Tuning the performance of PSCs using rare-earth elements
Sajid
Sajid
 ab,
Salem
Alzahmi
*ab,
Nouar
Tabet
ab,
Salem
Alzahmi
*ab,
Nouar
Tabet
 c,
Mohammad Y.
Al-Haik
d,
Mahmoud
Abdel-Hafiez
c,
Yousef
Haik
c,
Mohammad Y.
Al-Haik
d,
Mahmoud
Abdel-Hafiez
c,
Yousef
Haik
 ef and
Ihab M.
Obaidat
ef and
Ihab M.
Obaidat
 *c
*c
aDepartment of Chemical & Petroleum Engineering, United Arab Emirates University, Al Ain, P.O. Box 15551, United Arab Emirates. E-mail: s.alzahmi@uaeu.ac.ae
bNational Water and Energy Center, United Arab Emirates University, Al Ain, P.O. Box 15551, United Arab Emirates
cDepartment of Applied Physics and Astronomy, University of Sharjah, Sharjah, P.O. Box 27272, United Arab Emirates. E-mail: iobaidat@sharjah.ac.ae
dDepartment of Sustainable and Renewable Energy Engineering, University of Sharjah, Sharjah, 27272, United Arab Emirates
eDepartment of Mechanical and Nuclear Engineering, University of Sharjah, Sharjah, United Arab Emirates
fDepartment of Mechanical Engineering, The University of Jordan, Amman, Jordan
First published on 30th October 2024
Abstract
Perovskite solar cells (PSCs) are emerging and promising alternatives to the market-leading solar cells due to their high performance, low fabrication cost, and versatile material modification. There are still opportunities to enhance the performance of PSCs, such as regulating mismatched absorption and limiting degradation brought about by some parts of light. Rare-earth elements (REEs) have the potential to be useful in this context. Due to their diverse energetic transition orbitals, REEs can transform ultraviolet (UV) and infrared (IR) light into visible light, which helps to improve the photostability of PSCs in addition to allowing more light absorption. Furthermore, their reversible redox potential can help prevent degradation caused by the redox reaction of other functional materials used in PSCs. As interface modifiers, REEs have the ability to induce the formation of a donor–acceptor complex, which can overcome the interface barrier and enable high charge-carrier collecting ability. In this regard, we will scrutinize PSCs that utilize REEs as electron transporting materials, hole transporting materials, additives in perovskites, and interface modifiers. We also offer the possible future research directions and limitations of using REEs in PSCs for high stability and increased efficiency.
Introduction
Perovskite materials are the most appealing photovoltaic materials for next-generation solar cells because of their tunable bandgap,1 high absorption coefficient,2 appropriate ambipolar properties,3 suitability for use in a variety of devices,4 ease of solution processing,5 and high charge-carrier mobility,6 whether they are pure inorganic or hybrid perovskites.7–9 Thanks to their optoelectronic features, certified power conversion efficiency (PCEs) of perovskite solar cells (PSCs) have increased dramatically in recent years—from 3.8% to 26.7%.10,11 Unfortunately, the majority of efficient PSCs respond only to the visible spectrum of sunlight, which is between 400 and 800 nm, leaving out the ultraviolet (UV) and infrared (IR) components.12 Their insensitivity to UV and IR regions of the solar spectrum, which results in significant energy waste and thus indicates potential room for PCE improvement, is undoubtedly one of the main issues restricting their PCEs.13–15 Furthermore, because organic cations (in perovskites) and titanium dioxide (TiO2) are commonly employed as electron transporting materials (ETMs), UV illumination may probably induce the formation of oxygen vacancies and defects, leading to the disintegration of the used materials.16It is widely recognized that sunlight covers a broad range of wavelengths, spanning from the UV to IR (280–2500 nm) region of the spectrum. Perovskites exhibit a response to visible light, which extends from 400 nm to 800 nm and accounts for only 45–50% of the solar spectrum.12 The amount of radiation absorbed by PSCs and the spectral regions that can be used by the up-conversion (UC) and down-conversion (DC) processes in the AM 1.5G spectrum are displayed in Fig. 1a. In this context, rare-earth elements (REEs) (Fig. 1b) have recently emerged and can be used for achieving either DC or UC effects. As a result, REEs enhance PSC photostability in addition to enhancing the UV and near infrared (NIR) response.17,18 A modified solar cell with a UC layer could potentially achieve a theoretical efficiency limit of 47.6% for non-concentrated sunlight and 63.2% for concentrated sunlight.19 These theoretical efficiency limits were calculated based on assumptions of bifacial solar cells.20
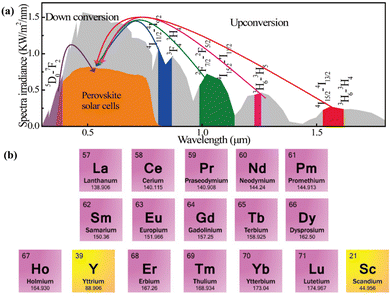 | ||
| Fig. 1 (a) The amount of light absorbed by PSCs in the AM 1.5G spectrum, as well as possible UC and DC effects that can use UV and IR spectrum regions. Reproduced from ref. 21 with permission from Elsevier, copyright 2018. (b) List of the REEs that can be used for UC and DC processes. Reproduced from ref. 22 with permission from MDPI, copyright 2024. | ||
Because several REEs, as shown in Fig. 1b, can be adequately doped to modify the electronic properties of materials, REEs are extensively used in the PSCs. In addition, the majority of REEs are in the 3+ or 2+ oxidation state and can form strong bonds with negatively charged ions. By coupling these REEs with other crystalline materials, one can alter the dynamics of crystallization and lessen intra-gap trap states. Additionally, REEs with the appropriate ionic radius can replace the elements in the crystal lattice, changing the target crystal's phase stability and unit cell parameters. Notably, REEs typically display two distinct luminescence processes, such as the f–d and f–f transitions. This characteristic turns those perfect phosphors capable of absorbing low-energy photons and emitting light in the UV to NIR range. Photoluminescent materials that show f–d transition display broadband and strong intensity and high luminescence efficiency. However, the f–f transition permits a narrow peak, low intensity, and comparatively less efficient luminescence. The REE-doped materials have promising application potential in optoelectronic devices due to their UC and DC properties. In particular, UC is an anti-Stokes nonlinear optical phenomenon that transforms several low-energy photons into high-energy photons. For example, the energy can be transferred to the nearby Er3+ and Tm3+ ions for UC emission after the REEs absorb the low-energy photons under NIR light excitation via excited state absorption, energy transfer between two ions, or cross-relaxation energy transfer in a typical UC system, as shown in Fig. 2(a) and (b).23 The wavelength range of the luminescent light falls between 400 and 700 nm, which is the wavelength range that perovskite responds to. One high-energy photon can be converted into several low-energy photons by the linear optical phenomenon known as DC. Fig. 2(c) and (d) show the schematic diagram a typical DC system and the basic DC process based on either one REE ion, I, or two REE ions, II, where I is an activator ion that can take in energy from I and II is a sensitizer ion with a broad bandgap that can absorb high energy photons. More details on the background UC/DC system can be found in the review article written by Shah et al.24 Furthermore, the presence of organic cations in most of the perovskites and the photocatalytic nature of TiO2 induce the formation of oxygen vacancies and defects in PSCs upon UV light exposure.25,26 By turning the UV part of the incident light into the visible range, the DC effects of REEs applied to PSCs can both boost the photocurrent and prevent the UV portion of the incident light from interacting with the perovskite/ETM.27,28
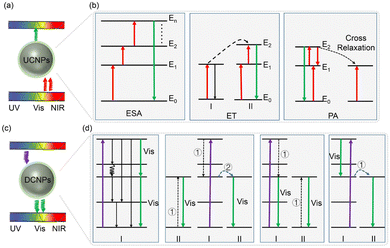 | ||
| Fig. 2 (a) Schematic illustration of a typical UC system and (b) UC processes in nanoparticles modified with REEs under NIR excitation. (c) The schematic diagram of a typical DC system and (d) a DC process showing how UV light is absorbed and how DC is produced on a single ion or two ions by the emission of two visible photons, where (1) represents relaxation and (2) represents energy transfer between the two ions. Note that PA stands for photon avalanche, ET for energy transfer, and SA for excited state absorption. Reproduced from ref. 23 with permission from IOP-Chinese Physical Society, copyright 2022. | ||
Considering the above-mentioned potential of the REEs, the following sections will scrutinize the use of REEs as interface modifiers, as additives in the perovskite layer, and as charge transport layers. In addition, every aspect of improving the performance of PSCs will be covered in detail.
REEs utilized in the perovskite layer
The quality of the perovskite layer is the main cause of PSC's high-performance; researchers tend to focus on lowering the density of defects, pinholes, and grain boundaries as well as enhancing the absorption range of the perovskite layer.29–31 The doping of REEs allows for the customization of the optoelectronic properties of the perovskites.32,33 For instance, due to their comparable ionic radii (Eu2+, 117 pm and Pb2+, 119 pm), REEs like Eu2+ are capable of partially replacing the metal cations of perovskites, leading to the formation of MAPb1−xEuxI3.34 This can form certain impurity energy levels through EuI2 that can significantly increase the absorption in the UV-vis wavelength region of MAPb1−xEuxI3. Furthermore, Eu2+ doping in the perovskite structure improves charge mobility and affects electronic orbitals, which enhances short-circuit current density (Jsc) and PCE.35 The photoluminescence of the perovskites may also be improved by the addition of REEs. For example, doping CsPbBr3 with Ce3+ leads to substantially increased photoluminescence response by improving the lattice order and enriching the conduction band-edge states.36 In addition, Ce3+ shows a d–f transition—which can be adjusted from the UV to the visible regions by introducing various coordinated environments—offering desirable optical properties.37,38 Furthermore, REEs based on chlorine help to stabilize the perovskite phases. Similar to the Pb2+ ionic radius (1.19 Å), Ce3+ has an ionic radius of approximately 1.034 Å, making it easier to replace Pb2+ and create bimetallic perovskites.39 Based on this, Li et al.40 looked into the effects of adding different concentrations of CeCl3 to the precursor solution on perovskite films and devices. The large grain size, enhanced carrier mobility, and superior crystallinity were all observed for the CeCl3-based perovskite films. Meanwhile, non-radiative recombination generation was decreased, the carrier migration length was increased, and device performance was enhanced through passivated interface defects and enhanced carrier transport ability. The PCE of the CeCl3-based device increased from 21.57% to 23.07%. When the unencapsulated sample was exposed to ambient conditions with 40–60% RH, the PCE of the CeCl3-based PSC remained at 87% for 1060 hours. The Eu–PbX2 complex can be utilized as a nucleus to encourage the growth of perovskites with uniformly distributed grain sizes and uniform surface coverage. It is produced by the strong coupling of the negatively charged halide plumbates (PbX3−, PbX42−, and PbX64−, X = I, Br) in the perovskite structure with Eu3+, as displayed in Fig. 3a and b.41,42 Here one-step conversion of a precursor to a perovskite with a larger formation energy barrier was changed to two-step conversion of a precursor to a complex and then to a perovskite with a decreased formation energy barrier. This enables the interaction between the REEs and the perovskite precursor in the solution, lowers the perovskite formation energy, and enables perovskite growth at low temperatures.43 Doping of EuCl3 and CsPbI3, for instance, demonstrated that room temperature and ambient conditions can stabilize the black perovskite phase of CsPbI3, which can be ascribed to the decreased grain size and crystal structure distortion due to the low temperature phase stabilization (Fig. 3c and d).44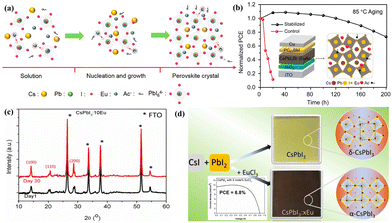 | ||
| Fig. 3 (a) Eu(Ac)3-doped perovskite crystal growth illustration and (b) the resulting PSC with long-term stability. Reproduced from ref. 41 with permission from American Chemical Society, copyright 2021. (c) Day 1 and Day 30 XRD patterns of the CsPbI3 film containing 10 mol% EuCl3. The film was kept in a dark, dry environment (10–20% RH). (d) Photographs of the as-prepared perovskite layers and optimized PCE. Reproduced from ref. 44 with permission from American Chemical Society, copyright 2018. | ||
Similarly, heterovalent neodymium cations (Nd3+) doped in perovskites demonstrated better film quality with substantially expanded charge carrier lifetimes, significantly increased and balanced charge carrier mobility, and greatly decreased trap-states.45 Consequently, PSCs fabricated using Nd3+-doped perovskites show a markedly reduced photocurrent hysteresis and a highly reliable PCE of 21.15%. Li et al.46 added scandium trifluoromethanesulfonate (Sc(OTF)3) as a versatile additive to the perovskite precursor solution to decrease the charge-carrier trapping and passivate non-radiative recombination centers of perovskite films. A superior perovskite film with large grains, high crystallinity, and few defects was produced as a result of the sulfonic group's ability to coordinate with lead ions, which slowed down the process of crystallization of the perovskite. In the meantime, Sc3+ doping in the perovskite precursor solution can allow Sc3+ to interact with the perovskite, enhancing film morphology and extending the lifetime of the charge carrier. Furthermore, the enhanced stability of the perovskite film was attributed to the immobilization of methylamine ions (MA+) due to the robust hydrogen bonding interaction between Sc(OTF)3 and MA+. PSCs with perovskite-doped Sc(OTF)3 (0.1 mg mL−1) demonstrated an impressive PCE of 20.63%, an open-circuit voltage (Voc) of 1.134 V, a Jsc of 21.71 mA cm−2, and a high fill factor (FF) of 83.80%. A straightforward hydrothermal method assisted by ethylenediamine tetraacetic acid disodium (EDTAD) was employed by Feng et al.47 to prepare REE-doped KMnF3 nanocrystals with a tunable size and a single red UC emission. An effective chelating agent, EDTAD, has the ability to modify the size, shape, and emission performance of nanomaterials. The prepared singular UC-REEs were used to improve perovskite growth and induce the formation of films with enhanced crystallinity, compact grains, and less defects in order to improve PSC photovoltaic metrics. The PCE of a PSC with KMnF3 (Yb3+, Er3+) increased to 19.11% with the optimum molar ratio of UC nanocrystals, compared to 14.94% for the pristine device.
Due to high charge recombination at interfaces or within perovskite films, as well as an inferior light-absorbance spectrum, the majority of inorganic perovskites, such as CsPbBr3-based PSCs, have low PCEs. Eu2+ doping of CsPbBr3 was done in this regard to increase PSC efficiency.48 The perovskite films doped with Eu2+ show smoother morphology and improved crystallinity. As a result, the HTM-free PSC with Eu2+ and an inexpensive carbon electrode demonstrated increased stability and efficiency. Particularly, by optimizing the doping concentration of Eu2+, the PCE increased from 5.66% to 7.28% with a high Voc of 1.45 V. Furthermore, the storage stability shows that PCE performed exceptionally well without encapsulating in air with a relative humidity (RH) of 70–80% (Fig. 4a–f). Duan et al.49 also developed lattice-doped CsPbBr3 films with REEs such as lanthanide ions (La3+, Ce3+, Nd3+, Sm3+, Eu3+, Gd3+, Tb3+, Ho3+, Er3+, Yb3+, and Lu3+) in order to overcome this problem. The incorporation of Ln3+ and Sm3+ ions into the perovskite lattice resulted in longer carrier lifetimes and larger grains, which in turn led to improved performance of the HTM-free CsPbBr3-based PSCs. Under 1-sun illumination, these PSCs achieved an ultrahigh Voc of 1.594 V and a champion efficiency of 10.14% (Fig. 4g and h). In the meantime, these devices demonstrated improved thermal stability at 80 °C for 60 days and high stability under 80% RH in an air atmosphere over 110 days (Fig. 4i and j).
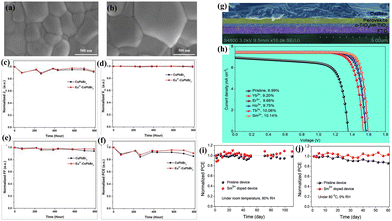 | ||
| Fig. 4 Surface morphology of (a) pristine and (b) Eu-doped perovskites, and (c)–(f) performance of the control and Eu-doped PSCs. Reproduced from ref. 48 with permission from Wiley-VCH GmbH, copyright 2020. (g) A cross-sectional SEM image of a HTM-free all-inorganic PSC, and (h) the J–V curves of the PSCs based on different perovskite absorbers. Stability of Sm3+ doped and pristine PSCs over time without encapsulation at (i) 25 °C and 80% RH and (j) 80 °C and 0% RH. Reproduced from ref. 49 with permission from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, copyright 2018. | ||
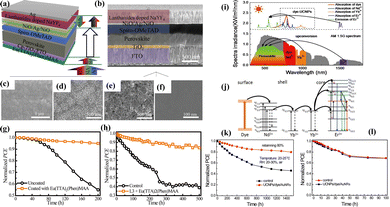
For utilization of REE-doped UC nanoparticles in the perovskite layer of PSCs, inadequate integration between the nanoparticles and the perovskite precursor is typically an obstacle to address. The majority of methods for creating high-quality REE-doped UC nanoparticles use long-chain alkyl molecules as ligands, such as oleic acid or oleylamine. This invariably creates an insulating barrier around each nanoparticle, preventing the perovskite from directly accessing the surface of the REE-doped UC nanoparticles and reducing the ability of the nanoparticles to mix with the perovskite precursor. To deal with this problem, Meng et al.50 devised a ligand-exchange technique that allows REE-doped UC nanoparticles to switch their phase from a nonpolar solvent (like n-hexane) to a variety of polar media (like DMF). This is achieved by substituting specially designed species for the long chain alkyl ligands on the surface of the nanoparticles. Methylammonium iodine (MAI) was employed as a ligand to produce MAI-capped β-NaYF4:Yb,Er rather than long-chain alkyl molecules capped on REE-doped UC nanoparticles. In addition to spectral conversion, β-NaYF4:Yb,Er supplied extra nucleation sites to support the heteroepitaxial growth of the perovskite crystals, which led to the formation of a perovskite layer embedded with β-NaYF4:Yb,Er that had much larger crystal grains. A PSC with a β-NaYF4:Yb,Er-embedded perovskite demonstrated a high PCE of 19.70% when illuminated with AM 1.5G sunlight and 0.37% when illuminated only with NIR light. Since the doped REE ions (Yb3+ and Er3+) show intrinsic 4f–4f electronic transitions, β-NaYF4:Yb, Er has intrinsically weak and narrow-band absorption.51 In order to boost and expand the spectral absorption, Lai et al.52 used NIR dye sensitization of β-NaYF4:Yb,Er to enhance the UC of the doped REE ions. In this way, the dye molecules function as antennas, absorbing incident light and concurrently passing their excitation energy to the up-converting REE ions through Förster-type energy transfer. β-NaYF4:Yb,Er was sensitized with a hydrophilic IR806 dye to create IR806-β-NaYF4:Yb,Er. A two-step ligand-exchange procedure was developed to replace the OAm ligands on the surface of β-NaYF4:Yb,Er to increase the stability of IR806-UCNCs in DMF, as displayed in Fig. 5. Adding IR806-β-NaYF4:Yb,Er to a PSC converts wide band NIR (beyond 800–1000 nm) into visible spectral response for the perovskite layer. The PSC that was integrated with IR806-β-NaYF4:Yb,Er demonstrated an outstanding efficiency of 17.49%, indicating an improvement of 29% in comparison to the control PSC (13.52%). The enhanced energy transfer from UC nanoparticles to perovskites, a rise in NIR absorption, and the uniform, pinhole-free perovskite film morphology with bigger crystal grains were all credited with improving device performance.
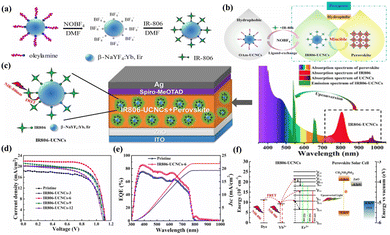 | ||
| Fig. 5 (a) The hydrophilic IR806-UCNC schematic preparation using a two-step ligand-exchange procedure. (b) The suggested plan for creating a well-miscible perovskite precursor incorporating IR806-sensitized UCNCs. (c) Activated perovskite for a PSC through broadband NIR sunlight harvesting and spectral conversion into the visible range. The right side displays the emission spectrum of IR806-UCNCs as well as the absorption spectra of the perovskite, IR806, and UCNCs. (d) Planar PSC J–V curves measured under AM 1.5G sunlight with varying IR806-UCNC additive amounts. (e) The pristine and IR806-UCNC-6-based PSC IPCE spectra. (f) Schematic energy transfer in a planar PSC with IR806-UCNCs integrated. Reproduced from ref. 52 with permission from Elsevier B.V. copyright 2017. | ||
The performances of the PSCs are summarized in Table 1. Overall, the factors that have contributed to better PSC performance include the REEs’ ability to broaden the PSC's response range to the NIR/UV region and convert these spectral ranges to visible light, which boosts absorption and raises PSC's photocurrent. In addition, high crystallinity, dense grains, and low defect counts could be found in perovskite films by adding REEs to the precursor. REEs can improve perovskite films, which can lower carrier recombination, extend carrier lifetime, and boost mobility, by efficiently filling in gaps and holes on grain boundaries and removing perovskite surface defects.
| Composition | PSC design | J sc (m A cm−2) | V oc (V) | FF (%) | PCE (%) | Stability (h) | Conditions | Ref. |
|---|---|---|---|---|---|---|---|---|
| MAPb1−xEuxI3 | FTO/NiO/MAPb1−xEuxI3/PCBM/BCP/Ag | 21.5 | 1.02 | 76.4 | 16.7 | 288 | 25 °C, 25 ± 5% RH, unencapsulated | 34 |
| CsPbI2Br(Eu(Ac)3) | ITO/NiO/CsPbI2Br(Eu(Ac)3)/PC61BM/Cu | 15.28 | 1.119 | 67.26 | 11.51 | 500 | 85 °C, 40% RH, unencapsulated | 41 |
| CsPbI3:xEu | FTO/c-TiO2/CsPbI3:xEu/spiro/Au | 11.1 | 0.898 | 68 | 6.8 | n/a | n/a | 44 |
| MAPbI3:xNd3+ | ITO/PEO-doped PEDOT:PSS/MAPbI3:xNd3+/LiSPS/PC61BM/Al | 24.33 | 1.04 | 83.6 | 21.15 | n/a | n/a | 45 |
| MAPbI3-xClx: Sc(OTF)3 | ITO/P3CT-Na/MAPbI3-xClx:Sc(OTF)3/C60/BCP/Ag | 21.71 | 1.134 | 83.80 | 20.63 | n/a | n/a | 46 |
| MAPbI3:KMnF3:Yb3+,Er3+ | FTO/c-TiO2/m-TiO2/MAPbI3:KMnF3:Yb3+,Er3+/spiro/Ag | 23.14 | 1.09 | 75.77 | 19.11 | n/a | n/a | 47 |
| CsPb1−xEuxBr3 | FTO/c-TiO2/m-TiO2/CsPb1−xEuxBr3/carbon | 6.33 | 1.4523 | 79.19 | 7.28 | 800 | 70–80% RH unencapsulated | 48 |
| CsPb0.97Sm0.03Br3 | FTO/c-TiO2/m-TiO2/CsPb0.97Sm0.03Br3/carbon | 7.48 | 1.594 | 85.1 | 10.14 | 2640 | 25 °C, 80% RH unencapsulated | 49 |
| MAPbI3:β-NaYF4:Yb,Er | ITO/ZnO/MAPbI3:β-NaYF4:Yb,Er/spiro/Ag | 22.71 | 1.15 | 75.39 | 19.70 | n/a | n/a | 50 |
| MAPbI3:IR806-β-NaYF4:Yb,Er | ITO/ZnO/MAPbI3:IR806-β-NaYF4:Yb,Er/spiro/Ag | 21.96 | 1.12 | 71.14 | 17.49 | n/a | n/a | 52 |
| CeCl3:Cs0.1FA0.85MA0.05Pb(I0.98Br0.02)3 | FTO/NiOx/CeCl3:Cs0.1FA0.85MA0.05Pb(I0.98Br0.02)3/C60/SnO2/Ag | 24.38 | 1.14 | 83.21 | 23.07 | 1060 | 40–60% RH, unencapsulated | 40 |
REEs utilized in ETMs
Using two or multi-photon UC/DC conversion steps, certain REEs can convert photons, whether they are UV or NIR, into the light-responsive range of perovskites. For example, doping Er3+ into the TiO2 layer allows for the formation of the TiO2 crystals and, because of the natural f–f transitions, turns NIR radiation into visible light, as illustrated in Fig. 6a–c.53 This can encourage the excitation of perovskites with NIR light and greatly increase the amount of photocurrent in PSCs. The energy band alignment in PSC functional layers can also be adjusted by REEs. Gao et al.,54 for instance, demonstrated how doping La3+ into TiO2 increases the Fermi level of TiO2 by scavenging oxygen and causing vacancies. This, in turn, increases the Voc and FF while decreasing the PSC series resistance. Consequently, a PCE of 15.42% was obtained in the La-doped-TiO2-based PSC. He et al.55 described an in situ method for preparing monodisperse NaYF4:Yb/Er as the mesoporous (m) electrode to replace m-TiO2 in PSCs. The first step in creating uniform NaYF4:Yb/Er was to use a double hydrophilic star-like poly(acrylic acid)-block-poly(ethylene oxide) (PAA-b-PEO) di-block copolymer as a nano-reactor. This gave the product its solubility and allowed for the modification of the film porosity during production. After NaYF4:Yb/Er was added as the mesoporous electrode, the efficiency rose to 17.8% and then to 18.1% when exposed to NIR radiation. Similarly, Li et al.56 created a mesoscopic layer for PSCs by incorporating NaYbF4:Ho3+ into ZrO2. This allowed for the dual-functional effects, which include the reduction of the charge carrier recombination and the harvesting of NIR light and its conversion to visible light. With optimized addition of NaYbF4:Ho3+ (40 wt%), the best-performing device displayed a PCE of 14.32%. The enhanced NIR light harvesting resulting from the integration of NaYF4:Yb3+,Tm3+/NaYF4 core–shell nanoparticles (NYF NPs) with TiO2 led to increased photocurrent densities, resulting in a PCE of 16.9% for the NYF-TiO2-fabricated PSC. Liang et al.57 reported that the NaYF4:Yb3+,Tm3+@TiO2 core–shell emits visible and UV light at 345, 362, 450, 477, and 650 nm upon excitation at 980 nm. As demonstrated in Fig. 6d, excitable electrons from the 1I6 and 1D2 levels of Tm3+ in NaYF4 could directly inject from Tm3+ to the conduction band (CB) of TiO2 through the hetero-interface, reducing charge recombination and promoting charge extraction kinetics. These levels of Tm3+ in NaYF4 match appropriately with the CB of TiO2. Hence, through Yb3+ ions, the NIR light pumps electrons to the higher excited-state states of Tm3+. From there, energy transfer takes place via two processes: (1) direct electron injection via the heterojunction into the CB of TiO2 and (2) UC and emission of visible photons that are absorbed by perovskites. These processes improved the PCE of an as-fabricated PSC from 13.98% to 16.27%. By co-doping more Li+, Shi et al.58 were able to further enhance the up-conversion emissions for Ho3+–Yb3+ co-doped TiO2. By maximizing the concentration of Yb3+ and Li+, the Ho3+–Yb3+–Li+ tri-doped TiO2, the best UC fluorescent material, was produced. The PCEs of the solar cells with Ho3+–Yb3+–Li+ tri-doped TiO2 modification raised to 16.9% from 15.5%. Guo et al.59 synthesized NaYF4:Yb3+/Er3+/Sc3+@NaYF4 UC nanoparticles with a core–shell structure. The NIR light was converted to red and Sc3+ improved green light by the doped REE ions Yb3+ and Er3+ and the UC luminescence efficiency. This increases the solar irradiation's absorption range and creates additional photocurrent in the solar cells. The even NaYF4 shell passivated the surface defects of NaYF4:Yb3+/Er3+/Sc3+, causing a noticeable increase in the intensity of UC emission. As a result, the TiO2-based device showed an efficiency of 17.44%, while the NaYF4:Yb3+/Er3+/Sc3+ mesoporous layer-based PSC attained a high PCE of 20.19%.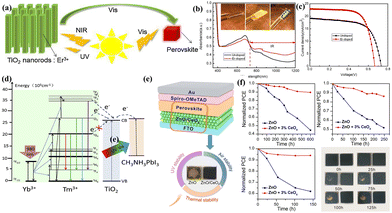 | ||
| Fig. 6 (a) Perovskite/Er-doped TiO2 performance enhancement mechanisms across the whole solar spectrum. (b) UV-vis-IR absorption spectra; the insets show pictures of the un-doped (middle), Er-doped (right), and bare FTO (left) in an infrared oven. (c) The J–V characteristic curves of as-prepared devices. Reproduced from ref. 53 with permission from Elsevier, copyright 2018. (d) Diagrammatic representation of the energy transfer process in the core–shell composites containing Tm3+@TiO2 and the NaYF4:Yb3+ and perovskite. Reproduced from ref. 57 with permission from Elsevier, copyright 2018. (e) Schematic illustration of the as-prepared PSC and stability enhancement. (f) PSC stability in 45% RH ambient air at room temperature in the dark, under UV irradiation in an inert atmosphere, and at 85 °C in an inert atmosphere, as well as photos of perovskite films with (right-side) and without CeOx doping (left-side) at different times at 85 °C in an inert atmosphere. Reproduced from ref. 60 with permission from American Chemical Society, copyright 2019. | ||
By preventing high-energy light damage and reducing spectral mismatch losses, REEs added as DCs to PSCs can enhance the photostability and PCEs of the associated solar cells. The luminescent DC abilities of REEs for UV prevention and conversion served as the model for this strategy. There are two principal approaches to introduce DC REEs into PSCs: the first is to introduce the REEs directly into the functional layers of the cells, and the second is to place an extra layer on the back/front of the glass substrates.61–64 For example, Meng et al.60 successfully fabricated a CeOx-doped ZnO ETM in PSCs, as shown in Fig. 6e and f. CeOx can be added to improve the stability and cell performance of PSCs by regulating the unmatched energy levels, poor UV stability, and chemical compatibility between ZnO and the perovskite. CeOx/ZnO-based PSCs consequently displayed a PCE of 19.5%. Moreover, ZnO/CeOx-based PSCs demonstrated noticeably improved moisture and thermal and UV stability in comparison to PSCs based on ZnO. Zhang et al.65 further utilized Sm3+/Eu3+ co-doped TiO2 as an ETM in PSCs. Here the Eu3+ ions were utilized as the DC material to boost UV light use and shield the breakdown of the perovskite layer from UV light. The Sm3+ ions were used as the sensitizer to enhance the DC ability of Eu3+ ions by expanding the excitation wavelength range for Eu3+ emission due to the energy transfer from Sm3+ to Eu3+. The optimized devices show a better PCE of 19.01%, greatly enhanced light, long-term stability and UV stability. Guo et al.66 used a reflux technique to create an ellipsoid-shaped, single-crystalline phase, Sc3+ doped brookite TiO2 nanoparticles with lengths of 20–30 nm and diameters of 10–15 nm and specific surface areas greater than 120 m2 g−1. By spin coating and annealing at 150 °C, a compact SnO2 layer and an Sc3+ tailored brookite TiO2 mesoporous layer were deposited. This resulted in a less-defective and high-quality mesoporous scaffold, which in turn developed a less-void and high-quality perovskite layer. The PSC performance was significantly improved by the retarded oxygen vacancy defect of the binder-free brookite TiO2, the sinter-free low-temperature processing, energy level adjustment by doping Sc3+, and the hole blocking of the compact SnO2 layer. The PSC based on a 5 mol% Sc3+ tailored SnO2/TiO2 scaffold not only showed excellent UV stability, maintaining 86.6% of its initial PCE after 50 hours of UV light exposure, but it also demonstrated a high-stabilized PCE of 21.75% (Fig. 7). There has been a lot of interest in SnO2 as an ETM to replace TiO2 in PSCs. Yet, it is difficult for the SnO2-based PSCs to match the TiO2-based device's performance. It has been demonstrated that doping REE ions Ln3+ (Sc3+, Y3+, and La3+) into SnO2 nano-spheres with a mesoporous structure can improve the long-term stability and photovoltaic performance of PSCs.67 The formation of dense and large-sized perovskite crystals was facilitated by Ln doping, which enhances charge transport dynamics and facilitates interfacial contact between the ETM and the perovskite. The Ln dopant minimizes the trap state density, lowers the charge transport resistance, and optimizes the perovskite layer's energy level. The optimized mesoporous PSC consequently displayed a PCE of 20.63% and long-term stability under 1-sun radiation for 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h under 30% RH ambient conditions.67 In the same way, Ce doping in SnOx can improve energy level alignment and conductivity with the perovskite layer, facilitating extraction of charges and transport capabilities.68 Additionally, placing a DC layer (SrAl2O4:Eu2+,Dy3+) (SAED), prepared by pulsed laser deposition, enhances PSC's UV photostability.69 Following the SAED adaptation, light-induced deep trap states in the perovskite layer were lowered and UV light-induced damage was impeded. The improved light capture and decreased carrier recombination of SAED led to an improved PCE of 17.8%. For a duration of 2600 hours, the unencapsulated SAED-based PSCs demonstrated long-term stability under ambient conditions.
h under 30% RH ambient conditions.67 In the same way, Ce doping in SnOx can improve energy level alignment and conductivity with the perovskite layer, facilitating extraction of charges and transport capabilities.68 Additionally, placing a DC layer (SrAl2O4:Eu2+,Dy3+) (SAED), prepared by pulsed laser deposition, enhances PSC's UV photostability.69 Following the SAED adaptation, light-induced deep trap states in the perovskite layer were lowered and UV light-induced damage was impeded. The improved light capture and decreased carrier recombination of SAED led to an improved PCE of 17.8%. For a duration of 2600 hours, the unencapsulated SAED-based PSCs demonstrated long-term stability under ambient conditions.
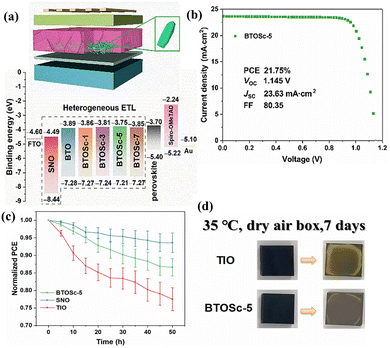 | ||
| Fig. 7 (a) Schematic illustration of the solar cell and corresponding energy levels of the used materials; TiO2 (BTO) and the brookite TiO2 doped with x mol% Sc3+ (BTOSc-x: x = 1, 3, 5, 7). (b) J–V curves of the BTOSc-5-based solar cell. (c) Normalized PCEs of the unencapsulated PSCs based on different ETMs under UV illumination (60 mW cm−2) for 50 h. (d) Photographs of the TIO and BTOSc-5 based perovskite films before and after UV irradiation for 7 days. Reproduced from ref. 66 with permission from Elsevier, copyright 2020. | ||
Table 2 summarizes the research that reports the REEs used in ETMs of PSCs. The use of REE-based DC/UC materials as separate ETMs or doped into the ETMs will efficiently alter the ETM's Fermi energy level, reduce the charge-carrier recombination, boost carrier transport efficiency, and increase the photocurrent of PSCs, as scrutinized in earlier sections. Furthermore, REEs can boost the absorption of UV/NIR light and protect the perovskite layer from UV/NIR light degradation, which enhance the photostability of PSCs.
| Composition | PSC design | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Stability (h) | Conditions | Ref. |
|---|---|---|---|---|---|---|---|---|
| La-doped TiO2 | FTO/c-TiO2/m-La-doped-TiO2/MAPbI3/spiro/Au | 21.30 | 1.05 | 68.92 | 15.42 | 120 | 40–50% RH, 25 °C, unencapsulated | 54 |
| NaYF4:Yb/Er | FTO/c-TiO2/m-NaYF4:Yb/Er/MAPbI3/spiro/Au | 23.1 | 1.06 | 73.8 | 18.1 | n/a | n/a | 55 |
| m-ZrO2:NaYbF4:Ho | FTO/c-TiO2/m-TiO2/m-ZrO2:NaYbF4:Ho/FA0.4MA0.6PbI3/spiro/Au | 25.16 | 0.973 | 58.7 | 14.32 | 500 | 35% RH, unencapsulated | 56 |
| m-TiO2:NaYF4:Yb3+,Tm3+/NaYF4 | FTO/c-TiO2/m-TiO2:NaYF4:Yb3+,Tm3+/NaYF4/MAPbI3/spiro/Au | 21.7 | 1.10 | 70.6 | 16.9 | n/a | n/a | 70 |
| NaYF4:Yb3+,Tm3+@TiO2 | FTO/c-TiO2/m-NaYF4:Yb3+,Tm3+@TiO2/MAPbI3/spiro/Au | 21.69 | 1.09 | 68.88 | 16.27 | n/a | n/a | 57 |
| Ho3+–Yb3+–Li+-doped TiO2 | FTO/c-TiO2/m-Ho3+–Yb3+–Li+-doped TiO2/MAPbI3/spiro/Au | 21.65 | 1.11 | 75.05 | 16.9 | n/a | n/a | 58 |
| NaYF4:Yb3+/Er3+/Sc3+ | FTO/c-TiO2/m-NaYF4:Yb3+/Er3+/Sc3+/MAPbI3/spiro/Au | 22.91 | 1.14 | 77.04 | 20.19 | 400 | 40% RH, unencapsulated | 59 |
| CeOx-doped ZnO | FTO/CeOx:ZnO/MAPbI3/spiro/Au | 23.64 | 1.09 | 75.67 | 19.52 | 600 | 45% RH, 25 °C, unencapsulated | 60 |
| Ce:SnOx | FTO/SnOx/Ce:SnOx/FA:MA:PbI3:PbBr3/spiro/Au | 21.98 | 1.08 | 64.4 | 15.77 | n/a | n/a | 68 |
| TiO2:Sm3+,Eu3+ | FTO/TiO2:Sm3+,Eu3+/MAPbI3/spiro/Au | 22.47 | 1.10 | 76.9 | 19.1 | 600 | 25–28 °C, 30–55% RH, unencapsulated | 65 |
| SnO2/TiO2:Sc3+ | ITO/SnO2/TiO2:Sc3+/(FA0.83MA0.17)0.95Cs0.05Pb(I0.9Br0.1)3/spiro/Au | 23.63 | 1.145 | 80.38 | 21.75 | 50 | 35 °C, unencapsulated | 66 |
| SnO2:Y3+ | FTO/c-SnO2/m-SnO2:Y3+/CsFAMAPbI3/spiro/Au | 23.61 | 1.117 | 78.16 | 20.63 | 400 | 30% RH, unencapsulated | 67 |
| SrAl2O4:Eu2+, Dy3+ | FTO/c-TiO2/SrAl2O4:Eu2+, Dy3+/MAPbI3-xClx/ZnPc/spiro/Au | 21.7 | 1.09 | 75.8 | 17.8 | 2600 | 16–25 °C, 25–30% RH, unencapsulated | 69 |
REEs utilized in HTMs
In PSCs, the HTMs are thought to be an essential layer for achieving excellent results.71 High stability, desirable compatibility, excellent charge-carrier mobility, a range of deposition techniques, and low production costs are required when using PSCs, regardless of whether they are inorganic or organic HTMs.71 Therefore, numerous researchers looked into various strategies for modifying the optoelectronic features of HTMs. Numerous REEs have been investigated as additives in the fabrication of the commonly used inorganic HTMs, i.e. NiO, including Ce3+, Nd3+, Eu3+, Tb3+, Yb3+, and La3+.72,73 In view of the large ionic radius variance in the REE ions and Ni, it turned out that these REE ions were barely able to enter the NiO crystal lattice. Instead, the dopants were primarily found at the thin-film surface or the interstitial site, which might help to reduce the defect states. In this regard, Y3+ ions were added by Hu et al.74 as dopants during the NiO-HTM preparation process in PSCs. It has been found that the small ionic radius of Y3+ allows for its effective incorporation into the NiO crystal lattice. Due to the occupied 4d orbital of Y3+, Y3+ doping can modify the electrical conductivity and electronic configurations of NiO.75 This can lead to a notable increase in photogenerated current when compared to a PSC that uses pristine NiO. To find the REE that is best for HTM doping, Chen et al.72 reported the doping of a number of REEs (Ce, Nd, Eu, Tb, and Yb) into NiOx. By comparing a PSC fabricated using 3% Eu:NiOx to other REE ion-doped and pristine NiOx-PSCs, it was found that the former had the best PCE of 15.06%. Higher perovskite quality, superior hole extraction, decreased interfacial recombination rate, and improved electronic conductivity were all credited with the appropriate REE-modified device's increased photovoltaic performance (Fig. 8a–d). Furthermore, Eu:NiOx-based PSCs perform better than pristine devices in the steady-state current density test and normalized long-term stability measurements. When not in use, the devices were kept under ambient conditions of 25–55% RH and 25–35 °C. Due to its (i) ability to adjust the energy band level, (ii) strong binding strength to oxygen, which can scavenge oxygen, and (iii) potential to inhibit perovskite degradation under illumination, La doping in NiOx has also demonstrated excellent results.76 A PSC with an La–NiOx HTM showed a 15.46% PCE. Furthermore, the La–NiOx solar cell demonstrated excellent stability in a vacuum desiccator environment for 50 days. | ||
| Fig. 8 (a) NiOx and Eu:NiOx film UPS spectra. (b) NiOx and Eu:NiOx film carrier concentration and mobility. (c) Using NiOx and Eu:NiOx in the steady-state Jsc of PSCs under maximum power points for 40 minutes. The picture of the actual device is shown in the inset. (d) Normalized PCE of PSCs with NiOx and Eu:NiOx under ambient conditions. Reproduced from ref. 72 with permission from Elsevier, copyright 2019. PTAA films on the CsPbI3 film containing different amounts of NaLuF4:Yb,Er@NaLuF4. (e) Reflectance spectra and (f) absorption spectra. Reproduced from ref. 77 with permission from American Chemical Society, copyright 2019. (g) The J–V characteristics of PSCs with 0.10 wt% Li(Gd,Y)F4:Yb,Er under AM 1.5G and additional 980 nm laser irradiance. (h) The photocurrent density–time (J–t) curves measured at 0.80 V under AM 1.5G and 980 nm laser irradiance. Reproduced from ref. 78 with permission from Elsevier B.V. copyright 2019. | ||
Additionally, a few study groups combined UC materials based on REEs with organic HTMs. NaLuF4:Yb,Er@NaLuF4 core–shell nanoparticles, for instance, were added to the PSC's PTAA HTM.77 The extremely low luminosity efficiency of UC nanoparticles was the reason for the hardly any UC influence for PCE, despite the absorption spectrum being expanded to the NIR range. It was concluded that the light scattering effect was primarily responsible for the longer optical path length and higher photoelectric current based on the reflectance and absorption spectra (Fig. 8e and f). Various weight ratios of monodisperse and extremely bright Li(Gd, Y)F4:Yb3+, Er3+ were incorporated into the spiro-OMeTAD-based HTM.78 Under 1-sun illumination, it was shown that the PSC based on spiro-OMeTAD with 0.1wt% Li(Gd,Y)F4:Yb3+,Er3+ produced a PCE of 18.34% (Fig. 8g and h). Using the Li(Gd,Y)F4:Yb,Er based HTM, the PSC also showed a clear NIR response to a further 980 nm NIR laser light.
As previously indicated, one promising method for producing high-efficiency PSCs is to use UC luminescent materials to broaden photoelectric response to the NIR region. Yet, issues with device modification and the lack of highly effective UC materials still exist. In this case, the local surface plasmon resonance (LSPR) effect enables NaCsWO3 to absorb NIR light, which can be exploited to increase the UC luminescence of REE-doped UC nanoparticles. NaCsWO3 was used as the LSPR centre by Xu et al.79 to synthesize NaCsWO3@NaYF4@NaYF4:Yb,Er nanoparticles (Fig. 9). At 2.8 mmol% NaCsWO3, the UCL intensity increased by more than 124 times. Subsequently, the PCE increased to 18.89% when these effective UC materials were utilized to alter the perovskite film in PSCs in addition to being doped into spiro-OMeTAD. Recently, Zhang et al.80 synthesized copper-doped lanthanum hydroxide (LaxCuy(OH)3) nanorods as a novel type of HTM for PSCs. Cu2+ dopants showed better energy level alignment at HTM/perovskite interfaces, despite the fact that the valence band edge of La(OH)3 was marginally out of alignment with that of the perovskite layers. Cu2+ ions, which act as an acceptor dopant for La(OH)3, also improved LaxCuy(OH)3 hole transport capability. Consequently, for LaxCuy(OH)3 based PSCs, Cu2+ dopants increased PCE from 11.3% to 20.4%.
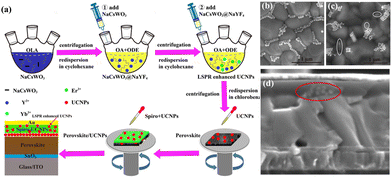 | ||
| Fig. 9 (a) A schematic diagram showing how the LSPR-enhanced UCNPs, NaCsWO3 nanorods, and NaCsWO3@NaYF4 nanoparticles are synthesized; how UCNPs are spin-coated to alter the perovskite films; and how UCNPs and spiro are combined to form the HTM. Top-view SEM images. (b) Perovskite/UCNP film and (c) perovskite/UCNP/spiro + UCNP film. (d) PSC cross-sectional SEM images showing the ITO/SnO2/perovskite/UCNPs/spiro + UCNPs/Au structure. Reproduced from ref. 79 with permission from American Chemical Society, copyright 2021. | ||
The stability and photovoltaic parameters of the PSCs fabricated using REE-doped HTMs are listed in Table 3. The usage of REEs can be attributed mainly to the following factors, according to the discussion above: to improve charge-carrier transport, REEs can: (1) change the energy band levels of the HTM; (2) increase the spectral response range of the perovskite, which boosts the photocurrent of PSCs; and (3) modify the perovskite film by eliminating surface defects and efficiently filling in gaps and holes at the grain boundary, which lowers carrier recombination; and (4) when REEs are added to PSCs, they increase light reflection, lengthen the light path, and further increase light absorption.
| Composition | PSC design | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Stability (h) | Conditions | Ref. |
|---|---|---|---|---|---|---|---|---|
| Eu:NiOx | FTO/Eu:NiOx/MAPbI3/PCBM/Ag | 21.96 | 1.03 | 66.6 | 15.06 | 240 | 25–55%, RH and 25–35 °C, encapsulated | 72 |
| Y:NiOx | FTO/Y:NiOx/MAPbI3/PC61BM/Au | 23.81 | 1.00 | 68.0 | 16.31 | n/a | n/a | 74 |
| La:NiOx | FTO/La:NiOx/MAPbI3/PC61BM/BCP/Ag | 21.02 | 1.01 | 73.0 | 15.46 | 1200 | Stored in moisture-free desiccator, unencapsulated | 76 |
| NaLuF4:Yb,Er@NaLuF4-doped PTAA | FTO/TiO2/CsPbI3/NaLuF4:Yb,Er@NaLuF4-doped PTAA/Au | 19.17 | 1.113 | 74.33 | 15.86 | n/a | n/a | 77 |
| Li(Gd, Y)F4:Yb3+,Er3+:spiro | FTO/c-TiO2/m-TiO2/MAPbI3/Li(Gd, Y)F4:Yb3+, Er3+:spiro/Au | 23.14 | 1.10 | 72.07 | 18.34 | n/a | n/a | 78 |
| NaCsWO3@NaYF4@NaYF4:Yb,Er:spiro | ITO/SnO2/FAI:MABr:MACl/NaCsWO3@NaYF4@NaYF4:Yb,Er/NaCsWO3@NaYF4@NaYF4:Yb,Er:spiro/Au | 23.26 | 1.08 | 76 | 18.89 | n/a | n/a | 79 |
| LaxCuy(OH)3 | FTO/LaxCuy(OH)3/Cs0.175FA0.750MA0.075Pb(I0.880Br0.120)3/PCBM/BCP/Ag | 22.2 | 1.12 | 82 | 20.4 | n/a | n/a | 80 |
REEs utilized as interface modifiers
Due to the numerous defects in PSC's functional layers, including poor interface contact, inferior stability, and carrier recombination, REEs have also been employed as interface modifiers to address these problems. For instance, due to the imperfections at the NaYF4 exterior, certain parasitic charge recombination processes may happen if NaYF4 is loaded directly within the PSC. Qi et al.81 prepared a core–shell structure of NaYF4:Yb,Er@SiO2 crystals by employing a thin insulating layer of SiO2 to stop NaYF4 from being directly exposed to prevent the unintended imperfection states. The TiO2 layer was covered with the NaYF4:Yb,Er@SiO2 layer, which could increase the photogenerated current density in PSCs by converting less energetic NIR light into visible light and suppressing interfacial non-radiative charge-carrier recombination. Finally, a high PCE of 14.52% in an HTM-free PSC was achieved. Furthermore, the Sr2CeO4:Eu3+ (SCOE) layer can be added to PSCs to enhance photovoltaic efficiency and UV light stability. In comparison to control devices (i.e., those without a SCOE layer), the SCOE-coated PSCs demonstrated a high current density of 23.70 mA cm−2 and a high PCE of 18.95%, which were increased by 9% and 14.15%, respectively.63 Furthermore, under UV light exposure for 70 hours, the SCOE-based PSCs maintain 80% of their initial PCE, and in an ambient environment with 20–25% RH for 75 days, they retain 78% of their initial PCE value. In order to enhance the UV stability of the PSC, Jia et al.82 deposited NaYF4:Eu3+ on the back of the FTO-substrate. High-energy UV photons could be absorbed by the NaYF4:Eu3+ layer and transformed into visible light. The layer reduced the photocatalytic activity of TiO2 in addition to extending the PSCs' spectral response range. With enhanced UV stability for 10 hours, the device with the NaYF4:Eu3+ layer had the best PCE at 20.17%. It is believed that the UC nanomaterial dual sensitizer will improve the UC effect and increase the amount of NIR photons that are absorbed. For instance, NaYF4 can be employed as the host material and the REEs ions Nd3+ and Yb3+ as sensitizers to enhance the absorption of the perovskite layer by converting NIR photons at approximately 808 nm and 980 nm into visible light (Fig. 10a–c). As a result, the PCE increased from 17.95% to 19.23% and the photocurrent density of PSCs was improved from 20.87 mA cm−2 to 22.74 mA cm−2 with REEs doped UC nanoparticles with dual-sensitizers (NaYF4:Nd3+/Yb3+/Er3+).83 Furthermore, the detailed analysis of adding Yb3+/Er3+ co-doped KY7F22 to a PSC's front-side (FTO/perovskite) interface or rear-side (perovskite/HTM) interface revealed 6.1% and 6.5% of averaged PCE improvement, respectively84 (Fig. 10d–i). The fabricated substrate's low transparency is the cause of the front-side PCE enhancement's lower value. It was shown that the density of Yb3+/Er3+ co-doped KY7F22 can be adjusted by varying its concentration in solution during the deposition process, resulting in a uniform and highly crystalline layer that can be fabricated onto either the front-side or rear-side. Furthermore, it was found that low concentration was unsuitable for the intended UC effect and that high concentration of the solution produces a large insulating cluster (increases resistance).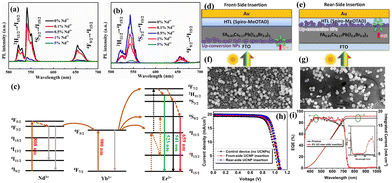 | ||
| Fig. 10 (a) PL spectra of UCNPs excited by a 980 nm laser with varying Nd3+ ratios. (b) PL spectra of 808 nm laser-excited UCNPs with various Nd3+ ratios. (c) A schematic diagram showing the energy transitions and UC energy level diagrams of the NaYF4:Nd3+/Yb3+/Er3+ system. Reproduced from ref. 83 with permission from Elsevier B.V. copyright 2020. (d) and (e) Schematics showing the interface where UCNPs were deposited and the solar cell architectures. SEM images of UCNPs placed (f) on the perovskite layer and (g) on the FTO. (h) J–V characteristics of the PSC with and without Yb3+ and Er3+ co-doped KY7F22 NPs inserted at the perovskite/HTM (rear-side) or FTO/perovskite (front-side) interfaces under AM 1.5G illumination. (i) EQE spectra of two planar ETM-free PSCs with and without KY7F22:Yb3+, Er3+. The zoomed-in view of the 750–1000 nm spectrum is displayed in the inset figure. Reproduced from ref. 84 with permission from American Chemical Society, copyright 2018. | ||
PSCs with full spectrum response have also been fabricated by adding UC and DC layers to use sunlight's NIR and UV ranges. The effective plasmon-enhanced NaYF4:Yb3+,Er3+/NaYF4:Yb3+,Tm3+/Ag composite layers placed on the rear-side of the PSCs, based on the NiO/Ag/NiO transparent electrode (Fig. 11a–h), led to a significant increase in PCE from 16.1% to 19.2% under 1.5 W cm−2 sunlight illumination.21 By adding a UV to a visible DC layer (Eu(TTA)2(Phen)MAA) to the front of the devices, the PCE was further increased to 19.5%. The most noticeable current density value (27.1 mA cm−2) was the result of the combined influence of UC and DC effects. The insertion of REEs on the front and back of the devices has significantly increased both the light stability under illumination and the long-term stability in humid environments. IR-783 dye-sensitized core–shell structures NaYF4:Yb3+,Er3+@NaYF4:Yb3+, and Nd3+ UC were synthesized by Bi et al.85 and coupled with films of plasmonic Au nanorods. As a result, the luminescence quantum yield increased from 0.2 to 1.2% and the UC luminescence (UCL) intensity was increased by approximately 120 times (Fig. 11i–l). Following the assembly of the composite UCNPs on the SnO2 layer, the PCE increased under AM 1.5G irradiation from 19.4 to 20.5%. With the use of a sun concentrator (1 W cm−2), the device's PCE can be increased even further to 21.1%. It was shown that the enhancement of PCE under typical sunlight irradiation is caused by the UC nanoparticles’ scattering effect as well as the IR to visible UCL.
 | ||
| Fig. 11 (a) A schematic representation of a PSC. (b) A cross-sectional SEM image of the PSC. (c)–(f) Top-view SEM images of the MAPbI3, NiO/Ag/NiO, and NaYF4:Yb3+,Er3+ and Ag films. (g) UV stability under UV irradiation of Eu(TTA)2(Phen)MAA coated and uncoated PSCs. It is estimated that UV light has an intensity of 10 mW cm−2. (h) The devices’ stability under ambient conditions with continuous sunlight without encapsulation. Reproduced from ref. 21 with permission from Elsevier, copyright 2018. (i) The AM 1.5G spectrum displays the spectral regions that can be used through the UC process as well as the fraction absorbed by PSCs. (j) UC populating schematic for the composite film of UCNPs, IR-783 dye, and AuNRs under simulated solar excitation. (k) Device stability in an air environment, both with and without UCNPs, IR-783 dye, and AuNR composite film. (l) Device light stability under AM 1.5G irradiation with and without UCNPs, IR-783 dye, and AuNR composite film. Reproduced from ref. 85 with permission from American Chemical Society, copyright 2020. | ||
Jiang et al.64 coated the back of FTO with Eu-4,7-diphenyl-1,10-phenanthroline (Eu-complex) to improve the PCE and UV-stability of the PSCs. Due to the Eu-complex's ability to re-emit UV light in the visible range (between 300 and 380 nm), the PSC coated with the Eu-complex showed an improvement of 11.8% in Jsc and 15.3% in efficiency when compared to the control PSC. Similarly, the PCE and UV stability of a PSC with a transparent layer of an Sm3+:Ce3+ ion doped P2O5–Li2O–ZnO–Sm2O3–CeO2 glass-ceramic waveguide, which served as the glass substrate for PSCs, were improved.86 The DC layer filters the UV light and transforms it into low energy photons. These photons not only broaden the spectral response range but also prevent the TiO2 from engaging in photo-catalysis, which enhances the photogenerated current and PSC operational stability. The green light region is typically where Er3+-doped nanoparticle emissions are restricted, which limits the optimization of Er3+ in PSCs. In this case, the UC emissions can be extended to the UV, visible, and NIR by doping Tm3+ and Yb3+ with NaYF4.87 The UC luminescence spectrum of NaYF4:Tm3+,Yb3+ was determined to extend from UV to visible blue light regions when excited by a 980 nm NIR laser. In addition to the extra photocurrent generation resulting from the NIR photon up-conversion of NaYF4:Tm3+,Yb3+, it has been found that the top and bottom layers of NaYF4:Tm3+,Yb3+ enhanced the charge dynamic behaviors and film morphology, resulting in improved device performance (Fig. 12a).
 | ||
| Fig. 12 (a) The J–V characteristic curves of the control device and NaYF4:Tm3+,Yb3+ modified PSC. Inset shows the schematic diagram of the device. Reproduced from ref. 87 with permission from Royal Society of Chemistry, copyright 2019. (b) A schematic representation of the perovskite film with the Eu-MOF effect. (c) PSCs’ stability performance as a function of N2 storage time, using spiro-OMeTAD as the HTM. (d) Air conditions of temperature between 10 and 20 °C and a humidity of roughly 20 to 40%. (e) PSC stability performance after continuous heating at 85 °C based on PTAA. (f) The MPP tracking of the device, which uses a white LED lamp to emit continuous light and is measured at 0.94 V and one sun illumination, based on PTAA. Reproduced from ref. 88 with permission from Wiley-VCH GmbH, copyright 2021. | ||
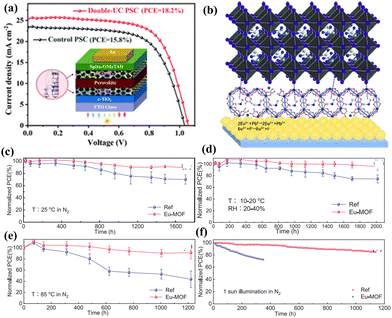
REEs can also be used as metal–organic frameworks (MOFs) to form a porous structure. Because such structures are composed of composite materials, they may be able to benefit from the synergistic effects of each component. First, the perovskite film could be efficiently passed through by metal ions; second, because of the conjugation of the framework, most of them could filter UV light. To increase device stability, Dou et al.,88 for instance, inserted an ultrathin Eu-MOF layer at the interface between the ETL and the perovskite layer (Fig. 12b–f). In the MOF, organic ligands and Eu ions can both lower the number of defects and enhance carrier transport. Moreover, the Eu-MOF in perovskite films can enhance light utilization and lessen degradation under UV light because of the Förster resonance energy transfer effect. Meanwhile, the perovskite films’ tensile strain was converted to compressive strain by the Eu-MOF. Consequently, the related devices attain a 22.16% PCE. Furthermore, under 30% relative humidity, the devices maintain 96% of their initial PCE after 2000 hours. To inhibit interfacial defects, Shi et al.89 recently introduced CeO2 into the interface between the perovskite and spiro-OMeTAD. CeO2 can speed up the oxidation process of spiro-OMeTAD to form a donor–acceptor complex at this interface, which can overcome the interface barrier for the high hole collecting ability. This is due to nearest-neighbour interaction of CeO2 with spiro-OMeTAD. This heterojunction exhibits metal conduction behavior at the interface due to the bond formation between lead and oxygen. In order to balance the extraction of electrons and holes by their respective electrodes and reduce device hysteresis while increasing efficiency and stability, CeO2 can be inserted between the perovskite and spiro-OMeTAD. As a result, the CeO2-based PSC showed a PCE of over 23% and maintained more than 87% of the original value after 2570 h of storage at 20–30% RH.
The photovoltaic parameters and stability of the PSCs with REEs as interface modifiers are summarized in Table 4. Overall, REEs enhance charge transport, minimize defects, and optimize interface properties, thereby augmenting the PCEs and stability of the PSCs. REEs are useful in raising the overall quality of PSCs due to their ability to alter electrical and optical properties.
| Composition | PSC design | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Stability (h) | Conditions | Ref. |
|---|---|---|---|---|---|---|---|---|
| NaYF4:Yb,Er@SiO2 | FTO/TiO2/NaYF4:Yb,Er@SiO2/MAPbI3/carbon | 20.72 | 1.018 | 65.2 | 14.52 | 720 | Stored in an ambient environment | 81 |
| NaYF4:Nd3+/Yb3+/Er3+ | FTO/TiO2/NaYF4:Nd3+/Yb3+/Er3+/MAPbI3/spiro/Au | 22.74 | 1.15 | 73.25 | 19.23 | n/a | n/a | 83 |
| Yb3+/Er3+ co-doped KY7F22 | FTO/TiO2/FA0.83Cs0.17Pb(I0.6Br0.4)3/NaYF4:Nd3+/Yb3+/Er3+/spiro/Au | 20.1 | 1.10 | 75.6 | 16.8 | n/a | n/a | 84 |
| Eu(TTA)2(Phen)MAA/NaYF4:Yb3+,Er3+/NaYF4:Yb3+,Tm3+/Ag | Eu(TTA)2(Phen)MAA/FTO/TiO2/MAPbI3/spiro/NiO-Ag-NiO/NaYF4:Yb3+,Er3+/NaYF4:Yb3+, Tm3+/Ag | 26.3 | 1.04 | 70.2 | 19.5 | 500 | 30–35% RH, unencapsulated | 21 |
| IR-783-NaYF4:Yb3+,Er3+@NaYF4:Yb3+,Nd3+:Au | ITO/SnO2/IR-783-NaYF4:Yb3+,Er3+@NaYF4:Yb3+,Nd3+:Au/MAPbI3/spiro/Au | 23.50 | 1.17 | 74.6 | 20.5 | 1500 | 20–25 °C, 20–30% RH, unencapsulated | 85 |
| Sr2CeO4:Eu3+ | FTO/c-TiO2/Sr2CeO4:Eu3+/CsFAMAPb(BrI)3/spiro/Au | 23.7 | 1.06 | 75.53 | 18.95 | 1800 | 20–25 °C, 25–30% RH, unencapsulated | 63 |
| NaYF4:Eu3+ | NaYF4:Eu3+/FTO/c-TiO2/Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3/spiro/Au | 23.22 | 1.127 | 77.1 | 20.19 | 240 | 25 °C, 25% RH, unencapsulated | 82 |
| Eu-4,7-diphenyl-1,10-phenanthroline | Eu-4,7-diphenyl-1,10-phenanthroline/FTO/TiO2/MAPbI3/spiro/Au | 19.30 | 1.03 | 73.54 | 15.3 | 240 | UV illumination, unencapsulated | 64 |
| NaYF4:Tm3+,Yb3+ | FTO/c-TiO2/NaYF4:Tm3+,Yb3+/Cs0.05(MA0.17FA0.83)99.5Pb(I0.83Br0.17)3/NaYF4:Tm3+,Yb3+/spiro/Au | 25.46 | 1.06 | 67.5 | 18.2 | 846 | 20–25% RH, 25 °C, unencapsulated | 87 |
| Eu-MOF | ITO/SnO2/Eu-MOF/(FA, MA, Cs)Pb(I, Br)3/spiro/Au | 24.19 | 1.14 | 76 | 21.2 | 2000 | 20–40% RH, 10–20 °C, unencapsulated | 88 |
| CeO2 | FTO/SnO2/perovskite/CeO2/spiro/Au | 24.54 | 1.16 | 81.5 | 23.21 | 2570 | 20–30% RH, unencapsulated | 89 |
Conclusions and outlook
It is concluded from the above discussion that the REEs are employed for the reduction of defect states, improvement of charge mobility and alignment of energy levels, modification of thin-film morphology to prevent pinhole formation and nanoparticle aggregation, and regulation of the nanostructure of materials for scaffold layers. Furthermore, through substitutional or interstitial doping, REEs can adjust the photo-physical characteristics of perovskites, reducing trap defects and increasing light harvesting and luminescence. Because of the strong interaction between the REE ions and the charged ions in the precursor, REEs can control perovskite crystallization with optimized thin-film morphology and lower formation energy. Due to their reduced defect states, customized grain size, altered lattice parameters, and restored redox capability, REEs stabilize the perovskite phase. By transforming UV and NIR light into visible light, the UC and DC effects of REEs can increase the range of light responsiveness. Hindering UV-radiation can also prevent light-induced degradation and improve the PSC's stability. Therefore, the best REEs for PSCs must meet a number of requirements before being used in PSCs: (1) there must be a significant spectral matching between the perovskites and the REEs. To maximize the desired spectral response in the cell, the excitation range of the UC and DC through REEs should be as broad as possible in the NIR and UV, respectively, and the emission light should fall within the peak absorption range of the PSC; (2) a lower UC threshold and effective UC/DC luminescence; (3) the REE layer must be nearly transparent in the PSC wavelength range to reduce the layer's absorption loss to visible light and to guarantee good optical transmission; (4) the chemical and thermal stability of the REEs is required. (5) Neither the fabrication nor the integration of REEs into PSCs nor any exposure of the extremely sensitive perovskites above or below them should damage them; (6) when adding REEs to PSCs, methods of preventing parasitic charge recombination should be established;90,91 and (7) to investigate new opportunities for the REEs, some of the already synthesized REE-based perovskites, such as CsYI3, CsEuBr3, CsEuCl3, CsEnI3, and (C4H9NH3)2EuI4, should be tried in the PSC architecture.92–95Despite the fact that REEs in PSCs have many advantages, there are still limitations. For example, compared to other materials, REEs can be more costly and hard to come by. This may result in significant PSC production expenses. Appropriate distribution and concentration control are necessary for the integration of REEs in PSCs. In order to guarantee their consistent and efficient integration, sophisticated techniques will be needed. Certain requirements for processing may be required for some REEs to be successfully incorporated into the PSCs as additives or interface modifiers. It might be essential to adjust the fabrication methods for this, as they may not be easily adapted or appropriate for existing fabrication facilities. High performance of PSCs depends on compatibility, preventing adverse reactions and minimizing phase separation because unanticipated chemical coupling between REEs and other PSC materials may occur. REEs can improve stability temporarily, but it is often not clear how they will affect the perovskite layer and mainly the performance of the device in the end. Research is still needed to make sure that adding them would not negatively influence the PSC's robustness and long lifespan.
Author contributions
Conceptualization: S. S., I. M. O., and S. A.; initial writing: S. S., I. M. O.; all authors contributed equally to correction, editing, and validation. All contributors have approved the final version of the manuscript.Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors acknowledge the UAEU-National Fund research program for providing financial support under grant no. 12R186.Notes and references
- N. K. Elangovan, R. Kannadasan, B. B. Beenarani, M. H. Alsharif, M.-K. Kim and Z. H. Inamul, Recent Developments in Perovskite Materials, Fabrication Techniques, Band Gap Engineering, and the Stability of Perovskite Solar Cells, Energy Rep., 2024, 11, 1171–1190 CrossRef.
- C. L. McCleese, M. C. Brennan, N. Episcopo, L. Sun, N. Hong, C. V. Ramana, T. A. Grusenmeyer and P. R. Stevenson, Optical Dispersion Data Analysis of Single-Crystal CH3NH3PbBr3 for Optimized Perovskite Solar Cell Active Layer Absorptance, Adv. Photonics Res., 2024, 2400019 CrossRef CAS.
- T. Kim and B. Park, Ambipolar Charge Carrier Transport Properties at the S-Benzyl-l-Cysteine-Induced 2D/3D Halide Perovskite Interface, Chem. Mater., 2024, 36(2), 675–681 CrossRef CAS.
- R. Tian, S. Zhou, Y. Meng, C. Liu and Z. Ge, Material and Device Design of Flexible Perovskite Solar Cells for Next-generation Power Supplies, Adv. Mater., 2024, 2311473 CrossRef CAS PubMed.
- M. T. Hoang, Y. Yang, N. D. Pham and H. Wang, Ecofriendly Solution Processing of Perovskite Solar Cells Using Water, J. Phys. Chem. Lett., 2024, 15(26), 6880–6889 CrossRef CAS.
- L. Shi, Z. Yang, Y. Zhang, Z. Ai, Y. Bao, T. Ma, L. Qin, G. Cao, C. Wang and X. Li, Correlating Carrier Dynamics with Performance in Perovskite Solar Cells, Sol. RRL, 2024, 8(2), 2300752 CrossRef CAS.
- X. Liu, Y. Wang, Y. Wang, Y. Zhao, J. Yu, X. Shan, Y. Tong, X. Lian, X. Wan, L. Wang, P. Tian and H.-C. Kuo, Recent Advances in Perovskites-Based Optoelectronics, Nanotechnol. Rev., 2022, 11(1), 3063–3094, DOI:10.1515/ntrev-2022-0494.
- W. Liu, Y. Duan, Z. Zhang, J. Gao, S. Li, Z. Fink, X. Wu, Z. Ma, A. Saeki, T. P. Russell and Y. Liu, Rational Organic Subcell Engineering Enables Efficient Organic-Perovskite Tandem Solar Cells, ACS Energy Lett., 2023, 8(10), 4514–4523, DOI:10.1021/acsenergylett.3c01681.
- L. Zhang, J. Gao, Z. You, Q. Li, M. Liu, Z. Ma and Y. Liu, A Multifunctional Phosphorylcholine-Based Polymer Reduces Energy Loss for Efficient Perovskite Solar Cells, J. Mater. Chem. C, 2022, 10(44), 16781–16788, 10.1039/D2TC03039J.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells, J. Am. Chem. Soc., 2009, 131(17), 6050–6051 CrossRef CAS PubMed.
- M. A. Green, E. D. Dunlop, M. Yoshita, N. Kopidakis, K. Bothe, G. Siefer and X. Hao, Solar Cell Efficiency Tables (Version 63), Prog. Photovolt. Res. Appl., 2024, 32(1), 3–13, DOI:10.1002/pip.3750.
- B. S. Richards, Enhancing the Performance of Silicon Solar Cells via the Application of Passive Luminescence Conversion Layers, Sol. Energy Mater. Sol. Cells, 2006, 90(15), 2329–2337, DOI:10.1016/j.solmat.2006.03.035.
- Y. Chen, T. Chen and L. Dai, Layer-by-Layer Growth of CH3NH3PbI3−Cl for Highly Efficient Planar Heterojunction Perovskite Solar Cells, Adv. Mater., 2015, 27(6), 1053–1059, DOI:10.1002/adma.201404147.
- H. Lian, Z. Hou, M. Shang, D. Geng, Y. Zhang and J. Lin, Rare Earth Ions Doped Phosphors for Improving Efficiencies of Solar Cells, Energy, 2013, 57, 270–283, DOI:10.1016/j.energy.2013.05.019.
- B. Li, F. Tian, X. Cui, B. Xiang, H. Zhao, H. Zhang, D. Wang, J. Li, X. Wang, X. Fang, M. Qiu and D. Wang, Review for Rare-Earth-Modified Perovskite Materials and Optoelectronic Applications, Nanomaterials, 2022, 12(10), 1773, DOI:10.3390/nano12101773.
- P. Zhu, C. Chen, J. Dai, Y. Zhang, R. Mao, S. Chen, J. Huang and J. Zhu, Toward the Commercialization of Perovskite Solar Modules, Adv. Mater., 2024, 2307357 CrossRef CAS.
- S. B. Jaffri, K. S. Ahmad, I. Abrahams and M. A. Habila, Sustainability Aggrandized Performance of [Sm-Eu-Tm]: CsPbI1. 8Br1. 2 Perovskite Material in Fully Ambient Solar Cells, Supercapacitors, and Electro-Catalysis, Mater. Sci. Eng. B, 2024, 301, 117153 CrossRef CAS.
- X. Zhang, Y. Wang, X. Wu, F. Wang, Q. Ou and S. Zhang, A Comprehensive Review on Mechanisms and Applications of Rare-Earth Based Perovskite Nanocrystals, Chin. J. Chem., 2024, 42(9), 1032–1056 CrossRef CAS.
- X. Huang, S. Han, W. Huang and X. Liu, Enhancing Solar Cell Efficiency: The Search for Luminescent Materials as Spectral Converters, Chem. Soc. Rev., 2013, 42(1), 173–201, 10.1039/C2CS35288E.
- T. Trupke, M. A. Green and P. Würfel, Improving Solar Cell Efficiencies by Up-Conversion of Sub-Band-Gap Light, J. Appl. Phys., 2002, 92(7), 4117–4122, DOI:10.1063/1.1505677.
- H. Li, C. Chen, J. Jin, W. Bi, B. Zhang, X. Chen, L. Xu, D. Liu, Q. Dai and H. Song, Near-Infrared and Ultraviolet to Visible Photon Conversion for Full Spectrum Response Perovskite Solar Cells, Nano Energy, 2018, 50, 699–709, DOI:10.1016/j.nanoen.2018.06.024.
- T. Behrsing, V. L. Blair, F. Jaroschik, G. B. Deacon and P. C. Junk, Rare Earths—The Answer to Everything, Molecules, 2024, 29(3), 688, DOI:10.3390/molecules29030688.
- L. Cang, Z. Qian, J. Wang, L. Chen, Z. Wan, K. Yang, H. Zhang and Y. Chen, Applications and Functions of Rare-Earth Ions in Perovskite Solar Cells, Chin. Phys. B, 2022, 31(3), 38402, DOI:10.1088/1674-1056/ac373a.
- S. A. A. Shah, M. H. Sayyad, J. Sun and Z. Guo, Recent Advances and Emerging Trends of Rare-Earth-Ion Doped Spectral Conversion Nanomaterials in Perovskite Solar Cells, J. Rare Earths, 2022, 40(11), 1651–1667, DOI:10.1016/j.jre.2021.12.001.
- T. Chen, J. Xie and P. Gao, Ultraviolet Photocatalytic Degradation of Perovskite Solar Cells: Progress, Challenges, and Strategies, Adv. Energy Sustain. Res., 2022, 3(6), 2100218, DOI:10.1002/aesr.202100218.
- S. S. Azad, R. Keshavarzi, V. Mirkhani, M. Moghadam, S. Tangestaninejad and I. Mohammadpoor-Baltork, Stability Enhancement of Perovskite Solar Cells Using Multifunctional Inorganic Materials with UV Protective, Self Cleaning, and High Wear Resistance Properties, Sci. Rep., 2024, 14(1), 6466, DOI:10.1038/s41598-024-57133-8.
- M. M. Osman, A. Q. Alanazi, T. I. Alanazi, M. H. Alkahtani, A. M. El-naggar, A. A. Albassam, A. M. Aldhafiri, M. Al-Gawati, M. Almalki, S. M. Alenzi and M. D. Mensi, Enhanced Performance of Perovskite Solar Cell via Up-Conversion YLiF4:Yb, Er Nanoparticles, Sol. Energy Mater. Sol. Cells, 2024, 273, 112955, DOI:10.1016/j.solmat.2024.112955.
- K. Yamamoto, A. Suzuki, R. Okumura, A. Enomoto, I. Ono, T. Oku, S. Fukunishi, T. Tachikawa and T. Hasegawa, Effects of Neodymium or Ytterbium Addition to CH3NH3PbI3 Perovskite Solar Cells Inserted with Decaphenylcyclopentasilane, Results Surf. Interfaces, 2024, 15, 100224, DOI:10.1016/j.rsurfi.2024.100224.
- Z. Yuan, Y. Yang, Z. Wu, S. Bai, W. Xu, T. Song, X. Gao, F. Gao and B. Sun, Approximately 800-Nm-Thick Pinhole-Free Perovskite Films via Facile Solvent Retarding Process for Efficient Planar Solar Cells, ACS Appl. Mater. Interfaces, 2016, 8(50), 34446–34454, DOI:10.1021/acsami.6b12637.
- T. Niu, J. Lu, R. Munir, J. Li, D. Barrit, X. Zhang, H. Hu, Z. Yang, A. Amassian and K. Zhao, Stable High-performance Perovskite Solar Cells via Grain Boundary Passivation, Adv. Mater., 2018, 30(16), 1706576 CrossRef.
- S. Sajid, S. Alzahmi, I. B. Salem and I. M. Obaidat, Guidelines for Fabricating Highly Efficient Perovskite Solar Cells with Cu2O as the Hole Transport Material, Nanomaterials, 2022, 12(19), 3315, DOI:10.3390/nano12193315.
- M. Hou, X. Guo, M. Han, J. Zhao, Z. Wang, Y. Ding, G. Hou, Z. Zhang and X. Han, Enhanced Stability of FA-Based Perovskite: Rare-Earth Metal Compound EuBr2 Doping, Chin. Phys. B, 2024, 33(4), 47802, DOI:10.1088/1674-1056/ad23d5.
- A. Alotaibi, F. Alsardi, F. Alshwikhat, M. Aldossary, F. S. Almarwani, F. J. Talidi, S. A. Almenhali, S. F. Almotawa, Y. A. Alzahrani, S. Alenzi, A. Alanazi and M. Alkahtani, Fabrication of Erbium-Doped Upconversion Nanoparticles and Carbon Quantum Dots for Efficient Perovskite Solar Cells, Molecules, 2024, 29(11), 2556, DOI:10.3390/molecules29112556.
- X. Wu, H. Li, K. Wang, X. Sun and L. Wang, CH3NH3Pb1−xEuxI3 Mixed Halide Perovskite for Hybrid Solar Cells: The Impact of Divalent Europium Doping on Efficiency and Stability, RSC Adv., 2018, 8(20), 11095–11101, 10.1039/C7RA12754E.
- A. Suzuki and T. Oku, Effects of Mixed-Valence States of Eu-Doped FAPbI3 Perovskite Crystals Studied by First-Principles Calculation, Mater. Adv., 2021, 2(8), 2609–2616, 10.1039/D0MA00994F.
- J. Yin, G. H. Ahmed, O. M. Bakr, J.-L. Brédas and O. F. Mohammed, Unlocking the Effect of Trivalent Metal Doping in All-Inorganic CsPbBr3 Perovskite, ACS Energy Lett., 2019, 4(3), 789–795, DOI:10.1021/acsenergylett.9b00209.
- F. Ferraro and R. Arratia-Pérez, Bonding, Energetic, Electronic Delocalization and Optical Properties of MCp3 Complexes, Where M= Sc, Y, La, Ac, Lu, Ce, Yb and Th, Chem. Phys. Lett., 2012, 554, 219–224 CrossRef CAS.
- L. Wang, P. Fang, Z. Zhao, Y. Huang, Z. Liu and Z. Bian, Rare Earth Complexes with 5d–4f Transition: New Emitters in Organic Light-Emitting Diodes, J. Phys. Chem. Lett., 2022, 13(12), 2686–2694, DOI:10.1021/acs.jpclett.2c00400.
- Z. Song, W. Xu, Y. Wu, S. Liu, W. Bi, X. Chen and H. Song, Incorporating of Lanthanides Ions into Perovskite Film for Efficient and Stable Perovskite Solar Cells, Small, 2020, 16(40), 2001770 CrossRef CAS.
- Y. Li, Y. Xu, K. Du, C. Zhou, J. Ding, L. Li and N. Yuan, Cerium Rare-Earth Ions Reinforced Built-in Electric Field to Enable Efficient Carrier Extraction for Highly Efficient and Stable Perovskite Solar Cells, J. Alloys Compd., 2024, 1005, 176066, DOI:10.1016/j.jallcom.2024.176066.
- L. Chen, W. Wu, J. Wang, Z. Qian, R. Liu, Y. Niu, Y. Chen, X. Xie and H. Zhang, Lanthanide Stabilized All-Inorganic CsPbI2Br Perovskite Solar Cells with Superior Thermal Resistance, ACS Appl. Energy Mater., 2021, 4(4), 3937–3944, DOI:10.1021/acsaem.1c00311.
- W. Xiang, Z. Wang, D. J. Kubicki, W. Tress, J. Luo, D. Prochowicz, S. Akin, L. Emsley, J. Zhou and G. Dietler, Europium-Doped CsPbI2Br for Stable and Highly Efficient Inorganic Perovskite Solar Cells, Joule, 2019, 3(1), 205–214 CrossRef CAS.
- J. Wang, L. Chen, Z. Qian, G. Ren, J. Wu and H. Zhang, Optimal Intermediate Adducts Regulate Low-Temperature CsPbI2Br Crystallization for Efficient Inverted All-Inorganic Perovskite Solar Cells, J. Mater. Chem. A, 2020, 8(47), 25336–25344, 10.1039/D0TA07663E.
- A. K. Jena, A. Kulkarni, Y. Sanehira, M. Ikegami and T. Miyasaka, Stabilization of α-CsPbI3 in Ambient Room Temperature Conditions by Incorporating Eu into CsPbI3, Chem. Mater., 2018, 30(19), 6668–6674, DOI:10.1021/acs.chemmater.8b01808.
- K. Wang, L. Zheng, T. Zhu, X. Yao, C. Yi, X. Zhang, Y. Cao, L. Liu, W. Hu and X. Gong, Efficient Perovskite Solar Cells by Hybrid Perovskites Incorporated with Heterovalent Neodymium Cations, Nano Energy, 2019, 61, 352–360, DOI:10.1016/j.nanoen.2019.04.073.
- S. Li, L. Zhu, Z. Kan, Y. Hua and F. Wu, A Multifunctional Additive of Scandium Trifluoromethanesulfonate to Achieve Efficient Inverted Perovskite Solar Cells with a High Fill Factor of 83.80%, J. Mater. Chem. A, 2020, 8(37), 19555–19560, 10.1039/D0TA07567A.
- Z. Feng, Z. Wu, Y. Hua, G. Zhu, X. Chen and S. Huang, Controlled Growth of Perovskite KMnF3 Upconverting Nanocrystals for Near-Infrared Light-Sensitive Perovskite Solar Cells and Photodetectors, J. Mater. Sci., 2021, 56(25), 14207–14221, DOI:10.1007/s10853-021-06173-w.
- S. K. Karunakaran, G. M. Arumugam, W. Yang, S. Ge, S. N. Khan, Y. Mai, X. Lin and G. Yang, Europium (II)-Doped All-Inorganic CsPbBr3 Perovskite Solar Cells with Carbon Electrodes, Sol. RRL, 2020, 4(11), 2000390, DOI:10.1002/solr.202000390.
- J. Duan, Y. Zhao, X. Yang, Y. Wang, B. He and Q. Tang, Lanthanide Ions Doped CsPbBr3 Halides for HTM-Free 10.14%-Efficiency Inorganic Perovskite Solar Cell with an Ultrahigh Open-Circuit Voltage of 1.594 V, Adv. Energy Mater., 2018, 8(31), 1802346, DOI:10.1002/aenm.201802346.
- F.-L. Meng, J.-J. Wu, E.-F. Zhao, Y.-Z. Zheng, M.-L. Huang, L.-M. Dai, X. Tao and J.-F. Chen, High-Efficiency near-Infrared Enabled Planar Perovskite Solar Cells by Embedding Upconversion Nanocrystals, Nanoscale, 2017, 9(46), 18535–18545, 10.1039/C7NR05416E.
- J.-C. Boyer and F. C. J. M. van Veggel, Absolute Quantum Yield Measurements of Colloidal NaYF4: Er3 +, Yb3+ Upconverting Nanoparticles, Nanoscale, 2010, 2(8), 1417–1419, 10.1039/C0NR00253D.
- X. Lai, X. Li, X. Lv, Y.-Z. Zheng, F. Meng and X. Tao, Broadband Dye-Sensitized Upconverting Nanocrystals Enabled near-Infrared Planar Perovskite Solar Cells, J. Power Sources, 2017, 372, 125–133, DOI:10.1016/j.jpowsour.2017.10.067.
- H. Zhang, Q. Zhang, Y. Lv, C. Yang, H. Chen and X. Zhou, Upconversion Er-Doped TiO2 Nanorod Arrays for Perovskite Solar Cells and the Performance Improvement, Mater. Res. Bull., 2018, 106, 346–352, DOI:10.1016/j.materresbull.2018.06.014.
- X.-X. Gao, Q.-Q. Ge, D.-J. Xue, J. Ding, J.-Y. Ma, Y.-X. Chen, B. Zhang, Y. Feng, L.-J. Wan and J.-S. Hu, Tuning the Fermi-Level of TiO2 Mesoporous Layer by Lanthanum Doping towards Efficient Perovskite Solar Cells, Nanoscale, 2016, 8(38), 16881–16885, 10.1039/C6NR05917A.
- M. He, X. Pang, X. Liu, B. Jiang, Y. He, H. Snaith and Z. Lin, Monodisperse Dual-Functional Upconversion Nanoparticles Enabled Near-Infrared Organolead Halide Perovskite Solar Cells, Angew. Chem., Int. Ed., 2016, 55(13), 4280–4284, DOI:10.1002/anie.201600702.
- Y. Li, L. Zhao, M. Xiao, Y. Huang, B. Dong, Z. Xu, L. Wan, W. Li and S. Wang, Synergic Effects of Upconversion Nanoparticles NaYbF4:Ho3+ and ZrO2 Enhanced the Efficiency in Hole-Conductor-Free Perovskite Solar Cells, Nanoscale, 2018, 10(46), 22003–22011, 10.1039/C8NR07225F.
- J. Liang, H. Gao, M. Yi, W. Shi, Y. Liu, Z. Zhang and Y. Mao, β-NaYF4:Yb3 +, Tm3 + @TiO2 Core-Shell Nanoparticles Incorporated into the Mesoporous Layer for High Efficiency Perovskite Solar Cells, Electrochim. Acta, 2018, 261, 14–22, DOI:10.1016/j.electacta.2017.12.112.
- W. Shi, Z. Zhang, J. Qin, Y. Zhang, Y. Liu, Y. Liu, H. Gao and Y. Mao, Interface Modification by Up-Conversion Material of Ho3 + -Yb3 + -Li+ Tri-Doped TiO2 to Improve the Performance of Perovskite Solar Cells, J. Alloys Compd., 2018, 754, 124–130, DOI:10.1016/j.jallcom.2018.04.283.
- Q. Guo, J. Wu, Y. Yang, X. Liu, J. Jia, J. Dong, Z. Lan, J. Lin, M. Huang, Y. Wei and Y. Huang, High Performance Perovskite Solar Cells Based on β-NaYF4:Yb3 +/Er3 +/Sc3 + @NaYF4 Core-Shell Upconversion Nanoparticles, J. Power Sources, 2019, 426, 178–187, DOI:10.1016/j.jpowsour.2019.04.039.
- R. Meng, X. Feng, Y. Yang, X. Lv, J. Cao and Y. Tang, Cerium-Oxide-Modified Anodes for Efficient and UV-Stable ZnO-Based Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2019, 11(14), 13273–13278, DOI:10.1021/acsami.9b01587.
- Y. Qiao, S. Li, W. Liu, M. Ran, H. Lu and Y. Yang, Recent Advances of Rare-Earth Ion Doped Luminescent Nanomaterials in Perovskite Solar Cells, Nanomaterials, 2018, 8(1), 43, DOI:10.3390/nano8010043.
- W. Chen, Q. Luo, C. Zhang, J. Shi, X. Deng, L. Yue, Z. Wang, X. Chen and S. Huang, Effects of Down-Conversion CeO2:Eu3+ Nanophosphors in Perovskite Solar Cells, J. Mater. Sci. Mater. Electron., 2017, 28(15), 11346–11357, DOI:10.1007/s10854-017-6928-0.
- N. U. Rahman, W. U. Khan, S. Khan, X. Chen, J. Khan, J. Zhao, Z. Yang, M. Wu and Z. Chi, A Promising Europium-Based down Conversion Material: Organic–Inorganic Perovskite Solar Cells with High Photovoltaic Performance and UV-Light Stability, J. Mater. Chem. A, 2019, 7(11), 6467–6474, 10.1039/C9TA00551J.
- L. Jiang, W. Chen, J. Zheng, L. Zhu, L. Mo, Z. Li, L. Hu, T. Hayat, A. Alsaedi, C. Zhang and S. Dai, Enhancing the Photovoltaic Performance of Perovskite Solar Cells with a Down-Conversion Eu-Complex, ACS Appl. Mater. Interfaces, 2017, 9(32), 26958–26964, DOI:10.1021/acsami.7b10101.
- B. Zhang, Z. Song, J. Jin, W. Bi, H. Li, C. Chen, Q. Dai, L. Xu and H. Song, Efficient Rare Earth Co-Doped TiO2 Electron Transport Layer for High-Performance Perovskite Solar Cells, J. Colloid Interface Sci., 2019, 553, 14–21, DOI:10.1016/j.jcis.2019.06.003.
- Q. Guo, J. Wu, Y. Yang, X. Liu, W. Sun, Y. Wei, Z. Lan, J. Lin, M. Huang, H. Chen and Y. Huang, Low-Temperature Processed Rare-Earth Doped Brookite TiO2 Scaffold for UV Stable, Hysteresis-Free and High-Performance Perovskite Solar Cells, Nano Energy, 2020, 77, 105183, DOI:10.1016/j.nanoen.2020.105183.
- Q. Guo, J. Wu, Y. Yang, X. Liu, Z. Lan, J. Lin, M. Huang, Y. Wei, J. Dong and J. Jia, High-Performance and Hysteresis-Free Perovskite Solar Cells Based on Rare-Earth-Doped SnO2 Mesoporous Scaffold, Research, 2019, 2019, 4049793, DOI:10.34133/2019/4049793.
- D. Lu, M. Jamshidi, C. Dun, J. J. Urban, J. M. Gardner and L. Belova, Inkjet-Printed Ce-Doped SnOx Electron Transport Layer for Improved Performance of Planar Perovskite Solar Cells, Mater. Adv., 2024, 5(15), 6270–6276, 10.1039/D4MA00094C.
- C. Chen, H. Li, J. Jin, X. Chen, Y. Cheng, Y. Zheng, D. Liu, L. Xu, H. Song and Q. Dai, Long-lasting Nanophosphors Applied to UV-resistant and Energy Storage Perovskite Solar Cells, Adv. Energy Mater., 2017, 7(20), 1700758 CrossRef.
- M. Que, W. Que, X. Yin, P. Chen, Y. Yang, J. Hu, B. Yu and Y. Du, Enhanced Conversion Efficiency in Perovskite Solar Cells by Effectively Utilizing near Infrared Light, Nanoscale, 2016, 8(30), 14432–14437, 10.1039/C6NR03021A.
- S. Sajid, S. Alzahmi, I. B. Salem, J. Park and I. M. Obaidat, Inorganic Hole Transport Materials in Perovskite Solar Cells Are Catching Up, Mater. Today Energy, 2023, 37, 101378, DOI:10.1016/j.mtener.2023.101378.
- X. Chen, L. Xu, C. Chen, Y. Wu, W. Bi, Z. Song, X. Zhuang, S. Yang, S. Zhu and H. Song, Rare Earth Ions Doped NiOx Hole Transport Layer for Efficient and Stable Inverted Perovskite Solar Cells, J. Power Sources, 2019, 444, 227267, DOI:10.1016/j.jpowsour.2019.227267.
- X. Yin, J. Zhai, P. Du, N. Li, L. Song, J. Xiong and F. Ko, 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D NiO Nanowall Hole-Transporting Layer for the Passivation of Interfacial Contact in Inverted Perovskite Solar Cells, ChemSusChem, 2020, 13(5), 1006–1012, DOI:10.1002/cssc.201903025.
D NiO Nanowall Hole-Transporting Layer for the Passivation of Interfacial Contact in Inverted Perovskite Solar Cells, ChemSusChem, 2020, 13(5), 1006–1012, DOI:10.1002/cssc.201903025. - Z. Hu, D. Chen, P. Yang, L. Yang, L. Qin, Y. Huang and X. Zhao, Sol-Gel-Processed Yttrium-Doped NiO as Hole Transport Layer in Inverted Perovskite Solar Cells for Enhanced Performance, Appl. Surf. Sci., 2018, 441, 258–264, DOI:10.1016/j.apsusc.2018.01.236.
- P. Qin, A. L. Domanski, A. K. Chandiran, R. Berger, H.-J. Butt, M. I. Dar, T. Moehl, N. Tetreault, P. Gao, S. Ahmad, M. K. Nazeeruddin and M. Grätzel, Yttrium-Substituted Nanocrystalline TiO2 Photoanodes for Perovskite Based Heterojunction Solar Cells, Nanoscale, 2014, 6(3), 1508–1514, 10.1039/C3NR05884K.
- S. Teo, Z. Guo, Z. Xu, C. Zhang, Y. Kamata, S. Hayase and T. Ma, The Role of Lanthanum in a Nickel Oxide-Based Inverted Perovskite Solar Cell for Efficiency and Stability Improvement, ChemSusChem, 2019, 12(2), 518–526, DOI:10.1002/cssc.201802231.
- L. Liang, M. Liu, Z. Jin, Q. Wang, H. Wang, H. Bian, F. Shi and S. Liu, Optical Management with Nanoparticles for a Light Conversion Efficiency Enhancement in Inorganic γ-CsPbI3 Solar Cells, Nano Lett., 2019, 19(3), 1796–1804, DOI:10.1021/acs.nanolett.8b04842.
- X. Deng, C. Zhang, J. Zheng, X. Zhou, M. Yu, X. Chen and S. Huang, Highly Bright Li(Gd,Y)F4:Yb,Er Upconverting Nanocrystals Incorporated Hole Transport Layer for Efficient Perovskite Solar Cells, Appl. Surf. Sci., 2019, 485, 332–341, DOI:10.1016/j.apsusc.2019.04.226.
- F. Xu, Y. Sun, H. Gao, S. Jin, Z. Zhang, H. Zhang, G. Pan, M. Kang, X. Ma and Y. Mao, High-Performance Perovskite Solar Cells Based on NaCsWO3@ NaYF4@NaYF4:Yb,Er Upconversion Nanoparticles, ACS Appl. Mater. Interfaces, 2021, 13(2), 2674–2684, DOI:10.1021/acsami.0c19475.
- J. Zhang, Q. Yang, C. Gao, X. Wang, C. Gao and X. Liu, Copper Doped Lanthanum Hydroxide Nanorods as a Low Temperature Processable Hole Transport Material for Perovskite Solar Cells, J. Power Sources, 2024, 590, 233797, DOI:10.1016/j.jpowsour.2023.233797.
- F. Qi, Y. Xiao, Z. Yu, P. Liu, S. Kong, F. Li, H. Zhang, Y. Wang and X.-Z. Zhao, Performance Enhancement of Hole-Transport Material Free Perovskite Solar Cells with TiO2 Nanorods Modified with SiO2/NaYF4:Yb,Er@SiO2 for Upconversion and Charge Recombination Suppression, Org. Electron., 2019, 73, 152–158, DOI:10.1016/j.orgel.2019.06.009.
- J. Jia, J. Dong, J. Lin, Z. Lan, L. Fan and J. Wu, Improved Photovoltaic Performance of Perovskite Solar Cells by Utilizing Down-Conversion NaYF4:Eu3+ Nanophosphors, J. Mater. Chem. C, 2019, 7(4), 937–942, 10.1039/C8TC05864D.
- M. Wang, Y. Wu, F. Juan, Y. Li, B. Shi, F. Xu, J. Jia, H. Wei and B. Cao, Enhanced Photocurrent of Perovskite Solar Cells by Dual-Sensitized β-NaYF4:Nd3 +/Yb3 +/Er3+ up-Conversion Nanoparticles, Chem. Phys. Lett., 2021, 763, 138253, DOI:10.1016/j.cplett.2020.138253.
- M. Schoenauer Sebag, Z. Hu, K. de Oliveira Lima, H. Xiang, P. Gredin, M. Mortier, L. Billot, L. Aigouy and Z. Chen, Microscopic Evidence of Upconversion-Induced Near-Infrared Light Harvest in Hybrid Perovskite Solar Cells, ACS Appl. Energy Mater., 2018, 1(8), 3537–3543, DOI:10.1021/acsaem.8b00518.
- W. Bi, Y. Wu, C. Chen, D. Zhou, Z. Song, D. Li, G. Chen, Q. Dai, Y. Zhu and H. Song, Dye Sensitization and Local Surface Plasmon Resonance-Enhanced Upconversion Luminescence for Efficient Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2020, 12(22), 24737–24746, DOI:10.1021/acsami.0c04258.
- P. Song, P. Zhu and C. Zhang, Sm3+–Ce3+-Doped Glass-Ceramic Waveguide as Reduced Ultraviolet Light Induced Degradation and Improved Photon Harvesting for Perovskite Solar Cells, J. Alloys Compd., 2018, 731, 1009–1013, DOI:10.1016/j.jallcom.2017.10.143.
- D. Ma, Y. Shen, T. Su, J. Zhao, N. U. Rahman, Z. Xie, F. Shi, S. Zheng, Y. Zhang and Z. Chi, Performance Enhancement in Up-Conversion Nanoparticle-Embedded Perovskite Solar Cells by Harvesting near-Infrared Sunlight, Mater. Chem. Front., 2019, 3(10), 2058–2065, 10.1039/C9QM00311H.
- J. Dou, C. Zhu, H. Wang, Y. Han, S. Ma, X. Niu, N. Li, C. Shi, Z. Qiu, H. Zhou, Y. Bai and Q. Chen, Synergistic Effects of Eu-MOF on Perovskite Solar Cells with Improved Stability, Adv. Mater., 2021, 33(39), 2102947, DOI:10.1002/adma.202102947.
- C. Shi, S. Xiao, Z. Wang, W. Xiang, R. Wu, X. Yu, L. Ma, Z. Qin, L. Xiong, Q. Liu, X. Jiang, G. Fang and P. Qin, Interface Modification via Rare Earth Material to Assist Hole Migration for Efficiency and Stability Promotion of Perovskite Solar Cells, Sol. RRL, 2023, 7(21), 2300461, DOI:10.1002/solr.202300461.
- L. Liang, Y. Liu and X.-Z. Zhao, Double-Shell β-NaYF4:Yb3 +, Er3 +/SiO2/TiO2 Submicroplates as a Scattering and Upconverting Layer for Efficient Dye-Sensitized Solar Cells, Chem. Commun., 2013, 49(38), 3958–3960, 10.1039/C3CC41252K.
- Z. Wang, Z. Quan and J. Lin, Remarkable Changes in the Optical Properties of CeO2 Nanocrystals Induced by Lanthanide Ions Doping, Inorg. Chem., 2007, 46(13), 5237–5242, DOI:10.1021/ic0701256.
- B. J. Moon, S. J. Kim, S. Lee, A. Lee, H. Lee, D. S. Lee, T. Kim, S. Lee, S. Bae and S. H. Lee, Rare-earth-element-ytterbium-substituted Lead-free Inorganic Perovskite Nanocrystals for Optoelectronic Applications, Adv. Mater., 2019, 31(33), 1901716 CrossRef.
- F. Alam, K. D. Wegner, S. Pouget, L. Amidani, K. Kvashnina, D. Aldakov and P. Reiss, Eu2+: A Suitable Substituent for Pb2+ in CsPbX3 Perovskite Nanocrystals?, J. Chem. Phys., 2019, 151(23), 231101, DOI:10.1063/1.5126473.
- J. Huang, T. Lei, M. Siron, Y. Zhang, S. Yu, F. Seeler, A. Dehestani, L. N. Quan, K. Schierle-Arndt and P. Yang, Lead-Free Cesium Europium Halide Perovskite Nanocrystals, Nano Lett., 2020, 20(5), 3734–3739, DOI:10.1021/acs.nanolett.0c00692.
- D. B. Mitzi and K. Liang, Preparation and Properties of (C4H9NH3)2EuI4:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Luminescent Organic−Inorganic Perovskite with a Divalent Rare-Earth Metal Halide Framework, Chem. Mater., 1997, 9(12), 2990–2995, DOI:10.1021/cm970352d.
A Luminescent Organic−Inorganic Perovskite with a Divalent Rare-Earth Metal Halide Framework, Chem. Mater., 1997, 9(12), 2990–2995, DOI:10.1021/cm970352d.
| This journal is © The Royal Society of Chemistry 2024 |
