35, 76–81 CrossRef CAS PubMed.
- F. A. Kunrath, O. L. Casagrande, L. Toupet and J.-F. Carpentier, Synthesis and reactivity in salt metathesis reactions of trivalent [La(TpMe2)2X] (X = Cl, I) complexes: Crystal structures of [La(TpMe2)2Cl] and [La(TpMe2)2(κ2-pzMe2)], Polyhedron, 2004, 23, 2437–2445 CrossRef CAS.
- N. Marques, A. Sella and J. Takats, Chemistry of the lanthanides using pyrazolylborate ligands, Chem. Rev., 2002, 102, 2137–2160 CrossRef CAS PubMed.
- M. A. J. Moss, R. A. Kresinski, C. J. Jones and W. J. Evans, Polypyrazolylborate derivatives of the lanthanides. The syntheses of oxidation state(II) complexes, Polyhedron, 1993, 12, 1953–1955 CrossRef CAS.
- A. C. Hillier, X. Zhang, G. H. Maunder, S. Y. Liu, T. A. Eberspacher, M. V. Metz, R. McDonald, A. Domingos, N. Marques, V. W. Day, A. Sella and J. Takats, Synthesis and structural comparison of a series of divalent Ln(TpR,R')2 (Ln = Sm, Eu, Yb) and trivalent Sm(TpMe2)2X (X = F, Cl, I, BPh4) complexes, Inorg. Chem., 2001, 40, 5106–5116 CrossRef CAS PubMed.
- M. Kühling, C. Wickleder, M. J. Ferguson, C. G. Hrib, R. McDonald, M. Suta, L. Hilfert, J. Takats and F. T. Edelmann, Investigation of the “bent sandwich-like” divalent lanthanide hydro-tris(pyrazolyl)borates Ln(TpiPr2)2 (Ln = Sm, Eu, Tm, Yb), New J. Chem., 2015, 39, 7617–7625 RSC.
- H. Qi, Z. Zhao, G. Zhan, B. Sun, W. Yan, C. Wang, L. Wang, Z. Liu, Z. Bian and C. Huang, Air stable and efficient rare earth Eu(II) hydro-tris(pyrazolyl)borate complexes with tunable emission colors, Inorg. Chem. Front., 2020, 7, 4593–4599 RSC.
- M. Suta, M. Kühling, P. Liebing, F. T. Edelmann and C. Wickleder, Photoluminescence properties of the “bent sandwich-like” compounds [Eu(TpiPr2)2] and [Yb(TpiPr2)2] - Intermediates between nitride-based phosphors and metallocenes, J. Lumin., 2017, 187, 62–68 CrossRef CAS.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter, Nat. Photon., 2019, 13, 678–682 CrossRef CAS.
- K. R. Naveen, H. Lee, R. Braveenth, D. Karthik, K. J. Yang, S. J. Hwang and J. H. Kwon, Achieving high efficiency and pure blue color in hyperfluorescence organic light emitting diodes using organo-boron based emitters, Adv. Funct. Mater., 2021, 32, 2110356 CrossRef.
- J. Bian, S. Chen, L. Qiu, R. Tian, Y. Man, Y. Wang, S. Chen, J. Zhang, C. Duan, C. Han and H. Xu, Ambipolar self-host functionalization accelerates blue multi-resonance thermally activated delayed fluorescence with internal quantum efficiency of 100%, Adv. Mater., 2022, 34, 2110547 CrossRef CAS PubMed.
- T. Huang, Q. Wang, G. Meng, L. Duan and D. Zhang, Accelerating radiative decay in blue through-space charge transfer emitters by minimizing the face-to-face donor-acceptor distances, Angew. Chem., Int. Ed., 2022, 61, e202200059 CrossRef CAS PubMed.
- S. Oda, B. Kawakami, M. Horiuchi, Y. Yamasaki, R. Kawasumi and T. Hatakeyama, Ultra-narrowband blue multi-resonance thermally activated delayed fluorescence materials, Adv. Sci., 2023, 10, 2205070 CrossRef CAS PubMed.
- J. Park, K. J. Kim, J. Lim, T. Kim and J. Y. Lee, High efficiency of over 25% and long device lifetime of over 500 h at 1000 nit in blue fluorescent organic light-emitting diodes, Adv. Mater., 2022, 34, 2108581 CrossRef CAS PubMed.
- H. Wang, Y. Liu, B. Yu, S. Song, Y. Zheng, K. Liu, P. Chen, H. Wang, J. Jiang and T. Li, A configurationally confined thermally activated delayed fluorescent two-coordinate Cu(I) complex for efficient blue electroluminescence, Angew. Chem., Int. Ed., 2023, 62, e202217195 CrossRef CAS PubMed.
- Z. P. Yan, L. Yuan, Y. Zhang, M. X. Mao, X. J. Liao, H. X. Ni, Z. H. Wang, Z. An, Y. X. Zheng and J. L. Zuo, A chiral dual-core organoboron structure realizes dual-channel enhanced ultrapure blue emission and highly efficient circularly polarized electroluminescence, Adv. Mater., 2022, 34, 2204253 CrossRef CAS PubMed.
- Y. H. Lee, W. Lee, T. Lee, J. Jung, S. Yoo and M. H. Lee, Achieving over 36% EQE in blue OLEDs using rigid TADF emitters based on spiro-donor and spiro-B-heterotriangulene acceptors, Chem. Eng. J., 2023, 452, 139387 CrossRef CAS.
- K. R. Naveen, H. Lee, L. H. Seung, Y. H. Jung, C. P. Keshavananda Prabhu, S. Muruganantham and J. H. Kwon, Modular design for constructing narrowband deep-blue multiresonant thermally activated delayed fluorescent emitters for efficient organic light emitting diodes, Chem. Eng. J., 2023, 451, 138498 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2234667 and 2234668. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc01456a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |