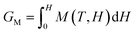Composition and microstructural control of Sm2Fe17N3 powders: a promising candidate for next-generation permanent magnets
Junjie
Xu†
a,
Yuhao
Yi†
a,
Boqian
Jia
a,
Hang
Xue
a,
Guang
Tian
b,
Zhenhui
Ma
 c and
Yanglong
Hou
c and
Yanglong
Hou
 *de
*de
aXi’an Rare Metal Materials Institute Co., Ltd, Xi’an 710000, China
bSchool of Physics, Peking University, Beijing 100871, China
cDepartment of Physics, Beijing Technology and Business University, Beijing 100048, China
dBeijing Key Laboratory for Magnetoelectric Materials and Devices, School of Materials Science and Engineering, Peking University, Beijing 100871, China. E-mail: hou@pku.edu.cn
eSchool of Materials, Shenzhen Campus of Sun Yat-Sen University, Shenzhen 518107, China
First published on 29th August 2024
Abstract
Sm2Fe17N3 powders have drawn much attention in both fundamental research and industrial applications due to their excellent magnetic properties. The magnetic properties of Sm2Fe17N3 powders mainly depend on the composition and microstructure. Herein, the intrinsic and extrinsic magnetic properties of Sm2Fe17N3 powders are introduced, where the correlation between the composition, microstructure, and magnetic properties is discussed. Based on the correlation, we provide a detailed description of the synthesis methods and regulation strategies for Sm2Fe17N3 powders, aiming to promote the design and preparation of Sm2Fe17N3 powders with better magnetic properties.
1. Introduction
Sm2Fe17N3 is expected to be a promising candidate for the next-generation rare-earth permanent magnets due to the huge anisotropy field (14 T), large saturation magnetization (1.54 T), and high Curie temperature (475 °C).1–3 In addition to the intrinsic magnetic properties comparable to or better than Nd2Fe14B, the resistivity of Sm2Fe17N3 is higher than that of Nd2Fe14B, which means lower current loss at high frequency. Thus, Sm2Fe17N3 is more suitable for high-frequency applications, such as magnets in electric vehicles.4–6Up to now, Sm2Fe17N3 has been widely used in the form of resin-bonded magnets because it decomposes at around 873 K, making sintering difficult.7,8 Nevertheless, it has been demonstrated that there is a high possibility of achieving high-performance sintered Sm2Fe17N3 magnets by a low-oxygen powder metallurgy process.9,10 Whether in the case of the resin-bonded or sintered Sm2Fe17N3 magnets, one of the most important issues is to prepare Sm2Fe17N3 powders with excellent coercivity, remanence, and maximum energy product.3 Generally, Sm2Fe17N3 powder is obtained by the nitridation of Sm2Fe17 powders. In the Sm–Fe binary system, Sm2Fe17 is formed by the peritectic reaction between solid Fe and liquid Sm–Fe, which would easily result in residual soft magnetic α-Fe, deteriorating the coercivity and remanence of Sm2Fe17N3.11,12 Thus, several techniques have been developed to prepare single-phase Sm2Fe17 powders, such as rapid-quenching,11,13 reduction–diffusion,14,15 hydrogenation–disproportionation–desorption–recombination (HDDR),16,17 and so on.18,19
The nitridation process directly determines the nitrogen content and distribution in Sm2Fe17Nx powders. The magnetic anisotropy would vary with the nitrogen content in Sm2Fe17Nx and it is considered that Sm2Fe17N3 would exhibit the best comprehensive magnetic properties.20 In addition to the nitrogen content, the nitrogen distribution is also important. Nonuniform nitrogen distribution in Sm2Fe17N3 would deteriorate the magnetic properties because the region with weaker magnetic anisotropy would act as nucleation centers of reverse domains. Therefore, Sm2Fe17N3 powder with homogenous nitrogen content has been pursued to achieve excellent magnetic properties.
The coercivity of Sm2Fe17N3 powders is mainly dominated by the nucleation of reverse domains. Thus, the coercivity of Sm2Fe17N3 powders is sensitive to the microstructure and the key to enhancing coercivity is to prohibit nucleation of reverse domains.21–23 In theory, Sm2Fe17N3 powders with a critical single-domain size and a smooth as well as undamaged surface would exhibit the largest coercivity.24–26 It should be noted that the size of Sm2Fe17N3 powders for the highest coercivity would deviate from the critical single-domain size due to surface oxidation and defects. Thus, many efforts have been made to regulate the size of Sm2Fe17N3 powders and inhibit surface oxidation for coercivity optimization.14,27
Herein, we will briefly introduce bonded Sm2Fe17N3 magnets as well as the intrinsic and extrinsic magnetic properties of Sm2Fe17N3 powders and then the efforts in the design and preparation of high-performance Sm2Fe17N3 powders will be discussed in detail. Last but not least, the prospects for Sm2Fe17N3 will be addressed.
2. Sm2Fe17N3 magnets
2.1. Bonded Sm2Fe17N3 magnets
MagValley, Sumitomo Metal Mining Co. Ltd, Nichia Corporation, and Daido Steel Co. Ltd have commercialized bonded Sm2Fe17N3 magnets, which are cheaper than bonded Nd–Fe–B magnets due to the lower cost of Sm. Specifically, bonded Sm2Fe17N3 magnets exhibit higher resistance to corrosion and heat than bonded Nd–Fe–B magnets. As a result, they have found widespread applications in products such as disk suction devices, automation robots, magnetic sensors, and more.Bonded Sm2Fe17N3 magnets are produced using compression and injection molding techniques, which are commonly employed for the production of other bonded rare-earth permanent magnets. Isotropically bonded Sm2Fe17N3 magnets have a maximum energy product ranging from 9 to 14 MGOe, which exceeds that of bonded Nd–Fe–B magnets (5–11 MGOe). Additionally, the irreversible flux loss for isotropically bonded Sm2Fe17N3 magnets without surface coating, when exposed to air at 120 °C and hot water at 70 °C for 1000 hours, is both less than 10%. In contrast, the irreversible flux loss for bonded Nd–Fe–B magnets under the corresponding environment is approximately 25% and 40%, respectively. Consequently, isotropically bonded Sm2Fe17N3 magnets are considered to have the most superior magnetic properties among the bonded permanent magnets. Despite the significant advancements in isotropically bonded Sm2Fe17N3 magnets, anisotropically bonded Sm2Fe17N3 magnets exhibit lower coercivity (15–17 kOe) and maximum energy product (12–16 MGOe) compared to anisotropically bonded Nd–Fe–B magnets (15–18 kOe, 21–23 MGOe).28 Therefore, it is essential to design and prepare high-performance Sm2Fe17N3 powders because the magnetic properties of these powders limit the performance of the resulting bonded magnets.
2.2. Intrinsic magnetic properties
It is well-known that the intrinsic magnetic properties rely on the chemical composition and crystal structure.29–31 Both Sm2Fe17 (Fig. 1a) and Sm2Fe17N3 (Fig. 1b) crystallize in rhombohedral structure with the space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m.28,32 The Fe sublattice in Sm2Fe17 exhibits basal-plane anisotropy, resulting in the basal-plane anisotropy of Sm2Fe17. The small distance of dumbbell Fe–Fe sites leads to negative exchange interactions, responsible for the low Curie temperature of Sm2Fe17 (135 °C). Therefore, Sm2Fe17 could not be applied as permanent magnets.
m.28,32 The Fe sublattice in Sm2Fe17 exhibits basal-plane anisotropy, resulting in the basal-plane anisotropy of Sm2Fe17. The small distance of dumbbell Fe–Fe sites leads to negative exchange interactions, responsible for the low Curie temperature of Sm2Fe17 (135 °C). Therefore, Sm2Fe17 could not be applied as permanent magnets.
 | ||
| Fig. 1 Side and top views of crystal structures of (a) Sm2Fe17, (b) Sm2Fe17N3, and (c) Sm2Fe17Nx (x > 3). | ||
The introduction of nitrogen atoms into the Sm2Fe17 unit cell would result in Sm2Fe17Nx with an unchanged unit cell structure and profoundly changed intrinsic magnetic properties. Generally, the nitrogen atom could only occupy the 9(e) octahedral interstices in Sm2Fe17 considering the radius of the nitrogen atom. Thus, the concentration of interstitial nitrogen in Sm2Fe17Nx would range from 0 to 3. With the increase of x, the magnetic properties become better and Sm2Fe17N3 would exhibit the best comprehensive magnetic properties. It should be noted that Sm2Fe17Nx with x > 3 has been obtained and the extra nitrogen atoms would occupy the 3b or 18 g interstitial sites along the c-axis (Fig. 1c). However, nitrogen atoms at 3b or 18 g interstitial sites would reduce the magnetic moment, Curie temperature, and easy-axis character.33
As shown in Fig. 1b, the Sm atoms (6c) are coordinated by the triangular interstitial nitrogen atoms (9e) in Sm2Fe17N3. A strong negative electric field gradient is created on Sm atoms, which changes the second-order crystal-field coefficient A20.34 The Sm–N bonding contributes to the axial anisotropy of the Sm sublattice and the room-temperature first-order anisotropy constant significantly increases from −0.8 MJ m−3 for Sm2Fe17 to 8.6 MJ m−3 for Sm2Fe17N3.35
In addition to the change of the anisotropy field, the introduction of nitrogen atoms to Sm2Fe17 would also enhance the Curie temperature and magnetic moment. The interstitial nitrogen atoms induce an increase in the average Fe–Fe distances, which leads to a high saturation magnetization of Sm2Fe17N3 (1.54 T). Besides, the increase of average Fe–Fe distance induces the increase of exchange interactions (from negative to positive), responsible for the high Curie temperature of Sm2Fe17N3 (475 °C).36 With a high anisotropy field, saturation magnetization, and Curie temperature, Sm2Fe17N3 could be applied as permanent magnets.
In spite of the enhancement in magnetic properties induced by interstitial N atoms in Sm2Fe17, interstitial C or B atoms could not change the magnetic properties profoundly, which is limited by the small absorption content. For Sm2Fe17Cx, the maximum amount of C atoms is at x = 1.5, which shows a volume expansion of 2.3% compared to Sm2Fe17, although the value of x can reach 2.3 or 2.5 due to the higher reactivity of C atoms in the gas phase. The absorption limit of B atoms is much lower in Sm2Fe17, typically around x = 0.2, leading to a volume expansion of 0.3%. The change in magnetic properties largely depends on the degree of lattice expansion. However, the lattice expansion induced by N or C atoms is far lower than that of N atoms. Thus, the introduction of B atoms would almost not change the magnetic properties. The increase in saturation magnetization and Curie temperature induced by C atoms is about half that of N atoms.37
2.3. Extrinsic magnetic properties
The magnetic properties of Sm2Fe17N3 powders are critical to the performance of their resin-bonded magnets. The coercivity, remanence, and maximum energy products of Sm2Fe17N3 powders are commonly concerned. The coercivity of anisotropic Sm2Fe17N3 powders is mainly controlled by the nucleation of reverse domains, which could be enhanced by the optimization of composition, particle size, or surface quality.1Concerning the composition, it is necessary to avoid the soft magnetic phases. The soft magnetic phase does not specifically refer to the α-Fe or Sm-rich phase but also contains a nitrogen-poor region in Sm2Fe17Nx powders. It is quite easy to induce the α-Fe or Sm-rich phase impurities during the preparation of Sm2Fe17 powders. Nitrogen atoms gradually diffuse into the interior of Sm2Fe17 powders during the nitridation process, which easily results in the inner core being poor in nitrogen. The nitrogen-poor region displays lower magnetic anisotropy, acting as a nucleation center during the magnetization reversal process.
The particle size of Sm2Fe17N3 powders depends on the magnetic domain structure and magnetic anisotropy energy. Magnetic domains arise from dipole interactions. When the particle is small enough, the energy for the domain wall is larger than the external magnetostatic energy, which means the formation of a single-domain particle. The diameter for the critical single-domain particle (Dsd) could be expressed as follows:
| Dsd ≈ 18(AK1)1/2/μ0Ms2 |
As the size multi-domain Sm2Fe17N3 powders decreases, the coercivity would increase. The coercivity would reach a maximum when the size reduces to Dsd where the particles have a single-domain structure. To reduce the size of single-domain Sm2Fe17N3 powders, the magnetic anisotropy energy (K1V, where V represents the volume) would decrease, and the coercivity would also decrease. Hence, the particle size of anisotropic Sm2Fe17N3 powders should be regulated to Dsd for magnetic properties optimization. It should be noted that Dsd would deviate from the theoretical value because of the surface defects.24
In terms of the surface, it should be smooth and undamaged to reduce nucleation of reverse domains. The reverse domain would nucleate by a spontaneous fluctuation and the nucleus volume is on the order of δw3 where δw is the domain wall width. Once the nucleus forms, it propagates; otherwise, it is pinned. Due to the small size of δw for Sm2Fe17N3 (3.7 nm), even the surface asperities would act as nucleation centers.39 Thus, the surface quality for Sm2Fe17N3 powders should be good enough to achieve higher magnetic properties.
Based on the understanding of the intrinsic and extrinsic magnetic properties of Sm2Fe17N3 powders, controlling the composition and microstructure of Sm2Fe17N3 powders is discussed in the subsequent section.
3. Preparation of Sm2Fe17N3 powders
Generally, Sm2Fe17N3 powders are prepared by nitriding Sm2Fe17 master alloy powders. Herein, the preparation and nitridation of Sm2Fe17 master alloy powders is discussed in detail.3.1. Preparation of Sm2Fe17 master alloy powders
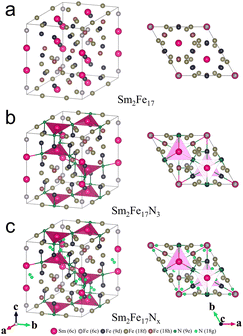
The common strategies for the preparation of Sm2Fe17 powders are rapid quenching, reduction–diffusion, and HDDR. As shown in Fig. 2a, Sm2Fe17 was formed by the peritectic reaction between liquid Sm–Fe (LSm–Fe) and solid Fe at 1010 °C, which grows along the Fe/LSm–Fe interface (Fig. 2b (I → II)). And the equation is LSm–Fe + Fe → Sm2Fe17. From II to III in Fig. 2b, the peritectic transformation occurs, and LSm–Fe and solid Fe transform directly into Sm2Fe17. As the Sm2Fe17 phase extends towards both LSm–Fe and solid Fe gradually (Fig. 2b (III → IV)), the peritectic reaction is complete. Thus, the peritectic reaction would cost a lot of time, easily resulting in an incomplete reaction. The incomplete peritectic reaction resulted in residual soft magnetic α-Fe and Sm-rich phases. The soft magnetic α-Fe acted as nucleation centers of the reverse domain, adversely affecting the magnetic properties of Sm2Fe17N3, while the Sm-rich phase partially decomposed into SmN and α-Fe during the subsequent nitridation process.11 Besides, the evaporation of Sm at high temperatures also made the preparation of single-phase Sm2Fe17 difficult.40–42 Therefore, obtaining single-phase Sm2Fe17 master alloy powders was essential for high-performance Sm2Fe17N3. In this section, Sm2Fe17 master alloy powders prepared by different methods will be overviewed.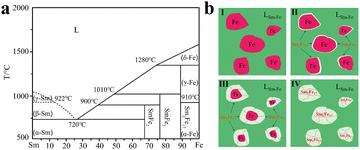 | ||
| Fig. 2 (a) Sm–Fe binary phase diagram, (b) schematic illustration of the peritectic reaction between LSm–Fe and solid Fe. | ||
Katter and co-workers prepared Sm2Fe17 by rapid quenching and then obtained Sm2Fe17Nx by nitridation for the first time.13 During the melting process, Sm evaporation led to a decrease in Sm concentration, leading to the formation of the SmFe9 phase with a TbCu7-type structure after quenching. Therefore, a moderate excess of Sm is required to compensate for Sm loss. Quenching rates would also affect the phase composition. With an increase in the wheel velocity, the amount of α-Fe would decrease. However, when the quenching rate is above 15 m s−1, the SmFe9 phase would be observed. Since the magnetic properties of SmFe9Nx are inferior to those of Sm2Fe17Nx, it is necessary to avoid the formation of the SmFe9 phase. In addition to SmFe9, α-Fe is always present in rapid-quenched ribbons and could be eliminated by annealing. However, the annealing process may result in Sm loss. The Sm2Fe17Nx powders prepared by rapid-quenched ribbons with the composition, quenching rate, annealing temperature, and time of Sm12Fe88, 60 m s−1, 730 °C, and 15 min, respectively, showed the best magnetic properties with the coercivity, remanence, and maximum energy product of 2.10 T, 0.73 T, and 8.24 MGOe, respectively. However, such powders were magnetically isotropic and remained soft α-Fe, resulting in a kink on the demagnetization curve.
To achieve better magnetic properties, Sm2Fe17N3 powders should be magnetically anisotropic and free of secondary phases (Sm-rich phase and α-Fe). It has been demonstrated that Sm2Fe17 ingots with fine columnar grains of the same orientation could be obtained by controlling the cooling rate and crystallization process, which lays the foundation for the preparation of anisotropic Sm2Fe17N3 powders.47 Concerning the secondary phase, it was expected that a strip-casting technique with an enhanced cooling rate could suppress α-Fe in Sm2Fe17 strips.48 Thus, the annealing process would also be simplified.
Kolodkin and co-workers prepared Sm2.4Fe17 ingots by induction melting, and then the strip-casting technique was followed, resulting in Sm2.08Fe17 strips.49 It was found that the volume of the secondary phase (Sm-rich phase and α-Fe) in Sm2.08Fe17 strips was lower than that in Sm2.4Fe17 ingots. The secondary phase in Sm2.08Fe17 strips could be eliminated by annealing at 1000 °C for 1 h, whereas α-Fe remained in the bulk Sm2.4Fe17 alloy even after annealing at 1050 °C for 20 h. In addition, the strip-casting process refined the grain size of Sm2Fe17, which could promote the nitridation process. The Sm2.08Fe17 strips were crushed and nitrided, obtaining Sm2.08Fe17N3 powders with a coercivity of 0.93 T.
Furthermore, Yang and co-workers prepared single-phase Sm2Fe17 strips using a strip-casting process without annealing the Sm2Fe17 ingot.50 After the nitridation process, Sm2Fe17N3 was ball-milled into powders with different diameters. As shown in Fig. 3a, the coercivity increased with a decrease in particle size and coercivity could reach 1.75 T when the particle size decreased to 1.3 μm. It should be noted that the relationship between coercivity and size was consistent with the aforementioned single-domain theory. In contrast, the saturation magnetization decreased with a decrease in particle size, which may be ascribed to surface oxidation. In addition, the temperature-dependent magnetic properties were also studied (Fig. 3b). The coercivity could increase to 3.61 T when the temperature decreased to 10 K. Increasing the temperature to 373 K, the saturation magnetization decreased to 128 emu g−1 and the remanence temperature coefficient was lower than that of Nd2Fe14B.
 | ||
| Fig. 3 (a) Dependencies of coercivity and saturation magnetization on particle size. (b) Hysteresis loops of the powders ground for 4 h at 10, 300, and 373 K. (a) and (b) Reproduced from ref. 50 with permission from the American Institute of Physics, copyright 2023. (c) The BSE image of the cross-section of nitrided SmFeCuN particles, (d) normalized demagnetization curves of the SmFeCuN powders. (c) and (d) Reproduced from ref. 11 with permission from Elsevier BV, copyright 2019. | ||
In addition to the optimization of the rapid quenching process, many efforts have been made to investigate nonmagnetic phases equilibrated with Sm2Fe17. Zhu and co-workers introduced Cu into the Sm–Fe system and achieved a three-phase region of SmCu, SmCu2, and Sm2Fe17.11 Thus, an ingot free of a α-Fe and Sm-rich phase could be obtained in the presence of nonmagnetic SmCu and SmCu2, which caused less damage to the magnetic properties of Sm2Fe17N3. The back-scattered electron (BSE) image (Fig. 3c) of the nitrided particles indicated the presence of nitrided Sm–Cu. The anisotropic Sm2Fe17N3 powders with Cu addition showed a coercivity and maximum energy product of 1.39 T and 31 MGOe, respectively (Fig. 3d).
Though single-phase anisotropic Sm2Fe17 powders could be achieved by a rapid quenching process, it seems that the elimination of the magnetic secondary phase still requires long-term annealing, which is not beneficial for industrial applications. Maybe, there is a compromise between the cost and magnetic performance by introducing some other elements into the Sm–Fe binary system to hinder the formation of soft magnetic secondary phases.
In the early stage, commercial micro-sized Sm2O3 and Fe were applied as precursors to verify the feasibility of the reduction–diffusion process.57 As shown in Fig. 4a, the precursors were mechanically milled with reduction agent (Ca or CaH2 powders) and then heated to a temperature higher than the melting point of Ca. Molten Ca would reduce Sm2O3 and then inter-diffusion of Sm and Fe would be responsible for the formation of Sm2Fe17, similar to the recombination of Sm and Fe in the HDDR process to some content. A moderate excess of Sm2O3 was required to compensate for Sm evaporation, critical to the generation of single-phase Sm2Fe17 powders without impurities (α-Fe or Sm-rich phases).58 It has been demonstrated that excessive Sm2O3 may lead to the formation of a Sm-rich phase, which could be washed with dilute acid.59 Besides, the amount of reduction agent should be larger than the theoretical value to guarantee that all Sm2O3 could be reduced.
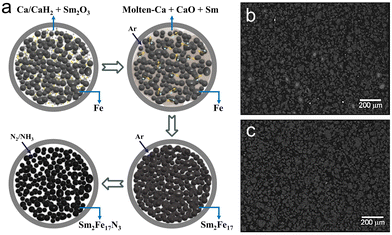 | ||
| Fig. 4 (a) Fabrication process for Sm2Fe17N3 powders by reduction–diffusion from commercial micro-sized Sm2O3 and Fe applied as precursors. BSE images of the cross-section for the Sm2Fe17N3 coarse powder converted from (b) washed Sm2Fe17 powders and (c) unwashed Sm2Fe17 powders. (b) and (c) Reproduced from ref. 60 with permission from the Institute of Physics, copyright 2011. | ||
The formation mechanism of Sm2Fe17 powders using the reduction–diffusion process has been investigated by Asaki and co-workers in detail.61 The reaction kinetics was strongly related to the reaction temperature, which affected the reduction rate of Sm2O3 and the state of Sm. At 1300 K, the reduction rate of Sm2O3 was not high enough and Sm existed in a solid state. Thus, the inter-diffusion of Sm and Fe resulted in the formation of Sm2Fe17 and the growth rate of Sm2Fe17 followed the parabolic law. When the temperature was above the melting point of Sm (1347 K), the reduction rate of Sm2O3 increased and Fe would dissolve in the Sm or Sm–Ca melt. Sm2Fe17 and SmFe2 would precipitate during the cooling process. Therefore, the high temperature may be not good for the formation of single-phase Sm2Fe17 because the reaction is similar to the peritectic reaction. Besides, the high temperature would lead to the growth and agglomeration of Sm2Fe17 powders, and the magnetic properties of Sm2Fe17N3 prepared by the nitridation of such Sm2Fe17 powders would be degraded. Therefore, the temperature for the reduction–diffusion process should be in the range of the melting points of Ca and Sm.
Intuitively, the by-product CaO and the residual reduction agent should be directly eliminated by water washing after the reduction–diffusion process. Due to the low solubility of generated Ca(OH)2, a long-term washing was necessary, which inevitably led to oxidation and corrosion of the obtained Sm2Fe17 powders.62,63 The surface oxidation and corrosion were not beneficial to nitridation. Although Sm2Fe17 powders were exposed to a careful nitridation process, it is still possible that some Sm2Fe17 powders may not be fully nitrided, which would deteriorate the coercivity, saturation magnetization, and squareness ratio. Fortunately, the nitridation process was modified by Ishikawa and co-workers, where Sm2Fe17 powders were nitrided without washing because the surface of such Sm2Fe17 powders was quite clean.60 The nitrogen distribution was studied by BSE imaging of the cross-section of coarse Sm2Fe17Nx powders. As shown in Fig. 4b, there were some white cores in the BSE image of Sm2Fe17Nx converted from washed Sm2Fe17, which corresponded to un-nitrided Sm2Fe17. In the BSE image of Sm2Fe17Nx prepared from unwashed Sm2Fe17 powders (Fig. 4c), white cores could almost not be observed. The ratio of the white core area to the whole powder area in Fig. 4b and c was 0.73% and 0.04%, respectively, which proved that unwashed Sm2Fe17 powders with a clean surface could promote the nitridation process dramatically. Hence, nitridation of unwashed Sm2Fe17 powders has been widely used for the preparation of Sm2Fe17N3 powders nowadays.
After the nitridation process, the by-product CaO and residual reduction agent still need to be removed. Using a direct water washing process, a metastable SmFe5 phase with poor hard magnetic properties was detected using synchrotron X-ray diffraction, which is attributed to the crystallization of the Sm-rich phase driven by the reaction heat between Ca and H2O.59 In addition, oxygen could stabilize SmFe5, and the higher the oxygen content, the worse the magnetic properties and heat resistance. To achieve higher magnetic performance, the washing process was carried out in a glove box and ethylene glycol was applied to eliminate CaO and Ca. However, the efficiency is far lower than that of the water-washing process. Fortunately, a much more practical strategy has been developed by Okada and co-workers.59 Before the washing process, the powder was subjected to dilute air at ambient temperature to slowly oxidize residual Ca. The slowly-oxidized powders could be directly washed with water or weak acid in air. The magnetic properties of Sm2Fe17N3 powders washed after pre-oxidation were better than those washed without pre-oxidation.
Concerning the mechanical mixing of Sm2O3, Fe, and Ca powders to prepare Sm2Fe17 powders, there remained two major problems. Insufficient mixing would result in impurities in the products due to the inadequate reaction. In addition, the size of the obtained Sm2Fe17 powders is quite large, unfavorable for the magnetic properties. Fortunately, the Sm–Fe–O precursors have been designed using the wet-chemistry method to control the phase and size of Sm2Fe17 and hinder agglomeration. The design of Sm–Fe–O precursors for Sm2Fe17N3 powders with high performance is discussed in the next section.
Under the H2 atmosphere, when the temperature increased to 250–300 °C, Sm2Fe17 would absorb H atoms, resulting in the formation of Sm2Fe17Hx. The higher the pressure of H2, the easier it is for H atoms to enter the lattice of Sm2Fe17. Increasing the temperature to 500 °C, the disproportionation reaction would proceed near the grain boundaries and SmH2 as well as α-Fe with a microcrystalline or amorphous structure would be generated. With a further increase in temperature, SmH2 and α-Fe fully crystallized. The grain size and morphology of SmH2 and α-Fe were essential to the control of the size and microstructure of recombined Sm2Fe17. Therefore, the disproportionation of Sm2Fe17Hx was conducted at around 750 °C to achieve a small grain size of SmH2 and α-Fe.17 Once the temperature was high enough, SmH2 and α-Fe could recombine to form Sm2Fe17 even in an H2 atmosphere, whereas high temperature induced grain growth.
To obtain recombined Sm2Fe17 powders with fine grain size, SmH2 and α-Fe with small grain sizes were subjected to vacuum conditions. When the temperature was higher than 600 °C, desorption of SmH2 and recombination of highly reactive Sm and α-Fe occurred almost simultaneously. Recombination of Sm and Fe was ascribed to the inter-diffusion of Sm and Fe, and a low temperature is necessary to hinder the grain growth.64
Even after the HDDR process, some un-recombined α-Fe and large recombined grains may be detected, whereas single-phase Sm2Fe17 powder with a uniform and fine grain is essential for the coercivity of Sm2Fe17Nx powders. Homma and co-workers have demonstrated that sufficient time for the recombination of Sm and α-Fe is a prerequisite to avoid residual α-Fe.65 However, the prolonged recombination process may lead to Sm loss and grain growth. Later, Kwon and co-workers applied the HDDR treatment repeatedly on the Sm2Fe17 powders to eliminate un-recombined α-Fe and large recombined grains as well as obtain homogeneous and fine Sm2Fe17 grains.66 Even though the strategy worked, the process cost too much time.
In order to fully achieve the recombination process in a short period and avoid residual α-Fe, some other elements have been introduced into the Sm–Fe binary system to regulate the disproportionation and recombination process, which might also affect the phase and microstructure of recombined Sm2Fe17.66,67 For example, Ga addition decreases the recombination temperature, making it much easier for full recombination, and the lower recombination temperature is beneficial for inhibiting grain growth.68
It has been anticipated that magnetically anisotropic Sm2Fe17N3 powders could be obtained by the HDDR process. However, there were almost no reports of anisotropic Sm2Fe17N3 powders prepared using the HDDR process. Fortunately, anisotropic Nd2Fe14B powders have been fabricated using the HDDR process. Strictly speaking, the grains in anisotropic Nd2Fe14B powders by the HDDR process were highly textured. The texture could be ascribed to the crystallographic orientation of Fe2B grains which “memorize” the orientation of initial Nd2Fe14B powders.69 It has been reported that the disproportionation of Sm2Fe17 could result in SmH2 with the same orientation whereas the orientation could not be maintained after the recombination process.70 Hence, more efforts are deserved to prepare anisotropic Sm2Fe17N3 powders with uniform and fine grain size using the HDDR process and the microstructure change during the HDDR process should be of special concern.
Mechanical alloying for the preparation of Sm2Fe17 powders has also been studied. The high-energy ball milling of Sm and Fe powders and the following nitridation would result in nanocrystalline Sm2Fe17N3 powders. Therefore, such Sm2Fe17N3 powders are magnetically isotropic.18,19,73 The coercivity of such Sm2Fe17N3 powders could reach up to 4.4 T due to the strong pinning.74 However, the remanence of such powders is not high, limiting the maximum energy product.
3.2. Nitridation
N atoms enter 9(e) octahedral interstices in the Sm2Fe17 lattice through a gas–solid reaction, resulting in the formation of Sm2Fe17Nx powders.75,76 First, N2 or NH3 is physically adsorbed on the surface of Sm2Fe17 powders and decomposed into N atoms with high activity. Then, N atoms diffused into Sm2Fe17 powders to form the nitrided phase. Finally, N atoms diffused from the fully nitrogenated phase to the poorly nitrogenated phase due to the concentration gradient and the diffusion of N atoms proceeded by voidal diffusion in Sm2Fe17.77,78 The N atoms in the 9(e) site jumped to the thermodynamically unstable 18(g) site and then to a new 9(e) site. Due to the anisotropic lattice structure of Sm2Fe17, the diffusion of N atoms was anisotropic.79 The diffusion in the c plane is isotropic and the diffusivity in the c plane was about 0.3 times that along the c axis. It should be noted that the diffusion of N atoms required energy to break the strong bonding between N atoms and neighboring atoms and the energy was almost independent of the nitrogen source.77Based on Fick's law, the size of Sm2Fe17 powders markedly affected the diffusion of N. Numerous reports have proved that Sm2Fe17 powders with a size of tens of micrometers could be nitride, and it has been demonstrated that the N diffusion coefficient increased with the decrease in particle size (5–500 μm).78 So many efforts have been made to optimize the nitridation process for the improvement of nitridation efficiency. Generally, the nitridation process was regulated by the nitrogen source, temperature, pressure, time, and magnetic field, which will be discussed in this section.
Using N2 as a nitrogen source, Sm2Fe17N2.7 powder that was not fully nitrided was usually achieved under atmospheric pressure due to the low activity of N2. Therefore, the increase in N2 pressure was investigated to increase the nitrogen content. Koyama and co-workers studied the nitridation of Sm2Fe17 under N2 with a pressure ranging from 0.01 to 6 MPa.81,82 As shown in Fig. 5a, with N2 pressure below 0.05 MPa, the nitridation process is mainly controlled by the diffusion of N. Thus, Sm2Fe17 powders were hard to be fully nitrided and N atoms in the obtained Sm2Fe17Nx powders were distributed in a gradient. Once the N2 pressure was above 0.1 MPa, the grain growth of the fully nitrided phase would be dominant and Sm2Fe17N3 could be achieved. In addition, higher pressure of N2 could inhibit the formation of α-Fe during the nitridation process. From an empirical point of view, the nitridation rate at N2 pressure of 0.1 MPa should be higher than that of 0.05 MPa. However, it was revealed that the nitrogen rate with the N2 pressure of 0.1 MPa was lower than that at 0.05 MPa in the early nitridation stage. In other words, low N2 pressure promoted the formation of incompletely nitrided Sm2Fe17Nx and high N2 pressure was beneficial to the formation of Sm2Fe17N3. Therefore, the nitridation efficiency could be raised by the combined pressure process with N2 pressure of 0.05 and 0.1 MPa in early and later stages rather than by just increasing N2 pressure.
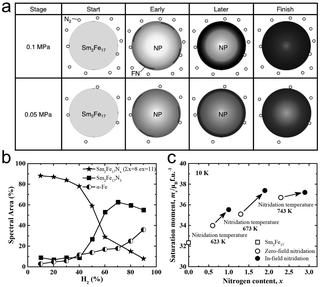 | ||
| Fig. 5 (a) Schematic of the nitridation process of Sm2Fe17 powders at different N2 pressure. (a) Reproduced from ref. 82 with permission from Elsevier, copyright 2022. (b) Effect of H2 content on the composition. (b) Reproduced from ref. 76 with permission from Elsevier, copyright 2004. (c) Effect of magnetic field on the nitrogen content and the saturation moment. (c) Reproduced from ref. 83 with permission from the Japan Institute of Metals, copyright 2020. | ||
In addition to increasing the N2 pressure, a mixture of N2 and H2 was also applied to adjust the nitrogen content and nitridation efficiency. As mentioned earlier, Sm2Fe17 powders would absorb H, which would induce strain, resulting in an incoherent interface between Sm2Fe17Nx and Sm2Fe17. The incoherent interface was beneficial to the diffusion of N atoms. Besides, the absorption of H would also lead to cracks in the powders, increasing the diffusion paths. It was thought that H atoms entered the lattice of Sm2Fe17, which resulted in lattice expansion, beneficial to N atoms diffusion. However, the introduction of H atoms did almost not change the activation energy for diffusion.78 Using the plasma of a mixture of N2 and H2 as a nitrogen source, Gama and co-workers obtained Sm2Fe17N3 powders with a nitridation temperature of 400 °C.76 As shown in Fig. 5b, when the concentration of H2 was low, poor-crystalline or amorphous Sm2Fe17N8 and Sm2Fe17N11 phases rich in nitrogen would be generated. Though excessive N atoms could be desorbed by annealing, they resulted in distortion or destruction of the crystal lattice. Increasing H2 concentration, the nitrogen-rich phase would reduce and Sm2Fe17N3 would become the dominant phase. However, the high H2 concentration may lead to the formation of α-Fe.
For higher nitridation efficiency, NH3 was applied as a nitrogen source, which decomposed into N2 with high activity and H2. Sm2Fe17N10 could be easily obtained under the NH3 atmosphere. To avoid irreversible lattice distortion induced by excessive N atoms, NH3 was mixed with H2 to suppress the activity of N atoms.84,85 Due to the high nitridation efficiency, the mixture of NH3 and H2 was widely used despite the potential dangers.
As mentioned earlier, the nitridation efficiency was improved by regulation of nitrogen source, plasma, pressure increase of nitrogen source, and so on. However, the nitridation process still needed several to dozens of hours and a little Sm2Fe17Nx may decompose during the nitridation process. Therefore, efforts are deserved for the optimization of the nitridation process to achieve short-time nitridation.
where M, T, and H represented the magnetic moment, temperature, and magnetic field, respectively.86,87 The Curie temperature of Sm2Fe17 is far lower than the nitridation temperature, so nitridation of Sm2Fe17 would theoretically become easier in favor of GM. Furthermore, the diffusion of N atoms from the fully nitrogenated phase to the poorly nitrogenated phase would also be enhanced by the external magnetic field for the magnetic moment of poor nitrogenated Sm2Fe17 was inferior to that of Sm2Fe17N3.
Koyama and co-workers performed the nitridation of Sm2Fe17 under a zero-field and in-field of 5 T.83 As shown in Fig. 5c, compared to Sm2Fe17Nx powders prepared under a zero-field, the magnetic field induced 0.4–0.6 N atoms increase per unit cell. Inspiringly, with the assistance of a magnetic field, Sm2Fe17N2.9 has been prepared at an N2 pressure of 0.1 MPa at 743 K, attributed to that GM approximately corresponded to a thermal energy of 35 K. For a more comprehensive study of the magnetic field effect, magnetostriction should also be considered, although it is not quite easy.
4. Magnetic properties optimization
As mentioned earlier, surface quality and particle size optimization of Sm2Fe17N3 powders could effectively inhibit the nucleation of the reverse domain, resulting in the enhancement of magnetic properties. In addition, some other elements are doped into Sm2Fe17N3 for higher remanence, coercivity, Curie temperature, oxidation resistance, and so on.4.1. Surface quality
Sm2Fe17 strips or ribbons should be roughly crushed for nitridation and then the nitrides were further pulverized for coercivity optimization. The crushing process would cause damage to the surface which is not beneficial to the magnetic properties.The surface quality of crushed Sm2Fe17 powders is not commonly concerned. Recently, it was pointed out that the pulverization process resulted in poor crystallinity and nanocrystalline grains on the surface of Sm2Fe17 powders even though the jet milling was carried out in a glove box.88 During the jet milling process, the impact, friction, and shear led to transient high temperatures at the powder surface. Due to that, residual oxygen in the jet-milling chamber would absorb on the surface and the region rich in Sm and O as well as α-Fe would generate. It is anticipated that the wet milling process is applied to crush Sm2Fe17 whereas the effect of solvent on the nitridation process is not studied.
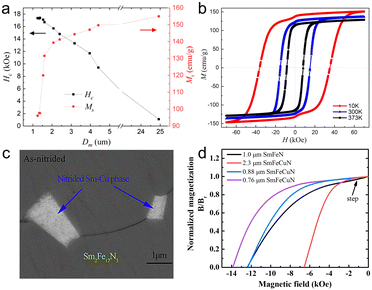
Sm2Fe17N3 powders with an average diameter (D50) ranging from 1.1 μm to 3.0 μm have been obtained by jet-milling in a very low oxygen atmosphere.89 With the decrease in powder size, the crystallinity became weaker, which was ascribed to the nanocrystalline region emerging at the surface induced by rapid heat during the milling process. Once the size was reduced to 1.3 μm, the best comprehensive magnetic properties could be achieved with a coercivity, remanence, and maximum energy product of 1.27 T, 147 emu g−1, and 43.6 MGOe, respectively. Although the magnetic properties are excellent, it could be further optimized because the poor crystallinity deteriorated the degree of alignment (DOA) of the particles, further optimization of the magnetic properties is possible. It should be noted that Sm2Fe17N3 powders with a size of 1.3 μm had a flake-like morphology which was not beneficial to magnetic alignment. Thus, DOA of such Sm2Fe17N3 powders by wet-milling to hinder transient surface heating and morphology optimization is required to increase mobility.
In order to deliver the heat generated by friction, Zhang and co-workers applied a cryo-milling system to crush the Sm2Fe17N3 powders.90 Compared to room-temperature milling, a low-temperature would result in a smaller particle size (Fig. 6a) and less nitrogen loss (Fig. 6b). More importantly, the hysteresis loop revealed that the low-temperature milling process would inhibit the formation of soft magnetic matter (Fig. 6c). Anyway, the low-temperature ball milling is an effective process to hinder the oxidation and nitrogen loss.
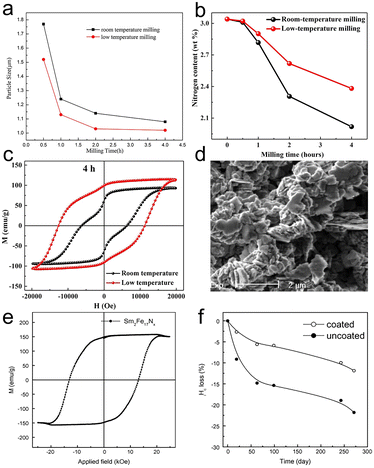 | ||
| Fig. 6 (a) The mean particle sizes of Sm2Fe17Nx nanoflakes milled at different times. (b) The nitrogen content of Sm2Fe17Nx nanoflakes milled at room temperature and low-temperature as a function of milling time. (c) Hysteresis loops of the magnetically aligned Sm2Fe17Nx nanoflakes milled for 4h at room temperature and low-temperature. (a)–(c) Reproduced from ref. 90 with permission from Elsevier, copyright 2020. (d) The morphology image of the milled Sm2Fe17N3 powders ground in heptane with 100 wt% OA, (e) The hysteresis loop of loosely packed Sm2Fe17N3 powders at room temperature, and (f) the curves of coercivity loss with time in air for coated and uncoated powders. (d)–(f) Reproduced from ref. 91 with permission from Elsevier, copyright 2014. | ||
Yang and co-workers studied the effect of the milling medium on the morphology and magnetic properties of Sm2Fe17N3 powders.91 Sm2Fe17N3 powders with a size of 100 μm were milled with the assistance of a solution of heptane and oleic acid, resulting in the formation of closely packed Sm2Fe17N3 powders with a dimensional size and thickness of about 0.8 μm and 0.1 μm, respectively (Fig. 6d). Oleic acid could promote the milling efficiency and was beneficial to the homogeneous pulverization and size reduction. However, the combination between oleic acid and Sm2Fe17N3 powders was found to be ineffectual. To improve the dispersibility of milled Sm2Fe17N3 powders, coarse Sm2Fe17N3 powder was milled in a mixture of heptane, oleic acid, and silane. The silane coupling agent was coated on the surface, resulting in the formation of loosely packed Sm2Fe17N3 powders. The loosely packed Sm2Fe17N3 powders showed comprehensive magnetic properties with coercivity, remanence, and maximum energy product of 1.30 T, 155 emu g−1, and 40 MGOe (Fig. 6e), respectively. The surface coating of Sm2Fe17N3 powders with silane improved the resistance to the external environment. Compared to Sm2Fe17N3 powders milled in heptane and oleic acid, the coercivity of silane-coated Sm2Fe17N3 powders decreased more slowly with time (Fig. 6f), further indicating the effective binding between silane and powder surface.
4.2. Particle size
Although pulverization of coarse Sm2Fe17N3 powders by ball milling was assisted by surfactants, surface defects, such as edges and strains, were hard to avoid, resulting in lower coercivity compared to Sm2Fe17N3 powders with similar size converted from Sm2Fe17 powders prepared by using a reduction–diffusion process.3 In addition, Sm2Fe17N3 powders might decompose during the ball milling process, deteriorating the magnetic properties.The nitridation process would almost not lead to powder growth and agglomeration due to the low nitridation temperature. Thus, the preparation of monodispersed Sm2Fe17 powders is a prerequisite for the fabrication of Sm2Fe17N3 powders with excellent magnetic properties. The high-temperature reduction–diffusion process easily led to the growth and sintering of Sm2Fe17 powders, and nitridation of such powders would result in Sm2Fe17N3 powders with poor magnetic properties. Generally, reduction–diffusion process optimization and precursor design have been applied to hinder the sintering and agglomeration of Sm2Fe17 powders.
The reduction–diffusion temperature regulation is quite easy, directly affecting the growth and sintering of Sm2Fe17 powders. Hono and co-workers studied the influence of the preparation temperature on the size of Sm2Fe17N3 powders.14 The Sm–Fe complex was obtained using a sol–gel method and then calcined to remove carbon in the atmosphere. Hydrogen reduction was followed to obtain a mixture of SmOx and Fe. With the mixture as a precursor, Sm2Fe17 powders were synthesized using the reduction–diffusion process and converted into Sm2Fe17N3 powders by nitridation. As displayed in Fig. 7a–c, Sm2Fe17N3 powders with sizes ranging from 0.69 to 3.49 μm could be obtained by regulating the calcination, hydrogen reduction, and reduction–diffusion temperatures. The low reaction temperature favored small particle size. At the calcination, hydrogen reduction, and reduction–diffusion temperatures of 500, 700, and 900 °C, respectively, Sm2Fe17N3 powders with a size of 0.69 ± 0.17 μm could be obtained (Fig. 7c) and exhibited a coercivity of 2.32 T (Fig. 7d). The large coercivity was attributed to the small particle size and the smooth surface. It should be noted that the Sauter mean diameter of the Sm2Fe17N3 powders, obtained by laser diffraction, was larger than that counted by the SEM image, indicating that agglomeration of Sm2Fe17 powders was hard to avoid by decreasing the temperature. It was inspired that the reduction–diffusion temperature decreased to below 600 °C.92 LiCl–KCl with a eutectic temperature of 352 °C was applied as a solvent for Ca, which could guarantee the reduction of Sm. Although Sm2Fe17 was not achieved, the low reduction–diffusion temperature was important to further hinder the growth and agglomeration.
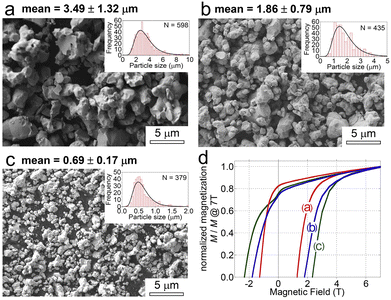 | ||
| Fig. 7 SEM images of the Sm2Fe17N3 particles with average sizes of (a) 3.49 μm, (b) 1.86 μm, and (c) 0.69 μm. (d) Demagnetization curves of Sm2Fe17N3 powders shown in Fig. 7a–c. (a)–(d) Reproduced from ref. 14 with permission from Elsevier, copyright 2016. | ||
To further reduce the size of Sm2Fe17N3 powders, precursor design is also an effective strategy. By ultrasonic spray pyrolysis and hydrogen reduction (USP–HR), Che and co-workers prepared the precursor in which Sm2O3 and α-Fe were embedded uniformly in particles with an average size of 0.48 μm, which could promote the reduction–diffusion process.93 After the reduction–diffusion and nitridation processes, Sm2Fe17N3 powders with an average size of 0.61 μm have been fabricated and exhibited a coercivity of 1.47 T. Recently, reduction–diffusion and nitridation of Sm–Fe–O precursors prepared using the UPS–HR process was carried out in a rotary furnace, which reduced the size distribution of Sm2Fe17N3. And the Sm2Fe17N3 powders with an average diameter of 0.4 μm showed a coercivity of 3.17 T.15
In addition to the UPS-HR process, coprecipitation was also applied to prepare Sm–Fe–O precursors with small particle sizes.59,94 The coprecipitated Sm(OH)3 and Fe(OH)3 were crushed until the mean size of secondary particles was smaller than 1 μm. Following the hydrogen reduction, reduction–diffusion, and nitridation process, submicron-sized Sm2Fe17N3 powders could be obtained. Sm2Fe17N3 powders with mean diameters of 0.6 μm (Fig. 8a) and 0.9 μm (Fig. 8b) have been obtained at reduction–diffusion temperatures of 900 °C and 950 °C. As shown in Fig. 8c, the Sm2Fe17N3 powders with a mean diameter of 0.6 μm exhibited a coercivity of 2.28 T, which was directly washed with water. The coercivity could be optimized to 2.81 T by slow-oxidization before washing to avoid the formation of metastable SmFe5. However, the saturation and remanence magnetizations were 134 and 100 emu g−1. The low remanence ratio was ascribed to the agglomeration.
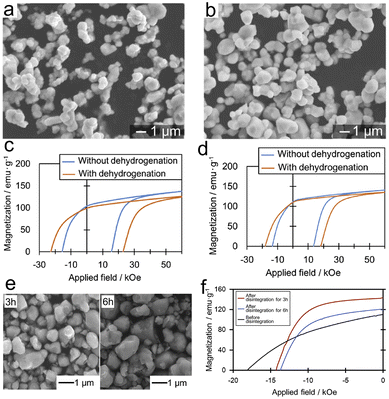 | ||
| Fig. 8 SEM images of Sm2Fe17N3 powders obtained at a reduction–diffusion temperature of (a) 900 °C and (b) 950 °C, and corresponding demagnetization curves with a mean diameter of (c) 0.6 μm and (d) 0.9 μm oriented in resin. (e) SEM images after disintegration and (f) demagnetization curves of the disintegrated powders. (a)–(f) Reproduced from ref. 94 with permission from Elsevier, copyright 2017. | ||
Compared to the Sm2Fe17N3 powders with a size of 0.6 μm, Sm2Fe17N3 powders with a mean diameter of 0.9 μm had a smaller coercivity of 1.81 T and a larger saturation and remanence magnetization of 142 and 110 emu g−1 (Fig. 8d), indicating the agglomeration of larger particles was partially inhibited. Even so, the maximum energy product of the Sm2Fe17N3 powders with a size of 0.9 μm was only 22.8 MGOe. To further enhance the remanence and maximum energy product, Sm2Fe17N3 powders with a size of 0.9 μm were subjected to ball milling for disintegration. As shown in Fig. 8e, the ball milling process effectively reduces the agglomeration of powders. After disintegration for 3 h, the Sm2Fe17N3 powders showed excellent comprehensive magnetic properties with coercivity, saturation magnetization, remanence, and maximum energy product of 1.43 T, 156 emu g−1, 141 emu g−1 and 42.8 MGOe, respectively (Fig. 8f), indicating that disintegration of the agglomerated powders promotes magnetic alignment. The decreased coercivity was attributed to the defects resulting from the ball milling. Inspiringly, the submicron-sized Sm2Fe17N3 powders displayed good thermal stability with a temperature coefficient of coercivity being −0.36%/K.
Upon the design of the precursor, efforts have been made to inhibit the agglomeration of submicron-sized Sm2Fe17N3 powders. Okada and co-workers employed a hydrothermal method to prepare Fe2O3/CaCO3, which was subsequently impregnated with Sm(NO3)3·6H2O. After the hydrogen reduction, reduction–diffusion, and nitridation process, the resulting Sm2Fe17N3 powders had a size of 0.6 μm, yet the remanence ratio was only 75%.95 It seems as though the precursor design did not work as expected. However, monodispersed submicron-sized SmCo5 powders have been obtained based on a similar precursor design.51,52 It is worthwhile to design an Sm–Fe–O precursor for the preparation of monodispersed Sm2Fe17N3 powders, which would possess both high coercivity and remanence.
4.3. Elemental substitution
As mentioned earlier, the introduction of other elements into the Sm–Fe binary system has been explored to prevent the formation of α-Fe and Sm-rich phases. Though the magnetic properties of Sm2(Fe1−xMx)17N3 are dominated by interstitial N atoms, where M represents the substitution elements, such as Co or Mn, substitution has a significant effect on the magnetic properties.96,97Co-substitution changes the lattice parameters, which is responsible for the optimization of the overall intrinsic magnetic properties with an appropriate substitution content. The Curie temperature and magnetocrystalline anisotropy could be enhanced by small Co contents, whereas the saturation magnetization is slightly optimized by Co substitution. The best comprehensive magnetic properties could be achieved when the content of Co is 20%.98
Different from Co doping, Mn substitution enhances the coercivity due to changes in the microstructure. Anisotropic Sm2Fe17N3 powders show a single-crystal feature, whereas anisotropic Sm2(Fe, Mn)17Nx powders have a cell-like structure.99–101 Sm2(Fe, Mn)17Nx powders consist of crystalline and amorphous phases. The crystalline phase is Sm2(Fe, Mn)17Nx, and the amorphous phase is rich in Mn and N. With the increase in nitrogen content, the fraction of the amorphous phase increases and the crystalline phase would be surrounded by the amorphous phase. Additionally, the magnetic domain would become smaller with the increase in nitrogen content. Magnetic domain walls would be pinned by the amorphous phase during the magnetic reversal process, thus enhancing the coercivity. Thus, Sm2(Fe, Mn)17Nx with x larger than 3 may display higher coercivity. It should be noted that crystalline Sm2(Fe, Mn)17Nx grains in Sm2(Fe, Mn)17Nx powders retain their orientation, ensuring that the powders are magnetically anisotropic. Despite the coercivity optimization, Mn substitution leads to a decrease in saturation magnetization.
Additionally, Sm can be partially substituted by Nd or Pr to alter the intrinsic magnetic properties of Sm2Fe17Nx.102,103 However, such substitutions of rare earth elements result in a decrease in the anisotropic field and Curie temperature to varying degrees, due to the easy plane anisotropy of Nd2Fe17Nx and Pr2Fe17Nx.
It is evident that the progress in the elemental substitution of Sm2Fe17N3 was not good enough to improve the magnetic properties. Referring to the great progress and wide applications of Nd2Fe14B-based magnets with elemental substitution,104–106 multi-element substitution may be a promising strategy for magnetic properties and thermal stability optimization.
From a global perspective, great progress has been made in high-performance anisotropic Sm2Fe17N3 powders by rapid-quenching and reduction–diffusion processes, whereas large-scale production of high-performance anisotropic Sm2Fe17N3 powders remains a great challenge, which is essential to the fabrication of bonded Sm2Fe17N3 magnets. The two methods suffer from complex and time-consuming processes, which suggest high costs. More importantly, controlling the composition and size of Sm2Fe17N3 powders is hard to achieve. Especially, the nitridation process, which is sensitive to the atmosphere, pressure, and surface of Sm2Fe17, could easily lead to uneven nitridation or the presence of soft magnetic Fe. So, the key to the mass production of high-performance anisotropic Sm2Fe17N3 powders may be the development of advanced equipment that could achieve homogeneous reaction and controlled loss of Sm.
5. Conclusion and perspective
In this review, we have summarized the fundamentals, synthesis methods, and regulation strategies of Sm2Fe17N3 powders, guiding the design and preparation of Sm2Fe17N3 powders with enhanced magnetic properties. The perspectives on Sm2Fe17N3 powders are discussed as follows.On the one hand, achieving precise control over the chemical composition, such as preparation of single-phase Sm2Fe17 without a significant loss of magnetic properties and enhancement of the nitridation efficiency, remains a significant challenge. The introduction of additional elements into the Sm–Fe binary system may offer opportunities to optimize the formation and nitridation of Sm2Fe17. Moreover, elemental substitution has the potential to enhance thermal stability and oxidation resistance. On the other hand, further investigations into microstructure control of Sm2Fe17N3 powders are essential for magnetic properties improvement. The ideal state for Sm2Fe17N3 powders would involve achieving a critical single-domain size with a smooth and non-oxidized surface. Therefore, concerted efforts should be directed towards attaining this ideal state, either through the pulverization of large powders or via the reduction–diffusion process.
Data availability
This is a review, the data should be found from original articles in the references.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2022YFB3505900), the Natural Science Basic Research Program of Shaanxi (2024JC-YBQN-0406), and the Qinchuangyuan Project of Shaanxi Province (QCYRCXM-2022-155, QCYRCXM-2022-267, QCYRCXM-2022-295).Notes and references
- J. M. D. Coey, Perspective and prospects for rare earth permanent magnets, Engineering, 2020, 6, 119–131 CrossRef CAS.
- R. W. McCallum, L. Lewis, R. Skomski, M. J. Kramer and I. E. Anderson, Practical aspects of modern and future permanent magnets, Annu. Rev. Mater. Res., 2014, 44, 451–477 CrossRef CAS.
- K. Takagi, Y. Hirayama, S. Okada, W. Yamaguchi and K. Ozaki, Novel powder processing technologies for production of rare-earth permanent magnets, Sci. Technol. Adv. Mater., 2021, 22, 150–159 CrossRef CAS PubMed.
- N. Imaoka, E. Kakimoto, K. Takagi, K. Ozaki, M. Tada, T. Nakagawa and M. Abe, Progress with insulating nanocomposites based on ferrite plating of Sm2Fe17N3 micropowders, J. Magn. Magn. Mater., 2019, 476, 613–621 CrossRef CAS.
- J. Cui, M. Kramer, L. Zhou, F. Liu, A. Gabay, G. Hadjipanayis, B. Balasubramanian and D. Sellmyer, Current progress and future challenges in rare-earth-free permanent magnets, Acta Mater., 2018, 158, 118–137 CrossRef CAS.
- T. Horikawa, M. Yamazaki, M. Matsuura and S. Sugimoto, Recent progress in the development of high-performance bonded magnets using rare earth-Fe compounds, Sci. Technol. Adv. Mater., 2021, 22, 729–747 CrossRef CAS PubMed.
- A. Hosokawa and Y. Hirayama, Cold consolidation and coercivity enhancement in Sm2Fe17N3 magnets by high-pressure torsion, Scripta Mater., 2023, 226, 115251 CrossRef CAS.
- C. Lu, J. Zhu, J. Gong and X. Gao, A method to improving the coercivity of sintered anisotropic Sm-Fe-N magnets, J. Magn. Magn. Mater., 2018, 461, 48–52 CrossRef CAS.
- K. Takagi, R. Soda, M. Jinno and W. Yamaguchi, Possibility of high-performance Sm2Fe17N3 sintered magnets by low-oxygen powder metallurgy process, J. Magn. Magn. Mater., 2020, 506, 166811 CrossRef CAS.
- W. Yamaguchi, R. Soda and K. Takagi, Role of surface iron oxides in coercivity deterioration of Sm2Fe17N3 magnet associated with low temperature sintering, Mater. Trans., 2019, 60, 479–483 CrossRef CAS.
- C. Lu, X. Hong, X. Bao, X. Gao and J. Zhu, Changing phase equilibria: a method for microstructure optimization and properties improvement in preparing anisotropic Sm2Fe17N3 powders, J. Alloys Compd., 2019, 784, 980–989 CrossRef CAS.
- V. Kraposhin, A. Kamynin, E. Khotulev and A. Everstov, Study of phase composition, structure and magnetic properties of high-coercivity alloys based on Sm2Fe17 system depending on technology of their manufacture, J. Phys. Conf. Ser., 2018, 1134, 012029 CrossRef.
- M. Katter, J. Wecker and L. Schultz, Structural and hard magnetic properties of rapidly solidified Sm-Fe-N, J. Appl. Phys., 1991, 70, 3188–3196 CrossRef CAS.
- Y. Hirayama, A. Panda, T. Ohkubo and K. Hono, High coercivity Sm2Fe17N3 submicron size powder prepared by polymerized-complex and reduction–diffusion process, Scripta Mater., 2016, 120, 27–30 CrossRef CAS.
- S. Okada, E. Node, K. Takagi and R. Hashimoto, Synthesis of ultra-high coercivity Sm2Fe17N3 powder by homogeneous reduction-diffusion with rotary furnace, J. Alloys Compd., 2023, 960, 170726 CrossRef CAS.
- M. Röhrig, R. G. T. Fim, L. T. Quispe, F. J. G. Landgraf, C. H. Ahrens, C. C. C. Plá and P. A. P. Wendhausen, On the feasibility of using Sm2Fe17Nx powders obtained via HDDR process for laser powder bed fusion of bonded permanent magnets, J. Magn. Magn. Mater., 2023, 565, 170273 CrossRef.
- J. Ye, Y. Liu, M. Li, S. Gao and M. Tu, Structural change in Sm2Fe17 alloys during homogenization and HD processing, Mater. Charact., 2007, 58, 108–113 CrossRef CAS.
- J. Pan, J. Shentu, C. He, D. Zeng, F. Gao, G. Dodbiba and T. Fujita, Preparation of temperature-sensitive rare-earth-iron alloy fine particles using mechanical alloying and sintering, IEEE Magn. Lett., 2023, 14, 1–5 Search PubMed.
- I. Nehdi, L. Bessais, C. Djega-Mariadassou, M. Abdellaoui and H. Zarrouk, X-ray and Mössbauer studies of Sm2Fe17-xCrx materials synthesized by mechanical alloying followed by an appropriate short annealing, J. Alloys Compd., 2003, 351, 24–30 CrossRef CAS.
- K. Kobayashi, T. Akiya and H. Kato, Magnetic anisotropy in Sm2Fe17Nx (0 < x < 3) of intermediate nitrogen concentrations, J. Alloys Compd., 2006, 408–412, 1404–1408 CrossRef CAS.
- L. Ye, F. Wang, Y. Liu, H. Zhou, L. Liu, Y. Ding, Y. Sun and A. Yan, New structural features and pinning-evolved coercivity mechanism: a potential route for developing high coercivities in anisotropic Sm-Fe-N material, J. Mater. Res. Technol., 2024, 30, 451–460 CrossRef CAS.
- J. M. D. Coey, P. Stamenov, S. B. Porter, M. Venkatesan, R. Zhang and T. Iriyama, Sm-Fe-N revisited; remanence enhancement in melt-spun Nitroquench material, J. Magn. Magn. Mater., 2019, 480, 186–192 CrossRef CAS.
- K. H. Kim, J. Kim, D. Shindo, I. Takashi and K. Ohmori, Magnetization process in Sm2Fe17N3 fine powders studied using Lorentz microscopy and electron holography, Mater. Trans., 2010, 51, 1302–1307 CrossRef CAS.
- J. Xu, K. Zhu, S. Gao and Y. Hou, Rare earth permanent magnetic nanostructures: chemical design and microstructure control to optimize magnetic properties, Inorg. Chem. Front., 2021, 8, 383–395 RSC.
- Z. Ma, J. Mohapatra, K. Wei, J. P. Liu and S. Sun, Magnetic nanoparticles: synthesis, anisotropy, and applications, Chem. Rev., 2023, 123, 3904–3943 CAS.
- S. Wang, J. Xu, W. Li, S. Sun, S. Gao and Y. Hou, Magnetic nanostructures: rational design and fabrication strategies toward diverse applications, Chem. Rev., 2022, 122, 5411–5475 CrossRef CAS PubMed.
- R. Skomski, K. H. Müller, P. A. P. Wendhausen and J. M. D. Coey, Magnetic reversal in Sm2Fe17Ny permanent magnets, J. Appl. Phys., 1993, 73, 6047–6049 CrossRef CAS.
- J. M. D. Coey and T. Iriyama, Bonded Sm-Fe-N permanent magnets, Woodhead Publishing, 2022, pp. 305–342 Search PubMed.
- E. A. Gorbachev, E. S. Kozlyakova, L. A. Trusov, A. E. Sleptsova, M. A. Zykin and P. E. Kazin, Design of modern magnetic materials with giant coercivity, Russ. Chem. Rev., 2021, 90, 1287 CrossRef.
- X. Yu, M. Yue, W. Liu, Z. Li, M. Zhu and S. Dong, Structure and intrinsic magnetic properties of MM2Fe14B (MM=La, Ce, Pr, Nd) alloys, J. Rare Earths, 2016, 34, 614–617 CrossRef CAS.
- Z. Li, M. Yue, X. Liu, H. Zhang, W. Liu, Y. Li and Z. Zhang, Tuning the structure and intrinsic magnetic properties of Ce2Fe14B alloys by elimination of CeFe2 with La substitution, J. Magn. Magn. Mater., 2020, 505, 166747 CrossRef CAS.
- S. Aich, D. Satapathy and J. Shield, Rapidly solidified rare-earth permanent magnets: processing, properties, and performance, CRC Press, 2017, 453–508 Search PubMed.
- S. Brennan, X. L. Rao, R. Skomski, N. Dempsey and J. M. D. Coey, Magnetic properties of Sm2Fe17Nx, x = 3.9, J. Magn. Magn. Mater., 1996, 157–158, 510–511 CrossRef.
- H. S. Li and J. M. Cadogan, Variation of A20 with nitrogen content in Sm2Fe17N3−δ compounds, Solid State Commun., 1991, 80, 905–908 CrossRef CAS.
- R. Skomski and D. J. Sellmyer, Anisotropy of rare-earth magnets, J. Rare Earths, 2009, 27, 675–679 CrossRef.
- C. N. Christodoulou and T. Takeshita, Preparation, structural and magnetic properties and stability of interstitial Sm2Fe17-carbonitrohydrides, J. Alloys Compd., 1993, 198, 1–24 CrossRef CAS.
- H. Horiuchi, U. Koike, H. Kaneko, T. Kurino and H. Uchida, Effects of N, C and B additions on the Sm2Fe17 crystal structure and magnetic properties, J. Alloys Compd., 1995, 222, 131–135 CrossRef CAS.
- J. Wang, W. Cai, J. Zheng, J. Zhu, X. Zhang, H. Chen, L. Qiao, Y. Ying, W. Li, J. Yu, J. Li, Y. Su and S. Che, Preparation of ultrafine Sm2Fe17N3 magnetic powders without ball milling, J. Mater. Res. Technol., 2023, 27, 6688–6695 CrossRef CAS.
- J. M. D. Coey, Magnetism and Magnetic Materials, Cambridge University Press, 2010, 1–617 Search PubMed.
- Z. Ma, H. Liu and Z. Luo, A general method for the large-scale preparation of multifunctional magnetic Sm-Fe/Co nanomaterials, Adv. Funct. Mater., 2023, 33, 2301350 CrossRef CAS.
- G. Deng, Q. Jing, X. Wang, G. He and X. Ye, Synthesis mechanism of Sm2Fe17 alloy produced in reduction-diffusion process, J. Rare Earths, 2010, 28, 420–424 CrossRef.
- R. Kuchi, D. Schlagel, T. A. Seymour-Cozzini, J. V. Zaikina and I. Z. Hlova, Exploiting mechanochemical activation and molten-salt-assisted reduction-diffusion approach in bottom-up synthesis of Sm2Fe17N3, J. Alloys Compd., 2024, 980, 173532 CrossRef CAS.
- Y. Luo, D. Yu, H. Li, W. Zhuang, Y. Mao, X. Chen, K. Li and Y. Liu, Structure and permanent magnetic properties of SmFex (x = 3-8) melt spun ribbons during heat treatment, J. Rare Earths, 2014, 32, 960–964 CrossRef CAS.
- Z. Sun, H. Zhang, S. Zhang, J. Wang and B. Shen, Structure and magnetic properties of Sm2Fe17-xMnx compounds, J. Phys. D: Appl. Phys., 2000, 33, 485 CrossRef CAS.
- M. Xing, J. Han, Y. Zhang, S. Liu, Z. Chen, C. Wang, J. Yang, H. Du, Y. Yang and M. Yue, Nitrogenation effect of Sm2Fe17 alloys prepared by strip casting technique, J. Appl. Phys., 2015, 117, 17A732 CrossRef.
- J. Yang, H. Chen, Y. Zhang, Y. Yang, X. Chen, S. Liu, C. Wang, J. Hang, H. Du, G. Lian and Y. Yang, Magnetostrictions of Sm2Fe17 and Sm2Fe17N3, IEEE Trans. Magn., 2011, 47, 3621–3624 CAS.
- G. Lin, W. Bi, X. Bao and W. Cao, Preparation of Sm2Fe17 columnar grains ribbons by rapid quenching, Adv. Mater. Res., 2014, 1004–1005, 367–370 CAS.
- M. Xing, J. Han, F. Wan, S. Liu, C. Wang, J. Yang and Y. Yang, Preparation of anisotropic Sm2Fe17NX magnetic materials by strip casting technique, IEEE Trans. Magn., 2013, 49, 3248–3250 CAS.
- D. A. Kolodkin, A. G. Popov, A. V. Protasov, V. S. Gaviko, D. Y. Vasilenko, S. Kavita, D. Prabhu and R. Gopalan, Magnetic properties of Sm2+αFe17Nx powders prepared from bulk and strip-cast alloys, J. Magn. Magn. Mater., 2021, 518, 167416 CrossRef CAS.
- D. Liang, W. Yang, X. Wang, Q. Xu, J. Han, S. Liu, C. Wang, H. Du, X. Zhu, T. Yuan, Z. Luo and J. Yang, Study of the anisotropic Sm2Fe17N3 powders with high performance, AIP Adv., 2023, 13, 025104 CrossRef CAS.
- C. Li, Q. Wu, Z. Ma, H. Xu, L. Cong and M. Yue, A novel strategy to synthesize anisotropic SmCo5 particles from Co/Sm(OH)3 composites with special morphology, J. Mater. Chem. C, 2018, 6, 8522–8527 RSC.
- H. Tang, M. A. H. Mamakhel and M. Christensen, Enhancing the coercivity of SmCo5 magnet through particle size control, J. Mater. Chem. C, 2020, 8, 2109–2116 RSC.
- J. Xu, K. Zhu, W. Li, X. Wang, Z. Yang, Y. Hou and S. Gao, First-order-reversal-curve analysis of rare earth permanent magnet nanostructures: insight into the coercivity enhancement mechanism through regulating the Nd-rich phase, Inorg. Chem. Front., 2021, 8, 1975–1982 RSC.
- Z. Ma, M. Yue, H. Liu, Z. Yin, K. Wei, H. Guan, H. Lin, M. Shen, S. An, Q. Wu and S. Sun, Stabilizing hard magnetic SmCo5 nanoparticles by N-Doped graphitic carbon layer, J. Am. Chem. Soc., 2020, 142, 8440–8446 CrossRef CAS PubMed.
- B. Shen, C. Yu, A. A. Baker, S. K. McCall, Y. Yu, D. Su, Z. Yin, H. Liu, J. Li and S. Sun, Chemical synthesis of magnetically hard and strong rare earth metal based nanomagnets, Angew. Chem., Int. Ed., 2019, 58, 602–606 CrossRef CAS PubMed.
- J. C. Boareto, J. Soyama, M. D. V. Felisberto, R. Hesse, A. V. A. Pinto, T. R. Taylor and P. A. P. Wendhausen, Sm2Fe17 prepared by calciothermic reduction-diffusion using different iron powders, Mater. Sci. Forum, 2007, 534–536, 1365–1368 CAS.
- S. Sugimoto, Current status and recent topics of rare-earth permanent magnets, J. Phys. D: Appl. Phys., 2011, 44, 064001 CrossRef.
- H. Chen, J. Zheng, L. Qiao, Y. Ying, L. Jiang and S. Che, Preparation of Sm2Fe17 alloy by reduction-diffusion process, Rare Met., 2018, 37, 989–994 CrossRef CAS.
- S. Okada, E. Node, K. Takagi, Y. Fujikawa, Y. Enokido, C. Moriyoshi and Y. Kuroiwa, Synthesis of Sm2Fe17N3 powder having a new level of high coercivity by preventing decrease of coercivity in washing step of reduction-diffusion process, J. Alloys Compd., 2019, 804, 237–242 CrossRef CAS.
- T. Ishikawa, K. Yokosawa, K. Watanabe and K. Ohmori, Modified process for high-performance anisotropic Sm2Fe17N3 magnet powder, J. Phys. Conf. Ser., 2011, 266, 012033 CrossRef.
- T. Tanabe, Y. Nagai, T. Kubota and Z. Asaki, Formation of Sm-Fe intermetallic compounds by the reduction-diffusion process with CaH2, Mater. Trans., 1992, 33, 1163–1170 CrossRef.
- J. Xu, J. Zheng, H. Chen, L. Qiao, Y. Ying, W. Cai, W. Li, J. Yu, M. Lin and S. Che, Corrosion mechanism of H2O on Sm2Fe17 and nitrogenation process of corroded Sm2Fe17, J. Rare Earths, 2019, 37, 638–644 CrossRef CAS.
- R. Matsuda, M. Matsuura, N. Tezuka, S. Sugimoto, T. Ishikawa and Y. Yoneyama, Preparation of highly heat-resistant Sm-Fe-N magnetic powder by reduction-diffusion process, Mater. Trans., 2020, 61, 2201–2207 CrossRef CAS.
- X. Zhao, Z. Zhang, W. Liu, Q. Xiao and X. Sun, Structural and magnetic properties of Sm-Fe-N magnets prepared by hydrogenation and nitrogenation processes, J. Magn. Magn. Mater., 1995, 148, 419–425 CrossRef CAS.
- M. Okada, K. Saito, H. Nakamura, S. Sugimoto and M. Homma, Microstructural evolutions during HDDR phenomena in Sm2Fe17Nx compounds, J. Alloys Compd., 1995, 231, 60–65 CrossRef CAS.
- K. Hae-Woong, Study of the effect of a repeated HDDR treatment on the coercivity of two rare-earth-transition metal hard magnetic materials, J. Phys. D: Appl. Phys., 2001, 34, 36 CrossRef.
- J. Sun, C. Cui, Y. Zhang, L. Li, J. Gao and Y. Liu, Structural and magnetic properties of Sm2Fe17-xNbx (x = 0-4) alloys prepared by HDDR processes and their nitrides, Rare Met., 2006, 25, 129–137 CrossRef CAS.
- A. Teresiak, O. Gutfleisch, N. Mattern, B. Gebel and K. H. Müller, Phase formation and crystal structure of Sm2Fe17-yGay compounds during hydrogen disproportionation and desorption recombination (HDDR-process), J. Alloys Compd., 2002, 346, 235–243 CrossRef CAS.
- H. Sepehri-Amin, T. Ohkubo, K. Hono, K. Güth and O. Gutfleisch, Mechanism of the texture development in hydrogen-disproportionation–desorption-recombination (HDDR) processed Nd-Fe-B powders, Acta Mater., 2015, 85, 42–52 CrossRef CAS.
- M. Xing, J. Han, Z. Lin, F. Wan, S. Liu, C. Wang, Q. Xu, J. Yang and Y. Yang, Self-organized nanorods in disproportionated Sm2Fe17 and NdFe10.5Mo1.5 alloys, J. Magn. Magn. Mater., 2013, 326, 201–204 CrossRef CAS.
- N. Mommer, M. Kubis, M. Hirscher, M. Gerlach, J. V. Lier, K. H. Müller and H. Kronmüller, Measurement of N and C diffusion in Sm2Fe17 by magnetic relaxation, J. Alloys Compd., 1998, 279, 113–116 CrossRef CAS.
- J. Wang, W. Li, X. Zhong, Y. Gao, W. Qin, N. Tang, W. Lin, J. Zhang, R. Zhao, Q. Yan and F. Yang, Study on high performance Sm2Fe17Nx magnets, J. Alloys Compd., 1995, 222, 23–26 CrossRef CAS.
- C. H. Lee and Y. S. Kwon, Effect of mechanical alloying on the formation of Sm2Fe17Nx compound, Met. Mater. Int., 2002, 8, 151–154 CrossRef CAS.
- W. Liu, Q. Wang, X. Sun, X. Zhao, T. Zhao, Z. Zhang and Y. Chuang, Metastable Sm-Fe-N magnets prepared by mechanical alloying, J. Magn. Magn. Mater., 1994, 131, 413–416 CrossRef CAS.
- H. Uchida, T. Kawanabe, S. Tachibana, K. Kinoshita, Y. Matsumura, M. Tada, H. H. Uchida, H. Kaneko, T. Kurino and M. Sato, Nitrogen absorption by Sm2Fe17, J. Ceram. Soc. Jpn., 2006, 114, 896–901 CrossRef CAS.
- J. D. Ardisson, R. C. Araujo, W. A. A. Macedo, A. I. C. Persiano and S. Gama, The effect of atmosphere composition in plasma nitrogenation of Sm2Fe17, J. Magn. Magn. Mater., 2004, 272–276, 1859–1861 CrossRef.
- C. N. Christodoulou and N. Komada, On the atomic diffusion mechanism and diffusivity of nitrogen atoms in Sm2Fe17, J. Alloys Compd., 1994, 206, 1–13 CrossRef CAS.
- H. Uchida, S. Tachibana, T. Kawanabe, Y. Matsumura, V. Koeninger, H. H. Uchida, H. Kaneko and T. Kurino, Diffusion behavior of N atoms in Sm2Fe17, J. Alloys Compd., 1995, 222, 107–112 CrossRef CAS.
- K. Kobayashi, M. Ohmura, Y. Yoshida and M. Sagawa, The origin of the enhancement of magnetic properties in Sm2Fe17Nx (0<x≲3), J. Magn. Magn. Mater., 2002, 247, 42–54 CrossRef CAS.
- C. Cui, J. Sun, R. Wang and Z. Liang, The effect of nitridation on the microstructure and lattice parameters of fine grained Sm2Fe17Nx, Superlattice. Microst., 2006, 39, 406–413 CrossRef CAS.
- H. Fujii, K. Tatami and K. Koyama, Nitrogenation process in Sm2Fe17 under various N2-gas pressures up to 6 MPa, J. Alloys Compd., 1996, 236, 156–164 CrossRef CAS.
- J. Takahashi, Y. Mitsui, M. Onoue, R. Kobayashi and K. Koyama, Nitridation kinetics of Sm2Fe17 probed using Mössbauer spectroscopy, J. Magn. Magn. Mater., 2022, 554, 169295 CrossRef CAS.
- K. Koyama, M. Onoue, R. Kobayashi, Y. Mitsui, R. Y. Umetsu and Y. Uwatoko, Magnetic field effect on nitrogenation of Sm2Fe17, Mater. Trans., 2020, 61, 1487–1491 CrossRef CAS.
- T. Iriyama, K. Kobayashi, N. Imaoka, T. Fukuda, H. Kato and Y. Nakagawa, Effect of nitrogen content on magnetic properties of Sm2Fe17Nx (0 < x < 6), IEEE Trans. Magn., 1992, 28, 2326–2331 CAS.
- Y. Wei, K. Sun, Y. Fen, J. Zhang, B. Hu, Y. Wang, X. Rao and G. Liu, Structural and intrinsic magnetic properties of Sm2Fe17Ny (y = 2-8), J. Alloys Compd., 1993, 194, 9–12 CrossRef CAS.
- Y. Mitsui, R. Y. Umetsu, K. Koyama and K. Watanabe, Magnetic-field-induced enhancement for synthesizing ferromagnetic MnBi phase by solid-state reaction sintering, J. Alloys Compd., 2014, 615, 131–134 CrossRef CAS.
- M. Onoue, R. Kobayashi, Y. Mitsui, R. Y. Umetsu, Y. Uwatoko and K. Koyama, In-field heat treatment effect on nitridation of Sm2Fe17, Mater. Trans., 2019, 60, 2179–2182 CrossRef CAS.
- A. Hosokawa, K. Suzuki, W. Yamaguchi and K. Takagi, Mechanism of anomalous α-Fe formation from stoichiometric Sm2Fe17 jet-milled powder during post-pulverization annealing, Acta Mater., 2021, 213, 116981 CrossRef CAS.
- A. Hosokawa, W. Yamaguchi, K. Suzuki and K. Takagi, Influences of microstructure on macroscopic crystallinity and magnetic properties of Sm-Fe-N fine powder produced by jet-milling, J. Alloys Compd., 2021, 869, 159288 CrossRef CAS.
- Y. Li, F. Wang, J. P. Liu, F. Wang, S. Wang and J. Zhang, Fabrication of remarkably magnetic-property-enhanced anisotropic Sm2Fe17Nx nanoflakes by surfactant assisted ball milling at low temperature, J. Magn. Magn. Mater., 2020, 498, 166191 CrossRef CAS.
- X. Ma, L. Li, S. Liu, B. Hu, J. Han, C. Wang, H. Du, Y. Yang and J. Yang, Anisotropic Sm-Fe-N particles prepared by surfactant-assisted grinding method, J. Alloys Compd., 2014, 612, 110–113 CrossRef CAS.
- J. Kim, S. Okada and K. Takagi, Low-temperature synthesis of Sm-Fe binary compounds by reduction-diffusion process using an eutectic salt solvent, Rare Met., 2023, 42, 1724–1731 CrossRef CAS.
- J. Zheng, S. Tian, K. Liu, W. Cai, Y. Tang, L. Qiao, Y. Ying, W. Li, J. Yu, Y. Liu and S. Che, Preparation of submicron-sized Sm2Fe17N3 fine powder by ultrasonic spray pyrolysis-hydrogen reduction (USP-HR) and subsequent reduction-diffusion process, AIP Adv., 2020, 10, 055119 CrossRef CAS.
- S. Okada, K. Suzuki, E. Node, K. Takagi, K. Ozaki and Y. Enokido, Preparation of submicron-sized Sm2Fe17N3 fine powder with high coercivity by reduction-diffusion process, J. Alloys Compd., 2017, 695, 1617–1623 CrossRef CAS.
- S. Okada, K. Suzuki, E. Node, K. Takagi, K. Ozaki and Y. Enokido, Improvement of magnetization of submicron-sized high coercivity Sm2Fe17N3 powder by using hydrothermally synthesized sintering-tolerant cubic hematite, AIP Adv., 2017, 7, 056219 CrossRef.
- B. Hu, X. Rao, J. Xu, G. Liu, F. Cao, X. Dong, H. Li, L. Yin and Z. Zhao, Magnetic properties of Sm2(Fe1-xMx)17Ny nitrides (M = Co, Ni, Al, Ti, V), J. Magn. Magn. Mater., 1992, 114, 138–144 CrossRef CAS.
- M. Matsuura, K. Yarimizu, Y. Osawa, N. Tezuka, S. Sugimoto, T. Ishikawa and Y. Yoneyama, Preparation of Mn-diffused Sm-Fe-N core-shell powder by reduction-diffusion process, J. Magn. Magn. Mater., 2019, 471, 310–314 CrossRef CAS.
- M. Katter, J. Wecker, C. Kuhrt, L. Schultz and R. Grössinger, Structural and intrinsic magnetic properties of Sm2(Fe1−xCox)17Ny, J. Magn. Magn. Mater., 1992, 114, 35–44 CrossRef CAS.
- K. Majima, M. Ito, S. Katsuyama and H. Nagai, Structure and magnetic properties of Sm2(Fe,Mn)17Nx coarse powders with high coercivity, J. Appl. Phys., 1997, 81, 4530–4532 CrossRef CAS.
- A. Hosokawa and K. Takagi, Multi-scale electron microscopy of overnitrided Sm-Fe-Mn-N powder, AIP Adv., 2018, 8, 055031 CrossRef.
- A. Yasuhara, H. S. Park, D. Shindo, T. Iseki, N. Oshimura, T. Ishikawa and K. Ohmori, Microstructures and magnetic domain structures in Sm2(Fe,Mn)17Nδ powders studied by analytical electron microscopy and Lorentz microscopy, J. Magn. Magn. Mater., 2005, 295, 1–6 CrossRef CAS.
- M. Katter, J. Wecker, C. Kuhrt, L. Schultz, X. C. Kou and R. Grössinger, Structural and intrinsic magnetic properties of (Sm1−xNdx)2Fe17N≈2.7 and (Sm1−xNdx)2(Fe1−zCoz)17N≈2.7, J. Magn. Magn. Mater., 1992, 111, 293–300 CrossRef CAS.
- Y. Zeng, Z. Lu, N. Tang, X. L. R. Zhao and F. Yang, Structural, magnetic and microscopic physical properties of (Sm, Pr)2Fe17 and their nitrides, J. Magn. Magn. Mater., 1995, 139, 11–18 CrossRef CAS.
- W. Chen, J. Jin, J. Yu, L. Zhou, B. Peng, S. Fu, X. Liu, G. Bai and M. Yan, Outstanding anti-corrosion performance in Nd-Fe-B permanent magnets by constructing a hydrophobic triplex surface coating, J. Mater. Chem. C, 2023, 11, 6884–6893 RSC.
- Z. Yu, X. Liu, J. He, J. Cao, W. Fan, Y. Wu, H. Yu, Z. Liu, Z. Wang and E. Niu, Tb-Cu grain boundary diffusion effects on single- and multi-main-phase Nd-Fe-B based magnets, J. Mater. Chem. C, 2023, 11, 645–655 RSC.
- J. Zhang, X. Liao, K. Xu, J. He, W. Fan, H. Yu, X. Zhong and Z. Liu, Enhancement in hard magnetic properties of nanocrystalline (Ce,Y)–Fe–Si–B alloys due to microstructure evolution caused by chemical heterogeneity, J. Mater. Chem. C, 2020, 8, 14855–14863 RSC.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |