Improving the broadband photocatalytic performance of TiO2 through a highly efficient optical converter†
Guotao
Xiang
 *a,
Yuanyuan
Yi
a,
Zhen
Liu
a,
Yanhong
Li
*a,
Yuanyuan
Yi
a,
Zhen
Liu
a,
Yanhong
Li
 a,
Zhiyu
Yang
a,
Yongjie
Wang
a,
Zhiyu
Yang
a,
Yongjie
Wang
 a,
Sha
Jiang
a,
Sha
Jiang
 a,
Xiao
Tang
a,
Xianju
Zhou
a,
Xiao
Tang
a,
Xianju
Zhou
 a,
Li
Li
a,
Xiaojun
Wang
a,
Li
Li
a,
Xiaojun
Wang
 *c and
Jiahua
Zhang
*c and
Jiahua
Zhang
 *b
*b
aDepartment of Mathematics and Physics, Chongqing University of Posts and Telecommunications, 2 Chongwen Road, Chongqing 400065, China. E-mail: xianggt@cqupt.edu.cn
bState Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, 3888 Eastern South Lake Road, Changchun 130033, China. E-mail: zhangjh@ciomp.ac.cn
cDepartment of Physics & Astronomy, Georgia Southern University, Statesboro, Georgia 30460, USA. E-mail: xwang@georgiasouthern.edu
First published on 13th August 2024
Abstract
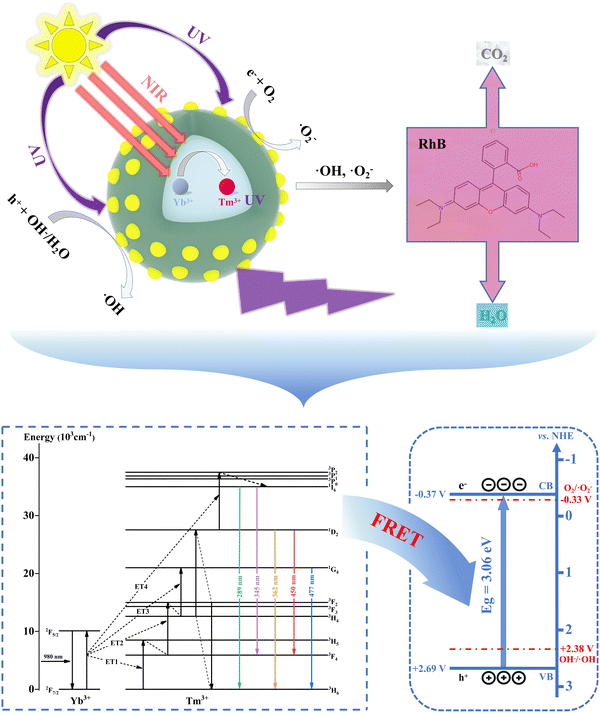
Developing efficient photocatalysts that respond to near-infrared (NIR) light holds significant scientific importance for improving the utilization of solar energy. In this study, the photocatalyst β-NaLuF4:Yb3+/Tm3+@TiO2 that simultaneously responds to both ultraviolet (UV) and NIR light is successfully synthesized. Instead of the conventional use of β-NaYF4:Yb3+/Tm3+, β-NaLuF4:Yb3+/Tm3+ upconversion (UC) nanoparticles (NPs) are employed as the NIR-UV light converter to achieve stronger UV UC luminescence. The data indicate that the photocatalytic degradation efficiency and rate of β-NaLuF4:Yb3+/Tm3+@TiO2 synthesized in this work are superior to those of NIR-driven photocatalysts based on β-NaYF4:Yb3+/Tm3+ under both NIR and sunlight irradiation. Further analysis of the UC emission spectra and the decay curves of the samples reveals an effective fluorescence resonance energy transfer (FRET) process between β-NaLuF4:Yb3+/Tm3+ and TiO2 after the combination, which makes the NIR-driven photocatalytic reactions happen. All results confirm that the present β-NaLuF4:Yb3+/Tm3+@TiO2 is an exceptionally efficient photocatalyst, owing to its responsiveness to both UV and NIR light.
Introduction
Environmental pollution and energy shortages have become two major challenges that urgently need to be addressed in today's world. Under solar irradiation, photocatalytic materials can not only decompose pollutants but also achieve water splitting for hydrogen production, making it one of the best solutions to tackle the above two problems simultaneously.1–6 Therefore, research on high-performance photocatalysts holds significant practical value for the sustainable development of humanity.7–11 Currently, the most widely used photocatalyst, TiO2, possesses advantages such as strong chemical stability, non-toxicity, low cost and so on. However, the bandgap width of TiO2 (3.2 eV) restricts its ability to utilize near-infrared (NIR) light for efficient photocatalytic reactions, which accounts for approximately 46% of solar radiation, resulting in a huge loss of solar power.12–17Rare earth ion-doped upconversion (UC) nanoparticles (NPs) possess the unique ability to transform NIR light into either ultraviolet (UV) or visible (Vis) light. This transforming ability enables their integration with photocatalysts to realize photocatalytic reactions under NIR illumination.18,19 For instance, Qin et al. reported a composite material of NaYF4:Yb3+/Tm3+ and TiO2, in which an effective fluorescence resonance energy transfer (FRET) process occurs between the UV emission level 1I6 and 1D2 of Tm3+ and TiO2.20 Then, the NIR-driven photocatalysis process is carried out based on this FRET process. Similar reports include β-NaYF4:Yb3+/Tm3+@ZnO, β-NaYF4:Yb3+/Tm3+@CdSe, β-NaYF4:Yb3+/Tm3+@Ag3PO4 and so on.21–27
As mentioned above, in the composite structures of UC materials and photocatalysts reported so far, β-NaYF4:Yb3+/Tm3+ is usually the preferred converter for NIR to UV light due to the high efficiency of β-NaYF4 in the UC process. However, β-NaLuF4, which shares a similar crystal cell structure and phonon energy with β-NaYF4, is also an extremely efficient UC matrix. Moreover, according to the intensity borrowing mechanism proposed by O. Guillot Noel et al., the unique electronic states at the valence band top of Lu-based compounds increase the vibrational intensity of the doped rare earth ions in the crystal.28 Therefore, the UC transition probability of the luminescent centers in Lu-based compounds is often higher than that of the corresponding Y-based compounds, giving rise to much stronger UC intensity appearing in Lu-based compounds. The validity of this theory has been substantiated by numerous reports.29,30 Nevertheless, currently, there are few reports on using β-NaLuF4:Yb3+/Tm3+ as a conversion agent for the NIR photocatalytic reaction.
Herein, β-NaLuF4:Yb3+/Tm3+ is selected as a NIR-UV light converter to combine with TiO2 for the aim of developing a high-performance NIR-driven photocatalyst. The FRET processes between β-NaLuF4:Yb3+/Tm3+ and TiO2 are thoroughly investigated using both steady-state and transient spectroscopy techniques. Additionally, rhodamine B (RhB) is employed as the mimical pollutant to evaluate the photocatalytic performance of β-NaLuF4:Yb3+/Tm3+@TiO2 under NIR light and sunlight irradiation, in which the results indicate that the sample developed in our work exhibits a much higher photodegradation efficiency and rate compared to the reported NIR-driven photocatalysts formed by β-NaYF4:Yb3+/Tm3+. Furthermore, a detailed study of the photoelectrochemical properties of the samples is conducted to elucidate the photocatalytic mechanism of β-NaLuF4:Yb3+/Tm3+@TiO2. The findings reveal that β-NaLuF4:Yb3+/Tm3+@TiO2 is an efficient photocatalytic material that can be driven by both UV and NIR light.
Experimental
Chemicals
LuCl3·6H2O, YbCl3·6H2O and TmCl3·6H2O are supplied by Beijing Founde Star Science & Technology Co, Ltd. Cetyltrimethylammonium bromide (CTAB), titanium diisopropoxide bis (acetylacetonate) (TDAA), isopropanol (IPA), ethylene diamine tetraacetic acid (EDTA) and benzoquinone (BQ) are provided by Shanghai Aladdin Biochemical Technology Co., Ltd. Cyclohexane, methanol, ethanol, NH4F, NH3·H2O (30 wt%) and NaOH are obtained from Chongqing Chuandong Chemical (Group) Co, Ltd. 1-Octadecene (ODE, 90%) and Oleic acid (OA, 90%) are acquired from Alfa Aesar. All the reagents are utilized directly in the experiments without additional purification.Preparation of β-NaLuF4:y% Yb3+/x% Tm3+ (UCNPs), x = 0.5, 1, 1.5, 2, y = 10, 20, 30, 40, 50, 60, 70
The UCNPs are synthesized by the solvothermal method. Specifically, the rare-earth chlorides are weighed according to their stoichiometric ratio using an electronic balance for the preparation of 1 mmol UCNPs and then transferred into a 100 mL three-neck flask containing 6 mL oleic acid (OA) and 15 mL octadecene (ODE). Nitrogen is employed as the shielding gas to prevent the occurrence of oxidation phenomena during the reaction process. Initially, the mixture in the three-neck flask is heated to 140 °C and maintained for 30 minutes, resulting in the formation of rare-earth oleate complexes. Following this, the mixture is allowed to gradually cool down to 50 °C, at which juncture 5 mL of a methanol solution containing 2.5 mmol of NaOH and 4 mmol of NH4F is introduced. Thereafter, the solution is heated up to 80 °C and sustained at this temperature for 30 minutes to facilitate the complete evaporation of methanol. Subsequently, the reactants are subjected to a temperature of 300 °C for a duration of 1.5 hours. After cooling naturally to ambient temperature, the end product is successfully synthesized. Finally, the UCNPs are washed with ethanol and cyclohexane and collected by centrifugation. The resulting centrifuged product is dispersed in 2 mL of cyclohexane for the subsequent coating process.Preparation of UCNPs@TiO2 composite structure
The preparation of the UCNPs@TiO2 composite structure involved the following steps: initially, UCNPs dissolved in cyclohexane are introduced into 20 mL of deionized water containing 0.1 g of CTAB, which is subsequently agitated until it achieved a white milky appearance. Then, the mixture is heated to 80 °C within a water bath, aiming to evaporate the cyclohexane and thus render the solution transparent. Once the solution is cooled to room temperature, the solution is washed using deionized water and centrifugated twice to yield CTAB-modified UCNPs. The sample is subsequently dispersed in 10 mL of isopropanol followed by mixing with another solution consisting of 10 mL isopropanol and 144 μL TDAA. Under continuous magnetic stirring, 0.3 mL NH3·H2O and 2.5 mL deionized water are added into the above solution. The mixture is maintained under magnetic stirring at room temperature for a period of 12 hours, and then collected by centrifugation and washed with deionized water and ethanol. After the mixture is dried in an oven at 70 °C for 12 hours, the mixture is annealed at 500 °C for 3 hours to form UCNPs@TiO2.Photocatalytic experiment
The photocatalytic efficacy of UCNPs@TiO2 is assessed through the degradation of RhB under the irradiation of diverse photosources, including a 300 W Xe lamp assembled with various optical filters and a 980 nm semiconductor laser. To be specific, 10 mg of UCNPs@TiO2 solid powder is dispersed in a 30 mL RhB solution with a concentration of 10 mg L−1. Then, the previously described mixture is transferred into a reaction vessel, where it is maintained under dark conditions for 1 hour to realize the adsorption–desorption equilibrium between UCNPs@TiO2 and RhB before illumination. During the process of photocatalytic experimentation, 1 mL of the liquid is extracted from the solution at the same time interval and then centrifuged to remove UCNPs@TiO2 from the solution. The UV-vis absorbance spectrum of the remaining liquid is tested to determine the degradation status of RhB. The photocatalytic performances of pure UCNPs, anatase TiO2 and the physical mixture of UCNPs and TiO2 (UCNPs/TiO2) are also evaluated using the above method. In addition, the UCNPs@TiO2 is recovered through centrifugation at the end of every cycle to evaluate its performance for repeated use.Characterization
The X-ray diffraction (XRD) data of the samples are obtained using an XD-2 X-ray diffractometer provided by Beijing Persee. The morphology and elemental analysis data are collected using a JEOL JEM 2100 transmission electron microscope (TEM) equipped with an energy-dispersive spectroscopy (EDS) analyzer. The absorption spectra are measured using a Cary 5000 UV-vis-NIR spectrophotometer. An FLS 1000 spectrometer equipped with a 980 nm laser as the excitation source is employed to measure the spectral data.Photoelectrochemical characterization is performed through a CHI760E-typed electrochemical workstation equipped with a conventional three-electrode setup in the 0.2 M Na2SO4 electrolyte. Here, a Pt plate electrode and saturated Ag/AgCl electrode serve as the counter electrode and reference electrode, respectively. Meanwhile, the prepared photocatalysts are layered onto FTO glass with an active area close to 1 cm2 to form the working electrode. A 300 W Xe lamp with an NIR filter (800–2500 nm) is employed as the light source for the experiments.
Results and discussion
Structure and morphology
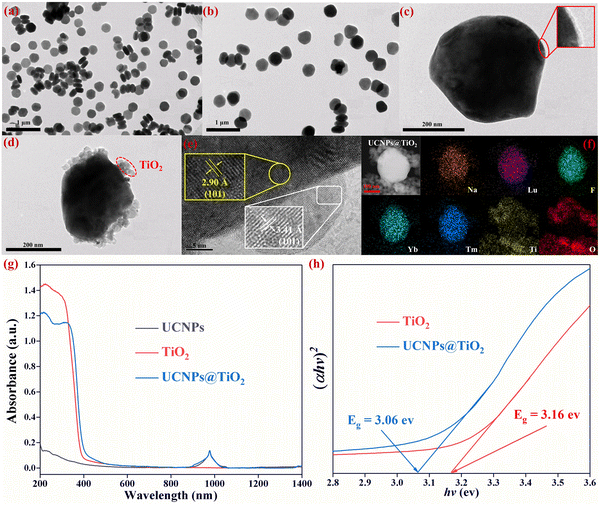
A two-step wet chemical method is used to synthesize the UCNPs@TiO2 composite structure photocatalyst, as presented in Fig. 1(a). First, CTAB serves as a surfactant to endow the as-prepared UCNPs with hydrophilic properties for utilization in the following stage. Next, TDAA is employed as the Ti source for the formation of UCNPs@TiO2, which is mixed with the CTAB-modified UCNPs in isopropanol along with the immission of NH3·H2O and deionized water under magnetic stirring at room temperature for 12 hours. The successful synthesis of the UCNPs@TiO2 composite material is demonstrated using XRD patterns. As shown in Fig. 1(b), each peak presented in the UCNPs and TiO2 corresponds precisely to β-NaLuF4 and anatase TiO2, referenced by JCPDS no. 27-0726 and JCPDS no. 21-1272, respectively. Furthermore, the XRD patterns of UCNPs@TiO2 distinctly reveal the coexistence of both UCNPs and TiO2 phases without the appearance of obvious extraneous peaks, verifying the purity of the composite NPs. The weak diffraction peak marked with an asterisk belongs to NaCl, which has no influence on the optical properties of the samples.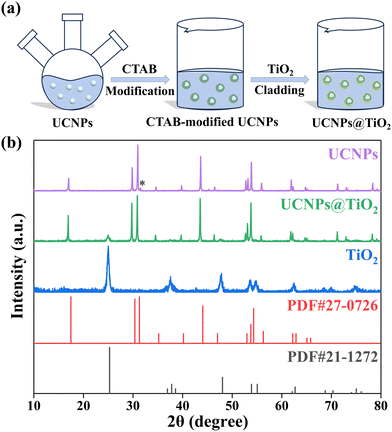 | ||
| Fig. 1 (a) Schematic representation of the preparation process of UCNPs@TiO2 composite structure. (b) XRD patterns of UCNPs, UCNPs@TiO2 and anatase TiO2. | ||
TEM characterization is performed to examine the morphology and structure of the as-synthesized NPs. The TEM image illustrated in Fig. 2(a) reveals that the UCNPs are uniformly dispersed with an average diameter of approximately 200 nm. Meanwhile, the size and morphology of the UCNPs remain almost unchanged after being modified by the hydrophilic CTAB because of the ultra-thin layer of CTAB molecules, as depicted in Fig. 2(b) and (c). Consequently, the CTAB-modified can be readily mixed with TDAA in isopropanol to form the UCNPs@TiO2 composite structure, as displayed in Fig. 2(d). Ulteriorly, the high-resolution TEM (HR-TEM) image of UCNPs@TiO2 is examined to shed light on the structural information of the composite NPs. As delineated in Fig. 2(e), the lattice fringe of the UCNPs is identified to be 2.90 Å, aligning with the (101) crystal plane of β-NaLuF4 (JCPDS No. 27-0726). Additionally, the interplanar spacing of TiO2 was found to be 3.41 Å, in perfect agreement with the (101) lattice plane of anatase TiO2 (JCPDS no. 21-1272). Element mapping of an individual UCNPs@TiO2 particle, shown in Fig. 2(f), vividly displays the distribution of the whole elements, encompassing Na, Lu, F, Yb, Tm, Ti and O. Notably, Na, Lu, F, Yb, and Tm predominantly occupy the inner of the particle, whereas Ti and O are primarily situated on the exterior. This situation of element distribution further confirms the successful formation of the UCNPs@TiO2 composite structure.
In order to gain insight into the absorption ability of the synthesized samples, UV-vis absorption spectra of UCNPs, TiO2 and UCNPs@TiO2 are measured. As presented in Fig. 2(g), the UCNPs only reveal a pronounced NIR absorption band peaked at about 980 nm, denoting the transition from 2F7/2 to 2F5/2 of Yb3+ ions. In contrast, anatase TiO2 is characterized by its strong absorption in the UV region with an edge near 400 nm, belonging to the band-band transition of TiO2. The UCNPs@TiO2 composite NPs integrate the absorption capacity in both UV and NIR regions mentioned above. Moreover, the UV absorption edge of the composite NPs experiences a slight red shift to a long wavelength direction, resulting from the composite structure between UCNPs and semiconductor.31,32 The bandgap (Eg) can be deduced by Tauc's equation:
| αhν = A·(hν − Eg)n/2 | (1) |
Optical properties
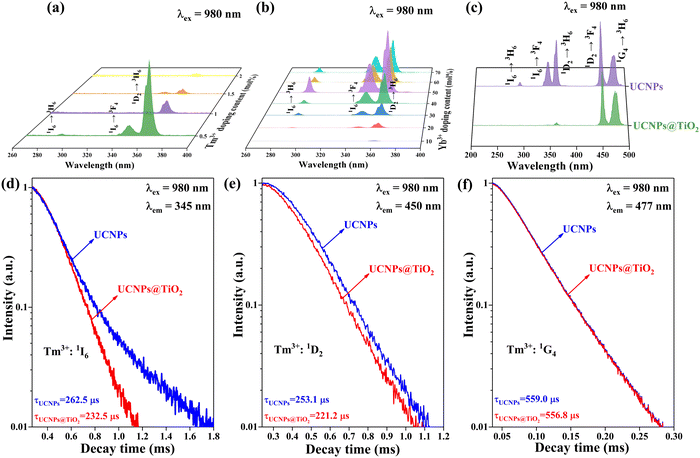
As illustrated in Fig. 3(a) and (b), under the excitation of 980 nm NIR light, Tm3+ can produce UC luminescence in the UV and blue light regions through the sensitization of Yb3+, including UV transition 1I6 → 3H6 at 289 nm, 1I6 → 3F4 at 345 nm and 1D2 → 3H6 at 362 nm as well as blue transition 1D2 → 3F4 at 450 nm and 1G4 → 3H6 at 477 nm. Notably, the UC emission in the UV region of UCNPs@TiO2 plays a crucial role in achieving NIR-driven photocatalytic reaction due to the bandgap width of TiO2. To obtain strong UV UC luminescence in Yb3+ and Tm3+ doped β-NaLuF4, a series of UCNPs doped with various concentrations of Yb3+ and Tm3+ are prepared, of which the corresponding XRD data are presented in Fig. S1 (ESI†). As shown in Fig. 3(a), with the doping concentration of Yb3+ fixed at 20%, a dependence of the luminescence intensity of UCNPs on the concentration of Tm3+ is detected, finding that the strongest UV UC emission is obtained at the Tm3+ doping concentration of 0.5%. Subsequently, through adjusting the doping concentration of Yb3+ in UCNPs with a fixed Tm3+ doping concentration of 0.5%, the strongest UV UC luminescence corresponds with the Yb3+ doping concentration of 50%, as shown in Fig. 3(b). Therefore, it can be determined that the optimal doping concentration of Yb3+ and Tm3+ is 50% and 0.5% respectively, which will be used in subsequent studies of UCNPs@TiO2 composite NPs.Fig. 3(c) reveals that the UV emissions of Tm3+ at 289 nm, 345 nm and 362 nm nearly vanish after integrating UCNPs with TiO2, while the intensity of blue UC emissions at 450 nm and 477 nm remains almost unchanged, indicating a significant alteration in UC luminescence of Tm3+ due to the introduction of TiO2. In order to explore the energy transfer (ET) mechanism between Tm3+ and TiO2, the decay curves of the relevant energy levels of Tm3+ in UCNPs and UCNPs@TiO2 are measured, including the 1I6, 1D2 and 1G4 levels. As can be seen from Fig. 3(d)–(f), after coating with TiO2, the lifetimes of Tm3+: 1I6 and 1D2 levels are significantly shortened, whereas the lifetime of the 1G4 level stays nearly constant. Here, the lifetime values are obtained from the integrated area of the corresponding normalized decay curves.33 This phenomenon suggests that the ET between the 1I6 and 1D2 levels of Tm3+ and TiO2 primarily occurs through a FRET process, rather than a radiation-reabsorption process, because of the unavailability of the reabsorption process on changing the lifetimes of the energy levels. Moreover, referring to the position of the 1I6 and 1D2 states in Tm3+ ions, the two energy levels reveal a good match with the bandgap of TiO2 indeed, allowing direct ET from the Tm3+ ions at the excited state of 1I6 and 1D2 to the semiconductor TiO2. This provides a good precondition for realizing an efficient photocatalytic process stimulated by NIR light. In the present work, the ET efficiencies from Tm3+: 1I6 and 1D2 level to TiO2 are calculated to be 11% and 13%, respectively, by using the following equation:34
| η = 1 − τT/τU | (2) |
Photocatalytic properties
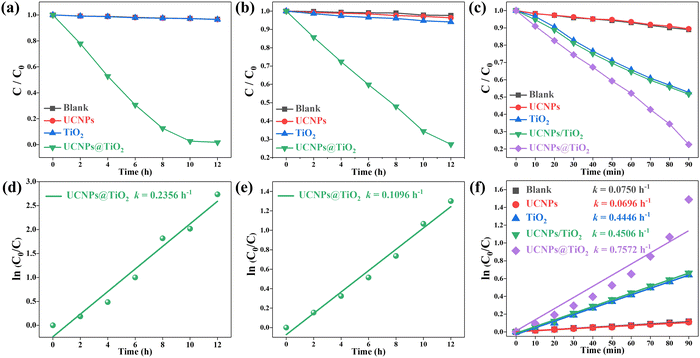
The photocatalytic performances of UCNPs@TiO2 can be evaluated through the photodegradation of RhB under various light irradiations, which can be regarded as the values of C/C0. Here, C0 represents the initial concentration of the RhB solution, while C denotes the concentration of the RhB solution following a period of illumination. Both of the two concentrations can be calibrated through the absorption spectra of the corresponding RhB solutions. As shown in Fig. 4(a), pristine TiO2 exhibits a quite poor photocatalytic activity under the irradiation of 980 nm laser, which is close to the performance of the control group without any photocatalysts. This inefficacy stems from the mismatch between the 980 nm wavelength and the bandgap width of anatase TiO2. The observed minor degradation of RhB in both experimental groups should be attributed to the thermal decomposition caused by the slight increase of solution temperature under the irradiation of a 980 nm laser. Similarly, the pure UNCPs show no ability to degrade RhB. Nevertheless, RhB is significantly degraded in the presence of UCNPs@TiO2 with a 97% reduction within 10 hours, benefiting from the efficient NIR-UV energy conversion through UCNPs followed by the effective FRET process from UCNPs to TiO2.The degradation rate k of RhB can be calculated using the Langmuir–Hinshelwood kinetics model as follows:
| −ln(C/C0) = kT | (3) |
The ability of a photocatalyst to harness sunlight for photocatalytic processes stands as a critical benchmark for its practical application. Therefore, the photocatalytic performance testing of the samples irradiated by simulated sunlight is conducted. Fig. 4(c) shows that the degradation effect of the pure UCNPs is virtually indistinguishable from that of the control group, suggesting that the degradation of RhB in the two groups is primarily due to thermal decomposition triggered by light exposure. Compared to anatase TiO2, the UCNPs@TiO2 composite material exhibited much stronger catalytic performance, degrading nearly 80% of RhB within 90 minutes, of which the degradation efficiency is 1.6 times higher than that of anatase TiO2. Moreover, the photocatalytic degradation rate constant of UCNPs@TiO2 is calculated to be 0.7572 h−1, significantly surpassing the value 0.4446 h−1 of anatase TiO2, as depicted in Fig. 4(f).
Additionally, to verify the pivotal role of the UCNPs@TiO2 composite structure in enhancing the photocatalytic performance of TiO2, the photocatalytic activity of the physically mixed UCNPs/TiO2 is detected (see Fig. 4(c)), showing only a negligible improvement in degradation of RhB compared to anatase TiO2 and is significantly lower than that of UCNPs@TiO2. The huge discrepancy in photocatalytic properties indicates that an effective FRET process is hard to be established between UCNPs and TiO2 in the physically mixed sample, in which only a small amount of NIR light can be utilized for photocatalytic reactions via the radiation-reabsorption process, demonstrating the significant advantages of the composite structure in markedly boosting the catalytic effectiveness of TiO2 through facilitating efficient FRET between UCNPs and TiO2.
The stability of the photocatalysts is another important criterion for its practical application. Thus, the cyclic degradation properties of the UCNPs@TiO2 are carried out. As shown in Fig. S2 (ESI†), the degradation effect of UCNPs@TiO2 on RhB only slightly decreases after three experiment cycles, evidencing its outstanding recyclability. Table S1 (ESI†) lists a series of typical NIR-responsive photocatalysts along with their associated parameters. Under the irradiation of 980 nm wavelength, the photocatalytic degradation efficiency and rate of UCNPs@TiO2 significantly surpass those of the composite materials based on β-NaYF4:Yb3+/Tm3+ and TiO2. Similarly, when exposed to simulated sunlight, the photocatalytic performance of UCNPs@TiO2 also outperforms other NIR-responsive photocatalytic materials, underscoring its potential value in photocatalytic applications.
Photoelectrochemical properties
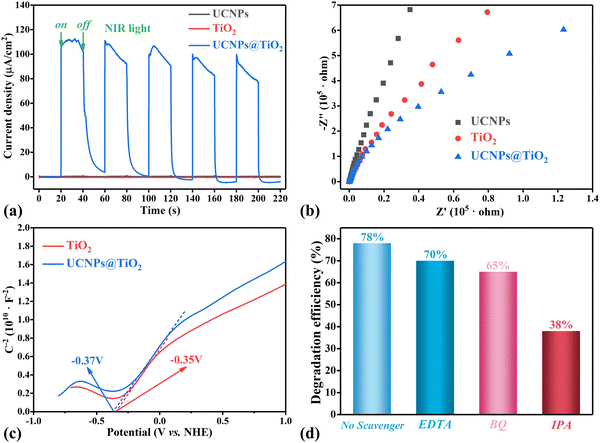
To delve deeper into the properties of photo-generated carrier separation and transport in the prepared samples induced by NIR light irradiation, the transient photocurrent response (TPR) and electrochemical impedance spectroscopy (EIS) are conducted. First, as illustrated in Fig. 5(a), a notably higher photocurrent density is observed in UCNPs@TiO2 with the periodic switching on and off of NIR light. In contrast, almost no photocurrent is detected in UCNPs and TiO2 individually, suggesting that TiO2 can effectively harness NIR light to facilitate the separation of electrons and holes for photocatalytic reactions after being integrated with UCNPs. Subsequently, as depicted in Fig. 5(b), the arc radii in EIS Nyquist plots of UCNPs, TiO2 and UCNPs@TiO2 decrease progressively, indicating that the lowest charge transfer resistance belongs to UCNPs@TiO2. These data collectively demonstrate that UCNPs@TiO2 exhibits superior photocatalytic activity under NIR light irradiation.Generally speaking, the conduction band potential (ECB) of n-type semiconductors closely aligns with their flat band (Efb). Therefore, the Mott–Schottky plots of the samples are detected under dark conditions to ascertain the position of the conduction band edge. The curves in Fig. 5(c) and Fig. S3 (ESI†) exhibit positive slopes, confirming that both anatase TiO2 and UCNPs@TiO2 are n-type characteristics. The Efb values of the two samples obtained from the intercept of the potential axis are −0.35 V and −0.37 V vs. the normal hydrogen electrode (NHE), respectively, which can be considered as their ECB values. Following this, using eqn (3):
| EVB = ECB + Eg | (4) |
Beyond that, it is widely recognized that the photocatalytic degradation of organic pollutants is primarily accomplished through active species such as photogenerated holes (h+), hydroxyl radicals (˙OH), and superoxide radicals (˙O2−). To determine the predominant active species produced by UCNPs@TiO2 under light illumination, EDTA, BQ and IPA are employed as the specific scavengers for h+, ˙O2− and ˙OH, respectively, in the photodegradation tests of RhB. As illustrated in Fig. 5(d) and Fig. S4 (ESI†), the addition of either EDTA or BQ results in only a minor decrease in the RhB degradation rate compared to the control group without any quenchers. However, the introduction of IPA leads to a significant reduction in the degradation rate from 78% to 38%, suggesting that ˙OH is the dominant reactive species produced by UCNPs@TiO2 under light exposure.
Photocatalytic mechanism
According to the detected reactive species and the UC emission of UCNPs@TiO2, the potential photocatalytic mechanism operating in UCNPs@TiO2 is presented in Fig. 6. Under sunlight irradiation, UV light is directly employed by TiO2 for photocatalytic reaction, while NIR light is converted to UV light via a series of UC processes and then utilized by TiO2. Thereinto, the UC processes initiate with Yb3+ ions absorbing the NIR component of sunlight and pumping to the 2F5/2 level. Subsequently, energy is transferred from the excited Yb3+ ions to the adjacent Tm3+ ions through ET1, ET2 and ET3 routes, enabling the population of Tm3+: 3H5, 3F2 and 1G4 levels. Due to the serious energy mismatch, Tm3+ ions at the 1G4 state are difficult to be excited to the 1D2 level through direct ET with Yb3+ ions. Instead, the population of the Tm3+: 1D2 level is primarily achieved through cross-relaxation processes, such as 3F2 + 3H4 → 3H6 + 1D2. The Tm3+ ions at the 1D2 level can be then populated to the 3P2 level involves an additional ET process (ET4), followed by multistep nonradiative relaxations to realize the population of 1I6 level. Thanks to the excellent matching of Tm3+: 1I6 and 1D2 levels with Eg of TiO2, an extremely efficient FRET process can occur from the Tm3+ ions at the above two states to the TiO2 adsorbed on the surface of UCNPs, allowing TiO2 to generate electrons in the CB and holes in the VB, respectively. More importantly, the suitable potentials of CB and VB in TiO2 compared with the standard redox potential of O2/˙O2− and OH−/˙OH make the electrons and holes easily to be reacted with O2 and OH−/H2O, respectively, resulting in the production of ˙O2− and ˙OH for photocatalysis.Conclusions
In summary, β-NaLuF4:Yb3+/Tm3+ NPs are selected as the efficient converters for NIR-UV light integrated with TiO2 to endow this semiconductor with photocatalytic ability under irradiation of NIR light. By analyzing the steady state and transient spectral data of the samples, a significant FRET process between the UV emission energy levels of Tm3+ (including 1I6 and 1D2) and TiO2 is identified after the successful combination of UNCPs and TiO2, which enables TiO2 to generate electrons and holes in the conduction and valence bands, respectively, for photocatalytic reaction under NIR irradiation. Compared to the reported NIR-responsive photocatalysts composed of the commonly used β-NaYF4:Yb3+/Tm3+ NPs, the UNCPs@TiO2 composite material exhibits much higher photodegradation efficiency and rate for pollutants under both NIR and simulated sunlight irradiation. The preparation of the UNCPs@TiO2 composite material based on β-NaLuF4:Yb3+/Tm3+ NPs not only paves the way for substantially raising the utilization efficiency of sunlight but also provides a new strategy for the design of NIR-driven photocatalysts.Data availability
The data used to support the findings of this study are available from the corresponding author upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (11704054, 52104392) and the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0366, csct2021jcyj-msxmX0578).References
- C. L. Torres-Martínez, R. Kho, O. I. Mian and R. K. Mehra, J. Colloid Interface Sci., 2001, 240(2), 525–532 CrossRef PubMed.
- A. Di Paola, E. García-López, G. Marcì and L. Palmisano, J. Hazard. Mater., 2012, 211, 3–29 CrossRef PubMed.
- D. Chen, Y. Cheng, N. Zhou, P. Chen, Y. Wang, K. Li, S. Huo, P. Cheng, P. Peng, R. Zhang, L. Wang, H. Liu, Y. Liu and R. Ruan, J. Cleaner Prod., 2020, 268, 121725 CrossRef CAS.
- S. Patial, P. Raizada, V. Hasija, P. Singh, V. K. Thakur and V. H. Nguyen, Mater. Today Energy, 2021, 19, 100589 CrossRef CAS.
- X. Zhang, J. Wang, X. X. Dong and Y. K. Lv, Chemosphere, 2020, 242, 125144 CrossRef CAS PubMed.
- P. Singh and A. Borthakur, J. Cleaner Prod., 2018, 196, 1669–1680 CrossRef CAS.
- A. Kubacka, M. Fernandez-Garcia and G. Colon, Chem. Rev., 2012, 112(3), 1555–1614 CrossRef CAS PubMed.
- S. N. Ahmed and W. Haider, Nanotechnology, 2018, 29(34), 342001 CrossRef PubMed.
- D. Bahnemann, Sol. Energy, 2004, 77(5), 445–459 CrossRef CAS.
- X. Qiu, Y. Zhang, Y. Zhu, C. Long, L. Su, S. Liu and Z. Tang, Adv. Mater., 2021, 33(6), 2001731 CrossRef CAS PubMed.
- D. Spasiano, R. Marotta, S. Malato, P. Fernandez-Ibanez and I. Di Somma, Appl. Catal., B, 2015, 170, 90–123 CrossRef.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13(3), 169–189 CrossRef CAS.
- Q. Guo, C. Zhou, Z. Ma and X. Yang, Adv. Mater., 2019, 31(50), 1901997 CrossRef CAS PubMed.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114(19), 9919–9986 CrossRef CAS PubMed.
- A. Meng, L. Zhang, B. Cheng and J. Yu, Adv. Mater., 2019, 31(30), 1807660 CrossRef PubMed.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63(12), 515–582 CrossRef CAS.
- S. Peiris and H. B. De Silva, J. Chin. Chem. Soc., 2021, 68(5), 738–769 CrossRef CAS.
- Q. Wu, X. Lai, W. Li, L. Luo and P. Du, J. Alloys Compd., 2023, 967, 171803 CrossRef CAS.
- Q. Wu, Q. Zhang, W. Li, L. Luo and P. Du, Chem. Eng. J., 2023, 475, 146192 CrossRef CAS.
- Y. Tang, W. Di, X. Zhai, R. Yang and W. Qin, ACS Catal., 2013, 3, 405–412 CrossRef CAS.
- W. Wang, W. Huang, Y. Ni, C. Lu and Z. Xu, ACS Appl. Mater. Interface, 2014, 6(1), 340–348 CrossRef CAS PubMed.
- W. Wang, M. Ding, C. Lu, Y. Ni and Z. Xu, Appl. Catal., B, 2014, 144, 379–385 CrossRef CAS.
- S. Duo, J. Zhang, H. Zhang, Z. Chen, C. Zhong and T. Liu, Opt. Mater., 2016, 62, 240–249 CrossRef CAS.
- X. Guo, C. Chen, S. Yin, W. Song, F. Shi and W. Qin, J. Photochem. Photobiol., A, 2015, 297, 14–19 CrossRef CAS.
- X. Guo, W. Song, C. Chen, W. Di and W. Qin, Phys. Chem. Chem. Phys., 2013, 15(35), 14681–14688 RSC.
- X. Bi, G. He, W. Di and W. Qin, Mater. Lett., 2016, 173, 187–190 CrossRef CAS.
- Y. Zhang, X. Lan, S. H. Park, L. Wang, D. Liu and J. Shi, Carbon, 2020, 169, 111–117 CrossRef CAS.
- Y. Li, J. Zhang, Y. Luo, X. Zhang, Z. Hao and X. Wang, J. Mater. Chem., 2011, 21, 2895–2900 RSC.
- J. Ouyang, D. Yin, X. Cao, C. Wang, K. Song and B. Liu, Dalton Trans., 2014, 43(37), 14001–14008 RSC.
- J. Ouyang, D. Yin, K. Song, C. Wang, B. Liu and M. Wu, J. Nanosci. Nanotechnol., 2015, 15(4), 2798–2803 CrossRef CAS PubMed.
- H. Anwer and J. W. Park, Appl. Catal., B, 2019, 243, 438–447 CrossRef CAS.
- C. Zhang, Z. Fu, F. Hong, J. Dou, T. Dong, Y. Zhang, D. Li, G. Liu, X. Dong and J. Wang, J. Solid State Chem., 2021, 300, 122248 CrossRef CAS.
- G. Xiang, M. Yang, Z. Liu, Y. Wang, S. Jiang, X. Zhou, L. Li, L. Ma, X. Wang and J. Zhang, Inorg. Chem., 2022, 61(13), 5425–5432 CrossRef CAS PubMed.
- C. Song, Z. Ye, G. Wang, J. Yuan and Y. Guan, ACS Nano, 2010, 4(9), 5389–5397 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc02695k |
| This journal is © The Royal Society of Chemistry 2024 |