Amide-functionalized lanthanide metal–organic frameworks: smart double ratiometric fluorescence sensing of thiodiglycolic acid and tunable luminescence†
Xu
Zhang
a,
Jiahui
Yu
a,
Xin
Li
a,
Chengqi
Jiao
*ab,
Yanyu
Zhu
*a,
Hanwen
Zheng
a and
Zhengang
Sun
 *a
*a
aSchool of Chemistry and Chemical Engineering, Liaoning Normal University, Dalian 116029, P. R. China. E-mail: jiaochengqi1989@163.com; summeryyzhu@163; szg188@163.com
bState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, P. R. China
First published on 11th October 2024
Abstract
Lanthanide metal–organic frameworks (Ln-MOFs) have excellent optical properties and structural diversities, providing a unique platform for the development of sensing and optical materials. Herein, two series of Ln-MOFs, [Ln(L)(DMF)(H2O)]·DMF (Ln-DMF, DMF = N,N-dimethylformamide), and [Ln(L)(DMA)(H2O)]·DMA (Ln-DMA, DMA = N,N-dimethylacetamide) (Ln = Eu, Gd, Tb, H3L = C6H3(CONH)3(C6H4)3(COOH)3), were fabricated using the same H3L ligand under solvothermal conditions, giving 3D supramolecular and 2D layered structures, respectively. The good water and acid–base stabilities of Tb-DMF and Tb-DMA provide prerequisites for their fluorescence sensing applications. Tb-DMF and Tb-DMA were used as double ratiometric fluorescence sensors for detecting thiodiglycolic acid (TDGA) with low LODs, excellent anti-interference, and visualization. Moreover, these sensors were applied to the detection of TDGA in real urine samples with satisfactory recoveries (96.34–110.75%). Then, two portable smartphone-based platforms were used to monitor TDGA with high precision. Moreover, the practicability and availability of smartphones in the TDGA sensing process were further improved by establishing logic gates. By adjusting the molar ratios of Eu3+, Gd3+, and Tb3+ ions, nine bi-metallic doped EuxTb1−x-DMA (x = 0.10–0.90) and one tri-metallic doped Gd0.95Eu0.03Tb0.02-DMA near white-light MOFs with a quantum yield of 25.73% were obtained. This study provides a strategy for the construction of multifunctional materials with double ratiometric fluorescence sensing and tunable luminescence.
1. Introduction
The rapid growth of population and the continuous revitalization of the industry have led to serious ecological deterioration and environmental pollution. Information leakage and counterfeiting have almost penetrated into every industry related to human security. Thus, the development of multifunctional materials is of great significance for biological monitoring and anti-counterfeiting techniques. Recently, lanthanide metal–organic frameworks (Ln-MOFs) have garnered considerable attention due to their remarkable structures, inherently spectroscopic features including a large Stokes shift, high color purity, long lifetime, and wide emission range, and are widely used in fields such as fluorescence sensing, tunable luminescence, anti-counterfeiting etc.1–18Vinyl chloride monomer (VCM) is an important raw material in the plastic industry, and long-term inhalation and exposure to the environment containing VCM can lead to cancer, hormone disorder, diabetes, and nerve damage.19 Thioglycolic acid (TDGA) is considered a biomarker for monitoring VCM exposure levels.20 The amount of TDGA excreted in urine is quantitatively related to the exposure level of VCM in the human body, and the threshold value for a healthy person is 20 mg L−1.21 Therefore, the detection of TDGA content in urine has vital clinical meaning for early diagnosis and disease monitoring of VCM exposure levels. Recently, some luminescent MOF-based sensors for detecting TDGA have been reported, all of which involve single-signal detection or ratiometric fluorescence detection.22–25 Compared to the former, ratiometric fluorescence sensors have received increasing attention due to their inherent self-calibrating function and high detection accuracy.26–31 Although current ratiometric fluorescence sensors can effectively monitor TDGA, their application is inhibited by shortcomings such as cumbersome synthesis processes and inability to achieve portable monitoring of TDGA. Due to the popularity of smartphones, digital imaging technology has become a potential analytical platform for developing portable sensors, providing more opportunities to improve traditional sensing technology and develop portable sensors for detecting TDGA.32–36 In addition, most of the reported MOF-based ratiometric fluorescence sensors rely on changes in two fluorescence signals. To further enhance the anti-interference ability of sensors during the sensing process, the construction of double ratiometric fluorescence sensors with the variations of multiple fluorescence signals has attracted greater attention because of their multiple self-calibration functions. To our knowledge, there are very few reports on MOF-based double ratiometric fluorescence sensors,37 and the aforementioned sensors for detecting TDGA have not been reported. Therefore, constructing a portable smartphone-assisted double ratiometric fluorescence sensor for detecting TDGA is a huge challenge.
In addition, Ln-MOFs can be utilized to fabricate light-emissive source materials due to their inherent luminescence.38 Most Ln3+ ions have similar ionic radii, coordination environments, and chemical activities, thus, the emission color of doped Ln-MOFs can be controlled by adjusting the molar ratio of two or three different Ln3+ ions in the reactions to obtain white-light emitting materials.4,39–44 Due to their diverse luminescent centers and good color adjustment flexibility, the doped Ln-MOFs are considered ideal phosphors for white-light emitting diodes (WLEDs).45–47 Recently, although some doped Ln-MOFs have been reported for use in WLED devices, most of them have low stabilities and low quantum yields. Therefore, it is necessary to develop high luminous efficiency and stable doped Ln-MOF materials. In addition, owing to the unique characteristic luminescence of Ln3+ ions, Ln-MOFs are highly considered as competitive fluorescent anti-counterfeiting materials as they can emit fluorescence that covered the entire visible region under excitation of ultraviolet (UV) light.48–52 Therefore, it is very significant to investigate the preparation and luminescence regulation of doped Ln-MOF materials.
To construct the aforementioned multifunctional Ln-MOF materials, the selection of ligand is crucial. The polycarboxylic acid ligand functionalized with amides (H3L = C6H3(CONH)3(C6H4)3(COOH)3) was selected due to the following factors: (1) based on theoretical calculations, the H3L ligand with appropriate energy levels cannot efficiently sensitize Ln3+ luminescence, resulting in incomplete transfer of the H3L ligand to the Ln3+ ion and providing a chance to obtain dual-emission Ln-MOFs; (2) because the –CONH– groups can serve as donors for hydrogen-bonding, it is speculated that the detection of TDGA can be achieved through hydrogen-bonding interactions between the –CONH– groups of H3L and the –COOH group in TDGA; (3) the solid-state emission spectrum of the H3L ligand is located in the cyan emitting region, providing a precursor for the study of tunable luminescence performance. Herein, two series of Ln-MOFs, [Ln(L)(DMF)(H2O)]·DMF (Ln-DMF, DMF = N,N-dimethylformamide), and [Ln(L)(DMA)(H2O)]·DMA (Ln-DMA, DMA = N,N-dimethylacetamide) (Ln = Eu, Gd, Tb), were solvothermally synthesized using H3L as the ligand. Tb-DMF and Tb-DMA can serve as low LOD, highly selective and anti-interference, visualized double ratiometric fluorescence sensors for detecting TDGA in actual urine samples with satisfactory recoveries. More importantly, considering the qualitative/quantitative requirements of on-site monitoring, a portable paper-based optical sensing platform and logic gate were developed by integrating a smartphone for visual sensing of TDGA, opening up a promising path for real-time detection of TDGA. Moreover, nine bi-metallic doped EuxTb1−x-DMA (x = 0.10–0.90) and one tri-metallic doped Gd0.95Eu0.03Tb0.02-DMA near white-light MOFs with high quantum yield were obtained by adjusting various molar ratios of Eu3+, Gd3+, and Tb3+ ions. Interestingly, the composite anti-counterfeiting films of Ln-DMA and doped EuxTb1−x-DMA were prepared, which show obvious excitation wavelength-dependent characteristics. Meanwhile, the structure–property relationships are also investigated.
2. Experimental section
2.1. Synthesis of [Ln(L)(DMF)(H2O)]·DMF (Ln-DMF)
A mixture of Ln(NO3)3·6H2O (0.05 mmol), H3L (0.10 mmol), DMF (3.0 mL) and H2O (3.0 mL) was heated in a Teflon-lined stainless steel autoclave (20.0 mL) at 140 °C for 72 h. After slowly cooling to room temperature, colorless block crystals of Ln-DMF were collected.2.2. Synthesis of [Ln(L)(DMA)(H2O)]·DMA (Ln-DMA)
The synthesis of Ln-DMA is similar to that of Ln-DMF, except that DMF was replaced by DMA.2.3. Synthesis of EuxTb1−x-DMA (x = 0.1–0.9)
The synthesis of Eu/Tb bi-metallic doped MOFs is similar to that of Ln-DMA except that Ln(NO3)3·6H2O was replaced by two different ratios of Eu(NO3)3·6H2O and Tb(NO3)3·6H2O, and the total amount of metal ions remained 0.05 mmol.2.4. Synthesis of Gd0.95Eu0.03Tb0.02-DMA
The Gd/Tb/Eu tri-metallic doped MOF was prepared using the same method as Ln-DMA by mixing 95% Gd(NO3)3·6H2O, 2% Tb(NO3)3·6H2O, and 3% Eu(NO3)3·6H2O, keeping the total amount of metal ions at 0.05 mmol.3. Results and discussion
3.1. Crystal structures of Ln-DMF and Ln-DMA
Ln-DMF (Ln = Eu, Gd, and Tb) is isostructural in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S1, ESI†), thus, Tb-DMF is selected as an example to analyze. The asymmetric unit of Tb-DMF contains one Tb3+ ion, one L3− anion, one coordinated water molecule, one coordinated DMF molecule and one free DMF molecule (Fig. 1a). The Tb3+ ion is eight-coordinated, linked to six Ocarboxyl atoms (O1, O2, O3A, O4A, O5B, and O6B) from three L3− anions, one O atom (O10) from a coordinated DMF molecule and one O atom (O11) from a coordinated water molecule. The Tb–O bond lengths range from 2.321(19) to 2.461(16) Å (Table S2, ESI†).53 Additionally, the L3− ligand adopts hexadentate chelation modes: μ3-η1:η1:η1:η1:η1:η1:η0:η0:η0 (Fig. S2a, ESI†). The adjacent Tb3+ ions are interconnected through L3− anions to form a two-dimensional (2D) layered structure with a rhombus-shaped window consisting of 59 atoms (Fig. 1b). The abundant O–H⋯O hydrogen bonds were formed between the amide groups (–CONH–) in the framework and coordinated water molecules. These layers are further linked by hydrogen-bonding interaction of O–H⋯O with a distance of 2.964 Å between the O9 atom from amide and O11 atom from coordinated water to form a three-dimensional (3D) supramolecular structure (Fig. 1c), and the guest DMF solvent molecules are arranged in an orderly manner on the channel. The abundant and ordered hydrogen bonds, combined with potential –CONH– recognition sites, make Tb-DMF a promising fluorescent sensing material.
(Table S1, ESI†), thus, Tb-DMF is selected as an example to analyze. The asymmetric unit of Tb-DMF contains one Tb3+ ion, one L3− anion, one coordinated water molecule, one coordinated DMF molecule and one free DMF molecule (Fig. 1a). The Tb3+ ion is eight-coordinated, linked to six Ocarboxyl atoms (O1, O2, O3A, O4A, O5B, and O6B) from three L3− anions, one O atom (O10) from a coordinated DMF molecule and one O atom (O11) from a coordinated water molecule. The Tb–O bond lengths range from 2.321(19) to 2.461(16) Å (Table S2, ESI†).53 Additionally, the L3− ligand adopts hexadentate chelation modes: μ3-η1:η1:η1:η1:η1:η1:η0:η0:η0 (Fig. S2a, ESI†). The adjacent Tb3+ ions are interconnected through L3− anions to form a two-dimensional (2D) layered structure with a rhombus-shaped window consisting of 59 atoms (Fig. 1b). The abundant O–H⋯O hydrogen bonds were formed between the amide groups (–CONH–) in the framework and coordinated water molecules. These layers are further linked by hydrogen-bonding interaction of O–H⋯O with a distance of 2.964 Å between the O9 atom from amide and O11 atom from coordinated water to form a three-dimensional (3D) supramolecular structure (Fig. 1c), and the guest DMF solvent molecules are arranged in an orderly manner on the channel. The abundant and ordered hydrogen bonds, combined with potential –CONH– recognition sites, make Tb-DMF a promising fluorescent sensing material.
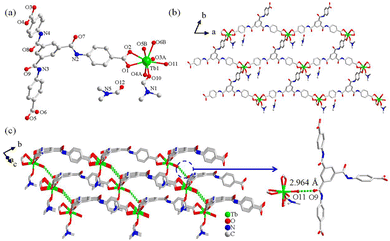 | ||
| Fig. 1 (a) Coordination environment of Tb-DMF. (A) 1 + x, 1 + y, +z; (B) −1 + x, 1 + y, 1 + z; (b) 2D layer of Tb-DMF; (c) 3D supramolecular structure of Tb-DMF. | ||
Ln-DMA (Ln = Eu, Gd, and Tb) is isostructural in the triclinic system with space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S3, ESI†). The coordinated environment of Ln3+ ions and the coordinated mode of the L3− ligand as well as the total structures of Ln-DMA are similar to those in Ln-DMF. The difference is that there is no weak interaction between the layers of Ln-DMA, thus, the overall structure of Ln-DMA is a 2D layer (Fig. 2 and Fig. S2b, ESI†). The guest DMA solvent molecules are arranged orderly between the layers. The Tb–O bond lengths range from 2.294(3) to 2.488(3) Å (Table S4, ESI†).53
(Table S3, ESI†). The coordinated environment of Ln3+ ions and the coordinated mode of the L3− ligand as well as the total structures of Ln-DMA are similar to those in Ln-DMF. The difference is that there is no weak interaction between the layers of Ln-DMA, thus, the overall structure of Ln-DMA is a 2D layer (Fig. 2 and Fig. S2b, ESI†). The guest DMA solvent molecules are arranged orderly between the layers. The Tb–O bond lengths range from 2.294(3) to 2.488(3) Å (Table S4, ESI†).53
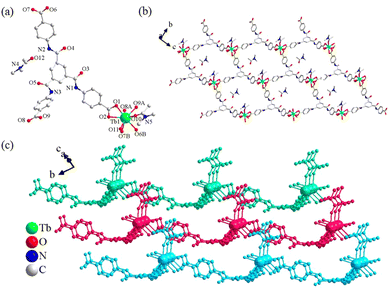 | ||
| Fig. 2 (a) Coordination environment of Tb-DMA. (A) 1 + x, −1 + y, −1 + z; (B) 2 + x, +y, −1 + z. (b) 2D layer of Tb-DMA; (c) 2D layer stacking structure of Tb-DMA. | ||
3.2. Purity and structural stability
To verity the phase purity of Ln-DMF and Ln-DMA, the powder X-ray diffraction (PXRD) patterns were explored. The experimental PXRD patterns of Ln-DMF and Ln-DMA correspond to the simulated PXRD, indicating that Ln-DMF and Ln-DMA samples exhibit phase purity (Fig. S3, ESI†). Meanwhile, the PXRD patterns of EuxTb1−x-DMA and Gd0.95Eu0.03Tb0.02-DMA match well with those of the simulated Ln-DMA, indicating that they are isostructural to Ln-DMA (Fig. S4, ESI†). To assess the structural stabilities of Tb-DMF and Tb-DMA, their powders were measured by PXRD under different conditions. First, the stabilities of Tb-DMF and Tb-DMA in air were tested by exposing the samples to air for 60 days. As shown in Fig. S5 (ESI†), the PXRD patterns of Tb-DMF and Tb-DMA remained unchanged, indicating that the structures of Tb-DMF and Tb-DMA remained stable for at least 60 days. Second, the water stabilities of Tb-DMF and Tb-DMA were tested by soaking them in water for 24 h and in boiling water for 4 h. Meanwhile, it can also be seen that Tb-DMF and Tb-DMA can maintain their framework integrity, demonstrating good water stability. Finally, the acid–base stabilities of Tb-DMF and Tb-DMA were also studied. All of the PXRD patterns and IR curves of the samples treated after 10 h in acid–base solutions of pH = 2–12 are consistent with the original ones, indicating the good acid–base resistance of Tb-DMF and Tb-DMA (Fig. S6 and S7, ESI†). These above results display the good water and acid–base stabilities of Tb-DMF and Tb-DMA. To validate the thermostabilities of Ln-DMF and Ln-DMA, thermogravimetric (TG) analyses of Ln-DMF and Ln-DMA were performed. As shown in Fig. S8 (ESI†), the TG curves of both Ln-DMF and Ln-DMA exhibit large plateaus before the initial weight loss, indicating that they have good thermostabilities. To further investigate the thermostabilities of Tb-DMF and Tb-DMA, the PXRD patterns were measured at the temperatures of 80–210 °C and 120–260 °C, respectively. As shown in Fig. S9 (ESI†), the PXRD patterns exhibit that the framework of Tb-DMF and Tb-DMA can remain intact below 200 °C and 250 °C, respectively. The above-mentioned results display that Tb-DMF and Tb-DMA have excellent physical and chemical stabilities, providing reliable prerequisites for sensing in water systems.3.3. Luminescence properties
The solid-state luminescence spectra of H3L, Ln-DMF and Ln-DMA were obtained. H3L exhibits a maximum emission at 460 nm (λex = 350 nm), which is due to the n–π* or π–π* transitions of the H3L ligand (Fig. S10, ESI†). As shown in Fig. 3a and b, Eu-DMF and Eu-DMA display ligand-based emission peaks and the characteristic peaks of Eu3+ ions (for Eu-DMF: 597 nm, 622 nm (λex = 351 nm); for Eu-DMA: 599 nm, 624 nm (λex = 350 nm)), which are assigned to the 5D0 → 7FJ (J = 1 and 2) transitions of Eu3+ ions. Under 365 nm UV light, they emit typical red luminescence and the correspondingly Commission Internationale de l'Éclairage (CIE) coordinates of Eu-DMF and Eu-DMA are (0.670, 0.330) and (0.675, 0.325), respectively (Fig. S11, ESI†). Complexes Gd-DMF and Gd-DMA exhibit broad ligand-based emission peaks centered at 452 nm (λex = 303 nm) and 448 nm (λex = 301 nm), respectively. Under 365 nm UV light, they emit cyan luminescence that can be distinguished by the naked eye and the correspondingly CIE coordinates of Gd-DMF and Gd-DMA are (0.132, 0.137) and (0.162, 0.194), respectively (Fig. 3c, d, and Fig. S11, ESI†). The emission spectra of Tb-DMF and Tb-DMA show weak ligand-based emission peaks and the characteristic emission peaks of Tb3+ ions (for Tb-DMF: 499 nm, 552 nm, 591 nm, and 628 nm (λex = 336 nm); for Tb-DMA: 497 nm, 551 nm, 590 nm, and 627 nm (λex = 340 nm)), corresponding to the 5D4 → 7FJ (J = 6, 5, 4, 3) of Tb3+ ions. They show green luminescence under UV light irradiation at 365 nm that can be distinguished by the naked eye, and their correspondingly CIE coordinates are (0.264, 0.725) and (0.232, 0.743), respectively (Fig. 3e, f, and Fig. S11, ESI†). Notably, the emission spectra of Eu-DMF, Tb-DMF, Eu-DMA and Tb-DMA all contain the ligand-based emissions, which means the incomplete energy transfer from H3L to Eu3+/Tb3+ ions via the “antenna effect”.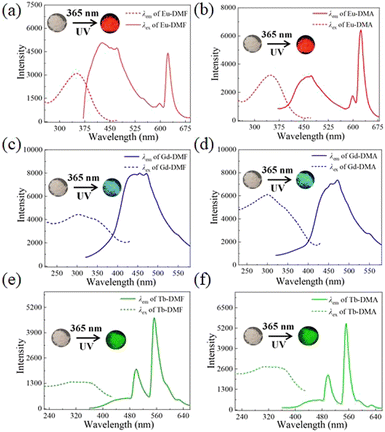 | ||
| Fig. 3 Solid-state luminescence spectra of Eu-DMF (a), Eu-DMA (b), Gd-DMF (c), Gd-DMA (d), Tb-DMF (e), and Tb-DMA (f). Inset: Fluorescence images under the 365 nm UV light. | ||
In addition, the luminescence lifetimes (τ) and quantum yields (Φ) of Ln-DMF and Ln-DMA were also tested. As shown in Fig. S12–S14 (ESI†), the τ values of Eu-DMF, Tb-DMF, Eu-DMA and Tb-DMA are 0.23, 0.61, 0.25 and 0.64 ms, and the corresponding Φ values are 13.83%, 18.27%, 13.96% and 28.21%, respectively. Moreover, the τ values of Gd-DMF and Gd-DMA are 14.78 and 49.25 ns, and the corresponding Φ values are 6.02% and 6.60%, respectively.
Then, the energy transfer processes of Ln-DMF and Ln-DMA were discussed. According to the solid-state UV-Vis absorption spectrum of H3L, the singlet-state (S1) energy level was calculated to be 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 412 cm−1 (340 nm) (Fig. S15a, ESI†). The triplet-state (T1) energy level of H3L was calculated to be 22
412 cm−1 (340 nm) (Fig. S15a, ESI†). The triplet-state (T1) energy level of H3L was calculated to be 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857 cm−1 using B3LYP/6-31G(d,p) time-dependent density functional theory (TD-DFT). According to Reinhold's empirical rule, the calculated energy gap [ΔE = E(S1) − E(T1)] of 6555 cm−1 exceeds 5000 cm−1, illustrating that the intersystem crossing (ISC) process is effective.54 The energy gap of 5357 cm−1 between the S1 and 5D0 for Eu3+ (17
857 cm−1 using B3LYP/6-31G(d,p) time-dependent density functional theory (TD-DFT). According to Reinhold's empirical rule, the calculated energy gap [ΔE = E(S1) − E(T1)] of 6555 cm−1 exceeds 5000 cm−1, illustrating that the intersystem crossing (ISC) process is effective.54 The energy gap of 5357 cm−1 between the S1 and 5D0 for Eu3+ (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) ions and the energy gap of 2357 cm−1 between the S1 and 5D4 for Tb3+ (20
500 cm−1) ions and the energy gap of 2357 cm−1 between the S1 and 5D4 for Tb3+ (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cm−1) ions are not within the range of 2500–4000 cm−1,55 indicating the incomplete energy transfer between H3L and Eu3+/Tb3+ ions (Fig. S15b, ESI†).
500 cm−1) ions are not within the range of 2500–4000 cm−1,55 indicating the incomplete energy transfer between H3L and Eu3+/Tb3+ ions (Fig. S15b, ESI†).
3.4. Double ratiometric fluorescence sensing for TDGA
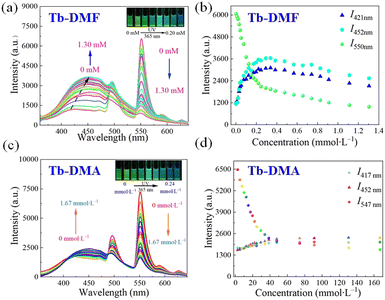
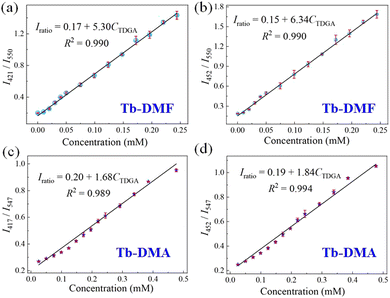
Considering the excellent structural stabilities and luminescence properties of Tb-DMF and Tb-DMA, we attempted to exploit them to detect TDGA molecules in water. As shown in Fig. S16 (ESI†), in the presence of TDGA, the emission peaks of Tb-DMF at 421 nm and Tb-DMA at 417 nm are changed and the intensities are enhanced, meanwhile, the emission peaks of Tb-DMF at 550 nm and Tb-DMA at 547 nm are quenched. It is noteworthy that the new emission peak appears at 452 nm in the emission spectra of both Tb-DMF and Tb-DMA. Given this result, Tb-DMF and Tb-DMA exhibit two intensity ratios (Tb-DMF: I421nm/I550nm, I452nm/I550nm, Tb-DMA: I417nm/I547nm, I452nm/I547nm), respectively, demonstrating their potential as double ratiometric fluorescent sensors for detecting TDGA. Meanwhile, the dispersibilities and time-dependent luminescent stabilities of Tb-DMF and Tb-DMA were also investigated. The intensity ratio (I421nm/I550nm) of the Tb-DMF suspension and the intensity ratio (I417nm/I547nm) of the Tb-DMA suspension basically remained unchanged within 120 minutes, indicating good dispersibilities and luminescent stabilities (Fig. S17, ESI†). The above results provide the foundation for TDGA detection in water systems.To further verify whether the proposed two double ratiometric sensing systems can quantitatively detect TDGA, the concentration-dependent fluorescence titration experiments were investigated. For Tb-DMF, with the increment of TDGA concentration from 0 to 1.30 mM, the intensities at 421 nm gradually enhanced, and the intensities of Tb3+ ions at 550 nm gradually decreased. Meanwhile, the new peak centered at 452 nm appeared and the intensities gradually enhanced. Thus, the intensities of the three emissions are calibrated to one another, indicating that Tb-DMF can achieve double ratiometric fluorescence sensing for TDGA (Fig. 4a and b). Under 365 nm UV light, the obvious changes in fluorescence color from green to blue can be observed, as represented by the variations of the CIE chromaticity diagram (Fig. 4a inset and Fig. S18a, ESI†). Tb-DMA displays a similar visual sensing process for TDGA as Tb-DMF, with significant changes in fluorescence intensities observed in peaks centered at 417, 452 and 547 nm (Fig. 4c, d, and Fig. S18b, ESI†), respectively. Meanwhile, good linear relationships between intensity ratios of Tb-DMF (I421nm/I550nm, I452nm/I550nm) and the concentrations of TDGA within 0.01–0.24 mM are expressed as I421nm/I550nm = 5.30CTDGA + 0.17 (R2 = 0.990) and I452nm/I550nm = 6.34CTDGA + 0.15 (R2 = 0.990) (Fig. 5a and b), and the good linear relationships between intensity ratios of Tb-DMA (I417nm/I547nm, I452nm/I547nm) and the concentrations of TDGA within 0.01–0.48 mM are expressed as I417nm/I547nm = 1.68CTDGA + 0.20 (R2 = 0.989) and I452nm/I547nm = 1.84CTDGA + 0.19 (R2 = 0.994). The limits of detection (LODs) for Tb-DMF are 0.43 μM (64.86 μg L−1) and 0.36 μM (54.30 μg L−1), and the corresponding LODs for Tb-DMA are 1.34 μM (202.11 μg L−1) and 1.22 μM (184.01 μg L−1), respectively (LOD = 3σ/s)37 (Fig. 5c and d), which are comparable with those of the reported TDGA sensors (Table S5, ESI†) and are much lower than the threshold value of 20 mg L−1 (0.13 mM). More importantly, Tb-DMF and Tb-DMA suspensions achieve the first double ratiometric fluorescence sensing for TDGA, further improving the anti-interference performance. Moreover, the fluorescence response times of Tb-DMF and Tb-DMA for TDGA are fast because the time-dependent emission intensities sharply decreased and reached stability within 3 min upon the addition of TDGA (Fig. S19, ESI†). Therefore, Tb-DMF and Tb-DMA suspensions are the efficient double ratiometric fluorescent sensors that can be used for visual monitoring of TDGA, which can meet the requirements for diagnosing VCM exposure levels.
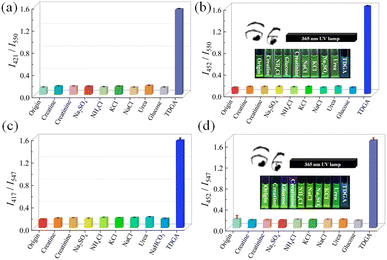
For practical applications, the selective experiment is very important. Meanwhile, TDGA as a biomarker generally exists in the human urine system, thus, a range of characteristic urine substances including KCl, NaCl, NH4Cl, urea, glucose, creatinine, creatine, and TDGA were incubated with Tb-DMF/Tb-DMA under identical conditions. As shown in Fig. 6 and Fig. S20 (ESI†), the negligible changes of intensity ratios (I421nm/I550nm, I452nm/I550nm, I417nm/I547nm, and I452nm/I547nm) are discovered when Tb-DMF/Tb-DMA is treated with other urine substances except TDGA, proving their high selectivity. Under a 365-nm UV lamp, it can be found that the green fluorescence of Tb-DMF and Tb-DMA suspensions turned blue after adding TDGA, however, the color of Tb-DMF and Tb-DMA suspensions after adding other interfering substances hardly changed under ultraviolet excitation, further proving the high selectivity and visual recognition effect of Tb-DMF and Tb-DMA suspensions to TDGA (Fig. 6b and d, inset).
Furthermore, the anti-interference experiment was also carried out. For Tb-DMF, other urine substances are added to the Tb-DMF suspensions containing TDGA, and the intensity ratios of I421nm/I550nm and I452nm/I550nm are basically unchanged compared to that induced by TDGA alone (Fig. 7a and b). For Tb-DMA, its anti-interference behavior (I417nm/I547nm, I452nm/I547nm) is similar to that of Tb-DMF (Fig. 7c and d), indicating that both Tb-DMF and Tb-DMA have high anti-interference ability. Based on the above results, Tb-DMF and Tb-DMA suspensions can be used for highly selective, sensitive, rapid, and visual double ratiometric sensing of TDGA in the water system.
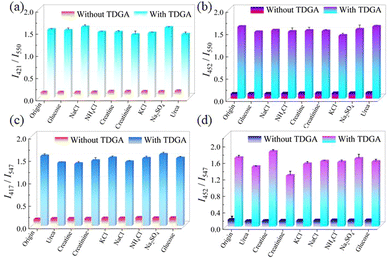 | ||
| Fig. 7 The intensity ratios of I421nm/I550nm (a) and I452nm/I550nm (b) of Tb-DMF and I417nm/I547nm (c) and I452nm/I547nm (d) of Tb-DMA with or without TDGA among various urine components. | ||
To assess the feasibility, effectiveness and actual detection abilities of Tb-DMF and Tb-DMA, first, the TDGA detection experiments in simulated urine samples spiked with different concentrations of TDGA were explored. For Tb-DMF, the measured concentrations of Tb-DMFvia these two detection pathways (I421nm/I550nm, and I452nm/I550nm) are basically consistent with the spiked concentrations. Moreover, the recoveries of TDGA are between 96.63% and 102.75%, and the relative standard deviations (RSD) are between 0.09% and 5.39% (Table S6, ESI†). Similarly, Tb-DMA also has satisfactory recoveries (96.34–110.75%) and low RSD (1.00–5.36%) via these two detection pathways (I417nm/I547nm and I452nm/I547nm) (Table S7, ESI†). Based on the efficient and reliable detection effect in simulated urine samples, we are prompted to investigate the detection of TDGA in the actual urine samples using the standard addition method. For Tb-DMF, as shown in Table S8 (ESI†), Tb-DMF can effectively detect TDGA in real urine samples, accompanied by good recoveries (96.65–107.27%) and RSD (0.05–0.48%). Meanwhile, Tb-DMA also shows satisfactory recoveries (100.21–108.70%) and low RSD (1.36–5.50%) (Table S9, ESI†). The above results indicate that Tb-DMF and Tb-DMA suspensions possess the abilities to detect TDGA in simulated and real urine samples.
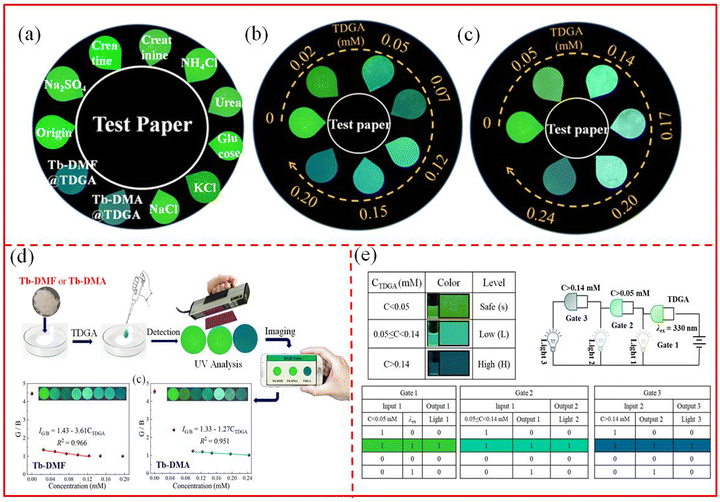
In order to push the actual application, luminescent test papers were also prepared. Under a 365 nm UV lamp, the initial green color of Tb-DMF and Tb-DMA changed to blue after the addition of TDGA, whereas the color remained unchanged after adding other co-existing substances (Fig. 8a). Besides, with the increase of the concentrations of TDGA, the color of the test paper changed from green to blue (Fig. 8b and c). The above results show that the proposed double ratiometric and colorimetric fluorescence sensors, Tb-DMF and Tb-DMA suspensions, can serve as ideal self-calibration platforms and potential portable devices for the sensing of TDGA in disease diagnosis.
3.5. Smartphone-assisted ultrafast and on-site detection of TDGA
To further improve human vision and detection accuracy in rapid on-site monitoring of TDGA, the fluorescent test paper coated with the Tb-DMF/Tb-DMA sensing platform, in combination with a portable smartphone, was developed. As shown in Fig. 8d, when TDGA with different concentrations was dropwise added to paper-based sensors containing Tb-DMF/Tb-DMA, it was observed that their green fluorescence gradually turned blue under 365 nm UV light. Subsequently, a photo was taken using a smartphone, and the color-piker application specialized in chromaticity analysis on the device was employed to convert the color information in the photo into an RGB signal.56 The good linear relationships between G/B values and the concentrations of TDGA are expressed as IG/B = 1.43–3.61CTDGA (R2 = 0.966) and IG/B = 1.33–1.27CTDGA (R2 = 0.951), and the LODs for TDGA are 44.06 and 45.11 μM, demonstrating that paper-based sensors have comparable detection capabilities to those of Tb-DMF and Tb-DMA suspensions. Therefore, the smartphone-assisted Tb-DMF and Tb-DMA paper-based sensors can accurately detect TDGA.To evaluate the feasibility of the smartphone-integrated paper-based sensors of Tb-DMF and Tb-DMA, the real-time detection of TDGA in actual urine samples was executed. Surprisingly, the proposed methods reveal satisfactory recoveries (Tb-DMF: 94.45–102.75%, Tb-DMA: 96.89–108.05%) and low RSD (Tb-DMF: 1.51–4.18%, Tb-DMA: 0.54–3.24%) (Table S10, ESI†). The above results indicated that the smartphone-integrated paper-based sensors of Tb-DMF and Tb-DMA possess good potential for ultrafast and on-site detection of TDGA.
3.6. Logic gate operation
Based on the high selectivity, sensitivity and visual effects of Tb-DMF and Tb-DMA, the portable TDGA fluorescence detectors based on logic gates were constructed in order to improve the practicality of smartphones. As shown in Fig. 8e, the logic detectors consisting of three output ports are used to monitor the fluorescence signals of Tb-DMF and Tb-DMA in response to the different concentrations of TDGA. The series logic systems have different fluorescence output signals S (safe), L (low), and H (high), corresponding to the recognizable concentrations of TDGA. The excitation wavelength (λex = 330 nm) and the concentration of TDGA were used as the dual inputs, and the variation of luminescence intensity served as the output. When the λex and TDGA coexist, gate 1 can form a complete pathway and light 1 lights up, emitting bright green light that can be distinguished by the naked eyes. With the increase of the concentrations of TDGA, the output of gate 1 is 1, the output of gate 2 in the series logic gate is 1, emitting blue-green light, indicating that the concentrations of TDGA are between 0.05 and 0.14 mM, which are at a low level. In this case, the presence of TDGA can cause harm to the human. When the concentrations of TDGA continue to increase, the gate 3 circuit is connected, leading to the gate 3 light to turn on and emit blue light, indicating that the concentrations of TDGA exceed 0.14 mM, which are at a very high level. Intelligent recognition is executed by a logic gate monitor to evaluate different output values (S, L and H) with different TDGA concentration gradients, which will be helpful for the real-time detection of VCM exposure in clinical practice.3.7. Luminescence quenching mechanism
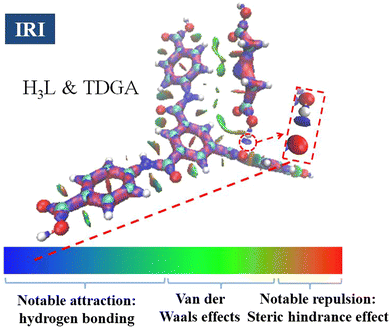
To investigate the mechanism of Tb-DMF and Tb-DMA as double ratiometric fluorescence sensors for TDGA, a series of experiments were explored. First, the PXRD patterns of Tb-DMF and Tb-DMA treated with TDGA are inconsistent with the primary patterns, indicating that the skeleton structures of Tb-DMF and Tb-DMA are changed, which is speculated to be caused by their interaction with TDGA (Fig. S21, ESI†). Second, the FT-IR spectra of Tb-DMF and Tb-DMA treated by TDGA displayed that the peaks (1670 for Tb-DMF and 1681 cm−1 for Tb-DMA) of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of amide (–CONH–) groups both moved to 1662 cm−1, which is attributed to the interaction between the –COOH group of TDGA and –CONH– groups of H3L in Tb-DMF/Tb-DMA (Fig. S22, ESI†). Afterwards, the interactions between TDGA and Tb-DMF/Tb-DMA were further examined by X-ray photo electron spectroscopy (XPS) (Fig. S23, ESI†). The results showed that after the addition of TDGA, the binding energy of O 1s shifted from 531.40 to 531.64 eV for Tb-DMF and from 531.40 to 531.58 eV for Tb-DMA, indicating that the density of the electron cloud around O changes due to the formation of O–H⋯O hydrogen bonds.57 Notably, since the variation in the binding energy of O 1s in Tb-DMF is larger than Tb-DMA, the detection effect of TDGA by the Tb-DMF sensor is superior to that of the Tb-DMA sensor. In order to further verify the formation of O–H⋯O during the TDGA sensing process, the interaction between the H3L ligand and TDGA was further studied via density functional theory (DFT). The diagram for the non-covalent interaction (NCI) between the H3L ligand and TDGA is shown in Fig. 9,58 and the blue part represents hydrogen-bonding interaction. Therefore, it can be seen that there is a strong hydrogen-bonding interaction of O–H⋯O between –COOH in TDGA and –CONH– in the H3L ligand, which further provides the theoretical support for the above conclusion.9,23 In addition, to further investigate whether TDGA can act by entering the pores or on the surface of Tb-DMF/Tb-DMA during the process of sensing, the N2 adsorption–desorption experiments of Tb-DMF/Tb-DMA samples before and after treatment with the TDGA solution were conducted at 77 K. As shown in Fig. S24a and c (ESI†), there are almost no obvious pores in Tb-DMF/Tb-DMA. Moreover, the pore size distribution of Tb-DMF/Tb-DMA samples before and after treatment with the TDGA solution did not change significantly (Fig. S24b and d, ESI†). The above results indicate that TDGA mainly interacts with the active sites on the surface of Tb-DMF/Tb-DMA and it is difficult for TDGA to enter the inner part of the Tb-DMF/Tb-DMA framework.
O stretching vibrations of amide (–CONH–) groups both moved to 1662 cm−1, which is attributed to the interaction between the –COOH group of TDGA and –CONH– groups of H3L in Tb-DMF/Tb-DMA (Fig. S22, ESI†). Afterwards, the interactions between TDGA and Tb-DMF/Tb-DMA were further examined by X-ray photo electron spectroscopy (XPS) (Fig. S23, ESI†). The results showed that after the addition of TDGA, the binding energy of O 1s shifted from 531.40 to 531.64 eV for Tb-DMF and from 531.40 to 531.58 eV for Tb-DMA, indicating that the density of the electron cloud around O changes due to the formation of O–H⋯O hydrogen bonds.57 Notably, since the variation in the binding energy of O 1s in Tb-DMF is larger than Tb-DMA, the detection effect of TDGA by the Tb-DMF sensor is superior to that of the Tb-DMA sensor. In order to further verify the formation of O–H⋯O during the TDGA sensing process, the interaction between the H3L ligand and TDGA was further studied via density functional theory (DFT). The diagram for the non-covalent interaction (NCI) between the H3L ligand and TDGA is shown in Fig. 9,58 and the blue part represents hydrogen-bonding interaction. Therefore, it can be seen that there is a strong hydrogen-bonding interaction of O–H⋯O between –COOH in TDGA and –CONH– in the H3L ligand, which further provides the theoretical support for the above conclusion.9,23 In addition, to further investigate whether TDGA can act by entering the pores or on the surface of Tb-DMF/Tb-DMA during the process of sensing, the N2 adsorption–desorption experiments of Tb-DMF/Tb-DMA samples before and after treatment with the TDGA solution were conducted at 77 K. As shown in Fig. S24a and c (ESI†), there are almost no obvious pores in Tb-DMF/Tb-DMA. Moreover, the pore size distribution of Tb-DMF/Tb-DMA samples before and after treatment with the TDGA solution did not change significantly (Fig. S24b and d, ESI†). The above results indicate that TDGA mainly interacts with the active sites on the surface of Tb-DMF/Tb-DMA and it is difficult for TDGA to enter the inner part of the Tb-DMF/Tb-DMA framework.
In addition, the luminescence decay curves of Tb-DMF and Tb-DMA in the presence and absence of TDGA were also investigated. The luminescence lifetimes of Tb-DMF and Tb-DMA were found to be 0.60 and 0.55 ms based on the intensities at 550 and 547 nm, respectively. After adding TDGA, the lifetimes change to 0.51 and 0.44 ms, respectively, indicating the occurrence of static quenching processes (Fig. S25, ESI†). In order to further verify the existence of the competitive absorption mechanism, the UV-Vis spectra of TDGA, Tb-DMF and Tb-DMA were recorded. As shown in Fig. S26 (ESI†), there was no significant overlap between the UV-Vis spectra of TDGA, Tb-DMF and Tb-DMA, indicating the absence of a competitive absorption mechanism.4 Furthermore, the molecular orbitals of H3L and TDGA were calculated at the B3LYP/6-31G(d,p) level via DFT. As shown in Fig. S27 (ESI†), the lowest unoccupied molecular orbital (LUMO) energy level of H3L is −2.29 eV, which is significantly lower than that of TDGA (−0.14 eV), thus, the photo-induced electron transfer (PET) process between H3L and TDGA is absent.59 Based on the above results, the luminescence quenching of TDGA is the synergistic result of the strong hydrogen-bonding interaction of O–H⋯O between the –COOH group of TDGA and –CONH– groups of H3L in Tb-DMF/Tb-DMA and static quenching.
3.8. Luminescence modulation and anti-counterfeiting applications of EuxTb1−x-DMA
Because Eu-DMA and Tb-DMA had strong red and green luminescence as well as the same coordination environment, an effective strategy was employed to fabricate multi-colored luminescent materials by doping diverse Ln3+ ions into MOFs. Therefore, a series of EuxTb1−x-DMA (x = 0.10–0.90) were synthesized by adjusting the molar ratio of Eu3+ and Tb3+ ions during the synthesis procedures. The PXRD patterns, TG curves and FT-IR spectra of EuxTb1−x-DMA coincide with those of Ln-DMA, suggesting that EuxTb1−x-DMA is isomorphic with Ln-DMA (Fig. S4, S28 and S29, ESI†). Meanwhile, all bimetallic EuxTb1−x-DMA complexes simultaneously exhibit the emissions of Eu3+, Tb3+, and H3L ligand upon excitation at 350 nm. With the molar ratio of Eu3+ increased from 0.1 to 0.9 in EuxTb1−x-DMA, the intensities of Eu3+ at 621 nm gradually increased and the intensities of Tb3+ at 546 nm gradually decreased, while the intensities of the H3L ligand remained basically unchanged (Fig. 10a). Interestingly, under a 365 nm UV lamp, the emission color of bimetallic EuxTb1−x-DMA powders, single crystals and suspensions changes from green to red, with the corresponding CIE coordinates ranging from (0.262, 0.726) to (0.644, 0.356) (Fig. 10b–e). In addition, the luminescence lifetimes and quantum yields of EuxTb1−x-DMA are shown in Fig. S30 and Table S11 (ESI†).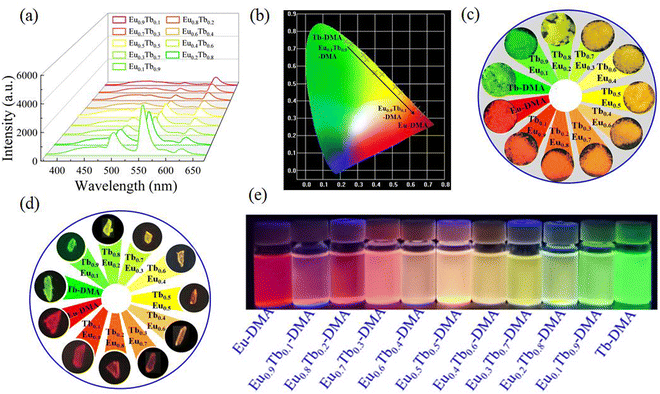 | ||
| Fig. 10 Luminescence emission spectra (a), CIE chromaticity diagram (b), photographs of powders (c), single crystals (d) and aqueous solutions (e) of EuxTb1−x-DMA under 365 nm UV light. | ||
Inspired by the unique characteristics of fluorescence modulation and excellent optical properties, EuxTb1−x-DMA can be used to prepare advanced anti-counterfeiting materials. The different excitation modes of luminescence can be determined and act as authentication information for anti-counterfeiting applications. To explore the potential anti-counterfeiting applications of EuxTb1−x-DMA, the emission spectra of EuxTb1−x-DMA (x = 1, 0.8, 0.6, 0.4, 0.2, 0) at different excitation wavelengths were recorded. Surprisingly, the emission spectra of EuxTb1−x-DMA display obvious excitation wavelength dependence characteristics. As shown in Fig. S31 (ESI†), with the excitation wavelengths increasing from 300 nm to 370 nm, the emission intensities of Eu3+ and Tb3+ ions also gradually increased, and the corresponding CIE coordinates gradually changed (Fig. S32, ESI†). Based on the above results, we speculate that EuxTb1−x-DMA can be used in anti-counterfeiting technology according to the excitation wavelength dependence characteristics. Therefore, a small logo was designed to simulate a new type of anti-counterfeiting graphic using EuxTb1−x-DMA and poly(methyl methacrylate) (PMMA). As shown in Fig. S33a (ESI†), the stamen and petals of a flower are composed of Eu-DMA, Eu0.8Tb0.2-DMA, Eu0.6Tb0.4-DMA, and Eu0.4Tb0.6-DMA, and the leaves and rhizome are composed of Tb-DMA and Eu0.2Tb0.8-DMA. Under a 365 nm lamp, the flower graphic displays red, yellow, orange-yellow, and orange petals, along with green leaves. However, under a 302 nm lamp, the flower graphic shows pale pink and blue petals, along with cyan leaves. The reversible information of the dependence on the excitation wavelength proves that EuxTb1−x-DMA@PMMA composite film materials are suitable for advanced information security applications.
3.9. Doped Eu/Gd/Tb-MOFs used for WLEDs
Considering the RGB strategy60 and the tunable luminescence of Ln-MOFs in the above research, it is probable to obtain a white luminescent material for WLEDs by adjusting the molar ratio of Eu3+, Gd3+, and Tb3+ ions added to the same reaction system. The introduction of Gd3+ions not only dilutes the emission of Eu3+ and Tb3+ ions but also enhances the intensity of the emission peak of the H3L ligand (Fig. S33b, ESI†). As we envisioned, a tri-metallic doped Ln-MOF, Gd0.95Eu0.03Tb0.02-DMA was successfully synthesized by accurately adjusting the Eu3+/Gd3+/Tb3+ molar ratio. Under a 365 nm lamp, the single crystal and aqueous solution of Gd0.95Eu0.03Tb0.02-DMA exhibit near white luminescence (Fig. S33b, inset, ESI†), and its CIE coordinate is (0.334, 0.353), which is very close to that of the ideal white light (0.33, 0.33) (Fig. S33c, ESI†). Compared with the reported white light doped Ln-MOF, Gd0.95Eu0.03Tb0.02-DMA possesses a higher quantum yield of approximately 25.73% (Table S12, ESI†). Therefore, Gd0.95Eu0.03Tb0.02-DMA can emit white light and can serve as a phosphor on the surface of commercial 365 nm UV-LEDs (Fig. S33c, inset, ESI†).4. Conclusions
In conclusion, two series of Ln-MOFs (Ln-DMF and Ln-DMA) with three-dimensional supramolecular frameworks and two-dimensional layered structures as well as doped Ln-MOFs were fabricated under different solvothermal conditions. Tb-DMF and Tb-DMA suspensions first achieve the double ratiometric fluorescence sensing of TDGA based on the synergistic effect of the strong hydrogen-bonding interaction of O–H⋯O between the –COOH group of TDGA and –CONH– groups of H3L in Tb-DMF/Tb-DMA and static quenching. Tb-DMF and Tb-DMA have the advantages of excellent anti-interference and low LOD (0.43 and 0.36 μM; 1.34 and 1.22 μM) in water, and have been successfully utilized for TDGA detection in actual urine systems with satisfactory recoveries. The proposed sensor simultaneously provides visible color signals for real-time detection and accurate readouts with the help of smartphone color recognition software. Among them, Tb-DMF displays higher detection accuracy than Tb-DMA because of the greater variation of the binding energy of O 1s in Tb-DMF before and after monitoring TDGA compared to that of Tb-DMA. In addition, nine bi-metallic doped EuxTb1−x-DMA (x = 0.10–0.90) and the tri-metallic doped Gd0.95Eu0.03Tb0.02-DMA were obtained. Interestingly, the luminescence of the composite films of Ln-DMA and doped EuxTb1−x-DMA possesses obvious excitation wavelength dependence characteristics, and the colors are adjustable and invisible under ambient light, enhancing their potential for anti-counterfeiting applications. This study provides a strategy for the construction of multifunctional materials with double fluorescence sensing with two SiC-calibration systems and tunable luminescence from the aspect of environmental protection and anti-counterfeiting techniques.Author contributions
Xu Zhang: experiment, data curation, writing – original draft preparation. Jiahui Yu: resources. Xin Li: resources. Chengqi Jiao: writing – review & editing, funding acquisition. Yanyu Zhu: review. Hanwen Zheng: review, funding acquisition. Zhengang Sun: investigation, funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21371085 and 22471112), the Scientific Research Fund of Liaoning Provincial Education Department (No. JYTQN2023267 and JYTMS20231055), the Dalian Youth Science and Technology Star Fund (No. 2023RQ030), and the State Key Laboratory of Fine Chemicals, Dalian University of Technology (No. KF2216), and the Special Fund for Basic Scientific Research Business Expenses of Undergraduate Universities in Liaoning Province (2024).References
- B. Yan, TrAC-Trend Anal. Chem., 2024, 170, 117430 CrossRef.
- B. S. Hou, S. Y. Yang, B. Li, G. F. Li, H. Y. Zheng, C. Qin, G. G. Shan, Z. M. Su and X. L. Wang, Inorg. Chem. Front., 2022, 9, 4376–4384 RSC.
- Y. L. Hong, S. W. Zuo, H. Y. Du, Z. Q. Shi, H. L. Hu and G. Li, ACS Appl. Mater. Interfaces, 2024, 16, 13745–13755 CrossRef PubMed.
- L. L. Ma, G. P. Yang, G. P. Li, P. F. Zhang, J. Jin, Y. Wang, J. M. Wang and Y. Y. Wang, Inorg. Chem. Front., 2021, 8, 329–338 RSC.
- P. P. Zhang, A. Y. Ni, J. J. Zhang, B. L. Zhang, H. J. A. Zhou, H. Zhao, S. Q. Liu, J. Ni and C. Y. Duan, Sens. Actuators, B, 2023, 384, 133624 CrossRef.
- C. Jia, S. Liang, Y. X. Wen, Z. Z. Xue, J. Pan, J. X. Hu and G. M. Wang, Cryst. Growth Des., 2024, 24, 2202–2209 CrossRef.
- Y. J. Cui, B. L. Chen and G. D. Qian, Coord. Chem. Rev., 2014, 273–274, 76–86 CrossRef CAS.
- Y. Zhang, S. Liu, Z. S. Zhao, Z. F. Wang, R. Y. Zhang, L. Liu and Z. B. Han, Inorg. Chem. Front., 2021, 8, 590–619 RSC.
- L. Yu, L. X. Feng, Z. Y. Wei, S. Wang, Y. M. Feng, Y. J. Shen, J. L. Cai, J. Y. Wu and Y. X. Xiao, Adv. Funct. Mater., 2023, 33, 2300309 CrossRef.
- T. L. M. Martinon and V. C. Pierre, Curr. Opin. Chem. Biol., 2023, 76, 102374 CrossRef.
- A. E. Psalti, D. Andriotou, S. A. Diamantis, A. Chatz-Giachia, A. Pournara, M. J. Manos, A. Hatzidimitriou and T. Lazarides, Inorg. Chem., 2022, 61, 11959–11972 CrossRef PubMed.
- X. R. Wang, Y. P. Jiang, A. Tissot and C. Serre, Coord. Chem. Rev., 2023, 497, 215454 CrossRef.
- T. Amiaud, V. Jubera and H. Serier-Brault, J. Mater. Chem. C, 2023, 11, 10951–10956 RSC.
- C. Sen, R. Singhaal, N. A. Ashashi, S. Devi, M. Ahmad and H. N. Sheikh, J. Mol. Struct., 2023, 1290, 135970 CrossRef.
- P. A. Demakov, A. A. Vasileva, S. S. Volynkin, A. A. Ryadun, D. G. Samsonenko, V. P. Fedin and D. N. Dybtsev, Molecules, 2021, 26, 5145 CrossRef CAS PubMed.
- K. Wang, Y. L. Zhu, T. F. Zheng, X. Xie, J. L. Chen, Y. Q. Wu, S. J. Liu and H. R. Wen, Anal. Chem., 2023, 95, 4992–4999 CrossRef CAS PubMed.
- Y. Li, B. L. Chai, H. Xu, T. F. Zheng, J. L. Chen, S. J. Liu and H. R. Wen, Inorg. Chem. Front., 2022, 9, 1504–1513 RSC.
- K. Wang, T. F. Zheng, J. L. Chen, H. R. Wen, S. J. Liu and T. L. Hu, Inorg. Chem., 2022, 61, 16177–16184 CrossRef CAS PubMed.
- B. Wang, L. P. Heng, Q. Sui, Z. Peng, X. Z. Xiao, M. H. Zheng, J. X. Hu, H. Fiedler, D. Barceló and G. Yu, Front. Environ. Sci. Eng., 2023, 17, 52 CrossRef CAS.
- Y. S. Liu, M. Y. Wang, Y. F. Hui, J. Y. Tian, J. X. Xu, Z. Y. Lu, Z. Y. Yang, H. Guo and W. Yang, ACS Sens., 2024, 9, 315–324 CrossRef CAS PubMed.
- C. W. Wang, H. Y. Chuang, K. W. Liao, M. L. Yu, C. Y. Dai, W. T. Chang, C. H. Tsai, H. C. Chiang and P. C. Huang, Environ. Int., 2019, 131, 104978 CrossRef PubMed.
- Y. C. Fan, X. Jiang, J. Che, M. F. Li, X. J. Zhang, D. J. Gao, J. Bi and Z. L. Ning, Molecules, 2022, 27, 6543 CrossRef PubMed.
- N. Wu, H. Guo, L. P. Peng, M. Y. Wang, Y. J. Cao, M. Yang, L. Sun and W. Yang, Sens. Actuators, B, 2021, 345, 130402 CrossRef.
- Z. X. Zhu, C. J. Wang, D. Luo, C. Liu, D. N. Liu, Y. M. Xiao, S. Chen and Y. Y. Wang, J. Coord. Chem., 2019, 72, 3526–3543 CrossRef.
- J. N. Hao, X. Y. Xu, X. Lian, C. Zhang and B. Yan, Inorg. Chem., 2017, 56, 11176–11183 CrossRef PubMed.
- S. Y. Wu, H. Min, W. Shi and P. Cheng, Multicenter Metal–Organic Framework-Based Ratiometric Fluorescent Sensors, Adv. Mater., 2020, 32, 1805871 CrossRef PubMed.
- Y. F. Zhao and D. Li, J. Mater. Chem. C, 2020, 8, 12739–12754 RSC.
- R. Q. Sun, P. Liu, Y. C. Ma and Q. L. Yang, Chem. Eng. J., 2024, 488, 150943 CrossRef CAS.
- X. Y. Zhai, P. F. Feng, N. Song, G. D. Zhao, Q. Y. Liu, L. L. Liu, M. Tang and Y. Tang, Inorg. Chem. Front., 2022, 9, 1406–1415 RSC.
- H. B. Rao, W. Liu, K. Q. He, S. Zhao, Z. W. Lu, S. X. Zhang, M. M. Sun, P. Zou, X. X. Wang, Q. B. Zhao, Y. Y. Wang and T. Liu, ACS Sustainable Chem. Eng., 2020, 8, 8857–8867 CrossRef CAS.
- K. F. Kayani, N. N. Mohammad, D. A. Kader, S. J. Mohammed, D. A. Shukur, A. H. Alshatteri, S. H. Al-Jaf, K. A. Abdalkarim and H. Q. Hassan, ChemistrySelect, 2023, 8, e202303472 CrossRef CAS.
- T. Kanwal, S. Rasheed, M. Hassan, B. Fatima, H.-M. Xiao, S. G. Musharraf, M. Najam-ul-Haq and D. Hussain, ACS Appl. Mater. Interfaces, 2024, 16, 1688–1704 CrossRef CAS.
- Y. J. Zhao, M. Y. Liu, S. Zhou, Z. Y. Yan, J. S. Tian, Q. J. Zhang and Z. Y. Yao, Food Chem., 2023, 425, 136449 CrossRef CAS PubMed.
- X. A. Zhang, Z. L. Wang, X. W. Huang, Q. L. Huang, Y. B. Wen, B. Li, M. Holmes, J. Y. Shi and X. B. Zou, Chem. Eng. J., 2023, 451, 138928 CrossRef CAS.
- H. L. Li, J. Y. Yang, X. C. Hu, R. Han, S. Wang and M. F. Pan, Chem. Eng. J., 2023, 473, 145401 CrossRef.
- W. Fu, X. Q. Fu, Z. M. Li, Z. F. Liu and X. Li, Chem. Eng. J., 2024, 489, 151225 CrossRef.
- W. W. Fan, X. L. Liu, Y. Cheng, S. S. Chang, L. J. Wang, Y. X. Liu, P. Liu, L. Y. Zheng and Q. E. Cao, ACS Appl. Mater. Interfaces, 2023, 15, 22590–22601 CrossRef PubMed.
- P. A. Demakov, A. A. Ryadun, P. V. Dorovatovskii, V. A. Lazarenko, D. G. Samsonenko, K. A. Brylev, V. P. Fedin and D. N. Dybtsev, Dalton Trans., 2021, 50, 11899–11908 RSC.
- Z. J. Hu, M. J. Tsai, Y. J. Tu and J. Y. Wu, Chem. – Eur. J., 2023, 29, e202300081 CrossRef PubMed.
- E. J. Gao, S. Y. Wu, J. Wang, M. C. Zhu, Y. Zhang and V. P. Fedin, Adv. Opt. Mater., 2020, 8, 1901659 CrossRef.
- M. Y. Huang, Z. X. Liang, J. L. Huang, S. Y. Zhang, Y. H. Wen and X. T. Wu, CrystEngComm, 2023, 25, 1637–1642 RSC.
- H. Brunckova, E. Mudra, L. Rocha, E. Nassar, W. Nascimento, H. Kolev, A. Kovalcikova, Z. Molcanova, M. Podobova and L. Medvecky, Appl. Surf. Sci., 2021, 542, 148731 CrossRef CAS.
- Y. Kitamura, H. Toshima, A. Inokuchi and D. Tanaka, Mol. Syst. Des. Eng., 2023, 8, 431–435 RSC.
- M. O. Barsukova, S. V. Cherezova, A. A. Sapianik, O. V. Lundovskaya, D. G. Samsonenko and V. P. Fedin, RSC Adv., 2020, 10, 38252–38259 RSC.
- A. Karmakar and J. Li, Chem. Commun., 2022, 58, 10768–10788 RSC.
- J. Othong, J. Boonmak, V. Promarak, F. Kielar and S. Youngme, ACS Appl. Mater. Interfaces, 2019, 11, 44421–44429 CrossRef CAS PubMed.
- Z. H. Sun, A. Khurshid, M. Sohail, W. D. Qiu, D. R. Cao and S. J. Su, Nanomaterials, 2021, 11, 2761 CrossRef CAS PubMed.
- X. M. Lu, Y. Tang, G. P. Yang and Y. Y. Wang, J. Mater. Chem. C, 2023, 11, 2328–2335 RSC.
- Y. Xie, G. T. Sun, J. W. Li and L. N. Sun, Adv. Funct. Mater., 2023, 33, 2303663 CrossRef CAS.
- H. F. Sha and B. Yan, J. Mater. Chem. C, 2022, 10, 7633–7640 RSC.
- Y. H. Cheng, M. J. Wu, Z. Y. Du, Y. Chen, L. Y. Zhao, Z. W. Zhu, X. B. Yu, Y. Y. Yang and C. H. Zeng, ACS Appl. Mater. Interfaces, 2023, 15, 24570–24582 CrossRef CAS PubMed.
- X. L. Yu, A. A. Ryadun, D. I. Pavlov, T. Y. Guselnikova, A. S. Potapov and V. P. Fedin, Angew. Chem., Int. Ed., 2023, 62, e202306680 CrossRef CAS PubMed.
- X. Zhang, D. Y. Liu, J. H. Yu, X. Li, H. W. Zheng, Y. N. Zhou, C. Y. Huang, Y. Y. Zhu, C. Q. Jiao and Z. G. Sun, Dalton Trans., 2023, 52, 8558–8566 RSC.
- F. J. Steemers, W. Verboom, D. N. Reinhoudt, E. B. van der Tol and J. W. Verhoeven, J. Am. Chem. Soc., 1995, 117, 9408–9414 CrossRef CAS.
- Z. S. Han, K. Y. Wang, Y. L. Chen, J. N. Li, S. J. Teat, S. H. Yang, W. Shi and P. Cheng, CCS Chem., 2022, 4, 3238–3245 CrossRef CAS.
- L. D. Yang, W. Hu, F. B. Pei, Z. W. Liu, J. Wang, Z. Y. Tong, X. H. Mu, B. Du, M. Z. Xia, F. Y. Wang and B. Liu, Food Chem., 2024, 438, 138068 CrossRef CAS PubMed.
- R. N. Wang, H. Zhang, J. Sun and Z. M. Su, Inorg. Chem. Front., 2024, 11, 269–277 RSC.
- T. Lu and Q. X. Chen, Chem.: Methods, 2021, 1, 231–239 CAS.
- K. Ren, S. H. Wu, X. F. Guo and H. Wang, Inorg. Chem., 2019, 58, 4223–4229 CrossRef CAS PubMed.
- J. J. Hu, Y. G. Li, H. R. Wen, S. J. Liu, Y. Peng and C. M. Liu, Inorg. Chem., 2022, 61, 6 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section (materials, measurements, luminescence sensing experiments), refinement data of crystals, selected bond lengths and angles, additional structural figures (Fig. S1–S33) and additional tables (Tables S1–S16). CCDC 2343224–2343229. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc03563a |
| This journal is © The Royal Society of Chemistry 2024 |